Abstract
Study Objectives:
Histamine neurons comprise a major component of the aminergic arousal system and significantly influence sleep-wake states, with antihistamines widely used as sedative hypnotics. Unlike the serotonergic and noradrenergic components of this arousal system, however, the role of histamine in the central control of respiratory motor activity has not been determined. The aims of this study were to characterize the effects of histamine receptor agonists and antagonists at the hypoglossal motor pool on genioglossus muscle activity across sleep and awake states, and also determine if histamine contributes an endogenous excitatory drive to modulate hypoglossal motor outflow to genioglossus muscle.
Design, Participants, and Interventions:
Thirty-three rats were implanted with electroencephalogram and neck electrodes to record sleep-wake states, and genioglossus and diaphragm electrodes for respiratory muscle recordings. Microdialysis probes were inserted into the hypoglossal motor nucleus.
Measurements and Results:
Histamine at the hypoglossal motor nucleus significantly increased tonic genioglossus muscle activity in wakefulness, non-REM sleep and REM sleep. The activating effects of histamine on genioglossus muscle activity also occurred with a histamine type-1 (H1) but not H2 receptor agonist. However, H1 receptor antagonism at the hypoglossal motor nucleus did not decrease genioglossus muscle activity in wakefulness or sleep.
Conclusions:
The results suggest that histamine at the hypoglossal motor pool increases genioglossus muscle activity in freely behaving rats in wakefulness, non-REM, and REM sleep via an H1 receptor mechanism.
Citation:
Bastedo T; Chan E; Park E; Liu H; Horner RL. Modulation of genioglossus muscle activity across sleep-wake states by histamine at the hypoglossal motor pool. SLEEP 2009;32(10):1313-1324.
Keywords: Sleep, genioglossus muscle, histamine, hypoglossal motor nucleus
THE AMINERGIC ASCENDING AROUSAL SYSTEM, COMPRISING SEROTONERGIC, NORADRENERGIC AND HISTAMINERGIC NEURONS, EXERTS IMPORTANT influences on behavior, alertness, and sleep-wake cycling.1–4 The serotonergic and noradrenergic components of this arousal system have been well characterized in the context of sleep1,4 and motor control,5 including modulation of medullary respiratory motor activity.6–11 In contrast, although histaminergic neurons are also known to significantly modulate behavioral arousal12,13 and sleep-wake states,4 the effects of histamine on respiratory motor activity have been little studied.
Histaminergic neurons project widely in the central nervous system, and the tuberomammillary nuclei in the posterior hypothalamus are one source of these projections.14–16 In addition to the major projections of histaminergic neurons to brainstem areas involved in sleep-wake regulation, histamine neurons also project to brainstem regions containing respiratory neurons and motoneurons.16,17 However, the physiological role of histamine in respiratory control is not well established. What is known is that histamine and histamine type-1 (H1) receptor agonists applied to the whole respiratory network in vitro leads to increased respiratory rate, whereas H2 receptor agonism has no effect.18 In vivo, systemically applied histamine increases the ventilatory response to hypercapnia in conscious goats,19 and unrestrained H1 receptor knockout mice show reduced respiratory rate responses to hypercapnia.20 However, because histamine is an important modulator of wakefulness,14,21 it is unknown whether these altered respiratory responses following manipulation of histamine levels were indicative of a role for histamine operating at the level of at the respiratory network per se and/or influencing breathing via altered sleep-wake states or alertness.
Importantly, however, although motoneurons are the final common pathway for the influence of the central nervous system on motor behavior,22 the effects of histamine on respiratory motor activity have not yet been characterized in vitro,5 and there has been only one study performed in vivo. That study reports activation of genioglossus (GG) muscle with histamine applied to the hypoglossal motor pool in cats, but the receptor subtypes mediating that effect and the responses to histamine receptor antagonists (to determine the presence of an endogenous excitatory histaminergic drive) were not investigated.23 Since histamine neurons show tonic activity in awake, freely behaving animals,12,24,25 histamine is positioned to contribute an endogenous excitatory input to respiratory motoneurons. Determination of histamine mechanisms operating at a respiratory motor pool has potentially important clinical relevance given that approximately 3% of North American adults use over-the-counter sleep-promoting agents, primarily antihistamines.26
We have developed an animal model for pharmacological manipulation of the hypoglossal motor pool in freely behaving rats to determine mechanisms modulating motor outflow to the GG muscle of the tongue.7,27 This model has helped to characterize the mechanisms modulating respiratory motor activity in vivo, with the GG being a relevant focus, as it is involved in maintaining an open airspace and preventing obstructive sleep apnea in humans.28 Since H1 and H2 receptors have been identified in the medulla,17,29,30 the aim of this study was to test the hypothesis that histamine at the hypoglossal motor pool will increase GG activity in wakefulness and sleep via an H1 and/or H2 receptor mechanism. We also tested the hypothesis that histamine contributes an endogenous excitatory drive to GG muscle.
METHODS
Procedures conformed to the recommendations of the Canadian Council on Animal Care and the University of Toronto Animal Care Committee approved the protocols. Rats were housed individually, maintained on a 12–12 hr light/dark cycle (lights on at 07:00), and had free access to food and water.
Anesthesia and Surgical Procedures
Experiments were performed on 33 male Wistar rats (mean body weight = 264 g, range 210–298 g). Sterile surgery was performed under anaesthesia induced with intraperitoneal ketamine (85 mg/kg) and xylazine (15 mg/kg) as described previously.7 Rats were intraperitoneally injected with buprenorphine (0.03 mg/kg) to minimize potential postoperative pain, atropine sulphate (1 mg/kg) to minimize airway secretions, and saline (3 mL, 0.9%) for fluid loading. An anaesthesia mask was placed over the snout and the rats breathed a 50:50 mixture of room air and oxygen. Any additional anaesthesia during surgery was then given by inhalation (isoflurane 0.2–2%). Effective anaesthesia was judged by abolition of pedal withdrawal and corneal blink reflexes. With the rats supine the ventral surface of the GG muscle was exposed via a submental incision and dissection of the overlying geniohyoid and mylohyoid muscles. Two insulated, multistranded stainless steel wires (AS631; Cooner Wire, Chatsworth, CA, USA) were implanted bilaterally into the GG muscle and secured with sutures and tissue glue. Observation of tongue movement in response to electrical stimulation (0.4–0.6 V) during surgery was also used to confirm electrode placements. We have also shown in previous experiments that tongue muscle activity is markedly decreased, and almost abolished, after section of the medial branches of the hypoglossal nerve, showing that recordings were predominantly from the GG muscle with these electrode placements.31 To record diaphragm electromyogram (EMG) activity, 2 insulated, multistranded stainless steel wires (AS636: Cooner Wire) were sutured onto the costal diaphragm via an abdominal approach. The size, configuration and placement of the GG and diaphragm electrodes were consistent across experiments. To further ensure adequate electrode placements during surgery, both the GG and diaphragm signals were monitored on chart (Grass Model 79D polygraph, 7P511 amplifiers) and loudspeaker (AM8 Audio Amplifier, Grass) to document respiratory-related activity. The GG and diaphragm wires were tunnelled subcutaneously to a small incision on the skull and the submental and abdominal incisions were closed with absorbable sutures.
The rats were placed in a stereotaxic apparatus (Kopf Model 962, Tujunga, CA, USA) with blunt ear bars. To ensure consistent positioning between animals, the flat skull position was achieved with an alignment tool (Kopf model 944). To record the electroencephalogram (EEG), 2 stainless steel screws (1.5-mm diameter) at-tached to insulated wires (30 gauge) were attached to the skull.7 Two insulated, multistranded stainless steel wires were also sutured onto the dorsal neck muscles to record neck muscle activity. Microdialy-sis guides (CMA/11, Chromatography Sciences Company Inc. St. Laurent, QC, CA) were inserted through a small hole drilled at the junction of the interparietal and occipital bones. The guides were implanted 13.97 ± 0.09 mm (mean ± SEM) posterior to bregma, 0.13 ± 0.01 mm lateral to the midline and aimed 3 mm above the hypoglossal motor nucleus by lowering to 6.90 ± 0.06 mm ventral to bregma. An internal cannula was placed inside the guide to keep it open until the day of experiment. At the end of surgery, the electrodes were con-nected to pins inserted into a miniature plug (STC-89PI-220ABS, Carleton University, Ottawa, Canada). The plug and microdialysis guides were then fixed to the skull with dental acrylic and anchor screws. Upon comple-tion of surgery, the rats were transferred to a clean cage and kept warm under a heating lamp until full recovery as judged by normal motor activity, drinking and eating. The rats were given soft food for the first day after surgery. The rats were then housed individually and recovered for an average of 7.5 days (range, 6-9 days) be-fore the experiments were performed.
Recording Procedures
For recordings, a lightweight shielded cable was connected to the plug on the rat's head. The cable was attached to a counterbalanced swivel that permitted free movement. All rats were studied in a noise-attenuated, electrically shielded cubicle (EPC-010, BRS/LVE Inc. Laurel, MD, USA) and supplied with fresh bedding, food and water. A video camera inside the cubicle allowed for continuous monitoring without disturbing the animal. For habituation, the rats were connected to the cable and swivel apparatus the day before the experiments.
The electrical signals were amplified and filtered (Super-Z head-stage amplifiers and BMA-400 amplifiers/filters, CWE Inc., Ardmore, PA, USA). The EEG was filtered between 1 and 100 Hz, whereas the neck, GG and diaphragm EMGs were filtered between 100 and 1000 Hz. The electrocardiogram was removed from the diaphragm EMG using an oscilloscope and electronic blanker (Model SB-1, CWE Inc.). The moving-time averages of the neck, GG, and diaphragm EMGs were also obtained (Model MA 821, CWE Inc., time constant = 200 ms). The raw EEG and GG signals and the moving-time averages of the GG, diaphragm, and neck EMGs were digitized and recorded on computer (Spike 2 software, 1401 interface, CED Ltd, Cambridge, UK).
Microdialysis
On the day of the experiments, the internal cannula was removed from the guide and the microdialysis probe (CMA/11 14/01) was inserted. The probes projected 3 mm from the tip of the guide and were therefore targeted to the hypoglossal motor nucleus. The probes were 240 μm in diameter with a 1-mm long membrane that had a 6000 Dalton cut-off. The rats were gently restrained during the insertion of the microdialysis probe through the guide, and showed no evidence of any discomfort during this procedure. In each rat, a burst of GG activity was observed when the probe initially penetrated the hypoglossal motor nucleus. This was useful as a preliminary confirmation of probe placement.7,27 The burst of GG activity observed during probe insertion was transient and typically lasted < 5 min and did not occur in the neck or diaphragm signals.7,27 Each probe was connected to FEP Teflon tubing (inside diameter, 0.12 mm) with this tubing connected to 1.0 mL syringes via a zero dead-space switch (Uniswitch, BAS, West Lafayette, IN, USA). The probes were continually flushed with artificial cerebrospinal fluid (ACSF) at a flow rate of 2.1 μL/min using a syringe pump and controller (MD-1001 and MD-1020, BAS). The composition (mM) of ACSF was: NaCl (125), KCl (3), KH2PO4 (1), CaCl2 (2), MgSO4 (1), NaHCO3 (25), and D-glucose (30). The ACSF was warmed to 37°C and bubbled with CO2 to a pH of 7.39 ± 0.002.
Protocol
All experiments began the morning of probe insertion (mean time = 08:19 h, range 08:09–08:34 h), and were performed during the day when the rats normally sleep. Signals were monitored continuously during microdialysis drug delivery of ACSF into the hypoglossal motor nucleus (i.e., control) and during drug administration (see below). For each condition, data were obtained for ≥ 2 full sleep cycles (i.e., periods containing quiet wakefulness, non-REM, and REM sleep). A period of at least 45 min was allowed to elapse before sleep-wake states and respiratory muscle activities were analyzed after probe insertion and switching the perfusion medium.7
Study 1: Histamine at the hypoglossal motor nucleus.
Experiments were performed in seven rats to determine if histamine at the hypoglossal motor nucleus increased GG activity in wakefulness, non-REM, and REM sleep. Respiratory muscle activities were recorded during dialysis of ACSF at the hypoglossal motor nucleus followed by dialysis delivery of histamine dihydrochloride dissolved in ACSF (1.0 mM, pH = 7.32 ± 0.02, Formula Weight (FW) = 184, Sigma, St. Louis, MO). In initial experiments in urethane-anesthetized rats this concentration of histamine produced robust increases in GG activity, and similar responses occurred in the conscious rats (see Results).
Study 2: H1 receptor agonism at the hypoglossal motor nucleus.
Experiments in a separate group of seven rats were performed to determine if dialysis delivery of an H1 receptor agonist to the hypoglossal motor nucleus would also increase GG activity across sleep-wake states. Respiratory muscle activities were recorded during dialysis of ACSF at the hypoglossal motor nucleus followed by dialysis delivery of 2-(2-pyridyl)ethylamine (PEA) (0.1 and 1.0 mM, pH = 7.61 ± 0.01 and 8.08 ± 0.02 respectively, FW = 122, Sigma). The concentrations were chosen because of preliminary studies in anesthetized rats, and with the concentration of 1 mM also corresponding to the concentration of histamine used in Study 1.
Study 3: H2 receptor agonism at the hypoglossal motor nucleus.
Experiments were performed in a total of 9 rats to determine if dialysis delivery of an H2 receptor agonist to the hypoglossal motor nucleus would also increase GG activity across sleep-wake states. Respiratory muscle activities were recorded during dialysis of ACSF at the hypoglossal motor nucleus followed by dialysis delivery of dimaprit dihydrochloride (0.1 and/or 1.0 mM, pH = 7.57 ± 0.03 and 7.54 ± 0.03 respectively, FW = 234, Tocris Bioscience, Bristol, UK). Six rats were studied with 0.1mM dimaprit and 6 were studied with 1 mM dimaprit, with 3 rats receiving both doses (i.e., a total of 9 rats were included in Study 3). The concentrations of dimaprit corresponded to those of histamine and PEA used above.
Study 4: H1 receptor antagonism at the hypoglossal motor nucleus.
Following identification of an H1 receptor-mediated excitation of hypoglossal motor outflow to GG muscle (see Results), further experiments were performed in 10 rats to determine if an endogenous H1-mediated tonic excitatory drive contributes to GG activation across sleep-wake states. Respiratory muscle activities were recorded during dialysis of ACSF at the hypoglossal motor nucleus followed by dialysis delivery of the H1 receptor antagonist diphenhydramine hydrochloride dissolved in ACSF (1.0 mM, pH = 7.55 ± 0.01, FW = 292, Sigma).
The concentration of the H1 receptor antagonist corresponded to the concentration of histamine and PEA used in Studies 1 and 2, and is consistent with our previous experiments using antagonists for other components of the aminergic arousal system.7,8 Moreover, this concentration of diphenhydramine was also chosen based on preliminary experiments using 6 isoflurane-anesthetized and tracheotomized rats (weight range 250–290 g) with microdialysis probes implanted into the hypoglossal motor nucleus (13.78 ± 0.03 mm posterior to bregma, 0.08 ± 0.03 mm lateral to the midline, and 9.72 ± 0.23 mm ventral to bregma), using techniques previously described.32,33 The microdialysis probes were perfused with ACSF for at least 30 min followed by 1 mM histamine with and without co-application of the H1 receptor antagonist. These preliminary studies showed that application of histamine to the hypoglossal motor nucleus caused a marked increase in tonic GG muscle activity (mean increase = 1080% ± 238% compared to the ACSF controls, t5 = 5.17, P = 0.007), but no significant increase in GG activity occurred in response to histamine in the presence of the H1 receptor antagonist diphenhydramine (t5 = 1.22, P = 0.291).
Tests for Each Experiment and Histology
At the end of each experiment, 5-hydroxytryptamine (5-HT, creatinine sulphate complex, Sigma, FW: 387, 10 mM) or histamine (1 mM) was applied to the hypoglossal motor nucleus as a positive control to confirm that it was still functional as judged by the expected increase in GG activity.7,27 The rats were then re-anesthetized with ketamine and xylazine (85 and 15 mg/kg respectively, as described above), and tongue movement was tested again in response to stimulation of the GG electrodes (0.3–0.9 V). The rats were then given an overdose of sodium pentobarbital by intraperitoneal injection (100 mg/100 g) and perfused intracardially with 50 mL of 0.9% saline and 40 mL of 10% formalin. To mark the lesion site left by the microdialysis probe, potassium permanganate (1%) was microinjected for 10 min at 2.1 μL/min via a microdialysis probe with the membrane cut at the tip.34 The medullary regions were blocked, transferred to 30% sucrose and cut in 50-mm coronal sections with a cryostat (Leica, CM 1850, Nussloch, Germany). Each section with the lesion site was mounted and stained with Neutral Red. Microdialysis sites were localized from the stained sections and marked on standard brain maps.35
Data Analysis
Analysis of EMG and EEG Signals
Analyses were performed as previously described.32 The EMGs were analyzed every 5-sec from the respective moving-time average signals (above electrical zero). Electrical zero was the voltage recorded with the amplifier inputs grounded. The GG and diaphragm signals were analyzed breath-by-breath, corresponding to approximately 7 to 10 breaths for each 5-sec epoch. For each breath in each 5-sec epoch, the analysis of the GG signal was time-locked to breathing as defined by the peak and trough of the diaphragm signal. Visual inspection of the computer-identified peaks and troughs of the diaphragm and GG signals was performed during the automated analysis, and any artefacts were discarded and not used in the analysis. Peak inspiratory GG activity was defined as the peak moving-time average of the GG signal spanning diaphragm inspiration. Tonic activity was defined as the minimal activity in expiration. For each 5-sec epoch, GG activity was then quantified as mean tonic activity (i.e., the mean minimal activity in expiration from the number of breaths in that epoch) and the mean amplitude of respiratory-related activity (i.e., mean peak inspiratory activity - tonic activity). Tonic GG activity included both basal tone and any background spontaneous activity that may occur during certain behaviors, e.g., twitches in REM sleep or behaviors in active wakefulness. Likewise, although clear phasic variations in GG activity can occasionally occur in behaviors such as REM sleep or active wakefulness, whether the activity that occurs in synchrony with the diaphragm is truly “respiratory” or random activation caused by behavioral or REM sleep processes cannot be determined with this method, but such activations do not affect measurements in quiet wakefulness or non-REM sleep.
Mean neck muscle activity, diaphragm amplitude, and respiratory rate were also calculated in consecutive 5-sec bins for all the periods of sleep and wakefulness in each rat. The EEG was sampled by computer at 500 Hz then analyzed on overlapping segments of 1024 samples, windowed using a raised cosine (Hamming) function and subjected to a fast Fourier transform to yield the power spectrum. The window was advanced in steps of 512 samples, and the mean power spectrum of the EEG signal for each 5-sec epoch was calculated. The power contained within 6 frequency bands was recorded as absolute power and as a percentage of the total power of the signal. The band limits were δ2 (0.5–2 Hz), δ1 (2–4 Hz), θ (4–7.5 Hz), α (7.5–13.5 Hz), β1 (13.5–20 Hz) and β2 (20–30 Hz); the ratio of high (20–30 Hz) to low (0.5–2 Hz) frequency activity was also calculated as a relative index of EEG activation.36 Overall, the analysis provided breath-by-breath measurements of GG and diaphragm activities, as well as mean neck and EEG frequencies. These variables were calculated and averaged for all the 5-sec bins occurring across sleep-wake states in each rat (see below).
Measurements across Sleep and Awake States
The moving-time averages of the GG, neck, and diaphragm EMGs were analyzed in established periods of quiet wakefulness, non-REM, and REM sleep as identified by standard EEG and EMG criteria.7 Data were selected for analysis at least 45 min after insertion of the microdialysis probe or switching between perfusion mediums, with the data analyzed over the subsequent 2 to 3 h. During this time, measurements were made during all periods of quiet wakefulness (i.e., in the absence of overt behaviors such as eating, drinking or grooming), non-REM sleep and REM sleep (> 30 s). Data were included in the analyses only if they were obtained during such unequivocal and clearly defined states. Data obtained during periods of active wakefulness (i.e., with movements and overt behaviors), and transitional states (e.g., drowsiness, arousals from sleep, and transitions from non-REM to REM sleep) were not included in the analysis. To minimize bias when selecting the periods for analysis, the EEG and neck EMG were scored without reference to the GG or diaphragm signals. Each rat served as its own control, with all interventions performed in one experiment, which allowed for consistent effects of experimental condition (i.e., ACSF or drug intervention) to be observed across sleep and awake states within and between rats. Accordingly, for each animal, an overall mean value was calculated for each variable for each sleep-wake state and for each drug delivered to the hypoglossal motor nucleus. These individual mean values obtained in each rat, themselves derived from the population of values that occurred for all of the 5-sec epochs that occurred in each sleep-wake state with each drug applied to the hypoglossal motor pool, were then used in the statistical analyses.
Statistical Analysis
For all comparisons, differences were considered significant if the null hypothesis was rejected at P < 0.05 using a 2-tailed test. Statistics were performed using analysis of variance with repeated measures (ANOVA-RM) and post hoc t-tests were performed using Bonferroni corrected P values. Data were tested for normality using the Kolmogorov-Smirnov test, and if any data set were not normally distributed then analyses were performed on the log transformed data.37 Analyses were performed using Sigmastat (Jandel Scientific, San Rafael, CA, USA). Data are expressed as mean ± SEM.
RESULTS
Location of Microdialysis Probes and Hypoglossal Motor Function
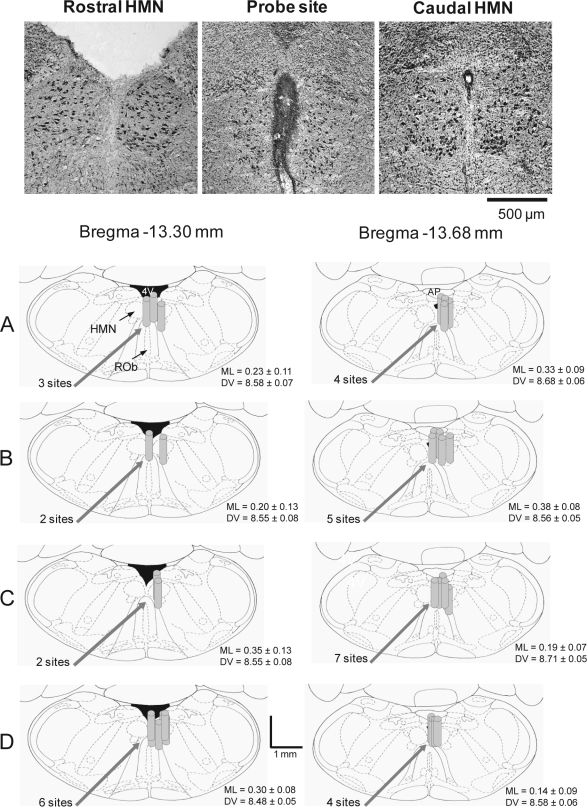
Figure 1 shows an example of a lesion site made by a microdialysis probe, as well as the distribution of individual microdialysis sites from all rats in the different experiments. Microdialysis probes were successfully implanted into, or immediately adjacent to, the hypoglossal motor nuclei in all animals. Figure 1 includes the mean stereotaxic coordinates for the center of each microdialysis site. There was no statistical difference in medial-lateral or dorsal-ventral coordinates between Studies 1–4 (each F3 < 1.55, P > 0.226, 1-way ANOVAs), and the coordinates also did not vary between the two stereotaxic planes (each F1 < 4.01, P > 0.056, 1-way ANOVAs). Tongue movement occurred in each rat in response to electrical stimulation of the GG electrodes both at the time of surgery and after the experiments, showing that these electrodes were in place throughout the study. Each rat also showed consistent GG responses to serotonin or histamine applied to the hypoglossal motor nucleus at the end of the experiments, indicating an intact motor pool that was able to respond to manipulation of neurotransmission.7,27
Figure 1.
Example and group data showing location of the microdialysis probes from all the experiments. The top images show histological sections with a lesion site made by the microdialysis probe immediately adjacent to both hypoglossal motor nuclei. Also shown are coronal diagrams from the rat medulla,35 illustrating the distribution of individual microdialysis sites from all rats administered (A) histamine, (B) the H1 receptor agonist 2-(2-pyridyl)ethylamine, (C) the H2 receptor agonist dimaprit, and (D) the H1 receptor antagonist diphenhydramine. The microdialysis probe locations are represented by gray cylinders which are drawn to scale. Overlap obscures some of the dialysis sites. The mean stereotaxic coordinates for the center of the probe sites are given in the medial-lateral (ML) and dorsal-ventral (DV) directions for each study and for each plane posterior to bregma. Abbreviations: AP, area postrema; HMN, hypoglossal motor nucleus; 4V, fourth ventricle.
Study 1: Histamine at the Hypoglossal Motor Nucleus
For Study 1, a total of 4921 5-sec epochs (i.e., a total of 6.83 h of data) were included in the analysis, of which 874 epochs were from periods of quiet wakefulness, 3386 epochs were from non-REM sleep, and 661 epochs were from REM sleep. Of these epochs, 2191 and 2730 were obtained with ACSF and histamine at the hypoglossal motor pool, respectively.
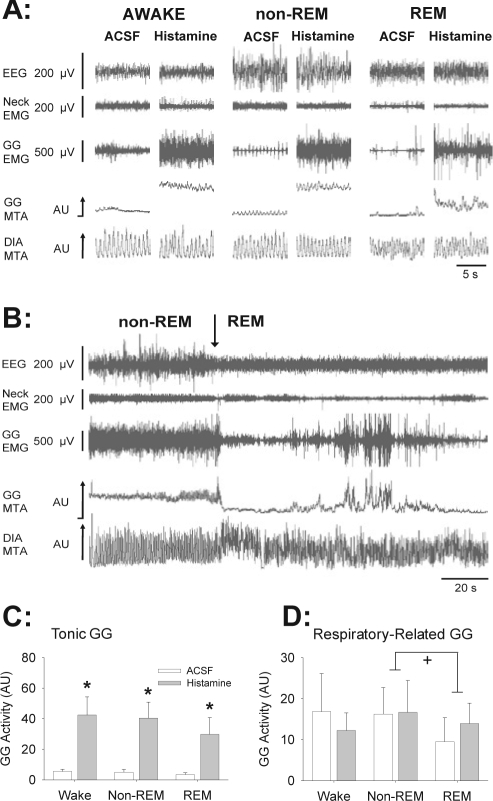
Figure 2A shows an example of GG responses to microdialysis delivery of histamine into the hypoglossal motor pool. Compared to ACSF controls, histamine increased tonic GG activity in wakefulness, non-REM, and REM sleep. The persistence of GG activation in REM sleep is notable because it is in contrast to the robust suppression and periods of complete abolition of GG activity that can occur in REM sleep with serotonin or α1 receptor agonism at the hypoglossal motor pool at equivalent or higher concentrations (10 and 1 mM, respectively).7,27 Nevertheless, despite the persistence of tonic GG activation in REM sleep in the presence of histamine, GG activity was still decreased compared to levels observed in the preceding period of non-REM sleep (e.g., see raw GG activity in Figure 2B).
Figure 2.
Stimulating effects of histamine at the hypoglossal motor pool on genioglossus (GG) muscle activity across sleep-wake states. (A) Traces show the electroencephalogram (EEG), neck electromyogram (EMG), GG, and diaphragm (DIA) EMG signals. The GG and DIA signals are also displayed as their moving-time averages (MTA) in arbitrary units (AU). The baseline of the integrator (i.e., electrical zero) is shown for the GG MTA. Compared to artificial cerebrospinal fluid (ACSF) controls, histamine increased tonic GG activity across all sleep-wake states. (B) Traces show that despite the persistence of tonic GG activation in REM sleep with histamine, GG activity was still decreased in REM compared to levels observed in the preceding period of non-REM sleep. (C and D) Group data showing a significant excitatory effect of histamine on tonic GG activity in wakefulness, non-REM, and REM sleep (C); but with no effects on respiratory related GG activity (D). The symbols * and + indicate P < 0.05 compared to ACSF controls, and between the indicated sleep-wake states, respectively. The suppression of respiratory-related GG activity in REM sleep (panel D) was independent of whether ACSF or histamine was applied to the hypoglossal motor pool. All data are shown as mean + SEM (n = 7 rats). The mean values for each individual rat were first calculated from the population of values that occurred for all the 5-sec epochs during each sleep-wake state with each drug applied to the hypoglossal motor pool. The means from each individual rat in each condition were then averaged to yield the grand means for the group which are shown in the figure. See text for further details.
GG Responses—Group Data
Figure 2C shows there was a significant stimulating effect of histamine on tonic GG activity (F1,6 = 11.52, P = 0.015, 2-way ANOVA-RM) that did not depend on prevailing sleep-wake state (F2,12 = 0.62, P = 0.554), i.e., the increase occurred across wakefulness, non-REM sleep, and REM sleep. Figure 2D shows that unlike tonic GG activity, there was no effect of histamine on respiratory-related GG activity (F1,6 = 0.0007, P = 0.980, 2-way ANOVA-RM). There was also a significant effect of sleep-wake state on respiratory-related GG activity (F2,12 = 4.19, P = 0.042, 2-way ANOVA-RM), that did not depend on whether ACSF or histamine was applied to the hypoglossal motor pool (F2,12 = 3.25, P = 0.074). Further analysis showed that respiratory-related GG activity in REM sleep was significantly reduced compared to non-REM sleep (t6 = 2.80, P = 0.048, post hoc paired t-test, Figure 2D). The effect of sleep-wake state on tonic GG activity was of borderline statistical significance (F2,12 = 3.79, P = 0.053).
Specificity of Responses
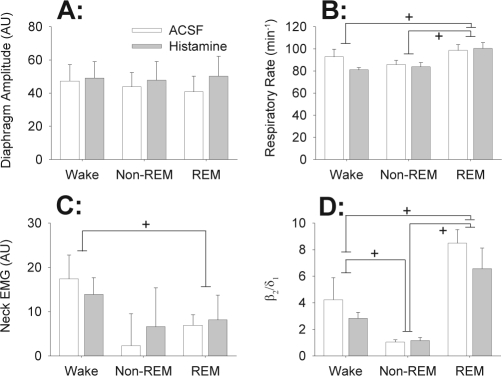
Figure 3 shows that the responses to histamine at the hypoglossal motor pool were specific to the GG muscle as there were no effects on diaphragm amplitude (F1,6 = 3.60, P = 0.107), respiratory rate (F1,6 = 1.86, P = 0.222, 2-way ANOVA-RM) or diaphragm minute activity (i.e., the product of respiratory rate and diaphragm amplitude, a surrogate for neural ventilation, F1,6 = 2.19, P = 0.189). Likewise, histamine at the hypoglossal motor pool also had no effect on the activity of the control postural muscle in the neck (F1,6 = 0.06, P = 0.819). EEG frequencies from 0.5 to 30 Hz, and the ratio of high (20–30 Hz) to low (0.5–2 Hz) frequency activity (i.e., the β2/δ1 ratio,36 Figure 3D), were also not different between histamine and ACSF (all F1,6 < 4.86, all P > 0.070).
Figure 3.
Responses to histamine at the hypoglossal motor pool were specific to the genioglossus muscle as there were no significant effects on (A) diaphragm amplitude, (B) respiratory rate, (C) neck muscle activity and (D) the ratio of high (20–30 Hz) to low (0.5–2 Hz) frequency activity in the electroencephalogram (i.e., the β2/δ1 ratio). All data are shown as mean + SEM (n = 7 rats). Data were averaged as described in Figure 2 and the Methods. The symbol + indicates P < 0.05 between the indicated sleep-wake states. The changes in these variables were independent of whether ACSF or histamine was applied to the hypoglossal motor pool. See text for further details.
The independent effects of sleep-wake states on respiratory variables (e.g., increased respiratory rates in REM sleep) and sleep data (e.g., changes in EEG frequencies and neck EMG) were typically as previously described.27,36,38 There was no significant effect of sleep-wake state on the amplitude of diaphragm activity (F2,12 = 0.542, P = 0.595, 2-way ANOVA-RM). In contrast, there was a significant effect of sleep-wake state on respiratory rate (F2,12 = 9.97, P = 0.003, 2-way ANOVA-RM), with respiratory rate in REM sleep being increased compared to both non-REM sleep and quiet wakefulness (both t6 > 3.52, P < 0.013, post hoc paired t-tests, Figure 3B). This effect of sleep-wake state on respiratory rate did not depend on whether ACSF or histamine was applied to the hypoglossal motor pool (F2,12 = 1.31, P = 0.305). Neck EMG also varied across sleep-wake states (F2,12 = 5.61, P = 0.019), with activity in REM sleep being significantly reduced compared to quiet wakefulness (t6 = 3.20, P = 0.023, Figure 3C). The ratio of high (β2, 20–30 Hz) to low (δ1, 2–4 Hz) frequency activity in the EEG was also significantly affected by sleep-wake states (F2,12 = 32.57, P < 0.001), with values in REM sleep being significantly increased compared to both quiet wakefulness and non-REM sleep (t6 > 3.40, P < 0.017), and values in quiet wakefulness also being increased compared to non-REM sleep (t6 = 4.64, P = 0.002, Figure 3D). These effects of sleep-wake state on EEG activity were not influenced by the presence of ACSF or histamine at the hypoglossal motor pool (F2,12 = 0.803, P = 0.471).
Study 2: H1 Receptor Agonism at the Hypoglossal Motor Nucleus
For Study 2, a total of 9982 5-sec epochs (i.e. a total of 13.86 h of data) were included in the analysis, of which 1167 epochs were from periods of quiet wakefulness, 7295 epochs were from non-REM sleep, and 1520 epochs were from REM sleep. Of these epochs, 3191, 3428, and 3363 were obtained with ACSF, 0.1, and 1.0 mM PEA at the hypoglossal motor pool, respectively.
As with histamine, the stimulating effects on GG activity also occurred with the H1 receptor agonist PEA, with increased GG activity in wakefulness, non-REM, and REM sleep (compare Figure 4A with Figure 2A). Figure 4B also shows that PEA increased both tonic and respiratory-related GG activities across sleep-wake states (F2,12 = 17.36, P < 0.001; and F2,12 = 5.58, P = 0.020, 2-way ANOVA-RM). Compared to the control levels of GG activity with ACSF at the hypoglossal motor pool, the stimulating effects of the H1 receptor agonist were statistically significant with 1 mM PEA for both tonic and respiratory-related GG activities (t6 = 5.27 and 2.94, respectively, P < 0.001 and < 0.04 respectively, post hoc paired t-tests) but not 0.1 mM PEA (each t6 < 0.30, each P = 1.00, post hoc paired t-tests). There was also a significant effect of sleep-wake state on tonic GG activity (F2,12 = 12.19, P = 0.001, 2-way ANOVA-RM), with activity in both quiet wakefulness and non-REM sleep being increased compared to REM sleep (both t6 > 4.27, P < 0.004, post hoc paired t-tests). Further analysis showed that the effect of sleep on tonic GG activity was influenced by the presence of ACSF or PEA at the hypoglossal motor pool (F4,23 = 3.42, P = 0.025, 2-way ANOVA-RM), with tonic GG activity in the presence of 1mM PEA also being increased in both quiet wakefulness and non-REM sleep compared to REM sleep (both t6 > 4.57, P < 0.001, post hoc paired t-tests, see Figure 4B). There was no effect of sleep-wake state per se on respiratory-related GG activity (F2,12 = 0.344, P = 0.716).
Figure 4.
Similar to the effects of histamine, H1 receptor agonism at the hypoglossal motor pool also increased GG activity across sleep-wake states. (A) Example traces showing that compared to ACSF controls, application of the H1 receptor agonist 2-(2-pyridyl)ethylamine (PEA), increased tonic GG activity across sleep-wake states. (B) Group data showing a significant stimulating effect of PEA on tonic and respiratory-related GG activities in wakefulness, non-REM, and REM sleep. The symbols * and + indicate P < 0.05 compared to ACSF controls, and between the indicated sleep-wake states, respectively. The suppression of tonic GG activity in REM sleep, compared to wakefulness and non-REM sleep (panel B), occurred with 1mM PEA only. See text for further details. All data are shown as mean + SEM (n = 7 rats). Data were averaged as described in Figure 2 and the Methods. Abbreviations are as for Figure 2.
The stimulating effects of PEA at the hypoglossal motor pool were also specific to the GG muscle as there were no effects on respiratory rate, diaphragm amplitude or diaphragm minute activity (all F2,12 < 1.87, all P > 0.197, 2-way ANOVA-RM). Likewise, PEA at the hypoglossal motor pool also had no effect on neck EMG activity (F2,12 = 2.33, P = 0.139), EEG frequencies from 0.5 to 30 Hz (all F2,12 < 2.14, all P > 0.159) and the ratio of high (20–30 Hz) to low (0.5–2 Hz) frequency EEG activity (F2,12 = 3.77, P = 0.053). The independent effects of sleep-wake states on respiratory variables (e.g., increased respiratory rates in REM sleep) and sleep data (e.g., changes in EEG frequencies and neck EMG) were as expected and similar to those reported in detail for Study 1.
Study 3: H2 Receptor Agonism at the Hypoglossal Motor Nucleus
For Study 3, a total of 7948 5-sec epochs (i.e. a total of 11.04 hrs of data) were included in the analysis, of which 1361 epochs were from periods of quiet wakefulness, 5086 epochs were from non-REM sleep, and 1501 epochs were from REM sleep. Of these epochs, 3628, 1969, and 2351 were obtained with ACSF, 0.1, and 1.0 mM dimaprit at the hypoglossal motor pool, respectively.
Figure 5A shows an example of the lack of GG response to microdialysis delivery of the H2 receptor agonist dimaprit into the hypoglossal motor nucleus. The group data in Figure 5B confirmed that there was no effect of either 0.1 or 1 mM dimaprit on tonic or respiratory-related GG activities (all F1,5 < 4.31, all P > 0.093, 2-way ANOVA-RM). Likewise there was no effect of dimaprit on respiratory rate, diaphragm amplitude, diaphragm minute activity, neck EMG activity, and the ratio of high to low EEG frequencies (all F1,5 < 6.08, all P > 0.057, 2-way ANOVA-RM). There was no significant effect of sleep-wake state on tonic GG activity (F2,10 < 1.28, P > 0.321, 2-way ANOVA-RM, Figure 5B), a lack of effect that did not depend on the presence of ACSF or dimaprit at the hypoglossal motor pool (F2,10 < 2.25, P > 0.159, 2-way ANOVA-RM). Nevertheless, there was a significant effect of sleep-wake state on respiratory-related GG activity in the 6 rats studied with ACSF and 0.1 mM dimaprit at the hypoglossal motor pool (F2,10 = 8.39, P = 0.007, 2-way ANOVA-RM), with activity in quiet wakefulness being significantly increased compared to REM sleep (t5 = 4.03, P = 0.007, post hoc paired t-test, Figure 5C). This effect of sleep state did not depend, however, on the presence of either ACSF or 0.1 mM dimaprit at the hypoglossal motor nucleus (F2,10 = 0.722, P = 0.510, 2-way ANOVA-RM), i.e., it occurred in both conditions. The effect of sleep-wake state on respiratory-related GG activity in the 6 rats studied with ACSF and 1mM dimaprit at the hypoglossal motor pool failed to reach statistical significance (F2,10 = 3.33, P = 0.078, 2-way ANOVA-RM). The independent effects of sleep-wake states on the other respiratory variables (e.g., increased respiratory rates in REM sleep) and sleep data (e.g., changes in EEG frequencies and neck EMG) were similar to those reported in detail for Study 1.
Figure 5.
Example traces (A) and group data (B) showing that H2 receptor agonism at the hypoglossal motor pool did not significantly alter GG activity. + indicates P < 0.05 between the indicated sleep-wake states, with the suppression of respiratory-related GG activity in REM sleep (panel C) being independent of whether ACSF or 0.1 mM dimaprit was applied to the hypoglossal motor pool. All data are shown as mean + SEM. Six rats were studied with ACSF and 0.1 mM dimaprit, and 6 were studied with ACSF and 1 mM dimaprit, with 3 rats receiving both doses. Data were averaged as described in Figure 2 and the Methods. Abbreviations are as for Figure 2. See text for further details.
Study 4: H1 Receptor Antagonism at the Hypoglossal Motor Nucleus
For Study 4, a total of 8913 5-sec epochs (i.e. a total of 12.38 h of data) were included in the analysis, of which 1518 epochs were from periods of quiet wakefulness, 6277 epochs were from non-REM sleep, and 1118 epochs were from REM sleep. Of these epochs, 4510 and 4403 were obtained with ACSF and the H1 receptor antagonist diphenhydramine at the hypoglossal motor pool, respectively.
Although both histamine and the H1 receptor agonist at the hypoglossal motor nucleus caused robust GG activation across sleep-wake states (Figures 2 and 4), there was no significant endogenous H1 receptor-mediated tonic excitatory drive to GG muscle because H1 receptor antagonism at the hypoglossal motor nucleus had no effect on tonic or respiratory-related GG activity (F1,9 = 3.25 and 0.343, respectively; P = 0.105 and 0.572, respectively, 2-way ANOVA-RM, see Figure 6). There were no nonspecific effects of H1 receptor antagonism at the hypoglossal motor pool as there were no changes in the control variables, i.e., respiratory rate, diaphragm amplitude, diaphragm minute activity, neck EMG activity, and EEG frequencies (all F1,9 < 3.54, each P > 0.093). There was no significant effect of sleep-wake state on tonic or respiratory-related GG activities (each F2,18 < 1.87, P > 0.182, 2-way ANOVA-RM). The independent effects of sleep-wake states on the other respiratory variables (e.g., increased respiratory rates in REM sleep) and sleep data (e.g., changes in EEG frequencies and neck EMG) were as expected and similar to those reported in detail for Study 1.
Figure 6.
Group data showing that H1 receptor antagonism at the hypoglossal motor pool did not significantly alter tonic (A) or respiratory-related (B) GG activity, indicating a minimal endogenously active H1 receptor mediated excitatory drive in these freely behaving animals across natural sleep-wake states. All data are shown as mean + SEM (n = 10 rats). Data were averaged as described in Figure 2 and the Methods.
DISCUSSION
Major Findings and their Interpretation
This paper contributes to the fields of physiology and sleep by showing that dialysis delivery of histamine and an H1 receptor agonist to the hypoglossal motor pool increases GG muscle activity in freely-behaving rats in wakefulness, non-REM, and REM sleep. The histamine receptor-mediated GG activation observed in wakefulness and non-REM sleep was also largely maintained in REM sleep (Figures 2 and 4), i.e., unlike the periods of atonia that occur in REM sleep despite on-going serotonin and α1 receptor agonism at the hypoglossal motor pool with similar or higher concentrations of agonists.7,27 Nevertheless, despite the significant GG activation produced by histamine and the H1 receptor agonist at the hypoglossal motor pool, there was not a tonic stimulating effect of endogenous histamine on hypoglossal motor activity in this intact behaving preparation in vivo, as judged by the lack of effect of dialysis delivery of an H1 receptor antagonist.
Histaminergic, noradrenergic and serotonergic neurons are the chief components of the ascending aminergic arousal system.1–4,14 That these aminergic neurons have tonic activity in vivo has made them candidates to mediate an endogenous excitatory drive to the hypoglossal motor pool. However, the control of respiratory motoneuron activity by these three major components of the aminergic arousal system has only recently begun to be characterized in vivo, with initial investigations focusing on the role of serotonergic8,9,39–42 and noradrenergic inputs.7,11 In contrast, the role of histamine had not been addressed. Nevertheless, there is one recent study, performed in cats, which has shown activation of GG muscle across sleep-wake states with histamine applied to the hypoglossal motor nucleus by microdialysis drug delivery at a concentration of 5 mM in the perfusion medium,23 i.e., as opposed to the 1 mM used in the present study. However, the receptor subtypes mediating that effect were not addressed, and the effects of an antagonist to determine the presence of an endogenous excitatory drive were not investigated. We have identified that histamine and H1 receptor agonism at the hypoglossal motor pool increases motor activity (Figures 2 and 4). Despite this robust GG activation when histamine is applied to the hypoglossal motor nucleus, however, endogenous histaminergic activity plays a minimal role in the normal modulation of GG activity, as activity did not decrease with concentrations of diphenhydramine that were sufficient to block histamine-induced excitation at the hypoglossal motor nucleus (see Methods).
H1 Receptor Antagonism and Potential Clinical Relevance
The lack of an endogenously active excitatory histaminergic drive to the hypoglossal motor pool is potentially clinically relevant given that antihistamines are the most widely used over-the-counter sleep-promoting agents.26 Accordingly, the results of this study suggest that antihistamines would not be expected to have adverse effects on GG muscle activity or upper airway mechanics, although we are not aware of a formal test of the latter or of any relevant case reports regarding potential worsening of obstructive sleep apnea with antihistamines. In rats, systemically administered diphenhydramine does not alter GG activity,43 in support of the results of the present study with dialysis delivery of diphenhydramine to the hypoglossal motor pool. Nevertheless, systemically administered diphenhydramine does delay arousal from sleep in response to respiratory stimulation,43 suggesting that the sedating effects of antihistamines could potentially worsen obstructive sleep apnea in certain individuals by lengthening the apneas. However, recent evidence suggests that suppression of arousal from sleep may not be detrimental in all obstructive sleep apnea patients (especially if there is no concomitant suppression of pharyngeal motor tone) because arousals themselves can promote ventilatory instability and recurrence of obstructions.44 Overall, these data with H1 receptor antagonism suggest a lack of an endogenously active excitatory histaminergic drive contributing to the normal maintenance of genioglossal motor tone. If this lack of an endogenously active excitatory histaminergic drive also applies to other motor pools, then it would fit with observations in narcoleptic dogs where episodes of muscle atonia during cataplexy are accompanied by cessations of noradrenergic activity despite continued activity of serotonergic and histaminergic neurons.12 These latter data have been taken to suggest that histaminergic neural activity contributes to mechanisms associated with wakefulness and consciousness but not motor tone per se.12
Histamine Receptor-Mediated Activation of Hypoglossal Motor Output and Potential Clinical Relevance
The results of the present study showed that an H1 but not H2 receptor agonist increased hypoglossal motor output to GG muscle (Figures 4 and 5). H1 receptor-mediated neuronal excitation is mediated by activation of Gq/11 protein and phospholipase C, which leads to the formation of the two second messengers—diacylglycerol and inositol-1,4,5-triphosphate (IP3)—thereby producing neuronal excitation via a variety of effector molecules such as increased intracellular Ca2+.14 These same second messengers pathways are also thought to be involved in the activation of hypoglossal motoneurons by serotonin type-2 (5-HT2) and α1 receptor agonists,45 so implicating a common downstream signalling mechanism underlying the aminergic modulation of hypoglossal motor activity. Since these aminergic systems share common downstream signalling mechanisms, it is relevant to note the presence of ongoing H1 receptor-mediated GG activation in REM sleep (Figures 2 and 4), unlike the periods of major suppression and complete atonia that typically occurs in REM despite serotonin and α1 receptor agonists at the hypoglossal motor pool, at similar or even higher concentrations (1 and 10 mM) than histamine.7,27
The mechanisms underlying this apparent difference of being able to sustain residual GG activity during REM sleep in the presence of agonists for these different components of the aminergic arousal system remains to be determined. However, this difference in response is potentially clinically relevant to pharmacological attempts to increase GG activity in sleep, especially REM, to prevent obstructive sleep apnea, which for the serotonin drugs attempted thus far have met with limited success.46,47 However, any attempted pharmacological manipulation of the noradrenergic and/or histaminergic neuronal systems with the aim of increasing pharyngeal muscle tone is likely to be severely hampered by the cardiovascular complications of obstructive sleep apnea in the case of noradrenergic agents48 and the wakefulness-promoting properties of histamine and H1 receptor agonists.4,12,14 In this respect, all previous studies in animals and humans aiming to increase pharyngeal muscle activity in sleep have targeted receptors on pharyngeal motoneurons via manipulation of extracellular neurotransmitters.49 However, in vitro experiments show that respiratory motoneuron excitability is dynamically modulated by a variety of intracellular signaling molecules that are amenable to manipulation.45,50 Based on these and other data it has also been suggested that modulation of intracellular targets downstream from surface receptors may offer new and alternative approaches to develop pharmacological strategies for obstructive sleep apnea.51 Such modulation of intracellular targets is possible in vivo with targeted delivery to the hypoglossal motor pool by microdialysis,52 but whether targeted manipulation of specific respiratory motor pools can be achieved with systemic approaches is a major challenge. If successful, the ability to increase pharyngeal muscle activity in non-REM and REM sleep to normal waking levels should be beneficial to the maintenance of upper airway patency and preventing obstructive sleep apnea, but with current approaches this goal has not been met.
In contrast to H1 receptors, H2 receptors are coupled to Gs protein which causes neuronal excitation via adenylyl cyclase and protein kinase A (PKA).14 Since we have shown previously that activation of the cyclic adenosine-3′ -5′ -monophosphate (cAMP)-PKA pathway at the hypoglossal motor pool increases GG activity in vivo,52 the lack of GG responses to H2 receptor activation at this motor pool (Figure 5) suggests that medullary H2 receptors30 are located outside the hypoglossal motor nucleus and were not influenced by the interventions. Of relevance, β-adrenergic, serotonin (5-HT4-7), and muscarinic (M1,3,5) receptors are also Gs protein coupled receptors that may underlie these responses to manipulation of the cAMP-PKA pathway.52
The predominant effect of histamine and H1 receptor agonists applied to the whole respiratory network in vitro is modulation of respiratory rate,18 with H1 receptor knockout mice also showing reduced respiratory rate responses to hypercapnic respiratory stimulation in vivo.20 However, the results of the present study also show that histamine and the H1 receptor agonist at the level of respiratory motoneurons produces clear increases in tonic GG activity (Figures 2 and 4). The effects on tonic GG activity are best explained by the tonic membrane depolarization also observed with serotonin and α1 receptor agonists in vitro and in vivo.5 We are unsure why histamine per se also did not augment respiratory-related GG activity as occurred with H1 receptor agonism, i.e., in addition to the increased tonic activity (Figure 2C vs. 4B). However, the main point of the paper is the demonstration that histamine at a mammalian motoneuron pool can increase motor activity, and for the hypoglossal motor pool, this was most clearly demonstrated as increased tonic activity.
Limitations and Other Considerations
With respect to the measurements of tonic and respiratory-related GG activities, tonic activity was quantified as the mean minimal activity in expiration, and respiratory-related activity was quantified as peak inspiratory activity minus tonic activity. In practice, this peak and trough detection was sufficient to adequately detect respiratory-related GG activity, as judged by the accompanying visual (albeit subjective) inspection of the computer-identified breath detections during off-line analysis. This quantification of respiratory-related GG activity from the EMG signal is typical in the field. It should be noted, however, that because of the quantification of peak activity in inspiration and minimal activity in expiration, the calculation of respiratory-related activity will inevitably include a component unrelated to respiration per se, simply due to subtraction of maximum and minimum values. This concern, however, does not affect the calculation of tonic GG activity. Indeed, increases in tonic activity were the most consistent and robust result of histamine and H1 receptor agonist application to the hypoglossal motor pool (Figures 2 and 4), so the main conclusions of this study are unaffected.
In addition to the identification of a histamine and H1 receptor-mediated activation of GG activity following interventions at the hypoglossal motor pool, the specificity of the responses was also indicated by the lack of changes in control variables. Such control variables included the amplitude of diaphragm and neck EMG activities (i.e., control respiratory and non-respiratory motor signals that should be unaffected by the interventions at the hypoglossal motor pool), respiratory rate (an indicator of the rhythmic activity generated by medullary respiratory neurons that should also be unaffected by interventions at the hypoglossal motor pool), and EEG activity (used as a marker of arousal state which may also indirectly influence GG activity). Importantly, unlike the significant GG activating effects observed with histamine and the H1 receptor agonist applied to the hypoglossal motor pool, there were no effects of the interventions on any of the aforementioned control variables. Overall, therefore, the most parsimonious explanation of the results reported in this paper is that the observed changes in GG activity were the result of specific effects occurring at the hypoglossal motor pool and not likely via indirect influences at other brainstem sites. Nevertheless, although the isolated changes in GG activity in response to the interventions at the hypoglossal motor pool strongly suggest that the responses to histamine were confined to influences at the motor nucleus, a justifiable criticism of the microdialysis method to locally apply these agents is that it is not possible to determine whether the observed changes in motor activity are exerted via pre- and/or post-synaptic receptor elements. The concern regarding an inability to separate pre- and/or post-synaptic effects is fully acknowledged.
It should also be noted that the evidence for histamine and H1 (but not H2) receptor effects at the hypoglossal motor pool was based on application of only one or two concentrations of agonist in addition to the ACSF controls. Applications of multiple concentrations to produce a concentration-response curve, as well as different agonists with different receptor affinities, were not performed to fully characterize the histamine receptor subtypes involved in the responses. These criticisms are also justified and fully acknowledged. Nevertheless, the data in their simplest form provide evidence for modulation of respiratory muscle activity caused by the presence of histamine at a distinct central nervous system motor pool, with the activating effect of histamine also occurring with an H1 receptor agonist.
Although there may be other justifiable criticisms or concerns regarding the experimental model or approach, we feel that these do not dispute the main observations outlined in this paper. The results of the present study show that histamine at the hypoglossal motor pool increases motor activity, and that this effect is most consistently observed as an increase in the tonic component of GG activity, with this activating effect of histamine also occurring with application of an H1 but not H2 receptor agonist to the hypoglossal motor pool.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563). RLH is supported by a Tier I Canada Research Chair in Sleep and Respiratory Neurobiology.
REFERENCES
- 1.Jones BE. Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 2nd ed. Philadelphia: Saunders; 1994. pp. 145–62. [Google Scholar]
- 2.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 4.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 5.Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep apnea: pathogenesis, diagnosis and treatment. New York: Dekker; 2002. pp. 99–154. [Google Scholar]
- 7.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–73. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 8.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–47. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 9.Sood S, Raddatz E, Liu X, Liu H, Horner RL. Inhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious rats. J Appl Physiol. 2006;100:1807–21. doi: 10.1152/japplphysiol.01508.2005. [DOI] [PubMed] [Google Scholar]
- 10.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–9. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 11.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–30. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–34. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–40. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 14.Haas H, Panula P. The role of histamine and the tuberomammillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–30. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 15.Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984;81:2572–6. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T, Taguchi Y, Shiosaka S, et al. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295:13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- 17.Iwase M, Homma I, Shioda S, Nakai Y. Histamine immunoreactive neurons in the brain stem of the rabbit. Brain Res Bull. 1993;32:267–72. doi: 10.1016/0361-9230(93)90187-g. [DOI] [PubMed] [Google Scholar]
- 18.Dutschmann M, Bischoff AM, Busselberg D, Richter DW. Histaminergic modulation of the intact respiratory network of adult mice. Pflugers Arch. 2003;445:570–6. doi: 10.1007/s00424-002-0904-z. [DOI] [PubMed] [Google Scholar]
- 19.Haxhiu MA, Cherniack NS, Altose MD, Kelsen SG. Effect of histamine on respiratory chemosensitivity in conscious goats. Respiration. 1983;44:411–8. doi: 10.1159/000194578. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K, Iwase M, Kimura H, Homma I. Central histamine contributes to the inspiratory off-switch mechanism via H1 receptors in mice. Respir Physiol Neurobiol. 2004;144:25–33. doi: 10.1016/j.resp.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke RE. Sir Charles Sherrington's the integrative action of the nervous system: a centenary appreciation. Brain. 2007;130:887–94. doi: 10.1093/brain/awm022. [DOI] [PubMed] [Google Scholar]
- 23.Neuzeret PC, Sakai K, Gormand F, et al. Application of histamine or serotonin to the hypoglossal nucleus increases genioglossus muscle activity across the wake-sleep cycle. J Sleep Res. 2009;18:113–21. doi: 10.1111/j.1365-2869.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 24.Strecker RE, Nalwalk J, Dauphin LJ, et al. Extracellular histamine levels in the feline preoptic/anterior hypothalamic area during natural sleep-wakefulness and prolonged wakefulness: an in vivo microdialysis study. Neuroscience. 2002;113:663–70. doi: 10.1016/s0306-4522(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–8. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States, 1970 through 1989. Arch Intern Med. 1991;151:1779–83. [PubMed] [Google Scholar]
- 27.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–81. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 29.Palacios JM, Wamsley JK, Kuhar MJ. The distribution of histamine H1-receptors in the rat brain: an autoradiographic study. Neuroscience. 1981;6:15–37. doi: 10.1016/0306-4522(81)90240-2. [DOI] [PubMed] [Google Scholar]
- 30.Traiffort E, Pollard H, Moreau J, et al. Pharmacological characterization and autoradiographic localization of histamine H2 receptors in human brain identified with [125I]iodoaminopotentidine. J Neurochem. 1992;59:290–9. doi: 10.1111/j.1471-4159.1992.tb08903.x. [DOI] [PubMed] [Google Scholar]
- 31.Morrison JL, Sood S, Liu X, et al. Glycine at the hypoglossal motor nucleus: genioglossus activity, CO2 responses and the additive effects of GABA. J Appl Physiol. 2002;93:1786–96. doi: 10.1152/japplphysiol.00464.2002. [DOI] [PubMed] [Google Scholar]
- 32.Steenland HW, Liu H, Horner RL. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J Neurosci. 2008;28:6826–35. doi: 10.1523/JNEUROSCI.1019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steenland HW, Liu H, Sood S, Liu X, Horner RL. Respiratory activation of the genioglossus muscle involves both non-NMDA and NMDA glutamate receptors at the hypoglossal motor nucleus in-vivo. Neuroscience. 2006;138:1407–24. doi: 10.1016/j.neuroscience.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Sun M-H, Hildenbrandt L, Curran AK, Darnall R, Chen G, Filiano J. Potassium permanganate can mark the site of microdialysis in brain sections. J Histotechnol. 2000;23:151–4. [Google Scholar]
- 35.Paxinos G, Watson C. 4th ed. San Diego: Academic Press; 1998. The rat brain in stereotaxic coordinates. [Google Scholar]
- 36.Hamrahi H, Chan B, Horner RL. On-line detection of sleep-wake states and application to produce intermittent hypoxia only in sleep in rats. J Appl Physiol. 2001;90:2130–40. doi: 10.1152/jappl.2001.90.6.2130. [DOI] [PubMed] [Google Scholar]
- Colton T. Boston: Little, Brown and Co.; 1974. Statistics in medicine. [Google Scholar]
- 38.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–87. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 39.Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- 40.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–8. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 41.Woch G, Davies RO, Pack AI, Kubin L. Behaviour of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J Physiol. 1996;490:745–58. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J Appl Physiol. 2007;102:2240–50. doi: 10.1152/japplphysiol.01229.2006. [DOI] [PubMed] [Google Scholar]
- 43.Park E, Younes M, Horner RL. Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep. 2008;31:355–65. doi: 10.1093/sleep/31.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 45.Feldman JL, Neverova NV, Saywell SA. Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades. Respir Physiol Neurobiol. 2005;147:131–43. doi: 10.1016/j.resp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: implications for obstructive sleep apnoea. Respir Res. 2001;2:286–94. doi: 10.1186/rr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veasey SC. Pharmacotherapies for obstructive sleep apnea: how close are we? Curr Opin Pulm Med. 2001;7:399–403. doi: 10.1097/00063198-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–65. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 49.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol. 2008;164:179–96. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Richter DW, Lalley PM, Pierrefiche O, et al. Intracellular signal pathways controlling respiratory neurons. Respir Physiol. 1997;110:113–23. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 51.Greer JJ. Are biogenic amines involved in controlling upper airway patency during REM sleep? Am J Respir Crit Care Med. 2005;172:1237–8. doi: 10.1164/rccm.2508007. [DOI] [PubMed] [Google Scholar]
- 52.Aoki CR, Liu H, Downey GP, Mitchell J, Horner RL. Cyclic nucleotides modulate genioglossus and hypoglossal responses to excitatory inputs in rats. Am J Respir Crit Care Med. 2006;173:555–65. doi: 10.1164/rccm.200509-1469OC. [DOI] [PubMed] [Google Scholar]