Abstract
Study Objectives:
To analyze sleep architecture of children with dyslexia, by means of conventional parameters and EEG spectral analysis and to correlate sleep parameters and EEG spectra with neuropsychological measures.
Design:
Cross-sectional study involving validated sleep questionnaires, neuropsychological scales, and polysomnographic recordings.
Setting:
Sleep laboratory in academic center.
Participants:
Sixteen subjects with developmental dyslexia (mean age 10.8 years) and 11 normally reading children (mean age 10.1 years). All the subjects underwent overnight polysomnographic recording; EEG power spectra were computed from the Cz derivation and spindle density was calculated during sleep stages N2.
Intervention:
N/A
Measurements and Results:
Dyslexic children showed an increase in power of frequency bands between 0.5–3 Hz and 11–12 Hz in stage N2 and between 0.5–1 Hz in stage N3; they also showed significantly increased spindle density during N2. The power of the sigma band in N2 was positively correlated with the Word reading and MT reading tests; similarly, spindle density was significantly correlated with the Word reading test. The increased spindle activity and EEG sigma power in dyslexic subjects were found to be correlated with the degree of dyslexic impairment.
Conclusions:
The correlation found between sleep spindle activity and reading abilities in developmental dyslexia supports the hypothesis of a role for NREM sleep and spindles in sleep-related neurocognitive processing.
Citation:
Bruni O; Ferri R; Novelli L; Terribili M; Troianiello M; Finotti E; Leuzzi V; Curatolo P. Sleep spindle activity is correlated with reading abilities in developmental dyslexia. SLEEP 2009;32(10):1333-1340.
Keywords: Dyslexia, reading disability, sleep spindles, EEG spectral analysis
DEVELOPMENTAL DYSLEXIA IS THE MOST FREQUENT LEARNING DISABILITY WITH NEUROBIOLOGICAL ORIGIN, CHARACTERIZED BY A DIFFICULTY IN accurate and/or fluent word recognition and by poor spelling and decoding abilities. Developmental dyslexia reflects an unexpected difficulty in reading in children and adults who appear to have all capabilities (intelligence, motivation, exposure to reasonable reading instruction) needed to turn print into meaning.1
People with dyslexia show a general impairment in all subprocesses involved in the grapheme–phoneme conversion, resulting in highly inadequate reading performance and associated linguistic deficits in spelling, writing, and spoken language. Etiology and pathophysiology of dyslexia are still unknown and different hypotheses conceptualize dyslexia as phonological, attentional, or auditory in nature or linked to a deficit of the cerebellar magnocellular system.2
Functional MRI (fMRI) studies have demonstrated that, in contrast to typical readers, dyslexic readers show a disruption of the posterior reading systems in the left parieto-temporal and occipito-temporal areas, but develop compensatory systems in the left anterior and right anterior areas and in the right-hemisphere homolog of the left-hemisphere visual word-form area (VWFA). Hypothetically, these ancillary systems allow the dyslexic reader to decode words, but slowly and not automatically.3
In order to trace a marker of dyslexia from a neurophysiological point of view, several studies have evaluated electroencephalographic (EEG) abnormalities in dyslexic children during phonological, semantic and orthographic assignments and found an overall increase in slower rhythms (i.e., delta and theta bands) and a decrease in faster waves (especially alpha).4,5 This pattern has been interpreted as generically due to a maturational lag in brain development in these subjects. Penolazzi et al.6 confirmed the fMRI findings3 because they showed higher levels of relative percentage of delta EEG activity in dyslexic children, compared to controls, in resting conditions and greater delta band amplitude in the frontal regions during the phonological task, as compared to both semantic and orthographic tasks.
Although in the last years important evidence has supported the hypothesis that sleep plays a key role in the processes of learning and memory7 and particularly in children,8 almost no studies are available on sleep in children with dyslexia. There is only one report in the literature that has evaluated EEG during sleep in children with reading disabilities9 showing an alteration of sleep architecture characterized by an increase in slow wave sleep (SWS), a decrease in REM sleep and a longer REM sleep latency in children with reading disabilities compared to controls.
Because of this lack of knowledge, we decided to analyze sleep in children with developmental dyslexia in order to characterize their sleep structure by means of the computation of the classic sleep parameters in addition to spectral sleep EEG analysis and to evaluate the differences between them and normal healthy controls.
METHOD
Participants
For the purpose of this study, we recruited 19 subjects with developmental dyslexia (11 girls, 8 boys; mean age: 10.3 years, range 8–16) and 11 normally reading children (6 girls, 5 boys; mean age: 10.1 years, range 7–16) previously recruited by advertisement from the community.
Patients with dyslexia were consecutively recruited at the Child Neuropsychiatry Clinic of the University of Rome “Tor Vergata”. All of them had normal or corrected-to-normal vision, no history of serious physical health problems or of epileptic seizures or mental retardation; none of them was taking medications of any type. All subjects were right-handed. Handedness was controlled by asking the subjects about the hand they use in different tasks such as handwriting, throwing a ball, brushing teeth, etc. A subject was considered as right-handed if he/she indicated to use the right hand for all of these different activities.
The clinical diagnosis of dyslexia was made according to standard criteria defined by the DSM-IV:10 full-scale IQ ≥ 85; no neurobehavioral, sensorial, or socioeconomic problems and defective performances on Italian standard tests for the assessment of reading skills with reading abilities, at least 2 standard deviations below age mean for speed and accuracy. Dyslexic children suffering from attention deficit disorder with hyperactivity (ADHD) were excluded from the experiment to avoid interfering and confounding effects.
Age-matched controls were retrospectively enrolled from our database of sleep recordings for the comparison of sleep structure; normal healthy children were selected with no history of organic or mental illness and without neurological or psychiatric disabilities. All of them attended regular primary, middle or secondary/high schools, with normal performances.
All participants fulfilled the Sleep Disturbance Scale for Children (SDSC)11 to evaluate the presence of sleep disorders. The SDSC is a standardized questionnaire and consists of six factors representing the most common areas of sleep disorders in childhood and adolescence: difficulty in initiating and maintaining sleep (DIMS), sleep breathing disorders (SBD), arousal disorders (DA), sleep-wake transition disorders (SWTD), disorders of excessive somnolence (DOES), and sleep hyperhydrosis (SHY). Based on this scale, 3 dyslexic children were excluded because they had pathological scores in the DIMS subscale (2 with insomnia and 1 with a phase-delay syndrome). None of the control children had scores in the clinical range for the total SDSC scale and for any of its 6 subscales. Therefore, after the exclusion of subjects with potential comorbidity with sleep disturbances the group of dyslexic children was composed of 16 subjects (10 girls, 6 boys; mean age: 10.8 years, range 8–16).
All parents of subjects participating to the study gave their written consent. The study was conducted in accordance to the regulations of the local ethics committee and to the principles expressed in the declaration of Helsinki.
Measures
Reading Abilities
Reading abilities were evaluate using:
MT (Memory and Learning Transfer) Reading test
The participants had to read a passage aloud with a 4-minute time limit; speed (time in seconds per syllable read) and accuracy (number of errors, adjusted for the amount of text read) were scored. To measure comprehension, the participants read a second passage without a time limit and responded to 10 multiple-choice questions. Stimulus materials (and related reference norms) varied by grade.12
Word and non-word reading test
This test, derived from The Battery for Evaluating Dyslexia and Dysorthography,13 assesses speed and accuracy (expressed in number of errors) in reading printed word lists (4 lists of 24 words) and non-words lists (3 lists of 16 non-words), and provides grade norms from the second to the last grade of junior high school. The dyslexia group obtained significantly lower scores on word (both in terms of accuracy and speed) and non-word (only in terms of speed) reading tasks.
Word, non-word and sentences writing test
This test, derived from The Battery for Evaluating Dyslexia and Dysorthography,13 assesses accuracy (expressed in number of errors) in writing word lists (48 words), non-words lists (24 non-words) and sentences (12 sentences) dictated by the examiner.
Wechsler Intelligence Scale for Children
Intelligence estimates (IQ) were obtained using the Wechsler Intelligence Scale for Children - Third Edition Revised (WISC-IIIR) which consists of standardized, individually administered tests that yield 3 intelligence measures: verbal IQ (VIQ), performance IQ (PIQ), and a full-scale (verbal + performance) IQ (FSIQ). In normal controls, these measures have a mean of 100 (S.D. 15). The IQ testing was performed before the sleep study at the initial evaluation.
Polygraphic Sleep Recordings
For this study, subjects underwent an overnight polysomnographic (PSG) recording in the Sleep Laboratory of the Department of Developmental Neurology and Psychiatry of the Sapienza University of Rome, after one adaptation night, in order to avoid the first-night effect.
EEG recordings and electrode placement were performed according the international 10-20 system and the PSG montage included at least 11 EEG channels (Fz, Cz, Pz, Fp1, Fp2, C3, C4, T3, T4, O1, O2) referenced to the contralateral mastoid, left and right electrooculogram (EOG), chin electromyogram (EMG), left and right tibialis anterior muscle EMG and electrocardiogram (ECG). All recordings started at the subjects' usual bedtime and continued until spontaneous awakening.
All parents were asked to sign a consent form approved by the institution in which sleep recordings were carried out.
Sleep Stage Scoring and Periodic Leg Movement Analysis
Sleep was subdivided into 30-second epochs and sleep stages were scored according to the recent American Academy Sleep Medicine criteria.14
The following conventional sleep parameters were evaluated:
Time in bed (TIB);
Sleep period time (SPT): time from sleep onset to sleep end;
Total sleep time (TST): the time from sleep onset to the end of the final sleep epoch minus time awake;
Sleep latency (SL): the start of the first epoch scored as any stage other than stage W, in minutes;
Stage R latency (RL): time from sleep onset to the first stage R epoch;
Number of stage shifts/hour (SS/h);
Number of awakenings/hour (AWN/h);
Sleep efficiency (SE%): the percentage ratio between total sleep time and time in bed (TST/TIB*100);
Percentage of SPT spent spent in stage W (W%) and in sleep stages N1 (N1%), N2 (N2%), N3 (N3%), and stage R (R%)
Arousal Index: number of arousals14 per hour in NREM, REM and Total sleep
Finally, periodic leg movements during sleep (PLMS) were detected following the recent criteria established by the AASM14 and WASM-IRLSSG15 and the PLMS index was calculated as the number of leg movements included in a series of 4 of more, separated by more than 5 and less than 90 s, per hour of sleep.
Power Spectra Analysis
Power spectra were calculated for the Cz-A2 channel using the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy), after Welch windowing (wn = 1 – ((n1/2(N – 1))/1/2 (N+1))2), in order to minimize the truncation error and reduce spectral leakage by suppressing sidelobes,16 by means of the Fast Fourier Transform (FFT),17 on 2-sec EEG epochs sampled at 200 Hz. Fifteen 2-sec epoch FFTs were performed for each 30-sec artifact-free sleep epoch and their results averaged. Subsequently, the power spectrum was calculated for frequencies between 0.5 and 30 Hz with a frequency step of 1 Hz and then averaged across the following bands: delta (0.5–4 Hz), theta (5–7 Hz), alpha (8–11 Hz), sigma (11–15 Hz), and beta (16–30 Hz). Individual averages were obtained, for each sleep stage, which were used for statistical analysis.
Sleep Spindle Density
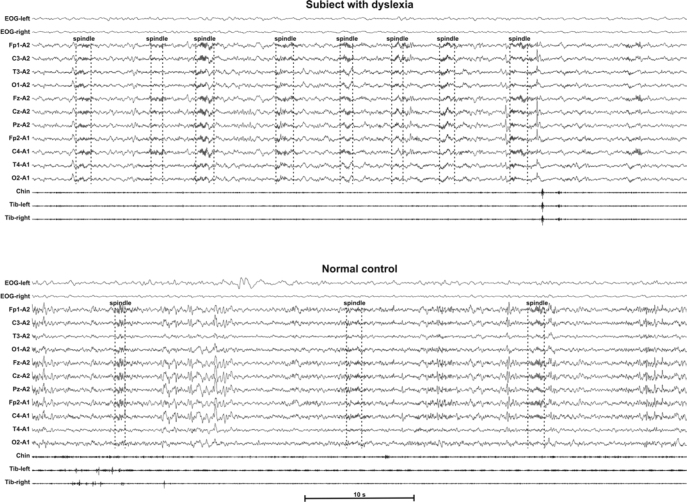
Sleep spindles were visually identified in the Cz-A2 channel in all epochs scored as stage N2 for the entire night as trains of distinct waves with frequency 11–16 Hz and with a duration > 0.5 s, usually maximal in amplitude using central derivations. However, sleep spindles in children usually occur at 2 different frequencies and 2 different scalp locations: 11.0–12.75 Hz over the frontal and 13.0–14.75 Hz over the centroparietal electrodes.18 In this study, all spindles included in the count had an amplitude exceeding 10 μV and had typical waxing-waning morphology. Figure 1 shows some examples of visual spindle detection in a patient with dyslexia and in a normal control. Spindle density was calculated as the ratio of the number of sleep spindles counted in stage N2 to the number of minutes of stage N2.
Figure 1.
Examples of visual spindle detection in a patient with dyslexia (top tracing) and in a normal control (bottom tracing).
Statistical Analysis
The comparisons between sleep architecture parameters, sleep EEG power spectra parameters, and spindle density obtained in children with dyslexia and normal controls, was conducted using the Bonferroni-corrected nonparametric Mann-Whitney test for independent data sets.
The Spearman correlation was used in order to evaluate the relationship between sleep EEG power spectra and cognitive measures. Differences and correlations were considered as statistically significant when P < 0.05. The commercially available software STATISTICA (StatSoft, Inc. 2004, version 6. www.statsoft.com) was used for all statistical tests.
RESULTS
Behavioral/Cognitive Measures
The IQ mean scores in children with dyslexia were: Full-Scale IQ 93.9 (range 85-115), Verbal IQ 90 (range 85-113), Performance IQ 98.9 (range 85-129).
The Standard Deviation scores on MT Reading test ranged from 1.21 to 3.39 (mean 2.11), on word reading test from 1.34 to 2.73 (mean 2.11) and on non-word reading test from 0.81 to 2.86 (mean 2.0).
Sleep Architecture
Table 1 shows the statistical comparison between the sleep architecture parameters obtained from children with dyslexia and normal controls. Several statistically significant differences were found between the 2 groups. Number of stage shifts per hour of sleep and number of stage R periods were significantly lower in dyslexic children vs. controls. Stage N2% was significantly higher (48.8% vs. 43.5%) and N3% significantly lower (21.5% vs. 25.25%) in dyslexic children with respect to controls. The analysis of arousals showed only a slight decrease of the arousal index in dyslexic children, during all sleep stages, which reached statistical significance only during REM sleep.
Table 1.
Sleep Architecture in Patients with Dyslexia and Control Subjects
| Dyslexia (n = 16) |
Controls (n = 11) |
Mann-Whitney test |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | “U” | P ≤ | |
| TIB, min | 557.97 | 33.12 | 547.14 | 43.10 | 80.50 | NS |
| SPT, min | 528.44 | 33.24 | 515.73 | 40.37 | 74.00 | NS |
| TST, min | 519.66 | 37.87 | 508.77 | 39.01 | 76.50 | NS |
| SL, min | 19.63 | 18.62 | 22.23 | 15.12 | 70.00 | NS |
| RL, min | 117.09 | 49.29 | 107.59 | 32.29 | 84.00 | NS |
| SS/h | 5.43 | 1.06 | 8.31 | 2.04 | 14.00 | 0.0003 |
| AWN/h | 0.20 | 0.29 | 0.31 | 0.24 | 60.50 | NS |
| SE% | 93.19 | 5.12 | 93.07 | 3.35 | 80.00 | NS |
| Number of stages R | 6.56 | 1.46 | 9.27 | 2.15 | 26.00 | 0.002 |
| W% | 1.67 | 3.21 | 1.33 | 1.41 | 60.00 | NS |
| N1% | 5.71 | 2.45 | 8.23 | 4.15 | 52.00 | NS |
| N2% | 48.82 | 3.85 | 43.55 | 5.34 | 36.00 | 0.01 |
| N3% | 21.49 | 3.57 | 25.17 | 3.81 | 34.50 | 0.008 |
| R% | 22.29 | 3.58 | 21.71 | 5.19 | 82.00 | NS |
| NREM Arousal index | 6.39 | 1.88 | 7.21 | 5.67 | 70.00 | NS |
| REM Arousal index | 5.05 | 2.53 | 7.81 | 2.63 | 39.00 | 0.015 |
| Total Arousal index | 6.11 | 1.74 | 7.29 | 2.26 | 63.00 | NS |
TIB = Time in bed; SPT = sleep period time; TST = total sleep time; SL = sleep latency; SS/h = stage shifts per hour; AWN/h = awakenings per hour; SE = sleep efficiency.
All but one dyslexic subjects had PLMS index < 5/hour; however, the remaining subject only had PLMS index = 5.6.
Power Spectral Analysis and Spindles Density
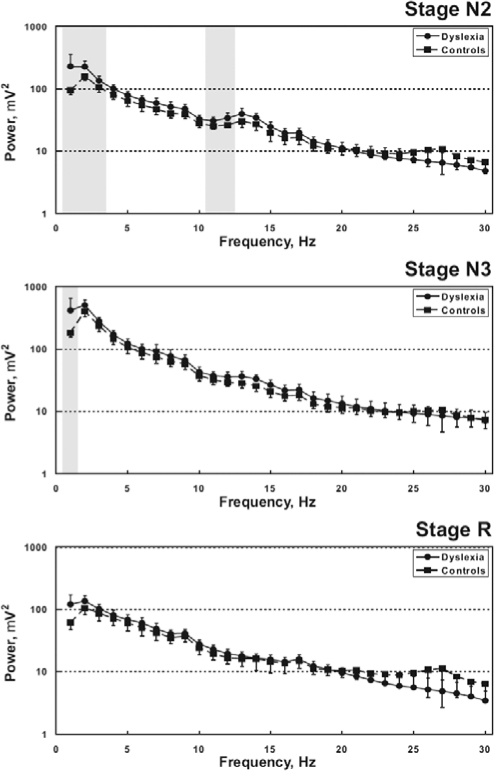
The FFT analysis revealed significant differences between the two groups of subject, as shown in Figure 2. Dyslexic children showed an increase in power of frequency bands 0.5–3 Hz and 11–12 Hz during stage N2 and 0.5–1 Hz during stage N3. No significant differences were found for sleep stage R.
Figure 2.
Comparison between the grand-average power spectra obtained from sleep stages N2, N3 and R, in our groups of children. Values are shown as mean and standard error (whiskers); grey-shaded areas indicate significant difference between the two spectra.
Dyslexic children showed also a significant increase in spindles density during stage N2 (6.2 vs. 3.5; P < 0.0001), while spindle mean duration was similar between the 2 groups (1.04 vs. 1.06, NS).
Correlation Between Sleep Parameters, Power Spectra and Reading Tests
In subjects with dyslexia, Spearman correlations were computed between sleep parameters or sleep power bands (Delta, Theta, Alpha, Sigma, Beta) during each sleep stage (N2, N3, R) or spindle density in N2 and the results of the reading tests (MT, Word and Non-Word reading test), writing test (Word, Non-Word and Sentences reading test) and WISC (table 2).
Table 2.
Correlations Between EEG Band Spectral Power in Stage N2 or Spindle Density and Cognitive Measures in Patients with Dyslexia
| Delta | Theta | Alpha | Sigma | Spindle density | |
|---|---|---|---|---|---|
| MT Reading test | 0.04 | 0.18 | 0.13 | 0.55 | 0.17 |
| Word Reading test | 0.05 | 0.01 | 0.11 | 0.63 | 0.66 |
| Non-Word Reading test | −0.16 | −0.17 | −0.22 | 0.24 | 0.26 |
| Word writing | −0.02 | −0.14 | 0.03 | 0.23 | 0.41 |
| Non-Word writing | −0.07 | 0.03 | 0.05 | 0.31 | 0.28 |
| Sentences writing | −0.08 | −0.09 | −0.03 | 0.05 | 0.18 |
| Verbal IQ | 0.02 | −0.04 | −0.02 | 0.05 | −0.04 |
| Performance IQ | −0.09 | −0.18 | −0.09 | 0.03 | 0.03 |
| Full scale IQ | 0.04 | −0.05 | 0.00 | 0.05 | −0.06 |
Bold values are significant at P < 0.05
No significant correlations were found between sleep parameters and the cognitive/behavioral measures. Of the different power bands, only the sigma band power in N2 was positively correlated with the Word Reading test (r = 0.63; P = 0.02) and MT reading test (r = 0.55; P = 0.03) while no significant correlations were found with the Non-Word Reading test; also, a positive significant correlation was found between spindle density and the Word Reading test (r = 0.66; P = 0.01). EEG band power in stages N3 and R did not correlate significantly with cognitive measures.
DISCUSSION
To our knowledge, this is the first study designed to analyze sleep architecture and EEG power spectra of the entire sleep in children with dyslexia. The main result of this study is the clear increase of spindle activity and sigma power in dyslexic subjects which seems to be correlated with their degree of dyslexic impairment. These findings, never reported before in the literature, seem to be consistent with the most recent reports on the role of sleep and of specific phasic events during non-rapid eye movement (NREM) sleep on learning and memory.19–21
A limitation of the study was that we recruited the normal healthy control group from our database of sleep recordings and we did not have cognitive testing measures for them. This can potentially change the results since it is possible that other minor neurocognitive impairments which might have been overseen are also associated with changes in EEG power spectra and sleep architecture.
The analysis of sleep architecture parameters showed only few changes in dyslexic patients, similar to those reported in the previously published single study on this population.9 We found a decrease in number of stage shifts (indicating an increased sleep stability), in number of stage R periods and in stage N3 percentage, but a slight increase in stage N2. Mercier et al.9 found the same decrease in number of REM periods and an increase of stage 2 NREM and of SWS during the first NREM cycle.
The FFT analysis revealed a specific pattern of sleep EEG power in dyslexic children represented by a clear increase in power of two specific frequency bands during stage N2: the low-frequency band at 0.5–3 Hz and at 11–12 Hz (slow sigma) while, during stage N3, a difference was found in the very-low-frequency band at 0.5–1 Hz. Dyslexic children showed also a significant increase in visually detected spindle density during stage N2, while spindle mean duration was similar to that of normal controls. The increase in the 11–12 Hz frequency band probably corresponds to the low frequency spindles peaking over the frontal brain regions. This finding is further supported by the increase in spindle density in stage N2.
Three neural systems subserve reading: an anterior system in the region of the inferior frontal gyrus (Broca's area), which is believed to serve articulation and word analysis, and two posterior systems, one in the parieto-temporal region, which is believed to serve word analysis, and a second in the occipito-temporal region (the visual word-form area or VWFA), which is believed to serve for the rapid, automatic, fluent identification of words. Non-impaired readers activate all three left hemisphere neural systems while dyslexic readers seem to have a disruption in the posterior neural systems but compensate by developing anterior systems in both left and right hemispheres and the posterior homolog of the VWFA in the right hemisphere. Functional MRI studies support the existence of this disruption of the posterior reading systems in the left parietotemporal and occipito-temporal areas of dyslexic readers, and the presence of compensatory systems in left anterior and right anterior areas and the right hemisphere homolog of the left hemisphere VWFA. These ancillary systems would allow the dyslexic reader to decode words, but slowly and not automatically.3 This compensatory frontal activation has been reported also during wakefulness by EEG spectral analysis by Penolazzi et al.6 that found higher levels of EEG delta band in both anterior and posterior sites, and a greater delta amplitude over dyslexic children's anterior sites during the phonological tasks.
It has been demonstrated that intensive evidence-based reading intervention brings significant changes in brain organization: children with dyslexia who receive intervention not only improve their reading but also show an increase in activation in the anterior system as well as in the parieto-temporal and occipito-temporal systems, compared to their pre-intervention brain activation patterns.22 Therefore the compensatory activation over the frontal regions could account for the increased EEG power in the low-frequency band at 0.5–3 Hz during N2 and in very-low-frequency band at 0.5–1 Hz during N3 because the generators of the sleep EEG low-frequency band (0.25–2.5 Hz) are localized mostly over the frontal midline cortex.23
In our group of subjects with dyslexia we did not find significant correlations between classic sleep parameters and cognitive/behavioral measures and between sleep EEG spectral data or spindles and tests for memory or cognitive performance, in agreement with to Schabus et al.24 and in contrast with Nader and Smith,25 and Bodizs et al.26
Schabus et al.24 showed that the most robust between-group differences were found if measures of spindle “intensity” (i.e., spindle activity: spindle duration = amplitude) were used, with weaker (but still significant) effects for sigma power and non-significant effects for the number of detected sleep spindles and traditional spindle density. This might explain the diverging results, showing no significant relationships (with tests of memory or cognitive performance) for the traditional measures of spindle counts and spindle densities that we employed. Moreover, Schabus et al.24 believed that the measures of spindle activity (duration of each detected spindle = mean amplitude of the spindle in the 11–16 Hz frequency band) should be the best indicators of highly efficient thalamo-cortical and/or hippocampal-neocortical networks. In future studies, spindle activity measures as well as spindle topography might disclose more interesting relationships between sleep spindles and learning.
We found a significant correlation between sigma band power in N2 and MT or Word reading tests and between spindle density in N2 and Word reading test; this indicates that the increase in spindles and sigma power is correlated to a more severe degree of dyslexic impairment.
As stated by Schabus et al.,33 previous studies on sleep and memory have been relying on “macroscopic” estimates of sleep, i.e. amount of REM or SWS, without specific findings. As of today, different studies give importance to transient or phasic events for different types of off-line memory processing during NREM sleep: sleep-spindles,29,30 slow waves or delta bursts.23,28 Recent research suggests that these oscillatory neuronal activities during NREM sleep are involved in sleep-dependent memory consolidation.27 Learning before sleep modulates the regional expression of slow wave activity (SWA)28 and spindle density29–33 during subsequent NREM sleep and also correlates with overnight improvement in memory performance.34
Several reports support the role of sleep spindles in procedural and declarative learning.8,29,30 Holdmoser et al.35 hypothesized that increasing spindle band power might also enhance declarative memory performance in a word-pair association task and the spindle increase does not seem to be related to an increase of stage 2 NREM.
Therefore, when a subject succeeds in elaborate encoding of newly acquired information, this should be reflected by an increase in spindle activity and, subsequently, increased memory performance.
In our children we found an enhancement of sigma activity at 11–12 Hz, probably reflecting an increase of the slow spindles. Some reports suggests the existence of two types of spindles:36 at 11.0–12.75 Hz over the frontal areas and at 12.5–14.5 Hz, over the centroparietal regions. The peak frequency of the centroparietal spindles increases linearly with age, whereas the incidence of frontal spindles abruptly increases during early adolescence.37,38 It is still an open question as to whether these 2 sources contribute differentially to memory consolidation. Unfortunately, most behavioral learning studies did not differentiate between slow and fast spindles nor used a consistent frequency range when relating the expression of sleep spindles to the sleep-dependent consolidation of declarative (12–15 Hz,29 11.5–16 Hz,30 11–16 Hz,39 11.25–13.75 Hz41) or procedural memory (12–16 Hz,31 12–14 Hz,42 12–16 Hz32).
Although recent data33 did not show a specific role of both slow and fast spindles in the processing of declarative word pairs, a previous study indicated an increase of low-frequency spindles over the left frontocentral areas, correlated with verbal declarative memory, and fast spindles over the parietal areas, after spatial declarative learning.39 Gais et al.29 indicated that the largest learning-induced differences in spindle activity can be observed at frontal leads, although spindle activity is generally highest at central electrodes. A recent study, however, provides evidence that both slow (≤ 13 Hz) and fast (≥ 13 Hz) sleep spindles are differently influenced by recent changes in neuronal plasticity and might play a different role for plasticity during sleep:34 the slow spindles predominantly reflect cortico-cortical coupling, while fast spindles are more closely associated with thalamocortical coupling.40
It has been demonstrated that spindles indicate the repeated activation of thalamocortical or hippocampo-cortical networks that have been suggested to be the basis for reorganization and consolidation of memory traces43: spindles may promote the formation of thalamocortical networks by providing endogenous signals with repetitive and synchronized activity44 and could provide the necessary conditions important for plastic modifications which underlie memory formation.43,45
Thus, the learning-dependent increase in spindles might indicate the efficiency of information processing dependent on thalamocortical communication. Maintenance and encoding of new information, in a more complex, system may require higher thalamocortical activity or a more efficient thalamocortical system that may be reflected in the higher number of sleep spindles.
We might hypothesize that the increase in slow spindles (sigma power at 11–12 Hz) in our dyslexic subjects reflects the enhancement of cortico-cortical coupling since they have to transfer information continuously from the disrupted left-hemisphere posterior neural systems to the ancillary anterior systems, in the left and right hemispheres, and the posterior homolog of the VWFA in the right hemisphere. Therefore, the increase of sigma power and spindles in dyslexic children might be related to a genetically more efficient thalamocortical system or to the obliged early adoption of acquired strategies in order to compensate their specific learning disability or to the intensive stimulation linked to cognitive rehabilitation. Moreover, the increase in SWA in N2 and N3 might represent the activation of the ancillary frontal areas.
In conclusion, based on our results, the increase in “slow” spindles and in EEG sigma power during sleep might be regarded as a neurophysiological marker of dyslexia; moreover, based on the clear correlation between reading test performance and EEG sigma power and/or spindle density, it is possible to hypothesize the presence of a relationship between the increase in spindles or sigma power and the severity of dyslexic impairment. Future research should be devoted to the confirmation of this correlation in large independent groups of dyslexic subjects and to the detailed evaluation of the differential anteroposterior topography of spindles, in order to confirm or not the frontal activation reported by fMRI also at a neurophysiological level, during sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Shaywitz S. Overcoming dyslexia: a new and complete evidence-based program for reading problems at any level. New York: Alfred A. Knopf; 2003. [Google Scholar]
- 2.Ramus F. Neurobiology of dyslexia: a reinterpretation of the data. Trends Neurosci. 2004;27:720–6. doi: 10.1016/j.tins.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Shaywitz SE, Shaywitz BA. Paying attention to reading: The neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–49. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- 4.Klimesch W, Doppelmayr M, Wimmer H, et al. Theta band power changes in normal and dyslexic children. Clin Neurophysiol. 2001;112:1174–85. doi: 10.1016/s1388-2457(01)00545-4. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca LC, Tedrus GM, Chiodi MG, Cerqueira JN, Tonelotto JM. Quantitative EEG in children with learning disabilities: analysis of band power. Arq Neuropsiquiatr. 2006;64:376–81. doi: 10.1590/s0004-282x2006000300005. [DOI] [PubMed] [Google Scholar]
- 6.Penolazzi B, Spironelli C, Angrilli A. Delta EEG activity as a marker of dysfunctional linguistic processing in developmental dyslexia. Psychophysiology. 2008;45:1025–33. doi: 10.1111/j.1469-8986.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 7.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–43. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15:373–7. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- 9.Mercier L, Pivik RT, Busby K. Sleep patterns in reading disabled children. Sleep. 1993;16:207–15. doi: 10.1093/sleep/16.3.207. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 11.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5:251–61. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornoldi C, Colpo G. Prove di lettura MT per la scuola elementare-2. Firenze: Organizzazioni Speciali; 1998. [Google Scholar]
- 13.Sartori G, Job R, Tressoldi PE. The Battery for Evaluating Dyslexia and Dysorthography. Firenze: Edizioni O.S.; 1995. [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes. The art of scientific computing. Cambridge: Press Syndicate of the University of Cambridge; 1989. [Google Scholar]
- 17.Cooley JW, Tukey OW. An algorithm for the machine calculation of complex Fourier series. Math Comput. 1965;19:297–301. [Google Scholar]
- 18.Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clin Electroencephalogr. 1999;30:39–43. doi: 10.1177/155005949903000203. [DOI] [PubMed] [Google Scholar]
- 19.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–78. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 20.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–24. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 21.Ferri R, Huber R, Aricò D, et al. The slow-wave components of the cyclic alternating pattern (CAP) have a role in sleep-related learning processes. Neurosci Lett. 2008;27(432):228–31. doi: 10.1016/j.neulet.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Shaywitz B, Shaywitz S, Blachman B, et al. Development of left occipito-temporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry. 2004;55:926–33. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Ferri R, Bruni O, Miano S, Terzano MG. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP) Sleep Med. 2005;6:29–36. doi: 10.1016/j.sleep.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Schabus M, Hoedlmoser K, Gruber G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosc. 2006;23:1738–46. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 25.Nader R, Smith C. The relationship between stage 2 sleep spindles and intelligence. Sleep. 2001;24:A160. [Google Scholar]
- 26.Bodizs R, Kis T, Lazar AS, et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 2005;14:285–92. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 27.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–13. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 28.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 29.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–34. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 31.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 32.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schabus M, Hoedlmoser K, Pecherstorfer T, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–35. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergmann TO, Moelle M, Marshall L, Kaya- YildizL, Born J, Roman Siebner H. A local signature of LTP- and LTD-like plasticity in human NREM sleep. Eur J Neurosc. 2008;27:2241–49. doi: 10.1111/j.1460-9568.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoedlmoser K, Pecherstorfer T, Gruber G, et al. Instrumental conditioning of human sensorimotor rhythm (12-15 Hz) and its impact on sleep as well as declarative learning. Sleep. 2008;31:1401–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Anderer P, Klosch G, Gruber G, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103:581–92. doi: 10.1016/s0306-4522(01)00028-8. [DOI] [PubMed] [Google Scholar]
- 37.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [Review] [DOI] [PubMed] [Google Scholar]
- 38.Scholle S, Zwacka G, Scholle HC. Sleep spindle evolution from infancy to adolescence. Clin Neurophysiol. 2007;118:1525–31. doi: 10.1016/j.clinph.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–78. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 40.Doran SM. The dynamic topography of individual sleep spindles. Sleep Res Online. 2003;5:133–9. [Google Scholar]
- 41.Schmidt C, Peigneux P, Muto V, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–82. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121:1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–45. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- 44.Jenni OG, Borbely AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol. 2004;286:528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- 45.Rosanova M, Ulrich D. Pattern-specific associative long term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]