Abstract
Study Objectives:
To determine the impact of alcoholism on sleep architecture and sleep EEG power spectra in men and women with uncomplicated alcoholism.
Design and Participants:
42 alcoholics (27 men) and 42 controls (19 men) screened for medical, psychiatric, and sleep problems participated in a full night of polysomnography following an adaptation night. Data were collected from multiple scalp sites and subjected to power spectral analysis. Sleep architecture and EEG spectral power measures were evaluated for the effects of diagnosis and sex using age as a covariate.
Results:
Compared with controls, alcoholics had less slow wave sleep and increased proportions of stage 1 and REM sleep. Spectral analysis of NREM sleep showed reduced levels of slow wave activity (SWA, 0.3–4 Hz) and slow θ (theta) power (4–6 Hz) in alcoholics. The differences in SWA extended across the slow band (0.3–1 Hz) and all δ (delta) frequencies and were most prominent over frontal scalp regions. No group differences were seen in the power spectra of REM sleep. Women had more SWA and θ power than men, but there were no sex by diagnosis interactions for any measures, suggesting that alcoholism does not differentially influence men and women.
Conclusion:
Long-term alcoholism affects sleep even after long periods of abstinence in both men and women. Measures of frontal slow wave activity were particularly sensitive markers of this long-lasting effect. Sleep EEG measures would thus seem to provide a functional correlate of the changes in brain structure seen in frontal cortex of long-term alcoholics.
Citation:
Colrain IM; Turlington S; Baker FC. Impact Of Alcoholism On Sleep Architecture And EEG Power Spectra In Men And Women. SLEEP 2009;32(10):1341-1352.
Keywords: Alcoholism, sleep, K-complex, N550, delta, sex
ALCOHOLISM IS A MAJOR HEALTH PROBLEM WITH AN ESTIMATED LIFETIME PREVALENCE IN THE UNITED STATES OF APPROXIMATELY 14%.1 IT IS DISPROPORTIONATELY represented in patients seeking treatment in hospitals and is thought to be present in up to 20% of patients in private hospitals, up to 35% of patients in municipal or teaching hospitals, and 50% of patients in Veterans Administration hospitals.2 Self-reported sleep problems are ubiquitous in those suffering from alcohol abuse and dependence, with rates of insomnia being extremely high.3 The sleep disturbances last long after successful detoxification and have been viewed as a potential pathway to relapse, when alcohol is consumed to facilitate sleep onset.4
In laboratory studies there is a general trend for alcoholics to have objectively defined sleep disturbances, which include increased frequency of awakenings, increased REM pressure,5 and decreased slow wave sleep (SWS).6 While several studies have focused on REM pressure abnormalities,5,7–10 SWS is also clearly impacted by alcoholism.5,7,9–18 Indeed, a meta-analysis of 8 studies of sleep in alcoholic men19 showed substantial reductions in SWS as the most consistent finding. Few studies have included female alcoholics, and none have had sufficient numbers to allow investigation of potential sex differences in the impact of alcoholism on sleep.
Comparison of alcoholics and controls using a combination of polysomnography and spectral analysis of sleep EEG has only been conducted in 2 sets of studies as far as we are aware. Gann et al.10 conducted a study comparing 29 alcoholic men and 11 alcoholic women with 30 control subjects. Alcoholics were consecutively recruited from enrolments in an inpatient treatment program. The major focus of the study was to evaluate the impact of a cholinergic REM sleep induction tests using an acetylcholinesterase inhibitor (galanthamine hydrobromide) compared to placebo. Subjects were studied for 3 consecutive nights, the first serving as an adaptation and screening night. An evaluation of data from placebo nights indicated that alcoholics had significantly reduced sleep efficiency, increased number of wake periods, and percentages of wakefulness and stage 1 sleep throughout the night compared with controls.10 Alcoholics also showed significantly reduced stage 2 sleep, SWS, and latency to the onset of REM sleep, but increased REM pressure compared to controls. Data from the adaptation nights were reported in Gann et al.20 Fewer differences between alcoholics and controls were reported, although the differences in the percentages of stage 1, stage 2, and SWS, as well as reduced REM onset latency and increased REM pressure were found. Data from both the adaptation and placebo nights were reanalyzed in Feige et al.,9 in which alcoholic subjects were subdivided into those who were shown to relapse 6 months after initial study and those who remained abstinent. The SWS effect seen previously in the adaptation night, held for both groups of alcoholics. Interestingly, spectral analysis of stage 2 and REM sleep data only showed significant group differences in data from the adaptation nights. Group effects were seen for high-frequency β (beta) activity in REM sleep and γ (gamma) activity in stage 2 sleep. Due to an unbalanced representation of women in the abstaining and relapsing groups, it was not possible to conduct an analysis of the impact of sex on these effects.
In a careful study of sleep and sleep homeostasis in European and African American alcoholic men, Irwin and colleagues evaluated sleep over 5 nights: adaptation/screening night, 2 baseline nights, a night when sleep was restricted to the second half of the sleep period, and a recovery night immediately following the sleep restriction night. Based on analysis of the first baseline night, alcoholics had significantly reduced sleep efficiency and sleep onset latency, significant increased percentage of stage 1 sleep, and significantly reduced SWS compared to controls.18 The percentage of REM sleep was not different, but showed a trend to be higher in alcoholics. REM onset latency was reduced, and both REM density and the duration of the first REM period were significantly increased in alcoholics. Spectral analysis of the EEG during NREM sleep revealed significantly reduced δ (delta) power (0.75–4 Hz) across the entire night in alcoholics, particularly in the first NREM period. Total power in the measured spectrum was also reduced, and there was a trend for θ power to be reduced in the first NREM period in alcoholics.
An analysis of the second baseline night (night 3) and the recovery night (night 5) were reported in.17 Similar results were obtained to those reported in18 with alcoholics having increased stage 1 and REM sleep, and decreased stage 2 and SWS relative to controls. Spectral analysis showed alcoholics to have significantly higher β activity across the entire night (a trend at baseline and significant on the recovery night), and significantly reduced δ power. Importantly there was a differential impact of sleep deprivation on SWS recovery as a function of both alcohol dependence and ethnicity. European American controls showed a larger stage 4 rebound than African American controls, with essentially no rebound being shown in either alcoholic group.
Based on these studies, there is some evidence for elevated high-frequency activity and reduced slow wave activity (SWA, < 4 Hz) during sleep in alcoholic men. Further support for a potential impact of alcoholism on SWA comes from a recent study from our group21 of sleep evoked potentials (ERPs), in which evoked δ frequency components during stage 2 sleep were compared between men and women with alcoholism and age- and sex-matched controls. The incidence and amplitude of the evoked δ frequency responses were significantly reduced in the alcoholic subjects, with the amplitude reduction being apparent only over prefrontal and frontal scalp sites.
More studies have focused on spectral analysis of awake resting eyes closed EEG in an attempt to characterize differences between alcoholics and control subjects, and many of these studies have much larger subject numbers and data collected from more electrodes than those conducted during sleep due to the relative ease of data collection. Studies have found that alcoholics have less δ power22 especially over frontal scalp regions.23 Faster activity (θ through β or γ) has been found to be elevated in alcoholics.24–26
The present study sought to evaluate the impact of alcoholism on sleep in men and women. Our first hypothesis was that alcoholism would be associated with decreased SWS and increased REM sleep pressure, indicated by reduced latency to REM sleep and increased REM sleep in men and women. Our second hypothesis was that alcoholism would be associated with reduced SWA during NREM and REM sleep in both men and women, with the effect seen to be more prominent over frontal scalp sites.
METHODS
Subjects
The study sample has been previously described.21 Briefly, potential participants underwent medical and psychiatric prescreening. All alcoholic participants and none of the controls met DSM-IV criteria for alcohol dependence. One hundred and five subjects underwent a screening polysomnogram (PSG), and 21 were excluded due to the presence of a clinically significant sleep disorder (AHI > 15, periodic leg movement arousal index > 10). Eighty-four subjects completed the study, 42 abstinent long-term alcoholics (27 men) who met DSM-IV criteria for alcohol dependence and 42 controls (19 men). Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).27 Data from 9 subjects (6 alcoholic men, 2 alcoholic women, 1 control man) were excluded from power spectral EEG analysis because of artifact in one or more of the electrode sites. The present data were collected from a night that was non-consecutive to either the adaptation night or the night of event-related potential data collection published previously.21 Data collection occurred on average 8 nights after the previous night in the laboratory and 16 nights after initial adaptation.
PSG analyses in the present paper were conducted on the same group of 84 subjects as previously described.21 Characteristics of the 34 alcoholics and 41 controls with data available for power spectral analysis are presented in Table 1. As with data from the entire sample, there were no significant effects of alcoholism diagnosis or sex on age or BMI. As expected, estimated lifetime alcohol consumption was significantly higher in alcoholics than in controls (F1,74 = 99.85, P < 0.001) and significantly higher in men than in women (F1,74 = 12.53, P = 0.001), with the sex difference more pronounced in the alcoholics (F1,74 = 9.00, P = 0.004). Alcoholics had shorter sobriety prior to testing than controls as indicated by the Mann-Whitney test (Z = −2.871, P = 0.002). The range of sobriety in alcoholics was 30 to 719 days and in controls was 1 to 10454 days.
Table 1.
Participant Characteristics Presented as Means (Standard Deviations) for all Controls and Alcoholics and then Separately for Men and Women in each Diagnostic Group
| Age | BMI | ESS | PSQIa | Sobrietyd | EACabc | |
|---|---|---|---|---|---|---|
| All | ||||||
| Control | 51.1 (9.7) | 25.2 (4.7) | 4.5 (3.0) | 4.4 (2.4) | 737.6 (2076.0) | 54.4 (84.7) |
| Alcoholic | 49.1 (8.5) | 25.6 (4.7) | 6.0 (3.3) | 7.1 (2.8) | 196.3 (189.2) | 1294.7 (784.8) |
| Women | ||||||
| Control | 51.0 (9.8) | 24.1 (3.4) | 4.1 (3.1) | 3.8 (2.3) | 1024.7 (2532.3) | 27.2 (36.7) |
| Alcoholic | 46.9 (9.7) | 25.9 (5.6) | 5.8 (2.7) | 6.7 (2.4) | 258.0 (217.1) | 843.7 (339.3) |
| Men | ||||||
| Control | 51.2 (9.8) | 26.6 (5.7) | 5.0 (2.7) | 5.1 (2.4) | 324.9 (1097.5) | 90.2 (114.9) |
| Alcoholic | 50.5 (7.7) | 25.4 (4.2) | 6.1 (3.7) | 7.3 (3.1) | 156.2 (161.8) | 1607.2 (885.5) |
Data are presented for age in years, body mass index (BMI) in kg/m2, Epworth Sleepiness Scale (ESS),58 Pittsburgh Sleep Quality Index (PSQI),27days since last drink before the study (Sobriety), and lifetime estimated alcohol consumption (EAC) in kg.59
Alcoholics vs. Controls, P < 0.001;
men vs. women P < 0.01;
diagnosis by sex interaction P < 0.01.
Alcoholic vs Controls, P < 0.01 (nonparametric)
Procedure
Data Acquisition and Analysis
The study was approved by the Internal Review Board at SRI International. Electroencephalographic (EEG), electro-oculographic (EOG), and electromyographic (EMG) recordings were made using Neuroscan Synamp amplifiers and Scan 4.3 software (Compumedics Neuroscan, El Paso, TX, USA). Electrodes for EEG recordings were placed at Fz, FCz, Cz, CPz, Pz, and O2 sites according to the international 10-20 system and were cross-referenced to A1 or A2. EEG signals were digitized at a sampling rate of 1000 Hz, high-pass filtered at 0.01 Hz, and low-pass filtered at 200 Hz. Data were refiltered offline with a bandpass of 0.3–30 Hz for visual scoring of the PSG. Thirty-sec epochs were scored according to standard criteria28 by 2 scorers blind to diagnosis of the subjects. Interrater reliability of sleep scoring was set at 0.90 with discrepancies resolved by a third scorer. Time in bed (TIB) refers to the time from lights-out to lights-on. Total sleep time (TST) is the time spent asleep minus in-bed wakefulness during the TIB. Sleep efficiency was calculated as a percentage of TST during TIB. Sleep onset latency was taken as the time from lights-out to the first three epochs of any stage of sleep. The time between sleep onset and the first epoch of REM sleep was defined as REM sleep onset latency (ROL). Time spent in each sleep stage was expressed as a percentage of TST.
Quantitative EEG Analysis of NREM Sleep
Power spectral analysis was performed using PASS PLUS EEG analysis software (Delta Software, St. Louis, MO.) on all EEG signals. Data were refiltered with a bandpass of 0.15–60 Hz. A fast Fourier transform (FFT) routine was performed on 30-s epochs of 4-s Welch tapered windows with 2.0-s overlap, resulting in a frequency resolution of 0.25 Hz. Spectral power density (μV2/Hz) was calculated for the 0.3–1 Hz frequency bin and for subsequent 1-Hz frequency bins (identified by their lower boundary value) until 50 Hz. Power spectra were also averaged for the main frequency bands: SWA (defined in this study as 0.3 to < 4 Hz comprising slow and δ [delta] rhythms); θ (theta) (4 to < 8 Hz); α (alpha) (8 to < 12 Hz); σ (sigma) (12 to < 15 Hz); β1 (beta1) (15 to < 23 Hz); β2 (beta2) (23 to < 30 Hz), and γ (gamma) (30 to < 50 Hz).
Epochs containing movement or artifact were excluded using a fully automated process described by Feige et al.9,29 Briefly, the total (0.3– 50 Hz) and γ-band (30– 50 Hz) log power of each epoch was related to the corresponding median-filtered value (the median of values in the 5 min preceding and 5 min following the epoch), and an epoch was excluded if the deviation was larger than the difference between the median and the first quartile of all median-filtered values across the night. In this way, artifacts restricted to the low frequencies (such as EOG events) or higher frequencies (such as EMG contamination) were eliminated in a data-driven way.29 Power spectra were averaged across NREM sleep (stages 2, 3, and 4) for the entire night and also for the first NREM sleep period. We also calculated spectral power density during stage 2 sleep only, to exclude the influence of different amounts of SWS, as recommended by some.29 Since results of the analysis of stage 2 sleep were the same as the analysis of NREM sleep, they are not reported. Power spectra were also averaged for all REM sleep across the whole night.
Statistical Analysis
All results are reported as means ( ± SD) unless otherwise indicated. Univariate analyses of variance (ANOVAs) were used to investigate changes in visually scored PSG variables according to diagnosis and sex. Power density values for each of the broad frequency bands were normalized with a logarithmic transformation before being subjected to a mixed model ANOVA, with diagnosis and sex as between-groups factors, electrode site as a repeated measures, and age as a covariate. When Maunchly's test of sphericity showed significance for site, degrees of freedom were adjusted using the Greenhouse-Geisser correction, but original degrees of freedom are reported. Relationships between visually scored sleep variables and all-night power spectra variables for NREM and REM sleep with age and alcohol consumption variables were explored with the Pearson correlation.
RESULTS
Pittsburgh Sleep Quality Index
The PSQI27 was significantly higher in alcoholics than controls (F1,79 = 22.5, P < 0.001), with no effect of sex or age and no sex by diagnosis interaction. A multiple analysis of covariance (MANCOVA) model was run with sex and diagnosis as between group factors and age a covariate for the individual subscale scores of the PSQI: Subjective Sleep Quality; Sleep Latency; Sleep Duration; Habitual Sleep Efficiency; Sleep Disturbances; Use of Sleep Medications; and Daytime Dysfunction. There were significant differences between alcoholics and controls on all subscales (P < 0.05) apart from the use of sleep medications. In all cases the alcoholics had higher scores (i.e., worse perceived sleep) than the controls. Sex differences were apparent for the sleep disturbance (P = 0.033), use of sleep medications (P = 0.005), and daytime dysfunction subscales (P = 0.035), with women reporting less sleep disturbance, more medication use, and more daytime dysfunction. The only significant sex by diagnosis interaction was for sleep disturbance (P = 0.046) where the effect of diagnosis was larger in the women than in the men.
Sleep Variables Derived from Visual Scoring
Variables derived from visual scoring of the polysomnogram are shown in Table 2.
Table 2.
Variables derived from visual scoring of the polysomnogram in 42 alcoholics and 42 age-matched controls
| Controls |
Alcoholics |
|||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Total recording time | 446.2 ± .05 | 436.8 ± 58.3 | 453.7 ± 65.4 | 461.8 ± 67.8 |
| Total sleep timeg | 397.0 ± 59.9 | 378.7 ± 70.2 | 417.0 ± 73.2 | 398.2 ± 68.0 |
| Sleep efficiencyd | 89.0 ± 6.3 | 86.7 ± 11.1 | 91.6 ± 5.9 | 86.2 ± 7.3 |
| Wake after sleep onsetg | 37.3 ± 30.2 | 45.1 ± 36.7 | 27.2 ± 18.6 | 43.5 ± 25.5 |
| Sleep onset latency | 10.4 ± 9.3 | 11.3 ± 14.5 | 8.0 ± 10.2 | 16.0 ± 17.1 |
| REM latency | 93.6 ± 46.5 | 79.2 ± 69.6 | 84.6 ± 57.6 | 95.0 ± 80.5 |
| Number of wake periodsc | 16.5 ± 5.8 | 22.1 ± 7.9 | 15.9 ± 5.9 | 25.4 ± 12.5 |
| Stage 1 (%)b | 5.6 ± 2.6 | 6.2 ± 2.9 | 6.3 ± 4.4 | 8.5 ± 3.9 |
| Stage 2 (%)e | 61.7 ± 6.8 | 62.1 ± 9.6 | 58.2 ± 7.1 | 60.6 ± 10.2 |
| SWS (%)a,f | 12.1 ± 4.9 | 12.0 ± 6.3 | 11.1 ± 6.5 | 6.6 ± 5.2 |
| REM (%)b,g | 20.6 ± 4.2 | 19.9 ± 7.5 | 24.4 ± 6.2 | 24.3 ± 7.6 |
diagnosis, P < 0.01;
diagnosis, P < 0.05;
sex P < 0.0001;
sex, P < 0.05;
age, P < 0.0001;
age, P < 0.01;
age, P < 0.05.
Time in bed (total recording time) was similar in controls and alcoholics. There was a significant effect of diagnosis for SWS% (F1,79 = 9.3, P = 0.002), with alcoholics having a smaller percentage of SWS than controls. Alcoholics had more REM sleep% (F1,79 = 6.5, P = 0.012) and stage1 sleep percentage (F1,79 = 4.2, P = 0.04) than controls. Sex differences were apparent, with women having better sleep efficiency (SE%) (F1,79 = 4.7, P = 0.033) and fewer in-bed wake periods (F1,79 = 14.5, P < 0.0001) than men. Finally, age had a significant effect on several variables. Minutes of wake after sleep onset (WASO) increased with age (F1,79 = 5.5, P = 0.02) and age related declines were seen in TST (F1,79 = 4.6, P = 0.035) and the percentages of stage 2 sleep (F1,79 = 13.8, P < 0.0001), SWS% (F1,79 = 10.8, P = 0.002), and REM sleep (F1,79 = 6.4, P = 0.01).
All-Night NREM Sleep EEG Power Spectra
SWA
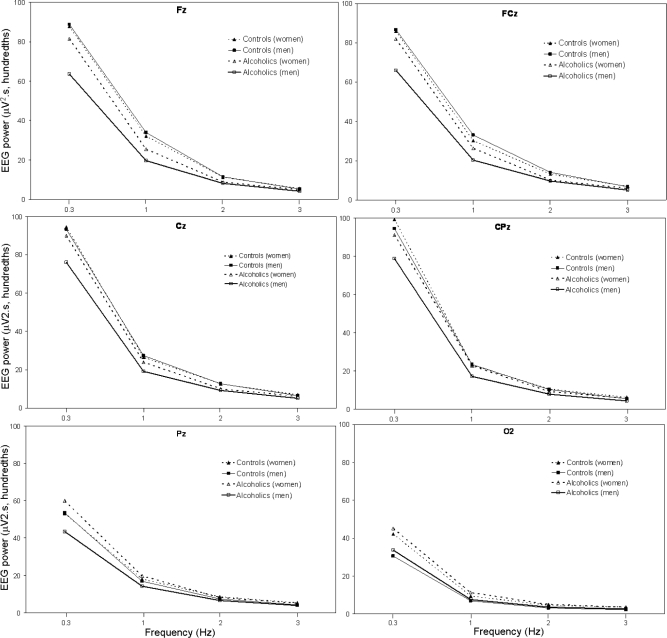
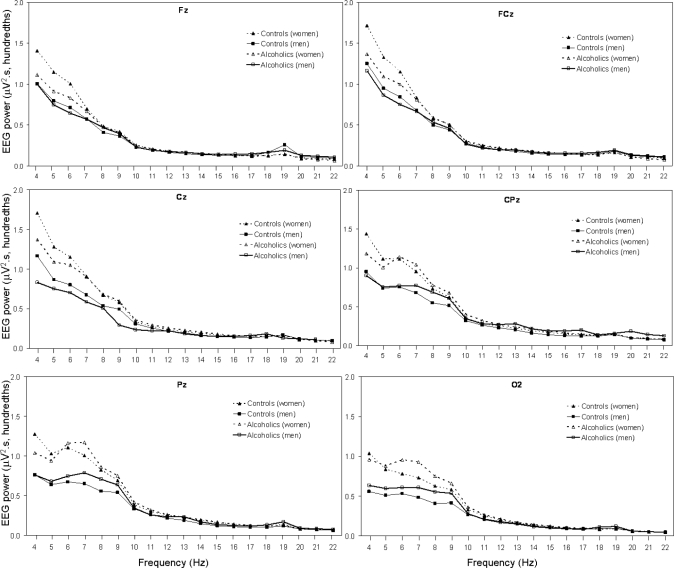
Figure 1 shows the average absolute EEG SWA in the frequency range from 0.3 to < 4 Hz for 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) during NREM sleep in alcoholic and control men and women. There was a significant effect of diagnosis for SWA (0.3 to < 4 Hz), with alcoholics having less SWA than controls (F1,70 = 4.0, P = 0.048). There was a significant main effect of sex (F1,70 = 6.7, P = 0.012) and a significant site by sex interaction effect (F5,350 = 3.6, P = 0.041), with women having higher SWA than men overall and especially at Pz and O2 regardless of diagnosis. Although male alcoholics appeared to show a more prominent difference in SWA compared with male controls than female alcoholics did compared with female controls (Figure 1), the diagnosis by sex interaction effect was not significant. There was a significant effect of site (F5,350 = 13.7, P < 0.001), with power appearing to be higher in the frontal and central sites and a significant diagnosis by site interaction (F5,350 = 9.2, P = 0.001), with the diagnosis difference appearing largest at frontal sites. Finally, SWA decreased significantly with age (F1,70 = 10.2, P = 0.002).
Figure 1.
All-night NREM sleep SWA (0.3 to < 4 Hz) at 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) for 34 alcoholics (21 men) and 41 controls (18 men). Values are averaged within the 0.3–1-Hz bin, and within each 1-Hz frequency bin thereafter. Bins are identified by their lower boundary. See text for details of statistical comparisons.
Theta
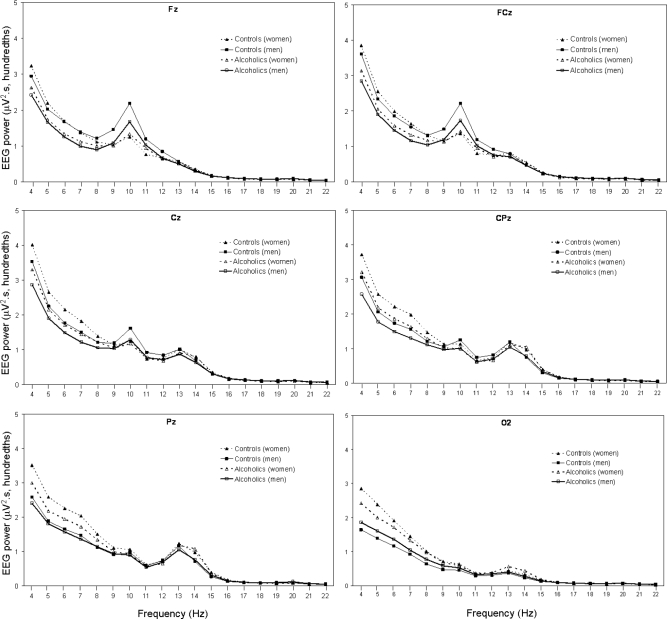
Figure 2 shows the average absolute EEG power density in the frequency range from 4 to < 23 Hz for 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) during NREM sleep in alcoholic and control men and women. There was a peak in α (8–12 Hz) power density prominent at Fz and FCz and a peak in σ (12–15 Hz) power density prominent at CPz and Pz sites (Figure 2). There was a significant main effect of diagnosis for θ power (F1,70 = 4.5, P = 0.038), with alcoholics having lower values than controls. There was also a significant main effect for sex (F1,70 = 8.4, P = 0.005) and a significant sex by site interaction effect (F5,350 = 7.8, P = 0.001), with women having greater θ power than men, apparent in the parietal and occipital sites. Age was not a significant factor in the ANOVA model.
Figure 2.
All-night NREM sleep spectral EEG power in the θ– β 1 bands (4 to < 23 Hz) at 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) for 34 alcoholics (21 men) and 41 controls (18 men). Values are averaged within each 1-Hz frequency bin. Bins are identified by their lower boundary. See text for details of statistical comparisons.
Alpha
There was a significant site effect (F5,350 = 4.1, P = 0.021) for α activity, which was higher at frontal sites (Figure 2). Main effects for diagnosis, sex, and age were not significant. There was a significant site by sex interaction effect (F5,350 = 10.6, P < 0.0001), with the frontal dominance appearing more pronounced in men than women.
Sigma
The main effect for diagnosis for σ power was not significant. There was a significant site effect (F5,350 = 7.8, P = 0.001), with larger values at central and parietal sites overall. The age effect was significant (F1,70 = 4.0, P = 0.049), with σ power declining with age.
Beta
There was a significant site effect for β1 power density (F5,350 = 6.3, P = 0.002), which was most prominent at central and parietal sites. None of the other main effects or interactions were significant. Similarly, there was a significant site effect for β2 power density (F5,350 = 3.7, P = 0.04), which was most prominent at Cz and CPz. There were no significant main or interaction effects for γ power during NREM sleep.
Single Bands
SWA comprises both slow ( < 1 Hz) and δ rhythms (1–4 Hz). Since there are differences in the cellular correlates of slow and δ rhythms they can be considered as 2 distinct processes,30–32 which may be differently affected by alcoholism. We therefore investigated where differences lay within the broad SWA band (0.3 to < 4 Hz) and the θ band (4 to < 8 Hz), by performing diagnosis by sex by site ANOVAs, with age as a covariate, to compare spectral power during all-night NREM sleep in the 0.3 to < 1 Hz band, and in the 1-Hz bands thereafter, up to 8 Hz. Results of the ANOVAs are shown in Table 3.
Table 3.
Significant Effects of Diagnosis by Sex by Site ANOVAs (with Age as a covariate) for Spectral Power within the δ and θ Bands for All-Night NREM Sleep
| Frequency (Hz) | Diagnosis Effect |
Sex Effect |
Derivation Effect |
Age Effect |
Diagnosis × Derivation interaction |
Sex × Derivation interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1,70 | P | F1,70 | P | F5,350 | P | F1,70 | P | F5,350 | P | F5,350 | P | |
| 0.3 to ≤ 1 | - | ns | 5.1 | 0.03 | 4.6 | 0.02 | 12.4 | <0.01 | 3.8 | 0.04 | - | ns |
| 1 to ≤ 2 | 7.2 | < 0.01 | - | ns | 17.4 | < 0.001 | 8.6 | < 0.01 | 18.9 | < 0.001 | 6.6 | < 0.01 |
| 2 to ≤ 3 | 6.8 | 0.01 | - | ns | 14.1 | < 0.001 | 5.2 | 0.03 | 14.4 | < 0.001 | 10.5 | < 0.001 |
| 3 to ≤ 4 | 5.1 | 0.03 | 8.7 | < 0.01 | 7.2 | < 0.01 | 8.8 | < 0.01 | 5.6 | < 0.01 | 8.8 | <0.01 |
| 4 to ≤ 5 | 5.7 | 0.02 | 8.1 | < 0.01 | - | ns | 5.8 | 0.02 | - | ns | 8.6 | < 0.01 |
| 5 to ≤ 6 | 4.7 | 0.03 | 6.8 | 0.01 | - | ns | - | ns | - | ns | 7.8 | < 0.01 |
| 6 to ≤ 7 | - | ns | 6.0 | 0.02 | - | ns | - | ns | - | ns | 6.8 | < 0.01 |
| 7 to ≤ 8 | - | ns | 6.1 | 0.02 | - | ns | - | ns | 3.9 | 0.02 | 5.9 | < 0.01 |
There were no significant Diagnosis × Sex × Derivation or Age × Derivation effects. ns, not significant.
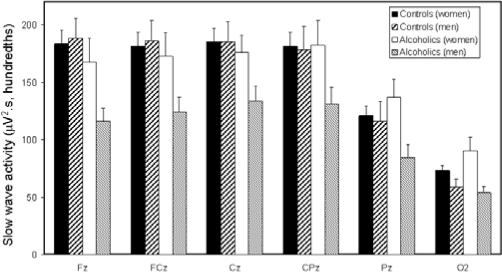
The 0.3 to < 1 Hz band did not show a significant main effect of diagnosis (P = 0.08), but there was a significant site by diagnosis effect (Table 3). Alcoholics had less power in the 0.3 to < 1 Hz band at frontal and central sites compared with controls (Figure 1). There was a significant main effect for sex, with women having higher power density than men. The sex by diagnosis interaction effect was not significant. There was a significant site effect with power appearing higher at frontal and central sites than parietal and occipital sites (Figure 1). Finally, slow wave power declined significantly with age.
There were significant diagnosis and diagnosis by site interaction effects for power in the 1 to < 2 Hz, 2 to < 3 Hz, and 3 to < 4 Hz bands. Alcoholics had less power than controls in all 3 δ bands at frontal and central sites. The diagnosis difference was no longer apparent at Pz and O2. There was a significant sex by site interaction, with women having higher power density in the 3 δ bands at Pz and O2. There were significant site effects, with slow wave power declining from frontal to occipital sites. Finally, power within each of the δ frequency bands declined significantly with age.
Compared to controls, alcoholics had lower power in the 4 to < 5 Hz and 5 to < 6 Hz bands, regardless of site. Compared to men, women had greater power in these 2 bands at CPz, Pz, and O2, but not at other sites. Similarly, women had greater power within the 6 to < 7 Hz and 7 to < 8 Hz bands, compared with men, at CPz, Pz, and O2 sites only. There were no significant diagnosis effects in the 6 to < 7 Hz or 7 to < 8 Hz bands, but there was a significant diagnosis by site interaction effect for 7 to < 8 Hz, with alcoholics having lower power at frontal and central sites.
EEG Power Spectra for the First NREM Sleep Episode
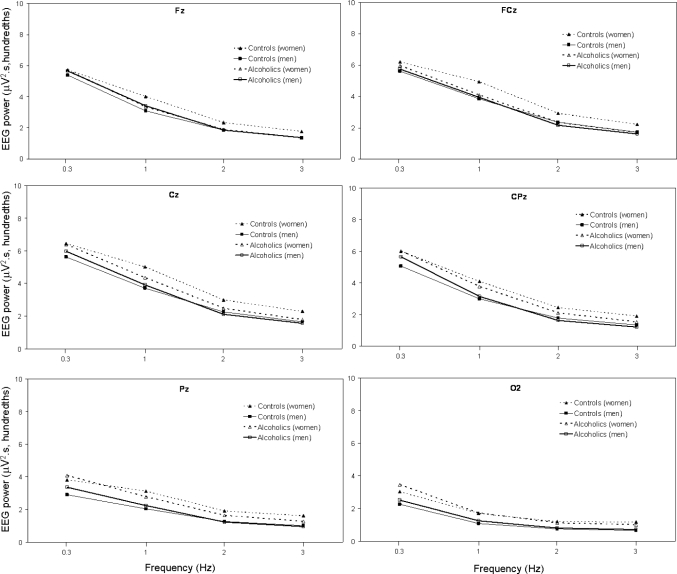
Average SWA at 6 electrode sites for the first NREM sleep period of the night for alcoholic and control men and women are shown in Figure 3. Results were similar as for the whole night analysis. SWA showed a significant effect of diagnosis (F1,70 = 6.0, P = 0.017) and a significant site by diagnosis interaction (F5,350 = 6.7, P = 0.003), with the lower levels of SWA in alcoholics being most evident at frontal sites. There was a significant main effect for sex (F1,70 = 6.6, P = 0.012) and a significant site by sex interaction effect (F5,350 = 4.0, P = 0.026), with women having greater SWA overall, most evident at Pz and O2. As evident in Figure 3, male alcoholics clearly showed the most prominent reduction in SWA, but the diagnosis by sex interaction effect was not significant (P = 0.1). There was a significant site effect overall (F5,350 = 11.0, P < 0.001), with SWA being highest at the frontal sites. Age was a significant factor (F1,70 = 10.5, P = 0.002), with SWA decreasing with age.
Figure 3.
Average SWA during the first period of NREM sleep at 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) for 34 alcoholics (21 men) and 41 controls (18 men). See text for statistical comparisons.
Theta power in the first NREM episode also showed a significant effect of diagnosis (F1,70 = 6.3, P = 0.014) and a significant site by diagnosis interaction effect (F5,350 = 3.5, P = 0.042), with alcoholics appearing to have less θ power at all sites except O2. There was a significant main effect of sex (F1,70 = 6.3, P = 0.006) and a significant site by sex interaction effect (F5,350 = 8.8, P = 0.001), with women having greater θ power, most apparent at central, parietal, and occipital sites. Age was also significant (F1,70 = 4.4, P = 0.039), with θ power decreasing with age.
There was a significant site effect for α power (F5,350 = 7.4, P = 0.001), which was highest at frontal sites, and a significant site by sex interaction effect (F5,350 = 11.2, P < 0.001), with men appearing to have greater α power at frontal sites. None of the other main effects approached significance.
There was a significant diagnosis by site effect for σ power (F5,350 = 3.3, P = 0.043), with alcoholics appearing to have less σ power in the first NREM period at frontal and central sites. There was a significant site effect (F5,350 = 6.1, P = 0.004) and a significant site by sex interaction effect (F5,350 = 3.3, P = 0.046), with men appearing to have more σ power at frontal sites but women appearing to have more σ power at parietal and occipital sites.
There was a significant site effect for β1 (F5,350 = 5.5, P = 0.005), which was most prominent at central and parietal sites. There was a site by sex interaction effect (F5,350 = 3.7, P = 0.0027) with women have more frontal power, but no diagnosis, sex, or age main effects. There was a significant site effect for β2 (F5,350 = 3.9, P = 0.034), which was most prominent at Cz and Pz. There were no significant effects for γ.
All-Night REM Sleep EEG Power Spectra
There was a significant site effect (F5,350 = 8.4, P = 0.003) for SWA in REM sleep (see Figure 4), which was highest at frontal sites. Age was a significant factor (F1,70 = 10.8, P = 0.002) with SWA decreasing with age. Neither the diagnosis effect nor any of the interaction effects were significant.
Figure 4.
All-night REM sleep SWA (0.3 to < 4 Hz) at 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) for 34 alcoholics (21 men) and 41 controls (18 men). Values are averaged within the 0.3–1-Hz bin, and within each 1-Hz frequency bin thereafter. Bins are identified by their lower boundary. Statistical analysis was performed on the averaged δ band. See text for details.
There was a significant site effect (F5,350 = 9.8, P < 0.001) for θ power (Figure 5), which was highest at FCz and Cz. There was also a significant sex effect (F1,70 = 12.3, P = 0.001) and site by sex interaction effect (F5,350 = 3.6, P = 0.03), with θ power appearing higher in women than in men, particularly at CPz and Pz. None of the other effects was significant for θ power although the site by diagnosis interaction effect tended to be significant (F5,350 = 2.95, P = 0.06), with θ power appearing higher in controls than alcoholics at frontal and central sites.
Figure 5.
Average all-night REM sleep spectral EEG power in the θ–β1 bands (4 to < 23 Hz) at 6 sites (Fz, FCz, Cz, CPz, Pz, and O2) for 34 alcoholics (21 men) and 41 controls (18 men). See text for statistical comparisons.
For α power in REM sleep, there was a significant sex effect (F1,70 = 5.2, P = 0.025), with α power being higher in women, but none of the other effects was significant. None of the effects was significant for σ or β1 power. There was a significant site effect for β2 power (F5,350 = 3.9, P = 0.034), which was greatest at FCz and Cz. There were no significant effects for γ power in REM sleep.
Influence of Lifetime Alcohol Consumption and Prior Sobriety on Alcoholism Effects
Due to the significant sex differences in estimated total lifetime alcohol consumption (EAC) and the period sobriety prior to testing, correlations between these measures and the key variables displaying alcoholism effects (PSQI, SWS, REM sleep, and SWA at Fz) were assessed separately in men and women using linear regression analysis with age as an additional predictor variable. Days sober prior to testing did not significantly predict any variables in alcoholic men or women. In alcoholic women, EAC significantly predicted the PSQI score (r = 0.544, age: ns.; EAC: P = 0.001). In alcoholic men, EAC significantly predicted the PSQI score (r = 0.347, age: ns.; EAC: P = 0.027) and age and EAC predicted the percentage of SWS sleep (r = 0.613, age: P < 0.001; EAC: P = 0.012). The model also predicted the percentage of REM sleep, but only the age coefficient was significant (r = 0.391, age: P = 0.023; EAC: ns.).
DISCUSSION
Alcoholics had increased amounts of stage 1 and REM sleep and decreased SWS relative to controls. SWA in NREM sleep was significantly reduced by alcoholism at frontal and central sites as was θ at all sites in both men and women. There were no significant sex by diagnosis interactions effects, indicating that alcoholism does not differentially affect sleep in men and women. There were no observed differences in sleep efficiency, sleep onset latency, REM onset latency, or the amount of wakefulness in the sleep period.
Studies that have shown decreased SE in alcoholics have typically studied patients with shorter periods of sobriety, ranging from 16–46 days5,7,18,20,33 Studies examining alcoholics after longer periods of abstinence failed to find differences in SE compared with controls.7,16,34 The subjects in the present study had an average of 179 days sober before the study with a large range (10 to 719 days). While less SWS and more stage 1 sleep are easily interpreted as being reflective of poorer sleep quality, a higher percentage of REM sleep in abstinent alcoholics is more difficult to explain. There is a substantial literature that describes REM sleep abnormalities in alcoholism in the context of an increase in REM pressure.5,7–10 It is reasonable to expect increased REM pressure in actively drinking or recently detoxified alcoholics, given that REM sleep is suppressed with high doses of alcohol.35 It is, however, less clear why it should be increased in those who have been abstinent for a long time. In any case, in the present study, REM onset latency (one of the key markers of REM pressure) was not affected by alcoholism. Possible alternative explanations of increased REM sleep are: first, that it represents a predisposition to altered sleep rather than a consequence of alcohol abuse; and second, that it represents alterations in brain chemistry induced by the abuse that do not recover with abstinence. Differentiation between these possibilities will require careful study of sleep in those with a genetic familial risk of alcoholism along the lines of the investigations conducted in wake EEG and evoked potentials.36
Spectral analysis of the sleep EEG revealed significantly reduced SWA in NREM sleep across the whole night and in the first NREM period in alcoholics relative to controls. Analysis of single frequency bands showed that alcoholics had reduced power in the slow ( < 1 Hz) band as well as in each of the 1 to < 2, 2 to < 3, and 3 to < 4 Hz δ bands. The observed decrease in SWA is consistent with the findings of Irwin et al.18 and with studies evaluating spectral properties of EEG in awake subjects22,23 in terms of the overall impact of alcoholism and the frontal distribution of the alcoholic-control difference.23 Interestingly, however, SWA during REM sleep did not differ between alcoholics and controls, showing that the reduction in SWA in alcoholism is sleep-state specific.
Theta power was lower in alcoholics compared with controls during all-night NREM sleep and in the first NREM period, and tended to be lower during REM sleep. Analysis of single bands indicated that the impact of alcoholism was limited to activity < 6 Hz. These results generally support those reported from Irwin's laboratory,17,18 and are able to extend them in a number of ways. First, our results show that the effects of alcoholism on SWA and θ power are apparent in women as well as men. Second, the impact on SWA is evident in NREM sleep but not REM sleep. Third, the effects of alcoholism are more prominent in anterior scalp locations. However, the present data do not show the significant elevation of β activity during NREM sleep seen in Irwin's study.17 Increases in high frequency β and γ activity in alcoholics in both NREM and REM sleep were also reported by Feige et al.9 There are important differences in methods and subject characteristics between those in the present study and previous studies,9,17 including differences in the periods of sobriety before subjects were studied, which may contribute to different results between studies, as previously discussed. There was a significant increase in β power only during recovery sleep following sleep deprivation in alcoholics studied by Irwin et al.,17 suggesting that a challenge to the sleep regulatory system may be needed to uncover effects of alcoholism on the high-frequency band during sleep. In the other study of power spectra during sleep in alcoholics,9 increased high-frequency activity in alcoholics was found only on an adaptation night.
In the present study neither estimated alcoholic consumption nor days of sobriety correlated with frontal δ power. The lack of correlation between SWA and alcohol consumption variables represents an important difference from the evoked δ frequency responses reported previously by our group.21 In that study, based on the same subjects investigated in the present report, longer sobriety correlated with increased N550 (r = − 0.44, P = 0.002) and P900 Fz (r = 0.369, P = 0.008) amplitudes, where these values represent the negative and positive peaks in single evoked δ waveforms. Comparison of the 2 data sets would seem to indicate that the evoked δ frequency responses are more sensitive measures of brain recovery with abstinence than the EEG power density measures from the present study. Estimated lifetime alcohol consumption was significantly related to the scores on the PSQI, with higher lifetime consumption predicting less sleep satisfaction. In alcoholic men, estimated lifetime consumption also predicted %SWS, with higher consumption being associated with less SWS. The PSQI data from the present study are consistent with the previously reports of alcoholics having increased dissatisfaction with sleep relative to controls using this measure.37–39 The values are generally lower than those previously reported in untreated recently detoxified alcoholics37–40 (but see Hornyak et al.41); however, they are consistent with the values seen post-treatment or after several months of abstinence.37,40 Taken together with the previously published data, the significant relationship with lifetime consumption but not with sobriety immediately prior to assessment, would argue for a pharmacologically mediated effect on perceived sleep quality that has only partial recovery with acute detoxification.
We are the first to evaluate topographic differences in EEG spectral power during sleep in alcoholics compared to controls. SWA was maximal over the frontal scalp regions as has previously been shown in studies of young normal individuals.42 This frontal distribution supports positron emission tomography30 and high density EEG array43 studies, implying a role for frontal cortex in the generation of SWA during sleep. The impact of alcoholism on SWA was greatest for frontal scalp regions, which is consistent with our previous results from the same subjects on a different night showing that evoked δ frequency responses are most reduced in alcoholics relative to controls at frontal sites.21 Both data sets probably reflect the differential impact of alcoholism on frontal cortical regions.
The impact of alcohol abuse on δ power at frontal and prefrontal sites is possibly due to a reduction in available neurons in prefrontal cortex. Uncomplicated alcoholism (i.e., not associated with medical or neurological sequelae such as cirrhosis, dementia, or amnesia) has been shown to produce reduced numbers of superior prefrontal cortex (PFC) neurons as measured in postmortem cells counts44–47 and to preferentially reduce gray matter volume in PFC as measured with in vivo MRI.48 There is also evidence of diminished metabolism49 and perfusion50 in frontal cortex of alcoholics.
Our results also represent the first systematic comparison of sleep and sleep EEG in men and women with alcoholism. Only 2 previous sets of studies have incorporated women,9,15,20 and neither of these presented sex by diagnosis analyses. We found significant effects of sex for many of the studied variables but no significant sex by diagnosis interactions. Women had better sleep efficiency, reduced number of wake periods, and a trend to have more SWS, which supports previous findings.51–54 Women showed elevated SWA during all-night NREM sleep and in the first NREM episode, and a trend for increased SWA in REM sleep, as shown by others.55–57 They had higher levels of θ activity in NREM and REM sleep and higher levels of α activity in REM sleep than men. Other studies that have used spectral analysis of the sleep EEG have also indicated that women have higher θ and α power during NREM sleep55–57 and during REM sleep56,57 than men. These sex differences in the sleep EEG may reflect differences in physical attributes, such as skull thickness56 or physiological differences, possibly related to cerebral metabolic rates.51 Women also differed from men in terms of their alcohol use, having lower levels of estimated lifetime alcohol consumption and longer periods of sobriety prior to testing than men. It is possible that these differences contributed to the main effect of sex on sleep and EEG variables, however it would be more likely that they produce a sex by diagnosis interaction which was not the case for any variable. What is clear from the present data is that regardless of the possible difference in magnitude, women show the same general pattern of alcoholism-related change in the SWS and in the slow frequency EEG during sleep, just as they showed the same pattern of effect as alcoholic men when evoked δ frequency responses were evaluated.21
Summary
Long-term alcohol abuse impacts sleep even after long periods of abstinence in both men and women. Measures of low-frequency EEG are sensitive markers of this long-lasting alcohol abuse effect, particularly when measured over frontal scalp regions. Sleep EEG measures would thus seem to provide a functional correlate of the changes in brain structure seen in frontal cortex of long-term alcoholics.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Colrain has received industry-supported research support. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Ali Yilmaz for his assistance with data analysis.
Financial Support: The work was supported by NIH grant AA14211.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual for Psychiatric Disorders IV Text Revision. Washington DC: American Psychiatric Press; 2000. [Google Scholar]
- 2.Arciniegas DB, Beresford TP. Neuropsychiatry: an introductory approach. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 3.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 4.Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20:279–86. doi: 10.1016/S0893-133X(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 5.Gillin J, Smith T, Irwin M, Kripke D, Schuckit M. EEG sleep studies in “pure” primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol Psychiatry. 1990;27:477–88. doi: 10.1016/0006-3223(90)90439-9. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers CL. Alcohol and sleep. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAA's Neuroscience and Behavioral Research Portfolio. Bethesda, MD: U.S. Department of Health and Human Services; 2000. pp. 417–36. [Google Scholar]
- 7.Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–802. [PubMed] [Google Scholar]
- 8.Thompson P, Gillin J, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiatry. 1995;38:831–6. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Feige B, Scaal S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31:19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 10.Gann H, Feige B, Hohagen F, van Calker D, Geiss D, Dieter R. Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol Psychiatry. 2001;50:383–90. doi: 10.1016/s0006-3223(01)01172-6. [DOI] [PubMed] [Google Scholar]
- 11.Allen R, Wagman A. Do sleep patterns relate to the desire for alcohol? In: Grosss MM, editor. Alcohol intoxication and withdrawal. New York: Plenum Press; 1975. pp. 495–508. [Google Scholar]
- 12.Wagman A, Allen R. Effects of alcohol ingestion and abstinence on slow wave sleep of alcoholics. In: Gross MM, editor. Alcohol intoxication and withdrawal. New York: Plenum Press; 1975. pp. 453–66. [DOI] [PubMed] [Google Scholar]
- 13.Othmer O, Dauhaday W, Goodin D, et al. Sleep and growth hormone secretion in adults. J Clin Psychiatry. 1982;43:411–4. [PubMed] [Google Scholar]
- 14.Rundell O, Williams H, Lester B. Sleep in alcoholic patients: longitudinal findings. In: Gross MM, editor. Alcohol intoxication and withdrawal. New York: Plenum Press; 1975. pp. 389–402. [Google Scholar]
- 15.Le Bon O, Verbanck P, Hoffmann G, et al. Sleep in detoxified alcoholics: Impairment of most standard sleep parameters and increased risk for sleep apnea, but not for myoclonias- A controlled study. J Stud Alcohol. 1997;58:30–6. doi: 10.15288/jsa.1997.58.30. [DOI] [PubMed] [Google Scholar]
- 16.Williams HL, Rundell OH., Jr. Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5:318–25. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 17.Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–41. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- 18.Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24:1376–84. [PubMed] [Google Scholar]
- 19.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 20.Gann H, Feige B, Fasihi S, van Calker D, Voderholzer U, Riemann D. Periodic limb movements during sleep in alcohol dependent patients. Eur Arch Psychiatry Clin Neurosci. 2002;252:124–9. doi: 10.1007/s00406-002-0371-8. [DOI] [PubMed] [Google Scholar]
- 21.Colrain IM, Crowley KE, Nicholas CL, Padilla M, Baker FC. The Impact of alcoholism on sleep evoked delta frequency responses. Biol Psychiatry. 2008 Dec 4; doi: 10.1016/j.biopsych.2008.10.010. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25:332–40. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- 23.Saletu-Zyhlarz G, Saletu B, Anderer P, et al. Nonorganic insomnia in generalized anxiety disorder. 1. Controlled studies on sleep, awakening and daytime vigilance utilizing polysomnography and EEG mapping. Neuropsychobiology. 1997;36:117–29. doi: 10.1159/000119373. [DOI] [PubMed] [Google Scholar]
- 24.Rangaswamy M, Porjesz B, Chorlian DB, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52:831–42. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- 25.Rangaswamy M, Porjesz B, Chorlian DB, et al. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003;27:607–15. doi: 10.1097/01.ALC.0000060523.95470.8F. [DOI] [PubMed] [Google Scholar]
- 26.Fein G, Allen J. EEG spectral changes in treatment-naive, actively drinking alcoholics. Alcohol Clin Exp Res. 2005;29:538–46. doi: 10.1097/01.alc.0000159107.08471.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington DC: U.S. Government Printing Office; 1968. [Google Scholar]
- 29.Feige B, Voderholzer U, Riemann D, Hohagen F, Berger M. Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol. 1999;110:1965–74. doi: 10.1016/s1388-2457(99)00147-9. [DOI] [PubMed] [Google Scholar]
- 30.Dang-Vu TT, Desseilles M, Laureys S, et al. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow ( < 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–99. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell IG, Higgins LM, Darchia N, Feinberg I. Homeostatic behavior of fast Fourier transform power in very low frequency non-rapid eye movement human electroencephalogram. Neuroscience. 2006;140:1395–9. doi: 10.1016/j.neuroscience.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Snyder S, Karacan I. Sleep patterns of sober chronic alcoholics. Neuropsychobiology. 1985;13:97–100. doi: 10.1159/000118169. [DOI] [PubMed] [Google Scholar]
- 34.Adamson J, Burdick JA. Sleep of dry alcoholics. Arch Gen Psychiatry. 1973;28:146–9. doi: 10.1001/archpsyc.1973.01750310116019. [DOI] [PubMed] [Google Scholar]
- 35.Aldrich M National Institute on Alcohol Abuse and Alcoholism Research Monograph 33 Alcohol Problems and Aging: U.S. Department of Health and Human Services. Effects of alcohol on sleep. 1998:281–300. [Google Scholar]
- 36.Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Pasmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Friedmann PD, Rose JS, Swift R, Stout RL, Millman RP, Stein MD. Trazodone for sleep disturbance after alcohol detoxification: a double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2008;32:1652–60. doi: 10.1111/j.1530-0277.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–51. [PubMed] [Google Scholar]
- 39.Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addict Biol. 2003;8:455–62. doi: 10.1080/13556210310001646439. [DOI] [PubMed] [Google Scholar]
- 40.Currie SR, Clark S, Hodgins DC, El-Guebaly N. Vol. 99. (Abingdon, England): Addiction; 2004. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics; pp. 1121–32. [DOI] [PubMed] [Google Scholar]
- 41.Hornyak M, Haas P, Veit J, Gann H, Riemann D. Magnesium treatment of primary alcohol-dependent patients during subacute withdrawal: an open pilot study with polysomnography. Alcohol Clin Exp Res. 2004;28:1702–9. doi: 10.1097/01.alc.0000145695.52747.be. [DOI] [PubMed] [Google Scholar]
- 42.Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 43.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–70. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–61. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 45.Harper C, Kril JJ. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–9. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- 46.Harper CG, Kril JJ, Daly JM. Are we drinking our neurones away? Br Med J. 1987;294:534–6. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 48.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 49.Samson Y, Baron JC, Feline A, Bories J, Crouzel C. Local cerebral glucose utilisation in chronic alcoholics: a positron tomographic study. J Neurol Neurosurg Psychiatry. 1986;49:1165–70. doi: 10.1136/jnnp.49.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hata T, Meyer JS, Tanahashi N, et al. Three-dimensional mapping of local cerebral perfusion in alcoholic encephalopathy with and without Wernicke-Korsakoff syndrome. J Cereb Blood Flow Metab. 1987;7:35–44. doi: 10.1038/jcbfm.1987.6. [DOI] [PubMed] [Google Scholar]
- 51.Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently? J Sleep Res. 1997;6:211–5. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 52.Goel N, Kim H, Lao RP. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int. 2005;22:905–15. doi: 10.1080/07420520500263235. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi R, Kohsaka M, Fukuda N, Honma H, Sakakibara S, Koyama T. Gender differences in the sleep of middle-aged individuals. Psychiatry Clin Neurosci. 1998;52:186–7. doi: 10.1111/j.1440-1819.1998.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 54.Webb WB. The measurement and characteristics of sleep in older persons. Neurobiol Aging. 1982;3:311–9. doi: 10.1016/0197-4580(82)90019-7. [DOI] [PubMed] [Google Scholar]
- 55.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 56.Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: Visual scoring and spectral analysis. Sleep. 1989;12:500–7. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- 57.Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E. Sex differences in delta and alpha EEG activities in healthy older adults. Sleep. 2005;28:1525–34. doi: 10.1093/sleep/28.12.1525. [DOI] [PubMed] [Google Scholar]
- 58.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 59.Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–7. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]