Abstract
Study Objectives:
Certain respiratory control characteristics determine whether patients with collapsible upper airway develop stable or unstable breathing during sleep, thereby influencing the severity of obstructive apnea (OSA). These include arousal threshold (TA), response to transient hypoxia and hypercapnia (Dynamic Response) and the increase in respiratory drive required for arousal-free airway opening (TER). We wished to determine whether these characteristics are inherent or are acquired during untreated OSA.
Design:
TA, Dynamic Response, and TER were measured in patients with severe OSA before and after treatment with continuous positive airway pressure (CPAP). Changes observed after treatment were deemed to have been acquired during untreated OSA.
Setting:
University-based sleep laboratory.
Patients:
15 patients with severe OSA.
Interventions:
(1) 30-sec alterations in inspired gases during sleep on CPAP. (2) Brief dial-downs of CPAP (dial-downs) both during air breathing and when ventilation was increased to different levels.
Measurements and Results:
TA: the increase in ventilation associated with a 50% probability of arousal (TA50). Dynamic Response: the increase in ventilation on the 5th breath following breathing 3% CO2 in 11% to 15% O2. TER: the increase in ventilation prior to dial-downs that was associated with an arousal-free airway opening during dial-down. CPAP therapy (10.5 ± 4.3 months) resulted in marked reduction in Dynamic Response (131% ± 95% to 52% ± 34% baseline ventilation, P < 0.005), a decrease in TA50 (134% ± 78% to 86% ± 47% baseline ventilation, P < 0.05), and no change in TER.
Conclusions:
TER may be an inherent characteristic. Untreated OSA results in an increase in dynamic response to asphyxia and an increase in arousal threshold.
Citation:
Loewen A; Ostrowski M; Laprairie J; Atkar R; Gnitecki J; Hanly P; Younes M. Determinants of Ventilatory Instability in Obstructive Sleep Apnea: Inherent or Acquired? SLEEP 2009;32(10):1355-1365.
Keywords: Ventilatory stability, arousal threshold, apnea-hypopnea index, asphyxia, plasticity, respiratory control, effective recruitment threshold
WE HAVE RECENTLY DESCRIBED A NUMBER OF PHYSIOLOGICAL PARAMETERS THAT ARE IMPORTANT DETERMINANTS OF VENTILATORY STABILITY DURING sleep in patients with obstructive sleep apnea (OSA) and whose alteration contributes to the severity of this disorder:
Effective Recruitment Threshold (TER): It has long been believed that arousal is required to open the upper airway in patients with OSA. However, recent evidence strongly suggests that most OSA patients are capable of opening, and maintaining open, their airway without arousal if chemical drive is allowed to rise sufficiently. Specifically: (1) Many OSA patients have periods of stable breathing during sleep.1 (2) The temporal association between arousal and upper airway opening is more consistent with an incidental than a causal relationship.2 (3) The obstructive events that typically occur upon withdrawal of continuous positive pressure (CPAP dial-downs) can be prevented if chemical drive is increased a threshold amount before dial-down.3 This threshold has been called the effective recruitment threshold (TER).3,4 A high TER promotes instability (and, hence, a higher apnea-hypopnea index) because the high respiratory muscle output at the time of opening results in a ventilatory overshoot when the airway opens. This reduces the stimulus required to keep the airway open, thereby promoting recurrence of airway closure.3,4 The determinants of this threshold are not known, but it may be expected that the magnitude of TER is in part related to the severity of the pharyngeal mechanical abnormality (see Discussion).
Arousal Threshold (TA): Although OSA patients have a higher arousal threshold than normal subjects,5,6 nonetheless, most patients still arouse in response to very small, and certainly non-threatening, changes in blood gas tensions.2,3 This impairs their ability to raise chemical drive sufficiently to keep the airway open without arousal. The occurrence of unnecessary arousals at the time of opening accentuates the ventilatory overshoot, further destabilizing breathing.2–4
Dynamic Response to Asphyxia: Another factor that promotes recurrence of obstructive apnea is a high dynamic response to the changes in blood gas tensions that occur during obstructive events. Because of the lung-to-carotid circulatory delay, chemical drive continues to increase for 1 to 2 breaths following upper airway opening.3 A fast rate of increase in chemical drive in this interval would accentuate the overshoot by increasing the likelihood of arousal and by promoting more complete opening of the airway.
In a recent study,3 the passive mechanical properties of the pharynx, reflected by the pressure at which the upper airway closes (closing pressure), and these physiological parameters of ventilatory stability (TER, TA, and dynamic response to blood gas changes) were evaluated in a group of patients with severe OSA (apnea-hypopnea index = 91 ± 24 per h). Each variable spanned a wide range across patients, and the variables did not correlate with one another. Thus, the mechanism of instability varies considerably among patients. One question that arises is whether the abnormalities in these physiological parameters that lead to instability are inherent or acquired as a result of years of untreated OSA. More specifically, does untreated OSA alter these determinants of ventilatory stability in a way that promotes more severe OSA? In the present study, this question was addressed by repeating the measurement of these physiological parameters in 15 of the 21 original patients3 following ≥ 5 months of CPAP therapy.
METHODS
Patient Population
The subjects represent a subgroup of patients studied earlier, before the institution of CPAP, using identical methods.3 Patient selection has been described in detail previously.3 Briefly, patients suspected of having OSA during home cardiopulmonary monitoring and who were free of exclusion criteria (dialysis-dependent renal failure, congestive heart failure, severe COPD, previous stroke, and use of sedatives or antidepressants) underwent comprehensive diagnostic polysomnography. Once the diagnosis of severe OSA was confirmed, 21 patients participated in the research study which included measurement of closing pressure, TER, TA, and dynamic response to blood gas changes. The results of this study have been reported.3 Patients were then placed on CPAP therapy following titration during attended polysomnography. Patients who were compliant with CPAP were invited to return for a follow-up study which involved reassessment of closing pressure, TER, TA, and dynamic response to blood gas changes. The Conjoint Health Research Ethics Board at the University of Calgary approved the study and all patients signed an informed consent.
Polysomnography and Dial-Down Study
This was identical to the pre-CPAP study, which has been described previously.3 Patients were instrumented for measurement of electroencephalogram (three leads), electrooculogram, submental electromyogram, leg movements, electrocardiogram, respiratory excursions, oxygen saturation, PCO2 at the airway, and snoring. CPAP was applied via nasal mask connected to a multipurpose ventilator research prototype that allowed reduction of CPAP to 1.0 cm H2O.1,2,7 Respiratory flow was recorded from a pneumotachograph inserted in the hose of the ventilator. Mask pressure was recorded from a side port in the mask. All signals were sampled at 120 Hz and recorded using a Windaq data acquisition system (DATAQ Instruments, Akron, OH).
Every effort was made to study patients in the same body position as in the baseline, pre-CPAP dial-down study. However, 4 patients could not comply; 3 patients who slept in the lateral position at baseline slept in the supine position at follow-up, and one patient who slept supine at baseline slept in the lateral position at follow-up. Three patients could not sleep during the first study until they were administered a hypnotic (5 mg zopiclone in 2 patients, and 20 mg zaleplon in 1 patient). These 3 patients were given the same dose of medication during the follow-up study. CPAP pressure was titrated to correct OSA, flow limitation, and snoring (holding pressure). This level was maintained thereafter. If flow limitation appeared during ventilatory stimulation, the pressure was increased to correct this.
Measurement of Closing Pressure, TER, TA, and Dynamic Response to Blood Gas Changes
Once stable sleep was established, brief (3 breaths) dial-downs to different pressures were made to determine the pressure at which the airway closed (closing pressure). Typically, at this pressure, some dial-downs resulted in apnea, while others resulted in severe flow limitation. During subsequent dial-downs, pressure was always reduced to closing pressure. If complete obstruction was not observed at 1 cm H2O, subsequent dial-down pressure was 1 cm H2O. Dial-down for 3 breaths was applied either while breathing room air (air dial-downs; Figure 1A) or 30 sec after changing the inspired gas composition (gas dial-downs; Figures 1B and C). If arousal occurred within 30 sec of changing the inspired gas mixture, dial-down was not performed. Five inspired gas mixtures were applied in random order prior to dial-downs: 3%, 6%, and 9% CO2 in air; 3% CO2 in either 15% or 11% O2; and 1% CO2 in either 15% or 11% O2. The choice of which oxygen mixture to use was based on the degree of oxyhemoglobin desaturation produced by the 15% oxygen mixture; we targeted a drop in oxygen saturation of 5% to 10%. The same O2 concentration used in the initial study was used in the follow-up study. In patients in whom arousals occurred frequently with 3% CO2, we used CO2 concentrations of 2%, 4%, and 6%. Details of the gas delivery system have been outlined previously.3
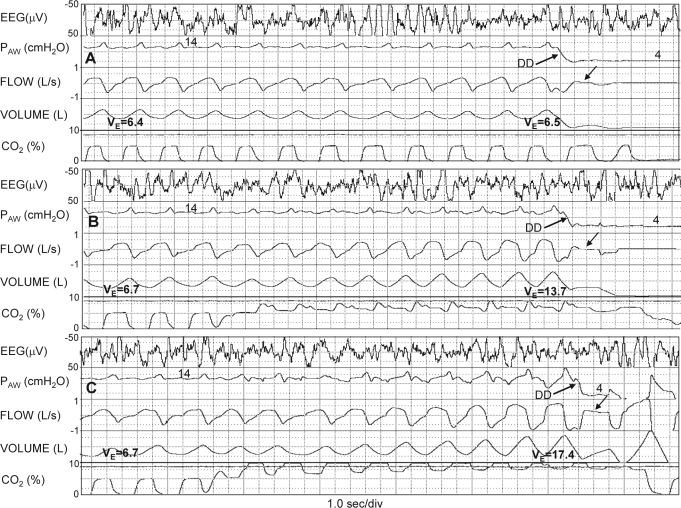
Figure 1.
Polysomnographic recordings illustrating the basic experimental procedures. A) Patient breathing air. Airway pressure (PAW) was reduced during sleep from holding pressure (14 cm H2O) to the pressure that resulted in near complete obstruction (Dial-down; upgoing arrow). B) Ventilation was increased from a baseline of 6.7 L/min to 13.7 L/min prior to dial-down by breathing a CO2-enriched mixture starting 30 sec before Dial-down. Note that flow during Dial-down was unchanged from the level obtained during air Dial-down (downgoing arrow). C) Ventilation was increased to an even higher level (17.4 L/min) before Dial-down. Note that there is substantial flow during the dial-down (downgoing arrow) indicating that at this level of chemical drive the dilator muscles could effectively maintain an open airway at the same airway pressure that resulted in near obstruction at lower levels of chemical drive. DD, dial-down; EEG, electroencephalogram.
Analyses
Analysis was limited to periods of NREM sleep. A full description of analysis methodology has been reported previously.3 Briefly, flow was corrected for leaks and was integrated to provide volume. Tidal volume, respiratory rate, minute ventilation, and mean inspiratory flow rate (tidal volume/ inspiratory duration) were measured breath-by-breath from the integrated flow tracing. The average of 10 breaths preceding dial-down or preceding a change in gas mixture is reported as the “baseline” value. End-tidal PCO2 (PETCO2) was measured in the expiratory phase during which CPAP was reduced at the onset of the dial-down. This expiratory phase invariably provided a technically adequate expiratory CO2 trace from which to estimate PETCO2. A senior certified technologist (MO) inspected the 3-channel electroencephalogram and electromyogram data and determined if an arousal occurred during an intervention and, if so, identified the onset of the arousal. Standard criteria were used to identify arousals,8 except that the 3-sec duration criterion for scoring an arousal was waived. In previous studies2,3 the accuracy of this manual scoring of arousals was confirmed with spectral analysis of the electroencephalogram and heart rate analysis.
Passive Pharyngeal Mechanics
The 3-breath dial-downs to different pressure levels were used for this purpose. Flow during the first dial-down breath was plotted against dial-down pressure. Linear regression analysis was performed.1,9 When the pressure intercept was ≥ 1, it was reported as closing pressure. When flow at the lowest pressure used (1 cm H2O) was still > 0 (i.e., hypopnea only), we reported the flow during the dial-down at 1 cm H2O instead of using the back-extrapolation technique.4
Dynamic Ventilatory Response to Hypoxia and Hypercapnia
Breath-by-breath ventilatory data between the onset of gas delivery and dial-down or arousal (whichever came first), were grouped according to the gas mixture used. Chemical drive achieved at any breath after the onset of gas delivery is reported as the difference in ventilation between the breath in question and the corresponding baseline values and is expressed both as L/min and as % of baseline ventilation. Only responses that were not associated with arousals are reported. The average response at breath 5 following a change to a given gas mixture was used to compare responses between different gases, different patients, and pre- vs. post CPAP therapy, since values for ventilation after the fifth breath were often invalidated by an arousal from sleep. Responses during hypoxic exposures were matched for the O2 concentration used (i.e., 11% or 15% O2).
Arousal Threshold
Some gas mixtures resulted in arousal before the end of the intervention while others did not. Minute ventilation values preceding dial-downs or preceding arousal for all tests in each patient were arranged in ascending order. Ventilation values associated with arousal were identified. A Kaplan-Meier curve10 was generated that reflected the probability of having no arousal as a function of ventilation reached (Figure 2). TA is reported as the ventilation associated with 50% probability of arousal (TA50) and also the value that could not be exceeded without arousal (TA0, Figure 2).
Figure 2.
Kaplan-Meier plot in a single patient showing the probability of arousal not occurring at different levels of chemical drive. At baseline ventilation (≈5 L/min) the probability of no arousal is very high. At some ventilation level (TA0) the probability of avoiding arousal is zero. Arousal threshold is expressed as the increase in ventilation above baseline at which the probability of arousal is 50% (TA50). Note the reduction in TA0 and TA50 following CPAP therapy in this patient.
Effective Recruitment Threshold (TER)
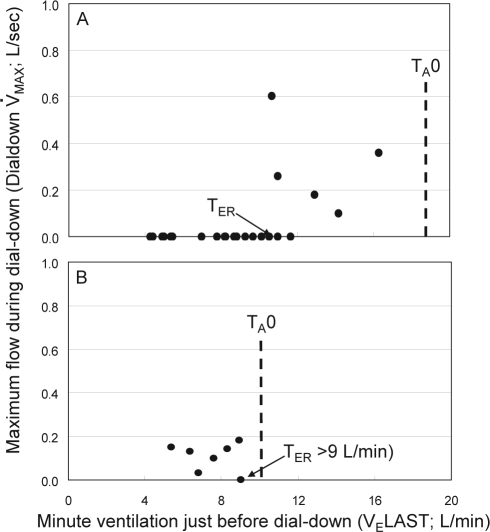
We obtained 2 values from each dial-down where there was no arousal before and during at least the first dial-down breath: (a) Minute ventilation in the last breath before dial-down (VELAST). The difference between VELAST and baseline ventilation provided the increase in chemical drive preceding the dial-down. (b) The highest flow value (V̇MAX) during the first 2 breaths after the dial-down (Dial-down V̇MAX). If an arousal occurred during breath 2, peak flow of the first dial-down breath was reported. Dial-down V̇MAX was plotted against VELAST (Figure 3). Typically, there was a range of chemical drive over which Dial-down V̇MAX was not different from that obtained during air breathing (i.e., baseline drive). Above a certain chemical drive, Dial-down V̇MAX was higher than the values obtained at baseline drive (Figure 3A), indicating that at this drive level, pharyngeal dilators were activated sufficiently to open the airway.3,4 A significant increase in Dial-down V̇MAX was defined as a value > mean + 2 SD of the baseline values. We required ≥ 2 values to be significant. The lowest chemical drive level at which a significant increase in Dial-down V̇MAX occurred was identified (Figure 3A). The difference between this value and baseline ventilation was the increase in chemical drive required for effective recruitment of upper airway dilator muscles (TER). Determination of TER was clearly limited by the maximum increase in ventilation that could be achieved without arousal (i.e., TA0, Figure 2). When TA0 was < TER, there was no significant increase in Dial-down MAX over the entire range of ventilation tested (Figure 3B). In this case, TER was reported as > X, where X is the highest ventilation reached without arousal that was not associated with an increase in Dial-down MAX (e.g., Figure 3B).
Figure 3.
Method of determining Effective Recruitment Threshold (TER). A) Maximum flow observed during dial-downs (Dial-down V̇MAX) is plotted against minute ventilation in the last breath before dial-down. Individual data points are obtained from observations such as those shown in Figure 1. Below a certain ventilation, Dial-down V̇MAX does not increase as ventilation is increased. Above that level, Dial-down V̇MAX is higher than the level observed at baseline respiratory drive. The ventilation above which Dial-down V̇MAX is > mean + 2 SD of the values obtained during air dial-downs is identified. The difference between this value and baseline ventilation is TER. Note that in this patient, before CPAP therapy (panel A), it was possible to test for TER over a wide ventilation range because arousal threshold was high. TA0 represents the highest ventilation that could be reached without arousal. Following CPAP therapy (panel B), arousal threshold was considerably lower making it impossible to reach the pre-CPAP TER level. Accordingly, it is possible to state that TER did not decrease substantially, but not possible to determine whether it increased following CPAP therapy.
Statistics
All values are reported as mean ± SD. The paired t-test was used to compare post- and pre-CPAP values.
RESULTS
The patients were predominantly obese, middle-aged males (Table 1). They had severe OSA, and their compliance with CPAP, which was assessed by electronic download of CPAP use (Encore Pro) for one month (2 weeks in one patient) prior to the second dial-down study, was excellent. There was no change in body mass index between baseline and follow up studies (38.9 ± 5.8 vs. 38.7 ± 7.2 kg/m2).
Table 1.
Patient Characteristics and Diagnostic Polysomnography Data1
| Number | 15 |
| Gender | 12M/3F |
| Age (years) | 46.7 ± 13.5 |
| Body mass index (kg/m2) | 39 ± 7 |
| Apnea/hypopnea index (/h) | 83 ± 32 |
| Mean O2 saturation (%) | 89 ± 2.9 |
| Minimum O2 saturation (%) | 70 ± 10 |
| Respiratory arousal index (/h) | 64 ± 27 |
| Duration of CPAP use (months) | 10.5 ± 4.3 |
| Average CPAP use (% of days) | 91 ± 11 |
| Average daily use CPAP (h) | 6.3 ± 1.2 |
| CPAP pressure (cm H2O) | 11.7 ± 1.6 |
Polysomnography data are during NREM sleep
Baseline Ventilatory Data on Optimal CPAP
Table 2 compares the ventilatory data obtained during the 2 dial-down studies. All data were obtained while the patient was asleep on optimal CPAP. Respiratory rate was slightly but significantly higher post CPAP therapy, but tidal volume was the same. Minute ventilation showed a nonsignificant tendency to be higher. PETCO2 was significantly lower after CPAP therapy, and oxygen saturation tended to be higher. The relatively low oxygen saturation and PETCO2 values in both studies reflect the high altitude in Calgary.
Table 2.
Baseline Ventilatory Data on CPAP
| Pre-CPAP Therapy | Post-CPAP Therapy | P value | |
|---|---|---|---|
| VT (L) | 0.46 ± 0.09 | 0.46 ± 0.09 | 0.431 |
| RR (/min) | 16.2 ± 2.5 | 16.9 ± 2.5 | 0.010 |
| VE (L/min) | 7.3 ± 1.8 | 7.6 ± 1.5 | 0.097 |
| VT / TI (L/sec) | 0.29 ± 0.08 | 0.32 ± 0.08 | 0.083 |
| PETCO2 (mm Hg) | 34.4 ± 2.7 | 31.8 ± 2.4 | 0.003 |
| O2 Sat (%) | 93.4 ± 1.3 | 94.5 ± 2.4 | 0.056 |
VT, tidal volume; RR, respiratory rate; VE, minute ventilation; VT/TI, mean inspiratory flow rate; PETCO2, end-tidal PCO2; O2Sat, oxyhemoglobin saturation.
Passive Pharyngeal Mechanics
Optimal CPAP did not change between the initial CPAP titration and reassessment during the follow-up study (12.3 ± 2.5 vs. 11.9 ± 1.7 cm H2O; P = 0.32). In 10 patients, closing pressure was ≥ 1.0 before CPAP therapy. There was no change in closing pressure in these patients after therapy (2.7 ± 1.8 vs. 2.4 ± 1.7 cm H2O, P = 0.25). In the other 5 patients, V̇MAX at 1.0 cm H2O was 0.18 ± 0.06 L/sec before CPAP therapy and 0.18 ± 0.12 L/sec after CPAP therapy (NS).
Dynamic Ventilatory Response to Hypoxia and Hypercapnia
Table 3 summarizes the responses to the different gas mixtures at breath 5 following the change in inspired gases. The ventilatory response to all gas mixtures decreased markedly following CPAP therapy, with the post CPAP values being about half what they were pre-therapy. This occurred despite the fact that the changes in PETCO2 were similar in the 2 studies, and, presumably because of the lower ventilatory response, the changes in oxygen saturation were more pronounced in the second study (Table 3). Figure 4 shows individual responses to the 2 most frequently used gas mixtures. Patients with the highest responses pre-CPAP sustained the greatest decreases post therapy, with the result that the response became modest in all patients after CPAP therapy. There was no correlation between the pre-therapy ventilatory response or the change in ventilatory response with therapy and any of the following pre-therapy variables: Epworth Sleepiness Scale, the apnea-hypopnea index, arousal index, or average magnitude of desaturation during respiratory events in the initial polysomnography study.
Table 3.
Ventilatory Responses to Inspired Gas Mixtures Before and After CPAP Therapy
| Gas mixture | N | ΔPETCO2 |
ΔO2Sat |
Dynamic Response |
Dynamic Response (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-CPAP | Post-CPAP | Pre-CPAP | Post-CPAP | Pre-CPAP | Post-CPAP | Pre-CPAP | Post-CPAP | ||
| 2% CO2 | 6 | 5.4 (2.0) | 4.2 (3.1) | 0.0 (0.8) | 0.2 (0.6) | 3.7 (1.5) | 2.4 (1.2)* | 47 (15) | 28 (15)* |
| 3% CO2 | 14 | 7.8 (2.5) | 7.1 (1.8) | 0.0 (1.0) | −0.9 (1.1)* | 3.8 (2.2) | 2.2 (1.3)*** | 54 (27) | 28 (13)*** |
| 6% CO2 | 15 | 14.2 (2.8) | 12.8 (2.8) | 0.2 (1.1) | −0.5 (0.7)* | 5.6 (3.5) | 4.1 (2.1)* | 80 (50) | 53 (26)** |
| 1% CO2 in (11-15)%O2 | 14 | 1.6 (1.4) | 2.0 (1.9) | −5.2 (2.3) | −7.0 (3.5) | 3.2 (2.2) | 1.6 (1.7)*** | 48 (35) | 22 (23)*** |
| 3% CO2 in (11-15)%O2 | 14 | 5.5 (2.3) | 5.3 (2.3) | −4.4 (1.8) | −5.0 (1.5) | 7.7 (4.8) | 3.8 (2.9)*** | 131 (95) | 52 (34)*** |
Values in brackets are standard deviation; ΔPETCO2, change in end-tidal PCO2 during the gas challenge; O2Sat, change in oxyhemoglobin saturation during the gas challenge; Dynamic Response, change in minute ventilation at breath 5 relative to baseline; Dynamic Response(%),Dynamic Response as % baseline. Numbers in brackets are SD.
P < 0.05;
P < 0.01;
P < 0.005 for differences between pre- and post-CPAP therapy.
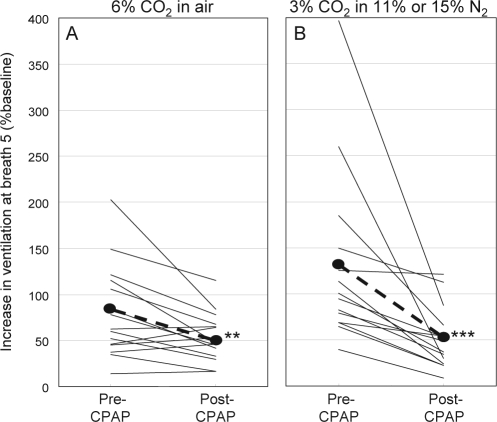
Figure 4.
Effect of CPAP therapy on the dynamic (5th breath) ventilatory response to breathing 6% CO2 or 3% CO2 in mild hypoxia.
Arousal Threshold
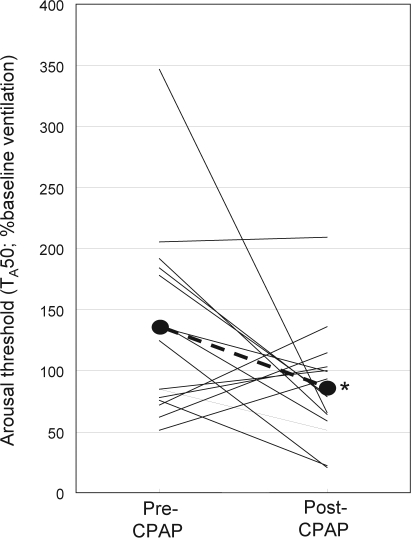
With the exception of one patient in whom arousal threshold did not change, the arousal threshold (TA50) decreased markedly in the 8 patients with the highest TA50 before therapy (Figure 5). In the 7 patients whose TA50 was low before therapy, the changes were small and bi-directional. On average, TA50 decreased from 134% ± 78% baseline to 86% ± 47% baseline (P < 0.05). Neither the pre-therapy arousal threshold nor the change in arousal threshold with therapy was correlated with the Epworth Sleepiness Scale before therapy, or with the apnea-hypopnea index, arousal index. or average magnitude of desaturation during respiratory events in the initial polysomnography study.
Figure 5.
Change in arousal threshold following CPAP therapy. Arousal threshold is the increase in chemical drive (expressed as % baseline) that is associated with a 50% probability of arousal (TA50).
Effective Recruitment Threshold (TER)
Assessment of changes in TER was complicated by the fact that arousal threshold was not the same in the 2 tests (Figure 5). As indicated earlier, arousal threshold (TA) determines the upper limit of the range of ventilation in which TER can be assessed. Table 4 shows the results. In 5 patients, TER was below TA in both the baseline and follow-up studies and hence could be determined in both cases (patients 1–5, Table 4). In these patients, average TER was not significantly different (60% ± 14% vs. 60% ± 22% baseline). In one patient (#6) TER was clearly higher post-CPAP, while in another (#7) it was lower. In the remaining 8 patients, a comparison could not be made, either because TA in one test was close to or below (e.g., Figure 3) TER in the other test (patients 8–11) or because TER was > TA in both tests (patients 12–15).
Table 4.
Effective Recruitment Threshold*
| Patient | Pre-CPAP | Post-CPAP |
|---|---|---|
| 1 | 36 | 35 |
| 2 | 69 | 43 |
| 3 | 58 | 59 |
| 4 | 69 | 71 |
| 5 | 67 | 92 |
| 6 | 43 | > 158 |
| 7 | > 118 | 57 |
| 8 | 116 | > 41 |
| 9 | 81 | > 84 |
| 10 | 80 | > 98 |
| 11 | > 96 | 113 |
| 12 | > 211 | > 119 |
| 13 | > 178 | > 71 |
| 14 | > 60 | > 76 |
| 15 | > 69 | > 93 |
values are the increase in chemical drive (%baseline) required to open the airway without arousal
DISCUSSION
The main findings from this study are that long-term use of CPAP is associated with: (1) no change in passive pharyngeal mechanical properties, (2) marked reduction in dynamic ventilatory responses to CO2 and hypoxia, (3) reduction in arousal threshold in those patients with a high arousal threshold prior to CPAP therapy and, (4) likely, no change in effective recruitment threshold. Because the changes observed after relief of OSA likely reflect characteristics acquired in untreated OSA prior to therapy, our findings indicate that sustained severe OSA is associated with an increase, at times dramatic, in dynamic ventilatory responses, and in arousal threshold in some patients.
Passive Pharyngeal Mechanics
By use of imaging11,12 and acoustic techniques,13 several investigators have reported an increase in pharyngeal dimensions in awake OSA patients following long-term use of CPAP. This was attributed to resolution of edema and inflammation of the pharynx that had developed secondary to the long-standing vibration and trauma associated with snoring and repetitive collapse.11–14 Consequently, we expected to find an improvement in passive mechanical properties of the pharynx following CPAP therapy. This was not the case. One potential explanation for the different findings is the possibility that the part of the pharynx subjected to the most trauma (negative pressure) is located downstream (i.e., caudad) from the critical site of collapse. Thus, resolution of the inflammatory changes associated with this trauma may be limited to a part of the pharynx that is not relevant to its dynamic behavior. Alternatively, pharyngeal dimensions during wakefulness may not faithfully reflect the passive properties of the pharynx because they incorporate the action of pharyngeal dilator muscles, which are quite active in the awake state.15,16 Our study was conducted during sleep under conditions (CPAP dial-down) where dilator muscle activity is minimal.14,9,17–19 Finally, it is possible that long-standing untreated OSA may result in structural changes (e.g., fibrosis) that are not reversible during several months of CPAP therapy. Regardless of the reason, the current study indicates that the intrinsic collapsibility of the pharynx during sleep is not altered following several months of CPAP therapy.
Dynamic Ventilatory Responses
The role of ventilatory responses to hypercapnia and hypoxia in the pathogenesis of OSA has been a subject of considerable interest for many years. Numerous studies have compared the responses in OSA patients to control subjects,20–23 as well as the responses in the same OSA patients before and after CPAP therapy.24–30 All of these studies were performed while subjects were awake. The results have been highly variable. Compared to “control” subjects, OSA patients were reported to have similar20,22 or decreased21 ventilatory responses to CO2, while the responses to hypoxia were reported to be higher,20 not significantly higher,22 or lower.21,23 The reported changes in these awake responses following CPAP therapy were also highly variable, ranging from an increase27,30 to no change24,26,28,29 in the ventilatory response to CO2 and no change26 or decrease25,29,30 in the response to hypoxia.
The previous studies cited above used conventional re-breathing techniques during which the stimulus was confined to pure hypoxia or hypercapnia, and the blood gas changes evolved over several minutes of wakefulness. In contrast, the stimulus during OSA is invariably a combination of hypoxia and hypercapnia (asphyxia), which typically evolves over only 10 to 30 sec (the duration of the obstructive events) of sleep. The relationship between ventilatory responses to slowly evolving pure hypercapnia or hypoxia and rapidly evolving combined hypercapnia and hypoxia is extremely complex,31 and may be further confounded by the differences in sleep/wake state. Consequently, it is difficult to relate the results of earlier studies using these techniques to the pathogenesis of OSA.
The current study and the related earlier report3 represent the first attempt at measuring ventilatory responses to combined hypoxia and hypercapnia during sleep (i.e., without the influence of behavioral responses) with a time pattern that reflects the fast changes in chemical stimuli during obstructive respiratory events. Thus, an obstructive apnea or severe hypopnea (i.e., tidal volume < dead space) represents a complete cessation of alveolar ventilation, which is equivalent to rebreathing one’s own expired gas (≈6% CO2 in 14% O2) for the duration of the hypopnea/apnea (typically < 30 sec with an average duration of 18 sec1). This was the type of mixed hypoxic and hypercapnic challenge presented to subjects in the current study, except for the fact that breathing 6% CO2 in a hypoxic mixture caused arousal from sleep so often and so quickly that we had to change to a weaker mixed stimulus (3% CO2 in 11% to 15% O2). The response to this mixture approximates the response to the chemical changes associated with a mild/moderate hypopnea, where alveolar ventilation is reduced by about half and oxygen saturation decreases 4% to 5%.
We also examined the response to pure hypoxia and hypercapnia to determine whether they were different from the responses observed with this combined hypoxia and hypercapnia. Thus, the 1% CO2 in 11% to 15% O2 mixture resulted in the same degree of hypoxemia as the reference mixture (≈5% reduction in oxygen saturation) but with minimal changes in PETCO2, while the 2% and 3% CO2 in air mixtures resulted in similar hypercapnia but with minimal changes in oxygen saturation (Table 3). Because the responses were measured on optimal CPAP, the confounding influence of a variable upper airway resistance during sleep was eliminated and all responses began from the same eupneic baseline.
As was reported before,3 the dynamic ventilatory response to combined hypoxia and hypercapnia was extremely high in OSA patients prior to CPAP therapy. Thus, in response to chemical changes comparable to those occurring during a mild/moderate hypopnea (i.e., response to 3% CO2 with ≈4% decrease in oxygen saturation), ventilation increased an average of 7.7 L/min (131% baseline) above baseline by breath 5 (Table 3, Figure 4B). It was not clear whether this high dynamic response was inherent to these patients (i.e., premorbid) or was acquired as a result of years of untreated OSA. The current results unequivocally show that, on average, the dynamic response to combined hypoxia and hypercapnia decreases dramatically to less than half the pre-CPAP value following CPAP therapy. By extension, it can be concluded that, on average, the dynamic ventilatory response to combined hypoxia and hypercapnia increases markedly in the course of OSA, but that the magnitude of increase is highly variable.
In contrast to the highly variable reported effect of CPAP therapy on awake ventilatory response to CO2,24,26,27–30 the dynamic response to all levels of CO2 stimulation also decreased substantially in our patients following CPAP therapy (Table 3, Figure 4A). In our experiments the response was measured 5 breaths (≈20 sec) after the change in the inspired gas mixture and was, accordingly, almost completely mediated by peripheral chemoreceptors. In previous studies in awake patients, the response during the rebreathing experiments incorporated a large contribution from central chemoreceptors. We found that, following CPAP therapy, the steady state PETCO2 was slightly, but significantly, lower than pre-CPAP levels (Table 2). Some investigators have reported similar reductions in awake, steady state PCO2 in normocapnic OSA patients following CPAP therapy.28,30 Since normoxic steady state PCO2 is determined primarily by central chemoreceptors,31 these data indicate that sustained OSA paradoxically decreases central chemoreceptor sensitivity to CO2 with this effect being reversed by CPAP.
In summary, our data indicate that untreated OSA results in selective enhancement of ventilatory responses to CO2 and hypoxia mediated by peripheral chemoreceptors with a paradoxical attenuation of central chemoreceptors’ responsiveness to CO2. These findings provide a possible explanation for the contradictory results obtained previously in awake subjects, in that subtle differences in experimental protocol may have resulted in different contributions from peripheral and central chemoreceptors to the ventilatory response.
The selective increase in responses mediated by peripheral chemoreceptors in the course of untreated OSA and its resolution following CPAP therapy is almost certainly related to plasticity in the peripheral chemoreceptors and their central pathways. Thus, chronic intermittent hypoxia in experimental animals results in an increase in their responsiveness to acute hypoxia32,33 via serotonin-dependent mechanisms.32 It is of interest to note that the change in dynamic response with CPAP therapy was not correlated with the frequency or severity of desaturation prior to therapy. This suggests that the response of the chemical control system to repeated asphyxia is not a simple function of the frequency and severity of such episodes.
The reason for attenuation of central chemoreceptor response to CO2 during untreated OSA is unclear. Untreated OSA may be expected to alter steady-state CO2 response in a number of ways, which include: (1) Average PCO2 across the night may be elevated, which would tend to increase PCO2 in the absence of the load. (2) Sleep deprivation depresses the ventilatory response to CO2.34 (3) Recurrent hypoxia, particularly when associated with hypercapnia or pharyngeal obstruction, elicits sustained increases in respiratory drive (long-term facilitation).35–38 Evidently, the net effect of these opposing influences is a mild suppression of steady-state respiratory drive.
The increased dynamic ventilatory response to asphyxia with untreated OSA should result in greater ventilatory instability and a higher apnea-hypopnea index.7,39 In patients in whom the chemical drive required to open the airway reflexly is less than arousal threshold (i.e., TER < TA) there is the potential for stable breathing to develop since arousal may be averted, leading to less of a ventilatory overshoot.2,4 However, because blood gas tensions continue to deteriorate at the chemoreceptor for a breath or two after pharyngeal opening, arousal may still occur in these patients if the dynamic response to asphyxia is high.4 Even in patients in whom arousal must occur before opening (i.e., TER > TA), the time to arousal (i.e., hypopnea duration) will be shorter in patients with a high dynamic response, with a consequent increase in AHI and arousal index.
A number of studies have reported that the apnea-hypopnea index following a single night without CPAP is less than what it was before therapy was initiated,40,41 implying that the beneficial effects of CPAP outlast the immediate period of its application. This has been attributed to resolution of upper airway edema, with consequent improvement in pharyngeal mechanics. Given that our study did not demonstrate any change in mechanical properties (closing pressure), but showed a clear reduction in dynamic responses to asphyxia, it is more likely that the “carry-over effect” of CPAP is related to reduction in the dynamic ventilatory responses to hypoxia and hypercapnia.
Arousal Threshold
We found that overall arousal threshold decreased following CPAP therapy (Figure 5). By extension, this indicates that arousal threshold increases in the course of untreated OSA. This is consistent with two previous reports in which arousal threshold was found to increase following discontinuation of CPAP6 and to decrease following institution of CPAP therapy.42 However, considering individual responses, Figure 5 shows that the effect of CPAP on TA and, by extension, the increase in TA during untreated OSA, varies greatly among patients. Specifically, 7 of the 8 patients with the highest TA before treatment (those with TA > 100%) sustained a marked decrease in TA. The 7 patients with TA < 100% at baseline underwent small and bi-directional changes, with no net change. This implies that only a subpopulation of severe OSA patients responds to untreated OSA with large increases in arousal threshold, while in another subpopulation the arousal threshold remains low.
The most obvious explanation for the increase in arousal threshold during untreated OSA is that it is a response to the associated sleep fragmentation. However, we found no correlation between the pre-therapy arousal threshold and the arousal index or apnea-hypopnea index during clinical polysomnography. This suggests that individuals differ substantially in how arousal threshold is altered in response to severe sleep fragmentation.
An increase in TA in the course of untreated OSA may or not be beneficial, depending on TER.4 When TER is relatively low, an increase in TA may make it possible for the airway to open without arousal (i.e., with less ventilatory overshoot), thereby increasing the possibility of a stable response following obstructive events.2–4 However, if TER is very high, an increase in TA may simply result in prolongation of respiratory events and more severe oxygen desaturation.
Effective Recruitment Threshold (TER)
The reason(s) why chemical drive must increase a threshold amount before the dilator muscles can open the pharynx is not clear. Possible mechanisms include:4 (1) Dilator muscle activity does not increase until chemical drive increases a threshold amount above eupneic drive because the negative pressure reflex is not engaged until negative pharyngeal pressure exceeds a threshold amount, or because the “recruitment threshold” of the dilators is higher than that of pump muscles, as has been described in experimental animals43–45 and humans.46,47 (2) The dilator muscles may be activated, but a threshold force must be generated before the upper airway can expand, with such a mechanical threshold being related to closing pressure, if it is greater than zero, to adhesiveness of apposed pharyngeal surfaces, or to negative effort dependence. (3) Weakness of the dilator muscles due to inflammation and denervation, which has been described,48,49 or to reduced gain of the negative pressure reflex due to impairment of pharyngeal mechanoreceptor function,50–52 may also contribute by requiring greater dilator activation before the mechanical threshold can be reached.
Given the changes in arousal threshold between the baseline and follow-up studies, it was possible to determine what happened to TER in only 7 patients. In these patients, TER clearly increased in one (#6), decreased in another (#7), and changed very little, in both directions, in the remaining five (#1–5, Table 4). Accordingly, the evidence in these 7 patients points to no net change. In the remaining 8 patients, the results are consistent with no change in TER or, at least, that it continued to be high; in 7 of 8 patients it was still > 70% (# 9–15, Table 4). Thus, the preponderance of evidence is in favor of no net effect of CPAP therapy on the mechanisms responsible for TER. In the current study, the mechanical properties of the pharynx remained unchanged, making it unlikely that the contribution of a mechanical threshold to TER (mechanism B, above) was altered. Thus, by a process of elimination, our results suggest that the neuromuscular mechanisms that contribute to TER (mechanisms A and C, above) are either inherent to the patient or are unalterably changed during the untreated phase of OSA
Net Effect of Untreated OSA on Stability Factors
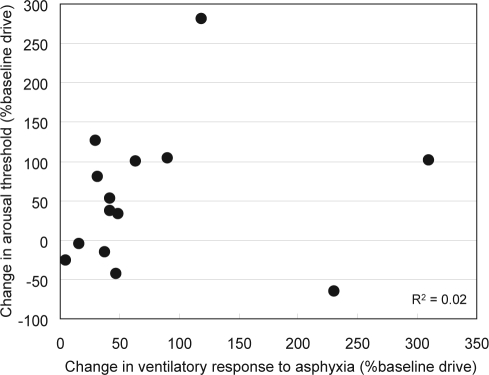
By making the reasonable assumption that the changes observed following CPAP therapy represent a reversal of the changes acquired during the untreated phase of OSA, it may be concluded that untreated OSA results in: (1) an increase in the dynamic response to asphyxia, which occurs in all patients but to a highly variable degree (Figure 4) and (2) an increase in arousal threshold that affects only some patients (Figure 5). As indicated earlier, the increase in dynamic response to asphyxia is undesirable in that it increases the likelihood of a post-respiratory event ove--shoot, thereby promoting instability, and it also increases the frequency of obstructive events when the system is unstable. By contrast, the increase in arousal threshold can be stabilizing in patients with a low TER because it increases the chance of the airway opening without arousal with, consequently, a milder overshoot. Considering that the two responses operate in opposite directions, it was interesting to see the relationship between them in individual patients. Figure 6 is a plot of the change in arousal threshold versus the change in ventilatory response to 3% CO2 in hypoxia (breath 5) in individual patients.
Figure 6.
Estimated effect of untreated obstructive sleep apnea (OSA) on dynamic ventilatory response to asphyxia, and on arousal threshold, in individual patients. The effect of untreated OSA was assumed to be the opposite of the change observed following CPAP treatment. Ventilatory response to asphyxia is the increase in ventilation (expressed as % of baseline ventilation) during the 5th breath following a change in inspired gas from air to 3% CO2 in 15% or 11% O2. Arousal threshold is the increase in chemical drive (expressed as % baseline) that is associated with a 50% probability of arousal (TA50). Note the wide range of responses among patients and the lack of correlation between the 2 responses.
In order to describe the changes that occur with untreated OSA, we reversed the polarity of the change observed in either variable following CPAP therapy. For example, if TA decreased from 185% baseline ventilation to 80% baseline ventilation following CPAP therapy, it was assumed to have increased from 80% to 185% baseline, or 105% baseline, in the course of untreated OSA. This figure clearly shows no correlation between the two responses (r2 = 0.02). Thus, the mechanisms responsible for the two opposing responses are quite independent of each other, so that in some cases both variables may change in a destabilizing direction (decreased TA and increased dynamic response), while in others the net effect may be stabilizing (substantially increased TA with little change in dynamic response) or neutral. Since all of our patients had severe OSA prior to CPAP therapy (Table 1), the changes that occurred during untreated OSA in these patients must have had the net effect of producing, or maintaining, severe OSA. It can be envisioned, however, that in other patients the changes occurring in the early stages of OSA may attenuate its severity, resulting in an indefinite course of mild OSA. It is also clear that if an increase in TA succeeds in increasing stability (e.g. in cases with a relatively low TER), the increase in dynamic ventilatory response may also be mitigated because oxygen desaturation events would be fewer. This further increases the likelihood of OSA remaining mild. It is possible that such a scenario underlies the large number of subjects who have mild OSA in the face of a highly collapsible pharynx.1,53–55
In summary, the current study suggests that in the course of untreated OSA changes occur in two variables that affect ventilatory stability, namely an increase in arousal threshold, which occurs in only some patients, and an increase in dynamic respiratory response to asphyxia, which occurs in all patients but to a highly variable degree. The two responses have opposite effects on ventilatory stability and are not correlated with each other. As a result, the changes that occur in the course of untreated OSA may have a net beneficial or detrimental impact on the severity of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Hanley has participated in a speaking engagement for Respironics. Respironics has donated funds to the University of Calgary to support a training fellowship program in Sleep Medicine. Dr. Younes is the owner of YRT Ltd., a research and development company that develops improvements to mechanical ventilation. YRT develops no products used for the treatment of OSA. Dr. Younes has participated in speaking engagements for Respironics and Covidian and has consulted for Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by the Canadian Institutes of Health Research
REFERENCES
- 1.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 2.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 3.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 4.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 5.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–75. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 6.Berry RB, Kouchi KG, Der DE, Dickel MJ, Light RW. Sleep apnea impairs the arousal response to airway occlusion. Chest. 1996;109:1490–6. doi: 10.1378/chest.109.6.1490. [DOI] [PubMed] [Google Scholar]
- 7.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 8.American Sleep Disorders Association. EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 9.Schwartz AR, O’Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–6. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method) BMJ. 1998;317:1572. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortimore IL, Kochhar P, Douglas NJ. Effect of chronic continuous positive airway pressure (CPAP) therapy on upper airway size in patients with sleep apnoea/hypopnoea syndrome. Thorax. 1996;51:190–2. doi: 10.1136/thx.51.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan CF, Lowe AA, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144:939–44. doi: 10.1164/ajrccm/144.4.939. [DOI] [PubMed] [Google Scholar]
- 13.Corda L, Redolfi S, Taranto Montemurro L, La Piana GE, Bertella E, Tantucci C. Short- and long-term effects of CPAP on upper airway anatomy and collapsibility in OSAH. Sleep Breath. 2008 Sep 25; doi: 10.1007/s11325-008-0219-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol. 2005;99:2440–50. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- 15.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–94. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- 17.Launois SH, Feroah TR, Campbell WN, et al. Site of pharyngeal narrowing predicts outcome of surgery for obstructive sleep apnea. Am Rev Respir Dis. 1993;147:182–8. doi: 10.1164/ajrccm/147.1.182. [DOI] [PubMed] [Google Scholar]
- 18.Pierce R, White D, Malhotra A, et al. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J. 2007;30:345–53. doi: 10.1183/09031936.00063406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis. 1986;134:555–7. doi: 10.1164/arrd.1986.134.3.555. [DOI] [PubMed] [Google Scholar]
- 20.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–9. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 21.Osanai S, Akiba Y, Fujiuchi S, et al. Depression of peripheral chemosensitivity by a dopaminergic mechanism in patients with obstructive sleep apnoea syndrome. Eur Respir J. 1999;13:418–23. doi: 10.1183/09031936.99.13241899. [DOI] [PubMed] [Google Scholar]
- 22.Radwan L, Maszczyk Z, Koziej M, et al. Respiratory responses to chemical stimulation in patients with obstructive sleep apnoea. Monaldi Arch Chest Dis. 2000;55:96–100. [PubMed] [Google Scholar]
- 23.Tafil-Klawe M, Thiele AE, Raschke F, Mayer J, Peter JH, von Wichert W. Peripheral chemoreceptor reflex in obstructive sleep apnea patients; a relationship between ventilatory response to hypoxia and nocturnal bradycardia during apnea events. Pneumologie. 1991;45(Suppl 1):309–11. [PubMed] [Google Scholar]
- 24.Berthon-Jones M, Sullivan CE. Time course of change in ventilatory response to CO2 with long-term CPAP therapy for obstructive sleep apnea. Am Rev Respir Dis. 1987;135:144–7. doi: 10.1164/arrd.1987.135.1.144. [DOI] [PubMed] [Google Scholar]
- 25.Cistulli PA, Sullivan CE. Pathology of sleep apnea. In: Saunders NA, Sullivan CE, editors. Sleep and breathing. Lung Biol Health Dis. vol 71. New York: Marcel Dekker; 1994. pp. 405–48. [Google Scholar]
- 26.Lin CC. Effect of nasal CPAP on ventilatory drive in normocapnic and hypercapnic patients with obstructive sleep apnoea syndrome. Eur Respir J. 1994;7:2005–10. [PubMed] [Google Scholar]
- 27.Moura SM, Bittencourt LR, Bagnato MC, Lucas SR, Tufik S, Nery LE. Acute effect of nasal continuous positive air pressure on the ventilatory control of patients with obstructive sleep apnea. Respiration. 2001;68:243–9. doi: 10.1159/000050505. [DOI] [PubMed] [Google Scholar]
- 28.Soler JJ, Morales P, Benlloch E, Cordero PJ, Macián V. Long-term effects of nasal continuous positive airway pressure on ventilatory patterns of patients with obstructive sleep apnea syndrome. Arch Bronconeumol. 1997;33:172–8. doi: 10.1016/s0300-2896(15)30626-8. [DOI] [PubMed] [Google Scholar]
- 29.Spicuzza L, Bernardi L, Balsamo R, Ciancio N, Polosa R, Di Maria G. Effect of treatment with nasal continuous positive airway pressure on ventilatory response to hypoxia and hypercapnia in patients with sleep apnea syndrome. Chest. 2006;130:774–9. doi: 10.1378/chest.130.3.774. [DOI] [PubMed] [Google Scholar]
- 30.Tun Y, Hida W, Okabe S, et al. Effects of nasal continuous positive airway pressure on awake ventilatory responses to hypoxia and hypercapnia in patients with obstructive sleep apnea. Tohoku J Exp Med. 2000;190:157–68. doi: 10.1620/tjem.190.157. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham DJC, Robbins PA, Wolff CB. Integration of respiratory responses to changes in alveolar partial pressures of CO2 and O2 and in arterial pH. In: Cherniack NS, Widdicombe JG, editors. Handbook of physiology. The respiratory system: control of breathing. Bethesda, MD: American Physiological Society; 1986. pp. 475–528. [Google Scholar]
- 32.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, JR, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–8. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol Lond. 2004;560:577–86. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffman PL, Trontell MC, Mazar MF, Edelman NH. Sleep deprivation decreases ventilatory response to CO2 but not load compensation. Chest. 198(84):695–8. doi: 10.1378/chest.84.6.695. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GS, Baker TL, Nanda SA, et al. Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–75. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 36.Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol. 2003;94:53–9. doi: 10.1152/japplphysiol.00476.2002. [DOI] [PubMed] [Google Scholar]
- 37.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 38.Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol. 2008;160:259–66. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kribbs NB, Pack AI, Kline LR, et al. Effect of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 41.Sforza E, Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep. 1995;18:195–201. [PubMed] [Google Scholar]
- 42.Haba-Rubio J, Sforza E, Weiss T, Schröder C, Krieger J. Effect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSAS. Sleep Breath. 2005;9:12–19. doi: 10.1007/s11325-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 43.Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS. Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir Physiol. 1987;70:183–93. doi: 10.1016/0034-5687(87)90049-1. [DOI] [PubMed] [Google Scholar]
- 44.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle responses to CO2 in rats. J Appl Physiol. 2002;92:878–87. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 45.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–88. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 46.Pillar G, Malhotra A, Fogel RB, et al. Upper airway muscle responsiveness to rising PCO2 during NREM sleep. J Appl Physiol. 2000;89:1275–82. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- 47.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–9. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 48.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 49.Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1960–6. doi: 10.1164/ajrccm.159.6.9809052. [DOI] [PubMed] [Google Scholar]
- 50.Guilleminault C, Li K, Chen NH, Poyares D. Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest. 2002;122:866–70. doi: 10.1378/chest.122.3.866. [DOI] [PubMed] [Google Scholar]
- 51.Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:250–5. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep. 2005;28:585–93. doi: 10.1093/sleep/28.5.585. [DOI] [PubMed] [Google Scholar]
- 53.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002;359:1207–9. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 54.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 55.Kirkness JP, Schwartz AR, Schneider H, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. 2008;104:1618–24. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]