Abstract
Although opioids are known to influence sleep-wake regulation, the neuroanatomic substrate(s) mediating these effects remain unresolved. We hypothesized that the influence of opiates on sleep may be mediated, at least in part, by the ventrolateral preoptic nucleus (VLPO), a key cell group for producing behavioral sleep. By combining in situ hybridization for kappa and mu receptor mRNA with immunostaining of Fos expressed by VLPO cells during sleep we show that > 85% of sleep-active VLPO neurons contain mRNA for either or both opioid receptor. Microinfusions of a kappa receptor agonist into the VLPO region increased NREM sleep by 51% during the subjective night, whereas a mu receptor agonist increased wakefulness by 60% during the subjective day. The sleep- and wake- promoting effects of the kappa and mu agonists were blocked by prior administration of their respective antagonist. Combining retrograde tracing from the VLPO with immunohistochemistry for dynorphin (Dyn, the endogenous kappa receptor agonist) or endomorphin 1 (EM1, the endogenous mu receptor agonist) we show that the central lateral parabrachial subnucleus (PBcl) provides Dyn inputs to the VLPO, whereas hypothalamic histaminergic neurons provide EM1 inputs to the VLPO. In summary, results from the present study suggest that central opioid inputs to the VLPO may play a role in sleep-wake regulation and that the VLPO likely mediates the hypnotic response to high levels of opioid analgesics.
Keywords: DAMGO, preoptic area, body temperature
Introduction
The effects of opium, of which morphine is the main constituent alkaloid, on sleep and pain have been recognized for thousands of years. In humans and rodents, low doses of morphine decrease sleep behavior, as measured by electroencephalography (EEG) of non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, and sleep efficiency (Kay et al., 1979; Bronzino et al., 1982). In contrast, high doses of morphine induce sedation and are associated with slowing of the EEG in humans, monkeys and rodents (Greenwald and Roehrs, 2005; Mayo-Michelson and Young, 1993a; Kastin et al., 1991; Zuo et al, 2007). Remarkably, however, little is known about the mechanisms or neuroanatomic structure(s) through which opioids influence sleep.
The critical role for the preoptic area-basal forebrain in the regulation of sleep-wakefulness was first indicated in the early 20th century (von Economo, 1930). Work over the past decade has further revealed the nature of this circuitry, including the identification of a population of sleep-promoting neurons in the ventrolateral preoptic nucleus (VLPO) (Sherin et al., 1996; Szymusiak et al., 1998). Neurons of the VLPO are sleep-active, and loss of VLPO neurons (e.g., cell specific lesions) produces profound insomnia and sleep fragmentation (Lu et al., 2002). The VLPO is also reciprocally connected with major wake-promoting centers (i.e., the ‘ascending arousal system’) including, the noradrenergic locus coeruleus (LC), the histaminergic tuberomammillary nucleus (TMN), the serotoninergic dorsal and median raphe (DR and MR) and the dopaminergic ventral periaqueductal grey (vPAG) (Chou et al., 2002). The VLPO may therefore function as the neuroanatomic substrate through which opioids mediate, at least in part, their effects on sleep.
Thus far, five endogenous opioid peptides (endorphin, enkephalin, dynorphin, endomorphin, and nociceptin [orphanin FQ]), and four opioid receptors (mu, delta, kappa and ORL1) have been identified (see review Kosterlitz 1985). Dynorphin (Dyn), endomorphin 1 (EM1), enkephalin, and nociceptin preferentially bind kappa, mu, delta and ORL1 receptors, respectively, although endorphin appears to bind to mu and delta receptors with equally high affinity (Moran et al., 2000). In the forebrain medial preoptic region, kappa and mu receptor mRNAs are highly co-expressed whereas delta receptor mRNA is not expressed (Gulledge et al., 2000; Minami et al., 1993; Mansour et al., 1994). In addition, some galanin-ir cells in the ventral preoptic region contain kappa and mu receptor mRNA (Mitchell et al., 1997), providing support for the concept that VLPO cells may contain mu and kappa receptors.
To begin to evaluate a possible role for the VLPO in mediating the effects of opioids on sleep and wakefulness, we first determined if kappa and mu receptor mRNA was expressed in sleep-active cells of the VLPO. We next evaluated the sleep effects of direct micro-injection of opioids into the VLPO region. Finally, to define the opioid cell groups projecting to the VLPO (i.e., endogenous opioid source), we retrogradely traced opioid afferents to the VLPO. Results from the present study suggest that central opioid inputs to the VLPO may play a role in sleep-wake regulation and that the VLPO likely mediates the hypnotic response to high levels of opioid analgesics.
Results
Kappa and mu opioid receptor mRNAs are co-expressed in sleep-active VLPO cells
To determine whether VLPO neurons (both cluster and extended VLPO were analyzed; see Lu et al., 2000) expressing mu or kappa opioid receptor mRNA are also sleep active (i.e., Fos positive), we combined immunostaining of Fos and in situ hybridization of kappa or mu receptor (Figure 1 B and C, arrows) in five rats perfused at 10–11 am. Because there are very few Fos positive cells in the VLPO during wakefulness (Sherin et al., 1996; Gaus et al., 2002; Lu et al., 2002), we did not use waking animals. All five rats perfused during this period had spent at least 70% of the hour prior to the onset of perfusion in sleep (NREM = 63.4 ± 5.6 % and REM = 8.4 ± 3.2 %). We found that 87% of Fos-positive (sleep-active) cells in the VLPO cluster (of 31.4 ± 1.3 Fos-ir cells per section per side, 27.4 ± 1.0 were double-labeled) expressed kappa receptor mRNA (Figure 1). Similarly, 86% of Fos-ir neurons in the VLPO cluster also expressed mu receptor (26.6 ± 2.1 double-labeled cells out of 30.8 ± 1.7). Only about 20% of Fos-ir cells in the extended VLPO (Lu et al., 2000; 2002) expressed either kappa or mu receptor mRNA. Finally, although both kappa and mu receptor mRNA were seen in the median preoptic nucleus (MnPO), they rarely co-localized with Fos. VLPO and MnPO Fos counts were performed as previously described (Lu et al., 2000)
Figure 1.
VLPO neurons containing kappa or mu opioid receptors are also sleep-active. In A, a 10X image shows Fos induced by sleep and kappa receptor mRNA in the VLPO. Opioid kappa receptor mRNA (B, enlarged from A) and mu receptor mRNA shown in sliver grains (C) are expressed in most Fos positive cells (brown color). Arrows indicate double-labeled cells and arrowheads indicate cells labeled only for Fos. In D, the “*” in the parastrial area indicates the typical location of the cannula tip used for drug infusions. Panel E shows the location of the cannulae (6 cases) used to infuse drugs (see Figure 2) into the VLPO region. OC = optic chiasm.
VLPO kappa and mu receptors promote sleep and wakefulness, respectively
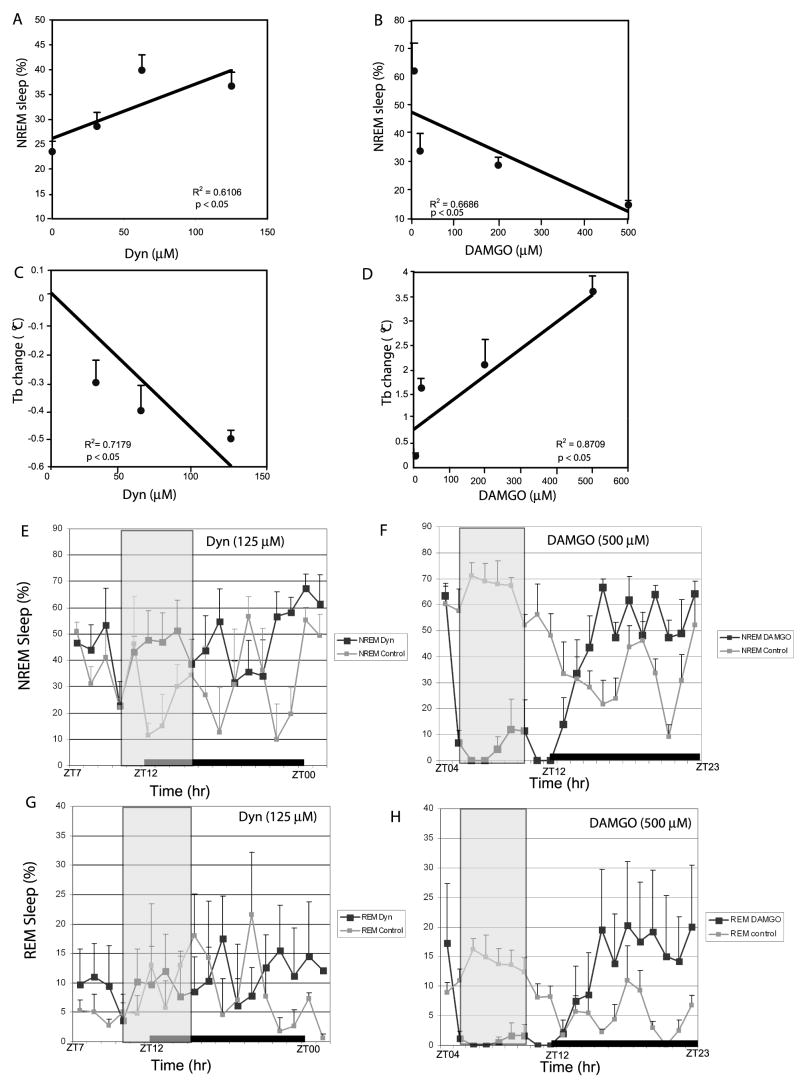
The typical location of cannula tip was within the parastrial area, which is located approximately ~0.2–0.8 mm dorsal to the VLPO (Figure 1D). As discussed below, drugs infused at this site could potentially affect the medial preoptic area as well. Infusion of Dyn (concentrations ranging from 31 μM to 125 μM) into the VLPO region significantly increased total NREM sleep time during the subjective night in a dose-dependent manner (Figure 2A and 2E, black lines), as compared to the saline controls (Figure 2A (0μM on y-axis) and 2E, grey lines). The same doses of Dyn did not significantly affect REM sleep (7.9% ± 3.2% (31 μM); 7.4% ± 1.3% (62 μM); 8.1% ± 3.12% (125 μM) vs. 6.7% ± 2.8% (control)) (Figure 2C). Dyn doses from 31 μM to 125 μM also dose-dependently decreased body temperature by as much as 0.5°C (Figure 2C). By contrast, infusion of DAMGO during the subjective day decreased total sleep time by as much as 60% (Figure 2B and 2F, black lines), as compared to the saline controls (Figure 2B (0μM on y-axis) and 2E, the grey lines). As shown in Figure 2H (500μM DAMGO shown), and unlike Dyn, the same doses of DAMGO also significantly reduced REM sleep during the infusion period (with a subsequent REM ‘rebound’ during the post-infusion period). DAMGO doses from 20μM to 500μM also dose-dependently increased body temperature by as much as 3.5°C, also in a dose-dependent manner (Figure 2D). Changes in NREM and REM behavior during both the infusion and post-infusion periods are shown for Dyn (125μM) and DAMGO (500μM) in Figures 2E-H (vertical grey box indicates infusion period and black bar indicates light-dark cycle).
Figure 2.
Dose-response relationships of opioids and sleep and body temperature. Dyn infusions into the VLPO region increased NREM sleep (A) and decreased body temperature (C) in a dose-dependent manner. By contrast, DAMGO infusions into the VLPO decreased NREM sleep (B) and increased body temperature (D), also in a dose-dependent manner. Correlation analysis indicated a strong linear relationship between NREM sleep and opioid dose for both Dyn (2A; p<0.05; R2=0.6106) and DAMGO (2B; p<0.05; R2=0.6686). Correlation analysis also indicated a strong linear relationship between Tb and opioid dose for both Dyn (2C; p<0.05; R2=0.7179) and DAMGO (2D; p<0.05; R2=0.8709). For A-D, the data point on the y-axis (0μM) represents the saline infusion control. (E and F) are group data (n = 6/group) showing NREM sleep (x ± SEM) changes prior, during and post infusion of Dyn at 125 μM (E) and (F,) DAMGO at 500 μM, respectively. Saline controls are also shown in E and F (gray line). REM sleep (x±SEM) changes prior, during and post infusion of (G, black lines) Dyn at 125μM and (F, black line) DAMGO at 500μM, respectively. Saline controls are also shown in G and F (gray lines). The gray box in E-H indicates the infusion period, and the black bar indicates the light-off period (1900–0700). ZT00 = 0700 or beginning of rest period and ZT12 = 1900 or beginning of active period (ZT = Zeitgeber Time).
Opioid receptors mediate sleep-wake effects of opioids in the VLPO
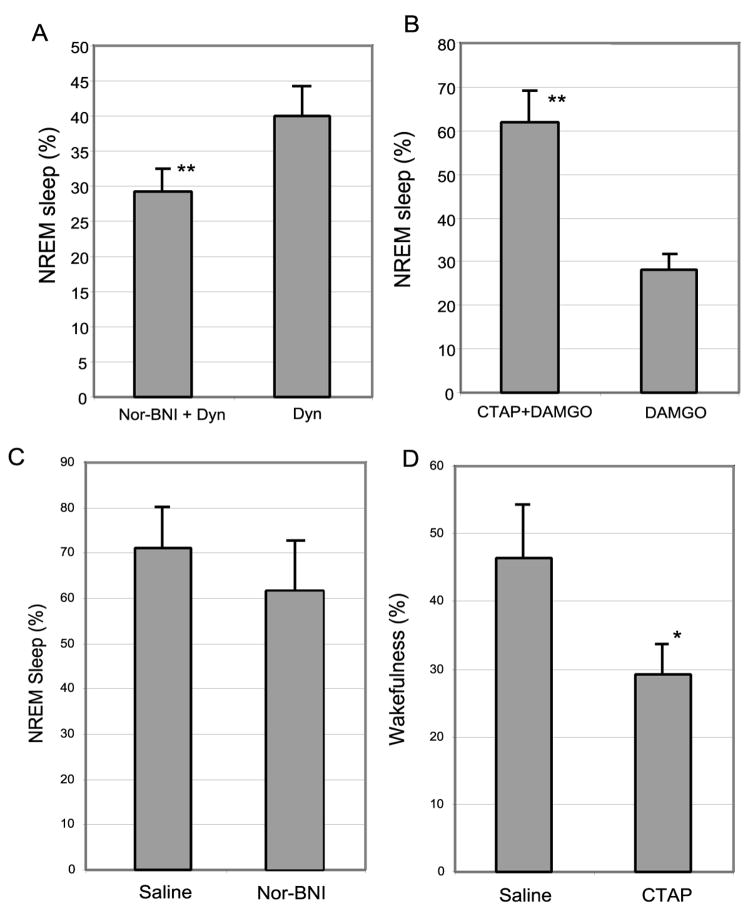
To determine whether the effects of opioids on sleep and wake behavior could be prevented by pre-administration of the opioid receptor antagonists, including the kappa receptor antagonist Nor-BNI (0.4 μM) or the mu receptor antagonist CTAP (1.0 mM) were infused for two hours prior to the six-hour infusions of Dyn (125 μM, 17:00 – 23:00) or DAMGO (500 μM, 11:00–17:00) in six additional rats. We found that these mu and kappa opioid receptor antagonists blocked the sleep-wake effects of Dyn and DAMGO, respectively (Figure 3 A and B).
Figure 3.
Opioid receptors mediate sleep-wake effects of opioids. Infusions of the kappa receptor antagonist Nor-BNI and mu receptor antagonist CTAP in the VLPO blocked the sleep and arousal effects of subsequent infusion of kappa and mu receptor agonists, respectively. As shown in Figure 2, Dyn (kappa agonist) was always infused from 5pm–11pm and DAMGO (mu agonist) from 11am–5pm. To antagonize the wake and sleep effects of Dyn and DAMGO, respectively, the kappa and mu antagonist (Nor-BNI and CTAP, respectively) were infused for two hrs prior to Dyn or DAMGO infusions (i.e., 3pm–5pm (Nor-BNI) or 9am–11am (CTAP)). The effects of pre-infusion of the antagonist are shown in A and B. In C, Nor-BNI was infused continuously from 11am–5pm (normal sleeping period) and compared with saline infusion controls. Nor-BNI blockade of endogenous kappa receptor activation did not produce a significant effect on sleep-wake. By contrast, in D, CTAP was infused continuously from 5pm–11pm (normal waking period) and, when compared with saline infusion controls, produced a significant (p<0.01) increase in sleep, suggesting a possible tonic wake-promoting influence of endogenous mu opioids.
Endogenous opioids in the VLPO region are involved in basal sleep-wake regulation
To determine the effects of endogenous opioids within the VLPO on sleep-wake regulation, we examined sleep changes after direct infusion of kappa or mu receptor antagonists into the VLPO.
We found that a 6 hour infusion of the kappa receptor antagonist Nor-BNI (40 μM, 17:00 – 23:00) increased wakefulness by 15%, comparing to saline control, but this difference did not reach statistical significance (p > 0.05, Figure 3C). By contrast, infusion of the mu receptor antagonist CTAP (200 μM) during 11:00 – 17:00 increased the total sleep by 40% (p < 0.01) compared to total sleep during saline infusion at the same period of the day (Figure 3D), suggesting a possible tonic wake-promoting influence of endogenous mu opioids.
Opioid afferent projections to the VLPO
To trace the sources of endogenous opioid inputs to the VLPO, rats received infusions of 1% CTB into the VLPO (AP, 0.4 mm, ML, 0.9 mm, DV 8.0 mm) and intraventricular injections of colchicine (11 μl containing 165 μg) one week later. These rats were perfused 40 hrs later. The brains were sectioned at 40 μm in a 1:4 series and sections were labeled with fluorescence immunohistochemistry for CTB and Dyn or for CTB and EM1.
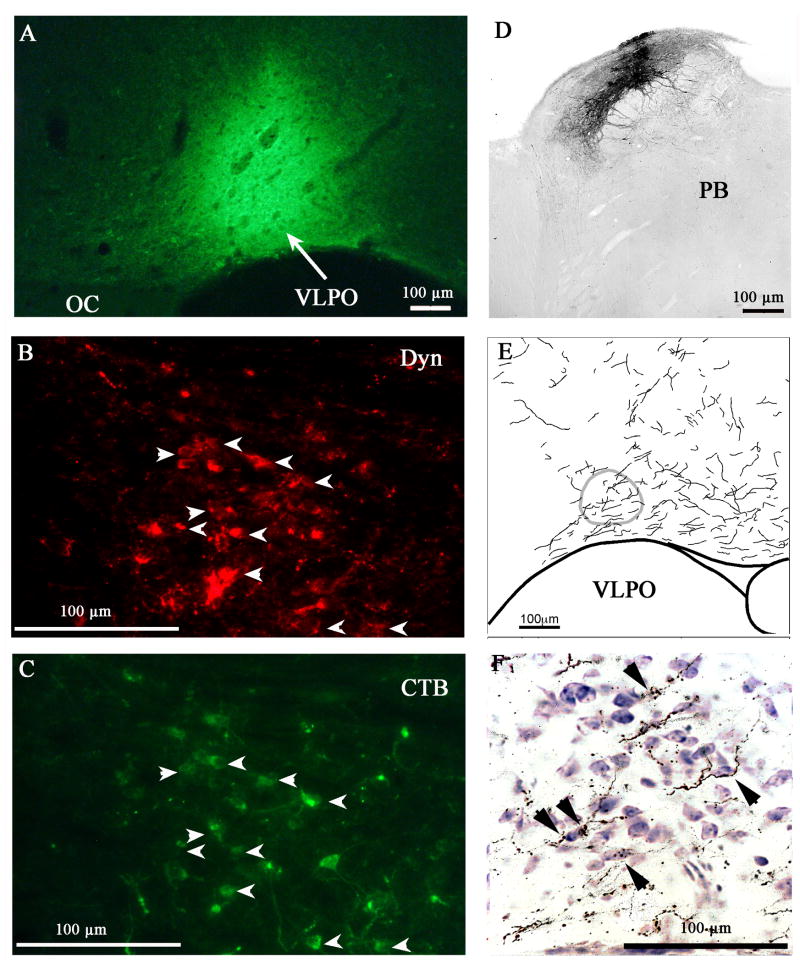
In three cases where CTB filled the VLPO, many of the Dyn-CTB dual-labeled cells were seen in the central lateral parabrachial nucleus (PBcl) ipsilateral to the injection site. In the PBcl, about 15 double-labeled cells were counted per ipsilateral section, which represented approximately 50% of the total CTB labeled cells in the ipsilateral side (Figure 4). Very sparse (i.e., one or two double-labeled cells per section) labeling was seen in several hypothalamic areas, including the ventral subparaventricular zone, arcuate nucleus, and perifornical area.
Figure 4.
Dyn-ir cells in the central lateral parabrachial nucleus (PBcl) project to the VLPO. CTB injected in the VLPO (A) retrogradely labels most dynorphin-ir cells in the PBcl (B, C). The anterograde tracer BD injected into the PBcl (D) gives rise to many efferent terminals in the VLPO (E), some of which (arrowheads) appose the Nissl-stained cell bodies in the VLPO (F).
To confirm the opioid projection to the VLPO, we injected the anterograde tracer BD (3nl) into the PBcl. The brain sections were immunostained for BD and counterstained with thionin. BD injections that filled the PBcl (Figure 4) showed that many BD labeled axon boutons apposed Nissl stained cell bodies in the VLPO (Figure 5). However, a control injection ventral to the PBcl showed few BD-labeled efferents in the VLPO region, as reported by Chou et al. (2002).
Figure 5.
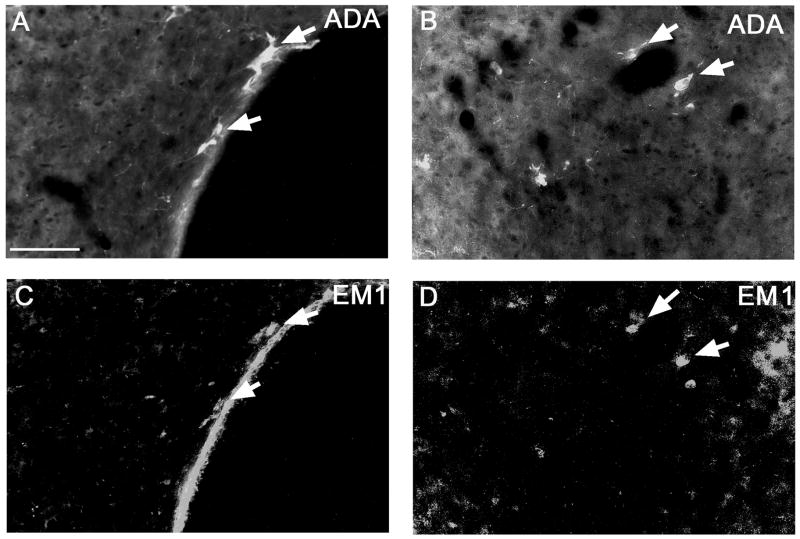
Endomorphin 1 (EM1) is expressed in the periventricular and dorsomedial hypothalamic histaminergic neurons. Double-labeling of ADA and EM1 is shown in the periventricular region (A, C) and in the dorsomedial hypothalamic region (B, D).
As previously reported in rats, EM1-ir cells were mainly distributed in the dorsomedial hypothalamus, periventricular hypothalamus and the nucleus of the solitary tract (Martin-Schild et al., 2001). On the basis of cell size and distribution, we hypothesized that EM1 in the hypothalamus might be co-localized with histamine. Dual-labeling of EM1 and adenosine deaminease (ADA, which is co-expressed with histamine (Yamamoto et al., 1990)) in colchicine injected rats (n = 2) indicated that 45% of ADA-ir cells in the periventricular and dorsomedial hypothalamus expressed EM1 and nearly 100% EM1-ir cells were ADA positive (Figure 5A, B).
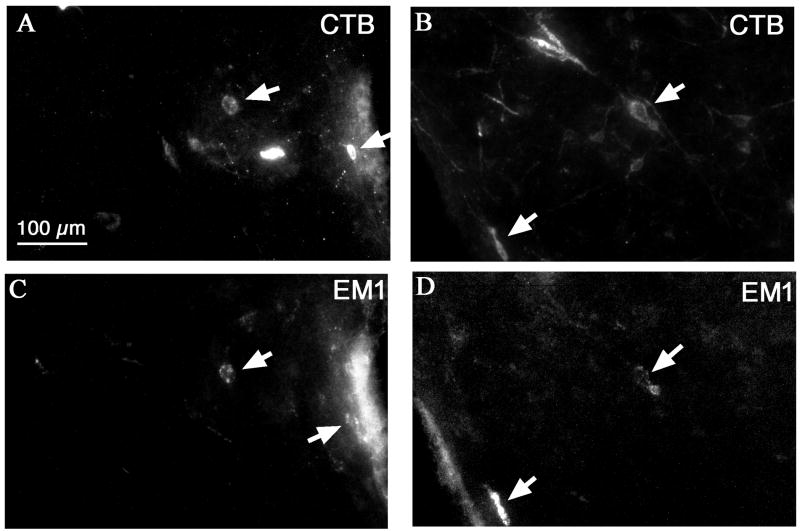
Fluorescence double-labeling of CTB injected into the VLPO and EM1 showed that about 5 EM-ir cells per ipsilateral section, i.e., 20% of total EM1-ir cells, in the medial hypothalamus were CTB positive (Figure 6C, D). By contrast, we found few CTB-EM1-ir cells in the nucleus of the solitary tract. A control study double-staining for CTB or ENK showed very few double-labeled neurons in the arcuate nucleus and the nucleus of the solitary tract. Since beta-endorphin is exclusively expressed in proopiomelanocortin neurons in the arcuate nucleus and the VLPO region lacks MC4 receptors (Kishi et al., 2003), it is unlikely that beta-endorphin neurons can significantly influence the activity of VLPO neurons.
Figure 6.
CTB injected into the VLPO retrogradely labels some EM1-ir cells in the dorsomedial hypothalamic region (A, B, CTB; C, D, EM1). Arrows indicate double-labeled cells.
In summary, the two main endogenous opioid inputs to the VLPO come from subpopulations of hypothalamic histaminergic EM1 neurons and PBcl Dyn neurons.
Discussion
The results of the present study demonstrate that opioid kappa and mu receptors are co-expressed in VLPO neurons. In addition, direct infusion of the kappa receptor agonist Dyn A (125 μM or less) or the mu receptor agonist DAMGO into the VLPO region increased sleep and wakefulness, respectively, in a dose-dependent manner. These effects were blocked by pre-injection of the respective kappa and mu receptor antagonists. Interestingly, discrete infusions of the mu, but not kappa, opioid receptor antagonist into the VLPO produced a significant increase in total sleep, suggesting a possible tonic wake-promoting influence by endogenous mu opioids. Finally, we identified two major endogenous opioid input sources to the VLPO: neurons in the PBcl that express Dyn and histaminergic neurons in the hypothalamus that express EM1. Together these data indicate a specific role of endogenous opioids in regulating sleep and wakefulness at the level of the VLPO and provide insight into the possible mechanism by which exogenous opioids such as morphine may influence the VLPO to exert hypnotic effects.
Opioid regulation of sleep-wake behavior
In rats, systemic low doses of morphine (<15 mg/kg) produce wakefulness, while high doses (>30 mg/kg) produce sleep (Zou et al., 2007; Bronzino et al., 1982; Mayo-Michelson and Young, 1993a; Tortella et al., 1978, 1979). Based upon the data from the present study, we propose that low-dose morphine primarily activates mu receptors in the VLPO, inducing wakefulness, whereas high-dose morphine activates kappa receptors in the VLPO, inducing sleep (sedation). As kappa and mu receptors are coupled to G proteins, which both activate an inwardly rectifying potassium conductance and inhibit voltage-gated calcium conductances (McFadzean, 1988; Moran et al., 2000), it is expected that DAMGO would inhibit sleep-active cells. By contrast, how Dyn stimulates the VLPO is not as yet understood. One possible mechanism is that Dyn acts presynaptically on incoming inhibitory terminals (e.g., GABAergic, serotoninergic, noradrenergic and cholinergic inputs), similar to the excitatory mechanism of adenosine in the VLPO (Morairty et al, 2004; Chamberlin et al., 2003). It is also possible that Dyn has a novel mechanism of stimulating neurons in the VLPO for sleep control (and in the preoptic area for temperature control, see below).
Although we focused here on roles of the VLPO in opioid control of sleep, other sleep-wake regulatory systems likely respond to opiates. It has been reported that stimulation of mu or kappa receptors in the locus coeruleus promotes NREM sleep (Garzon et al., 1995), and microinjection of mu receptor agonists including morphine into the nucleus of the solitary tract also produces slow-wave sleep (Reinoso-Barbero and de Andres, 1995). A recent in vitro brain slice study showed that morphine robustly stimulates the TMN neurons, a major arousal center, via a dis-inhibitory mechanism. Because the VLPO sends a major GABAergic projection to the TMN, this effect may also be mediated by mu receptors on GABAergic terminals from the VLPO (Eriksson et al, 2000).
Grasing and Szeto (1991) showed that bolus intravenous injection of naloxone, a mainly mu receptor antagonist, increased delta wave sleep for 90 min in rats. In our current study, infusion of the more selective mu receptor antagonist CTAP into the VLPO also promoted sleep, suggesting that endogenous mu receptor agonists (such as endomorphin) participate in maintaining arousal via the VLPO. The fact that infusion of the kappa receptor antagonist into the VLPO produced only minor effects on sleep suggests that endogenous kappa receptor agonists may play a limited role in sleep-wake regulation under normal physiologic conditions.
Opioid inputs to the VLPO
By combining anterograde and retrograde tracing methods, Chou and colleagues (2002) systematically identified neuronal inputs to the VLPO. Unfortunately, the study by Chou et al. did not determine if the cell groups providing afferent projections to the VLPO were opioidergic. Results from the present study identify for the first time the central lateral parabrachial nucleus (PBcl) and two discrete hypothalamic histaminergic cell groups as the main endogenous opioid sources projecting to the VLPO. Interestingly, despite the wide CNS distribution of enkephalin cells, we did not find any major projections to the VLPO. Similarly, endorphin is almost exclusively synthesized in the arcuate nucleus, which has only a small input to the VLPO (Chou et al., 2002) and thus it is unlikely that endorphins significantly influence the VLPO.
The PB is an interface between the medullary reflex systems and forebrain behavioral and integrative system that regulate autonomic functions (see review by Saper 1995) and overall arousal (Lu et al., 2006; Fuller and Lu, unpublished observations). The PBcl receives direct inputs from the dorsal horn of the spinal cord (Cechetto et al., 1985; Feil and Herbert, 1995) and medial part of the nucleus of the solitary tract (Fulwiler and Saper 1984). The latter, in turn, receives general visceral information via the vagus nerve. Results from the present study suggest that the PB may, under specific physiologic circumstances, influence the activity of VLPO neurons through Dyn projections.
Histaminergic neurons are exclusively found in the TMN. Due to its unique location and high cell density, the ventrolateral TMN (vlTMN) has been the focus of numerous studies. For example, vlTMN neurons containing GABA and histamine have long been implicated in arousal function, particularly in a novel environment (Parmentier et al., 2002; Takahashi et al., 2006). More specifically, it has been demonstrated that TMN cell activity (i.e., c-Fos and discharge) is strongly correlated with behavioral wakefulness (Vanni-Mercier et al., 2003; Scammell et al., 2000; Takahashi et al., 2006). In addition, injection of the GABA agonist muscimol into the TMN (and peri-TMN vicinity) causes hypersomnolence (Lin et al., 1989). Although VLPO neurons are innervated by the TMN, histamine does not inhibit VLPO neurons (Gallopin et al., 2004). We thus propose, but cannot conclude definitively, that hypothalamic histaminergic neurons may inhibit the VLPO by mu opioid receptor and/or GABA receptor activation.
Opioid regulation of body temperature
In parallel with the sleep-wake response to morphine, low doses of morphine (4–16 mg/kg, i.v) induce hyperthermia, whereas high dose (>30mg/kg, i.v) produce hypothermia (Martin and Morrison, 1978). Chen and colleagues (1996) previously showed that icv injection of antisense oligodeoxynucleotide against the mu receptor (nucleotides 1–18 of the coding region of the mu receptor) prevented morphine-induced hyperthermia, and icv injection of an antisense oligodeoxynucleotide against kappa receptors (4–21 of the coding region of the kappa receptor) blocked morphine-induced hypothermia. These blocking effects were later replicated by microdialysis of antisense oligodeoxynucleotides for mu and kappa opioid receptors into the preoptic area (Xin et al., 1997). Consistent with these observations, extracellular single cell recordings in the preoptic area showed that low-dose Dyn (<0.5 nM) stimulates warm-sensitive cells, and that this effect is blocked by a kappa receptor antagonist. By contrast, high doses of dynorphin (>10 nM) inhibit thermo-sensitive preoptic area neurons, and this effect is partially blocked by kappa receptor or mu receptor antagonists. Finally, DAMGO inhibits warm-sensitive cells in the preoptic area in brain slices in vitro (Yakimova et al., 1998). Consistent with these findings, we show here that focal infusions of mu and kappa receptor agonists dose-dependently cause hyperthermina and hypothermia, respectively. Thus, in the present study, it is likely that the opioids infused into the parastrial region diffused to surrounding regions such as the median preoptic nucleus (MnPO) and medial preoptic area (MPA); both areas contain GABAergic neurons that project to the medullary raphe pallidus and control regulatory and facultative thermogenesis by modulating sympathetic tone (Morrison, 2004). Based upon previous work in our laboratory in which VLPO lesions did not affect baseline temperature (Lu et al., 2000), it is unlikely that the VLPO is involved in mediating the temperature effects of the administered opiods. Rather, the increase in body temperature was likely mediated by inhibition of mu receptors in the MnPO and MPA and the reduction in body temperature by simulation of kappa receptors in MnPO and MPA.
In summary, our data indicate that endogenous opioids likely regulate sedative and arousal behaviors through projections to the VLPO. Our data also indicate that the VLPO mediates, at least in part, the arousal and hypnotic effects of exogenous opioids.
Experimental Procedures
Animals
Pathogen free adult male Sprague Dawley rats (275–300 grams, Harlan) were individually housed in standard plastic rat cages with ad libitum food (Lab Diet) and water. The cages were housed inside isolation chambers, which provided ventilation, controlled light intensity and timing (12:12, lights on at 0700), an ambient temperature of 22 ± 1°C, and visual isolation. All protocols were appr oved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School.
Sleep monitoring
EEG/EMG, cannula and sleep recordings
The surgery, EEG/EMG data collection and analyses have been described in detail previously (Lu et al., 2000 and Lu et al., 2002). In brief, after the rats were anesthetized with chloral hydrate (7% in saline, 350 mg/kg), the skulls were exposed. Four EEG screw electrodes were implanted into the skull, in the frontal (two) and parietal bones (two) of each side, and two flexible EMG wire electrodes were placed into the neck muscles. The free ends of the leads were soldered into a socket that was attached to the skull with dental cement, and the incision was then closed by wound clips. Immediately after the EEG screws were placed, a cannula (30 gauge, PlasticOne, Roanoke, Virginia) was lowered to a point ~0.5 mm dorsal to the VLPO (AP = 0.5 mm, ML =1.0 mm, DV=7.0 mm). Similar to the EEG screws and EMG wires, the cannula was then secured by dental cement. Following EEG/EMG and cannula installation, a biotelemetry transmitter (Data Sciences International, St. Paul, MN) was implanted i.p. for the recording of body temperature. During recovery from surgery the animals were kept on a heating pad, where they were monitored for irregularities in respiration, post-operative pain and the ability to return to sternal recumbency. To minimize post-surgical discomfort, all animals were provided with analgesia during the first 3 days post-op (Flunixamine, 0.5mg/kg) during health monitoring. On post-surgical day 7, the sockets were connected via flexible recording cables and a commutator to a Grass polygraph and computer. Following 3 days of adaptation to the recording cables (i.e., post-surgical day 10), sleep-wake recordings were initiated using an Apple Macintosh computer running the ICELUS sleep recording system (M. Opp, University of Michigan, Ann Arbor, MI). Each cage was placed on top of a telemetry receiver interfaced to a microcomputer data acquisition system and Tb values were recorded at 5-min intervals.
Sleep analysis
The EEG/EMG signals were amplified by Grass amplifiers (AstroMed Inc., West Warwick, RI) and the signals were filtered to exclude frequencies <0.5 or >35 Hz (EEG) and <10 or >35 Hz (EMG). The EEG/EMG data were digitized by an Apple Macintosh power computer running ICELUS 4.0 and wake-sleep states were manually scored in 12-second epochs. Wakefulness was identified by the presence of a desynchronized EEG and phasic EMG activity. NREM sleep consisted of high amplitude slow wave EEG together with a low EMG tone relative to wake. REM sleep was identified by the presence of regular theta activity coupled with low EMG tone relative to NREM sleep. The amount of time spent in wake, NREM sleep and REM sleep was determined for each hour, as previously described (Lu et al., 2000, 2002, 2006).
Fos and opioid receptor mRNA double labeling study
To determine whether sleep-active VLPO neurons contained mu or kappa receptors, we implanted EEG/EMG electrodes in five rats and perfused these rats at 10–11 am (i.e., at or near maximal sleep drive) two weeks later. 30μm brain sections were labeled for Fos and mu receptor mRNA or Fos and kappa receptor mRNA (For details, see histology).
Drug infusion studies
To examine the effects of kappa receptor activation on sleep and Tb, we measured EEG/EMG and Tb before (24 hours) and after (48 hours) six hours (17:00–23:00) of continuous unilateral microinfusion of Dyn (Dynorphin A (1–17), Sigma; pump rate 10 nl/min) into the VLPO region of six rats. As a baseline, sleep was recorded during a time-matched continuous unilateral saline microinfusion on the day prior to the first Dyn A injection. For the Dyn A infusions, each animal was treated with 3 different non-randomized dosages of Dyn A in saline (31 mM, 62 mM and 125 mM). A three-day recovery period was allowed between each dose.
To determine the effects of mu receptor activation in the VLPO on waking and Tb, we infused six additional implanted rats with DAMGO (pump rate, 10 nl/min) during the rest period (11:00 to 17:00). As a baseline, sleep was recorded during a time-matched continuous unilateral microinfusion of saline on the day prior to the first DAMGO injection. For the DAMGO infusions, each animal was treated with 3 different non-randomized dosages of DAMGO in saline (20μM, 200μM and 500μM). A three-day recovery was allowed between infusions.
In addition, in order to determine whether the effects of these opioids (i.e., Dyn A and DAMGO) on sleep and wake behavior can be blocked by pre-administration of the mu receptor agonist CTAP (D-Phe-Cys-Tyr-Arg-Pen -Thr-Thr-Nh2, 1.0 mM, Sigma) or the kappa receptor antagonist, nor-binaltorphimine (Nor-BNI, 40 nM, Sigma), we followed protocols similar to those described above, but infused the agonist for two hours prior to the six-hour infusions of Dyn (125 μM, 17:00 – 23:00) or DAMGO (20 μM, 11:00–17:00). Six (6) rats were used for each agonist. At the end of the experiments, all rats were perfused and tissues were Nissl stained to ascertain the location of the cannula tip and for histological analysis of the VLPO.
Histology
Tracer injections
Under chloral hydrate anesthesia (7% in saline, 350 mg/kg), a burr hole was made and a fine glass pipette containing 1.0 % Cholera toxin subunit B (CTB, Biological List) or 12% biotinylated dextran amine (BD, Molecular Probe) was lowered to the pre-determined targets, i.e., VLPO and cell populations projecting to the VLPO (Paxinos and Watson, 1986). A small volume of tracer (3–10 nl) was injected using an air pressure system (Lu et al., 2000; 2002). Incisions were closed with wound clips and animals were allowed to recover for 7 days (except when colchicine was co-administered, see below).
Perfusion
Animals were deeply anesthetized by chloral hydrate (500 mg/kg), then transcardially perfused with 50 ml saline followed by 500 ml 10% neutral phosphate buffered formalin. The brains were post-fixed in 10% formalin for 4 hours, and then cryoprotected in 20% sucrose in PBS overnight.
Immunohistochemistry
The frozen brains were sectioned at 40 μm or 30 μm (for dual labeling with in situ hybridization) on a freezing microtome, and sections spaced at 160 μm or 120 μm were divided into four series. Sections were washed in phosphate buffered saline (PBS) and incubated in primary antiserum (rabbit antibody agonist cFos, AB5, 1:50,000, antigen sequence: residues 4–17 from human cFos, Oncogene; goat polyclonal antibody agonist CTB, 1:50,000, #703, List Biological Laboratories; endomorphin 1 (EM1) [Tyr-Pro-Trp-Phe-NH2], rabbit, 1:5000, gift of Dr. James Zadina; adenosine deaminase (ADA), sheep, 1:1000, a gift of Dr. Rodney Kellems; rabbit polycolonal antibody agonist Dyn, H2620, 1:2000, antigen sequence, H-Tyr-Gly-GlyPhe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln-OH, Penninsula) in PBS containing 0.3% Triton X-100 (PBT) and 0.2% sodium azide for one day at room temperature. For single labeling, sections were then washed in PBS and incubated in biotinylated secondary antiserum (against appropriate species IgG, 1:1,000 in PBT) for one hour, washed in PBS and incubated in ABC reagents for 1 hour. Sections were then washed again and incubated in a 0.06% solution of 3, 3-diaminobenzidine tetrahydrochloride (DAB, Sigma) plus 0.02% H2O2. The immunocytochemical controls of these antibodies have been reported previously (Chou et al., 2003; Martin-Schild et al., 1999).
To double-label CTB and DYN, sections incubated with primary antisera against CTB (goat) and DYN (rabbit) were incubated with CY3 conjugated-donkey anti-goat serum (1:500, red color) and biotinylated donkey anti-rabbit serum (1:500) followed by streptavidin Alexa Fluor 488 (1:1000, green color). Using the same method, we double-labeled CTB + EM1 and EM1 + ADA. To verify that there was no cross-reaction of primary antibody with other secondary bodies, we also stained CTB plus secondary antiserum for DYN, and vice versa. Similar tests were performed for CTB and EM1, and EM1 and ADA. There was no cross-reaction.
Double-staining of in situ hybridization of opioid receptor mRNA and immunolabeling of Fos
We used the method previously described (Lu et al., 2002), to label Fos with an immunoperoxidase brown color and opioid receptor mRNA by autoradiography. Briefly, sections (30 mm thickness) with Fos staining (brown) were acetylated and hybridized overnight (55 °C) with a 35S-labeled cRNA probe synthesized from a plasmid containing the complete coding sequence of kappa or mu receptor (gift from Dr. S. Watson at University of Michigan. The probe sequence has been described in Xie and colleagues (1994). After a succession of one hour washes (2xSSC/1mM DTT, 50°C; 0.2xSSC/1mMDTT, 55°C; 0.2xSSC/1mM DTT, 60°C), the tissue was treated with RNase-A (Boehringer-Mannheim, Indianapolis) and washed under conditions of increasing stringency, including a 30 min wash at 60°C in 0.1× SSC. The tissue was then dehydrated in alcohols and air-dried. The sections were exposed to X-ray film (Eastman-Kodak) for 2–3 days, then the slides were dipped in Kodak NTB-2 emulsion and exposed for one month. Slides were developed in Kodak D-19, fixed, then dehydrated and coverslipped.
Cell counting
The number of Fos-ir cells and Fos-ir cells with silver-grains (indicating kappa and mu receptors mRNA) were counted within a box that covered the VLPO (AP −0.3 mm to −0.7 mm). The counting box size and location were as described previously (Lu et al., 2000). For cell quantification in other regions, we counted three adjacent sections in the region of interest.
Statistics
Student’s paired t-tests were used to determine significant differences (p<0.05) in NREM sleep and REM sleep in the control and treatment groups. We also performed correlation analysis to estimate the association between sleep and Tb responses and drug dosage (R2=square of correlation coefficient). All data are reported as mean±standard error of the mean (x±SEM).
Acknowledgments
We thank Quan Hui Ha and Mihn Ha for technical help and Drs. Clif Saper and Marshall Devor for editing and insight comments. This work is supported by NS 05169, VA Medical Research, MH55772, AG 09975, and HL60292.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Bronzino JD, Kelly ML, Cordova C, Gudz M, Oley N, Stern WC, Morgane PJ. Amplitude and spectral quantification of the effects of morphine on the cortical EEG of the rat. Electroencephalogr Clin Neurophysiol. 1982;3:14–26. doi: 10.1016/0013-4694(82)90102-x. [DOI] [PubMed] [Google Scholar]
- Chen XH, Geller EB, DeRiel JK, Liu-Chen LY, Adler MW. Antisense confirmation of mu- and kappa-opioid receptor mediation of morphine’s effects on body temperature in rats. Drug Alcohol Depend. 1996;43:119–24. doi: 10.1016/s0376-8716(96)01295-1. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240(2):153–60. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Arrigoni E, Chou TC, Scammell TE, Greene RW, Saper CB. Effects of adenosine on GABAergic synaptic inputs to identified ventrolateral preoptic neurons. Neuroscience. 2003;119:913–918. doi: 10.1016/s0306-4522(03)00246-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;8:289–294. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltro Campi C, Clarke GD. Effects of highly selective kappa-opioid agonists on EEG power spectra and behavioural correlates in conscious rats. Pharmacol Biochem Behav. 1995;51:611–6. doi: 10.1016/0091-3057(94)00384-u. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Reed TK. Ethanol effects on EEG spectra in monkeys: comparison to morphine and diazepam. Electroencephalogr Clin Neurophysiol. 1987;66:317–21. doi: 10.1016/0013-4694(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tajima A, Izumimoto N, Tajima C, Suzuki T, Saitoh A, Suzuki T, Narita M, Tseng L, Nagase H. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999;65:1685–94. doi: 10.1016/s0024-3205(99)00417-8. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Stevens DR, Haas HL. Opposite modulation of histaminergic neurons by nociceptin and morphine. Neuropharmacology. 2000;39:2492–8. doi: 10.1016/s0028-3908(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J Comp Neurol. 1995;353:506–28. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB. The neuroanatomy of sleep: sleep architecture, circadian regulation and regulatory feedback. J Biol Rhythms. 2006;21(6):482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–59. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Luppi PH, Rambert FA, Frydman A, Fort P. Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: an in vitro pharmacologic study. Sleep. 2004;27:19–25. [PubMed] [Google Scholar]
- Garzon M, Tejero S, Beneitez AM, de Andres I. Opiate microinjections in the locus coeruleus area of the cat enhance slow wave sleep. Neuropeptides. 1995;29:229–39. doi: 10.1016/0143-4179(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Grasing K, Szeto H. Naloxone causes a dose-dependent increase in total power and delta wave activity in the EEG of opioid-naive rats. J Pharmacol Exp Ther. 1991;259:464–468. [PubMed] [Google Scholar]
- Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–21. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- Guedes AG, Papich MG, Rude EP, Rider MA. Pharmacokinetics and physiological effects of two intravenous infusion rates of morphine in conscious dogs. J Vet Pharmacol Ther. 2007;30:224–33. doi: 10.1111/j.1365-2885.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- Gulledge CC, Mann PE, Bridges RS, Bialos M, Hammer RP., Jr Expression of mu-opioid receptor mRNA in the medial preoptic area of juvenile rats. Brain Res Dev Brain Res. 2000;119:269–76. doi: 10.1016/s0165-3806(99)00184-4. [DOI] [PubMed] [Google Scholar]
- Hayes AG, Birch PJ, Hayward NJ, Sheehan MJ, Rogers H, Tyers MB, Judd DB, Scopes DI, Naylor A. A series of novel, highly potent and selective agonists for the kappa-opioid receptor. Br J Pharmacol. 1990;101:944–8. doi: 10.1111/j.1476-5381.1990.tb14185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG, Fitzjarrell AT, Walker CF. Kappa opiate receptor agonists: effects on behavior and on brain function and structure in rhesus monkeys. Biol Psychiatry. 1984;19:1045–74. [PubMed] [Google Scholar]
- Kamerling SG, Martin WR, Wu KM, Wettstein JG. Medullary kappa hyperalgesic mechanisms II. The effects of ethylketazocine administered into the fourth cerebral ventricle of the conscious dog. Life Sci. 1983;33:1839–43. doi: 10.1016/0024-3205(83)90692-6. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457(3):213–35. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pearson MA, Banks WA. EEG evidence that morphine and an enkephalin analog cross the blood-brain barrier. Pharmacol Biochem Behav. 1991;40:771–4. doi: 10.1016/0091-3057(91)90084-f. [DOI] [PubMed] [Google Scholar]
- Kay DC, Pickworth WB, Neidert GL, Falcone D, Fishman PM, Othmer E. Opioid effects on computer-derived sleep and EEG parameters in nondependent human addicts. Sleep. 1979;2:175–91. doi: 10.1093/sleep/2.2.175. [DOI] [PubMed] [Google Scholar]
- Kosterlitz HW. The Wellcome Foundation lecture. Opioid peptides and their receptors. Proc R Soc Lond B Biol Sci. 1985;225:27–40. doi: 10.1098/rspb.1985.0048. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479(2):225–40. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PE, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during REM sleep. J Neurosci. 2002 doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–38. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Martin GE, Morrison JE. Hyperthermia evoked by the intracerebral injection of morphine sulphate in the rat: the effect of restraint. Brain Res. 1978;145:127–40. doi: 10.1016/0006-8993(78)90801-6. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–71. [PubMed] [Google Scholar]
- Mayo-Michelson L, Young GA. Genetic profiles of morphine-induced EEG, EEG power spectra, and behavior in two inbred rat strains. Brain Res Bull. 1993;30(1–2):79–84. doi: 10.1016/0361-9230(93)90041-9. [DOI] [PubMed] [Google Scholar]
- Mayo-Michelson L, Young GA. EEG, EEG power spectral, and behavioral differences in response to acute ethylketocyclazocine administration in two inbred rat strains. Brain Res Bull. 1993;31(3–4):345–51. doi: 10.1016/0361-9230(93)90226-2. [DOI] [PubMed] [Google Scholar]
- McFadzean I. The ionic mechanisms underlying opioid actions. Neuropeptides. 1988;11:173–80. doi: 10.1016/0143-4179(88)90072-8. [DOI] [PubMed] [Google Scholar]
- Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of mu and kappa opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport. 1997;8:3167–72. doi: 10.1097/00001756-199709290-00032. [DOI] [PubMed] [Google Scholar]
- Minami M, Hosoi Y, Toya T, Katao Y, Maekawa K, Katsumata S, Yabuuchi K, Onogi T, Satoh M. In situ hybridization study of kappa-opioid receptor mRNA in the rat brain. Neurosci Lett. 1993;162:161–4. doi: 10.1016/0304-3940(93)90585-9. [DOI] [PubMed] [Google Scholar]
- Moran TD, Abdulla FA, Smith PA. Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides. 2000;21:969–76. doi: 10.1016/s0196-9781(00)00235-7. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette NC, Young GL, Geller EB, Adler MW. Body Relationship between regulation of morphine-induced EEG effects and changes in naloxone sensitivity. Eur J Pharmacol. 1991;196:61–7. doi: 10.1016/0014-2999(91)90409-j. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Pickworth WB, Sharpe LG, Gupta VN. Morphine-like effects of clonidine on the EEG, slow wave sleep and behavior in the dog. Eur J Pharmacol. 1982;81:551–7. doi: 10.1016/0014-2999(82)90344-2. [DOI] [PubMed] [Google Scholar]
- Reinoso-Barbero F, de Andres I. Effects of opioid microinjections in the nucleus of the solitary tract on the sleep-wakefulness cycle states in cats. Anesthesiology. 1995;82:144–52. doi: 10.1097/00000542-199501000-00019. [DOI] [PubMed] [Google Scholar]
- Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–83. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. In: Paxinos G, editor. The Rat Nervous System. Academic Press; 1995. pp. 107–135. [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803(1–2):178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–8. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortella FC, Rose J, Robles L, Moreton JE, Hughes J, Hunter JC. EEG spectral analysis of the neuroprotective kappa opioids enadoline and PD117302. J Pharmacol Exp Ther. 1997;282:286–93. [PubMed] [Google Scholar]
- Tortella FC, Moreton JE, Khazan N. Electroencephalographic and behavioral tolerance to and cross-tolerance between D-Ala2-methionine-enkephalinamide and morphine in the rat. J Pharmacol Exp Ther. 1979;210(2):174–9. [PubMed] [Google Scholar]
- Tortella FC, Moreton JE, Khazan N. Electroencephalographic and behavioral effects of D-ala2-methionine-enkephalinamide and morphine in the rat. J Pharmacol Exp Ther. 1978;206(3):636–43. [PubMed] [Google Scholar]
- Vanni-Mercier G, Gigout S, Debilly G, Lin JS. Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophysiological study in freely moving cats. Behav Brain Res. 2003;144:227–241. doi: 10.1016/s0166-4328(03)00091-3. [DOI] [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- Vonvoigtlander PF, Lahti RA, Ludens JH. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–86. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie GX, Meng F, Mansour A, Thompson RC, Hoversten MT, Goldstein A, Watson SJ, Akil H. Primary structure and functional expression of a guinea pig kappa opioid (dynorphin) receptor. Proc Natl Acad Sci U S A. 1994;91:3779–83. doi: 10.1073/pnas.91.9.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Geller EB, Adler MW. Body temperature and analgesic effects of selective mu and kappa opioid receptor agonists microdialyzed into rat brain. J Pharmacol Exp Ther. 1997;281:499–507. [PubMed] [Google Scholar]
- Yakimova KS, Sann H, Pierau FK. Effects of kappa and delta opioid agonists on activity and thermosensitivity of rat hypothalamic neurons. Brain Res. 1998;786:133–42. doi: 10.1016/s0006-8993(97)01456-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ochi J, Daddona PE, Nagy JI. Ultrastructural immunolocalization of adenosine deaminase in histaminergic neurons of the tuberomammillary nucleus of rat. Brain Res. 1990;527:335–41. doi: 10.1016/0006-8993(90)91155-a. [DOI] [PubMed] [Google Scholar]
- Young GA, Hudson GM, Stamidis H, Steinfels GF. Interactions between U-50,488H and sigma receptor antagonists: EEG, EEG power spectral and behavioral correlates. Eur J Pharmacol. 1993;231:473–6. doi: 10.1016/0014-2999(93)90127-4. [DOI] [PubMed] [Google Scholar]
- Zuo YF, Wang JY, Chen JH, Qiao ZM, Han JS, Cui CL, Luo F. A comparison between spontaneous electroencephalographic activities induced by morphine and morphine-related environment in rats. Brain Res. 2007;1136:88–101. doi: 10.1016/j.brainres.2006.11.099. [DOI] [PubMed] [Google Scholar]