Abstract
Aims
The primary aim was to compare the efficacy of smoking cessation treatment using the combination of active nicotine patch plus active nicotine gum versus therapy consisting of active nicotine patch plus placebo gum in a sample of alcohol dependent tobacco smokers in an early phase of outpatient alcohol treatment. A secondary aim was to determine whether or not there were any carryover effects of combination nicotine replacement on drinking outcomes.
Design
Small scale randomized double-blind placebo controlled clinical trial with one-year smoking and drinking outcome assessment.
Setting
Two outpatient substance abuse clinics provided a treatment platform of behavioral alcohol and smoking treatment delivered in three months of weekly sessions followed by three monthly booster sessions.
Participants
Participants were 96 men and women with a diagnosis of alcohol abuse or dependence and smoking 15 or more cigarettes per day.
Intervention
All participants received open-label transdermal nicotine patch and were randomized to receive either 2 mg nicotine gum or placebo gum under double blind conditions.
Findings
Analysis of 1-year follow-up data revealed that patients receiving nicotine patch plus active gum had better smoking outcomes than those receiving patch plus placebo gum on measures of time to smoking relapse and prolonged abstinence at 12 months. Alcohol outcomes were not significantly different across medication conditions.
Conclusions
Results of this study were consistent with results of larger trials of smokers without alcohol problems showing that combination therapy (nicotine patch plus gum) is more effective than monotherapy (nicotine patch) for smoking cessation.
Keywords: smoking, smoking cessation, nicotine, alcoholism, tobacco
Although cigarette smoking prevalence among U.S. adults has declined to 21 percent (1), the majority of individuals with alcohol problems remain current smokers (2, 3). The negative health consequences of smoking among alcohol abusers are substantial. A longitudinal study of an alcohol treatment sample indicated that smoking killed more alcoholics than did alcohol (4). The United States Department of Health and Human Services (USDHHS) Clinical Guideline for Treating Tobacco Use and Dependence provided a consensus recommendation that smokers receiving treatment for chemical dependency should be provided smoking cessation treatments including both counseling and pharmacotherapy (5). These Guideline recommendations have not led to significant changes in clinical practice. Fuller and colleagues (6) recently surveyed 342 substance abuse treatment units and found that 235 (69%) offered no treatment for nicotine dependence.
Studies have evaluated smoking cessation interventions for individuals with alcohol and drug problems. Although one review found that smokers with past alcohol problems were as likely to stop smoking on a given quit attempt as smokers without alcohol problems (7), another review (8) concluded that smoking cessation rates tended to be low among individuals in early substance abuse recovery and increased with length of sobriety. A meta-analysis of 11 studies of smoking cessation during substance abuse treatment (9) found long term smoking abstinence rates of 7% in the intervention groups and 6% in the comparison conditions with a summary risk ratio of 1.00. These reviews, taken together with USDHHS clinical practice guideline recommendations, suggest the need to develop more effective smoking cessation interventions for use during alcohol and drug treatment with individuals in early recovery.
Smokers with alcohol problems might have a greater need for pharmacotherapy to assist cessation compared to smokers without alcohol problems. Smokers with a history of alcoholism tend to have more severe nicotine dependence (7). They reported a greater preference for active nicotine gum and self-administered more milligrams of nicotine than smokers without a history of alcoholism (10), suggesting that nicotine was more reinforcing in smokers with a history of alcoholism than in smokers without this history. Among alcohol dependent smokers who received concurrent alcohol and smoking cessation treatment, increased cigarette craving was a significant antecedent to both tobacco and alcohol relapse (11). This finding suggests the possibility that pharmacotherapy to control cigarette craving could reduce relapse risk for both substances.
It is reasonable to hypothesize that higher dose nicotine replacement may improve smoking cessation treatment efficacy for alcoholic smokers. However, a study conducted with smokers with a history of alcohol dependence found that high dose nicotine patch therapy (42 mg) was not more effective than standard 21 mg dose (12). It has been suggested that smokers might benefit from both a constant level of nicotine (nicotine patch) to relieve symptoms of withdrawal and craving, in combination with a self-administered, self-titrated, faster acting preparation (e.g., nicotine gum) for more immediate relief of breakthrough craving to smoke (13). Clinical trials suggest higher efficacy of this type of combined nicotine replacement therapy in studies of smokers without alcohol problems (14). Although combination nicotine replacement therapy is not FDA approved, the 2008 update of the DHHS Clinical Guideline for Treating Tobacco Use and Dependence (15) included the combination of nicotine patch and other ad libitum nicotine replacement on its list of recommended pharmacotherapies. The meta-analysis included in the Guideline update found that this combination yielded an odds ratio of 3.6 compared to placebo. This odds ratio appeared to be higher than any other monotherapy or combination therapy examined. A recent preference study compared combination nicotine treatments (patch/patch vs. lozenge/patch vs. inhaler/patch vs. gum/patch) and found that the combination of nicotine gum and patch received overall better preference ratings than the alternatives across measures of “ease of use”, “use under stress”, and “help to quit” (16).
The primary aim of our study was to compare the efficacy of smoking cessation treatment using active nicotine patch and active nicotine gum (NP+NG) versus therapy consisting of active nicotine patch and placebo nicotine gum (NP+PG) in a sample of alcohol dependent tobacco smokers in an early phase of outpatient alcohol treatment. A secondary aim was to determine whether or not there are any carryover effects of combination nicotine replacement on drinking outcomes.
Method
Participants
Although we sought to enroll 175 participants, only 96 men and women were enrolled at the Newington and West Haven campuses of the Veterans Affairs (VA) Connecticut Healthcare System. The trial was approved by institutional review boards at the VA Connecticut Healthcare System, Yale University School of Medicine and the University of Connecticut Health Center. The sample was recruited via radio and newspaper advertisements and by soliciting referrals from the VA substance abuse clinic. Participants were eligible for inclusion if they were at least 18 years of age, were English speaking, met DSM-IV criteria for current substance abuse or dependence based on the computer-administered Structured Clinical Interview for DSM-IV (SCID; 17), reported currently smoking 15 or more cigarettes per day with at least a 3-year smoking history, met American Society of Addiction Medicine (ASAM) patient placement criteria for Level 1 outpatient treatment (18), and reported current motivation to stop drinking, stop smoking, and attend a 16-session outpatient treatment program. Individuals were excluded if they had an allergy or hypersensitivity to nicotine or to adhesives used in transdermal delivery systems, weighed less than 100 lbs., had severe generalized skin disorder, active peptic ulcer, uncontrolled hypertension, insulin-dependent diabetes, active temporomandibular joint disease, history of clinically significant cardiovascular disease, were pregnant or lactating females, or females who were not practicing a medically accepted form of contraception, were taking medications known to influence alcohol or tobacco use (e.g., naltrexone, disulfiram, bupropion), were homeless or had unstable residence, were diagnosed with opiate or benzodiazepine abuse or dependence, or had a history of intravenous drug abuse within the past year.
Study Design
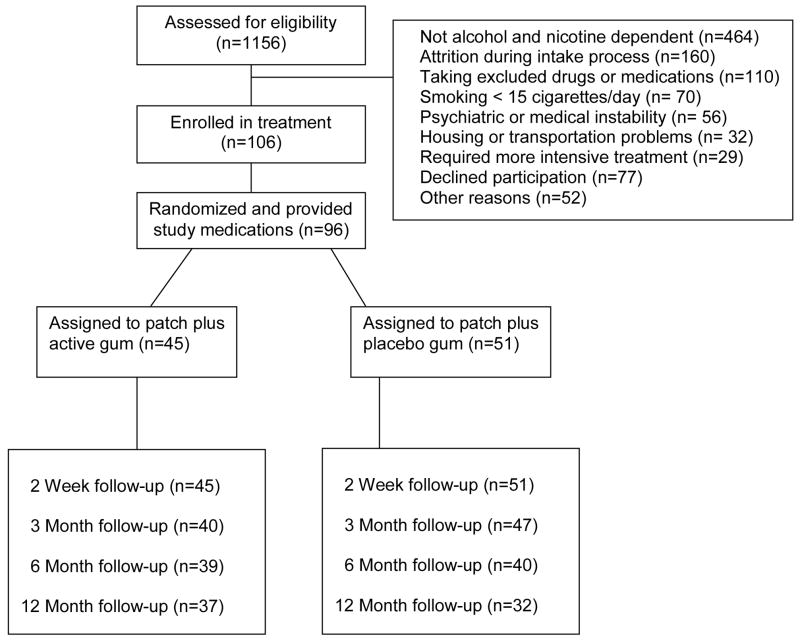
This study was a randomized, placebo controlled clinical trial comparing one group that received transdermal nicotine patch (open label) and 2 mg nicotine gum (NP+NG) versus another group that received nicotine patch (open label) and placebo gum (NP+PG). Gum was dispensed under double blind conditions. Both groups were provided a platform of behavioral alcohol and smoking treatment delivered in three months of weekly outpatient sessions followed by three monthly booster sessions. Research assessments were scheduled at 2 weeks, 3 months, 6 months, and 12 months after the target smoking quit date. Participant flow is illustrated in Figure 1. An Ecological Momentary Assessment (EMA) protocol was used to collect treatment process data during the two weeks before and after target smoking quit date and during a two-week period three months after target smoking quit date. EMA process results will be reported separately.
Figure 1.
Study profile.
Procedures
Combination nicotine replacement was evaluated in the context of a behavioral alcohol treatment and smoking cessation treatment platform. Participants entered the treatment protocol after signing an informed consent form, followed by completion of a battery of intake interviews and questionnaires and a medical history and physical examination. Alcohol detoxification, if necessary, was completed outside the treatment protocol prior to enrollment. Participants were randomized to study treatments using an urn randomization (19) computer program that balanced the two groups for history of previous substance use treatment, age, sex, baseline drinks/drinking day, and baseline cigarettes/day.
Assessment
Smoking and drinking parameters are the focus of this report. Research assistants who collected these data were blind to medication assignment and did not provide psychosocial treatments. The Form-90 (20), a calendar-based survey of daily drinking, was used to gather alcohol, drug, and tobacco frequency-of-use data for the 90 days prior to intake and each follow-up. The Form-90 was based on the timeline follow-back method (21) and has good test-retest reliability and validity for verifiable events (22).
The primary outcome variables were prolonged cigarette abstinence and time to smoking relapse. These measures were chosen based on recommendations of the abstinence outcome measures workgroup of the Society for Research on Nicotine and Tobacco (23). Smoking relapse was defined as smoking on 7 consecutive days or smoking at least once each week over 2 consecutive weeks. Prolonged smoking abstinence was defined as an absence of relapse after a 30 day grace period from the target quit date. This translated to 2 months abstinence prior to the 3 month follow-up, 5 months abstinence prior to the 6 month follow-up, and 11 months abstinence prior to the 12 month follow-up. Designation of smoking abstinence outcomes also required a breath carbon monoxide (CO) level < 10 ppm using the Breath CO (Vitalograph, Inc., Lenexa, KS) at the time of the endpoint assessment and at all previous assessments. The primary alcohol outcome was self-reported continuous alcohol abstinence for 90 days prior to the follow-up time point. Designation of alcohol abstinence outcomes also required an alcohol breathalyzer reading of 0 using the Alco-Sensor IV (Intoximeters, Inc., St. Louis, MO)
Treatment
Individual 60-minute treatment sessions were scheduled weekly for the first three months, then monthly for the next three months for a total of sixteen sessions. Therapists had a minimum of a masters or doctoral degree and one or more years experience in cognitive behavioral addiction therapy. The alcohol and tobacco interventions were distinct from one another, and did not emphasize the similarities between quitting smoking and stopping alcohol and drug use. This clinical model was chosen based on findings from Burling et al. (24) who reported that concurrent treatment of alcohol and tobacco that emphasized these similarities led to worse short-term drinking outcome compared with concurrent treatment that did not emphasize these similarities.
Alcohol treatment was based on the cognitive behavioral therapy manual developed for Project MATCH (25), with approximately 40–45 minutes of each session devoted to alcohol treatment. Components of this intervention included identifying alcohol antecedents, coping with alcohol urges, managing thoughts about alcohol, problem solving, drink refusal skills, planning for emergencies, communication and assertiveness training, and enhancing social support networks for alcohol abstinence.
The smoking cessation intervention was delivered in the same sessions as the alcohol treatment, with approximately 15 – 20 minutes of each session devoted to smoking cessation. Treatment employed behavioral elements that have been empirically supported according to the USDHHS smoking cessation practice guideline (5): problem solving/skills training, extra-treatment social support, and intra-treatment social support. A target smoking quit date was set for the fourth treatment session to allow three weeks to prepare for cessation. Interventions in these early sessions included motivational and behavioral strategies to initiate smoking cessation, and behavioral and cognitive management of nicotine withdrawal symptoms. The remaining eight weekly sessions were aimed at relapse prevention and maintenance of tobacco abstinence. Components of these sessions included identification of smoking antecedents, cognitive behavioral coping skills training, eliciting support networks for cessation, enhancing self-efficacy for cessation, and management of stress and weight concerns. Subjects in both the NP+NG and the NP+PG groups were instructed to use one 21 mg (Nicoderm CQ ®) nicotine patch daily for 8 weeks, followed by one 14 mg patch daily for 2 weeks, then followed by one 7 mg patch daily for 2 weeks, for a total of 12 weeks of nicotine patch therapy. Nicotine gum (2 mg uncoated mint Nicorette ®) or placebo gum was given for ad libitum use, with encouragement to use at least six pieces per day, up to a maximum of twenty pieces per day. The placebo gum (manufactured by Fertin Pharma A/S, Vejle, Denmark) contained 2.6% cayenne pepper to simulate the taste of nicotine. Use of the gum was encouraged for 24 weeks. Those who relapsed to smoking were encouraged to continue using patch and gum and to redouble their quit efforts.
Follow-up
Follow-up assessments were scheduled at 2 weeks and at 3, 6, and 12 months after the participant’s target smoking quit date. All participants randomized to treatment and provided nicotine replacement medications were considered part of an intent-to-treat sample, and were followed regardless of their retention in treatment. Participants were paid $75 for participating in each of the four follow-up meetings.
Nicotine patch and gum use were assessed at the 2-week, 3-month, and 6-month follow-up interviews. At these times participants reported patch and gum use over the previous 7 days (days used and average per day). Patch and gum use were also assessed at treatment sessions. If patch and gum use data were missing from a research follow-up, use was estimated from reports obtained at treatment sessions. Saliva samples were obtained at the 2-week follow-up to assess the impact of nicotine replacement therapy on cotinine levels.
A 17-item checklist format was used to obtain information about the most commonly reported adverse effects experienced in the past 7 days for nicotine patch and nicotine gum, with severity rated on a scale from 0 (not present) to 3 (severe). In addition, subjects were asked to report any other concerns that were recorded individually. This information was collected at each research appointment.
Data Analysis
Analysis of smoking outcomes
Because of the low frequency of abstinence at later follow-up points, the assumptions of logistic regression could not be met with our data when the Treatment Condition term was in the model. Chi-square analysis was therefore used to evaluate the effect of treatment condition on prolonged smoking abstinence (0, 1) at each follow-up point. Note that this analysis does not include covariate adjustment. Following the recommendations of West et al. (26), participants with missing data at each time point were coded as smokers. Cox regression analysis was used to examine time to relapse in days as a function of Treatment condition.
Analysis of drinking outcomes
A generalized estimating equations model (GEE, Proc GENMOD; 27) was used to examine reported alcohol abstinence across retrospective 90-day time windows assessed at 3, 6, and 12 months after target smoking quit date. This approach was used because it takes advantage of all available data (N = 96) by using maximum likelihood estimation procedures to estimate the parameters of the multivariate model (28, 29). Time to first drink and time to first heavy drinking day were analyzed using Cox model regression analyses.
Relation between nicotine gum use and outcome
Logistic regression was used to examine the association between a 7-day retrospective report of the frequency of gum use assessed 2 weeks after quit date and subsequent smoking outcomes at the 3, 6, and 12-month follow-ups.
Results
Study sample and randomization
Randomization began in January 2004, and the last participant follow-up was conducted in June 2007. The number of participants enrolled at the two treatment sites was 56 at Newington and 40 at West Haven. Of these 96, 45 were assigned to the NP+NG condition and 51 to the NP+PG condition. This study recruited individuals who were in the beginning phase of alcohol treatment. The mean number of days of alcohol abstinence of sample at time of enrollment was 9.02 (SD = 26.30), and 81 percent reported alcohol use in the week before enrollment. Demographics and baseline characteristics of the sample are shown in Table 1. The two treatment groups were balanced with respect to age, sex, race, baseline smoking rate and CO levels, alcohol and drug use, and veteran status. However, among 13 variables examined, Education, baseline Fagerstrom Test for Nicotine Dependence (FTND), and Center for Epidemiologic Studies Depression Scale (CES-D) scores were significantly different across treatment groups, with the active gum group having a higher level of education, nicotine dependence, and depressive symptoms. Participants at the two treatment sites were equivalent on demographic and baseline variables except for lower mean baseline FTND scores at Newington (M = 5.55, SD = 2.26) compared to West Haven (M = 6.45, SD = 2.53) and a lower percentage of Black participants at Newington (0%) compared with West Haven (12.5%).
Table 1.
Baseline and Demographic Characteristics by Treatment Condition
| Placebo Gum (n = 51) |
Active Gum (n = 45) |
|
|---|---|---|
| Age (M, SD) | 44.8 (10.1) | 45.1 (10.2) |
| Women (%) | 29.4 | 28.9 |
| Race (%) | ||
| White | 88.2 | 91.1 |
| Black | 3.9 | 6.7 |
| Hispanic | 3.9 | 2.2 |
| Other (bi-racial) | 3.9 | 0.0 |
| Education (% college degree)* | 25.5 | 51.1 |
| Baseline cigarettes/day (M, SD) | 25.0 (10.3) | 26.0 (9.16) |
| Carbon monoxide ppm (M, SD) | 29.7 (13.6) | 29.7 (13.4) |
| FTND (M, SD)* | 5.45 (2.17) | 6.47 (2.33) |
| 90-Day baseline alcohol PDA (M, SD) | 0.28 (0.26) | 0.28 (0.27) |
| 90-Day baseline alcohol PDH (M, SD) | 0.58 (0.32) | 0.56 (0.31) |
| 90-day baseline other drug use (% any use) | ||
| Cocaine | 13.7 | 13.3 |
| Cannabis | 19.6 | 26.6 |
| CES-D (M, SD)* | 15.5 (5.61) | 19.4 (8.67) |
| Veteran (%) | 29.4 | 33.3 |
Note. FTND = Fagerstrom Test for Nicotine Dependence, PDA = Percent Days Abstinent, PDH = Percent Days Heavy, CES-D = Center for Epidemiologic Studies Depression Scale.
p < .05.
Data Completeness
The average retention across groups for the prolonged CO-verified smoking abstinence outcome measure was 100% at 2 weeks, 91% at 3 months, 82% at 6 months, and 72% at 12 months. There were no between-treatment differences in percent of participants retained over the follow-up periods (all p’s > .05). In spite of follow-up attrition, we were able to determine time to smoking relapse for 100% of the sample because all of the attrition occurred after smoking relapse was reported.
Adherence to medications and behavioral interventions
Nicotine patch and gum use during treatment among smoking abstinent participants is shown in Table 2. There were no statistically significant differences between treatment groups in reported patch or gum use (all p’s > .10). Salivary cotinine samples were available for 35 of the 43 subjects who did not report a smoking relapse prior to the 2-week follow-up. Cotinine levels were significantly higher in the patch plus active gum condition (M = 407, SD = 250) than in the patch plus placebo gum condition (M = 245, SD = 106; t (33) = −2.05, p < .05). The mean number of treatment sessions attended out of a possible 16 sessions was as follows: placebo gum 10.3 sessions (SD = 3.75) and active gum 11.6 sessions (SD = 3.51). Mean attendance was not significantly different (p > .05) across gum conditions or across treatment sites.
Table 2.
Mean (and SD) Nicotine Patch and Gum Use among Abstinent Participants by Treatment Condition
| Patch plus Placebo Gum | Patch plus Active Gum | |
|---|---|---|
| Patches/wk at 2 weeks (n=33) | 6.89 (0.33) | 6.55 (1.61) |
| Patches/wk at 13 weeks (n=13) | 3.18 (3.03) | 3.53 (3.47) |
| Gum pieces/day at 2 weeks (n=33) | 6.61 (3.03) | 7.45 (3.73) |
| Gum pieces/day at 13 weeks (n=13) | 5.43 (5.23) | 4.06 (3.60) |
All placebo vs. active gum comparisons nonsignificant, p > .10.
Effectiveness of blind
In order to determine the effectiveness of the blind procedure, at the six month follow-up participants were asked, “Which type of gum were you taking during treatment?” The response options were “nicotine gum”, “placebo gum”, or “don’t know”. Eighty percent responded “don’t know” and the remaining 20 percent correctly guessed the gum contents only 50 percent of the time.
Adverse effects
Eight adverse events were reported that were rated as possibly related to nicotine patch and gum use, with four reports each in the placebo and active gum conditions. Only one event was systemic (reported nausea, diarrhea, and general rash on neck) and no events were rated severe. On the 17-item adverse effects checklist completed at the 2 week, 3 month, and 6 month follow-ups, two significant differences emerged between treatment conditions among the 51 comparisons examined: reported sore or bleeding gums (placebo 2.4% vs. active gum 15.8%) at the 2 week follow-up and reported aches in jaw muscles (placebo 0% vs. active gum 11.8%) at the 3 month follow-up. No participant reported discontinuing gum use due to adverse effects.
Smoking Outcomes
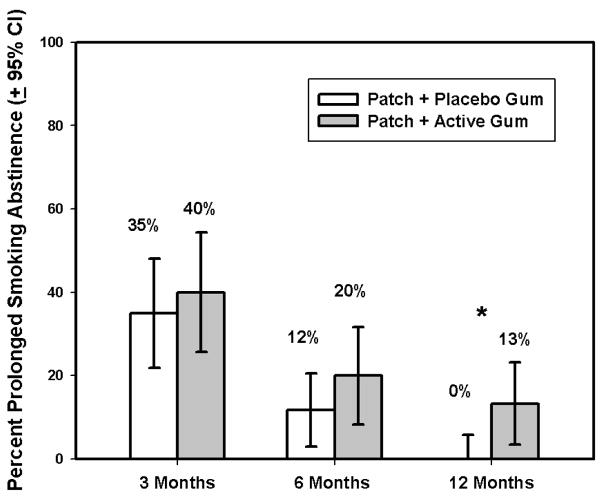
The rate of prolonged smoking abstinence by Gum Treatment Condition at each follow-up time point is shown in Figure 2. The Active Gum condition tended to yield more abstinence than did the Placebo condition, but this difference was not significant until the 12-month period (χ2 (1, N = 96) = 7.25, p < .01).
Figure 2.
Percent prolonged CO-verified smoking abstinence by gum condition across follow-ups.
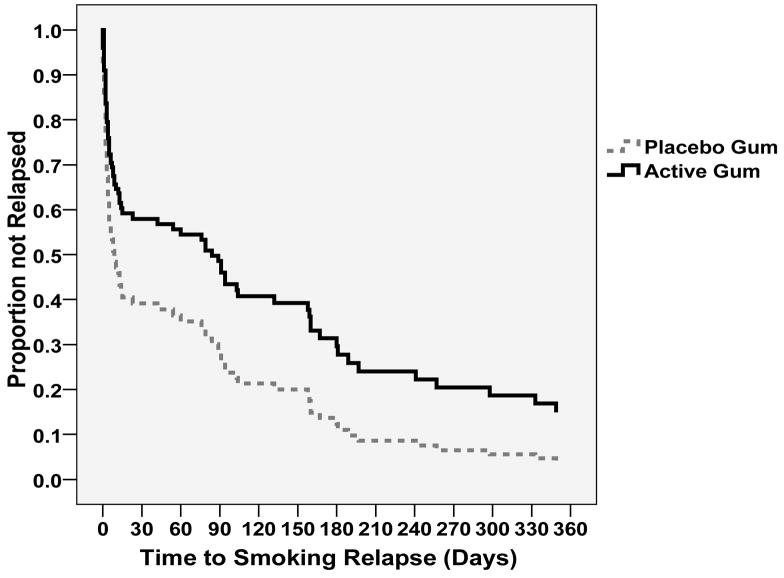
A Cox proportional hazards regression model of time to smoking relapse was conducted in which time to smoking relapse was evaluated by nicotine replacement treatment condition, and controlling for the set of covariates that differed between treatment conditions (i.e., education level, depression score, Fagerstrom score, and treatment site). The results indicated that, controlling for the covariates, treatment condition was a significant predictor of time to relapse, such that being in the Active Gum condition extended survival [B = −.57, se = .27, Wald χ2 = 4.47, p < .05; hazard ratio = 0.57, (95% CI = 0.34 to 0.96)]. The covariate-adjusted estimated survival functions by Treatment Condition are shown in Figure 3.
Figure 3.
Estimated survival functions for time to smoking relapse by gum condition.
Drinking Outcomes
Ninety-day abstinence rates for drinking at 3 months, 6 months, and 12 months were 28%, 32% and 32% for the placebo gum condition and were 45%, 38%, and 43% for the active gum condition. GEE models evaluating repeated abstinence rates as a function of Treatment condition, while controlling for covariates, showed no effect for Treatment (B = −0.14; se = 0.13, 95% CI: −0.40 to 0.20) for Time (B = −0.00005; se = 0.0004, 95% CI: 0.000 to 0.001), or for the interaction of Time X Treatment (B = −0.0000; se = 0.00005, 95% CI: 0.000 to 0.001). Likewise, a Cox proportional hazards regression model of time to first drink showed no effect on survival for Treatment condition [B = 0.17, se = .24, Wald χ2 = 0.53, p > .46; hazard ratio = 1.19, (95% CI = 0.74 to 1.91)].
Gum Use and Smoking Outcomes
To test the hypothesis that gum use drives subsequent smoking abstinence, we examined seven-day reported frequency of nicotine or placebo gum use at 2 weeks after target quit date as a predictor of CO-verified prolonged smoking abstinence in a subsample of participants who were cigarette abstinent at the 2-week time point (n = 37). Logistic regression analyses were conducted, controlling for the covariate set specified above. Gum use at 2 weeks was predictive of 3-month prolonged smoking abstinence (n = 37; B = .036; se = .014; Wald χ2 = 6.96; p = .008; OR = 1.04; 95% CI: 1.01 to 1.06), and 6-month prolonged abstinence (n = 32; B = .036; se = .018; Wald χ2 = 4.02; p = .045; OR = 1.04; 95% CI: 1.01 to 1.08), but not 12-month prolonged abstinence (n = 30; B = .032; se = .035; Wald χ2 = 0.82; p = .364; OR = 1.03; 95% CI: 0.97 to 1.10).
Discussion
In the present small clinical trial, patients receiving nicotine patch plus ad libitum nicotine gum showed better smoking outcomes than those receiving nicotine patch and placebo gum. The risk of smoking relapse (hazard ratio) in the combination NRT condition in the year after quit date was about half the relapse risk in the nicotine patch plus placebo gum condition. Four larger scale placebo-controlled studies of smoking cessation conducted among individuals without alcohol or drug problems also found better smoking outcomes for combination NRT conditions relative to a single form of active NRT plus placebo. One study compared nicotine patch plus nasal spray vs. patch plus placebo nasal spray (30), another compared nicotine patch plus gum vs. nicotine patch plus placebo gum (31), the third compared nicotine patch plus gum vs. placebo patch plus gum (32), and the forth compared nicotine inhaler plus patch vs. nicotine inhaler plus placebo patch (33). In three of these studies nicotine patch was used for longer durations than in the present study, ranging from 18 to 24 weeks.
Gum use in the present study was recommended for only six months after cessation, so the finding that significant differences between combination and single NRT conditions in smoking abstinence did not emerge until 12 months after treatment was not expected and is not easily explained. In other studies of combination v. single NRT the appearance of between-treatment differences has come at variable follow-up points, with one study showing differences between conditions at week 6 (32), one at week 12 (33), one at week 24 (31), and one study not showing any between-treatment differences until week 52 (30).
A meta-analysis found that the estimated odds ratio of combination NRT arms was 1.9 relative to nicotine patch therapy alone in predicting prolonged abstinence (15). The present study extended the evidence of efficacy of combination NRT to the population of patients receiving smoking cessation therapy concurrent with alcohol and drug treatment. This study also stands in contrast with a clinical trial of individuals with a history of alcoholism which did not find improved smoking outcomes with higher dose nicotine patch therapy (12). This suggests that the improved smoking outcomes seen after combination NRT depend on the combination of a constant dose of nicotine (patch therapy) along with rapid acting, self-titrating nicotine delivery (patch), and these results are not merely the due to higher nicotine dose levels.
There was no evidence that the combination NRT treatment with its higher rates of smoking abstinence had a detrimental impact on drinking outcomes relative to the single NRT condition. In fact, the nonsignificant difference in drinking outcomes was in the direction of higher posttreatment drinking rates in the single NRT condition than in the combination NRT condition. Results suggest that smoking treatments that produce higher smoking quit rates are not likely to elevate alcohol relapse risk. Note that the current study did not provide a test of whether or not providing smoking treatment concurrent with alcohol treatment elevated or reduced the risk of alcohol relapse compared with alcohol treatment with smoking treatment delayed until later in alcohol recovery. The present trial cannot rule out the possibility that smoking abstinence worsens outcomes in a minority of alcoholics in early recovery. The study design only allowed a test of the relative impact of single vs. combination NRT.
Clinical studies have not shown any increased safety concerns of combined nicotine patch plus gum regimens, compared with patch or gum alone for smoking cessation (31, 32). These studies employed almost 700 subjects, excluding only those smoking fewer than 10 cigarettes a day, and found a low rate of adverse effects that was not significantly different between single and combination nicotine replacement conditions. Results from the present study were consistent with the findings of a low rate of adverse effects of combination NRT. It seems that tobacco users can quickly learn not to exceed ad libitum NRT use that could generate nicotine levels that might produce adverse effects. If anything, the greater problem is that patients did not use enough ad libitum NRT to obtain optimal benefits. Although patients in the present study were advised to use 5 to 20 pieces of gum per day, the mean use soon after cessation was approximately seven pieces per day. Greater frequency of gum use early in treatment was predictive of later smoking abstinence even after controlling for early smoking status.
Limitations of the present study include the relatively small sample size for detecting secondary effects of combination NRT on drinking outcomes. This study was powered to detect only large effects. The smoking interventions were delivered in the context of a relatively low intensity alcohol treatment consisting of once weekly individual behavioral counseling sessions. Prior research on concurrent treatment for substance use and smoking cessation has been conducted in the context of more intensive inpatient or residential programs. The present results may or may not generalize to those treatment settings. Questions about the mechanism of action of combination NRT and processes leading up to relapse will be addressed in a forthcoming paper.
In conclusion, the outcome of smoking cessation intervention delivered concurrent with alcohol and drug treatment may be improved by providing a combination pharmacotherapy consisting of nicotine patch and gum. However, the relatively low long-term smoking quit rate seen even in the combination patch and gum condition suggests the need to continue to explore cessation interventions for this challenging population of smokers.
Acknowledgments
The project described was supported by award number R01 AA011197 and P50 AA1563 from the National Institute on Alcohol Abuse and Alcoholism and by a MIRECC award from the Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health, or the Department of Veterans Affairs. Thank you to Stephanie O’Malley for assistance with the research design and to James Yaffe for medical evaluation of research participants.
Footnotes
Trial registration: ClinicalTrials.gov identifier NCT00064844
Conflict of interest statement: Judith Cooney and Kevin Sevarino have worked as promotional speakers for Pfizer, Inc.
References
- 1.Centers for Disease Control and Prevention. Cigarette smoking among adults--United States, 2004. Morb Mortal Wkly Rep. 2005;54:1121 –4. [PubMed] [Google Scholar]
- 2.Hughes JR. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice. Washington, DC: U.S. Government Printing Office; 1995. pp. 171–85. NIAAA Research Monograph no. 95–3931. [Google Scholar]
- 3.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study . JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 4.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 5.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000. [Google Scholar]
- 6.Fuller BE, Guydish J, Tsoh J, Ried MS, Resnick M, Zammarelli L, et al. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. J Subst Abuse Treat. 2007;32:53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Sussman S. Smoking cessation among persons in recovery. Subst Use Misuse. 2002;37:1275–98. doi: 10.1081/ja-120004185. [DOI] [PubMed] [Google Scholar]
- 9.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery . J Consult Clin Psychol. 2004;72:1144–56. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR, Ross GL, Callas PW. Nicotine is more reinforcing in smokers with a past history of alcoholism than in smokers without this history . Alcohol Clin Exp Res. 2000;24:1633–8. [PubMed] [Google Scholar]
- 11.Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA. Concurrent brief versus intensive smoking intervention during alcohol dependence treatment . Psychol Addict Behav. 2007;21:570–5. doi: 10.1037/0893-164X.21.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalman D, Kahler CW, Garvey AJ, Monti PM. High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-week outcomes . J Subst Abuse Treat. 2006;30:213–7. doi: 10.1016/j.jsat.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Schneider NG. Nicotine therapy in smoking cessation: pharmacokinetic considerations . Clin Pharmacokinet. 1992;23:169–72. doi: 10.2165/00003088-199223030-00001. [DOI] [PubMed] [Google Scholar]
- 14.Sweeny CT, Fant RV, Fagerstrom KO, McGovern JF, Henningfield JE. Combination nicotine replacement therapy for smoking cessation: Rationale, efficacy, and tolerability . CNS Drugs. 2001;15:453–67. doi: 10.2165/00023210-200115060-00004. [DOI] [PubMed] [Google Scholar]
- 15.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- 16.Schneider NG, Koury MA, Cortner C, Olmstead RE, Hartman N, Kleinman L, et al. Preferences among four combination nicotine treatments. Psychopharmacology. 2006;187:476–85. doi: 10.1007/s00213-006-0449-5. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Gibbon M, Williams JBW, Spitzer RL. SCID screen patient questionnaire (SSPQ) and SCID screen patient questionnaire – extended (SSPQ-X) North Tonawanda, NY: Multi-Health Systems, Inc; 1999. [Google Scholar]
- 18.American Society of Addiction Medicine. ASAM patient placement criteria for the treatment of substance-related disorders. 2. Chevy Chase, MD: American Society of Addiction Medicine; 2001. [Google Scholar]
- 19.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Alcoholism treatment matching research: Methodological and clinical approaches. In: Donovan DM, Mattson ME, editors. J Stud Alcohol Suppl. Suppl No. 12. 1994. pp. 70–5. [DOI] [PubMed] [Google Scholar]
- 20.Miller WR, DelBoca FK. Measurement of drinking behavior using the Form-90 family of instruments . J Stud Alcohol. 1994;12:112–8. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 21.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–71. [Google Scholar]
- 22.Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: An instrument for assessing alcohol treatment outcome . J Stud Alcohol. 1997;58:358–64. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- 23.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations . Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 24.Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance-dependent inpatients . J Consult Clin Psychol. 2001;69:295–304. doi: 10.1037//0022-006x.69.2.295. [DOI] [PubMed] [Google Scholar]
- 25.Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, et al. NIAAA Project MATCH Monograph Series. 3. Rockville, MD: US Government Printing Office; 1992. Cognitive-behavioral coping skills therapy manual: a clinical research guide for therapists counseling patients with alcohol abuse and dependence. DHHS Publication No. (ADM) 92–1895. [Google Scholar]
- 26.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard . Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute. SAS/STAT software: changes and enhancements through V7 and V8. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 28.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes . Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 30.Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomized trial with six year follow up . BMJ. 1999;318:285–89. doi: 10.1136/bmj.318.7179.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial . Prev Med. 1995;24:41–7. doi: 10.1006/pmed.1995.1006. [DOI] [PubMed] [Google Scholar]
- 32.Puska P, Korhonen HJ, Vartiainen E, Urjanheimo EL, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation: a clinical trial in North Karelia . Tob Control. 1995;4:231–5. [Google Scholar]
- 33.Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation . Arch Intern Med. 2000;160:3128–34. doi: 10.1001/archinte.160.20.3128. [DOI] [PubMed] [Google Scholar]