Abstract
Background
The in vivo transplantation assay has become a valuable tool for assessing the osteogenic potential of diverse cell populations. It has required that cells are cotransplanted with a matrix into recipient animals using large incisions and extensive dissections. Here, we demonstrate that transplants of an osteogenic cell population, bone marrow stromal cells (BMSCs), are capable of assembling into mature bone organs when injected as suspensions of cells and a particulate matrix.
Methods
Human BMSCs, along with hydroxyapatite/tricalcium phosphate (HA/TCP) particles, were placed either into the dorsal subcutaneous space or onto the calvarium of immunodeficient mice, either via injection or via a wide operative exposure. Transplants were harvested from 7 to 110 weeks later; their histologic and mechanical properties and their cellular origin were analyzed.
Results
A total of 43 transplants were evaluated. The extent of new bone and hematopoiesis, the bone's adherence to the underlying mouse calvarium, and the bone elastic modulus and hardness were comparable between the two groups. In situ hybridization confirmed a human origin of the new bone.
Conclusions
Our data indicate that BMSCs and HA/TCP particles, when injected as a suspension, can assemble into mature bone organs, and that this bone has histologic and mechanical properties similar to bone formed in standard transplants delivered through a large incision. These results open the possibility for assessing the osteogenic capacities of cell populations, for modeling bone formation and repair and for treating bone deficits, all in the context of minimal surgical intervention or soft tissue disruption.
Introduction
The use of in vivo transplantation assays under defined experimental conditions has become a valuable standard for delineating osteogenic potential of cell populations as diverse as embryonic stem cells,1 amniotic stem cells,2 pericytes,3 circulating fibroblast-like cells,4 adipose tissue stem cells,5 and spleen stromal cells.6 These assays help delineate the differentiation potential of cell populations according to bone quantity (if any), histologic appearance, density, hardness, and stiffness, as well as the maintenance of hematopoiesis.7 They uniquely demonstrate whether the cell populations can recapitulate the entire bone organ or only specific subsets.8 These assays, which can be performed in heterotopic or orthotopic sites, require a highly invasive operation that includes long skin incisions and extensive tissue dissection. The operative requirements limit the utility of these models to superficial anatomic locations, and they necessitate a general rather than local anesthetic.9 Development of a minimally invasive technique could expand the utility of this assay.
The same logic applies to reconstructive surgery, whose goal is a return of normal function, including pain-free movement, normal motion, premorbid strength, and intact sensation. Yet, the process of surgically manipulating tissue is associated with significant scarring and pain, which delay the return of normal function. For this reason, surgical care in the past 2 decades has been revolutionized by the incorporation of minimally invasive surgical techniques. Laparoscopy and thoracoscopy have permitted the resection of hollow and solid organs through 1 cm incisions, cystoscopy allows for the treatment of bladder and kidney pathology without any skin incision, and arthroscopy can be used to treat joints from the hip to wrist. Yet, some problems are not amenable to endoscopic techniques, including the placement of large cortical bone grafts; here, innovative techniques are required. Thus, the development of a minimally invasive technique for placing cell–scaffold constructs in anticipation of new bone formation could have immediate clinical benefits.
The purpose of this study was to determine whether multipotent human osteogenic cells derived from the bone marrow could still form bone after transplantation into recipient animals via injection rather than a major transplantation operation. Transplants were formed by combining hydroxyapatite/tricalcium phosphate (HA/TCP) particles with ex vivo–expanded human bone marrow stromal cells (BMSCs).10,11 Transplants included mouse fibrin, which helped them maintain their shape when delivered through a standard incision. They were placed into immunocompromised recipient mice either in the subcutaneous space of the back (modeling heterotopic bone formation) or onto the calvarium (modeling bone augmentation), either by injection or by placement using a long surgical incision.
Materials and Methods
Transplant preparation, placement, and recovery
Surgical specimens were obtained containing fragments of normal unaffected bone with bone marrow from two patients undergoing reconstructive surgery. The patients were a girl and a boy, aged 14 years and 13 years, respectively, undergoing iliac crest bone harvest for correction of scoliosis. Tissue procurement proceeded in accordance with NIH regulations governing the use of human subjects (Protocols 94-D-0188). Multicolony-derived strains of BMSCs were derived from the bone marrow in a manner previously described.11 Briefly, a single-cell suspension of bone marrow cells was cultured in growth medium consisting of αMEM, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate (Invitrogen, Grand Island, NY), 10−8 M dexamethasone (Sigma, St. Louis, MO), 10−4 M L-ascorbic acid phosphate magnesium salt n-hydrate (Wako, Osaka, Japan), and 20% fetal bovine serum of a preselected lot (Equitech-Bio, Kerrville, TX). After 24 h, nonadherent cells were removed by extensive washing. The cells were then incubated at 37°C in an atmosphere of 100% humidity and 5% CO2; medium replacements were performed weekly.
Upon approaching confluency, BMSCs were trypsin-released and transferred into new flasks. BMSCs of passage 3 were pipetted into 1.8 mL polypropylene cryotubes (Nunc, Roskilde, Denmark), each previously loaded with a 40 mg aliquot of HA/TCP particles (Zimmer, Warsaw, IN). Using a sieve shaker (CSC Scientific, Fairfax, VA), only particles of size range 0.5–1.0 mm were isolated and used. These represented the specific size and shape offered in Zimmer's commercially available, FDA-approved product Collagraft™. Each tube received 1.0–3.5 million cells. We earlier demonstrated that 1 million BMSCs exceed the threshold for 40 mg HA/TCP transplants, above which a plateau is reached so that equally abundant bone formation takes place.12 The mixtures were incubated for 90 min at 37°C on a slowly rotating platform. They were then centrifuged at 200 g for 60 s, and the supernatant was discarded.
Each transplant then received mouse fibrinogen and mouse thrombin to form a disc-shaped cohesive mixture of cells and particles.13 Mouse fibrinogen (Sigma F4385) was reconstituted in sterile PBS at 3.2 mg/mL; mouse thrombin (Sigma T8397) was reconstituted in sterile 2% CaCl2 at 25 U/mL. HA/TCP particles with attached cells were combined first with 15 μL of fibrinogen and then with 15 μL of thrombin; brief but thorough mixing was performed after each component had been added. A fibrin gel typically formed within 1 min. The fibrinogen and thrombin were added to the transplants immediately prior to transplantation into the mice. Fibrin-exposed transplants delivered via the substantial surgical incision retained their shape and form during transplantation, while injected transplants lost their cohesion during the injection process.
Three-month-old immunocompromised Bg-Nu/Nu-Xid female mice (Harlan-Sprague Dawley, Indianapolis, IN) served as transplant recipients. All animals were cared for according to the policies and principles established by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals. Operations were performed in accordance to specifications of an approved NIH small-animal protocol (97-024). Mice were anesthetized with a combination of IP ketamine (140 mg/kg body weight) and IP xylazine (7 mg/kg body weight). Transplants were placed either in the subcutaneous space along the back or subperiosteally on the calvarium. Placement occurred either by a surgical approach using a 20 mm incision, which constitutes our standard procedure and encompassed the control group in these studies, or by an injection, which constituted our experimental group. Each mouse received multiple transplants through these approaches. In the injection group, a CORB Biopsy Needle (Zimmer) was advanced through the skin to the recipient site at either calvarium or subcutis, and the transplant advanced down the needle using a fine stylet, or surgical probe. These transplants were heavily disrupted during advancement, such that the particles and cells lost their initial relationships; in effect, they represented a suspension of individual particles with individual cells attached. The needle could be tunneled subcutaneously for a distance as long as 3 cm, and it could be redirected to multiple recipient sites from the same skin entrance site. In the open surgical approach, the transplants were placed directly under the incision, whether on the back or at the calvarium; the transplants maintained their shape due to the fibrin gel throughout the transplantation. Incisions were closed with surgical staples. Twenty-three mice were given a total of 43 transplants. The mice were sacrificed at intervals ranging from 7 to 110 weeks postoperatively with inhaled CO2, and their transplants were harvested.
Estimation of bone formation and bone union
The transplants were fixed in 4% phosphate-buffered formalin freshly prepared from paraformaldehyde. Following an overnight fixation at 4°C, the transplants were suspended in phosphate-buffered saline. The transplants were demineralized in buffered 10% EDTA, dehydrated, embedded in paraffin, and sectioned. Sections were deparaffinized, hydrated, and stained with hematoxylin and eosin (H&E). The H&E-stained sections were examined histologically, and the extent of bone within each transplant was scored on a semiquantitative, exponential scale by a blinded observer in a manner similar to that described previously.11 Transplants were scored on a scale of 0–4; a score of 0 corresponded to no bone formation, while a score of 4 was given to transplants with abundant bone formation occupying greater than one half of the section (Table 1A and Fig. 1). When the bone scores reported on this scale have been compared to histomorphometric measurements of tissue sections, a high correlation was previously observed between the bone score and the square root of the fraction of bone area to total transplant area (B/T) (r = 0.973).14 Bone score statistical analysis employed InStat 3.06 (GraphPad, San Diego, CA).
Table 1A.
Semiquantitative Scale for the Estimation of Bone Formation
| Score | Extent of bone present within the transplant |
|---|---|
| 0 | No bone evident |
| 1 | Minimal bone evident (one trabecula) |
| 2 | Weak bone formation, occupying only a small portion of the section |
| 3 | Moderate bone formation, occupying a significant portion but less than one half of the section |
| 4 | Abundant bone formation, occupying greater than one half of the section |
FIG. 1.
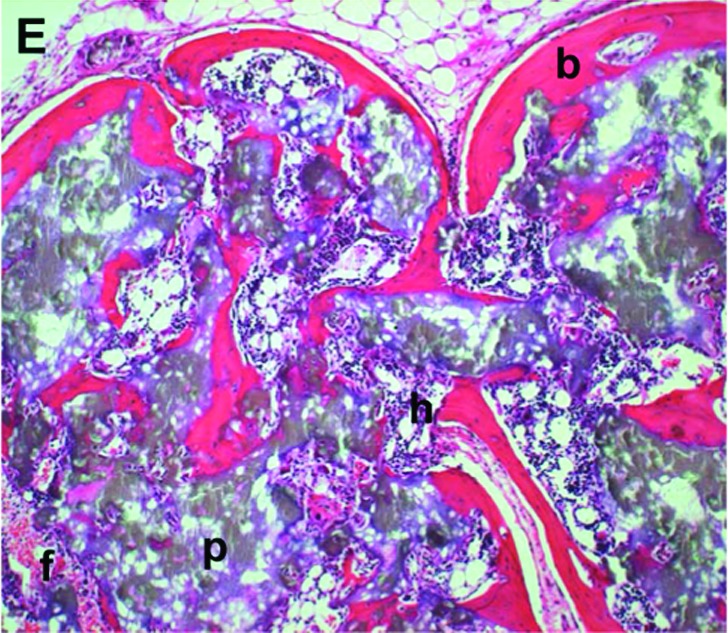
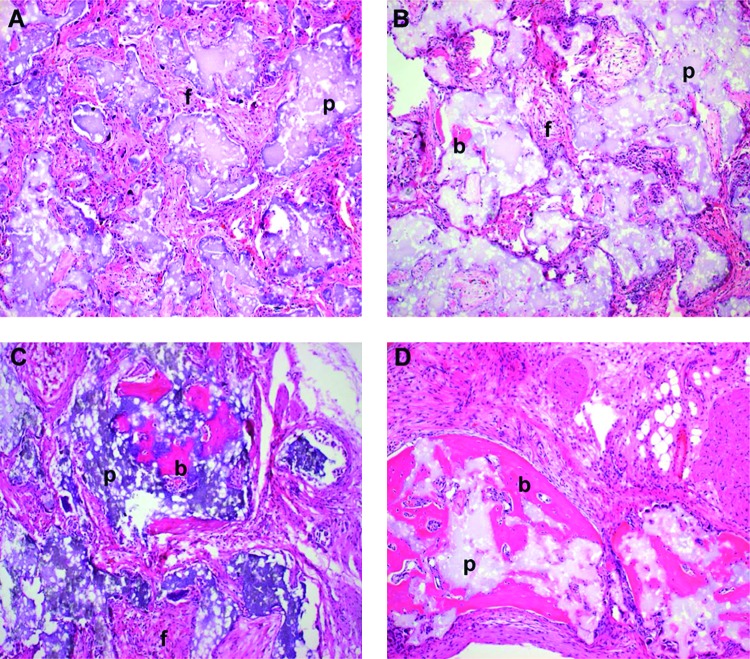
HA/TCP and BMSC transplants that have formed varying amounts of bone. (A) Transplant exemplifying a bone score of 0. No bone trabecula present. (B) Transplant exemplifying a bone score of 1. Only one bone trabecula present. (C) Transplant exemplifying a bone score of 2. Weak bone formation, with only a few trabeculae present. New bone does not bridge adjacent particles. (D) Transplant exemplifying a bone score of 3. Bone formation is appreciable but less than one half of the transplant. (E) Transplant exemplifying a bone score of 4. Abundant bone formation. Bone bridges adjacent particles. b, bone; f, fibrous connective tissue; p, particle; h, hematopoiesis. Magnification: 10×; stain: H&E; paraffin embedding following demineralization. Color images available online at www.liebertonline.com/ten.
Regarding the applicability of statistical comparisons of bone score data, in our previous publication,14 we demonstrated that a strong parametric relationship exists between the subjective (ordinally scaled variable) with the objective (quantitative histomorphometry measure, square root scale), producing a very high correlation (r = 0.86 or greater). This demonstrates that, although not perfectly, the rankings of the histomorphometric measures and the bone scores are preserved, at least to within small intervals of values for the histomorphometric measures for each bone score. There is some overlap among these ranges, but the misclassification rates are small (see Fig. 6 in Reference 14). Thus, although one will lose a small amount of statistical sensitivity in bone measurement, and thus some statistical power when making comparisons, the ordinally scaled bone score can be used as a reasonably valid proxy for the more intensive histomorphometric measure and thus can produce valid statistical results.
The degree of bony union between transplant and the mouse calvarium was evaluated by a blinded observer in a manner previously described.7 Union was scored on a scale of 0–4; a score of 0 corresponded to the absence of a palpable or histologic union between transplant and adjacent mouse bone, while a score of 4 was given to transplants which were grossly secure and where bony union was evident in greater than one half of the histologic sections (Table 1B).
Table 1B.
Semiquantitative Scale for the Estimation of Transplant–Bone Union
| Score | Extent of union between transplant and adjacent bone |
|---|---|
| 0 | No union evident by gross palpation |
| 1 | Union present grossly; fibrous union in all histologic sections |
| 2 | Union present grossly; bony union in one histologic section |
| 3 | Union present grossly; bony union occupying a significant portion but less than one half of the histologic sections |
| 4 | Union present grossly; bony union occupying greater than one half of the histologic sections |
Mechanical testing of calvarial transplants
Following sacrifice at 8 weeks, a pair of BMSC-HA/TCP transplants were dehydrated in ethanol, embedded undecalcified in methylmethacrylate, and sectioned with a microtome into 5-μm-thick sections. These sections were stained with Goldner's modified trichrome for the collection of architecture data with the light microscope. The methylmethacrylate-embedded BMSC-HA/TCP transplant samples were polished on one side with progressively finer grades of diamond paste, down to 0.1 μm grade, until a smooth bone surface was exposed.
For topographic imaging and discrete determination of the mechanical properties of individual trabeculae, a modified atomic force microscope (AFM; Nanoscope IIIa; Digital Instruments, Santa Barbara, CA) was used.7 The modification consisted of replacing the cantilever/tip assembly of the microscope with a transducer-driven head and tip (Triboscope Micromechanical Test Instrument; Hysitron, Minneapolis, MN) that allowed the microscope to operate both as an imaging and an indentation instrument as previously described.15 A sharp diamond Berkovich indenter with a radius of curvature < 100 nm was fitted to the transducer. The AFM piezo and respective control systems were used to image the surface of the sample to find a specific site of interest after which the load-displacement transducer was used to indent the sample while collecting the load displacement data. All indentations were performed with a trapezoidal load profile of 0.3 mm/s in time to a 150-μN maximum load. Elastic modulus and hardness were calculated from the unloading force/displacement slope at maximum load and the projected contact area at this load following the method of Doerner and Nix.16 After indentation, the AFM piezo was used to scan the indented area. However, because of the texture of the sample surface, it was difficult to distinguish the indents.
The AFM measurements were performed on different trabeculae on each specimen; specimens from two mice were analyzed. The elastic modulus (E) and hardness (H) were obtained by indentation at four different sets of sites on each specimen; each site underwent nine indentations, with an interval of 5 μm between successive indentations.
Identification of donor cells
The human-specific repetitive alu sequence, which comprises about 5% of the total human genome, can be applied for identification of human cells.17 We used in situ hybridization for the alu sequence to study the origin of tissues formed in the transplants. The digoxigenin-labeled probe specific for the alu sequence was prepared by PCR, including 1 × PCR buffer (Perkin Elmer, Foster City, CA), 0.1 mM dATP, 0.1 mM dCTP, 0.1 mM dGTP, 0.065 mM dTTP, 0.035 mM digoxigenin-11-dUTP (Boehringer Mannheim, Indianapolis, IN), 10 pmol of specific primers, and 100 ng of human genomic DNA. The following primers were created on the basis of previously reported sequences:18 sense, 5′-GTGGCTCACGCCTGTAATCC-3′; antisense, 5′-TTTTTTGAGACGGAGTCTCGC-3′. The method for in situ hybridization of HA/TCP containing transplants has been previously described.11 Sections deparaffinized with xylene and ethanol were immersed in 0.2 N HCl at room temperature for 7 min and then incubated in 1 mg/mL pepsin in 0.01 N HCl at 37°C for 10 min. After washing in PBS, the sections were treated with 0.25% acetic acid containing 0.1 M triethanolamine (pH 8.0) for 10 min and prehybridized with 50% deionized formamide containing 4× SSC at 37°C for 15 min. The sections were then hybridized with 1 ng/μL digoxigenin-labeled probe in hybridization buffer (1× Denhardt's solution, 5% dextran sulfate, 0.2 mg/mL salmon sperm DNA, 4× SSC, and 50% deionized formamide) at 42°C for 3 h after the denaturation step at 95°C for 3 min. After washing with 2× SSC and 0.1× SSC, digoxigenin-labeled DNA was detected by immunohistochemistry using antidigoxigenin alkaline phosphatase-conjugated Fab fragments (Boehringer Mannheim). Transplants harvested at 9 weeks were analyzed.
Results
A total of 43 transplants were evaluated, including 16 transplants to the calvarium and 27 transplants to the dorsum of the back. The boy donor contributed cells to 5 control and 14 injected transplants, while the girl donor's cells were used for 10 control and 14 injected transplants. BMSC number per transplant ranged from 1 million, among 7 control and 5 injected transplants, to 3.5 million, among 8 control and 23 injected transplants. The mean BMSC number per transplant was 2.4 million for the control transplants and 3.1 million for the experimental transplants (no significant difference), reflecting an inadvertent weighting of small BMSC-dose transplants toward the control group. As demonstrated below, this difference did not appear to influence bone formation rates.
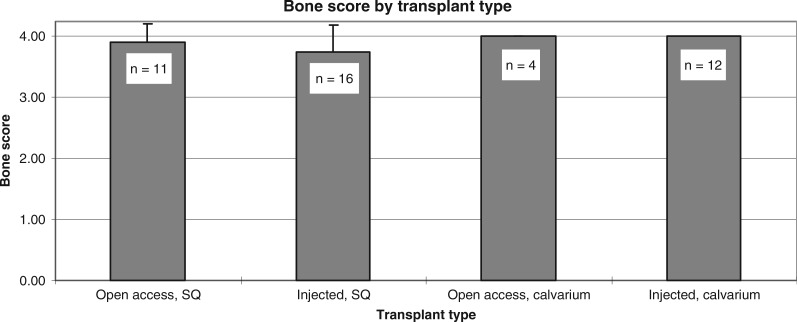
Bone scores among the transplants ranged from moderate (score = 3) to abundant (score = 4) (Fig. 2). Bone scores were comparable (p > 0.05) between subcutaneous and calvarial, as well as between the injected and standard surgical approaches. Bone scores among control transplants harvested during the first, second, and third years were 3.9, 4, and 4, respectively, and among injected transplants were 3.7, 4, and 4, respectively not significant (NS). Cell numbers were substantially higher than the minimal numbers required for good bone formation.12 Consequently, abundant bone formation appeared to occur independently of BMSC dosage; the few transplants with a bone score of 3 had received a high BMSC dose (3.5 million cells), while all transplants with 1.0 million BMSCs, whether in the control or experimental group, had bone scores of 4. Among transplants to the calvarium, union scores were comparable between standard (3.75, SD 0.50) and injection (3.94, SD 0.17) approaches (p > 0.05). With regard to the harvest time-line, 19 transplants were harvested from weeks 7 to 52, 17 transplants were harvested from weeks 53 to 104, and 7 transplants were harvested from weeks 105 to 110.
FIG. 2.
Bone score as a function of transplant delivery method and location. Data are inclusive of all time points. All transplants had high bone scores. SQ, subcutaneous.
Recipient mouse variability was controlled by placing in some mice both control and experimental transplants. In all but one mouse receiving both types of transplants, bone score was comparable between the two groups. In that single mouse, bone score was 3 in a control transplant but 4 in the experimental transplant. Also, since it has been demonstrated in BMSC heterotopic transplants that endothelial cells are of recipient origin while adventitial cells are of donor origin, both of these cell types are essential for new bone formation as well as for the formation of sinusoids and hematopoiesis-supporting territories.19 Because of each of these populations has a different origin yet are integral to successful bone formation, relative host and donor contributions should not differ between individual recipients and thus should not decide the outcome of bone formation.
Transplant histology
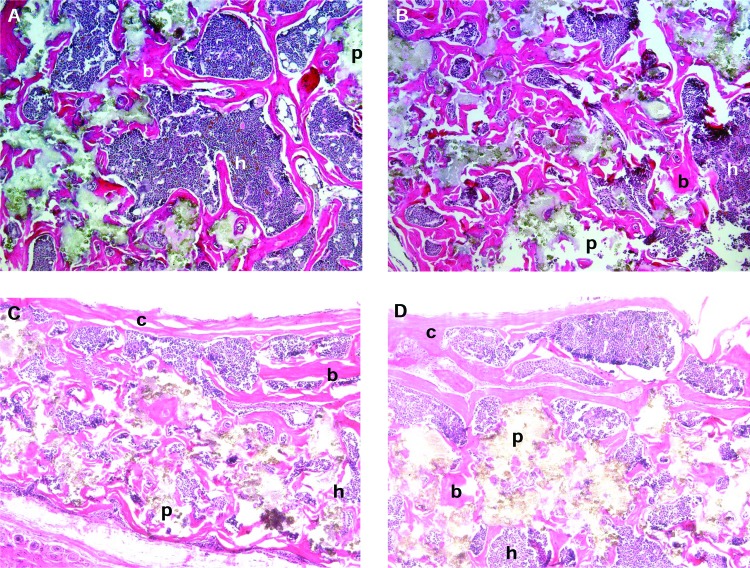
Bone morphology was comparable between the two delivery methods (Fig. 3A, B). HA/TCP particles were separated by lamellar bone. Bone formation was extensive, and in many areas, bone associated with individual particles appeared to coalesce, forming a rim of bone along the exterior surface of the transplant and a latticework of bone within the transplant. Transplants delivered by both techniques contained abundant hematopoietic tissue and occasional adipocytes, all of which were spatially associated with the new bone. A modicum of fibrovascular tissue devoid of bone or hematopoietic tissue was found among those transplants receiving a score of 3. This was distributed equally between transplants of the two delivery methods. All transplants and peritransplant tissues were characterized by the absence of an inflammatory reaction. Transplants placed on the calvarium exhibited osseous union between the transplants and the calvarium (Fig. 3C, D). Relative to young transplants, the oldest transplants exhibited reduced amounts of particle, increased amounts of hematopoiesis, increased amounts of bone at the periphery of the transplant, and loss of fibrous tissue (Fig. 3E).
FIG. 3.
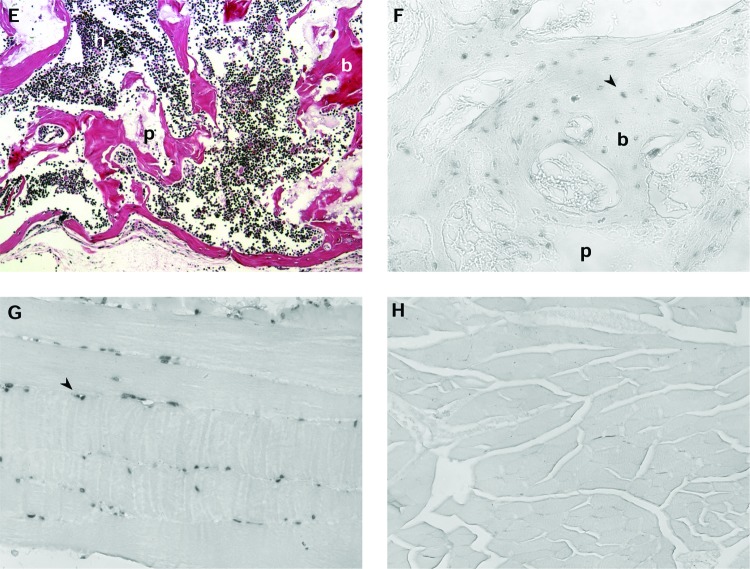
Human BMSC and HA/TCP transplants placed into mice. (A) Transplant placed subcutaneously via a standard 2 cm incision, harvested at 69 weeks. Abundant corticocancellous bone and hematopoiesis. Magnification: 10×. (B) Transplant placed subcutaneously via injection, harvested at 69 weeks. Magnification: 10×. (C) Transplant placed via a standard 2 cm incision that was made directly over the calvarium, harvested at 86 weeks. Magnification: 10×. (D) Transplant on the calvarium placed via an injection started in the skin of the midback, harvested at 83 weeks. Note bone union between transplant and calvarium. Magnification: 10×. (E) Transplant placed subcutaneously via a standard 2 cm incision, harvested at 107 weeks. Reduced particles, localization of bone to the transplant periphery (at the inferior edge of the image), and increased hematopoiesis relative to early transplants. Magnification: 10×. (F) Confirmation of the donor origin of the newly formed bone in a 9-week-old BMSC-containing transplant, placed via injection. In situ hybridization to alu is localized to osteocytes in the new bone, and is absent in the hematopoietic or peritransplant tissues. Magnification: 20×. (G) Human muscle, following in situ hybridization to alu. Signal is localized to nuclei. Magnification: 20×. (H) Mouse muscle, following in situ hybridization to alu. No signal is identified. Magnification: 20×. b, bone; h, hematopoietic tissue; p, particle; c, mouse calvarium; arrowheads, alu-positive cells. Stain (A–E): H&E; paraffin embedding following demineralization. Color images available online at www.liebertonline.com/ten.
Identification of donor cells
The human alu gene sequence was used to follow the fate of the transplanted cells. alu served as a marker for donor cell activity because it is not present in the mouse recipient cells. Unstained tissue sections from transplants were evaluated with a digoxigenin-labeled probe specific for the alu sequence. alu was detected in osteoblasts and osteocytes within both the cortical and trabecular components of the new bone, confirming that the osteogenic cells were of donor origin rather than originating from the local microenvironment (Fig. 3F). Alu was restricted to the new bone, was absent in the hematopoietic cells, and was absent from the peritransplant tissues. Concurrent positive and negative controls confirmed localization to human cells (Fig. 3G, H). These findings were consistent with our earlier studies.7,10,20
Mechanical characteristics of the transplants
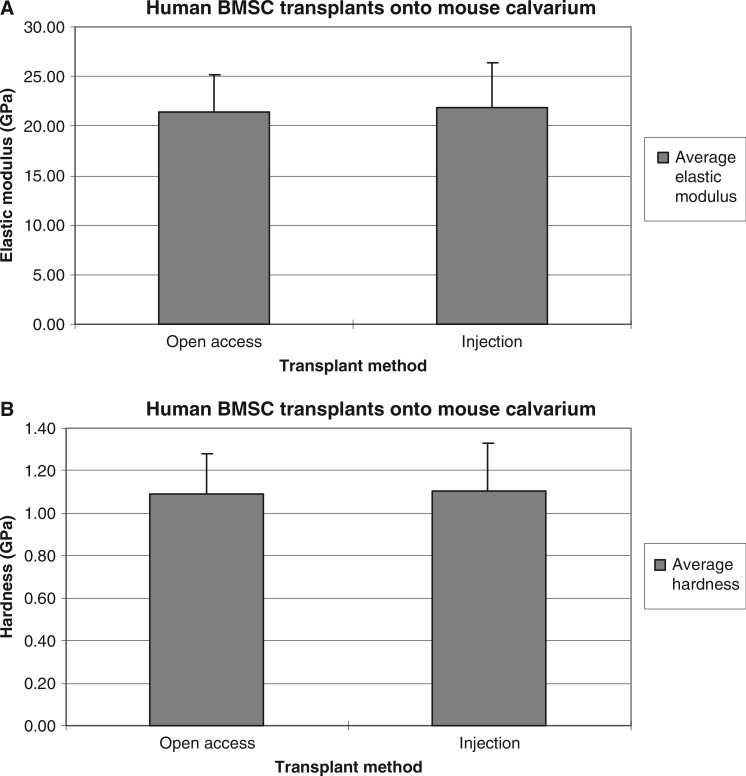
The trabeculae in these transplants measured approximately 100 μm in size, and were interspersed between HA/TCP particles and hematopoietic tissues. Consequently, they were too small to undergo traditional three-point bending regimens. Instead, AFM-based nanoindentation was used to measure the elastic modulus of bone from injection and open surgical transplants. Care was taken to avoid sampling residual HA/TCP particles, whose elastic modulus was two- to threefold greater than the bone's. Elastic modulus values of the BMSC-associated bone from open surgical and injected transplants were 21.37 (±2.82) and 21.62 (±3.07) GPa, respectively, while hardness values were 1.09 (±0.19) and 1.11 (±0.23) GPa, respectively (Fig. 4A, B). An unpaired t test with 95% confidence level indicated no significant difference in elastic modulus and hardness of bone between injected and open surgical transplants.
FIG. 4.
Mechanical properties of bone in human BMSC transplants on mouse calvarium, evaluated using AFM. Average values were comparable between both delivery methods. (A) Elastic modulus. (B) Hardness.
Discussion
Using currently existing approaches, very few cell types are able to form a new organ single-handedly, including both its cellular makeup and three-dimensional structure, upon in vivo transplantation as single-cell suspensions. Features of successful organogenesis include the creation of an anatomically normal three-dimensional structure, the recruitment of a vascular supply and of other relevant cell types from the recipient, and the ability to function physiologically. Thus far, successful examples of organogenesis by a cell suspension have been limited to hematopoietic stem cells (HSCs), which can reconstitute and proliferate to form a functional hematopoietic marrow, but unlike BMSCs, HSCs by themselves did not establish a three-dimensional structure but rather used a preexisting stromal meshwork. For the first time, our study demonstrates that another cell type, the human BMSC, is able to coordinate the formation of a complete three-dimensional bone/marrow organ following injection in vivo, and that this capability does not depend on the spatial organization established before transplantation. This newly formed bone is characterized by a lamellar corticocancellous mineral structure, functional osteoprogenitor cells, and support for a functional hematopoietic marrow. This minimally invasive technique can enhance the utility of BMSC transplantation as both an osteogenic assay and a clinical method for reconstructing bone deficits.
The in vivo transplantation approach has become a valuable tool for assessing the osteogenic potential of diverse cell populations, and has been validated in a number of studies by our and other groups. It offers significant advantages over in vitro assays of bone formation, because it allows for the formation of bone that is histologically and mechanically normal.7 Also, transplanted BMSCs can recreate the characteristics of pathologic bone and marrow, such as that seen in McCune-Albright syndrome and similar conditions, and in abnormal bone/marrow from transgenic animals.21–23 This tool also offers the possibility of high-throughput analyses through the use of noninvasive methods for estimating bone formation, including radiologic and fluorescence techniques.24 Yet, despite these advantages, a substantial limitation of this model to date has been the necessity of placing the transplants into recipient animals via a sizable operative procedure.7,9,24,25 This limitation has been addressed in the current study by the development of a technique for injecting the transplant material, including both BMSCs and HA/TCP particles, directly into a recipient site without a major operation. Bone in these injected transplants has histologic and material properties comparable to those of bone formed in transplants placed via large operations. The method of placing the transplant, whether via injection or a standard long incision open approach, did not affect the quantity, histologic character, hardness, or elastic modulus of newly formed bone, nor did it affect the quantity of hematopoietic tissue. Additionally, both approaches succeeded in achieving an osseous union between the transplant and underlying recipient calvarium. In situ hybridization for the gene sequence alu confirmed the human origin of the osteogenic cells in the early harvest transplants and therefore of the newly formed bone formed by these cells. From a clinical standpoint, the amount of bone resulting from transplantation into these healthy recipient mice is independent of transplant technique. In other clinical situations, such as irradiated or scarred recipient sites, the recipient cell contribution to bone formation might be impaired. Appropriate animal models will eventually help resolve this issue.
It should be mentioned that we transplanted greater than the minimal number of BMSCs necessary to elicit good bone formation. Previously, we identified the minimal number of transplanted BMSCs necessary for good bone formation for particular donors.12 One potential criticism of the current study is that the use of an abundant number of cells from young donors may have obscured a difference in bone formation between the two techniques, a difference which may have become more apparent had fewer cells from older donors been utilized. While not disputing the criticism, it should be emphasized that this study represents a first-order effort to demonstrate that cell injection does not fundamentally impair bone formation in our system.
To date, very few studies have described the in vivo injection of osteogenic cells. The available studies do not include key features of our study, such as (1) injection of human BMSCs in conjunction with an appropriate matrix, (2) comparison of bone formed in injected transplants with bone formed in surgically placed transplants, and (3) long-term observation of transplants. For instance, Trojani et al. described combining mouse BMSCs with an HA/TCP matrix, and then injecting this into mouse recipients.26 Transplants were examined after 4 and 8 weeks, and showed good bone formation. Unlike our study, human cells were not used, a control set of surgically placed transplants was not compared, and transplants were examined at a very early time point. Similarly, Yamada et al. injected rat MSCs + β-TCP into the rat subcutis and examined the transplants after 8 weeks, observing good bone formation.27 In contrast to these two studies, the features employed in our study are important toward the clinical realization of these techniques, and they demonstrate that injection of cells is as efficacious as surgical placement. In separate studies employing the same model, Ito et al. and Yamada et al., injected autologous dog BMSCs in a platelet-rich plasma (PRP) gel into noncritical-sized mandibular defects, and these transplants were compared to PRP alone, cancellous bone + marrow, and no treatment. No calcium phosphate–containing matrices were used, and transplants were examined only as late as 12 weeks.28,29 Healing occurred in the control group, and bone formation by the BMSCs was not significantly different from that by cancellous bone +marrow, bringing into doubt the appropriateness of this model for demonstrating the activity of BMSCs. Our study, in contrast, used a model in which bone fails to form unless the cells and matrices are appropriately prepared and combined. This is of importance to the study of human BMSCs, which optimally form bone when combined with a mineral matrix.10 In summary, our study is the first to combine the observations we consider critical toward the clinical use of an injectable bone technique.
Our data indicate that an intrinsically osteogenic cell population will be able to form bone and a hematopoietic microenvironment when it is injected in vivo in conjunction with an appropriate matrix, and that this bone has histologic and mechanical properties comparable to surgically placed transplants. The success of injected BMSC transplants offers both investigative and clinical benefits. From a research perspective, injected transplants allow cell placement with minimal disruption of the surrounding tissues and without the need for incising the skin in the vicinity of the transplant. For instance, they permit a study of bone formation with minimal influence of soft tissue inflammation. Additionally, compared to the standard surgical approach, injected transplants minimize the risk of infection, involve fewer surgery- and anesthesia-related complications, and, taken together, permit more laboratories to perform this in vivo osteogenic assay.
We included in this study a bone onlay model, in which we tested the ability of surgically placed and injected transplants to form a bone union with the underlying mouse calvarium. Regardless of technique used, bone union rates were uniformly high. Critical to the success of this technique is the removal of the endogenous periosteum, which when left intact interferes with creation of a bony union.7,30 The ability to inject engineered bone has immediate clinical significance to the treatment of mandibular atrophy or spinal disease, where endoscopic efforts at placing conventional bone graft are already being investigated.31–33
The analysis of bone mechanical properties is an important parameter for assessing phenotypes produced by stem cells, but which is often neglected in studies of this type. AFM has great utility in assessing the properties of minute tissues, including the small trabeculae in BMSC transplants, where it can detect differences in hardness and modulus not discernible by traditional multipoint bending.7,34 We have also demonstrated using AFM that human BMSC transplants form bone with a modulus and hardness comparable to endogenous mouse bone in the same animals.7 In contrast, the modulus and hardness of HA/TCP particles are significantly higher than those of bone. In this study, we used AFM to demonstrate that the bone mechanical properties were comparable between the two sets of transplants, just as we had demonstrated that the histologic properties were comparable.
From a clinical perspective, this technique represents a novel description of injected osteoprogenitor cell placement for the purpose of the engineering new bone. While surgical care has been revolutionized by minimally invasive (endoscopic) operative techniques, repair of bone defects still requires the placement of bone graft through large incisions. It would be reasonable to envision that certain bone graft operations might be replaced by the injection of BMSC transplants. These would include procedures where BMSCs have successfully closed bone defects in experimental models.7,9,35–37
In summary, we have created a new approach that combines the bone-forming ability of culture-expanded BMSCs with minimal access surgical techniques. The successful formation of corticocancellous bone via injection may potentially offer new research and clinical opportunities. This study represents a first effort to transplant BMSCs via injection, and additional studies are necessary before translating this to the patient or using it as an osteogenic assay.
Acknowledgments
The authors are indebted to Zimmer for its gift of HA/TCP particles. Albert Kingman, Ph.D., provided invaluable statistical assistance; Vijay Tiwari, M.B.B.S., assisted with the image analysis; and Ms. Katherine Huang assisted with the in situ hybridization. This research was supported in part by the University of California–San Francisco Research Evaluation and Allocation Committee and in part by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, NIH, DHHS.
References
- 1.Bielby R.C. Boccaccini A.R. Polak J.M. Buttery L.D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 2.De Coppi P. Bartsch G., Jr. Siddiqui M.M. Xu T. Santos C.C. Perin L. Mostoslavsky G. Serre A.C. Snyder E.Y. Yoo J.J. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. Epub 2007 January 7. [DOI] [PubMed] [Google Scholar]
- 3.Doherty M.J. Ashton B.A. Walsh S. Beresford J.N. Grant M.E. Canfield A.E. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 4.Kuznetsov S.A. Mankani M.H. Gronthos S. Satomura K. Bianco P. Robey P.G. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicok K.C. Du Laney T.V. Zhou Y.S. Halvorsen Y.D. Hitt D.C. Cooper L.F. Gimble J.M. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 6.Derubeis A.R. Mastrogiacomo M. Cancedda R. Quarto R. Osteogenic potential of rat spleen stromal cells. Eur J Cell Biol. 2003;82:175–181. doi: 10.1078/0171-9335-00300. [DOI] [PubMed] [Google Scholar]
- 7.Mankani M.H. Kuznetsov S.A. Wolfe R.M. Marshall G.W. Robey P.G. In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells. 2006;24:2140–2149. doi: 10.1634/stemcells.2005-0567. Epub 2006 June 8. [DOI] [PubMed] [Google Scholar]
- 8.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 9.Mankani M.H. Kuznetsov S.A. Shannon B. Nalla R.K. Ritchie R.O. Qin Y. Robey P.G. Canine cranial reconstruction using autologous bone marrow stromal cells. Am J Pathol. 2006;168:542–550. doi: 10.2353/ajpath.2006.050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebsbach P.H. Kuznetsov S.A. Satomura K. Emmons R.V. Rowe D.W. Robey P.G. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 11.Kuznetsov S.A. Krebsbach P.H. Satomura K. Kerr J. Riminucci M. Benayahu D. Robey P.G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 12.Mankani M.H. Kuznetsov S.A. Robey P.G. Formation of hematopoietic territories and bone by transplanted human bone marrow stromal cells requires a critical cell density. Exp Hematol. 2007;35:995–1004. doi: 10.1016/j.exphem.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Kuznetsov S.A. Mankani M.H. Robey P.G. Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation. 2000;70:1780–1787. doi: 10.1097/00007890-200012270-00018. [DOI] [PubMed] [Google Scholar]
- 14.Mankani M.H. Kuznetsov S.A. Fowler B. Kingman A. Robey P.G. In vivo bone formation by human bone marrow stromal cells: effect of carrier particle size and shape. Biotechnol Bioeng. 2001;72:96–107. doi: 10.1002/1097-0290(20010105)72:1<96::aid-bit13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Marshall G.W., Jr. Balooch M. Gallagher R.R. Gansky S.A. Marshall S.J. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Doerner M.F. Nix W.D. A method for interpreting the data from depth-sensing indentation instruments. J Mater Res. 1986;1:601–609. [Google Scholar]
- 17.Jacobsen P.F. Daly J. A method for distinguishing human and mouse cells in solid tumors using in situ hybridization. Exp Mol Pathol. 1994;61:212–220. doi: 10.1006/exmp.1994.1038. [DOI] [PubMed] [Google Scholar]
- 18.Matera A.G. Hellmann U. Hintz M.F. Schmid C.W. Recently transposed Alu repeats result from multiple source genes. Nucleic Acids Res. 18:6019–6023. doi: 10.1093/nar/18.20.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I. Tagliafico E. Ferrari S. Robey P.G. Riminucci M. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Gronthos S. Mankani M. Brahim J. Robey P.G. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianco P. Kuznetsov S.A. Riminucci M. Fisher L.W. Spiegel A.M. Robey P.G. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha-mutated skeletal progenitor cells. J Clin Invest. 1998;101:1737–1744. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmbeck K. Bianco P. Caterina J. Yamada S. Kromer M. Kuznetsov S.A. Mankani M. Robey P.G. Poole A.R. Pidoux I. et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 23.Kuznetsov S.A. Riminucci M. Ziran N. Tsutsui T.W. Corsi A. Calvi L. Kronenberg H.M. Schipani E. Robey P.G. Bianco P. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J Cell Biol. 2004;167:1113–1122. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mankani M.H. Kuznetsov S.A. Avila N.A. Kingman A. Robey P.G. Bone formation in transplants of human bone marrow stromal cells and hydroxyapatite-tricalcium phosphate: prediction with quantitative CT in mice. Radiology. 2004;230:369–376. doi: 10.1148/radiol.2302011529. [DOI] [PubMed] [Google Scholar]
- 25.Mankani M.H. Krebsbach P.H. Satomura K. Kuznetsov S.A. Hoyt R. Robey P.G. Pedicled bone flap formation using transplanted bone marrow stromal cells. Arch Surg. 2001;136:263–270. doi: 10.1001/archsurg.136.3.263. [DOI] [PubMed] [Google Scholar]
- 26.Trojani C. Boukhechba F. Scimeca J.C. Vandenbos F. Michiels J.F. Daculsi G. Boileau P. Weiss P. Carle G.F. Rochet N. Ectopic bone formation using an injectable biphasic calcium phosphate/Si-HPMC hydrogel composite loaded with undifferentiated bone marrow stromal cells. Biomaterials. 27:3256–3264. doi: 10.1016/j.biomaterials.2006.01.057. Epub 2006 February 28. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y. Boo J.S. Ozawa R. Nagasaka T. Okazaki Y. Hata K. Ueda M. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg. 2003;31:27–33. doi: 10.1016/s1010-5182(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 28.Ito K. Yamada Y. Nagasaka T. Baba S. Ueda M. Osteogenic potential of injectable tissue-engineered bone: a comparison among autogenous bone, bone substitute (Bio-oss), platelet-rich plasma, and tissue-engineered bone with respect to their mechanical properties and histological findings. J Biomed Mater Res A. 2005;73:63–72. doi: 10.1002/jbm.a.30248. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y. Ueda M. Naiki T. Takahashi M. Hata K. Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 30.Krebsbach P.H. Mankani M.H. Satomura K. Kuznetsov S.A. Robey P.G. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 31.Picetti G.D. Pang D. Thoracoscopic techniques for the treatment of scoliosis. Childs Nerv Syst. 2004;20:802–810. doi: 10.1007/s00381-004-0934-2. Epub 2004 September 4. [DOI] [PubMed] [Google Scholar]
- 32.Jahng T.A. Fu T.S. Cunningham B.W. Dmitriev A.E. Kim D.H. Endoscopic instrumented posterolateral lumbar fusion with Healos and recombinant human growth/differentiation factor-5. Neurosurgery. 2004;54:171–180. doi: 10.1227/01.neu.0000097516.00961.eb. [DOI] [PubMed] [Google Scholar]
- 33.Engelke W. Deckwer I. Endoscopically controlled sinus floor augmentation. A preliminary report. Clin Oral Implants Res. 1997;8:527–531. doi: 10.1034/j.1600-0501.1997.080612.x. [DOI] [PubMed] [Google Scholar]
- 34.Pelled G. Tai K. Sheyn D. Zilberman Y. Kumbar S. Nair L.S. Laurencin C.T. Gazit D. Ortiz C. Structural and nanoindentation studies of stem cell-based tissue-engineered bone. J Biomech. 2007;40:399–411. doi: 10.1016/j.jbiomech.2005.12.012. Epub 2006 March 9. [DOI] [PubMed] [Google Scholar]
- 35.Bruder S.P. Kraus K.H. Goldberg V.M. Kadiyala S. 44th Annual Meeting, Orthopaedic Research Society. Vol. 23. New Orleans, LA: Orthopaedic Research Society; 1998. Critical-sized canine segmental femoral defects are healed by autologous mesenchymal stem cell therapy; p. 147. [Google Scholar]
- 36.Bruder S.P. Jaiswal N. Ricalton N.S. Mosca J.D. Kraus K.H. Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355:S247–S256. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 37.Bruder S.P. Kraus K.H. Goldberg V.M. Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]