SUMMARY
The C. albicans ALS (agglutinin-like sequence) family includes 8 genes (ALS1 to ALS7 and ALS9) that share a common general organization, consisting of a relatively conserved 5’ domain, a central domain of tandemly repeated sequence units, and a 3’ domain of relatively variable length and sequence. Working from the hypothesis that the cell-surface glycoproteins encoded by the ALS genes mediate contact between the fungal cell and host surfaces, we systematically constructed a set of C. albicans mutant strains, each lacking one of the ALS sequences, and analyzed the mutant strain phenotypes in assays that emphasize adhesion. ALS9 is unique within the ALS family due to extensive allelic sequence variation within the 5’ domain that may result in functional differences between proteins encoded by ALS9-1 and ALS9-2. Deletion of ALS9 significantly reduces C. albicans adhesion to human vascular endothelial cell monolayers. The mutation was complemented by reintegration of a wild-type copy of ALS9-2, but not ALS9-1, suggesting allelic functional differences. Complementation of the mutation with a gene fusion between the 5’ domain of ALS9-2 and the tandem repeats and 3’ domain of ALS9-1 also restored wild-type adhesion levels. Analysis of the als9Δ/als9Δ mutant phenotype in other assays demonstrated no significant difference from a control strain for adhesion to buccal epithelial cells or laminin-coated plastic plates. The als9Δ/als9Δ mutant did not show significant differences from the control for adhesion to or destruction of cells in the reconstituted human epithelium (RHE) disease model, or for cell wall defects, germ tube formation or biofilm formation in a catheter model. Analysis of ALS9 allelic frequency in a collection of geographically diverse clinical isolates showed a distinct preference for ALS9-2 allelic sequences, both within the 5’ and 3’ domains of the ALS9 coding region. These data suggest greater selective pressure to maintain the ALS9-2 allele in C. albicans isolates and imply its greater relative importance in host-pathogen interactions.
INTRODUCTION
Shortly after its identification, the first C. albicans ALS gene, ALS1, was found to belong to a larger family of eight related genes (Hoyer et al., 1995; Hoyer et al., 1998; Hoyer, 2001). Because of sequence similarities between Als1p and the S. cerevisiae cell surface adhesive glycoprotein alpha-agglutinin, it was hypothesized that the Als proteins are adhesins (Hoyer et al., 1995). Isolation of ALS1 and ALS5 based on their ability to confer adhesive function on the relatively non-adherent S. cerevisiae supported this hypothesis (Gaur & Klotz, 1997; Fu et al., 1998). We have taken a systematic approach to investigating Als protein function that involves generating C. albicans mutant strains, each lacking the coding region for a single ALS gene. Consistent with our initial hypothesis, phenotypic testing of these C. albicans mutants has focused on adhesion assays and measured the contributions of Als1p and Als3p (Zhao et al., 2004), Als2p and Als4p (Zhao et al., 2005) to interactions between C. albicans and host surfaces.
Completion of the eight ALS gene sequences highlighted common features within the family (Hoyer, 2001). In the center of each coding region is a domain composed entirely of tandemly repeated copies of a highly conserved 108-bp unit. The number of 108-bp copies present varies considerably between alleles. Flanking this central domain are a 5’ domain of approximately 1.3 kb that is 55–90% conserved across the ALS family and a 3’ domain of variable length and sequence. The tandem repeat region and 3’ domain encode serine/threonine-rich amino acid sequences that are heavily glycosylated in the mature Als protein (Kapteyn et al., 2000). These sequence features are consistent with a model of Als protein structure that includes a relatively non-glycosylated N-terminal domain that is displayed on the C. albicans cell surface by the remainder of the mature protein that forms an extended stalk, due to its heavy glycosylation. For Als proteins that display adhesive activity in C. albicans and/or S. cerevisiae, data support the conclusion that the majority of adhesive activity resides within the N-terminal domain (Loza et al., 2004; Zhao et al., 2006). However, recent work in S. cerevisiae suggests that the tandem repeat and C-terminal domain of Als5p may have more than a structural role (Rauceo et al., 2006).
The focus of this work is C. albicans ALS9, which is localized on chromosome 6, immediately downstream of the coding regions for ALS5 and ALS1 (Zhao et al., 2003). Analysis of ALS gene expression showed that ALS9 is transcribed under a wide variety of in vitro growth conditions and in both human clinical specimens and animal disease models (Cheng et al., 2005; Green et al., 2004; 2005a,b; 2006). Under the in vitro conditions assayed by real-time RT-PCR, however, maximal ALS9 transcript copy number was approximately 10 to 1000-fold less than the maximal copy number observed for other ALS genes such as ALS1, ALS2 or ALS3 (Green et al., 2005b). Perhaps the most notable feature of ALS9 is its allelic variability, which was defined based on the sequences of the two ALS9 alleles from C. albicans strain SC5314 (ALS9-1 = GenBank accession number AY269423; ALS9-2 = GenBank accession number AY269422). In general, the ALS family is known for its large degree of allelic variability, since each ALS gene exhibits this feature within the central tandem repeat domain (Hoyer, 2001). Certain ALS genes also display allelic sequence variability within the 5’ domain (ALS5; Hoyer & Hecht, 2001) or 3’ domain (ALS7; Zhang et al., 2003), however, ALS9 is the only gene with extensive sequence variability within each coding region domain (Zhao et al., 2003). The 5’ domain sequences of ALS9-1 and ALS9-2 from strain SC5314 vary by 11% at the nucleotide level (16% at the amino acid level). Within the 3’ domain, allele ALS9-2 encodes two sequence blocks, called Variable Block 1 (VB1) and Variable Block 2 (VB2), which are absent from the 3’ domain of allele ALS9-1 (Zhao et al., 2003). In the course of our work to assay Als9p function, we found different functional conclusions for the two ALS9 alleles when tested in adhesion assays. Here, we associate adhesive function with Als9-2p and demonstrate that the ALS9-2 allele is more prevalent in C. albicans clinical isolates.
METHODS
Construction of a C. albicans als9Δ/als9Δ strain
ALS9 coding regions were deleted using the PCR product-directed method of Wilson et al. (2000). General methods for creating alsΔ/alsΔ strains were described by Zhao et al. (2004). Details specific to the ALS9 studies are presented here. A diagram of the ALS9 alleles from strain SC5314 is shown in Fig. 1. An ALS9 disruption cassette was amplified from the plasmid pDDB57 template (a generous gift from Aaron Mitchell, Columbia University) with primers ALS9-5DR and ALS9-3DR (Table 1). The disruption cassette was transformed into C. albicans strain CAI4 (Table 2), Transformants were selected as described previously (Zhao et al., 2004), and screened by Southern blot. The Southern blot probe, which hybridizes to nucleotides −473 to −55 upstream of ALS9, was PCR amplified from SC5314 genomic DNA (Table 2) with primers ALS9upF and ALS9upR (Table 1). Correct transformants were screened by PCR using primer pair VB2F and VB2R (Table 1) to confirm Southern blot results and determine which ALS9 allele was deleted. Strain 1976, from which the ALS9-2 allele was deleted, was grown on medium containing 5-fluoroorotic acid (5-FOA; Boeke et al., 1984) to select a Uri− strain (Wilson et al., 2000). Strain 1978 (Table 2) was identified by Southern blotting and used for deletion of the ALS9-2 allele with the PCR-generated disruption cassette as described above. Southern blotting indicated that both ALS9 alleles were deleted in strain 2028. The deletion region extends from 15 bp upstream of the ALS9 coding region to 29 bp downstream of the ALS9 stop codon. A Uri− version of strain 2028, named 2030 (Table 2), was used for subsequent transformation steps.
Fig. 1.
Diagram of (a) ALS9-1, (b) ALS9-2, and (c) the ALS9-2/ALS9-1 fusion. The locations of the ALS domains are shown below ALS9-1. The ALS9-2/ALS9-1 fusion encodes amino acids 1 to 420 from Als9-2p fused to amino acids 421 to 1854 from Als9-1p. Capital letters show the approximate location of PCR primers used for allelic genotyping reactions. Primers E and F amplify a 3’ domain region (speckled) that contains Variable Block 1 (VB1). Primers G and H amplify a region (basketweave) that contains Variable Block 2 (VB2). Solid shading in each region denotes the extra nucleotides that comprise VB1 and VB2.
Table 1.
Oligonucleotide primers used in this study
| Primer name | Forward/Reverse | Label in Fig. 1* | Primer sequence (5’ – 3’) |
|---|---|---|---|
| ALS9-5DR | F | CTGTTGACATTGATAAAAAAAATATATAAAAGGCATCTATTTCCATGCAGAG | |
| GGACGTGTGTTTTCCCAGTCACGACGTT | |||
| ALS9-3DR | R | AACTACAAAAAGAAAACCGAAAAGACAAAATATAATAACATCACGAAGAAAT | |
| AATAAAAGTGTGGAATTGTGAGCGGATA | |||
| ALS9upF | F | TTTTTCTTTTCCTTGTTTTCC | |
| ALS9upR | R | TTGAAATTCTTGGTGATTAAG | |
| VB2F | F | G | CATCATTTGTGTCTACAACTGCTG |
| VB2R | R | H | GAACCCTTTGTTTCTGAATATGG |
| QRT9-1F | F | CATTTTACTCATCAGAAGCACAGTCG | |
| QRT9-1R | R | AGGTATTACTCGAATCAATTTCTAAACTCG | |
| QRT9-2F | F | AATATTTCACTCGTCAGAACCACATTAT | |
| QRT9-2R | R | AATCACTGGAATCAAAGTCTGAGCTAT | |
| ALS9dnF2 | F | ACCTTCTATTTAGAGATAGTGA | |
| ALS9dnR | R | CTCTCAAATGACGCGACTCATG | |
| ALS9FullF | F | CCCCCTAGGGGAAAAAAAAATGAGAGAAAAAAAGGAA | |
| ALS9R | R | CCCCTCGAGTCACTATCTCTAAATAGAAGG | |
| ALS9URCF | F | CCCACTAGTATAGGAATTGATTTGGATGG | |
| ALS9dnRCR2 | R | CCCAAGCTTCTCTCAAATGACGCGACTCATG | |
| ALS9F2 | F | CCCCCTAGGGGAAAAAAAAATGAGAGAAAAAAAGGAA | |
| 1499-PinAIR | R | CCCCTCGAGTTTACCGGTGGGATTAGTATAAGTAGTGG | |
| 2096-PinAIFm | F | CCCACCGGTTCTGTAGATACTGTTATTGTCGAAC | |
| ALS9-XhoIR | R | CCCCTCGAGTTAAATAAACAAAAATAATATTGTGACCA | |
| ALS9-1F | F | A | CAACTCATTAACTTGGGCCAATG |
| ALS9-1R | R | B | GAAGTTCGACAATAACAGTATCTACA |
| ALS9-2F | F | C | TGACTCATTGACATGGACTAGAT |
| ALS9-2R | R | D | GAATTTGCACAATAACAGTGTCTATG |
| VB1F | F | E | CGCTATGTCAACACCACAATCTCC |
| VB1R | R | F | TGGAACGTAGGAACTAACATGACC |
The location for some primers within the ALS9 coding region is shown in Fig. 1 where letter labels are used instead of primer names.
Table 2.
C. albicans strains used in this study
| Strain | Parent | Genotype | Doubling time (h) |
Source/ reference |
|---|---|---|---|---|
| SC5314 | Wild-type clinical isolate | Gillum et al. (1984) | ||
| CAI4 | SC5314 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 | Fonzi & Irwin (1993) | |
| CAI12 | CAI4 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434/IRO1 URA3 | 1.59 ± 0.04 | Porta et al. (1999) |
| 1976 | CAI4 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 ALS9-1/als9-2Δ-URA3 | Zhao et al. (2003) | |
| 1978 | 1976 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 ALS9-1/als9-2Δ-ura3 | This study | |
| 2028 | 1978 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 als9-1Δ/als9-2Δ-URA3 | 1.55 ± 0.04 | This study |
| 2030 | 2028 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 als9-1Δ/als9-2Δ-ura3 | This study | |
| 2740 | 2030 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 als9-1Δ/als9-2 ALS9-1-URA3 | 1.60 ± 0.04 | This study |
| 2064 | 2030 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 als9-1Δ/als9-2 ALS9-2-URA3 | 1.61 ± 0.04 | This study |
| 2767 | 2030 | iro1-ura3Δ∷λimm434/iro1-ura3Δ∷λimm434 als9-1Δ/als9-2 ALS9-1-ALS9-2∷URA3 | 1.58 ± 0.04 | This study |
Real-time RT-PCR analysis was used to assess transcriptional levels for each ALS9 allele in the strains constructed for this study. Methods for the assay and data analysis were described previously (Green et al., 2005b). Primers QRT9-1F and QRT9-1R detected ALS9-1 transcript specifically, while primers QRT9-2F and QRT9-2R were specific for ALS9-2 (Table 1). In strain CAI12, which is wild-type for ALS9, transcription of ALS9-1 was 1.1 ± 0.2-fold of ALS9-2 transcript levels, suggesting that the alleles were transcribed equally. In strain 2028, transcription of ALS9-1 was below the detection limit of this assay, while transcription of ALS9-2 relative to strain CAI12 was 0.005 ± 0.003. These results are consistent with the deletion of both ALS9 alleles in strain 2028.
Re-integration of wild-type ALS9 alleles into the als9Δ/als9Δ strain
Because the large sequence differences between ALS9-1 and ALS9-2 might reflect functional differences in the encoded proteins, each ALS9 allele was reintegrated separately into the als9Δ/als9Δ strain. To construct the ALS9-2 reintegration cassette, primers ALS9dnF2 and ALS9dnR (Table 1) were used to amplify 710 bp of sequence downstream of ALS9. This fragment was digested with SstII-NgoMIV and cloned into plasmid pUL (Zhao et al., 2004) to yield plasmid 1320. Full length ALS9-2 with 602 bp of upstream sequence was amplified from SC5314 with primers ALS9FullF and ALS9R (Table 1) and cloned into pCRBlunt (Invitrogen) to yield plasmid 1499. Primers ALS9URCF and ALS9dnRCR2 (Table 1) were used to amplify the URA3 gene and ALS9 downstream sequence cassette from plasmid 1320. This product was digested with SpeI-HindIII and cloned into plasmid 1499 downstream of ALS9-2 to yield plasmid 1541. The ALS9-1 reintegration cassette was produced by amplifying full-length ALS9-1 with 475 bp of upstream sequence (primers ALS9F2 and ALS9R; Table 1) from strain 1976, which has an ALS9-2 deletion. The PCR product was digested with AvrII-XhoI and cloned into plasmid 1320 (Zhao et al., 2004) to yield plasmid 2729. Digestion with AvrII-NgoMIV released the ALS9-1 cassette from plasmid 2729 and the ALS9-2 cassette from plasmid 1541. These fragments were transformed individually into strain 2030 to yield strain 2740 (ALS9-1 reintegrated) and strain 2064 (ALS9-2 reintegrated). Correct transformants were confirmed by Southern blot. DNA sequencing of both the transformation cassette and of PCR-amplified sequences from the correct transformants verified the integrity of the ALS9 alleles in these strains. PCR was used to confirm integration of ALS9-1 under control of its own promoter in strain 2740 and integration of ALS9-2 under control of its own promoter in strain 2064. Real-time RT-PCR (Green et al., 2005b) was used to demonstrate that transcription levels from the reintegrated genes were similar to the levels in the CAI12 control strain. Transcription of ALS9-1 in strain 2740 was 1.8 ± 0.2 compared to strain CAI12, while transcription of ALS9-2 in strain 2740 was 0.001 ± 0.001. Relative to strain CAI12, transcription of ALS9-1 in strain 2064 was 0.005 ± 0.004 while transcription of ALS9-2 was 1.1 ± 0.3.
Construction of a C. albicans strain that produces a cell-surface Als9-2p/Als9-1p fusion protein
Approximately 500 bp of ALS9-2 upstream sequence and the 5’-most 1266 bp of the ALS9-2 coding region were amplified from plasmid 1499 using primers ALS9F2 and 1499-PinAIR (Table 1). Primer 1499-PinAIR includes a silent mutation (T to C at nt 1260 of the ALS9-2 coding region) that creates a PinAI site. The PCR product was digested with AvrII-XhoI and cloned into plasmid 2729 to replace the ALS9-1 sequence. The resulting construct was called plasmid 2760. The tandem repeat and 3’ domains of ALS9-1 were amplified from plasmid 2096 using primers 2096-PinAIFm and ALS9-XhoIR (Table 1). The same silent mutation described above was included in the 2096-PinAIFm primer to create a PinAI site in the PCR product. The PCR product was digested with PinAI-XhoI and cloned into plasmid 2760 to yield the ALS9-2/ALS9-1 fusion plasmid 2765. This construct encodes a chimeric Als9 protein that includes amino acids 1 to 420 of Als9-2p followed by amino acids 421 to 1854 of Als9-1p. The fusion construct was verified by DNA sequencing. Digestion with AvrII-NgoMIV released the fragment that included the fused genes and the URA3 selectable marker, flanked by ALS9 upstream and downstream sequences to direct its integration into strain 2030 (Table 2). Southern blotting identified the correct transformant (strain 2767; Table 2). PCR demonstrated that the fusion construct was integrated under control of the ALS9-2 promoter. The real-time RT-PCR primers for ALS9-1 were used to measure transcription of the fused allele relative to ALS9-1 transcription in strain CAI12. Transcript levels for the fused allele were 1.8 ± 0.5, indicating similar transcriptional activity between the strains.
Germ tube formation assay
The germ tube assay format was changed from the one used in our previous work in order to require fewer input cells and to include multiple time points. Strains for testing were streaked from −80°C glycerol stocks to YPD plates (1% yeast extract, 2% peptone, 2% glucose with 2% agar for plates), incubated 24 h at 37°C and stored at 4°C for no more than one week. A single C. albicans colony was inoculated into 10 ml YPD and incubated 16 h at 37°C with 200 rpm shaking. Cells were washed in Dulbecco’s phosphate-buffered saline without calcium or magnesium (DPBS; Cambrex catalog number 17-512Q), counted and 5 × 105 added to 3 ml of either RPMI 1640 with L-gluatmine (RPMI; Invitrogen catalog number 11875-085) or Lee medium (Lee et al., 1975) pre-warmed to 37°C in a 25 ml Erlenmeyer flask. The culture flasks were incubated at 37°C with 200 rpm shaking for 20, 40 or 60 min. The entire volume of the flask was collected by vacuum filtration over a 1µm pore-size, polycarbonate filter (Fisher Scientific). Filters were washed with 5 ml of DPBS to remove residual medium. Filters were removed from the vacuum filtration unit, inverted onto glass slides and dried. Following removal of the filter from the slide, slides were heat fixed, stained with crystal violet for 2 min, washed with tap water and dried. C. albicans yeast with a germ tube longer than one diameter of the mother cell were considered positive. The percentage of cells forming germ tubes was calculated from visually counting all cells present on 5 randomly selected fields on the slide. Approximately 200 cells were counted from each slide. Duplicate assays for each condition were conducted on two different days. Statistical significance was assessed using PROC MIXED in SAS (SAS Institute) with P ≤ 0.05 considered significant.
Testing for cell wall defects
C. albicans strains were tested for defects in the C. albicans cell wall by growing them on YPD agar plates containing one of the following compounds: NaCl (1 M, 1.5 M), SDS (0.02%, 0.1%), Calcofluor White (Sigma; 20, 50 µg ml−1), Congo Red (Sigma; 100, 250 µg ml−1), or Hygromycin B (Invitrogen; 75, 200 µg ml−1). C. albicans strains were grown to saturation overnight at 37°C and 200 rpm shaking. Cells were harvested, washed in DPBS, counted and resuspended at 1 × 108 cells ml−1. Ten-fold serial dilutions were prepared and 5 µl of each spotted onto the YPD agar plates with the appropriate test substance included. Plates were incubated 24 h and growth of the test strains compared to growth of a CAI12 positive control.
Other phenotypic assays
Key strains used in this analysis were evaluated to ensure that their growth rate was not statistically different from that of the CAI12 control strain (Table 2), and that the degree of cellular aggregation observed did not increase compared to CAI12. Zhao et al. (2004) described methods for these analyses. Formation and analysis of biofilm on a silicone elastomer catheter substrate followed the method of Zhao et al. (2006). Methods for measuring adhesion to vascular endothelial cells, adhesion to buccal epithelial cells, and relative pathogenicity in the reconstituted human epithelium (RHE) tissue model are published (Zhao et al., 2004). Adhesion and cellular aggregation assays used C. albicans yeast forms inoculated from a YPD stock plate and incubated 16 h at 37°C as described above. Methods for real-time RT-PCR analysis of ALS gene expression were published (Green et al., 2005b).
Adhesion to laminin was evaluated in a six-well plate format using modifications of the fibronectin adhesion assay described by Zhao et al. (2004). Briefly, laminin (Sigma catalog number L-2020) was diluted to 10 µg ml−1 in DPBS and 1 ml added to each tissue culture well. Plates were incubated overnight at 37°C. C. albicans yeast forms were cultured in YPD as described above, washed in DPBS and resuspended at a density of 500 cells ml−1. Laminin solution was removed immediately before the assay and each well rinsed twice with 2 ml DPBS. One ml of the yeast suspension was added to each coated well and plates incubated at 37°C for 30 min. Washing and counting of adherent colonies, calculation of percentage adherence, experimental replication and statistical analysis were the same as described for the fibronectin adhesion assay (Zhao et al., 2004).
Collection of clinical C. albicans isolates and PCR genotyping reactions
The collection of 196 isolates used in this study was obtained from three previously analyzed populations (Pujol et al., 1997, 2002; Blignaut et al., 2002). The collection included 100 bloodstream isolates, 81 oral isolates, 10 vaginal isolates and 5 from other body sites. PCR genotyping was used to determine which ALS9 domains were present in each strain. Primers ALS9-1F and ALS9-1R were used to detect the ALS9-1 5’ domain while primers ALS9-2F and ALS9-2R were used to detect the ALS9-2 5’ domain (Table 1; Fig. 1). The size of Variable Block 1 (VB1) was determined using primers VB1F and VB1R (Table 1; Fig. 1). A smaller product is characteristic of the ALS9-1 prototype allele as defined in strain SC5314 (GenBank accession number AY269423) while a larger product is associated with the ALS9-2 allele (GenBank accession number AY269422; Zhao et al., 2003). The presence of both PCR product sizes suggests that both allelic sequences are present, but does not associate a specific VB1 sequence with a particular 5’ domain. Similarly, the size of Variable Block 2 (VB2) was assessed using primers VB2F and VB2R with a smaller product amplified from the ALS9-1 allele and a larger product from the ALS9-2 allele (Fig. 1). Amplification of both product sizes was observed for heterozygous strains. PCR products were separated on agarose gels using a SC5314 standard to judge relative fragment sizes and assign allelic designations.
RESULTS
Effect of ALS9 deletion on C. albicans adhesion
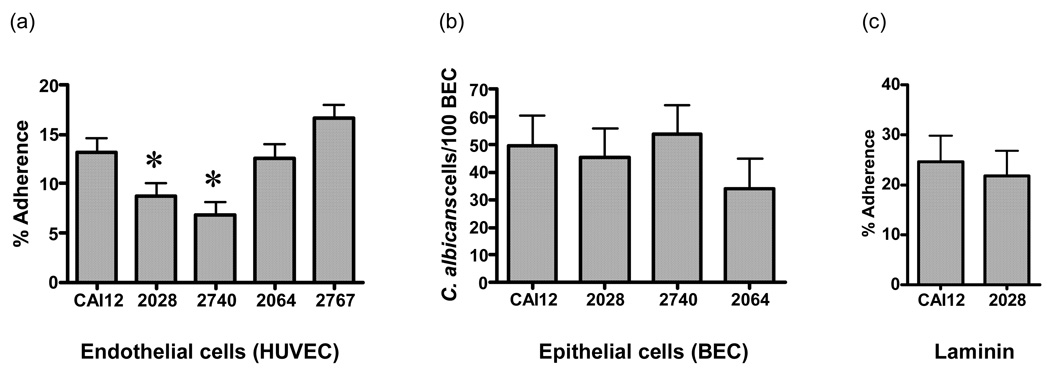
Our main goal in phenotypic analysis of the als9Δ/als9Δ mutant (strain 2028) was to assess its adhesion to cell types relevant to C. albicans host-pathogen interactions. These assays used C. albicans yeast forms that were grown at 37°C in YPD medium for 16 h as described in the Methods. ALS9 is transcribed in C. albicans cells grown in this manner (Green et al., 2005b), suggesting the conditions are appropriate for assessing Als9p function. The als9Δ/als9Δ strain was significantly less adherent to human vascular endothelial cell monolayers compared to the control strain CAI12 (P = 0.05; Fig. 2a). Although reintegration of a wild-type ALS9-1 allele did not complement the mutant phenotype (P = 0.02), transformation of ALS9-2 into the mutant strain restored wild-type adhesion levels (Fig. 2a). These results suggest functional differences between the proteins encoded by the ALS9 alleles. One possible explanation for this result is that the reintegrated ALS9-1 allele had defects and did not produce a viable protein. Since antibodies specific for Als9p are not available, other verification steps were pursued. DNA sequencing of ALS9-1 that was PCR amplified from the reintegrant strain verified that its sequence was identical to the sequence derived from the parental strain. Further, the adhesion phenotype of strain 1976 was tested. Strain 1976 is hemizygous (ALS9-1/als9-2) and was derived during construction of the als9Δ/als9Δ mutant (Table 1). Compared to the percent adherence for the CAI12 control (15.8 ± 1.6), percent adherence for the hemizygous strain 1976 (9.7 ± 1.6; P = 0.005) was similar to percent adherence for the mutant strain 2028 (9.1 ± 1.6; P = 0.003) and ALS9-1 reintegrant strain 2740 (9.9 ± 1.6; P = 0.006). These results are consistent with the conclusion that there is an unequal contribution of these ALS9 alleles to adhesion between C. albicans and human vascular endothelial cells.
Fig. 2.
Adhesion assay data. Histograms showing the adherence of C. albicans control (CAI12), als9Δ/als9Δ (2028), als9Δ/als9Δ∷ALS9-1 (2740), als9Δ/als9Δ∷ALS9-2 (2064) and als9Δ/als9Δ∷ALS9-1/ALS9-2 fusion (2767) strains to (a) vascular endothelial cell monolayers, (b) buccal epithelial cells and (c) laminin-coated plastic plates. Adhesion assays used C. albicans yeast forms that were grown for 16 h in YPD at 37ºC as described in Methods. Asterisks indicate significant differences from wild-type adhesion (P ≤ 0.05).
A fusion construct between the N-terminal domain of Als9-2p (amino acids 1 to 420) and the tandem repeats and C-terminal domain of Als9-1p (amino acids 421 to 1854) was constructed in C. albicans als9Δ/als9Δ strain 2028 (Fig. 1). The resulting strain, called 2767, had wild-type adhesion levels suggesting that the fusion protein restored the missing Als9p function. This result suggests that adhesive function for Als9-2p largely resides within the N-terminal domain.
The als9Δ/als9Δ mutant was also tested for adhesion to buccal epithelial cells (Fig. 2b) and laminin-coated plastic plates (Fig. 2c). Testing for adhesion to laminin was prompted by a report that S. cerevisiae becomes adherent to laminin when overexpressing ALS9 (Sheppard et al., 2004). In each assay, deletion of ALS9 did not have a significant effect on the phenotype of the C. albicans strain suggesting that although Als9-2p may play a measurable role in adhesion to endothelial cells, the Als9 proteins do not appear to be important for buccal cell or laminin adhesion.
Other phenotypic analyses of the C. albicans als9Δ/als9Δ strain
The C. albicans als9Δ/als9Δ strain 2028 was tested in other assays to assess its phenotype. Inoculation of the buccal reconstituted human epithelium (RHE) model with the als9Δ/als9Δ mutant and a control strain showed no difference between the strains with respect to epithelial destruction, adherence, or hypha formation (Fig. 3). Comparison between the als9Δ/als9Δ strain and the CAI12 control strain showed no differences in growth on YPD agar that contained cell wall stressing agents including NaCl, SDS, Calcofluor White, Congo Red or Hygromycin B. Lack of an effect of these strains on growth of the als9Δ/als9Δ mutant suggests that cell wall structure and glycosylation are not affected by ALS9 deletion (Elorza et al., 1983; Kopecka & Gabriel, 1992; Dean, 1995; Machi et al., 2004). The als9Δ/als9Δ mutant strain also did not exhibit any significant differences in germ tube formation over the course of 1 h when grown in RPMI 1640 or Lee medium (Table 4). Finally, although growth of the als9Δ/als9Δ strain in a model of biofilm formation on a silicone elastomer catheter surface (Kuhn et al., 2002) resulted in decreased biomass compared to the CAI12 control strain (1.21 ± 0.16 vs. 1.4 ± 0.16 mg, respectively), this difference was not significant statistically (P = 0.06). Compensatory changes in expression of other ALS genes in the als9Δ/als9Δ strain were ruled out using real-time RT-PCR analysis of ALS gene expression (Green et al., 2005b).
Fig. 3.
Light micrographs of buccal RHE inoculated with either (a) the C. albicans control (CAI12) or (b) the als9Δ/als9Δ mutant strain (2028) and grown for 8 h at 37ºC. The similar appearance of RHE following incubation with either strain suggested that Als9p does not contribute to epithelial damage in this experimental model.
Table 4.
Allelic frequencies for ALS9 domains in a Allelic frequencies for ALS9 domains in a geographically diverse collection of clinical isolates
| 5’ domain | 3’ domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALS9-1 | ALS9-2 | Variable Block 1 (VB1) | Variable Block 2 (VB2) | |||||||||
| Source | Pos. | Neg. | Pos. | Neg. | Both | ALS9-1 | ALS9-2 | Neither | Both | ALS9-1 | ALS9-2 | Neither |
| Bloodstream | 51 | 49 | 100 | 0 | 50 | 2 | 49 | 0 | 36 | 1 | 64 | 1 |
| Oral | 45 | 36 | 81 | 0 | 46 | 2 | 31 | 1 | 13 | 3 | 63 | 0 |
| Vaginal | 4 | 6 | 10 | 0 | 5 | 0 | 4 | 1 | 1 | 1 | 8 | 0 |
| Other | 4 | 1 | 5 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 4 | 0 |
| Total | 104 | 92 | 196 | 0 | 104 | 5 | 85 | 2 | 51 | 5 | 139 | 1 |
Genotyping of clinical isolates
Endothelial cell adhesion assays showed a clear difference in function between Als9-1p and Als9-2p (Fig. 2). In a previous study, PCR genotyping of the ALS9 alleles in 30 C. albicans clinical isolates showed that, in each strain, at least one allele had ALS9-2 sequences in the 5’ domain (Zhao et al., 2003). This analysis was expanded to 196 C. albicans clinical isolates from various body sites (Table 4). PCR primers were used that could distinguish between the ALS9-1 and ALS9-2 sequences within the 5’ domain and also within the VB1 and VB2 regions of the 3’ domain (Table 1; Fig. 1). Within the 5’ domain, approximately half of the isolates were positive for ALS9-1 while all isolates had at least one ALS9-2 allele (Table 4). Therefore, among the C. albicans isolates tested, approximately half were heterozygous (ALS9-1/ALS9-2) while the others appeared to be ALS9-2 homozygotes, although it is formally possible for these strains to encode other ALS9 alleles. Within Variable Block 1 of the 3’ domain, ALS9-2 sequences were common while ALS9-1 sequences were rather rare (Table 4). Nearly half of the C. albicans isolates examined had both ALS9-1 and ALS9-2 sequences within VB1. Within the Variable Block 2 region, ALS9-2 sequences were far more common than ALS9-1 sequences, and nearly one-quarter of the strains examined had both ALS9-1 and ALS9-2 sequence types (Table 4). Within both VB1 and VB2, a low number of strains had sequences that did not conform to either the ALS9-1 or ALS9-2 types that were defined from strain SC5314. This result suggests even greater sequence variability within the ALS9 3’ domain.
DISCUSSION
Phenotypic assays of the C. albicans als9Δ/als9Δ mutant strain showed that the most notable effect of deleting ALS9 was a significant decrease in adhesion to human vascular endothelial cell monolayers. Reintegration of the ALS9-2 allele complemented the mutation, while the ALS9-1 allele did not. Fusion of the Als9-2p N-terminal domain (amino acids 1–420) to the tandem repeats and C-terminal domain of Als9-1p (amino acids 412–1854) produced a protein that was also capable of complementing the mutant phenotype. This observation is consistent with the conclusion that adhesive function resides within the Als9p N-terminal domain. In previous work, fusion of the Als3p N-terminal domain (amino acids 1 to 430) to the C-terminal 323 amino acids of S. cerevisiae alpha-agglutinin was sufficient to increase adhesion of a C. albicans als3Δ/als3Δ strain to buccal epithelial cells (Zhao et al., 2006). Although the majority of adhesive activity is believed to be associated with the N-terminal domain, experiments that involve overexpression of ALS genes in S. cerevisiae suggest that some adhesive activity may be associated with other domains in Als proteins (Loza et al., 2004; Rauceo et al., 2006).
Assay of C. albicans alsΔ/alsΔ mutant strains and overexpression of ALS genes in S. cerevisiae are the two main methods that have been utilized to evaluate Als protein function (Loza et al., 2004; Rauceo et al., 2006; Zhao et al., 2004; 2005). In some cases, results from the two methods are in agreement, however more often, the two methods provide different views of Als protein function. Advantages of the S. cerevisiae system include working in a tractable model system using relatively simple genetic manipulations, and the ability to address hypotheses regarding adhesive function in a low adhesion background. These advantages are gained at the expense of differences in genetic code (Santos & Tuite, 1995) and glycosylation patterns (Herrero et al., 2002), the tendency to draw functional conclusions from testing a single ALS allele, the need to utilize artificial gene expression levels and patterns, the potential for protein overproduction to create cell wall artifacts including mislocalized products, the inability to address C. albicans-specific Als protein function and/or interaction of Als proteins with others, and the limited ability to address non-adhesive phenotypes. While these disadvantages of S. cerevisiae-based studies are not present when working with the native C. albicans, C. albicans is inherently sticky, which complicates adhesion assays. Working in C. albicans also requires an understanding of ALS gene expression patterns to select assay conditions, and phenotypic changes may be masked by compensatory gene expression changes (Zhao et al., 2005). Differences in results between the C. albicans- (Fig. 2) and S. cerevisiae-based (Sheppard et al., 2004) approaches to studying Als9p function are obvious. When overexpressed in S. cerevisiae, ALS9 did not confer adhesion to vascular endothelial cell monolayers (Sheppard et al., 2004). Because the allele tested was not specified, it is possible that ALS9-1 was used, which would account for the difference in results compared to our C. albicans analysis (Fig. 2). Overexpression of ALS9 in S. cerevisiae, which presumably coats the cell surface in heterologous protein, resulted in adhesion to laminin (Sheppard et al., 2004). Although statistically significant, this percent adhesion was very low (approximately 3%) suggesting, at best, a minimal contribution to laminin adhesion from the Als9 protein.
A key issue in extrapolating S. cerevisiae-based Als protein functional data to the C. albicans system is how well the S. cerevisiae model mimics native C. albicans. High-level overexpression of ALS genes in S. cerevisiae would best mimic C. albicans growth stages where a specific ALS gene is highly expressed. While some ALS genes are known to undergo large changes in expression level from nearly undetectable to very high (ALS1 and ALS3, for example; Zhao et al., 2004), others are appear to be transcribed only at very low levels (ALS6 and ALS7; Green et al., 2004; 2005a; 2005b; 2006). ALS9 ranks in the middle of this ALS gene expression hierarchy, clearly transcribed under all of the conditions tested, but to date, observed at expression levels that are well below those of the most highly expressed ALS genes (Green et al., 2004; 2005a; 2005b; 2006). Protein abundance and localization on the cell surface are almost unknown for each of the Als proteins. Many variations in Als protein localization can be envisioned, from large areas or small focal patches of high protein concentration, to diffuse arrangements of a sparse number of proteins across the cell surface. Knowing the Als protein localization on the C. albicans cell surface would provide a better understanding of how well the S. cerevisiae model mimics the native system.
In many of the phenotypic assays conducted, the als9Δ/als9Δ strain failed to show a difference from the wild-type control strain. One potential explanation for these results is compensatory expression of other C. albicans ALS genes to confer a wild-type phenotype. This possibility was suggested by work that showed a relationship between expression of ALS2 and ALS4 where reduction in transcriptional level of one gene resulted in increased transcription of the other (Zhao et al., 2005). Compensatory ALS gene expression in response to ALS9 deletion was ruled out by real-time RT-PCR analysis (Green et al., 2005b). Compensatory expression of non-ALS genes is possible, but if applicable to the als9Δ/als9Δ mutant, would have to involve genes that function in all processes tested except endothelial cell adhesion. These limitations decrease the likelihood that compensatory expression is a factor in functional analyses of the als9Δ/als9Δ strain.
Within the ALS family, ALS9 is the most extreme example of allelic variability that has been described. While all eight ALS genes have variability in the number of tandem repeat copies within the central coding domain, sequence variability in the non-tandem-repeat domains is less documented. Examples include ALS5, which has sequence variability within the 5’ domain that surpassed the mean sequence variability present in a representative list of C. albicans genes (Hoyer & Hecht, 2001), and ALS7, which has a complex repeated structure within the 3’ domain that lends considerable allelic variability to this locus (Zhang et al., 2003). For ALS3, the presence of 12 instead of 9 tandem repeat copies in the central domain resulted in a higher percentage adherence of C. albicans to both endothelial and epithelial cell monolayers (Oh et al., 2005). For ALS9, allele-specific function is associated with the large sequence differences (11% at the nucleotide level; 16% at the amino acid level) within the ALS9 5’ domain. These differences may affect key residues within the Als9p N-terminal domain that are involved in binding to endothelial cells. Structural analysis of the Als N-terminal domain will answer such questions and could exploit the sequence and functional differences described here to better understand Als9p interaction with host cells.
Table 3.
Percentage of C. albicans cells forming germ tubes over time in different growth media*
| Growth Medium | Time (min) | CAI12 | 2028 | Strain 2064 | 2740 | 2767 |
|---|---|---|---|---|---|---|
| RPMI 1640 | 20 | 8.7 ±0.5 | 8.1 ±0.5 | 8.3 ±0.5 | 9.1 ±0.5 | 9.0 ±0.5 |
| 40 | 51.3 ± 1.1 | 51.1 ± 1.1 | 50.9± 1.1 | 49.7 ±1.1 | 50.3 ±1.1 | |
| 60 | 90.6 ±0.4 | 90.1 ±0.4 | 90.2 ±0.4 | 90.0 ±0.4 | 89.5 ±0.4 | |
| Lee | 20 | 7.4 ±0.3 | 6.3 ±0.3 | 6.9 ±0.3 | 6.6 ±0.3 | 6.6 ±0.3 |
| 40 | 27.7 ±1.3 | 26.4 ±1.3 | 27.5 ±1.3 | 27.5 ±1.3 | 26.0 ±1.3 | |
| 60 | 40.6 ±2.0 | 40.9 ±2.0 | 39.8 ±2.0 | 40.8 ±2.0 | 41.0±2.0 |
Duplicate assays were conducted on two different days. Means and standard errors of the mean are reported. There were no significant differences between the CAI12 control strain and any of the other strains for germ tube formation percentage at each time point (P > 0.05 for each comparison).
ACKNOWLEDGEMENTS
We thank Aaron Mitchell for plasmid pDDB57 and Michael Pfaller and David Soll for the collection of C. albicans clinical isolates. This research was supported by Public Health Service grant DE14158 from the National Institute of Dental and Craniofacial Research, National Institutes of Health. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515-01 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- Blignaut E, Pujol C, Lockhart S, Joly S, Soll DR. Ca3 fingerprinting of Candida albicans isolates from human immunodeficiency virus-positive and healthy individuals reveals a new clade in South Africa. J Clin Microbiol. 2002;40:826–836. doi: 10.1128/JCM.40.3.826-836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, Lacroute F, Fink GR. A positive selection for mutants lacking orotidine 5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wozniak K, Wallig MA, Fidel PL, Jr., Trupin SR, Hoyer LL. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect Immun. 2005;73:1656–1663. doi: 10.1128/IAI.73.3.1656-1663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean N. Yeast glycosylation mutants are sensitive to aminoglycosides. Proc Natl Acad Sci. 1995;92:1287–1291. doi: 10.1073/pnas.92.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorza MV, Rico H, Sentandreu R. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J Gen Microbiol. 1983;129:1577–1582. doi: 10.1099/00221287-129-5-1577. [DOI] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans . Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Rieg G, Fonzi WA, Belanger PH, Edwards JE, Jr, Filler SG. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect Immun. 1998;66:1783–1786. doi: 10.1128/iai.66.4.1783-1786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur NK, Klotz SA. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect Immun. 1997;65:5289–5294. doi: 10.1128/iai.65.12.5289-5294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans genes for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Green CB, Cheng G, Chandra J, Mukherjee P, Ghannoum MA, Hoyer LL. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology. 2004;150:267–275. doi: 10.1099/mic.0.26699-0. [DOI] [PubMed] [Google Scholar]
- Green CB, Zhao X, Hoyer LL. Use of green fluorescent protein and reverse transcription-PCR to monitor Candida albicans agglutinin-like sequence gene expression in a murine model of disseminated candidiasis. Infect Immun. 2005a;73:1852–1855. doi: 10.1128/IAI.73.3.1852-1855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Zhao X, Yeater KM, Hoyer LL. Construction and real-time RT-PCR validation of Candida albicans PALS-GFP reporter strains and their use in flow cytometry analysis of ALS gene expression in budding and filamenting cells. Microbiology. 2005b;151:1051–1060. doi: 10.1099/mic.0.27696-0. [DOI] [PubMed] [Google Scholar]
- Green CB, Marretta SM, Cheng G, Faddoul FF, Ehrhart EJ, Hoyer LL. RT-PCR analysis of Candida albicans ALS gene expression in a hyposalivatory rat model of oral candidiasis and in HIV-positive human patients. Med Mycol. 2006;44:103–111. doi: 10.1080/13693780500086527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero AB, Uccelletti D, Hirschberg CB, Dominguez A, Abeijon C. The Golgi GDPase of the fungal pathogen Candida albicans affects morphogenesis, glycosylation, and cell wall properties. Eukaryot Cell. 2002;1:420–431. doi: 10.1128/EC.1.3.420-431.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL. The ALS gene family of Candida albicans . Trends Microbiol. 2001;9:176–180. doi: 10.1016/s0966-842x(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Hecht JE. The ALS5 gene of Candida albicans and analysis of the Als5p N-terminal domain. Yeast. 2001;18:49–60. doi: 10.1002/1097-0061(200101)18:1<49::AID-YEA646>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Scherer S, Shatzman AR, Livi GP. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol Microbiol. 1995;15:39–54. doi: 10.1111/j.1365-2958.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998;33:451–459. doi: 10.1007/s002940050359. [DOI] [PubMed] [Google Scholar]
- Kapteyn JC, Hoyer LL, Hecht JE, Muller WH, Andel A, Verkleij AJ, Makarow M, Van Den Ende H, Klis FM. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. 2000;35:601–611. doi: 10.1046/j.1365-2958.2000.01729.x. [DOI] [PubMed] [Google Scholar]
- Kopecka M, Gabriel M. The influence of Congo red on the cell wall and (1–3)-β-D-glucan microfibril biogenesis in Saccharomyces cerevisiae . Arch Microbiol. 1992;158:115–126. doi: 10.1007/BF00245214. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70:878–888. doi: 10.1128/iai.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Buckley HR, Campbell CC. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans . Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Loza L, Fu Y, Ibrahim AS, Sheppard DC, Filler SG, Edwards JE., Jr Functional analysis of the Candida albicans ALS1 gene product. Yeast. 2004;21:473–482. doi: 10.1002/yea.1111. [DOI] [PubMed] [Google Scholar]
- Machi K, Azuma M, Igarashi K, Matsumoto T, Fukuda H, Kondo A, Ooshima H. Rot1p of Saccharomyces cerevisiae is a putative membrane protein required for normal levels of the cell wall 1,6-β-glucan. Microbiology. 2004;150:3163–3173. doi: 10.1099/mic.0.27292-0. [DOI] [PubMed] [Google Scholar]
- Oh S-H, Cheng G, Nuessen JA, Jajko R, Yeater KM, Zhao X, Pujol C, Soll DR, Hoyer LL. Functional specificity of Candida albicans Als3p proteins and clade specificity of ALS3 alleles discriminated by the number of copies of the tandem repeat sequence in the central domain. Microbiology. 2004;151:673–681. doi: 10.1099/mic.0.27680-0. [DOI] [PubMed] [Google Scholar]
- Porta A, Ramon AM, Fonzi WA. PRR1, a homolog of Aspergillus nidulans palF, control pH-dependent gene expression and filamentation in Candida albicans . J Bacteriol. 1999;181:7516–7523. doi: 10.1128/jb.181.24.7516-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Joly S, Lockhart SR, Noel S, Tibayrenc M, Soll DR. Parity among the randomly amplified polymorphic DNA method, multilocus enzyme electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans . J Clin Microbiol. 1997;35:2348–2358. doi: 10.1128/jcm.35.9.2348-2358.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Pfaller MA, Soll DR. Ca3 fingerprinting of Candida albicans bloodstream isolates from the United States, Canada, South America, and Europe reveals a European clade. J Clin Microbiol. 2002;40:2729–2740. doi: 10.1128/JCM.40.8.2729-2740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauceo JM, De Armond R, Otoo H, Kahn PC, Klotz SA, Gaur NK, Lipke PN. Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryot Cell. 2006;5:1664–1673. doi: 10.1128/EC.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MA, Tuite MF. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans . Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE., Jr Functional and structural diversity in the Als protein family of Candida albicans . J Biol Chem. 2004;279:30480–30489. doi: 10.1074/jbc.M401929200. [DOI] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Enloe BM, Mitchell AP. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruption. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Zhang N, Harrex AL, Holland BR, Fenton LE, Cannon RD, Schmid J. Sixty alleles of the ALS7 open reading frame in Candida albicans: ALS7 is a hypermutable contingency locus. Genome Res. 2003;13:2005–2017. doi: 10.1101/gr.1024903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Pujol C, Soll DR, Hoyer LL. Allelic variation in the contiguous loci encoding Candida albicans ALS5, ALS1 and ALS9 . Microbiology. 2003;149:2947–2960. doi: 10.1099/mic.0.26495-0. [DOI] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Cheng G, Green CB, Nuessen JA, Yeater K, Leng RP, Brown AJP, Hoyer LL. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparison between Als3p and Als1p. Microbiology. 2004;150:2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Yeater KM, Hoyer LL. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family Microbiology 2005. 151 1619 11630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Daniels K, Green CB, Oh S-H, Soll DR, Hoyer LL. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology. 2006;152:2287–2299. doi: 10.1099/mic.0.28959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]