Abstract
Epithelial-Mesenchymal Transition (EMT) is a process occurring during both embryogenesis and early stages of invasive cancer. Epithelial cells that undergo EMT become more migratory and invasive with a mesenchymal morphology. Herein we assess EMT induction in a mouse mammary epithelial cell line driven by Msx2, a homeobox-containing transcription factor important during mammary gland development. NMuMG cells, a normal mouse mammary epithelial cell line, stably-transfected with a Msx2 cDNA showed downregulation of an epithelial marker E-cadherin and upregulation of the mesenchymal markers vimentin and N-cadherin. Furthermore, overexpression of Cripto-1, a member of the epidermal growth factor-CFC protein family already known to be involved in EMT, was detected in Msx2-transfected cells. The expression of Cripto-1 was accompanied by activation of the tyrosine kinase c-Src pathway and an increase in the invasive ability of the cells. Functional assays also demonstrated inhibition of the invasive behavior of the Msx2-transfected cells by a c-Src specific inhibitor. Moreover, immunohistochemistry of human infiltrating breast carcinomas showed positive staining for Msx2 only in the infiltrating tumor cells while the non-infiltrating tumor cells were negative. These results suggest that Msx2 may play a significant role in promoting EMT in epithelial cells that acquire properties involved in tumor invasion.
Keywords: Msx2, EMT, mammary epithelial cells, Cripto-1
Introduction
Msx2 is a member of the vertebrate homologues of the Drososophila muscle-segment homeobox gene family (msh/Msx). This transcription factor is a major regulator of developmental pathways during embryogenesis of different organs including craniofacial structures, limb, neural tube and mammary gland (Alappat et al., 2003; Satoh et al., 2004). In all these different tissues, Msx2 is expressed at the sites of epithelial-mesenchymal inductive interactions mediating the signaling between the two different tissues (Alappat et al., 2003; Ramos and Robert, 2005; Satoh et al., 2004). In the mammary gland, Msx2 expression is hormonally regulated (Friedmann and Daniel, 1996; Phippard et al., 1996; Satoh et al., 2007) and promotes early branching morphogenesis of the mammary epithelium (Satoh et al., 2007). Homeobox genes, when deregulated, can contribute to carcinogenesis and Msx genes may be deregulated in many types of epithelial cancers such as mammary carcinomas (Abate-Shen, 2002).
An essential process occurring during embryogenesis is Epithelial-Mesenchymal Transition (EMT), whereby the polarized epithelial cells lose their polarity, disassemble their cell-cell junctional structures and remodel their cytoskeleton becoming migratory (Hay, 2005). In particular, the mammary gland tissue utilizes EMT to facilitate branching morphogenesis through which the developing gland migrates into and invades the fat pad (Fata et al., 2004). EMT is also considered a major mechanism for the conversion of early-stage tumors to invasive malignancies(Thiery, 2002). If epithelial tumor cells undergo EMT, they are facilitated to migrate from the primary tumor and to invade adjacent tissues: the migratory ability of the epithelial tumor cells is an index of aggressiveness and increased metastatic potential in different types of malignant tumors (Fuchs et al., 2002; Xue et al., 2003).
Cripto-1 is a signaling protein member of the epidermal growth factor (EGF)-CFC protein family that plays an important role during early embryonic development (Bianco et al., 2005). Cripto-1 is overexpressed in a variety of human carcinomas and has a potential role in EMT (Salomon et al., 1999; Strizzi et al., 2004). In vitro and in vivo studies have demonstrated that Cripto-1 overexpression in mouse mammary epithelial cells can enhance migration, branching morphogenesis and development of mammary hyperplasias and adenocarcinomas in mice (Wechselberger et al., 2001; Wechselberger et al., 2005). Cripto-1 can function as a co-receptor for the TGF-β–related protein Nodal, activating the Alk4-Smad dependent pathway (Bianco et al., 2002) but can also bind to glypican-1 and activate the tyrosine kinase c-Src pathway in a Nodal-independent manner. The activation of the tyrosine kinase c-Src is required for cell proliferation and migration of mammary epithelial cells (Bianco et al., 2003). In this paper, we show the ability of Msx2 to confer the biological and molecular characteristics of EMT on mammary epithelial cells through upregulation of Cripto-1 expression and activation of the c-Src pathway.
Materials and Methods
Cell lines, transfection and plasmids
NMuMG cells (ATCC, Manassas, VA) were cultured in DMEM (Invitrogen, Gaithersburg, MD) with 10% fetal bovine serum. NMuMG cells were transfected using Fugene HD Transfection Reagent (Roche Applied Science, Indianapolis, IN) with pEF4 (Empty Vector) or pEF4-Msx2. Stably transfected cells where selected in the presence of 500 μg/ml of Zeocin (Invitrogen).
RT-PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) and all cDNAs were prepared using 1 μg of RNA. PCR conditions and primer sequences for amplification of mouse Msx2 and mouse GAPDH were previously described (Satoh et al., 2007). The RT-PCR amplifications were performed using the following primer pairs: m-TGF-β1, forward 5′-GAAAACCAAAGACATCT CACAC-3′ and reverse 5′-GAATCGAAAGCCCTGTATTCC-3′; m-TGF-β3, forward 5′-GAAAACCAAAGCGACAGACC-3′ and reverse 5′-TTCCCCTAACCA ACCCACAC-3′; m-TGF-β-RII, forward 5′-ATCTCCACAGTGACCACACTCC-3′ and reverse 5′-ATCCGTCTGCTTGAACGACTCC-3′; m-Twist, forward 5′-CAGAGAAGGAGAAAATGGACAG-3′ and reverse 5′-GGACACAAACGAGT GTTCAG-3′; m-Snail, forward 5′-AGCCATAGAACTAAAGCCAAC-3′ and reverse 5′-AGGGGAACTATTGCATAGTCTG-3′; m-Slug, forward 5′-CAAAAACTGCTCC AAAACCTTC-3′ and reverse 5′-TCTCTCTCTCTCTCTCT CTCTC-3′; m-Cripto, forward 5′-TCCTCCAACGTTTTTAGCC-3′ and reverse 5′-GCAAGTCCCTCCAT TCAGACAG-3′; m-Nodal, forward 5′-AGTTTCATCCTACCAACCATGC-3′ and reverse 5′-ACCCACACTCCTCCACAATC-3′. The conditions for the PCR reaction were an initial denaturation of 95° C for 5 min, followed by 95° C for 1 min, 56° C for 1 min, 72° C for 1 min for 25 cycles with a final extension at 72° C for 10 min.
Protein extracts, western blot, and antibodies
Cells were lysed in Proteinase Inhibitor Cocktail (Roche) in the presence of 50mM Tris-HCl, pH 7.5, 250 mM NaCl, 1% NP-40, 5mM EDTA, 5mM EGTA, 25 mM NaF and, 1 mM orthovanadate. For western blot analysis 50-80 μg of proteins were separated by SDS-PAGE, blotted onto nitrocellulose membrane and detected with specific antibodies: rabbit anti-E-cadherin (H-108), rabbit anti-vimentin (C-20), rabbit anti-N-cadherin (H-63), goat anti-Cripto-1 (F-20), rabbit anti-ERK1 (K-23) were purchased from Santa Cruz, CA. Rabbit anti-phospho-FAK, rabbit anti-FAK, rabbit anti-phospho-Src, rabbit anti-c-Src, rabbit anti-phospho-Akt, rabbit anti-Akt, mouse anti-phospho-ERK, rabbit anti-β-catenin were purchased from Cell Signaling (Beverly, MA). Mouse anti-α-tubulin was purchased from Sigma (St. Louis, MO). Signal was detected using ECL Plus (GE Healthcare, Piscataway, NJ). Rabbit anti-Msx2 used for fluorescent immunocytochemistry and for immunohistochemistry was purchased from Affinity BioReagents (Golden, CO).
siRNA for m-Cripto1
ON-Target plus SMART pool siRNA for mouse TDGF-1 (m-Cripto1) and ON-Target plus Control Non-Targeting Pool were purchased from Dharmacon (Lafayette, CO). NMuMG-Msx2 cells were transfected following the manufacturer's protocol using DharmaFECT 4.
3D Matrigel culture and cell invasion assay
1×104 cells were plated onto a 24-well plate coated with a thin layer of growth factor-reduced (GFR) Matrigel Matrix (BD Biosciences, Bedford, MA). Phase-contrast photomicrographs were taken after 1 day and after 7 days using a 10× objective.
Cell invasion was evaluated using the BD BioCoat Matrigel Invasion Chamber (BD Biosciences). Approximately 4×104 cells were seeded in 5% FBS medium onto the chamber and incubated for 24h. The assay was performed following the manufacturer's instructions and invasion was determined by counting the stained cells on the lower surface of the filter by phase-contrast microscopy using a 4× objective (10 randomly selected fields). The same assay was performed adding c-Src inhibitor SU6656 (10 μM; Calbiochem, Gibbstown, NJ), Alk 4/5/7 inhibitor SB-431542 (10μM; Sigma) or DMSO as vehicle control to the medium both in the top and the bottom chambers at the final concentrations indicated.
Immunocytochemistry and immunohistochemistry analysis
NMuMG-EV and NMuMG-Msx2 were plated onto an 8-well chamber slide (Nunc, Rochester, NY). After 24h, cells were fixed with PBS-4% paraformaldehyde for 10 min at room temperature (RT) and permeabilized in PBS-0.5% Triton X-100. The cells were incubated for 1h at RT with the specific primary antibodies (1:200), followed by fluorescein-conjugated or rhodamine-conjugated secondary antibodies (AlexaFluor, Invitrogen). Nuclei were stained with Prolong Gold antifade reagent with DAPI (Invitrogen). The fluorescent immunocytochemistry assay used to detect Cripto-1 expression was performed under non-permeabilized conditions.
For immunohistochemistry, paraffin-embedded slides from four different infiltrating ductal carcinomas were deparaffinized in xylene, rehydrated in a series of graded ethanols and pre-digested with ready to use pepsin solution (Digest-All3, Invitrogen). The endogenous peroxidase activity was blocked with 3% H2O2. The slide was incubated for 1h at RT with the anti-Msx2 antibody (1:200). Immunostaining was carried out using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Color was developed with DAB (Vector) and the nuclei counterstained with hematoxylin.
For fluorescent immunohistochemistry, paraffin embedded sections from infiltrating ductal carcinomas (US Biomax, Inc., Rockville, MD) were prepared as described by the manufacturer. For co-localization of Msx2 with Cripto-1, slides were blocked with 5% normal goat serum and incubated sequentially with a polyclonal antibody against h-Msx2 (1:200; Affinity BioReagents, Golden, CO) and a monoclonal antibody against h-Cripto-1 (10 mg/ml; R&D Systems, Minneapolis, MN) for 1 hr at room temperature. Slides were washed extensively with TBS, then incubated simultaneously with goat anti-rabbit (1:1000; AlexaFluor 488, Invitrogen) and goat anti-mouse secondary antibody (1:1000; AlexaFluor 594, Invitrogen). For co-localization of Msx2 with phospho-Src, slides were blocked with 10% normal goat serum with 3% Triton and incubated sequentially with a monoclonal antibody against h-Msx2 (10 mg/ml; Stressgen, Ann Arbor, MI) and a polyclonal antibody against phospho-Src (1:1000; Cell Signaling Technology, Beverly, MA). Slides were mounted with Prolong Gold antifade reagent with DAPI (Invitrogen).
Statistics
Student's t-test was used to determine significance of the mean values obtained from the cell invasion assay. A P value of <0.05 was considered statistically significant.
Results
Msx2 causes EMT in NMuMG cells
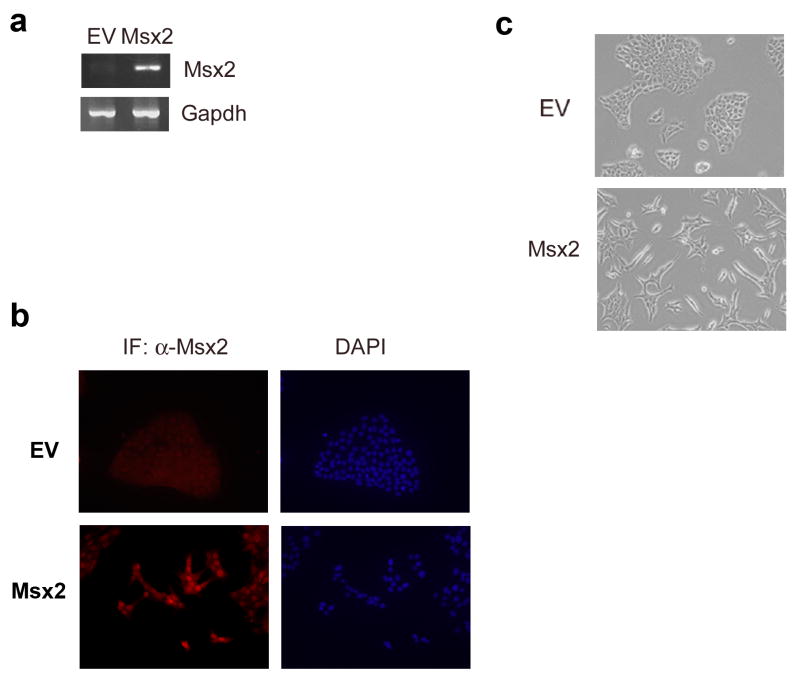
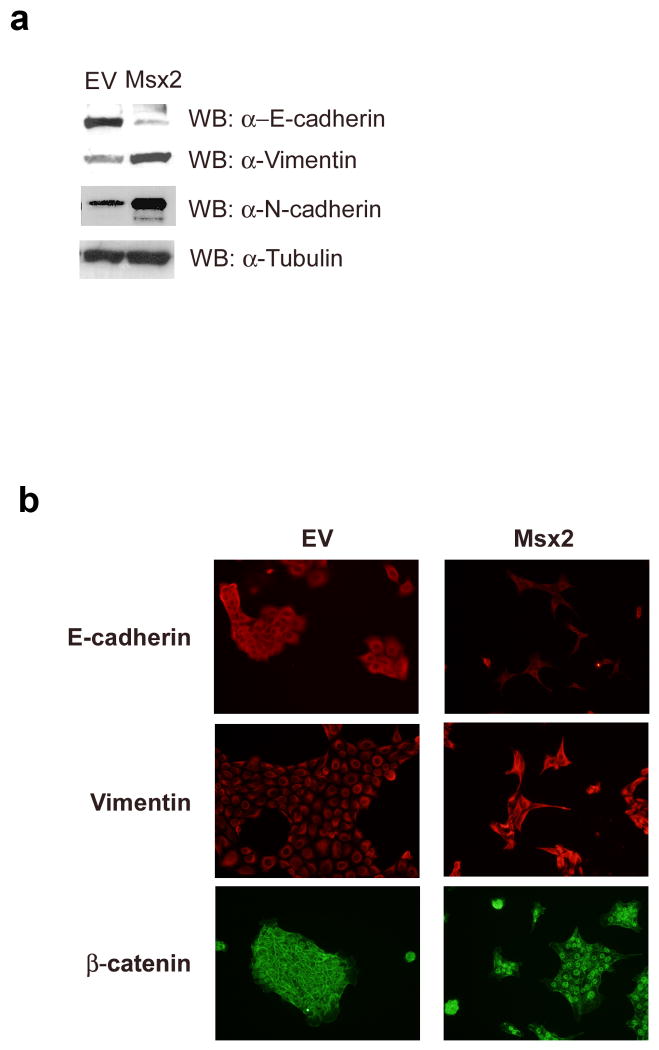
To investigate whether Msx2 plays a role in EMT, we stably transfected cDNA for Msx2 into NMuMG cells, a spontaneously immortalized normal mouse mammary epithelial cell line with undetectable Msx2 expression. Intriguingly, the overexpression of Msx2 (Figs.1a and 1b) induced morphological changes in these cells. Compared to the NMuMG cells stably transfected with empty vector (NMuMG-EV) which demonstrate a cobblestone-like phenotype, the NMuMG cells that were stably transfected with Msx2 (NMuMG-Msx2) appeared spindle shaped or fibroblast-like in monolayer culture (Fig.1c). Consistent with this observation, western blot analysis of cell extracts showed a reduction in expression of the epithelial marker E-cadherin and an increase in expression of the mesenchymal markers vimentin and N-cadherin in NMuMG-Msx2 cells (Fig. 2a). The changes in the expression of epithelial marker E-cadherin and mesenchymal marker vimentin were also observed by fluorescent immunocytochemical analysis (Fig.2b). In addition, the subcellular localization of β–catenin was altered with β–catenin now localized to the membrane in the NMuMG-EV cells, but became localized to the nucleus in the NMuMG-Msx2 cells. This translocation of β–catenin is a characteristic phenomenon during EMT (Lee et al., 2006) as well as possible activation of the canonical wnt signaling pathway (Cheng et al., 2008). Thus, morphological alterations from an epithelial to fibroblastic phenotype and expression of molecular markers in NMuMG cells stably expressing Msx2 were consistent with EMT.
FIGURE 1. Msx2 expression induces EMT-like morphological changes in NMuMG cells.
Msx2 expression levels were checked by (a) RT-PCR and by (b) fluorescent immunocytochemistry. (c) Phase-contrast photomicrographs of NMuMG cells stably transfected with empty vector (EV) and Msx2 (Msx2) grown on tissue culture plastic.
FIGURE 2. NMuMG-Msx2 cells express mesenchymal markers and loss of epithelial markers.
(a) Western blot analysis of E-cadherin, vimentin and N-cadherin in NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) cell total protein extracts. (b) NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) cells were fixed and processed for immunocytochemistry with antibodies recognizing E-cadherin, vimentin and β–catenin.
The Nodal independent pathway is involved in Msx2 induced EMT
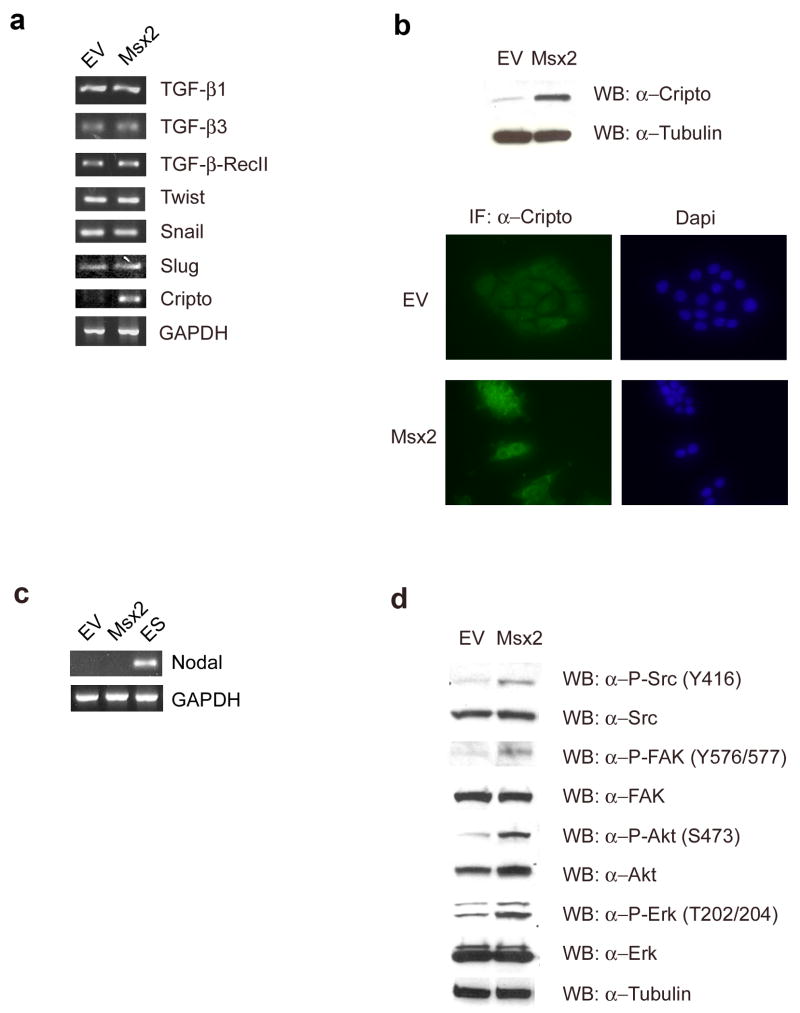
To elucidate the mechanism of Msx2-induced EMT, we examined the expression levels of different molecules involved in EMT. Interestingly, while we found no differences in expression levels of TGF-β1, TGF-β3, TGF-β RII, Twist, Snail or Slug (Fig. 3a), we found an overexpression of Cripto-1 in the NMuMG-Msx2 cells by RT-PCR (Fig. 3a), western blot analysis and by immunocytochemistry (Fig. 3b). To further determine the role of Cripto-1 in the Msx2-induced EMT, we investigated which intracellular pathway members were activated by Cripto-1. In particular, we assessed, by RT-PCR, the expression level of Nodal, a TGF-β–related protein that is a ligand that binds to the Cripto-1 co-receptor in both NMuMG-EV and NMuMG-Msx2 cells. No detectable Nodal was expressed in those cells (Fig. 3c). On the other hand, western blot analysis of protein extracts showed an increase of c-Src phosphorylation at tyrosine 416 which is the activation site, in the NMuMG-Msx2 cells (Fig. 3d). To verify the c-Src kinase activation, the phosphorylation of one of its substrates, FAK (Focal Adhesion Kinase) at tyrosine 576/577, was examined by western blot analysis. Furthermore, downstream components of the c-Src pathway such as Akt and MAPK were also activated through phosphorylation in the NMuMG-Msx2 cells (Fig. 3d). Thus, Cripto-1 and the activation of c-Src kinase/Nodal independent pathway are affected by Msx2 overexpression.
FIGURE 3. Involvement of Cripto-1/c-Src Kinase - Nodal independent pathway in Msx2-induced EMT.
(a). Expression levels of TGF-β1, TGF-β3, TGF-β-Receptor II, Twist, Snail, Slug and Cripto were detected by RT-PCR in NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) total RNA preparations. (b) Cripto-1 expression level was detected in NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) cell lysates by western blot analysis and by immunocytochemistry on fixed NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) cells. Nuclei were stained with DAPI. (c) Nodal expression level was detected by RT-PCR in NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) total RNA preparations. The PCR positive control for Nodal (ES) was performed using total RNA extracted from mouse embryonic stem cells. (d) The active c-Src kinase pathway was determined by western blot analysis of phosphorylated c-Src, FAK, Akt and Erk using specific antibodies. Total c-Src, FAK, Akt and Erk were also determined by western blot.
Msx2 expression can promote invasion by NMuMG cells
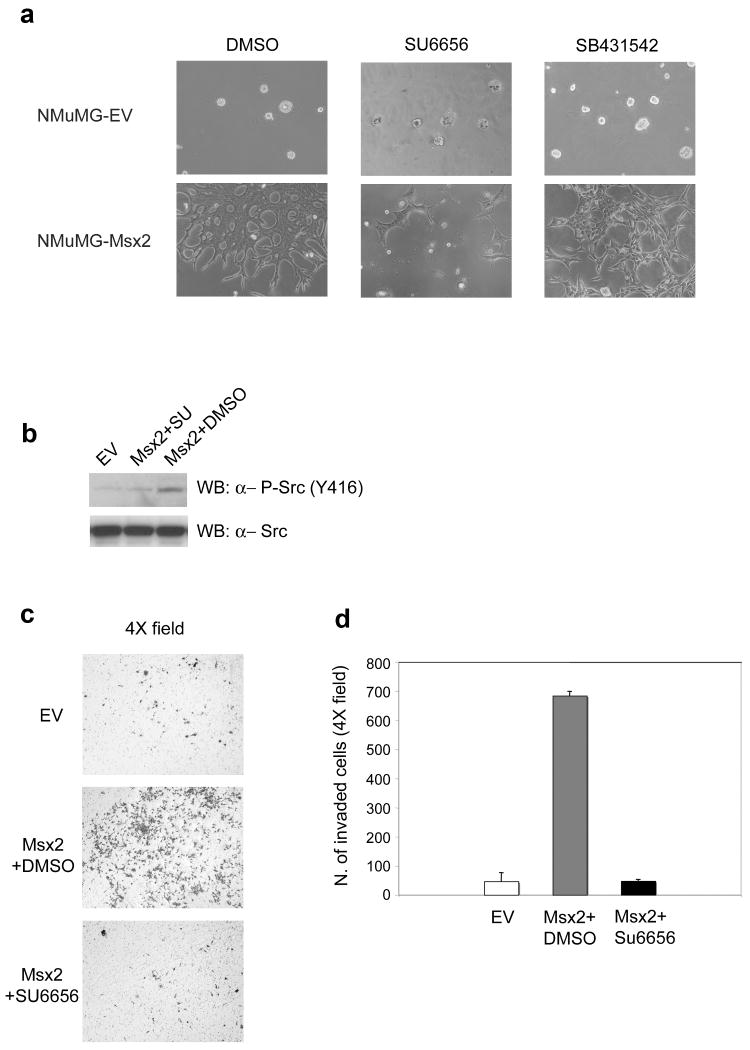
To assess whether the Msx2-overexpressing cells acquire greater invasive ability, we performed 3D Matrigel cultures of NMuMG-EV and NMuMG-Msx2 cells. Representative photomicrographs were taken 1 day (not shown) and 7 days (Fig. 4a) after the cells were plated on a Matrigel layer. No invasive potential was observed in NMuMG-EV cells during the entire observation period: they formed regular colonies without any cells invading the Matrigel layer. In contrast, the NMuMG-Msx2 colonies exhibited several lamellipodia-like structures that began to penetrate the Matrigel layer as early as after 1 day. After 7 days, most of the NMuMG-Msx2 cells had invaded the Matrigel layer and were growing on the bottom of the well (Fig. 4a). Interestingly, the presence of the c-Src inhibitor SU6656 greatly reduced the invasive behavior of the NMuMG-Msx2 after 7 days of Matrigel culture (Fig. 4a). Western blot analysis also demonstrated the inhibition of c-Src phosphorylation on tyrosine 416 in the presence of the inhibitor SU6656 (Fig. 4b). However, no difference in the invasive behavior of the NMuMG-Msx2 cells was observed in the presence of the ALK 4/5/7 inhibitor SB-431542 (Fig. 4a). This result confirms that the Nodal/ALK 4/5/7 pathway was not involved in the Msx2-induced EMT but that c-Src activation was necessary.
FIGURE 4. Msx2 expression causes NMuMG invasive ability in a c-Src kinase activity dependent manner.
(a) Photomicrographs of 3D Matrigel cultures of NMuMG-EV and NMuMG-Msx2 cells treated with DMSO as control and with c-Src inhibitor SU6656 (10μM) or with Alk4/5/7 inhibitor SB-431542 (10μM). (b) Western blot analysis using the phospho-specific antibody p-Src (Y416) on total cell lysates extracted from NMuMG-EV (EV) and NMuMG-Msx2 (Msx2) cells treated for 6h with c-Src inhibitor SU6656 (10μM) or with DMSO as control. (c) Photomicrographs of cells that invaded through the Matrigel invasion chamber. The cells were fixed and stained with crystal violet. Magnification at 4×. (d) Histogram represents the number of cells that invaded through the Matrigel invasion chamber, counted after fixation and crystal violet staining.
To quantify the invasive capacity of of NMuMG-Msx2 cells, we utilized the Boyden chamber invasion assay. NMuMG-EV and NMuMG-Msx2 cells were plated on Matrigel coated invasion chambers in the presence of DMSO (vehicle control) or the c-Src inhibitor SU6656. After 24 h, the cells were able to penetrate and traverse the Matrigel coat and were attached on the lower surface of the filter. The stained cells (Fig. 4c) were counted and showed that NMuMG-Msx2 cells have a significantly greater invasive potential than the NMuMG-EV cells (Fig. 4d). Moreover, the presence of the c-Src inhibitor SU6656 resulted in the complete suppression of the invasive phenotype displayed by the NMuMG-Msx2 cells (Fig. 4d). These results suggest that Msx2 overexpression can confer an invasive potential on the epithelial NMuMG cells through c-Src kinase activation which is independent of ALK pathway activation.
Inhibition of Cripto-1 expression or c-Src signaling reverts the expression of EMT markers
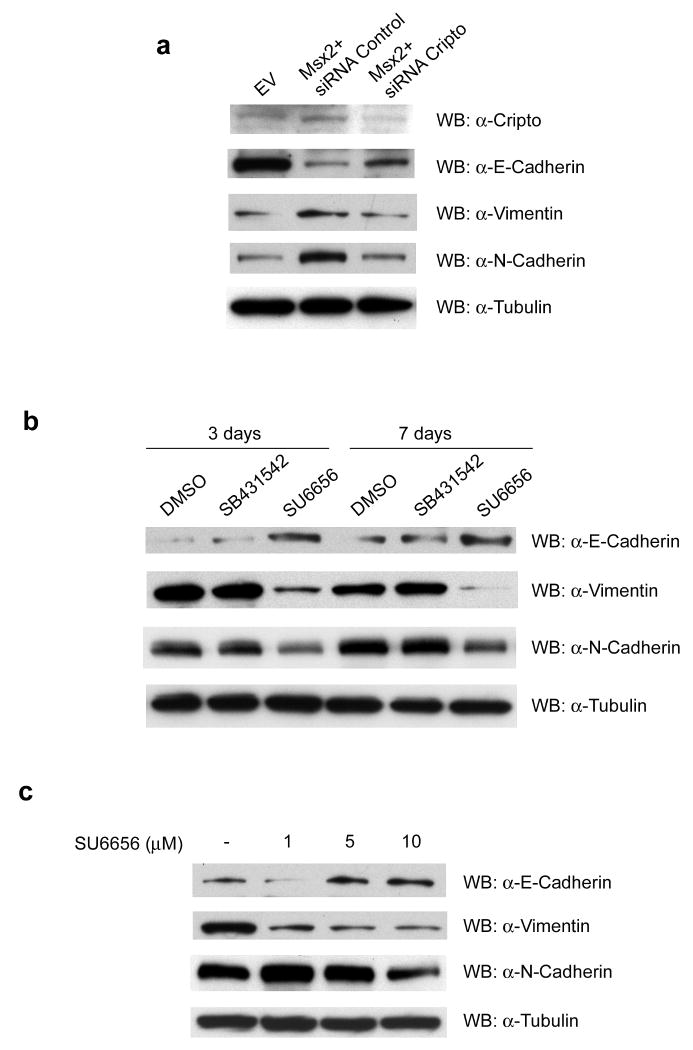
To further investigate the role of Cripto-1 in the Msx2-induced EMT in NMuMG cells, expression of Cripto-1 was knocked down using siRNA. NMuMG cells overexpressing Msx2 were transiently transfected with siRNA for Cripto-1 and 48h later, cell lysates were prepared and western blot analyses were performed. As depicted in Fig. 5a, the reduced expression of Cripto-1 in the NMuMG-Msx2 cells triggered the upregulation of the epithelial marker E-Cadherin. In contrast, the mesenchymal markers Vimentin and N-Cadherin were downregulated when compared to the same cells transfected with a non-targeting siRNA control.
FIGURE 5. Reduction of Cripto expression or incubation with c-Src inhibitor SU6656 are able to revert Msx2-induced EMT in NMuMG cells overexpressing Msx2.
(a) NMuMG-Msx2 were transiently transfected with siRNA for a non-target Control (Msx2+siRNA Control) or with siRNA for m-Cripto-1 (Msx2+siRNA Cripto): 48h post- transfection, cell lysates were prepared and western blot analysis were performed for the EMT markers E-Cadherin, Vimentin and N-Cadherin. (b) NMuMG-Msx2 cells were incubated in culture with DMSO (vehicle control), Alk4/5/7 inhibitor SB-431542 (10μM) or c-Src inhibitor SU6656 (10μM). After 3 or 7 days of incubation, cell lysates were prepared and western blot analysis were performed for EMT markers E-Cadherin, Vimentin and N-Cadherin. (c) NMuMG-Msx2 cells were incubated in culture with different concentrations of c-Src inhibitor SU6656 (1μM, 5μM and 10μM). After 4 days of incubation, cell lysates were prepared and western blot analyses were performed for the EMT markers E-Cadherin, Vimentin and N-Cadherin.
A similar trend of EMT markers expression was observed in NMuMG-Msx2 cells treated in culture with the c-Src specific inhibitor SU6656. Incubation with SU6656 (10 μM) induced the upregulation of the epithelial marker E-Cadherin and dowregulated the mesenchymal markers Vimentin and N-Cadherin (Fig. 5b). This reverting trend of EMT marker expression was not observed when NMuMG-Msx2 cells were treated with the ALK 4/5/7 inhibitor SB-431542 (10 μM) (Fig. 5b).
Moreover, the inverse expression of EMT markers induced by the c-Src inhibitor SU6656 was shown to be dose-dependent when NMuMG-Msx2 cells were treated in culture with 3 different doses of SU6656 (1, 5 and 10μM). As shown in Fig. 5c, only the Vimentin expression was affected with a dose as low as 1μM while higher doses of SU6656 were required to affect the expression of E-cadherin and N-cadherin.
These results further confirm the involvement of Cripto-1 expression and of the c-Src pathway in Msx2-induced EMT in NMuMG cells. The silencing of Cripto-1 expression and the inhibition of the Src pathway reverted the mesenchymal-like status of NMuMG-Msx2 cells to a more epithelial-like status.
Msx2 expression is observed in human infiltrating breast carcinoma versus non-infiltrating breast carcinoma
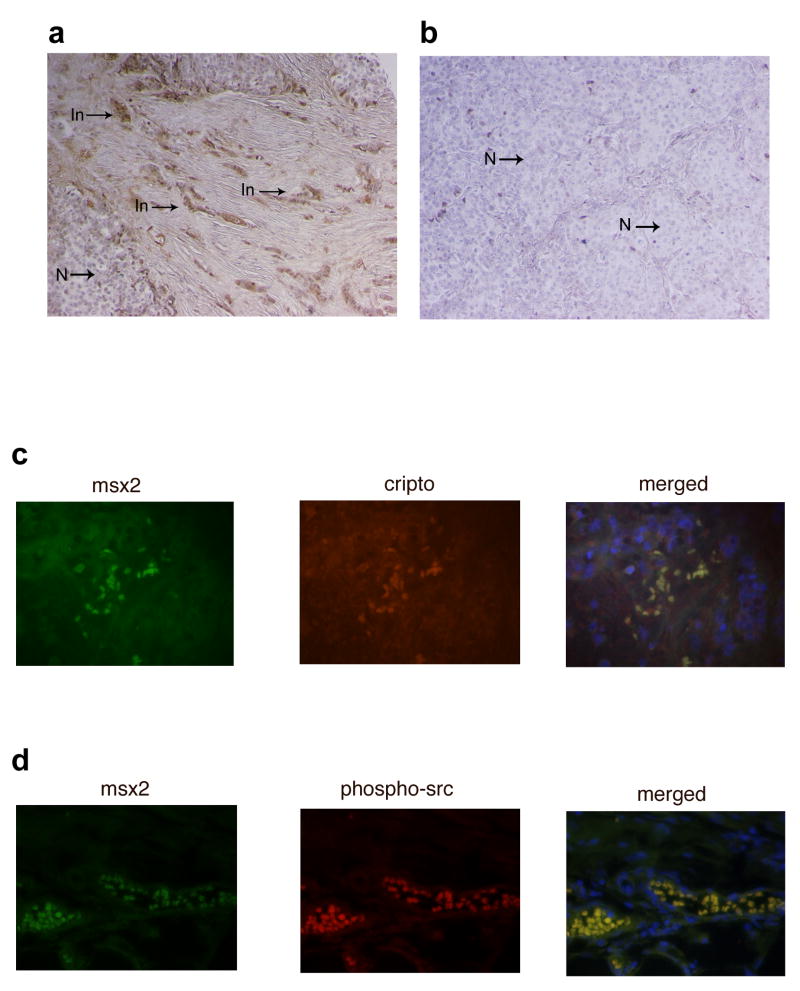
Sections of human breast cancer tissue were immunostained with Msx2 antibody. Interestingly, Msx2 expression was observed in sections from infiltrating breast carcinomas and was nearly undetected in sections from non-infiltrating breast carcinomas (Fig. 6a and b). In particular, sections from infiltrating breast carcinomas (in which the adjacent mesenchyme was also present) showed that the tumor cells that invaded into the adjacent mesenchyme strongly expressed Msx2 compared to the tumor cells that did not invade (Fig. 6a).
FIGURE 6. Msx2 is expressed only in the invasive tumor cells in human infiltrating breast carcinoma.
Immunohistochemical analysis for expression of Msx2 in human breast cancer tissue reveals (a) the overexpression of Msx2 only in the infiltrating tumor cells (In) indicated by the arrows versus the non-infiltrating tumor cells (N) in a human infiltrating breast carcinoma. (b) Expression of Msx2 was not detected in human non-infiltrating breast tumor cells (N). (c) Fluorescent immunohistochemistry to detect colocalization of Msx2 and Cripto-1 in human breast cancer tissue. (d) Fluorescent immunohistochemistry to detect colocalization of Msx2 and phospho-Src in human breast cancer tissue. These pictures are representative of four different infiltrating breast carcinomas.
In addition, the expression of Msx2 in human infiltrating breast carcinomas was accompanied by the expression of Cripto-1 and also by the phosphorylation of c-Src. By fluorescent immunohistochemistry on sections of human infiltrating breast carcinomas, Msx2 expression was colocalized with Cripto-1 (Fig. 6c) and also with the phosphorylated form of c-Src (Fig. 6d).
These data suggest a potential involvement of Msx2, together with Cripto-1 and the c-Src pathway, during the infiltration process occurring in human breast carcinoma, possibly by triggering EMT.
Discussion
The EMT process was originally identified during specific stages of embryonic development in which cells migrate and colonize to different embryonic territories (Nieto, 2001). In recent years, EMT was found to be an important event also during malignant tumor progression and metastasis (Thiery, 2002). EMT involves a series of events occurring in epithelial cells in which cell-cell and cell-extracellular matrix interactions are altered. In this respect, the cytoskeleton is reorganized to confer an ability to move, and a mesenchymal phenotype is gained. In normal epithelial tissue, the cells are bound to each other through tight intercellular junctions. The most important are the adherens junctions composed by the transmembrane molecule E-cadherin which binds to different catenins (β–, γ – and α–catenin) forming a bridge for connecting these to actin (Tsukita et al., 1992). EMT is characterized by the loss of intercellular contact, which is evidenced by a decrease in E-cadherin expression and disassembly of the adherens junction complex. This leads to a change in the subcellular localization of β–catenin. The morphology of the epithelial cells becomes more mesenchymal-like as the cells exhibit an increase in motility and invasiveness and mesenchymal markers such as vimentin and N-cadherin are upregulated (Lee et al., 2006).
The presence of the homeodomain protein Msx2 helps drive Bmp4-induced EMT in pancreatic cancer cell lines (Hamada et al., 2007) while the homeodomain protein HOXB7 can induce EMT in a immortalized normal mammary epithelial cell line (Wu et al., 2006). Additionally, Msx1 and Msx2 have been shown to be involved in the EMT occurring in endocardial cushion and cardiac valve development (Chen et al., 2008). In our study we demonstrate, for the first time that a normal mammary epithelial cell line that overexpresses Msx2 shows morphological and biochemical characteristics of EMT. Msx2-overexpressing cells presented a typical elongated and mesenchymal-like shape, accompanied by a decreased expression of the epithelial marker E-cadherin and a corresponding increase in the expression of mesenchymal markers, vimentin and N-cadherin. In addition, increased nuclear expression of β–catenin was detected suggesting relocalization of β–catenin to the nucleus, a common feature of aggressive cancer cells, including breast cancer cells (Nakopoulou et al., 2006).
The changes in cellular morphology and the disruption of cell-cell contacts in the Msx2 transfected cells conferred mobility and invasive ability. Our results demonstrated that NMuMG-Msx2 presented a greater invasive behavior compared to the NMuMG-EV across Matrigel-coated membranes. All of these data suggest that Msx2 may play a role in promoting EMT.
It is well documented that many growth factors such as TGF-β, HGF, VEGF, EGF and FGF, acting through tyrosine kinase receptors, induce EMT during embryogenesis but also during tumorigenesis (Kalluri and Neilson, 2003; Moustakas and Heldin, 2007; Thiery, 2002). Cripto-1, a member of the epithelial growth factor (EGF)-CFC family implicated in embryogenesis and in carcinogenesis, is yet another growth factor involved in EMT-induction (Salomon et al., 1999; Strizzi et al., 2004). Cripto-1 is highly expressed during early vertebrate development and expressed at very low levels in adult tissues. Cripto-1 also enhances migration, invasion and EMT in several mouse mammary epithelial cell lines (Bianco et al., 2005). In this study we found that Cripto-1 was overexpressed in the NMuMG cells transfected with Msx2, suggesting that Cripto-1 may be involved in the EMT occurring in those cells. However, the mechanism by which Msx2 induces the expression of Cripto-1 is still largely unknown. The Cripto-1 promoter (3 kilobases upstream of the translation start codon ATG) contains two potential Msx2-binding sites identified by the Genomatix Mat Inspector program suggesting that Msx2 may bind directly to the promoter thus activating transcription. It is also possible that Msx2 acts indirectly by interacting and inhibiting a negative regulator of Cripto-1 transcription bound to the promoter. These possibilities are currently under investigation.
To validate the hypothesis of a role for Cripto-1 in the Msx2-induced EMT, we looked at the activation of Cripto-1 downstream signaling molecules. A previous study demonstrated that c-Src Kinase is activated by Cripto-1 and that its activity is necessary for Cripto-1 dependent transformation and migration of mouse mammary epithelial cells through the downstream activation of MAPK/Akt (Bianco et al., 2003). The results from our study showed active phospho-c-Src and the downstream activation of FAK, Akt and MAPK in the NMuMG-Msx2 cells where Cripto-1 overexpression was detected. FAK is phosphorylated directly by c-Src in its specific sites in the NMuMG-Msx2 cells and its activation can contribute to initiate intracellular pathways leading to cell spreading (Burridge et al., 1992). Furthermore, increased FAK activity has been detected in mammary gland tumors overexpressing human Cripto-1 in a transgenic mouse model (Strizzi et al., 2004).
Additionally, another way in which Cripto-1 can work is through its binding as a co-receptor for Nodal, a TGF-β family member essential during embryogenesis, activating ALK4/7 signaling (Bianco et al., 2002). However, in the present study we found that the NMuMG-Msx2 cells lack Nodal expression confirming that the Cripto-1 pathway in these cells is Nodal-independent.
Further, our data support the crucial role of the activation of the Cripto-1/c-Src pathway in Msx2-induced EMT since the knockdown of Cripto-1 by siRNA or the presence of the c-Src inhibitor SU6656, which impairs c-Src activation, results in the reverted expression of the EMT markers in the NMuMG cells overexpressing Msx2. The NMuMG-Msx2 cells inhibited with either Cripto-1 si-RNA or treated with SU6656 showed an inverse relationship between signature EMT markers with a decrease in mesenchymal and an increase in epithelial protein expression. Moreover, inhibition of the c-Src pathway through the c-Src inhibitor SU6656 also caused loss of the NMuMG-Msx2 cells invasive behavior across Matrigel-coated membranes.
In contrast, the presence of the ALK4/5/7 inhibitor, SB-431542, did not interfere with the EMT markers expression nor with the invasive property of the NMuMG cells induced by Msx2 overexpression, thus confirming that the pathway Cripto-1/Nodal/ALK4/5/7 is not involved in Msx2-induced EMT.
It is widely accepted that the deregulation of the homeobox genes in adult tissues may contribute to carcinogenesis. Their principal function is essential for development of the organism during embryogenesis, but many examples of deregulated homeobox genes expressed in cancer define them also as “tumor modulators” (Abate-Shen, 2002). In particular, Msx2 plays a role in normal development of the mammary gland and overexpression of Msx2 was found in a variety of carcinoma cell lines of epithelial origin (Satoh et al., 2004).
In our study we detected an increase of Msx2 expression in human infiltrating breast carcinomas compared to non-infiltrating cancers. In particular, only the tumor cells that have infiltrated into the adjacent stroma strongly express Msx2 while the tumor cells located in a non-infiltrating region of the tumor demonstrate an absence or very low expression of Msx2. In those cells the expression of Msx2 was also accompanied by the expression of Cripto-1 and the phosphorylation of c-Src, confirming once again the involvement of the Cripto-1/c-Src pathway downstream from the expression of Msx2. These data suggest an involvement of Msx2 during the infiltration process: possibly Msx2 expression in the epithelial cancer cells contributes to the triggering of EMT, through the Cripto-1/c-Src pathway, and then to the invasiveness of the cancer cells themselves.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2(10):777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- Alappat S, Zhang ZY, Chen YP. Msx homeobox gene family and craniofacial development. Cell Res. 2003;13(6):429–442. doi: 10.1038/sj.cr.7290185. [DOI] [PubMed] [Google Scholar]
- Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol Cell Biol. 2002;22(8):2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. doi: 10.1016/S0070-2153(05)67003-2. [DOI] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63(6):1192–1197. [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Ishii M, Sucov HM, Maxson RE., Jr Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium. BMC Dev Biol. 2008;8(1):75. doi: 10.1186/1471-213X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283(29):20505–20522. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6(1):1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Y, Daniel CW. Regulated expression of homeobox genes Msx-1 and Msx-2 in mouse mammary gland development suggests a role in hormone action and epithelial-stromal interactions. Dev Biol. 1996;177(1):347–355. doi: 10.1006/dbio.1996.0168. [DOI] [PubMed] [Google Scholar]
- Fuchs IB, Lichtenegger W, Buehler H, Henrich W, Stein H, Kleine-Tebbe A, Schaller G. The prognostic significance of epithelial-mesenchymal transition in breast cancer. Anticancer Res. 2002;22(6A):3415–3419. [PubMed] [Google Scholar]
- Hamada S, Satoh K, Hirota M, Kimura K, Kanno A, Masamune A, Shimosegawa T. Bone morphogenetic protein 4 induces epithelial-mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J Cell Physiol. 2007;213(3):768–774. doi: 10.1002/jcp.21148. [DOI] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233(3):706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakopoulou L, Mylona E, Papadaki I, Kavantzas N, Giannopoulou I, Markaki S, Keramopoulos A. Study of phospho-beta-catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod Pathol. 2006;19(4):556–563. doi: 10.1038/modpathol.3800562. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The early steps of neural crest development. Mech Dev. 2001;105(1-2):27–35. doi: 10.1016/s0925-4773(01)00394-x. [DOI] [PubMed] [Google Scholar]
- Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, Woo I, Roberts-Clark D, Francis-West PH, Liu YH, Maxson R, Hill RE, Dale TC. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122(9):2729–2737. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- Ramos C, Robert B. msh/Msx gene family in neural development. Trends Genet. 2005;21(11):624–632. doi: 10.1016/j.tig.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Salomon DS, Bianco C, De Santis M. Cripto: a novel epidermal growth factor (EGF)-related peptide in mammary gland development and neoplasia. Bioessays. 1999;21(1):61–70. doi: 10.1002/(SICI)1521-1878(199901)21:1<61::AID-BIES8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Satoh K, Ginsburg E, Vonderhaar BK. Msx-1 and Msx-2 in mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9(2):195–205. doi: 10.1023/B:JOMG.0000037162.84758.b5. [DOI] [PubMed] [Google Scholar]
- Satoh K, Hovey RC, Malewski T, Warri A, Goldhar AS, Ginsburg E, Saito K, Lydon JP, Vonderhaar BK. Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene. 2007;26(54):7526–7534. doi: 10.1038/sj.onc.1210555. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201(2):266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4(5):834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, Wallace-Jones B, Montesano R, Salomon DS. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp Cell Res. 2001;266(1):95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B, Normanno N, Adkins H, Sanicola M, Salomon DS. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24(25):4094–4105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen H, Parker B, Rubin E, Zhu T, Lee JS, Argani P, Sukumar S. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66(19):9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- Xue C, Plieth D, Venkov C, Xu C, Neilson EG. The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 2003;63(12):3386–3394. [PubMed] [Google Scholar]