Abstract
We investigated the effect of joint immobilization on the postural sway during quiet standing. We hypothesized that center of pressure (COP), rambling, and trembling trajectories could be affected by joint immobilization. Ten young adults stood on a force plate during 60 s without and with immobilized joints (only knees constrained, CK; knees and hips, CH; and knees, hips and trunk, CT), with their eyes opened (EO) or closed (EC). The root mean square deviation (RMS, the standard deviation from the mean) and mean speed of COP, rambling, and trembling trajectories in the anterior-posterior and medial-lateral directions were analyzed. Similar effects of vision were observed for both directions: larger amplitude for all variables was observed in the EC condition. In the anterior-posterior direction, postural sway increased only when the knees, hips and trunk were immobilized. For the medial-lateral direction, the RMS and the mean speed of the COP, rambling, and trembling displacements decreased after immobilization of knees and hips and knees, hips and trunk. These findings indicate that the inverted pendulum model is unable to completely explain the processes involved in the control of the quiet upright stance in the anterior-posterior and medial-lateral directions.
Keywords: posture, quiet stance, inverted pendulum, joint constraint, postural control
1. Introduction
During upright quiet standing the body has often been modeled as a single inverted pendulum, pivoting about the ankle joints (Gage, Winter, Frank, & Adkin, 2004; Winter, Patla, Prince, Ishac, & Gielo-Perczak, 1998). The premise of the inverted pendulum model is that the body above the ankles behaves as a rigid structure. The use of the inverted pendulum model to represent balance maintenance in quiet standing has been supported by kinematic, kinetic (Gage, Winter, Frank, & Adkin, 2004; Karlsson & Persson, 1997), and EMG (Gatev, Thomas, Kepple, & Hallett, 1999) measures in both sagittal and frontal planes (Winter, Prince, Frank, Powell, & Zabjek, 1996). However, most of the kinematic studies have shown small angular displacement at joints above the ankle (Gage, Winter, Frank, & Adkin, 2004; Gatev, Thomas, Kepple, & Hallett, 1999). For example, Gage and collaborators (Gage, Winter, Frank, & Adkin, 2004) reported that knee joint movements were larger as compared to ankle joint displacement in quiet standing but they still considered the inverted pendulum model a good representation of quiet standing in humans. Conversely, it has also been claimed that small angular displacements at joints above the ankles play an important role in the control of quiet upright stance and its contribution should not be ignored (Aramaki, Nozaki, Masani, Sato, Nakazawa, & Yano, 2001; Bardy, Marin, Stoffregen, & Bootsma, 1999; Creath, Kiemel, Horak, Peterka, & Jeka, 2005; Day, Steiger, Thompson, & Marsden, 1993; Fitzpatrick, Rogers, & McCloskey, 1994; Krishnamoorthy, Yang, & Scholz, 2005; Nicholas, Doxey-Gasway, & Paloski, 1998).
Recently, Hsu and collaborators (Hsu, Scholz, Schoner, Jeka, & Kiemel, 2007) and Krishnamoorthy and colleagues (Krishnamoorthy, Yang, & Scholz, 2005) reported that stabilization of a reference position (assumed to be described by the position of the center of mass, COM) was achieved by coordination of several joints along the human “pendulum” when participants quietly stood on a normal and on a narrow base of support. Similar findings were observed when sudden perturbations were applied to the participants in quiet stance (Alexandrov, Frolov, Horak, Carlson-Kuhta, & Park, 2005), when supra-postural task were performed in quiet standing (Bardy, Marin, Stoffregen, & Bootsma, 1999; Bardy, Oullier, Bootsma, & Stoffregen, 2002; Bardy, Oullier, Lagarde, & Stoffregen, 2007), during fast-forward voluntary trunk bending movements (Alexandrov, Frolov, & Massion, 2001), and during whole-body voluntary movements (Freitas, Duarte, & Latash, 2006). In addition, Kuo and Zajac (Kuo & Zajac, 1993) found that the “ankle strategy” involving rotation about the ankles only (Nashner, 1981) requires more muscle activation than the “hip strategy” (two-segment inverted pendulum pivoting about the hip and ankle joints) for a given amount of horizontal acceleration. Hence, the hip strategy is more effective at controlling the COM position with minimal muscle activation when comparing to the “ankle strategy”.
Another approach to investigate the adequacy of the single pendulum model to describe the control of the upright stance and, concurrently, the role played by the joints above the ankle in this control is to prevent the movement of those joints while maintaining a quiet stance. To date, only two studies investigated the effect of preventing movements of the joints above the ankle on postural control (Aramaki et al., 2001; Fitzpatrick, Rogers, & McCloskey, 1994), and only Fitzpatrick and colleagues (1994) measured the amount of postural sway in the anterior-posterior direction after joint immobilization, whereas the effect of joint immobilization on medial-lateral direction was not assessed. They observed that when a rigid splint prevented the movement of all joints above the ankles, the magnitude of the ankle angular sway increased significantly.
The most common measure of postural sway in quiet standing is the center of pressure (COP) migration derived from force plate data. COP is the point of application of the resultant of vertical forces acting on the surface of support (Duarte & Zatsiorsky, 2002). Several methods have been proposed to decompose the COP displacement, commonly called stabilogram, with the intention to understand the mechanisms of postural control during quiet standing (Baratto, Morasso, Re, & Spada, 2002; Bottaro, Casadio, Morasso, & Sanguineti, 2005; Collins & De Luca, 1993; Dijkstra, 2000; Frank, Daffertshofer, & Beek, 2000; Winter, Patla, Prince, Ishac, & Gielo-Perczak, 1998; Zatsiorsky & Duarte, 1999, 2000). Zatsiorsky & Duarte (1999) proposed a method to decompose the COP trajectory based on the idea that the body sways due to two reasons: (a) migration of the reference point with respect to which the equilibrium is instantly maintained (rambling) and (b) oscillation of the body with respect to the moving equilibrium reference (trembling). They found that the trembling migration highly (and negatively) correlates with the horizontal ground reaction force while such a correlation does not exist for the rambling. These data were interpreted as evidence that the trembling represents a deviation from the instant equilibrium position corrected via the horizontal ground reaction forces inducing the center of mass acceleration in the direction opposite to the deviation while the central controller does not correct the COP migrations associated with the rambling. According to the rambling and trembling hypothesis, the rambling and trembling reflect two distinct processes contributing to the control of body sway. The rambling trajectory may be related to supraspinal processes that are involved in the control of the migration of the instant reference point. The trembling may be a result of both, the action of spinal reflexes and changes in the intrinsic mechanical properties of the muscles and joints. In particular, the authors suggested that the trembling was due to the moment at the ankle joints during quiet standing.
The analysis of the trajectories of the COP and its components during quiet standing while the joints above the ankle are immobilized could provide additional information regarding the effectiveness of the single pendulum model and also the role played by the above-the-ankle joints in the posture control. Therefore, the purpose of this study was to observe the contribution of the main joints (ankle, knee, hip, and joints of the trunk) to the control of quiet upright stance. Specifically, the current study investigated the COP, rambling and trembling behaviors when the knees, hips, and lower and medium portion of the trunk were constrained and the body balance had to be controlled exclusively by the non-constrained joints, especially by the ankle joints. We hypothesized that if the body behaves as a single inverted pendulum, the joint constraints will have a minor or no effect on the COP, rambling and trembling variables, whereas, significant changes in the COP, rambling, and, principally, in the trembling would indicate that the inverted pendulum model can not accurately explain the processes involved in the control of the quiet upright stance.
Because the hip abductors/adductors, in addition to the ankle invertors/evertors, have been considered responsible by controlling the medial-lateral movements (Winter, Prince, Frank, Powell, & Zabjek, 1996), it was expected that the hip joint constraints would also affect the body sway in the frontal plane as well. Specifically, we predicted an increase in postural sway in medial-lateral direction mainly when hip and trunk were immobilized. In addition, this study investigated the combined effect of immobilization and vision on the sway in both directions (sagittal and frontal); the subjects stood with their eyes open or closed.
2. Method
2.1. Participants
Ten healthy adults, 5 males and 5 females, without any known neurological and musculoskeletal disorders, as well as balance problems, participated in this study. The participants’ mean (±1 SD) age, body mass, and height were 28.3 (±4.6) years, 66.7 (±4.6) kg, and 169 (± 9) cm, respectively. Prior to testing the subjects signed the informed consent form in accordance with the policies established by the Office for Regulatory Compliance of The Pennsylvania State University.
2.2. Procedures
The participants were instructed to stay upright in bipedal stance as still as possible on a 40×80 cm force platform (model 4080S, Bertec, Worthington, OH, USA). They stood barefoot with their feet positioned side-by-side at a comfortable width (about shoulder width), and arms placed in a relaxed position at the body’s sides. Also, they were instructed to maintain the head erect and to look at a circular target (radius = 2.5 cm) positioned approximately 120 cm in front and at their eyes level.
During the experiment the participants were submitted to four joint immobilization conditions: no constraint (NC), constraint of the knees (CK), constraint of the hips and knees (CH), and constraint of the trunk, hips and knees (CT) and two visual conditions, open and closed eyes (OE and CE, respectively). Figure 1 depicts the different levels of immobilization. The joint constraints were applied for each limb separately.
Figure 1.
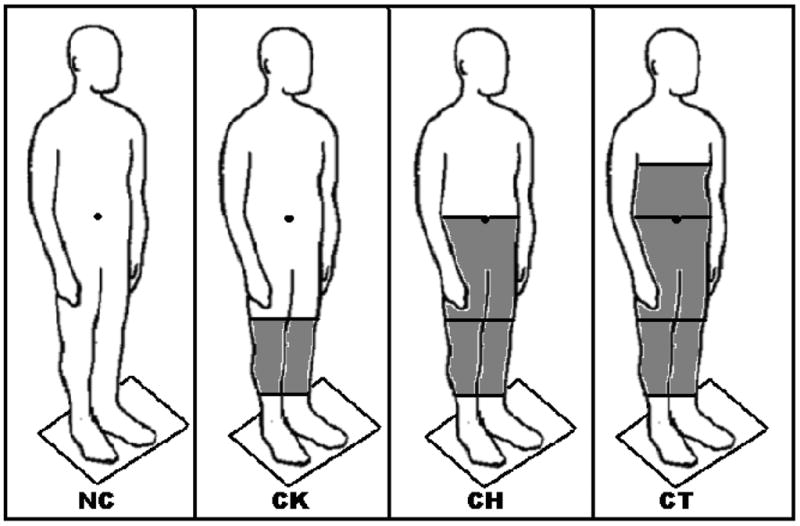
Schematic representation of the different immobilization conditions. No Constraint (NC), Constraint of the knee joints (CK), Constraint of the knee and hip joints (CH), and Constraint of the knees, hips, and trunk (CT).
The joints were tightly immobilized by splints, casts and duct tape. The knees constraints started from the ⅔ superior part of the shank and ended at the ⅔ inferior part of the thigh, being each knee immobilized separately. Four splints, one on anterior, one on posterior, one on medial, and one on lateral sides, were placed vertically around each knee. After the splints were positioned, cast tapes were placed to fix the splints and, finally duct tape was placed around each knee to increase the resistance and prevent any knee’s flexion-extension movement. The hip was immobilized from ⅓ of the proximal part of the thigh to ⅓ of the inferior part of the trunk at the navel level. In the same way as the knee constraints, splints were placed around the thighs, pelvis and the inferior portion of the trunk; fixed by cast tapes and reinforced by duct tape. Finally, the trunk was immobilized from the navel line to the sternum xiphoid process, also using splints, casts and duct tape. The immobilizations assured that joint movements around knees, hip and trunk were completely restricted.
Six trials were performed in each immobilization condition, being three trials with eyes open and three with eyes closed. The order in which each visual condition was presented in each trial was randomized across participants: a trial with open (close) eyes always followed by a trial with eyes closed (open). During each trial the subjects stood quietly for 60 s. Five participants started the experiment with the trunk, hip and knee immobilized (CTOE and CTCE condition) and had the immobilization hardware removed in a top-to-down sequence (CT – NC). The other five participants started without any constraint; the immobilization was applied in a down-to-top sequence (NC – CT). This procedure was used to avoid fatigue effects in body sway.
2.3. Data Acquisition and Analysis
Data were recorded using a PC with a 12-bit A/D board (model AT-MIO-64E-3, National Instruments Corporation, Dallas, TX, USA) controlled by a special code written in LabView software (LabView 6.1; National Instruments, Dallas, TX, USA). The force platform used during the experiment provided information about the three forces and three moment components. The signals were used to calculate the center of pressure (COP) displacement in the anterior-posterior (COPAP) and medial-lateral direction (COPML). The data were recorded with a sampling frequency of 100 Hz. The data analysis was performed using MATLAB software (MATLAB 6.5, The MathWorks, Natick, MA, USA). Data from horizontal forces (Fy and Fx) and COP (COPAP and COPML) were low-pass filtered with a zero-lag, fourth-order Butterworth filter with the cutoff frequency of 8 Hz.
Rambling and trembling were determined using the procedures described previously by Zatsiorsky and Duarte (Zatsiorsky & Duarte, 1999). A brief description of the analyses is provided below. At the instances when the horizontal force (Fhor) is zero (Fhor=0) the body is instantaneously in at equilibrium state (Zatsiorsky & King, 1998). The instances when Fhor changed its sign were determined using local linear interpolation of the Fhor time-series. Then, the instant equilibrium points (IEP, i.e. the COP locations at the instances when the horizontal forces were zero) were identified. After this procedure, the IEPs were used to compute the rambling and trembling trajectories. The IEPs, rambling and trembling were separately determined from COPAP and COPML time-series.
The rambling trajectory was established by interpolating the discrete IEPs with a cubic spline function. According to the rambling-trembling hypothesis, the rambling represents migration of the reference point on the supporting surface with respect to which the body equilibrium is maintained. The trembling represents the deviation of the COP from the equilibrium position and it was determined by subtracting the rambling trajectory from the COP trajectory [for more details see (Zatsiorsky & Duarte, 1999, 2000)]. Figure 2 depicts an example of the Fhor trajectory, the IEPs, COP, rambling and trembling for one trial in NCOE condition (anterior-posterior direction, 20 seconds).
Figure 2.
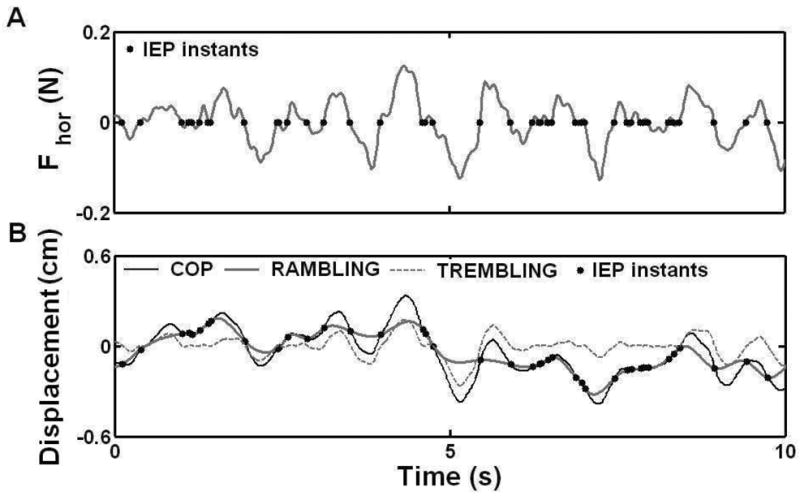
Sequence of operations used for COP trajectory decomposition. In A, the horizontal force and the instances when Fhor=0 (•) are presented. The COP displacement, the COP position at the IEP instants (•), rambling trajectory (estimated by the cubic spline interpolation of the COP position at the IEP instants), and trembling trajectory (the difference between COP and interpolated rambling trajectories) are presented in B. Subject SS, female, age 29; Ht 1.61 m; Wt 52 Kg.
The dependent variables analyzed in this study were root mean square deviation (RMS, the standard deviation from the mean) and mean speed of the COP, rambling, and trembling displacement in the anterior-posterior (AP) and medial-lateral (ML) directions. Before calculating these variables, the very low frequencies were removed from the COP, rambling and trembling time-series by subtracting their own best fit line (linear detrend) from their respective time-series. The RMS in both directions was determined by calculating the standard deviation of each signal, i.e. the oscillations of the signals around their mean values. The mean speed was determined by dividing the total distance along the signal trajectory by the total time of recording.
2.4. Statistical Analysis
Before performing the statistical analyses, we normalized, for each individual subject, the values of RMS and mean speed of COP, rambling and trembling in percentage of their respective values measured in NCOE condition. For each variable, we subtracted the value of each one of the immobilization condition by the value of the NCOE condition, divided the result by the value of the NCOE condition, and then multiplied by 100 [e.g., (((RMSCTOE − RMSNCOE)/RMSNCOE))*100) − NCOE= 0%]. The statistical analyses were performed by SPSS statistical package (SPSS version 10.1, Chicago, IL) using the normalized values averaged among trials for each subject for all dependent variables (RMS and mean speed of the COP, rambling, and trembling time-series). To test the effects of the joint immobilization (NC, CK, CH, CT) and vision (OE and CE) on the each dependent variable (RMS and mean speed of the COP, rambling, and trembling displacements) a total of four repeated measures two-way MANOVAs, two for AP and two for ML direction, were performed. When necessary, univariate analysis and post-hoc tests with Bonferroni corrections were carried out. The significance level was set at 0.05. However, marginal effects (p<.1) was also considered to further analysis.
3. Results
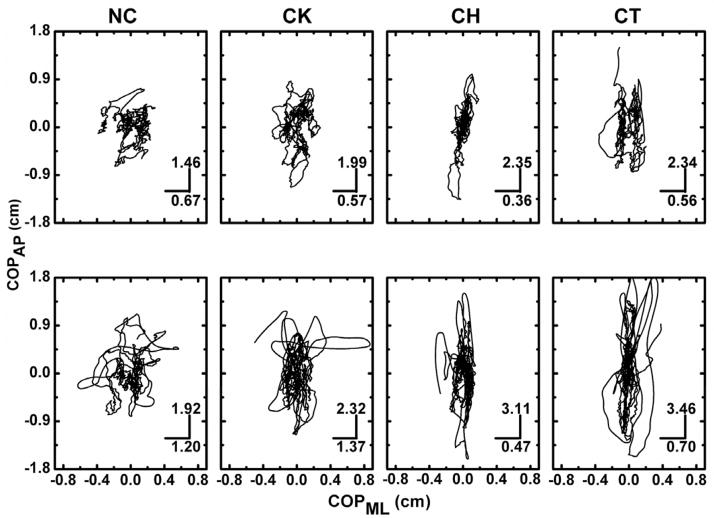
All subjects were able to stand when their joints were immobilized. However, changes in the postural sway due to joint immobilization were observed. Figure 3 illustrates a stabilogram with a typical COP trajectory in each joint immobilization and visual condition. As it can be seen in the figure 3, the COP displacements in the anterior-posterior (COPAP) direction and in the medial-lateral (COPML) direction were differently influenced by the joint immobilization. These results were observed for both visual conditions. Detailed statistical analyses of each variable of postural sway are presented below separately for each direction. Table 1 presents the non-normalized means and standard errors values in both directions.
Figure 3.
A representative example of the results of COP trajectory during all joints immobilization and visual conditions. Bars and numbers represent the standard deviation in the anterior-posterior (COPAP) and medial-lateral (COPML) directions. Subject SS, female, age 29; Ht 1.61 m; Wt 52 Kg.
Table 1.
Mean (±S.E.) of the COP, rambling, and trembling RMS and mean speed for four immobilizations and two visual conditions.
| Open Eyes |
Closed Eyes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | NC | CK | CH | CT | NC | CK | CH | CT | ||
| RMS (cm) | AP | COP | 0.286 ± 0.023 | 0.271 ± 0.021 | 0.282 ± 0.020 | 0.278 ± 0.017 | 0.368 ± 0.032 | 0.384 ± 0.037 | 0.396 ± 0.035 | 0.423 ± 0.031 |
| Rambling | 0.258 ± 0.021 | 0.239 ± 0.019 | 0.251 ± 0.019 | 0.246 ± 0.017 | 0.320 ± 0.030 | 0.322 ± 0.032 | 0.340 ± 0.035 | 0.353 ± 0.025 | ||
| Trembling | 0.076 ± 0.008 | 0.083 ± 0.009 | 0.085 ± 0.009 | 0.084 ± 0.007 | 0.114 ± 0.009 | 0.143 ± 0.016 | 0.134 ± 0.007 | 0.157 ± 0.015 | ||
| ML | COP | 0.132 ± 0.012 | 0.114 ± 0.012 | 0.082 ± 0.010 | 0.090 ± 0.014 | 0.164 ± 0.015 | 0.140 ± 0.015 | 0.101 ± 0.012 | 0.110 ± 0.016 | |
| Rambling | 0.115 ± 0.012 | 0.100 ± 0.012 | 0.071 ± 0.010 | 0.078 ± 0.014 | 0.140 ± 0.014 | 0.120 ± 0.015 | 0.088 ± 0.012 | 0.093 ± 0.016 | ||
| Trembling | 0.045 ± 0.003 | 0.041 ± 0.003 | 0.031 ± 0.003 | 0.032 ± 0.004 | 0.058 ± 0.004 | 0.052 ± 0.003 | 0.035 ± 0.004 | 0.042 ± 0.003 | ||
| Mean Speed (cm.s−1) | AP | COP | 0.484 ± 0.045 | 0.507 ± 0.041 | 0.527 ± 0.056 | 0.518 ± 0.060 | 0.656 ± 0.061 | 0.739 ± 0.062 | 0.713 ± 0.066 | 0.792 ± 0.068 |
| Rambling | 0.244 ± 0.014 | 0.349 ± 0.032 | 0.226 ± 0.025 | 0.243 ± 0.030 | 0.392 ± 0.020 | 0.285 ± 0.021 | 0.294 ± 0.029 | 0.327 ± 0.030 | ||
| Trembling | 0.426 ± 0.038 | 0.170 ± 0.020 | 0.416 ± 0.040 | 0.414 ± 0.045 | 0.187 ± 0.016 | 0.611 ± 0.049 | 0.580 ± 0.055 | 0.656 ± 0.066 | ||
| ML | COP | 0.275 ± 0.021 | 0.259 ± 0.025 | 0.234 ± 0.020 | 0.234 ± 0.021 | 0.318 ± 0.018 | 0.293 ± 0.024 | 0.251 ± 0.022 | ± 0.266 ± 0.019 | |
| Rambling | 0.172 ± 0.014 | 0.232 ± 0.019 | 0.157 ± 0.014 | 0.161 ± 0.015 | 0.291 ± 0.017 | 0.182 ± 0.020 | 0.163 ± 0.015 | 0.162 ± 0.015 | ||
| Trembling | 0.300 ± 0.030 | 0.423 ± 0.034 | 0.274 ± 0.028 | 0.266 ± 0.024 | 0.543 ± 0.052 | 0.317 ± 0.031 | 0.285 ± 0.026 | 0.291 ± 0.024 | ||
3.1. Anterior-Posterior direction
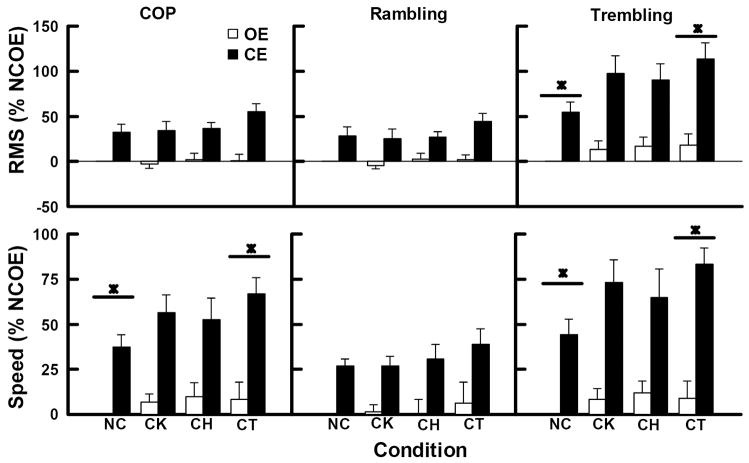
The results showed that postural sway in anterior-posterior (AP) direction was altered after immobilization of knee, hip, and lower and middle trunk joints. Figure 4 depicts the averaged normalized RMS and mean speed values of the COP (left-hand side), rambling (middle), and trembling (right-hand side) displacements in the AP direction.
Figure 4.
The RMS and Speed values of the COP, rambling and trembling in the anterior-posterior direction averaged across subjects. Values were normalized by the values of the NCOE condition (NCOE=0%). Error bars indicate the standard error of the means. * represents differences with p. < .05.
Effect of immobilization on the RMS
The AP RMS of the COP, rambling and trembling displacements averaged across joint immobilization and visual conditions are depicted in the upper panels of the figure 4. MANOVA revealed main effect of joint immobilization [Wilks’ Lambda=.477, F(9, 61)=2.4 p<.05,η2=.21], and vision [Wilks’ Lambda=.07, F(3,7)=31, p>.001, η2=.93], but revealed no interaction between joint immobilization and vision [Wilks’ Lambda=.634, F(9,61)=1.39, p>.05, η2=.14] on the RMS in AP direction. Univariate analyses revealed a main effect of joint immobilization on the RMS of the trembling displacement [F(3, 27)=4.29 p>.05, η2=.32], and a marginal main effect on the RMS of the COP [F(3, 27)=2.55 p=.076, η2=.22] and rambling [F(3, 27)=2.49 p=.082, η2=.22] displacements. Post hoc tests with appropriated Bonferroni corrections showed that the RMS of the trembling displacement was larger when the movement knee, hip and trunk was constrained (CT condition) as compared to when no constraint was imposed to the joints (NC condition). Although a marginal effect of joint immobilization on the RMS of the COP and rambling displacements were identified, no differences among joint immobilization conditions were observed. With respect to main effect of vision, univariate analysis indicated that the RMS of the COP [F(1,9)=56, p<.001, η2=.86], rambling [F(1,9)=27, p<.005, η2=.75], and trembling [F(1,9)=48 p<.001, η2=.84] displacements were larger when participants kept their eyes closed (CE) as compared to when participants kept their eyes open (OE).
Effect of immobilization on the mean speed
The AP mean speed of the COP, rambling and trembling displacements averaged across joint immobilization and visual conditions are depicted in the lower panels of the figure 4. For these variables, MANOVA revealed a marginal main effect of joint immobilization [Wilks’ Lambda=.572, F(9, 61)=1.75 p=.098, η2=.17], and a main effect of vision [Wilks’ Lambda=.09, F(3,7)=24, p>.001, η2=.91], but revealed no interaction between joint immobilization and vision [Wilks’ Lambda=.72, F(9,61)=.99, p>.05, η2=.10]. Univariate analyses revealed main effects of joint immobilization on mean speed of the COP [F(3, 27)=3.77 p=.022, η2=.29] and trembling [F(3, 27)=4.78 p=.008, η2=.35] displacements, but not on mean speed of the rambling [F(3, 27)=.88 p=.462, η2=.21] displacement. For both, COP and trembling, post hoc tests revealed that the mean speed of the COP and trembling displacements was larger in CT than in NC condition. Regarding main effect of vision, univariate analysis indicated that the main speed of the COP [F(1,9)=70, p<.001, η2=.89], rambling [F(1,9)=70, p<.001, η2=.89], and trembling [F(1,9)=56, p<.001, η2=.86] displacements was larger in CE than in OE condition.
3.2. Medial-Lateral direction
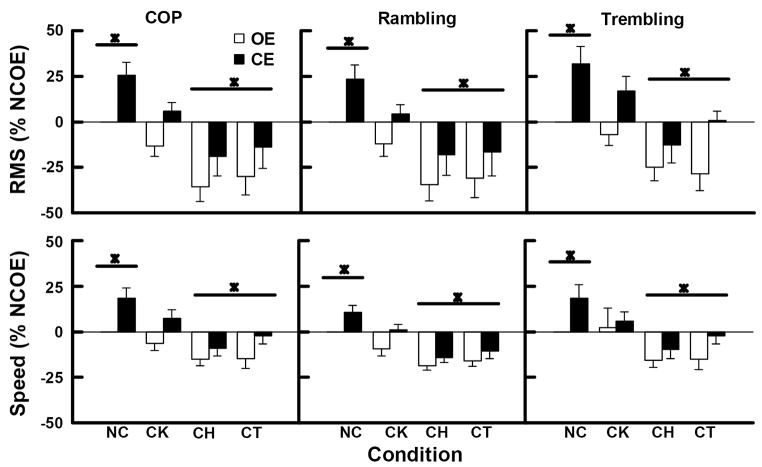
With respect to postural sway in medial-lateral (ML) direction, the results showed that when the participants had their knee and hip joints and their knees, hips and trunk immobilized their postural sway in ML direction was altered. Figure 5 depicts the averaged normalized RMS and mean speed values of the COP (left-hand side), rambling (middle), and trembling (right-hand side) displacements in the ML direction.
Figure 5.
The RMS and Speed values of the COP, rambling and trembling in the medial-lateral direction averaged across subjects. Values were normalized by the values of the NCOE condition (NCOE=0%). Error bars indicate the standard error of the means. * represents differences with p. < .05.
Effect of immobilization on the RMS
The ML RMS of the COP, rambling and trembling displacements averaged across immobilization and visual conditions are depicted in the upper panels of the figure 5. MANOVA revealed a significant effect of joint immobilization [Wilks’ Lambda=.346, F(9,61)=3.7, p<0.005, η2=.30], and vision [Wilks’ Lambda=.214, F(3,7)=8.55, p<.05, η2=.79] on RMS in ML direction. No interaction between joint immobilization and vision [Wilks’ Lambda=.713, F(9,61)=1, p>0.05, η2=.41] was observed. Univariate analysis revealed a significant effect of joint immobilization on the RMS of the COP [F(3, 27)=9.24 p<.001, η2=.51], rambling [F(3, 27)=7.02 p<.005, η2=.43], and trembling [F(3, 27)=10.6 p<.001, η2=.54] displacements. Post-hoc tests revealed that the RMS of COP, rambling and trembling displacements were smaller in CH and CT than in NC condition. Regarding vision, results revealed that RMS of COP [F(1,9)=23.73 p<.005, η2=.73], rambling [F(1,9)=17.2 p<.005, η2=.66], and trembling [F(1,9)=22.4 p<.005, η2=.71] were larger in CE than in OE condition.
Effect of immobilization on the mean speed
The ML mean speed of the COP, rambling and trembling displacements averaged across immobilization and visual conditions are depicted in the lower panels of the figure 5. MANOVA revealed significant effect of joint immobilization [Wilks’ Lambda=.35, F(9,61)=3.66, p<0.005, η2=.30], and vision [Wilks’ Lambda=.271, F(3,7)=6.27, p<.05, η2=.73], and no interaction between joint immobilization and vision [Wilks’ Lambda=.591, F(9,61)=1.63, p>0.05, η2=.16] on the mean speed. Univariate analysis revealed a significant effect of joint immobilization on the mean speed of the COP [F(3, 27)=10.4 p<.005, η2=.51], rambling [F(3, 27)=14.6 p<.001, η2=.43], and trembling [F(3, 27)=7.1 p<.05, η2=.54] displacements. Post-hoc tests revealed that the mean speed of the COP, rambling and trembling displacements were smaller in CH and CT than in NC condition. Regarding vision, results revealed that the mean speed of the COP [F(1,9)=24 p<.005, η2=.73], rambling [F(1,9)=16 p<.005, η2=.64], and trembling [F(1,9)=11 p<.01, η2=.55] were larger in CE than in OE condition.
4. Discussion
The results of the study (summarized in Table 2) showed, as expected, that postural sway was affected by the absence of visual information and, also by joint immobilization. In addition, the presence or absence of visual information affected similarly postural sway at each level of joint immobilization. Overall, independently of the direction (AP or ML), COP, rambling, and trembling displacements were larger and quicker when participants kept their eyes closed as compared to eyes open. Regarding joint immobilization, the results showed that it affects differently postural sway in the AP and ML directions. In the AP direction, COP and trembling displacements and speeds were larger than in the control condition (NC) only when the knees, hips and trunk were immobilized (CT). In contrast, in the ML direction, the COP, rambling and trembling displacements and speeds dramatically reduced after immobilization of the hip joints (CH and CT conditions). Due to the distinctive effect of joint immobilization in AP and ML directions, the results for each direction will be discussed separately.
Table 2.
Summary of the effects of joint immobilization on standing balance.
| Variables | Immob. level | COP | AP Ramb. | Tremb. | CoP | ML Ramb. | Tremb. |
|---|---|---|---|---|---|---|---|
| RMS | CK | = | = | = | = | = | = |
| CH | = | = | = | ↓ | ↓ | ↓ | |
| CT | = | = | ↑ | ↓ | ↓ | ↓ | |
| Mean Speed | CK | = | = | = | = | = | = |
| CH | = | = | = | ↓ | ↓ | ↓ | |
| CT | ↑ | = | ↑ | ↓ | ↓ | ↓ | |
Sign ↑ indicates that a given variable increased statistically significantly as compared with the condition without joint immobilization and open eyes (NCOE); sign ↓ indicates that the given variable decreased; sign = signifies no effect of joint immobilization.
Anterior-posterior direction
The available literature data on the mechanics of the body sway in the anterior-posterior direction are controversial. On the one hand, the results of some studies have indicated that the body sway is controlled mainly at the ankle joints without any significant participation of other joints or muscle groups in this control (Gage, Winter, Frank, & Adkin, 2004; Gatev, Thomas, Kepple, & Hallett, 1999; Karlsson & Persson, 1997; Winter, Patla, Prince, Ishac, & Gielo-Perczak, 1998). Considering the body above the ankles as a rigid structure, such studies have proposed the inverted pendulum model for describing the control during quiet standing. On the other hand, other studies have claimed that body sway is controlled by compensatory movements from several joints and not a single joint (Krishnamoorthy, Yang, & Scholz, 2005; Peterka, 2003). According to this view, all joints co-vary, i.e. act as a synergy, trying to control the COM displacement (Alexandrov, Frolov, & Massion, 2001; Creath, Kiemel, Horak, Peterka, & Jeka, 2005; Fitzpatrick, Rogers, & McCloskey, 1994; Freitas, Duarte, & Latash, 2006), and minimize the overall joint variance (Krishnamoorthy, Yang, & Scholz, 2005), which consequently, could reduce the muscle activity in a specific joint (for example, the ankle joint) (Kuo & Zajac, 1993). Thus, if this is the case, it could be expected an increase in postural sway when one or more joints were immobilized.
The results of the present study revealed that only after immobilization of the knees, hips and trunk a small but significant increase in postural sway in AP direction could be identified. These results lead us to refute the hypothesis that postural stability in AP direction can be achieved without significant motion of joints above the ankles and to question the idea that the inverted pendulum model can adequately describe the upright posture during unperturbed quiet standing in terms of its whole body sway. These results are in accordance with the results of Fitzpatrick and colleagues (1994) who reported a 52% increase in body sway (i.e. ankle joint sway) when subjects were splinted to limit the sway to the ankles than when the subjects stood freely. These authors suggested that, when all body segments are free to move, there is an increase in body stability and that the movements around the ankle joints are not the most determinant factor in body stability.
It is important to mention that in our current study differently of Fitzpatrick and colleagues (1994), we did not observe any change in RMS of the postural sway variable (i.e., COP). Whilst Fitzpatrick and colleagues (1994) assessed the RMS of the ankle angle sway (in radians) we assessed the RMS of the COP displacement (cm) in AP direction. Despite being different variables, we believe that both variables, while all joints above the ankle are constrained, may be highly related and it would not be the reason for the different results of these two studies. Therefore, a possible explanation for the different result is that Fitzpatrick and colleagues (1994) immobilized the upper trunk, arms and head while we kept them unconstrained. Although the upper trunk, arms and head has only a small contribution to the global COM/COP position, the obviation of head-trunk relative motion rather than the lower-limbs joint immobilization might have contributed to the increase in body sway. However, this is something yet to be investigated. Clearly, such lack of effect of joint mobilization is only possible in a quiet standing task, where the task offers no or little challenge to healthy individuals. It is well known that in a situation where the individual is perturbed during standing, stereotypical movements across all joints are typically observed (Nashner, Woollacott, & Tuma, 1979).
The observed effect of immobilization on COP speed still leads us to believe that increased body stability is achieved when more joints are free to move. One explanation is that joints’ immobilization would increase the level of activation of muscle groups located around the ankle joints which, consequently, could increase the apparent stiffness around ankle joint and prevent increase in postural sway. The increase in RMS and mean speed of the trembling trajectories supports the idea (i.e. increase of the apparent stiffness at the ankle joints) that the trembling may be a result of the action of spinal reflexes and changes in the intrinsic mechanical properties of the muscles. However, we were not able to measure muscle activity and, then, more studies are necessary to support this hypothesis.
Medial-lateral direction
One of the goals of this study was to identify the effects of joint immobilization on medial-lateral (ML) direction. The results surprisingly revealed a reduction of RMS and speed of the COP, rambling, and trembling displacements in ML direction when knee and hip and knee, hip and lower and middle trunk joints were immobilized. According to Winter and collaborators (Winter, Prince, Stergiou, & Powell, 1993) in the ML direction the magnitude of body sway is constrained at the hip joint as a result of the activation of hip adductors and abductors. Therefore, one would expect that the immobilization of the knee joint would not affect the postural sway in the ML direction because the most muscle groups around the knee are related to the motions in the anterior-posterior direction. On the other hand, the immobilization of knee and hip and knee, hip and trunk would cause an increase in postural sway in ML direction, due the fact that the ankle invertors and evertors alone would not be able to restrict sufficiently the COP displacement. Contradicting our expectations, we observed the opposite effect, i.e. a decrease in COP, rambling and trembling RMS and speed during the immobilization of knee and hip and knee, hip and trunk. Carpenter and collaborators (Carpenter, Frank, & Silcher, 1999; Carpenter, Frank, Silcher, & Peysar, 2001) verified that when people maintained their upright stance in a threatening situation they decreased displacement amplitude and increased mean frequency due to augmentation of the stiffness caused by increased muscle co-contraction. However, our result can not be explained by the increased joint stiffness at the ankle joints, for instance by co-contraction of the ankle joint muscles, and, consequently, reduced postural sway due the fact that postural sway speed also decreased.
A possible explanation for the decrease in sway in the ML direction after joint immobilization would be that, with the constraining of the hip movements, the individuals were less likely to use the mentioned load/unload mechanism resulting in lower COP displacement in the ML direction. This would be possible only if the displacement observed in the ML direction was not due to internally generated perturbations itself but due to self-motion and that we are also able to control our quiet standing posture in the ML direction without the use of the load/unload mechanism. The idea of self-motion to explain part of the body sway during quiet standing has indeed been proposed before as a possible exploratory mechanism (Roebuck, Simmons, Richardson, Mattson, & Riley, 1998). In addition, it might be possible that the load/unload mechanism using the hip joints is not an exclusive requirement for posture control in the ML direction during quiet standing, but only one of the strategies repertoire [e.g., the ankle eversion/inversion muscles can also participate in the control of standing (Winter, Prince, Frank, Powell, & Zabjek, 1996; Winter, Prince, Stergiou, & Powell, 1993)]. The load/unload mechanism using the hip joints has been shown to be essential for posture control in more challenging tasks involving weight transfers, such as gait initiation, walking, unconstrained standing, among others (Freitas, Duarte, & Latash, 2006). In this last view, clearly less sway would not mean better control, only that a different mechanism of control is being used during quiet standing.
Another related interpretation for the contrasting effects of joint immobilization on the sway in the AP and ML directions allow to suggest the following hypothesis. The ankle, knee, and hip joints show higher kinematic mobility in the AP direction resulting in a kinematically redundant system able to stabilize the center of mass location by co-varied changes in these joint angles (Freitas, Duarte, & Latash, 2006). Motor redundancy may be viewed as a useful feature that allows, in particular, to deal with secondary tasks and external perturbations (Latash, Scholz, & Schoner, 2007; Zhang, Scholz, Zatsiorsky, & Latash, 2008). Joint fixation may be viewed as an example of such a perturbation. Apparently, the kinematic redundancy of the postural chain allowed to keep the AP sway characteristics unchanged under joint immobilization. In contrast, there is limited joint mobility in the ML direction and, as such, the system may be viewed as less (or even non-) redundant. For such a system, any additional constraint may lead to an impossibility of using the earlier mode of control. Note that, although our manipulations were designed to lead to joint immobilization primarily in the AP direction, they likely limited joint mobility in the ML direction as well, partly by limiting muscle length changes for postural muscles with the lines of action in-between the AP and ML axes. If we assume that sway magnitude is to a large degree defined by a purposeful neural process [for example, as a possible exploratory mechanism (Roebuck, Simmons, Richardson, Mattson, & Riley, 1998)], this process may be suppressed if the controller is impaired in postural control. For example, patients with Parkinson’s disease can demonstrate decreased postural sway while postural instability is one of the cardinal features of this disorder (Romero & Stelmach, 2003).
Both interpretations given above consider, in a different degree, that the observed drop in the ML sway is a reflection of a purposeful strategy of the controller that suppresses the natural sway process that may by itself lead to loss of balance under the additional constraints. In addition, the results of the present study corroborate the idea that the control of the body sway in AP and ML directions during unperturbed upright stance are achieved by two distinct neuromuscular mechanisms that can be influenced by the outcome of each other’s action.
In summary, the findings of this study indicate that the inverted pendulum model is unable to completely explain the processes involved in the control of the quiet upright stance in the anterior-posterior and medial-lateral directions.
References
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biological Cybernetics. 2005;93(5):309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov AV, Frolov AA, Massion J. Biomechanical analysis of movement strategies in human forward trunk bending. II. Experimental study. Biological Cybernetics. 2001;84(6):435–443. doi: 10.1007/PL00007987. [DOI] [PubMed] [Google Scholar]
- Aramaki Y, Nozaki D, Masani K, Sato T, Nakazawa K, Yano H. Reciprocal angular acceleration of the ankle and hip joints during quiet standing in humans. Experimental Brain Research. 2001;136(4):463–473. doi: 10.1007/s002210000603. [DOI] [PubMed] [Google Scholar]
- Baratto L, Morasso PG, Re C, Spada G. A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Motor Control. 2002;6(3):246–270. doi: 10.1123/mcj.6.3.246. [DOI] [PubMed] [Google Scholar]
- Bardy BG, Marin L, Stoffregen TA, Bootsma RJ. Postural coordination modes considered as emergent phenomena. Journal of Experimental Psychology-Human Perception and Performance. 1999;25(5):1284–1301. doi: 10.1037//0096-1523.25.5.1284. [DOI] [PubMed] [Google Scholar]
- Bardy BG, Oullier O, Bootsma RJ, Stoffregen TA. Dynamics of human postural transitions. Journal of Experimental Psychology-Human Perception and Performance. 2002;28(3):499–514. [PubMed] [Google Scholar]
- Bardy BG, Oullier O, Lagarde J, Stoffregen TA. On perturbation and pattern coexistence in postural coordination dynamics. Journal of Motor Behavior. 2007;39(4):326–334. doi: 10.3200/JMBR.39.4.326-336. [DOI] [PubMed] [Google Scholar]
- Bottaro A, Casadio M, Morasso PG, Sanguineti V. Body sway during quiet standing: Is it the residual chattering of an intermittent stabilization process? Human Movement Science. 2005 doi: 10.1016/j.humov.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP. Surface height effects on postural control: A hypothesis for a stiffness strategy for stance. Journal of Vestibular Research-Equilibrium & Orientation. 1999;9(4):277–286. [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Experimental Brain Research. 2001;138(2):210–218. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- Collins JJ, De Luca CJ. Open-loop and closed-loop control of posture: a random-walk analysis of center-of-pressure trajectories. Experimental Brain Research. 1993;95(2):308–318. doi: 10.1007/BF00229788. [DOI] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neuroscience Letters. 2005;377(2):75–80. doi: 10.1016/j.neulet.2004.11.071. [DOI] [PubMed] [Google Scholar]
- Day BL, Steiger MJ, Thompson PD, Marsden CD. Effect of Vision and Stance Width on Human-Body Motion When Standing - Implications for Afferent Control of Lateral Sway. Journal of Physiology-London. 1993;469:479–499. doi: 10.1113/jphysiol.1993.sp019824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra TMH. A gentle introduction to the dynamic set-point model of human postural control during perturbed stance. Human Movement Science. 2000;19(4):567–595. [Google Scholar]
- Duarte M, Zatsiorsky VM. Effects of body lean and visual information on the equilibrium maintenance during stance. Experimental Brain Research. 2002;146(1):60–69. doi: 10.1007/s00221-002-1154-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK, McCloskey DI. Stable human standing with lower-limb muscle afferents providing the only sensory input. J Physiol. 1994;480(Pt 2):395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TD, Daffertshofer A, Beek PJ. Multivariate Ornstein-Uhlenbeck processes with mean-field dependent coefficients: Application to postural sway. Physical Review E. 2000;63(1):11905. doi: 10.1103/PhysRevE.63.011905. [DOI] [PubMed] [Google Scholar]
- Freitas SMSF, Duarte M, Latash ML. Two kinematic synergies in voluntary whole-body movements during standing. Journal of Neurophysiology. 2006;95(2):636–645. doi: 10.1152/jn.00482.2005. [DOI] [PubMed] [Google Scholar]
- Gage WH, Winter DA, Frank JS, Adkin AL. Kinematic and kinetic validity of the inverted pendulum model in quiet standing. Gait & Posture. 2004;19(2):124–132. doi: 10.1016/S0966-6362(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514(Pt 3):915–928. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WL, Scholz JP, Schoner G, Jeka JJ, Kiemel T. Control and estimation of posture during quiet stance depends on multijoint coordination. Journal of Neurophysiology. 2007;97(4):3024–3035. doi: 10.1152/jn.01142.2006. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Persson T. The ankle strategy for postural control--a comparison between a model-based and a marker-based method. Computer Methods and Programs in Biomedicine. 1997;52(3):165–173. doi: 10.1016/s0169-2607(96)01794-4. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Yang JF, Scholz JP. Joint coordination during quiet stance: effects of vision. Experimental Brain Research. 2005;164(1):1–17. doi: 10.1007/s00221-004-2205-6. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Zajac FE. Human Standing Posture - Multijoint Movement Strategies Based on Biomechanical Constraints. Progress in Brain Research. 1993;97:349–358. doi: 10.1016/s0079-6123(08)62294-3. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Toward a new theory of motor synergies. Motor Control. 2007;11(3):276–308. doi: 10.1123/mcj.11.3.276. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Analysis of stance posture in humans. In: ALL TOWE ES, editor. Motor coordination (Handbook of behavioral neurology) New York: Plenum; 1981. pp. 527–565. [Google Scholar]
- Nashner LM, Woollacott M, Tuma G. Organization of Rapid Responses to Postural and Locomotor-Like Perturbations of Standing Man. Experimental Brain Research. 1979;36(3):463–476. doi: 10.1007/BF00238516. [DOI] [PubMed] [Google Scholar]
- Nicholas SC, Doxey-Gasway DD, Paloski WH. A link-segment model of upright human posture for analysis of head-trunk coordination. J Vestib Res. 1998;8(3):187–200. [PubMed] [Google Scholar]
- Peterka RJ. Simplifying the complexities of maintaining balance. IEEE Engineering in Medicine and Biology Magazine. 2003;22(2):63–68. doi: 10.1109/memb.2003.1195698. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Richardson C, Mattson SN, Riley EP. Neuromuscular responses to disturbance of balance in children with prenatal exposure to alcohol. Alcoholism-Clinical and Experimental Research. 1998;22(9):1992–1997. [PubMed] [Google Scholar]
- Romero DH, Stelmach GE. Changes in postural control with aging and Parkinson’s disease. IEEE Eng Med Biol Mag. 2003;22(2):27–31. doi: 10.1109/memb.2003.1195692. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80(3):1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. Journal of Neurophysiology. 1996;75(6):2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Stergiou P, Powell C. Medial-Lateral and Anterior-Posterior Motor-Responses Associated with Center of Pressure Changes in Quiet Standing. Neuroscience Research Communications. 1993;12(3):141–148. [Google Scholar]
- Zatsiorsky VM, Duarte M. Instant equilibrium point and its migration in standing tasks: rambling and trembling components of the stabilogram. Motor Control. 1999;3(1):28–38. doi: 10.1123/mcj.3.1.28. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Duarte M. Rambling and trembling in quiet standing. Motor Control. 2000;4(2):185–200. doi: 10.1123/mcj.4.2.185. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, King DL. An algorithm for determining gravity line location from posturographic recordings. Journal of Biomechanics. 1998;31(2):161–164. doi: 10.1016/s0021-9290(97)00116-4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Scholz JP, Zatsiorsky VM, Latash ML. What do synergies do? Effects of secondary constraints on multidigit synergies in accurate force-production tasks. J Neurophysiol. 2008;99(2):500–513. doi: 10.1152/jn.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]