Abstract
Rationale
Dopamine D3 receptor mechanisms have been implicated in the abuse-related behavioral effects of cocaine.
Objectives
The purpose of this study was to investigate the effects of the D3 receptor partial agonist CJB 090 on the discriminative stimulus, reinforcing and priming effects of cocaine in squirrel monkeys. Studies were conducted to compare CJB 090’s effects on food-maintained behavior and species-typical unconditioned behaviors.
Methods
Monkeys were trained to: 1) discriminate cocaine from saline using a two-lever choice procedure, 2) self-administer cocaine on a second-order fixed-interval, fixed-ratio schedule of i.v. drug injection or 3) self-administer food on a comparable second-order schedule of food delivery. A final group of monkeys served in quantitative observational studies of unconditioned behaviors.
Results
In cocaine discrimination studies, pretreatment with CJB 090 significantly attenuated cocaine’s discriminative stimulus effects. CJB 090 also significantly attenuated the partial cocaine-like stimulus effects of the preferential D3 receptor agonist PD 128907, but not the preferential D2 receptor agonist sumanirole. CJB 090 did not attenuate either self-administration of cocaine or cocaine-induced reinstatement of extinguished drug-seeking at a dose that reduced responding maintained by food. CJB 090 did not induce scratching or biting (species-typical effects of D2/3 receptor agonists) or catalepsy (typical effect of D2/3 receptor antagonists).
Conclusions
The results provide no evidence that CJB 090 reduced either the reinforcing or priming effects of cocaine, but do suggest that CJB 090, acting via a D3 receptor mechanism, antagonized the discriminative stimulus effects of cocaine at a dose that did not induce adverse side effects.
Keywords: D3 receptor partial agonist, CJB 090, cocaine discrimination, cocaine-primed reinstatement of cocaine-seeking, cocaine self-administration, food-maintained behavior
Introduction
There is now a substantial body of literature suggesting a role for dopamine (DA) D3 receptor mechanisms in the abuse-related behavioral effects of cocaine (Le Foll et al. 2005, review). Previous studies have shown, for example, that drugs acting as preferential agonists at D3 receptors, such as PD 128907 and 7-OH-DPAT, partially reproduce the discriminative stimulus (DS) effects of cocaine in rats and monkeys (Acri et al. 1995; Spealman 1996); maintain i.v. self-administration when substituted for cocaine in these species (Nader and Mach 1996; Caine and Koob 1993) and reinstate extinguished cocaine-seeking behavior in rats (Self et al. 1996). Conversely, drugs acting as preferential antagonists or partial agonists at D3 receptors, such as SB-277011A, NGB 2904 and BP 897, can attenuate the discriminative stimulus effects of cocaine in mice (Beardsley et al. 2001); the reinforcing effects of cocaine in rats (Xi et al. 2005) and behavior either maintained by or reinstated by a cocaine-associated stimulus in rats (Pilla et al. 1999; Gilbert et al. 2005). In addition to this behavioral evidence D3 receptors are localized primarily in mesolimbic brain regions, particularly the nucleus accumbens and amygdala in humans (Murray et al. 1994; Hall et al. 1996), which are thought to mediate the abuse-related effects of cocaine. Furthermore, D3 receptors and D3 receptor mRNA are upregulated in the nucleus accumbens of cocaine overdose victims (Segal et al. 1997; Mash and Staley 1999).
Based on findings such as these, D3 receptor antagonists and partial agonists have been proposed as candidate medications to treat cocaine addiction (Heidbreder 2008; Newman et al. 2005, reviews). Consideration of D3 receptor partial agonists as potential pharmacotherapies for cocaine addiction may be especially attractive because of the dual agonist/antagonist properties of this category of drugs (Pulvirenti and Koob 1994, Bergman et al. 2000, Platt et al. 2002). Partial agonists can be characterized as drugs that bind to a receptor, but have submaximal capacity to activate its associated signal transduction mechanisms. As a result, partial agonists can exhibit agonist-like or antagonist-like properties depending on the neurotransmitter tone, receptor reserve and presence of exogenous ligands (Ariens 1983; Kenakin 1997). D3 partial agonists might therefore be expected to function primarily as antagonists in the presence of cocaine, when DA synaptic concentrations and binding at D3 (and other DA) receptors are high, and as weak agonists in the absence of cocaine, when DA activity is no longer stimulated. Additionally, because of low agonist efficacy, D3 partial agonists would be expected to have reduced abuse liability compared to full agonists (Beardsley et al. 2001) and may induce less severe motor effects compared to antagonists (Platt et al. 2002). Extrapyramidal side effects of D3 partial agonists also may be limited due to the predominant localization of D3 receptors in mesolimbic brain regions (Levant 1997).
To date, the most extensively studied D3 partial agonist is BP 897, which can reduce drug-seeking either maintained by or reinstated by cocaine-associated cues (Pilla et al. 1999; Gilbert et al. 2005) and prevent the development and expression of cocaine conditioned place preference in rats (Duarte et al, 2003). Other recently developed D3 partial agonists also have pharmacological profiles suggestive of potential therapeutic utility (Grundt et al. 2005; Gyertyan et al. 2007). One such drug CJB 090 (Newman et al. 2003), exhibits high D3 receptor affinity (Ki ~ 1 nM) and a ≥60-fold selectivity for D3 over other DA receptor subtypes in vitro (Grundt et al. 2005). Based on pharmacological magnetic resonance imaging with a closely related ligand (PG01037; (E)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)but-2-enyl)-4-(pyridin-2-yl)benzamide, Grundt et al. 2007), CJB 090 would be expected to penetrate the brain and localize predominantly in D3 receptor rich brain regions. In a previous study with rhesus monkeys, CJB 090 was found to attenuate the DS effects of cocaine and reduce cocaine self-administration, while showing no cocaine-like effects when tested alone (Martelle et al. 2007).
The purpose of this study was to investigate the possible cocaine-antagonist effects of CJB 090 in squirrel monkeys whose behavior was: 1) controlled by the DS effects of cocaine, 2) maintained under a second-order schedule of i.v. cocaine self-administration, and 3) extinguished and then reinstated by priming injections of cocaine along with restoration of a cocaine-paired stimulus. Comparison studies were conducted using a second-order schedule of food reinforcement and quantitative observations of unconditioned behavior. Because CJB 090 significantly attenuated the DS effects of cocaine, additional studies using this procedure were conducted with the preferential D3 receptor agonist PD 128907 (Pugsley et al. 1995) and the preferential D2 receptor agonist sumanirole (McCall et al. 2005), both of which partially substituted for the DS effects of cocaine.
Materials and methods
Subjects
Twenty-four male and 2 female adult squirrel monkeys (Saimiri sciureus) weighing 0.7 to 1.1 kg were studied in daily experimental sessions. Male monkeys were housed singly and female monkeys were pair-housed in a climate-controlled vivarium where they had unlimited access to water. Animals in cocaine self-administration, reinstatement, and observation experiments had unlimited access to food (Harlan Teklad Monkey Diet, Madison, WI) in their home cages. Animals in food self-administration and cocaine discrimination experiments were maintained at approximately 90% of their free-feeding body weights by adjusting access to food in the home cages. All monkeys were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington, DC, 1996). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Surgical Procedures
Monkeys in cocaine self-administration and reinstatement experiments were implanted with chronic venous catheters using the surgical procedures described by Platt et al. (2005). Briefly, under isoflurane anesthesia and aseptic conditions, one end of the catheter was passed via a femoral or jugular vein to the level of the right atrium. The distal end was then passed under the skin to a mid-scapular exit site. Catheters were flushed daily with 0.9% saline solution and sealed with obturators when not in use. Monkeys wore nylon mesh jackets (Lomir Biomedical, Toronto, Canada) to protect the catheter.
Apparatus
In studies involving cocaine discrimination, cocaine and food self-administration, and cocaine-induced reinstatement of drug-seeking, daily sessions were conducted in ventilated, sound-attenuated chambers with white background noise (MED Associates, St. Albans, VT). Within the chambers, monkeyss at in Plexiglas chairs (MED Associates) facing apanel equipped with response levers and red and white stimulus lights. In experiments involving catheterized subjects, catheters were connected to syringe pumps (MED Associates) located outside the chamber. The pumps were programmed to deliver drug or vehicle solutions into the catheter at a rate of 0.18 ml/s for 1 sec. In cocaine discrimination and food self-administration experiments, 190-mg sucrose pellets (Bioserve, Frenchtown, NJ)could be delivered to a receptacle in the front panel of the chair. Experiments were controlled and data were recorded via interfaces (Med Associates) and PC-compatible computers located in an adjacent room. Behavioral observation studies were conducted in a ventilated, transparent Plexiglas arena (114 × 122 × 213 cm) situated in a lighted room, separate from other animals (Platt et al. 2000). The arena was equipped with perches, suspended plastic chains, and a wood-chip bedding to permit a range of species-typical behaviors. A video camera was positioned 1 m in front of the chamber to record a subject’s behavior during the session.
Cocaine Discrimination
Six monkeys were trained to discriminate cocaine from vehicle injections using procedures described by Spealman et al. (1991). Briefly, 10 consecutive responses (FR10) on one lever (counter balanced across subjects) produced a sucrose pellet if cocaine (0.3 or 0.42 mg/kg, i.m., depending on the subject) was injected, whereas 10 consecutive responses on the other lever produced a pellet if vehicle was injected. Each response on the inappropriate lever reset the FR10 requirement. Delivery of each sucrose pellet was followed by a10-sec timeout (TO) during which the chamber was dark and lever pressing had no programmed consequences. During training sessions, this procedure was repeated 10 times per component, and the number of components per session (n=1–4) was varied daily in an irregular order. In the event that lever pressing was not maintained at a rate sufficient to obtain all 10 available sucrose pellets, the component ended automatically after 5 min. Each component was preceded by an extended (10-min) TO, during which vehicle was administered 5 min before the beginning of the n-1 component(s) of the session and cocaine was administered 5min before the nth component. Drug testing began when monkeys made ≥90% of responses on the injection-appropriate lever for at least three consecutive sessions. Test sessions consisted of three (sumanirole) or four (cocaine and PD 128907) components, each preceded by an extended TO (10-min for cocaine and PD 128907; 30-min for sumanirole due to its slower onset of effect; cf. McCall et al. 2005). In each component, completion of the FR10 on either lever resulted in delivery of 1 pellet. Dose-response functions for cocaine, CJB 090, PD 128907 and sumanirole were determined using a cumulative-dosing procedure (Spealman et al. 1991). Incremental doses of a test drug (0.25–0.5 log unit increments) were injected during the TO periods that preceded sequential components of a test session, resulting in a three- or four-point cumulative dose-response function determined in a single session. In experiments involving drug pretreatments, i.m. injections of CJB 090 or vehicle were administered 5 min before the start of the test session.
Cocaine Self-administration
A separate group of five monkeys were trained to self-administer cocaine under a second-order fixed-interval, fixed-ratio [FI (FR)] schedule of i.v. cocaine injection using procedures similar to those described by Adewale et al. (2006). Monkeys self-administered cocaine under this schedule for at least 8 months before testing began. Each experimental session was signaled by the illumination of a white light, and completion of each FR10 produced a 2-sec change in illumination from white to red. This cycle was repeated throughout a 5-min FI, and the first FR10 completed after the expiration of the FI produced the 2-sec red light paired with an i.v. injection of cocaine. A 60-sec TO followed each cocaine injection. If the FR requirement was not completed within 8 min after expiration of the FI, the TO was started automatically without an injection of cocaine. Daily sessions ended after ten cycles of the second-order schedule or a maximum session length of 90 min. Once baseline performance stabilized (no monotonic trend in response rate over at least 3 consecutive sessions), the dose of self-administered cocaine was varied over a 10-fold range (0.03–0.3 mg/kg/injection), with each dose tested for at least five consecutive sessions and until responding was stable. Each SA test session was preceded by pretreatment (5-min) with either CJB 090 or vehicle.
Food Self-administration
Five monkeys were trained to respond under a second-order FI (FR) schedule of food presentation with schedule parameters identical to the second-order schedule of cocaine injection described above (Platt et. al. 2001). Once baseline performance stabilized (see above) pretreatment studies with CJB 090 and vehicle were begun. Monkeys were pretreated with either 17.8 mg/kg of CJB 090 or vehicle i.m. 5 min before the start of each food SA session for at least five consecutive sessions.
Reinstatement of Cocaine-seeking
Six monkeys were used to evaluate the effects of CJB 090 on the reinstatement of extinguished cocaine-seeking using procedures similar to those described by Khroyan et al. (2000). Briefly, subjects were first trained under a second-order FI(FR) schedule of i.v. cocaine self-administration (0.18–0.30 mg/kg/injection) similar to the one described above except that the FI was 10 min in length and sessions consisted of five components. After a period of stable responding (defined above) cocaine-seeking was extinguished by substituting vehicle for cocaine injections and omitting presentations of the cocaine-paired stimulus. Extinction sessions were conducted daily until response rates declined to 10% or less of the rate maintained by cocaine self-administration for at least three consecutive sessions. Reinstatement tests began once this criterion was met. Reinstatement tests used the same schedule parameters as the second-order schedule described above except that vehicle rather than cocaine was available for self-administration. On reinstatement test days, monkeys received an i.m. pretreatment of either CJB 090 or vehicle 5 min before the test session followed by an i.v. priming injection of cocaine (0.1–1.0 mg/kg) immediately before the session. To separate the effects of CJB 090 on conditioned cue-induced reinstatement versus cocaine-primed reinstatement, separate tests were conducted in which subjects were pretreated with test drug prior to a saline rather than a cocaine prime. Tests involving different dose combinations of priming and either CJB 090 or vehicle pretreatment were separated by two or more extinction sessions without drug administration.
Observation Studies
Behavioral observations were conducted in four monkeys using procedures described by Platt et al. (2000). Briefly, after habituation to the observation arena, handling and injection procedures, 30-min observation sessions were conducted daily, during which the animal’s behavior was videotaped. Video recordings were scored by trained observers, who were not informed about the drug treatment conditions. Ten behavioral categories adapted from Platt et al. (2000) were scored in each session: locomotion, object exploration, foraging, self-grooming, scratching, visual scanning, sleep posture, static posture and vocalization. Behaviors were scored by recording the presence or absence of each behavioral category in 15-s intervals during three 5-min observation periods across the session (0–5, 12–17, and 24–29 min). Frequency scores were calculated as the number of 15-s intervals in which a particular behavior was observed. In addition, during the 6th, 18th and 30th min of each session, the monkeys were removed from the observation arena and evaluated for ataxia (defined as the inability to balance and/or grasp a 1.0 cm diameter stainless steel pole held horizontally) and muscle rigidity (defined as resistance to hind limb extension) using a three-point rating scale (Platt et al. 2000).
Drugs
Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO), PD 128907 hydrochloride (Sigma Aldrich) and sumanirole maleate (Pfizer Inc, Groton, CT) were dissolved in 0.9% saline solution. CJB 090 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide) hydrochloride was synthesized in the Medicinal Chemistry Section, NIDA-Intramural Research Program, Baltimore MD according to the methods described in Newman et al. 2003) was dissolved in sterile distilled water containing 12% β-cyclodextran. No i.m. injection volumes exceeded 0.3ml.
Data Analysis
In drug discrimination studies, responses made on cocaine and saline levers during each component were recorded and used to calculate the percentage of responses on the cocaine lever and the rate of lever pressing. ED50 values for the discriminative stimulus effects of drugs and drug combinations were calculated by linear regression of the log-dose response curves. In studies involving cocaine and food self-administration and cocaine-induced reinstatement of drug-seeking, response rates were determined for individual across sequential components of the session as well as for the entire session. In observation studies, scores for each behavior were averaged across the three 5-min observation periods of the session. In each type of experiment, data from individual subjects were averaged to obtain group means and were analyzed using repeated measures ANOVAs and Bonferroni t-tests for planned comparisons (significance level p < 0.05).
Results
DS effects of cocaine and CJB 090
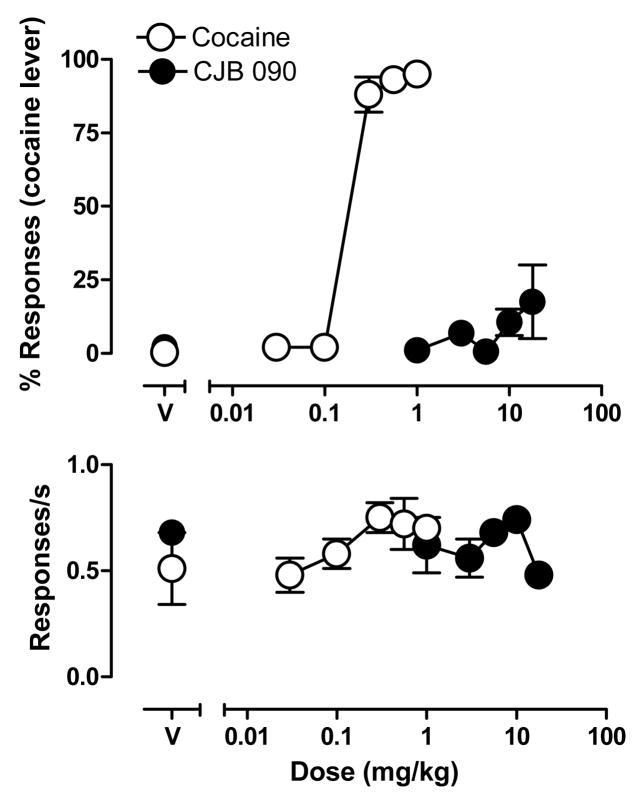
Under training conditions, responding on the cocaine lever averaged 93% after administration of the training dose of cocaine, whereas administration of saline engendered an average of only 6% responding on the cocaine lever. The average rate of responding was similar after administration of cocaine or saline except for one subject that responded at a lower rate after administration of cocaine comjpared to saline. Under test conditions, administration of cumulative doses of cocaine (0.03–1.0 mg/kg) produced dose-related increases in the percentage of cocaine-lever responding, with the two highest doses resulting in >90% of responses on the cocaine lever (Fig. 1, top). In contrast, cumulative doses of CJB 090 (1.0–17.8 mg/kg) generated a maximum of only 17.5% cocaine-lever responses, which was not significantly greater than responding engendered by saline (p>0.05, Bonferroni t-tests). Compared to saline, neither cocaine nor CJB 090 had a significant effect on the rate of responding regardless of dose (Fig. 1, bottom).
Figure 1.
Percentage of responses on the cocaine lever (top) and response rate (bottom) as a function of cumulative dosing of cocaine or CJB 090 in squirrel monkeys trained to discriminate cocaine from vehicle. Points are means ± SEM (n=6). Points above V show effects of vehicle.
Attenuation of DS effects of cocaine by CJB 090
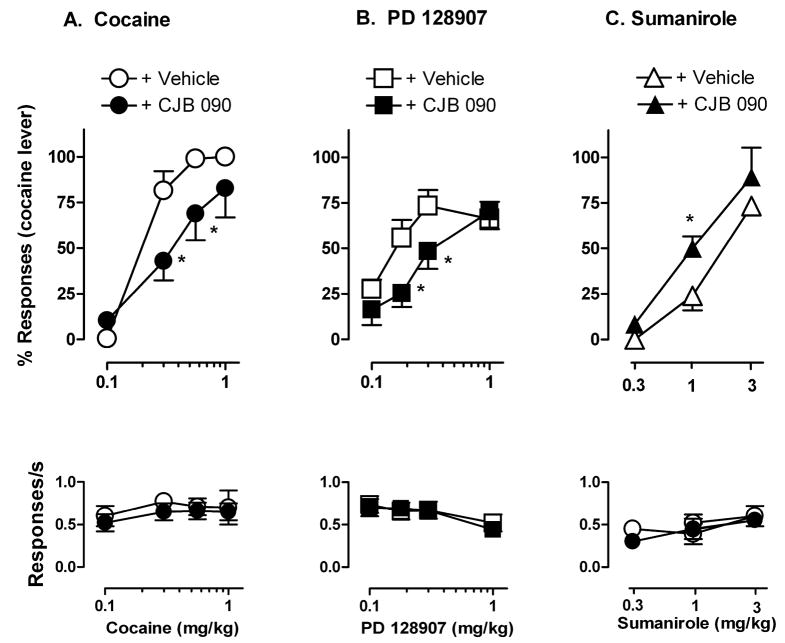
Pretreatment with CJB 090 (17.8 mg/kg) attenuated the DS effects of cocaine, resulting in an overall rightward shift in the cumulative dose-response curve (Figure 2A, top). Two-way repeated measures ANOVA revealed a significant effect of cocaine dose [F (4, 60) = 67; p<0.001], CJB 090 dose [F (3, 60) = 4.5; p=0.02], and a significant CJB 090 x cocaine interaction [F (12, 60) = 2.05; p=0.04] on the percentage of cocaine-lever responses. Bonferroni t-tests showed that, compared to vehicle pretreatment, CJB 090 produced a significant reduction in the percentage of cocaine-lever responses engendered by 0.3 and 0.56 mg/kg cocaine, along with a significant 3.6-fold increase in the average ED50 (Table 1). Attenuation of the DS effects of cocaine by CJB 090 was not accompanied by significant effects on response rate (Fig. 2A, bottom). Pretreatment with lower doses of CJB 090 (3.0 and 10.0 mg/kg) had no significant effect on either the percentage of cocaine-lever responses or response rate engendered by any dose of cocaine (not shown).
Figure 2.
Effects of A) cocaine, B) PD 128907, and C) sumanirole after 5 min i.m. pretreatment with vehicle or CJB 090 (17.8 mg/kg) in monkeys trained to discriminate cocaine from vehicle. Points are means ± SEM (n=5 or 6). * p<0.05 comparing percentage of cocaine lever responding after vehicle treatment, Bonferroni t-test.
Table 1.
ED50 values (doses estimated to engender 50% drug-lever responses) for cocaine, PD 128907 and sumanirole following pretreatment with vehicle or 17.8 mg/kg of CJB 090. Values are means (± SEM) determined from individual dose-response functions of squirrel monkeys (n=5 or 6).
| Agonist | ED50 (mg/kg) |
|
|---|---|---|
| Vehicle | CJB 090 (17.8 mg/kg) | |
| Cocaine | 0.11 ± 0.01 | 0.40 ± 0.06* |
| PD 128907 | 0.17 ± 0.02 | 0.55 ± 0.14* |
| Sumanirole | 2.26 ± 0.24 | 1.63 ± 0.17† |
significant increase compared to vehicle pretreatment (p<0.05).
significant decrease compared to vehicle pretreatment (p<0.05).
Cocaine-like DS effects of dopamine agonists and modulation by CJB 090
Both the preferential D3 agonist PD 128907 and the preferential D2 agonist sumanirole engendered dose-related increases in cocaine-lever responding, reaching average maximums of 73% (± 8.8) after 0.3 mg/kg PD 128907 and 73% (± 4.7) after 3.0 mg/kg sumanirole (Fig. 2B and C, top). Neither PD 128907 nor sumanirole significantly altered the rate of responding over the dose range tested (Fig. 2B and C, bottom). Pretreatment with CJB 090 (17.8 mg/kg) attenuated the cocaine-like DS effects of PD 128907, resulting in an overall rightward shift in the cumulative dose-response curve (Fig. 2B, top). Two-way repeated measures ANOVA revealed a significant effect of PD 128907 [F (3, 15) = 16; p<0.001], CJB 090 [F (1, 15) = 7.8; p=0.04], and a significant CJB 090 x PD 128907 interaction [F (3, 15) = 4.7; p=0.02] on the percentage of cocaine lever responses. Compared to vehicle pretreatment, CJB 090 produced a significant reduction in the percentage of cocaine-lever responses at doses of 0.18 and 0.3 mg/kg PD 128907 (p<0.05), along with a significant 3.2-fold increase in the average ED50 (Table 1; p<0.05). Significant attenuation of cocaine-like DS effects of PD 128907 by CJB 090 was not accompanied by significant effects on response rate (Figure 2B, bottom).
In contrast to the findings with PD 128907 and cocaine, pretreatment with CJB 090 (17.8 mg/kg) did not attenuate the cocaine-like DS effects of sumanirole (Fig. 2C, top). ANOVA revealed a significant effect of sumanirole dose [F (2, 8) = 73.6; p<0.001], but not of CJB 090 or their interaction. Planned comparisons showed that pretreatment with CJB 090 produced a significant increase rather than decrease in the percentage of cocaine-lever responding engendered by 1.0 mg/kg sumanirole (p<0.05), along with a significant 1.4-fold decrease in the average ED50 (Table 1). Pretreatment with CJB 090 had no significant effect on the rate of responding at any dose of sumanirole.
Effects of CJB 090 on cocaine and food self-administration
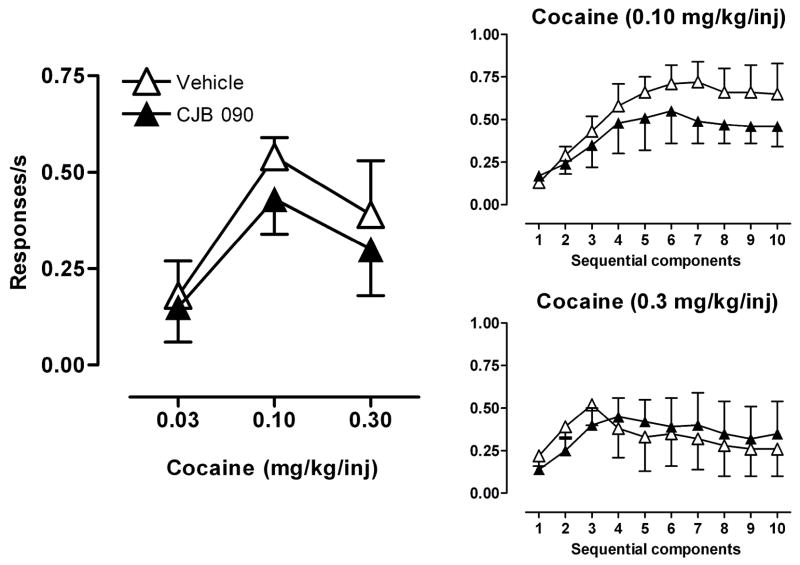
Cocaine self-administration was maintained in all five monkeys under the second-order schedule of i.v. drug injection throughout the study. As the dose of cocaine was increased from 0.03 to 0.3 mg/kg/injection, the average rate of responding first increased and then decreased, resulting in a biphasic dose-response curve (Fig. 3, left). Pretreatment with 17.8 mg/kg CJB 090 did not markedly alter the overall shape of the cocaine dose-response curve, and two-way repeated measures ANOVA revealed no significant effect of pretreatment with CJB 090 on response rates engendered during cocaine self-administration. Analysis of response rates across the ten sequential components of the cocaine self-administration session showed that for all cocaine doses the average rate of responding was lowest at the beginning of the session, increased to a maximum later in the session, and then remained relatively constant or decreased as the session progressed. As illustrated for the two highest doses of cocaine, this within-session pattern was not markedly affected by pretreatment with CJB 090 (Fig. 3, right), and planned comparisons showed no significant effect of CJB 090 compared to vehicle pretreatment during any portion of the session. When the intermediate cocaine dose (0.1 mg/kg) was available, CJB 090 (30 mg/kg) pretreatment resulted in an average of 0.48 responses/sec (±0.16) which was not significantly different from the mean rate of 0.60 responses/sec (±0.09) engendered after vehicle pretreatments (data not shown). Since this higher dose of CJB 090 failed to alter the rate of cocaine self-administration and produced emesis on multiple occasions, the effects of doses greater than 17.8 mg/kg of CJB 090 on a cocaine self-administration full dose-response curve were not systematically tested.
Figure 3.
Effects of repeated pretreatment with CJB 090 (17.8 mg/kg) or vehicle on response rate under the second-order schedule of i.v. cocaine injections. Right panel: Comparison of response rates after pretreatments during sequential components of cocaine (0.10 or 0.30 mg/kg/inj) self-administration. Data are means ± SEM (n=5).
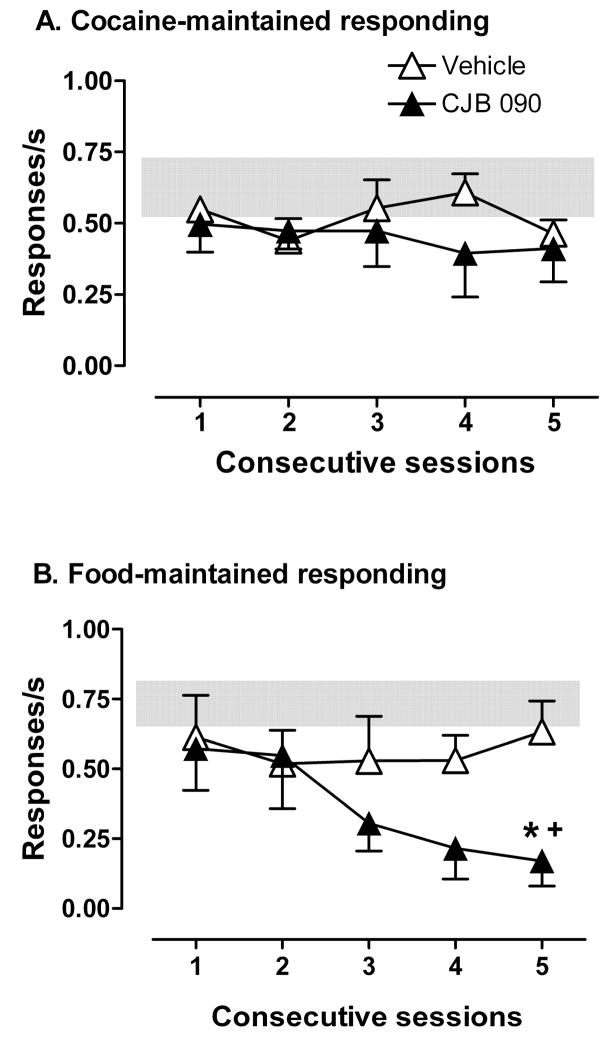
Under baseline conditions, food self-administration was maintained at an average response rate of 0.73 (± 0.10) responses/s, which was comparable to the average rate maintained by the maximally effective dose of cocaine [0.64 (± 0.16) responses/s at 0.10 mg/kg/injection]. As shown in Figure 4 (open symbols), pretreatment with vehicle before each of five consecutive test sessions had no systematic effect on the average rate of responding maintained by either food or cocaine self-administration. Daily pretreatment with 17.8 mg/kg CJB 090, however, produced a gradual decline in the rate of responding maintained by food presentation (Fig. 4B) but not cocaine injection (Fig. 4A). ANOVA of response rates during food self-administration revealed an effect of CJB 090 that approached significance [F (1,12) =9.6; p=0.05], and a significant day x CJB 090 interaction F (4,12) =3.6; p=0.04]. Bonferroni t-tests revealed that daily pretreatment with CJB 090 produced a significant reduction in food-maintained responding on day 5, compared to either vehicle pretreatment on day 5 (asterisk, p< 0.05) or CJB 090 pretreatment on day 1 (cross, p< 0.05). Analysis of response rates across the ten sequential components of the food self-administration session on day 5 showed that, compared to vehicle pretreatment, CJB 090 produced a significant reduction in food-maintained responding throughout the session with the exception of component 3 (not shown).
Figure 4.
Comparison of mean response rates across 5 days of pretreatment with CJB 090 (17.8 mg/kg) or vehicle during the availability of A) 0.1 mg/kg/inj of cocaine (n=5) or B) sucrose pellets (n=4). * p<0.05 comparing mean response rates on the corresponding day after vehicle treatment. + p<0.05 comparing mean response rates after CJB 090 treatment on day 1.
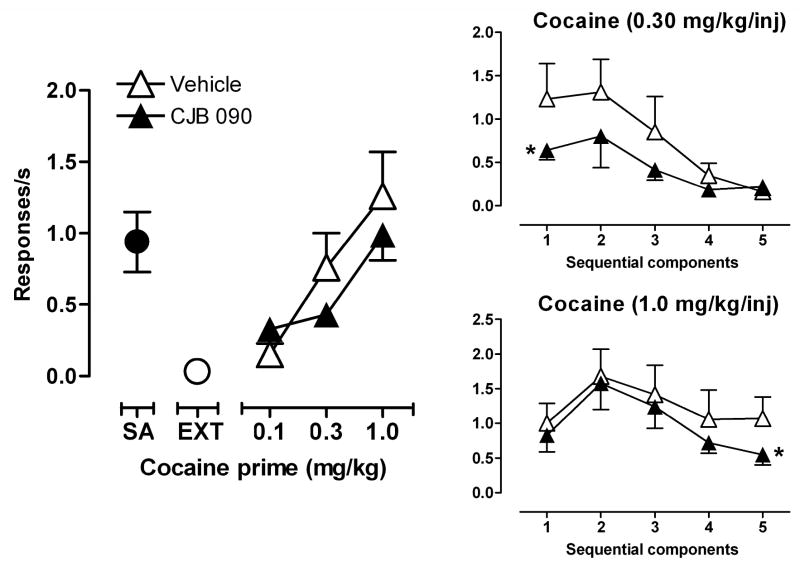
Effects of CJB 090 on reinstatement of drug-seeking
During extinction sessions in which saline was substituted for cocaine and the cocaine-paired stimulus was omitted, responding declined to <10% of the rate maintained by cocaine self-administration in all subjects (Fig. 5, compare points at SA and EXT). Following vehicle pretreatment, priming with cocaine (0.1–1.0 mg/kg) accompanied by restoration of the cocaine-paired stimulus produced a dose-related reinstatement of cocaine-seeking, reaching a maximum response rate that approached the rate maintained by cocaine self-administration. Compared to vehicle, pretreatment with 17.8 mg/kg of CJB 090 before cocaine priming did not significantly alter the average rate of responding induced by any priming dose (Fig. 5, left). Analysis of the within-session pattern of responding revealed that response rates induced by cocaine priming were typically highest during the first and/or second components of the session and then declined as the session progressed (Fig. 5, right). Compared to vehicle pretreatment, CJB 090 (17.8 mg/kg) had no significant effect on cocaine-primed reinstatement of responding during most components of the session. The two exceptions were a significant reduction in response rate during the first component after priming with 0.3 mg/kg cocaine and during the last component after priming with 1.0 mg/kg cocaine (p<0.05).
Figure 5.
Effects of CJB 090 on reinstatement of drug-seeking induced by cocaine priming and the presentation of a cocaine cue. Point above SA: response rate during cocaine SA. Point above EXT: response rate during extinction of cocaine-seeking behavior. Right panel: Comparison of response rates after pretreatments during sequential components after priming with 0.30 or 1.0 mg/kg cocaine. * p<0.05 comparing response rates for the corresponding component after vehicle treatment (n=6), Bonferroni t-test.
Preliminary observations from three monkeys revealed that pretreatment with CJB 090 (30 mg/kg) resulted in a similar lack of effect on overall response rates after a cocaine prime of 0.3 mg/kg and induced vomiting. CJB 090 (30 mg/kg) pretreatment resulted in an average of 0.46 responses/sec (±0.22) which was not significantly different from the mean rate of 0.55 responses/sec (±0.28) engendered after vehicle pretreatments (data not shown). During reinstatement sessions induced by the conditioned cue alone i.e. by a saline prime, the rate of responding was low and similar to the rates achieved during extinction, averaging 0.08 responses/sec (±0.07) after vehicle pretreatments and 0.07 responses/sec (±0.11) after pretreatment with CJB 090 (17.8 mg/kg) in four monkeys (data not shown).
Effects of CJB 090 on unconditioned behaviors
Compared to vehicle, CJB 090 (3.0–17.8 mg/kg) had no significant effect on locomotion, object exploration, foraging, self-grooming, scratching, visual scanning, sleep posture, static posture, vocalization, or other recorded behaviors. CJB 090 also did not induce muscle rigidity or ataxia. Doses of CJB 090 >17.8 mg/kg were not tested systematically due to emesis in an initial observation.
Discussion
In the present study the D3 partial agonist CJB 090 attenuated the DS effects of cocaine and the cocaine-like DS effects of the D3 agonist PD 128907. CJB 090 did not, however, attenuate the cocaine-like DS effects of the D2 agonist sumanirole, nor did it reduce the reinforcing or priming effects of cocaine at a dose that attenuated food-reinforced behavior.
Our finding that CJB 090 reduced the DS effects of cocaine and PD 128907 to a similar degree (3.6-and 3.2-fold increase in ED50, respectively), but did not reduce the effects of sumanirole under the same conditions has two main implications. First, these findings are consistent with the hypothesis, developed originally from studies with D3 agonists (Acri et al. 1995; Spealman 1996), that D3 receptor mechanisms play an integral role in the transduction of cocaine’s DS effects. Second, the different effects of CJB 090 combined with PD 128907 vs. sumanirole, support previous speculation that CJB 090 has in vivo as well as in vitro selectivity for the D3 compared to the D2 receptor subtype (Grundt et al. 2005; Martelle et al. 2007). Although the dose of CJB 090 (17.8 mg/kg) needed to attenuate the DS effects of cocaine was somewhat higher than might be expected on the basis of its in vitro binding affinity (Grundt et al. 2005) or its in vivo potency in rhesus monkeys (Martelle et al. 2007), this dose did not produce changes in the rate of responding and had no significant effect on unconditioned behaviors or motor coordination in observational studies. These findings suggest that the attenuation of the DS effects of cocaine by CJB 090 was not the result of a non-specific disruptive effect on behavior. The lower effective doses (1.0–3.0 mg/kg, i.v.) in Martelle et al. (2007) compared to ours likely reflect the different routes of administration (i.v. vs. i.m.) and/or subject species (rhesus vs. squirrel monkey).
Consistent with the findings of Martelle et al. (2007), CJB 090 did not exhibit cocaine-like DS effects when tested alone. Although there appears to be an increasing trend in the dose-response function for CJB 090 (see fig. 1), higher doses could not be safely tested due to potential toxicity. The absence of significant substitution of CJB 090 for cocaine in both the present study and the study by Martelle et al. (2007) probably reflects the low intrinsic activity of CJB 090 compared to D3 receptor full agonists, which partially reproduce the DS effects of cocaine in monkeys (present study; Spealman 1996).
Sumanirole exhibits ~ 200-fold selectivity D2/D3 selectivity in vitro (McCall et al. 2005). The partial sumanirole generalization to cocaine observed in this study appears to be typical of other DA D2-selective agonists including (+)-PHNO and quinpirole which yielded average responses on the cocaine-associated lever of only 68% in squirrel monkeys (Spealman et al. 1991) and 40–70% in rats (Witkin et al. 1991). An unexpected outcome of the present study however, was that pretreatment with CJB 090 produced a significant increase in cocaine-lever responding engendered by the intermediate sumanirole dose along with a modest, though significant (1.4-fold) decrease in ED50. The mechanism(s) underlying this apparent enhancement of the effects of sumanirole by CJB 090 are unknown. It is noteworthy that a similar enhancement of D2 receptor-mediated effects by inhibition of D3 receptors has been observed in other studies with monkeys and may indicate an inhibitory role for D3 receptors in the regulation of D2 receptor activity. Millan et al. (2008) for example, reported that in parkinsonian monkeys, locomotor activity induced by the D2 agonist ropinrole was enhanced after administration of the partial D3 antagonist, S33138. Consistent with these results, D3 antagonists also have been found to enhance amphetamine-induced locomotion in mice (Pritchard et al. 2007) and augment haloperidol-induced catalepsy in rats (Gyertyan and Saghy 2007). Along these same lines, knockout mice lacking D3 receptors exhibit enhanced cocaine-induced locomotion compared to wild-type controls (Xu et al. 1997). It is therefore possible that attenuation of D3 receptor activity by CJB 090 in our study resulted in D2 receptor disinhibition leading to the enhanced cocaine-like DS effects of sumanirole. Alternatively, these findings could be explained by the intrinsic activity of CJB 090 at D2 receptors or its reasonably high binding affinities at 5-HT receptors (Grundt et al. 2007). It is possible that at the doses we tested, CJB 090 induced weak D2 agonist effects or 5-HT antagonist effects which could have enhanced the cocaine-like DS effects of sumanirole.
In the cocaine self-administration experiments CJB 090 did not significantly reduce the overall rate of responding under the second-order schedule of i.v. drug injection, nor did it have significant effects across the ten sequential components of the session. These findings differ from those reported by Martelle et al. (2007) in rhesus monkeys using a different type of second-order schedule [FR(FI) with two components/session]. In their study, CJB 090 significantly reduced the rate of responding during the second component of the self-administration session. These different results could reflect a number of factors, including the different schedule parameters, which resulted in markedly different frequencies of cocaine injection (nominally 10 injections/h vs. 2 injections/h), the different doses and routes of administration for CJB 090 (17.8 mg/kg i.m. vs. 3.0 mg/kg i.v.) or the different species (squirrel monkeys vs. rhesus monkeys). Nevertheless, the lack of effect of CJB 090 on cocaine self-administration in our study mirrors the results of previous studies showing limited or no effect of other D3 partial agonists in attenuating self-administration of cocaine in rodents (Pilla et al. 1999; Campiani et al. 2003; Gyertyan et al. 2007) suggesting limitations on the contribution of D3 receptor mechanisms in the reinforcing effects of cocaine.
Findings such as those discussed above have prompted speculation that D3 receptors play a more prominent role in the motivational salience of cocaine-associated stimuli than in the reinforcing effects of cocaine per se and that reduced D3 receptor activity may serve primarily to reduce cocaine-seeking that is either maintained or reinstated by cocaine-associated cues. For example, the D3 partial agonists BP-897 and RGH-237 can attenuate cue-induced reinstatement of cocaine-seeking in rats (Pilla et al. 1999; Cervo et al. 2003; Gilbert et al. 2005; Gyertyan et al. 2007). In the present study however, cocaine-primed reinstatement was more robust compared to the modest effects of restoration of the conditioned cue alone and in some cases the latter resulted in response rates that were similar to rates during extinction conditions. It appears that the significance of the conditioned cue in inducing reinstatement to cocaine-seeking may be dependent on the length of training and cue exposure experience. For example, a trend was observed in which monkeys with a longer history of cue-induced reinstatement yielded lower response rates compared to less experienced monkeys in the same study. Further, all of the aforementioned studies used rodents to examine the role of DA D3 receptor partial agonists in cue-induced reinstatement. Typically, rodents undergo training periods of weeks to months whereas the squirrel monkeys in this study underwent longer training periods (6–12 months before testing). Our method of acute exposure to cocaine (priming) in order to study reinstatement of drug-seeking was chosen because in monkeys, it resulted in more robust behavior, than cue-induced reinstatement.
CJB 090 had only minimal effects on cocaine-primed reinstatement of drug-seeking in this study. These apparently disparate findings between the present study and the cue-induced reinstatement studies using rodents could be due to differences in the reinstatement procedures used. Reinstatement of cocaine-seeking in rodent studies was achieved by restoring the cocaine-paired stimulus in the absence of cocaine priming, whereas robust reinstatement in the present study was achieved by concurrent presentation of the cocaine-paired stimulus and an i.v. priming injection of cocaine. Previous studies have shown that cocaine-related cues can induce modest increases in DA release in the nucleus accumbens of rats as determined by in vivo microdialysis (Weiss et al. 2000) and in the dorsal striatum in humans detected by PET imaging (Wong et al. 2006). These findings indicate an increase in the level of extracellular DA available for binding at D3 (and other DA) receptors. Administration of cocaine also induces a large increase in extracellular DA as a result of DA transport inhibition. It is possible that the summation of DA levels induced by cocaine priming and restoration of the cocaine-paired stimulus in our study resulted in a greater degree of D3 receptor activation (and reinstated drug-seeking) than could be overcome consistently by CJB 090. Even under these demanding conditions, however, CJB 090 significantly reduced drug-seeking reinstated in the initial component of the session after priming with an intermediate dose of cocaine and in the last component of the session after priming with a higher dose.
Genetic and pharmacological manipulations of DA receptor activity indicate a role for DA in food reinforcement and the regulation of food intake (see Palmiter 2007 for review). Recent evidence suggests that D3 receptors may play an important role in these processes. For example, the D3 antagonist SB-277011A recently has been shown to inhibit food-maintained responding and to decrease food intake in obese rats (Thanos et al. 2008). Knockout mice lacking the D3 receptor gene increase leptin production (~2-fold) compared to wild-type mice (McQuade et al. 2004) and exhibit reduced food intake (Benoit et al. 2003), further supporting a role for D3 receptors in regulating food-maintained behavior. In our study repeated treatment with CJB 090 resulted in a gradual decrease in food-maintained responding over a five-day treatment period. This finding may reflect a selective effect of CJB 090 on food-reinforced behavior because cocaine-reinforced behavior was unaffected by CJB 090 using the same treatment regimen. It should be noted however, that repeated administration of CJB 090 could have resulted in sensitization to potential emetic effects (observed at doses greater than 17.8 mg/kg) which might serve as the mechanism mediating its ability to reduce food-maintained behavior. It is possible that gastrointestinal issues may not be as disruptive to cocaine self-administration explaining its lack of effect on cocaine-maintained behavior.
In summary, our drug discrimination findings show that CJB 090, acting most likely via a D3 receptor mechanism, antagonized the DS effects of cocaine at a dose that did not induce adverse side effects. Although these findings illustrate functional antagonism of one aspect of cocaine’s abuse-related behavioral profile, CJB 090 did not antagonize the reinforcing effects of cocaine or consistently block cocaine-induced reinstatement of drug-seeking. Based on these findings, CJB 090 does not appear to be a promising candidate for the treatment of active cocaine abuse or the prevention of relapse. However, the different effects of CJB 090 on cocaine and PD 128907 vs. sumanirole discrimination and its selective effects in reducing food-maintained vs. drug-maintained behavior suggest that this compound may be a useful pharmacological tool for exploring the role of D3 receptors in the DS effects of cocaine and in the regulation of food-intake.
Acknowledgments
The authors thank Jianjing Cao in the Medicinal Chemistry Section, NIDA-IRP, NIH for the synthesis of CJB 090, Shana Langer and Kristen Bano for technical contributions and Donna Reed for editorial assistance.
Support This work was supported by United States Public Health Service Grants DA011054, DA017700, NIDA-IRP and RR00168.
References
- Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikström H, Katz JL, Witkin JM. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacol. 1995;281:R7–9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Ariens EJ. Intrinsic activity: partial agonists and partial antagonists. J Cardiovasc Pharmacol. 1983;5:S8–S15. [PubMed] [Google Scholar]
- Beardsley PM, Sokoloff P, Balster RL, Schwartz JC. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav Pharmacol. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Benoit SC, McQuade JA, Clegg DJ, Xu M, Rushing PA, Woods SC, Seeley RJ. Altered feeding responses in mice with targeted disruption of the dopamine-3 receptor gene. Behav Neurosci. 2003;117:46–54. doi: 10.1037/0735-7044.117.1.46. [DOI] [PubMed] [Google Scholar]
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology (Berl) 2000;153:67–84. doi: 10.1007/s002130000567. Review. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Campiani G, Butini S, Trotta F, Fattorusso C, Catalanotti B, Aiello F, Gemma S, Nacci V, Novellino E, Stark JA, Cagnotto A, Fumagalli E, Carnovali F, Cervo L, Mennini T. Synthesis and pharmacological evaluation of potent and highly selective D3 receptor ligands: inhibition of cocaine-seeking behavior and the role of dopamine D3/D2 receptors. J Med Chem. 2003;46:3822–3839. doi: 10.1021/jm0211220. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiébot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Kiss B, Gal K, Laszlovszky I, Horvath A, Gemesi LI, Saghy K, Pasztor G, Zajer M, Kapas M, Csongor EA, Domány G, Tihanyi K, Szombathelyi Z. Effects of RGH-237 [N-{4-[4-(3-aminocarbonyl-phenyl)-piperazin-1-yl]-butyl}-4-bromo-benzamide], an orally active, selective dopamine D(3) receptor partial agonist in animal models of cocaine abuse. J Pharmacol Exp Ther. 2007;320:1268–1278. doi: 10.1124/jpet.106.107920. [DOI] [PubMed] [Google Scholar]
- Gyertyan I, Saghy K. The selective dopamine D3 receptor antagonists, SB 277011-A and S 33084 block haloperidol-induced catalepsy in rats. Eur J Pharmacol. 2007;572:171–174. doi: 10.1016/j.ejphar.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Dijkstra D, Wikstrom H, Wise LD, Pugsley TA, Sokoloff P, Pauli S, Farde L, Sedvall G. Autoradiographic localisation of D3-dopamine receptors in the human brain using the selective D3-dopamine receptor agonist (+)-[3H]PD 128907. Psychopharmacology (Berl) 1996;128:240–247. doi: 10.1007/s002130050131. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets. 2008;7:410–421. doi: 10.2174/187152708786927822. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacological analysis of drug-receptor interaction. 3. Lippincott-Raven; New York: 1997. [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB2904 and CJB090, the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Mash DC, Staley JK. D3 dopamine and kappa opioid receptor alterations in human brain of cocaine-overdose victims. Ann N Y Acad Sci. 1999;877:507–522. doi: 10.1111/j.1749-6632.1999.tb09286.x. [DOI] [PubMed] [Google Scholar]
- McCall RB, Lookingland KJ, Bédard PJ, Huff RM. Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson’s disease. J Pharmacol Exp Ther. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
- McQuade JA, Benoit SC, Xu M, Woods SC, Seeley RJ. High-fat diet induced adiposity in mice with targeted disruption of the dopamine-3 receptor gene. Behav Brain Res. 2004;151:313–319. doi: 10.1016/j.bbr.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Svenningsson P, Ashby CR, Jr, Hill M, Egeland M, Dekeyne A, Brocco M, Di Cara B, Lejeune F, Thomasson N, Munoz C, Mocaër E, Crossman A, Cistarelli L, Girardon S, Iob L, Veiga S, Gobert A. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent. II. A neurochemical, electrophysiological and behavioral characterization in vivo. J Pharmacol Exp Ther. 2008;324:600–611. doi: 10.1124/jpet.107.132563. [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berl) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-(4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl)arylcarboxamides as novel dopamine D(3) receptor antagonists. Bioorg Med Chem Lett. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3379. doi: 10.1021/jm040190e. Review. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD. Intravenous self-administration techniques in monkeys. Curr Protoc Neurosci. 2005;Chapter 9(Unit 921) doi: 10.1002/0471142301.ns0921s32. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. Review. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Modulation of cocaine and food self-administration by low-and high-efficacy D1 agonists in squirrel monkeys. Psychopharmacology (Berl) 2001;157:208–216. doi: 10.1007/s002130100779. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Dissociation of cocaine-antagonist properties and motoric effects of the D1 receptor partial agonists SKF 83959 and SKF 77434. J Pharmacol Exp Ther. 2000;293:1017–1026. [PubMed] [Google Scholar]
- Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA, Xu M, Zhang J, Richtand NM. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol Biochem Behav. 2007;86:718–726. doi: 10.1016/j.pbb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikström H, Whetzel SZ, Georgic LM, Cooke LW, DeMattos SB, Corbin AE, Glase SA, Wise LD, Dijkstra D, He¤ner TG. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther. 1995;275:1355–1366. [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci. 1994;15:374–379. doi: 10.1016/0165-6147(94)90158-9. Review. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1996;278:1128–1137. [PubMed] [Google Scholar]
- Spealman RD, Bergman J, Madras BK, Melia KF. Discriminative stimulus effects of cocaine in squirrel monkeys: involvement of dopamine receptor subtypes. J Pharmacol Exp Ther. 1991;258:945–953. [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, Ashby CR, Jr, Gardner EL, Volkow ND. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Nichols DE, Terry P, Katz JL. Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther. 1991;257:706–713. [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasić JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]