Abstract
Acute airway inflammation is associated with enhanced production of nitric oxide (NO•) and altered airway epithelial barrier function, suggesting a role of NO• or its metabolites in epithelial permeability. While high concentrations of S-nitrosothiols disrupted transepithelial resistance (TER) and increased permeability in 16HBE14o- cells, no significant barrier disruption was observed by NONOates, in spite of altered distribution and expression of some TJ proteins. Barrier disruption of mouse tracheal epithelial (MTE) cell monolayers in response to inflammatory cytokines was independent of NOS2, based on similar effects in MTE cells from NOS2-/- mice and a lack of effect of the NOS2-inhibitor 1400W. Cell pre-incubation with LPS protected MTE cells from TER loss and increased permeability by H2O2, which was independent of NOS2. However, NOS2 was found to contribute to epithelial wound repair and TER recovery after mechanical injury. Overall, our results demonstrate that epithelial NOS2 is not responsible for epithelial barrier dysfunction during inflammation, but may contribute to restoration of epithelial integrity.
Keywords: transepithelial resistance, inflammation, LPS, oxidant, wound repair, occludin, claudin
INTRODUCTION
One of the primary functions of the respiratory epithelium is to generate a physical barrier to minimize the entry of inhaled allergens, irritants or microorganisms, and chronic inflammatory disorders of the respiratory tract are typically associated with epithelial injury and increased permeability due to loss of barrier integrity [1, 2]. Among the factors that maintain epithelial polarity and barrier function are tight junctions (TJ), which comprise a complex of integral and peripheral membrane proteins, creating specialized apical borders that prevent the diffusion of soluble mediators or proteins between apical and basolateral cell surfaces [3]. The regulation of TJ function is complex and incompletely understood, but several studies have suggested that epithelial barrier function and TJ proteins are dysregulated in models of allergic airway inflammation [4, 5], and various inflammatory mediators are capable of disrupting TJ protein expression or distribution [6–8], and thereby contribute to epithelial barrier dysfunction and injury.
Among the inflammatory mediators that might affect airway epithelial integrity is nitric oxide (NO•), which is generated by various nitric oxide synthase (NOS) isoforms, including inducible NOS (NOS2) within the airway epithelium. NOS2 is expressed in airway epithelia under normal conditions, but is known to be induced by various stimuli, including pro-inflammatory cytokines, resulting in enhanced production of NO• in the airway [9, 10]. Elevated production of NO• and/or its metabolites may affect structural and functional proteins, and can interfere with the activation of various signaling pathways and transcription factors involved in pro-inflammatory gene expression [10]. Indeed, a number of studies examining epithelial injury, either in response to bacterial infection or by stimulation with inflammatory cytokines, have implicated NO• in processes that contribute to inflammation and/or injury [7, 11–13]. For example, pulmonary edema during in vivo endotoxemia was reported to depend in part on NOS2, and in vitro studies with the airway epithelial cell line Calu-3 implicated NOS2 in cytokine-induced epithelial permeability [12]. However, others failed to demonstrate involvement of NOS2-derived NO• in epithelial injury during inflammation [14, 15], and several studies suggested that NOS2 may in fact be protective against epithelial injury during oxidative stress or bacterial infection [16–19]. For example, apical stimulation of isolated rat alveolar type II cells with LPS enhanced epithelial resistance to oxidative stress, which was dependent on induction of NOS2 and elevated NO• production [18].
These various disparities in the role of NOS2 or NO• on epithelial function may be related to cell-type or species-dependent differences, and/or the use of airway or alveolar epithelial cell lines that do not fully recapitulate epithelial biology in vivo. Moreover, most of the claims regarding the involvement of NOS2 or NO• were based on studies with pharmacological inhibitors that may lack specificity, or relied on experiments in which NO• donor compounds were used at conditions that were not necessarily physiologically relevant. For this reason, we evaluated the ability of NO• to disrupt epithelial TJ proteins and barrier function in immortalized human bronchial epithelial cell line 16HBE14o- or primarily mouse tracheal epithelial (MTE) cells, using patho-physiologically relevant concentrations of NO•. Moreover, we determined the role of NOS2 in epithelial barrier dysfunction or recovery in response to pro-inflammatory cytokines or under conditions of oxidative or mechanical stress, using selective NOS2 inhibition or by comparison of MTE cells from normal and NOS2-deficient mice. Collectively, our results indicate that epithelial NOS2 does not significantly contribute to epithelial barrier dysfunction during inflammation, but rather appears to promote epithelial recovery following injury.
EXPERIMENTAL PROCEDURES
Cell Culture
Experiments were performed with the SV40-transformed human bronchial epithelial cell line 16HBE14o-, kindly provided by Dr. D. Gruenert [20]. Cells were grown in minimum essential medium (MEM) (Invitrogen; Carlsbad, CA) supplemented with 10% FBS (Invitrogen), 2 mM L-glutamine, and 100 U/100 μg/mL Pen/Strep (Sigma-Aldrich; St. Louis, MO). Cells were plated in Transwell culture inserts (PET membrane pore size 0.4 μm; Corning; Corning, NY), 8-well chamber slides (Nalge Nunc International; Rochester, NY), or 24-well culture plates (Corning), each coated with a solution consisting of LCH basal medium (Invitrogen), BSA (Invitrogen), bovine collagen I (BD laboratories; Franklin Lakes, NJ) and human fibronectin (Sigma), and grown to confluence prior to experimentation. Studies of epithelial barrier function were initiated when transepithelial electrical resistance (TER) was >300 Ω·cm2.
Additional experiments were performed using primary mouse tracheal epithelial (MTE) cells, which were isolated from either C57BL/6J or Nos2tm1Lau/J mice (Jackson Laboratory; Bar Harbor, ME), essentially as described by Wu and Smith [21]. Briefly, tracheas were isolated, filled with MEM media containing 0.1% Protease 14 (Sigma-Aldrich; St. Louis, MO), and incubated overnight at 4°C in MEM. Tracheas were then flushed with 5 ml of MEM containing 10% FBS, and dislodged cells were pelleted and cultured on rat tail collagen I gel (BD Biosciences, San Jose, CA) coated tissue culture flasks in DMEM/F12 media containing 20 ng/ml cholera toxin (List Biological Laboratories; Campbell, CA), 4 μg/ml insulin (Sigma), 5 μg/ml transferrin (Sigma), 5 μg/ml bovine pituitary extract (Invitrogen), 10 ng/ml EGF (Calbiochem; San Diego, CA), 100 nM dexamethasone (Sigma), 2 mM L-glutamine, and 50 U/50 μgl/ml Pen/Strep (Invitrogen). For experiments involving TER and permeability measurements, cells were seeded at a density of 2 × 105 cells/cm2 in Transwell culture inserts (Corning) coated with collagen I/fibronectin (Sigma) and cultured until TER >500 Ω·cm2. Alternatively, cells were plated in collagen I/fibronectin-coated 24-well plates, for additional experiments.
Cell Treatments
Cells in transwells or 24-well plates were treated with the S-nitrosothiols, S-nitroso-N-acetyl-penicillamine (SNAP; Calbiochem) or S-nitroso-L-cysteine (CSNO; prepared freshly before experimentation [22]), or with the NO-donor diethylenetriamine NONOate ((Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate; DETA-NO). Release of NO• from DETA-NO was monitored using an amiNO-FLAT Nitric Oxide Sensor and analyzed using inNO 3.0 software (Harvard Apparatus, Holliston, Massachusetts). Alternatively, cells were treated with the cytokines TNF-α (10 ng/mL), IFN-γ (100 ng/mL), and IL-1β (1 ng/mL) (Calbiochem), or with 10 μg/ml LPS (serotype E. coli 055:B5; Sigma), for the indicated times before functional or biochemical analyses. Where indicated, cells were pre-incubated with the NOS2 inhibitor 1400W (Alexis Biochemicals;San Diego, CA), the guanylyl cyclase inhibitor ODQ (Sigma), the protein kinase G (PKG) inhibitor KT 5823 (Calbiochem), or the proteasome inhibitor MG132 (Calbiochem), for 30 min prior to NO•-donor or cytokine treatment. Cellular NO• production was evaluated by measuring nitrite accumulation within the medium using the Griess assay [23]. For treatment of cells in Transwell inserts, all reagents were added to both apical and basolateral compartments.
Determination of Transepithelial Electrical Resistance (TER)
Barrier function of 16HBE14o- or MTE cells in transwell culture plates was measured as TER using an EVOM™ Epithelial Voltohmmeter (World Precision Instruments; Sarasota, FL). TER values were corrected for background resistance of the coated culture insert and medium.
Analysis of Epithelial Permeability
The permeability of 16HBE14o- or MTE monolayers was determined by measuring the transepithelial passage of the water-soluble fluorescent probe FITC-dextran (Mr 4 kDa, Sigma-Aldrich) from the apical to the basolateral compartment. Apical medium was replaced with 150 μL of medium containing 25 mg/mL FITC-dextran, prior to cell exposure to NO• donors or stimulation with cytokines or H2O2. Aliquots (30 μL) of the basolateral medium were collected at various time points for analysis of fluorescence using a Biotek Synergy HT plate reader (Biotek; Winooski, VT), and epithelial permeability was expressed as percent leakage of FITC-dextran from apical to basolateral compartments.
In Vitro Wound Assay
To investigate the effects of NO• on epithelial cell migration and wound repair, confluent MTE cell monolayers in 24-well plates were mechanically wounded using a sterile P-200 pipette tip, creating a linear scratch of 0.5 mm width. After washing to remove cell debris, fresh media was added to each well and appropriate reagents were re-administered. Wound closure was followed using an IX70 inverted microscope (Olympus; Center Valley, PA) and imaged wound areas were quantified using ImageJ software (NIH), for calculation of % wound closure [23]. In order to associate epithelial wound repair with restoration of barrier integrity, an alternative wound model was used in which MTE cells in Transwell inserts (TER >500 Ω·cm2) were wounded by applying a sterile glass Pasteur pipette, connected to a vacuum pump, to the center of the well, in an attempt to create a circular wound of defined size. Successful injury was evaluated by >90% loss of TER, after which cells were washed to remove cell debris, and fresh media and treatments were added to monitor recovery of TER over time.
Immunofluorescence Analysis of TJ Proteins
Cells grown on permeable supports were fixed with 4% PFA and washed twice with PBS. Cells were permeabilized with 0.05% Triton X-100 for 10 min at room temperature and blocked with PBS containing 2.5% BSA for 30 min, and incubated overnight (4°C) with monoclonal or polyclonal antibodies against zonula occludens (ZO)-1 (1 μg/mL), occludin (0.5 μg/mL), or claudin-1 (1 μg/mL) (Zymed; Carlsbad, CA). Negative controls were incubated with TBS-Triton containing 2.5% BSA alone instead of a primary antibody. After 3x washing with PBS, cells were incubated with Alexa Fluor 568-conjugated goat α-mouse/rabbit secondary antibodies (Invitrogen) for 30 min in the dark. Nuclei were stained with SYTOX (Molecular Probes Inc.; Eugene, OR) and inserts were mounted using Aquamount (Polysciences, Inc.; Warrington, PA). Images were captured using an inverted Bio-Rad MRC-1024 Confocal Laser Scanning Microscope with Lasersharp 2000 software (Bio-Rad, Hercules, CA, USA).
Western Blot Analysis
For total cellular protein extracts, cells were washed 3x with cold PBS and lysed in cold RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.1 mg/ml APMSF, 1.0 mM sodium orthovanadate, and 1x mammalian protease inhibitor cocktail; Sigma). Samples were sonicated on ice for 5–10 sec and spun at 14,000 rpm for 5 min to remove cell debris. Aliquots (20 μg protein) were loaded onto 7.5% (ZO-1) or 12% (claudin-1 and occludin) SDS-page gels, transferred to PVDF membranes, and blotted with primary antibodies against ZO-1 (1:500), occludin (1:1000), or claudin-1 (1:500) (Zymed). Primary antibodies were detected with HRP-conjugated secondary antibodies (Cell Signaling; Danvers, MA) and enhanced chemiluminescence (ECL) (Pierce, Rockford, IL).
RT-PCR Analysis
RNA was isolated from cells using an RNeasy mini Kit (Qiagen; Valencia, CA) according to the manufactures instructions. Complementary DNA (cDNA) was prepared from 1 μg of total RNA with M-MLV reverse transcriptase and Oligo(dT)15 primer (Invitrogen), and 5 μL of a 1:10 dilution of cDNA was amplified 33 times with Taq DNA polymerase (Invitrogen) and appropriate primer sets and visualized on 1% agarose gels stained with ethidium bromide. Gels were analyzed using GelDoc XR and Quantity One 1-D software (Bio-Rad Laboratories) and mRNA levels were normalized to β-actin. Primers used for cDNA amplification were: h-occludin: left 5′-tttgtgggacaaggaacaca-3′, right 5′-gcaggtgctctttttgaagg-3′; h-claudin-1: left 5′-gccccagtggaggatttact-3′, right 5′-agccagacctgcaagaagaa-3′; h-β-actin: left 5′-tgacggggtcacccacactgtgcccatcta-3′, right 5′-ctagaagcatttgcggtggacgatggaggg-3′; m-iNOS: left 5′-ccttgttcagctacgccttc-3′, right 5′-agttcgtccccttctcctgt-3′; m-β-actin: left 5′-tgttaccaactgggacgaca-3′, right 5′-tctcagctgtggtggtgaag-3′ (Operon; Huntsville, AL).
Statistical analysis
Quantitative data are presented as mean ± S.E.M. from 2–3 experiments performed in duplicate or triplicate. Statistical significance was evaluated using Minitab 15.1 (Minitab Inc., State College, PA.) and results were considered significant when p<0.05 unless noted otherwise.
RESULTS
Effects of NO• Donors on Epithelial Barrier Function
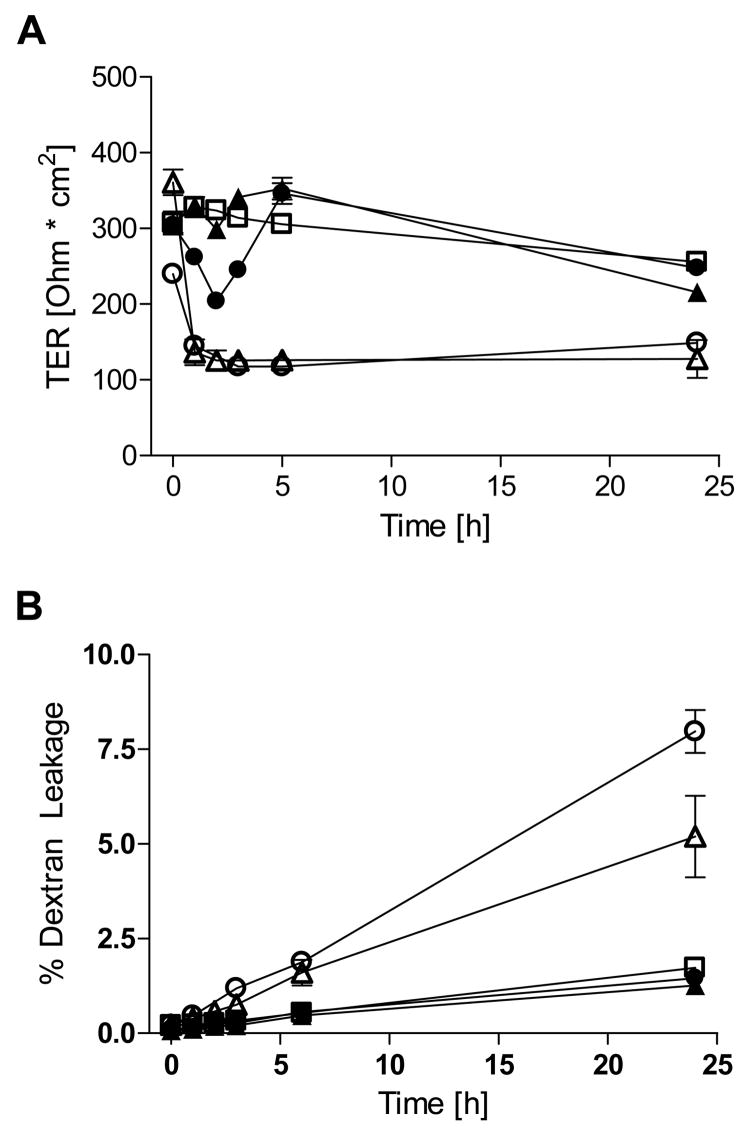
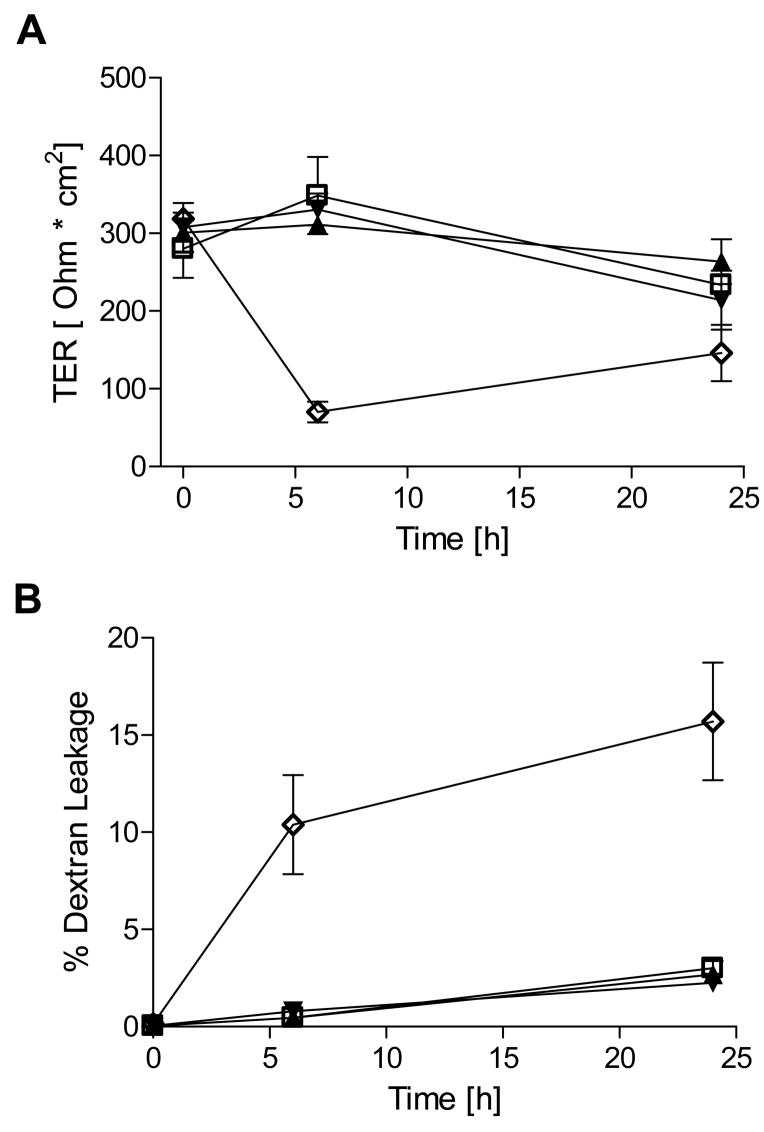
We first sought to confirm previous observations [24] that high concentrations of S-nitrosothiols are capable of reducing epithelial barrier function. Indeed, exposure of confluent 16HBE14o- monolayers (TER >300 Ω·cm2) to 1 mM SNAP induced a dramatic and persistent decrease of TER (Fig. 1A), as well as a marked increase in permeability to FITC-Dextran over 24 hrs (Fig. 1B). Similar exposure to CSNO resulted in a more transient loss of TER during the first 2–3 hrs, consistent with its limited chemical stability [25], and did not significantly enhance permeability to FITC-Dextran over 24 hrs (Fig. 1). Because these high concentrations of S-nitrosothiols do not represent physiologically relevant conditions, we next exposed16HBE14o- cells to various NONOates that more closely mimic (patho)physiological fluxes of NO•. As shown in Fig. 2, exposure to DETA-NONOate (up to 500 μM) did not significantly decrease TER in 16HBE14o- cells, and did not increase epithelial permeability to FITC-dextran. Higher concentrations of DETA-NO (>1 mM) caused a modest and gradual decrease in TER, but also increased cytotoxicity as measured by lactate dehydrogenase release (data not shown). For comparison, cell treatment with 2 mM EGTA, which chelates Ca2+ and disrupts TJ’s [26], resulted in rapid loss of TER and increase FITC-dextran permeability (Fig. 2). Similarly, exposure of primary MTE cells to DETA-NO (500 μM) did not cause a significant change in TER over 24 hrs, compared with untreated controls (data not shown). Measurements of NO• concentrations during treatments with 100 or 500 μM DETA NONOate indicated steady-state levels of 80–100 and 250–300 nM NO•, respectively, which remained relatively constant over 24 hrs.
Figure 1. CSNO and SNAP decrease barrier function in human bronchial epithelial cells.
Effects of CSNO and SNAP (500 and 1000 μM) on (A) TER and (B) FITC-dextran (4 kDa) leakage were monitored in 16HBE14o- cells over 24 hrs. FITC-dextran permeability was assessed as apical to basolateral passage in polarized monolayers as measured by fluorescence (excitation at 492 nm, emission at 515 nm; n=4). □ = control; ▴ = CSNO 500 μM; • = CSNO 1000 μM; ▵ = SNAP 500 μM; ○ = SNAP 1000 μM.
Figure 2. Exposure to DETA NONOate does not cause barrier dysfunction in 16HBE14o- cells.
Effects of the indicated concentrations of DETA NONOate (100 and 500 μM) on (A) transepithelial electrical resistance and (B) FITC-dextran leakage were monitored in 16HBE14o- cells over 24 hrs. For comparison, loss of TER and increased FITC-dextran permeability upon TJ disruption by the Ca2+-chelator EGTA (2 mM) are shown (n=4). □ = control; ▴= DETA–NO 100 μM; ▾ = DETA – NO 500 μM; ⋄ = EGTA 2mM.
Effects of NO• on Regulation of TJ Proteins
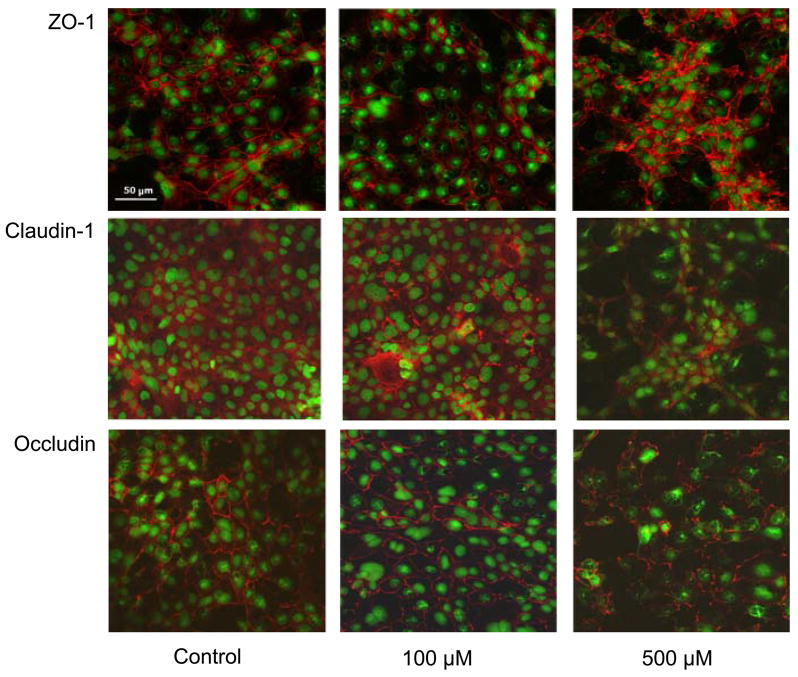
In addition to evaluating epithelial barrier function by exogenous NO• donors, we also explored whether NO• is capable of altering the overall expression and distribution of TJ proteins, as suggested previously [7]. As illustrated in Fig. 3, the TJ proteins occludin, claudin-1, and ZO-1 are localized at the apical portion of polarized 16HBE14o- cells and evenly distributed around the entire circumference of the cell. Cell exposure to 100 μM DETA-NO for 24 hrs did not significantly affect the distribution of these proteins, but exposure to 500 μM DETA-NO appeared to markedly disrupt their localization. As shown, ZO-1 staining was reorganized and no longer located strictly at the cell borders, and the distribution of both occludin and claudin-1 appeared punctuate and less abundant as compared to untreated controls (Fig. 3).
Figure 3. DETA NONOate causes altered TJ protein distribution in human bronchial epithelial cells.
Monolayers of 16HBE14o- cells in 8-well chamber slides were treated with DETA NONOate (100 or 500 μM) for 24 hrs, and TJ protein distribution was visualized using primary antibodies against ZO-1, claudin-1, or occludin, and Alexa Fluor 568-conjugated secondary antibody. Nuclei were counterstained with SYTOX green, and images of a single focal plane were captured using an inverted Bio-Rad MRC-1024 confocal laser-scanning microscope.
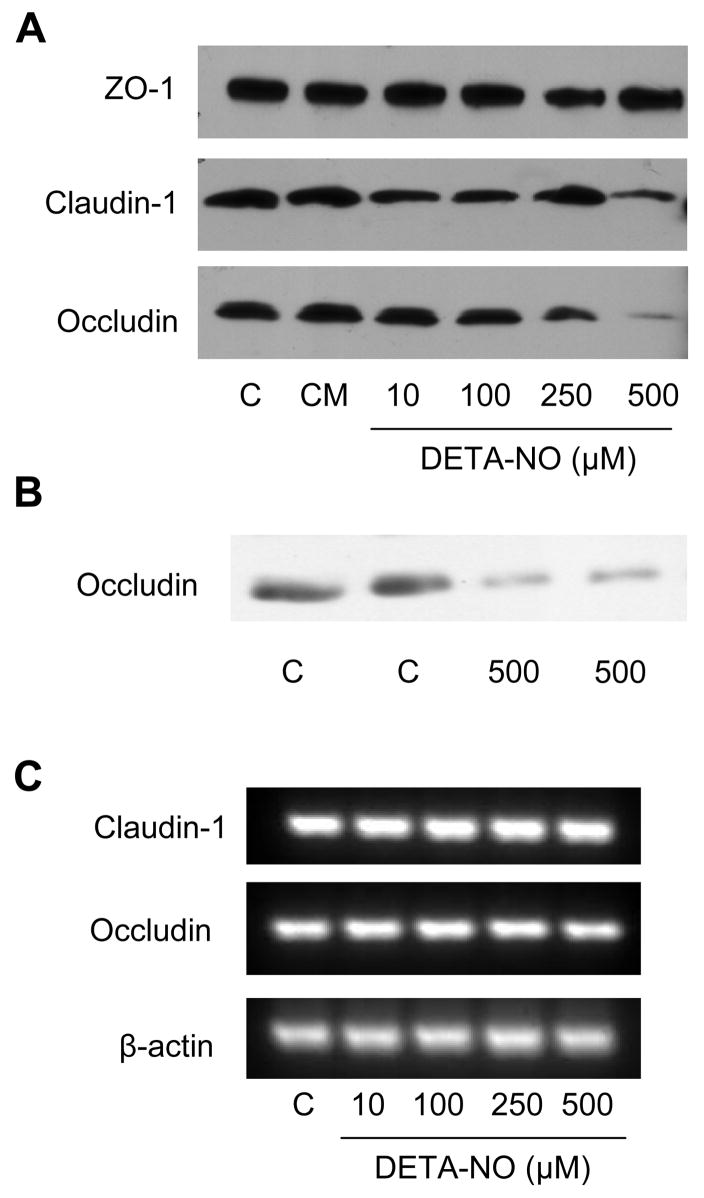
To determine if the observations obtained by confocal microscopy were due to changes in the overall expression of TJ proteins, extracts from DETA-NO-exposed cells were analyzed by western blotting. While no differences were observed in ZO-1 protein levels in response to up to 500 μM DETA-NO, overall levels of both occludin and claudin-1 were found to be reduced in a dose dependent manner (Fig. 4A). Occludin levels decreased at >100 μM DETA-NO, whereas claudin-1 started to decline using >250 μM DETA-NO. Similarly, DETA-NO exposure (500 μM) also reduced protein levels of occludin in MTE cells (Fig. 4B). These alterations in claudin-1 and occludin protein levels, were not accompanied by changes in occludin and claudin-1 mRNA expression (Fig. 4C), suggesting a post-translational mechanism. Suppression of occludin protein expression by DETA-NO was also not affected by 30 min pre-incubation with the soluble guanylyl cyclase inhibitor, ODQ (10 μM) or the protein kinase G (PKG) inhibitor KT 5823 (10 μM) (not shown), indicating that DETA-NO acts by a mechanism independent of cGMP production and activation of PKG. Finally, we speculated that DETA-NO might promote proteasomal degradation of occludin. However, DETA-NO-induced decrease in occludin levels were unaffected by pre-incubation with the proteasome inhibitor, MG 132 (1 or 10 μM) (not shown). In summary, these various findings indicate that epithelial cell exposure to exogenous NO•, at pathophysiologically relevant concentrations, results in redistribution and reduction of some TJ proteins, but these changes were not accompanied by significant alterations in epithelial TER or permeability.
Figure 4. Alterations in bronchial epithelial cell TJ protein expression by DETA NONOate.
Monolayers of 16HBE14o- cells (A) or MTE cells (B) were exposed to DETA NONOate or a mixture of TNF-α, IL-1β and IFN-γ for 24 hrs, and whole cell lysates were analyzed by western blot using antibodies against ZO-1, occludin, or claudin-1. (C) RT-PCR analysis of claudin-1 and occludin mRNA after exposure of 16HBE14o- cells to DETA NONOate. Representative blots of at least 3 experiments are shown.
Role of NOS2 in Epithelial Barrier Regulation by Inflammatory Cytokines
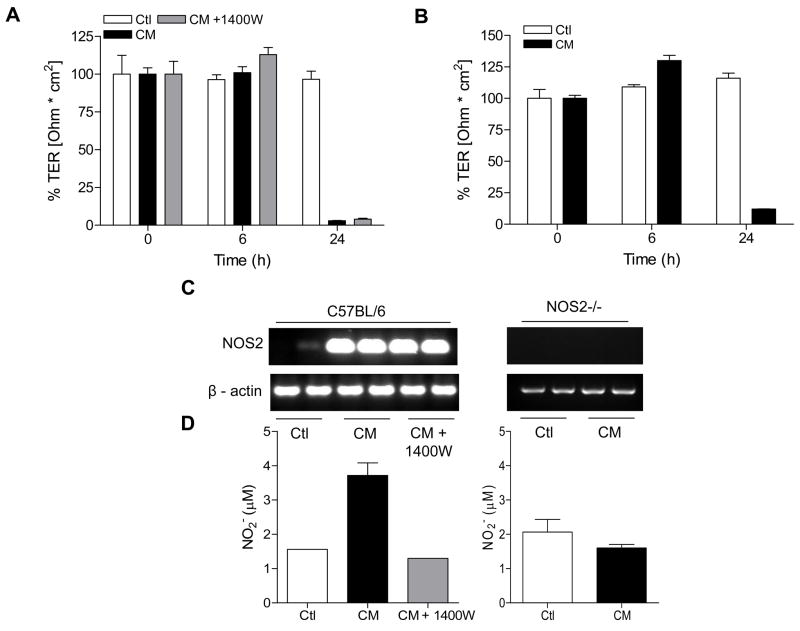
It is well appreciated that pro-inflammatory cytokines can disrupt barrier properties of intestinal and pulmonary epithelia [5, 6, 8, 27]. Moreover, previous studies have suggested that induction of NOS2 and production of NO• contribute to epithelial barrier dysregulation by altering the expression or distribution of TJ proteins [7, 12]. To address the potential contribution of endogenously produced NO• on airway epithelial barrier function, 16HBE14o- or MTE cells were stimulated with a mixture of cytokines (CM) known to induce epithelial expression of NOS2. Neither CM nor IFN-γ alone significantly altered TER of 16HBE14o- cells over 48 hrs (not shown), but treatment of MTE cells with CM resulted in markedly decreased TER in MTE cells after 24 hrs, which was accompanied by induction of NOS2 and increased NO• production (Fig 5A,C,D). Cytokine-induced TER loss was, however, not significantly affected in the presence of 1400W, a specific inhibitor of NOS2 which effectively inhibited CM-induced NO• production (Fig 5A,D). Moreover, induction of NOS2 with IFN-γ alone was not sufficient to induce significant changes in TER (not shown). To more directly assess the role of NOS2 in CM-induced TER loss, similar experiments were performed using MTE cells isolated from NOS2-/- mice. As shown in Fig. 5B, CM stimulation disrupted TER of NOS2-/- MTE cells with similar kinetics compared to MTE from control mice, indicating that TER loss is independent of NOS2. Consistent with earlier observations [12], CM stimulation also increased epithelial permeability in MTE cells, as measured by FITC-dextran leakage after 24 hrs, from 6% in untreated controls to 35% in CM treated wells. However, CM-induced FITC-dextran leakage was not prevented in the presence of 1400W (46% vs. 35% in controls; n=2), and CM stimulation similarly increased FITC-dextran permeability in MTE cells from NOS2-/- mice compared to MTE cells from WT mice (41% vs. 35% respectively, n=2). In addition, in contrast to the observed loss of TJ proteins by exogenous DETA-NO (Fig. 4), exposure of 16HBE14o- cells to CM did not alter the level of TJ proteins (Fig. 4). Collectively, these findings indicate that NO• production from endogenous epithelial NOS2 is not sufficient to induce alterations in epithelial barrier function during conditions of airway inflammation. In fact, the slight increases in CM-induced permeability to FITC-dextran in the presence of 1400W or in NOS2-/- MTE cells suggest that NOS2 may in fact be protective against epithelial barrier loss in response to cytokine stimulation.
Figure 5. Cytokine-induced TER loss in MTE cells is independent of NOS2.
MTE cells from C57Bl6/J (A) or NOS2-/- mice (B) were treated with a cytokine mixture (CM: 10 ng/mL TNF-α; 1 ng/mL IL-1β; 100 ng/mL IFN-γ) and TER was monitored over 24 hrs. Where indicated, the NOS2-specific inhibitor 1400W (100 μM) was added 30 min prior to CM stimulation. Data were expressed relative to initial TER for each well (n=4). Effects of CM stimulation on NOS2 mRNA expression or NO• production was assessed by RT-PCR (C) or analysis of nitrite accumulation in the media (D). White bars = control; Black bars = cytokine mix; Grey bars = cytokine mix + 1400W.
Does NOS2-Derived NO• Affect Epithelial Barrier Recovery upon Injury?
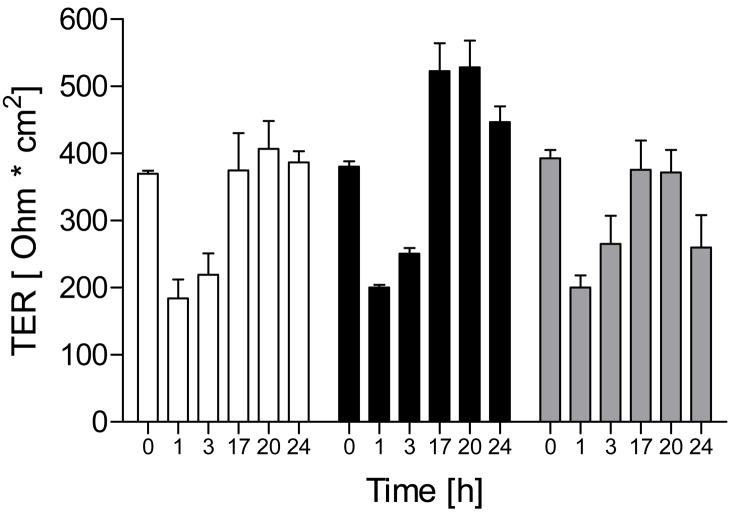
Contrary to claims that NO• can contribute to epithelial barrier disruption during inflammation, several studies have suggested that NO• may protect barrier function during infection or oxidative stress [16–19]. To determine whether NO• is capable of promoting restoration of disrupted barrier function, we incubated 16HBE14o- cells in MEM without FBS and supplemented with 2 mM EGTA, which results in TJ disruption [26], and followed TER restitution after replacing the EGTA medium with normal Ca2+-containing medium in the absence or presence of DETA-NO. As shown in Fig. 6, Ca2+ depletion for 24 hrs resulted in TER loss, which was restored to baseline levels upon addition of Ca2+-containing medium within 18 hrs. Administration of 100 μM DETA-NONOate during Ca2+ replenishment did not affect the rate of TER recovery, but resulted in slightly higher TER after 18 hrs, suggesting some improvement of barrier function in the presence of NO•. Higher concentrations of DETA-NO (500 μM) did not improve barrier function over controls (Fig. 6).
Figure 6. Effect of NO• on TER recovery after Ca2+ depletion.
16HBE14o- cells in Transwell inserts (TER >300 Ω·cm2) were rinsed in Ca2+-free HBSS and incubated in MEM without FBS in the presence of 2 mM EGTA to disrupt TJ’s [26], after which media was replaced with normal Ca2+-containing medium in the absence or presence of indicated concentrations of DETA-NO, and TER recovery was monitored. Results from a typical experiment in duplicate are shown. White bars = control; Black bars = DETA-NO 100 μM; Grey bars = DETA-NO 500 μM.
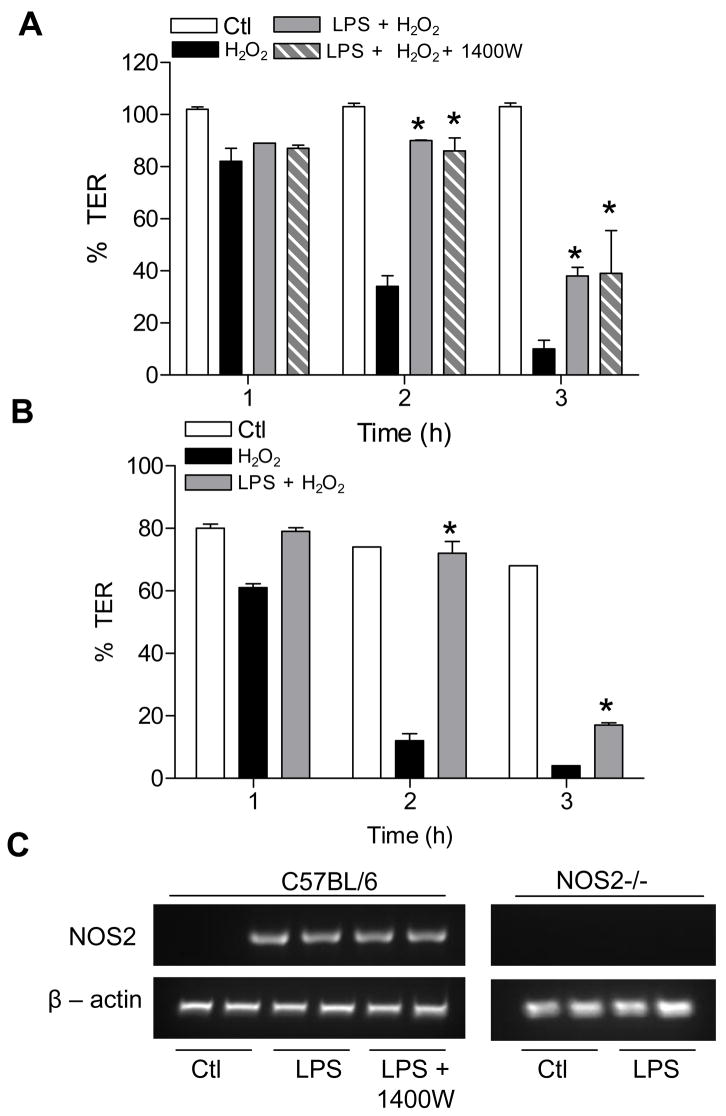
In the next set of studies, we determined whether epithelial NO• may protect against oxidant-induced barrier function, as suggested previously [16, 18]. To this end, we pre-incubated MTE cells from WT or NOS2-/- mice with LPS for 24 hrs, to induce NOS2 expression (Fig. 7C), and then assessed their sensitivity to TER loss or epithelial permeability in response to H2O2. Although treatment of MTE cells with H2O2 at concentrations up to 250 μM did not significantly affect TER over 24 hrs, exposure of MTE cells to 500 μM H2O2 resulted in rapid loss of TER within several hours (Fig. 7A), which was associated with increased FITC-Dextran permeability and decreased levels of occludin (not shown). Pre-incubation of MTE cells with LPS did not significantly affect basal TER or dextran permeability, but partially protected MTE cells from H2O2-induced barrier dysfunction, as measured by TER (Fig. 7A). Similarly, LPS preincubation also partially prevented H2O2-induced FITC-dextran permeability (not shown). However, comparable protective effects of LPS pre-incubation were observed in the presence of 1400W (Fig. 7A) or in MTE cells from NOS2-/- cells (Fig. 7B), indicating that the protective effects of LPS are independent of NOS2-derived NO•.
Figure 7.
LPS pre-incubation protects against H2O2-induced barrier loss independent of NOS2. MTE cells from C57Bl6/J (A) or NOS2-/- mice (B) were pre-treated with LPS (10 μg/mL) for 24 hrs, and subsequently exposed to H2O2 (500 μM) for 3 hrs during which TER was monitored. The NOS2 specific inhibitor 1400W (100 μM) was added 30 min prior to H2O2 exposure. (C) RT-PCR analysis of LPS-induced expression of NOS2 mRNA. Data were expressed relative to initial TER for each well. White bars = control; Black bars = H2O2; Grey bars = LPS + H2O2; Hatched bars = LPS +1400W + H2O2. *: p<0.05 vs treatment with H2O2 alone, using Repeated Measure ANOVA followed by a Fisher test as post-hoc (n=6).
Epithelial NOS2 Contributes to Wound Repair and Restoration of Barrier Function
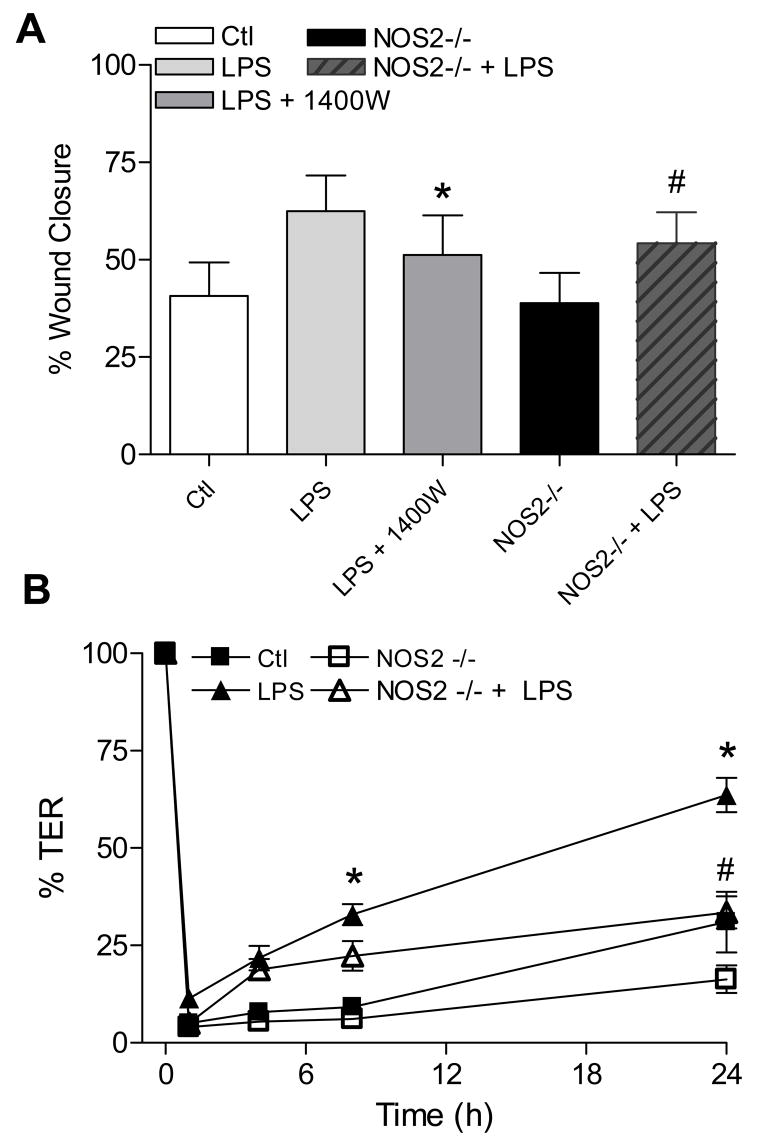
We previously demonstrated that NOS2-derived NO• contributes to airway epithelial repair after mechanical injury (24). To extend this finding, we performed scratch wound assays in MTE cell monolayers from WT or NOS2-/- mice. Because epithelial wound repair can be accelerated by LPS (25), we addressed the role of NOS2 in this process. MTE cell pretreatment with LPS 24 hrs prior to wounding enhanced the extent of wound closure by about 65% compared to untreated controls. Moreover, this positive effect of LPS pretreatment on wound closure was markedly attenuated in the presence of the NOS2-inhibitor 1400W or in MTE cells from NOS2-/- mice (Fig. 8A).
Figure 8. LPS promotes epithelial wound repair and TER restoration via NOS2 dependent mechanisms.
(A) MTE cell monolayers from C57Bl6/J or NOS2-/- mice in 24-well plates were pre-treated with LPS (10 μg/mL; 24 hrs), and subjected to linear scratch injury using a pipette tip. Wound areas were monitored over 24 hrs, and wound closure was expressed as a percentage of the initial wound area. *: p<0.1 vs. LPS treated cells; #: p <0.1 vs. corresponding C57Bl6/J cells, using paired t-test (n=12). (B) MTE cells in Transwell inserts were pre-treated with LPS (10 μg/mL; 24 hrs) before administration of circular wounds by application of a glass pasteur pipette attached to a vacuum pump for ~30 sec. Loss and recovery of TER was monitored over 24 hrs, and expressed relative to initial baseline TER (>500 Ω·cm2). *: p<0.05 vs. untreated C57Bl6/J cells; #: p<0.05 vs. corresponding LPS-treated C57Bl6/J cells, using Repeated Measure ANOVA followed by a Fisher test as post-hoc (n=4).
To determine whether these changes in wound closure translate to altered recovery of epithelial barrier integrity, MTE cells from either WT or NOS2-/- mice were plated in Transwell inserts until TER was >500 Ω·cm2. Wounds were then administered by placing a sterile glass Pasteur pipette, attached to a vacuum pump, onto the cell monolayer to create a defined circular wound. As shown in Fig. 8B, this resulted in an immediate 90–95% reduction in TER, which recovered to about 30% of initial TER in control MTE cells after 24 hrs. For comparison, TER recovery was attenuated in MTE cells from NOS2-/- cells (about 16% over the same time span). Consistent with its ability to increase wound repair in linear scratch assays (Fig. 8A), pre-treatment with LPS significantly improved the restitution of TER in similarly wounded MTE cells, and this effect was again attenuated in MTE cells from NOS2-/- mice. Taken together, these results extend our earlier findings and implicate an important role for NOS2 in LPS-mediated effects on epithelial repair and in restoring barrier integrity in response to injury.
DISCUSSION
The main objective of our studies was to address seemingly conflicting reports in the literature regarding the ability of NO• or its metabolites to either contribute to epithelial barrier dysfunction during inflammation or to promote epithelial barrier recovery during inflammation or oxidative stress. Overall, our results demonstrate that high concentrations of NO• or S-nitrosothiols are capable of impairing epithelial barrier function and altering TJ protein expression or distribution, as suggested previously [7, 11, 24], but indicate that pathophysiologically relevant concentrations of NO• (< μM) do not significantly impair epithelial barrier function. It is important to note that studies using high concentrations of S-nitrosothiols do not reflect relevant physiological levels of S-nitrosothiols (typically in the nM range), and that S-nitrosothiols possess redox properties different from NO• itself and therefore do not necessarily adequately mimic NO•-induced biological responses [28].
A role for NOS2-derived NO• in epithelial barrier dysfunction during endotoxemia has been implicated based on studies using pharmacological inhibitors of NOS or chemical NO• scavengers [7, 11, 12, 29]. Additionally, in vitro studies by Han et al. using Calu-3 epithelial monolayers indicated that cytokine-induced permeability to FITC-dextran (Mr 4 kDa) was blocked by the NOS2 inhibitor L-NIL, implicating a direct role for epithelial NOS2 [12]. Our studies do not support this claim, as they failed to demonstrate significant changes in cytokine-induced loss of TER or increased epithelial permeability in the presence of the NOS2-specific inhibitor 1400W or in MTE cells from NOS2-/- mice. In fact, our result suggested the opposite, as CM-induced epithelial permeability appeared to be enhanced if NOS2 was inhibited or absent. One difference between our studies and those of Han et al. [12] is that our experiments were conducted at pH 7.4 whereas they used a pH of 6.8, in an attempt to mimic the slightly acidic conditions at the airway surface. Analogous to our present findings, it was recently reported that cytokine-induced albumin leak of pulmonary microvascular endothelial cells (PMVEC) was independent of NOS, and could not be mimicked by exogenous NO• donors [30]. Moreover, these studies also pointed out that cytokine-induced PMVEC barrier loss in co-culture studies with neutrophils was NOS2-dependent, and related to neutrophil-dependent formation of reactive nitrogen species such as peroxynitrite (ONOO−) [30, 31]. Accordingly, the contributing role of NOS2 in neutrophil extravasation into the airways in models of acute lung injury appeared to be due to NOS2 originating from bone marrow-derived cells and not from parechymal (epithelial) cells [32, 33]. Indeed, our previous studies indicate that LPS stimulates NOS2-dependent pro-inflammatory cytokine production, most likely from alveolar macrophages [33], which in turn could be responsible for epithelial barrier changes that would facilitate neutrophil emigration. Thus, in spite of the limitations of our studies with isolated epithelial cells, which do not take into account interactions with other cell types, our present findings are consistent with those of Mehta and colleagues [30, 32] and confirm that epithelial/endothelial NOS activation does not directly increase paracellular permeability but may require the activation of inflammatory cell types which produce NO• and promote its metabolism to more reactive species.
Previous reports demonstrated the ability of NO• to regulate TJ proteins in intestinal or alveolar epithelial cells in vitro [7, 12, 34] or in pulmonary or hepatic tissues in endotoxemic mice, based on studies with either pharmacological NOS inhibitors or NOS2-deficient mice [12, 35]. However, these studies did not establish a direct relationship between NO•-dependent changes in TJ proteins and epithelial barrier function, and the effects of NO• may have been indirect, as discussed above. Our results indicate that exposure of 16HBE14o- monolayers to exogenous NO• caused some alterations in the distribution and overall levels of the TJ proteins occludin and claudin-1, but these changes were not associated with significant increase in epithelial permeability or loss of TER. Indeed, the overall importance of occludin for epithelial barrier function may be limited, since occludin-null mice are viable and do not appear to suffer major epithelial defects [36]. Moreover, knockdown of occludin in Madin-Darby canine kidney (MDCK) cells resulted in some changes in TJ regulation, but did not significantly affect TJ fence function or steady-state epithelial TER maintenance [37]. Similarly, effects of modest changes in claudin-1 levels, as observed in our study, may only have subtle consequences which might be masked by other members of the large claudin family [38, 39]. Given these considerations, it is perhaps not surprising that the relatively modest changes in TJ protein expression or distribution by NO• do not necessarily translate to significant alterations in overall epithelial barrier function [3]. Attempts to address the mechanisms by which NO• may suppress occludin indicated that this occurs by cGMP-independent mechanisms, perhaps related to post-translational or proteolytic processing [40–42], although it was not due to proteasomal degradation. In addition to proteolytic occludin processing, its biological function is also influenced by serine/threonine and tyrosine phosphorylation [40], events that may be subject to regulation by NO• [10]. Additionally, it has been suggested that occludin redistribution or degradation may also be mediated by activation of protein kinase A (PKA) [43], a pathway that may be activated by NO•. Because of the complex relationship between the various TJ proteins and overall epithelial permeability [3], their regulation by NO• was not further explored in the present study.
While our studies did not suggest a significant role of epithelial NOS2 in epithelial barrier dysfunction during infection or inflammation, they are in fact more consistent with several recent reports indicating that NOS2-derived NO• protects barrier integrity during infection or oxidative stress. First, although addition of exogenous NO• did not seem to accelerate TER recovery after TJ disruption by Ca2+ depletion, TER was restored to slightly higher levels (Fig. 6), suggesting a salutory role of NO• in barrier restoration after disruption. Similarly, previous studies have indicated that NO• can help prevent intestinal or alveolar epithelial barrier dysfunction in response to oxidant injury [16, 18]. We attempted to confirm and extend these findings to MTE cells, and observed that LPS pre-exposure of MTE cell monolayers (which induces NOS2 expression) protected these cells from H2O2-induced barrier dysfunction, measured by loss of TER or FITC-dextran permeability. However, this protective response was not prevented by the NOS2-selective inhibitor 1400W and was comparable in MTE cells from NOS2-/- mice, arguing against a role for NOS2. It is important to emphasize that, although both TER and paracellular permeability to neutral solutes are commonly used measures of epithelial barrier function, these parameters reflect different aspects of epithelial barrier function and are not necessarily directly related [3, 16]. Analysis of TER reflects the permeability to ions at a given time point, whereas the latter reflects the permeability to relatively large molecules over a period of time. Indeed, previous studies indicate that NOS2-derived NO• may protect against oxidant-induced paracellular epithelial permeability, but is less effective in protecting against TER loss [16, 18]. Nevertheless, our findings indicate that LPS pretreatment protects MTE cells from both loss of TER and increased FITC-dextran permeability in response to H2O2, which was in either case independent of NOS2. Although we cannot rule out the impact of different experimental conditions, the apparent discrepancies between our results and those of others [16, 18] likely reflect species- and cell-specific differences.
In spite of the relative lack of effect of NOS2 in LPS-mediated protection against H2O2-mediated TER loss and permeability in MTE cells, our results indicate that NOS2 does contribute to epithelial recovery of barrier function after mechanical injury (Fig. 8), which is related to NOS2-dependent effects on epithelial cell migration and wound closure [44]. Indeed, LPS pre-stimulation enhanced MTE cell migration and wound closure as well as TER recovery, which was partially dependent on the presence of NOS2. These findings are consistent with previous observations indicating that NOS2-derived NO• can promote epithelial cell migration by cGMP-dependent mechanisms, which may include activation of signaling cascades that regulate cytoskeletal alterations (e.g. by promoting phosphorylation of myosin light chain kinase) [45–47], as well as upregulation and activation of matrix metalloproteinase-9 (MMP-9), a critical mediator of epithelial wound repair [23]. With respect to the latter, NOS2 was recently found to localize at the leading edge of migrating trophoblasts in close proximity to MMP-9 [48], further confirming an association between NOS2 and MMP-9. Additionally, although cell migration is the primary mechanism of wound closure in such wound models, we cannot rule out the possibility that NO• might also improve wound closure and TER recovery by stimulating cell proliferation.
Collectively, these findings suggest that induction of epithelial NOS2 during pulmonary infection of inflammation is likely to present a protective response that facilitates recovery of epithelial injury or dysfunction as a result of the acute innate immune response. While airway epithelial NOS2 is recognized as a critical mediator of innate antimicrobial or anti-viral host defense [9], it is at present not known whether airway epithelial NOS2 also mediates epithelial barrier restoration following infection or injury. Recent studies of intestinal infection with C. parvum or S. aureus have suggested such a role, and showed that induction of epithelial NOS2 was protective, in part by improving epithelial barrier function [17, 19]. Interestingly, this protective effect of NOS2 has been linked to induction/activation of COX-2 and production of prostaglandin E2 [19]. NOS2 also activates COX2 and PGE2 in airway epithelial cells [10], and PGE2 is a positive regulator of epithelial cell migration and wound closure [49], thus it is plausible that similar mechanisms may be operative in airway epithelial responses to infection or inflammation, serving to limit epithelial injury and to promote restoration of epithelial function.
In summary, our results indicate that activation of airway epithelial NOS2 and production of NO• are not responsible for epithelial barrier dysfunction during airway inflammation, although it is possible that epithelial NO• might contribute by indirect mechanisms in concert with activated inflammatory cell types. Instead, epithelial NOS2 activation may contribute to restoration of barrier dysfunction following injury, by promoting wound repair processes, and may thereby be beneficial in preventing chronic airway inflammation and injury.
Acknowledgments
The authors wish to thank Douglas Taatjes for assistance with confocal microscopy, and Yvonne Janssen-Heininger and Amy Guala for their assistance and advice with MTE cell isolation and culture. This work was supported by NIH grants HL60812, HL068865, and HL074295.
List of Abbreviations
- DETA-NO
Diethylentriamine NONOate ((Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate)
- DPTA-NO
Dipropylenetriamine NONOate
- CSNO
S-nitroso-cysteine
- HBE
human bronchial epithelial
- MDCK
Madin-Darby canine kidney
- MTE
mouse tracheal epithelial
- NO•
nitric oxide
- NOS
nitric oxide synthase
- PMVEC
pulmonary microvascular endothelial cells
- SNAP
S-nitroso-N-acetyl-penicillamine
- TER
transepithelial electrical resistance
- TJ
tight junctions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayram H, Rusznak C, Khair OA, Sapsford RJ, Abdelaziz MM. Clinical & Experimental Allergy. 2002;32:1285–1292. doi: 10.1046/j.1365-2745.2002.01435.x. [DOI] [PubMed] [Google Scholar]
- 2.Knight DA, Holgate ST. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 3.Schneeberger EE, Lynch RD. Am J Physiol Cell Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 4.Cloutier MM, Guernsey L, Wu CA, Thrall RS. Am J Pathol. 2004;164:1849–1856. doi: 10.1016/S0002-9440(10)63743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans SM, Blyth DI, Wong T, Sanjar S, West MR. Am J Respir Cell Mol Biol. 2002;27:446–454. doi: 10.1165/rcmb.4776. [DOI] [PubMed] [Google Scholar]
- 6.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Fink MP, Delude RL. Shock. 2003;19:229–237. doi: 10.1097/00024382-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Zheng S, Dweik RA, Erzurum SC. Free Radic Biol Med. 2006;41:19–28. doi: 10.1016/j.freeradbiomed.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bove PF, van der Vliet A. Free Radic Biol Med. 2006;41:515–527. doi: 10.1016/j.freeradbiomed.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Forsythe RM, Xu DZ, Lu Q, Deitch EA. Shock. 2002;17:180–184. doi: 10.1097/00024382-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Am J Physiol Lung Cell Mol Physiol. 2004;286:L259–267. doi: 10.1152/ajplung.00187.2003. [DOI] [PubMed] [Google Scholar]
- 13.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 14.Harren M, Barmeyer C, Schmitz H, Fromm M, Horak I, Dignass A, John M, Wiedenmann B, Schulzke JD. Ann N Y Acad Sci. 2000;915:204–213. doi: 10.1111/j.1749-6632.2000.tb05243.x. [DOI] [PubMed] [Google Scholar]
- 15.Kubes P, Reinhardt PH, Payne D, Woodman RC. Am J Physiol. 1995;269:G34–41. doi: 10.1152/ajpgi.1995.269.1.G34. [DOI] [PubMed] [Google Scholar]
- 16.Katsube T, Tsuji H, Onoda M. Biochim Biophys Acta. 2007;1773:794–803. doi: 10.1016/j.bbamcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Rachlis A, Watson JL, Lu J, McKay DM. J Leukoc Biol. 2002;72:339–346. [PubMed] [Google Scholar]
- 18.Rose F, Guthmann B, Tenenbaum T, Fink L, Ghofrani A, Weissmann N, Konig P, Ermert L, Dahlem G, Haenze J, Kummer W, Seeger W, Grimminger F. J Immunol. 2002;169:1474–1481. doi: 10.4049/jimmunol.169.3.1474. [DOI] [PubMed] [Google Scholar]
- 19.Gookin JL, Duckett LL, Armstrong MU, Stauffer SH, Finnegan CP, Murtaugh MP, Argenzio RA. Am J Physiol Gastrointest Liver Physiol. 2004;287:G571–581. doi: 10.1152/ajpgi.00413.2003. [DOI] [PubMed] [Google Scholar]
- 20.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 21.Wu R, Smith D. In Vitro. 1982;18:800–812. doi: 10.1007/BF02796504. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Hogg N. PNAS. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Am J Respir Cell Mol Biol. 2007;36:138–146. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu DZ, Lu Q, Deitch EA. Shock. 2002;17:139–145. doi: 10.1097/00024382-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Peterson LA, Wagener T, Sies H, Stahl W. Chem Res Toxicol. 2007;20:721–723. doi: 10.1021/tx700095u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothen-Rutishauser B, Riesen FK, Braun A, Gunthert M, Wunderli-Allenspach H. J Membr Biol. 2002;188:151–162. doi: 10.1007/s00232-001-0182-2. [DOI] [PubMed] [Google Scholar]
- 27.Ahdieh M, Vandenbos T, Youakim A. Am J Physiol Cell Physiol. 2001;281:C2029–2038. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- 28.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Fink MP, Yang R, Delude RL. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 30.Shelton JL, Wang L, Cepinskas G, Sandig M, Scott JA, North ML, Inculet R, Mehta S. Microvasc Res. 2007;74:23–31. doi: 10.1016/j.mvr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 32.Mehta S. Vascul Pharmacol. 2005;43:390–403. doi: 10.1016/j.vph.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet A. Am J Physiol Lung Cell Mol Physiol. 2004;286:L198–209. doi: 10.1152/ajplung.00136.2003. [DOI] [PubMed] [Google Scholar]
- 34.Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Gastroenterology. 1997;113:1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Am J Physiol Gastrointest Liver Physiol. 2004;286:G126–136. doi: 10.1152/ajpgi.00231.2003. [DOI] [PubMed] [Google Scholar]
- 36.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Am J Physiol Cell Physiol. 2005;288:C1231–1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 38.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Van Itallie CM, Anderson JM. Proc Am Thorac Soc. 2004;1:38–41. doi: 10.1513/pats.2306013. [DOI] [PubMed] [Google Scholar]
- 40.Feldman GJ, Mullin JM, Ryan MP. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 42.Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, Shen HD. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 43.Kawedia JD, Jiang M, Kulkarni A, Waechter HE, Matlin KS, Pauletti GM, Menon AG. J Pharmacol Exp Ther. 2008;326:829–837. doi: 10.1124/jpet.107.135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zahm JM, Kaplan H, Herard AL, Doriot F, Pierrot D, Somelette P, Puchelle E. Cell Motil Cytoskeleton. 1997;37:33–43. doi: 10.1002/(SICI)1097-0169(1997)37:1<33::AID-CM4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 45.Jadeski LC, Chakraborty C, Lala PK. Int J Cancer. 2003;106:496–504. doi: 10.1002/ijc.11268. [DOI] [PubMed] [Google Scholar]
- 46.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Mol Cell Biol. 2003;23:5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris LK, McCormick J, Cartwright JE, Whitley GS, Dash PR. Exp Cell Res. 2008;314:1765–1776. doi: 10.1016/j.yexcr.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Savla U, Appel HJ, Sporn PH, Waters CM. Am J Physiol Lung Cell Mol Physiol. 2001;280:L421–431. doi: 10.1152/ajplung.2001.280.3.L421. [DOI] [PubMed] [Google Scholar]