Abstract
Muramyl dipeptide (MDP), the NOD2 agonist, induces NF-κB and MAPK activation leading to the production of anti-microbial and pro-inflammatory molecules. MDP is internalized into acidified vesicles in macrophages. However, the endocytic mechanism of MDP uptake that induces NOD2 signaling is unknown. We now report the identification of an endocytosis pathway dependent on clathrin and dynamin that mediates MDP internalization and NOD2 activation. Intracellular MDP uptake was inhibited by chlorpromazine, a drug that disrupts clathrin-dependent endocytosis, but not by compounds that block pinocytosis or cellular entry via scavenger or mannose receptors. In contrast, MDP uptake and NOD2-dependent signaling were unimpaired in macrophages deficiency in PepT1, a peptide transporter previously implicated in MDP internalization. Both chlorpromazine and knockdown of clathrin expression by RNA interference attenuated MDP-induced NF-κB and MAPK activation. Furthermore, MDP uptake and NOD2-dependent signaling were impaired by inhibition of dynamin, a GTPase required for budding of clathrin-coated vesicles from the plasma membrane. Finally, bafilomycin A, a specific inhibitor of the vacuolar proton pump, blocked MDP accumulation in acidified vesicles and cytokine responses, suggesting that vacuolar maturation is important for MDP-induced NOD2 signaling. These studies provide evidence for a clathrin- and dynamin-dependent endocytosis pathway that mediates MDP uptake and NOD2 activation.
Keywords: Monocyte, macrophage, Inflammation, signal transduction
INTRODUCTION
NOD2 is a member of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family (1). Several NLRs including NOD2 function as intracellular pattern-recognition receptors that sense microbial moieties that are shared by a large number of bacteria (2). NOD2 is activated by peptidoglycan (PGN)-derived molecules containing muramyl dipeptide (MDP) that are produced by both Gram-negative and Gram-positive bacteria (3, 4). Upon MDP recognition, NOD2 induces the activation of the transcription factor NF-κB and the mitogen-activated protein kinases (MAPKs) via the serine-threonine kinase RICK (also called RIP2) (3, 5-8). Recent studies have demonstrated that K63-linked regulatory ubiquitination of RICK is essential for the recruitment of TAK1 (9, 10), a kinase required for the activation of the MAPKs and the I-κBα kinase (IKK) complex (11). The importance of NOD2 in inflammatory homeostasis is underscored by the observation that mutations in the NOD2 gene increase the susceptibility to inflammatory disorders, including Crohn's disease and Blau's syndrome (12-15). Although the precise mechanisms by which NOD2 mutations promote disease remain unclear, several studies have demonstrated that Crohn's disease-associated NOD2 variants are deficient in MDP recognition whereas those linked to Blau's syndrome exhibit constitutive activity (3, 16).
The NOD2 signaling pathways induced by MDP stimulation have been largely defined (1, 2). However, the cellular mechanism that mediates MDP uptake to induce NOD2 activation and signaling is poorly understood. In intestinal epithelial cell lines, there is evidence that MDP can be internalized through the peptide PepT1 transporter, but it is unclear if this mechanism is involved in MDP-induced signaling in cells such as macrophages that normally express NOD2 (17-20). After MDP exposure, macrophages internalized the NOD2 agonist in acidified vesicles (22). However, the endocytic pathway responsible for MDP uptake is unknown. Furthermore, it remains unclear whether endocytosis of MDP is important for NF-κB and MAPK activation induced via NOD2. In these studies, we have identified clathrin- and dynamin-dependent endocytosis, but not the peptide PepT1 transporter, as the mechanism for the uptake of MDP which is critical for MDP-induced NOD2 activation and signaling.
MATERIALS AND METHODS
Mice and Cells
C57BL/6 mice were purchased from the Jackson Laboratory. PepT1 knockout (KO) mice in C57BL/6 background generated by homologous recombination have been described (21). Mice were housed in a pathogen-free facility. The animal studies were conducted under approved protocols by the University of Michigan Committee on Use and Care of Animals. Bone marrow-derived macrophages were prepared as described (23). Human monocytes were purified from peripheral blood mononuclear cells of healthy volunteers by adherence to plastic dishes (24). Briefly, venous blood was drawn from the cubital vein into EDTA tubes and mononuclear cells were isolated by density centrifugation of blood diluted 1:2 in PBS over Ficoll-Paque (Pharmacia Biotech). Cells were washed twice in PBS and suspended in culture medium (RPMI 1640) supplemented with antibiotics, 10mM L-glutamine and 10mM Pyruvate. Mononuclear cells were incubated at 2-3 × 106 /ml in plastic dishes for 1 hr, washed to remove non-adherent cells and adherent cells recovered by scraping in PBS without Ca++ and Mg++ and replated in complete medium. Human HEK293T cells were cultured in Dulbecco's modified Eagle's medium + 10% fetal bovine serum + penicillin/streptomycin.
Reagents and Plasmids
Ultrapure LPS from Escherichia coli 0111:B4 was purchased from Invivogen. Human TNF-α was purchased from Roche. MDP (Ac-muramyl-Ala-D-Glu-NH2) was purchased from Bachem. MDP labeled with alexa-488 (MDP-Alexa488) and rhodamine B (MDP-Rhodamine) have been described (22). Fluorescent low density lipoprotein (LDL-BODIPY) was purchased from Invitrogen. Bafilomycin A, chlorpromazine (CPZ), dimethilamyloride, polyinosinic acid and mannans from Sacharomyces cerevesiae were purchased from Sigma. Dynasore was synthesized by Dr. Henry Pelishand and generously provided by Dr. Tom Kirchhausen (Harvard Medical School) (25). The luciferase NF-κB reporter assay using the NOD2 expression construct, pMXp-HA-NOD2, the luciferase reporter plasmid pBVIx-Luc, and the pEFBOS-βgal used for normalization have been reported (26). Plasmid expressing dominant negative dynamin II (K44A), dynaminK44A, and the parental control plasmid were provided by Dr. Theodora Ross (University of Michigan Medical School). Lentiviral constructs expressing small hairpin RNAs ( shRNA ) (shRNA1: CCGGGCCCAAATGTTAGTTCAAGATCTCGAGATCTTGAACTAACATTTGGGCTTTTT; shRNA2: CCGGGCCAATGTGATCTGGAACTTACTCGAGTAAGTTCCAGATCACATTGGCTTTTT) for silencing the human clathrin heavy chain gene were purchased from Sigma.
cDNA synthesis and real-time PCR
Total RNA was extracted from cultured macrophages derived from WT and PepT1 KO mice using the RNeasy Plus Mini Kit (Qiagen) and cDNA was synthesized using SuperScripIII Reverse Transcriptase (Invitrogen) according to manufacturer's instructions. Real-time PCR was performed using TaqMan PCR Master Mix (Applied Biosystems). The PCR conditions were as follows: initial denaturation for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The primer sequences were: PepT1 Forward, CTTGGAGCCACCACAATGG; PepT1 Reverse, ACAGAATTCATTGACCACGATGA; PepT1 Probe, 5'-/56-FAM/-TTGCTTCGGTTACCCGTTGAGCATCT -/36-TAMSp/-3 '; PepT1Standard , CCGGAGCCTTGGAGCCACCACAATGGGGATGTCCAAGTCTCGGGGTTGCTTCGGTTA CCCGTTGAGCATCTTCTTCATCGTGGTCAATGAATTCTGTGAAAGA; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Forward, GAGACAGCCGCATCTTCTTGT; GAPDH Reverse, CACACCGACCTTCACCATTTT; GAPDHProbe , 5'-/56-JOE/-CAGTGCCAGCCTCGTCCCGTAGA-/36-TAMSp/-3 '; G A P D H S t a n d a r d , TCCCTGTTCCAGAGACAGCCGCATCTTCTTGTGCAGTGCCAGCCTCGTCCCGTAGAC AAAATGGTGAAGGTCGGTGTGAACGGATTTG.
Luciferase Reporter Gene Assays
HEK293T cells were plated at 4 × 104 cells/ml in 24-well plates and transfected with plasmid DNA using Lipofectamine PLUS (Invitrogen) in triplicate. Transfected cells were stimulated for 16-18 h with 100 ng/ml MDP or 10 ng/ml of human TNF-α, lysed in 1x reporter lysis buffer (Promega) and cell extracts assayed for luciferase and β-galactosidase activity (26). The luciferase activity was normalized for transfection efficiency using β-galactosidase values.
Clathrin heavy chain gene knockdown
HEK293T cells were plated at 4 × 104 cells/ml in 24-well plates and transfected with 500 ng/well of the indicated shRNA plasmid using Lipofectamine PLUS (Invitrogen). After 48 hrs, cells were transfected with plasmid DNA for luciferase reporter gene assays and stimulated as described before.
Immunoblotting
For analysis of Iκ-Bα, p38, JNK and ERK phosphorylation, cells were treated for 30 min with medium alone or medium containing chlorpromazine or dynasore, and then stimulated with MDP (10 μg/ml) for the time indicated in the figures. Immunoblotting was performed using phospho-specific antibodies (Cell Signaling Technology) as described (8). For analysis of clathrin heavy chain gene knockdown, HEK293T cells were plated in 6-well plates and were transfected with 2 μg/well of control shRNA plasmid, shRNA(-), or shRNA targeting the clathrin heavy chain. 48 h after transfection, cell lysates were prepared and immunoblotted with anti-clathrin heavy chain antibody or anti-β-actin antibody as a control (BD Biosciences).
Inhibitor treatments
In the different MDP-uptake or MDP-stimulation experiments, bafilomycin A was used at 10 nM (27), CPZ was used at 5-20 μM (27, 28), dimethylamiloride at 500 μM (28), polyinosinic acid at 50 μg/ml (28), mannans at 1 mg/ml (30), and dynasore at 80 μM (25). Cells were pretreated with each inhibitor for 30 min and then further incubated with the corresponding stimuli, in the presence of the inhibitor, for the time indicated in figure legends. Dynasore experiments were performed in media without serum.
Cytotoxicity assay
The percentage of macrophage death was determined by measurement of the release of LDH after incubation with the CytoTox 96 nonradioactive cytotoxicity assay (Promega). The absorbance at 490 nm was measured, and the percentage of cell death was calculated as follows: [(experimental release - spontaneous release) / (maximum release-spontaneous release)] × 100, where 'spontaneous release' is that found in untreated macrophages and 'maximum release' is the value obtained after lysis of macrophages with a solution of 0.1% Triton-X100.
Flow cytometry
Macrophages were plated at 5 × 105 cells/well and treated with medium alone or medium containing the indicated inhibitors for 30 min, and then incubated for 1 hour with MDPAlexa488 (20 μg/ ml) or with LDL-BODIPY (1 μg/ml). Cells were fixed and analyzed using a FACSCalibur Cytometer (BD Biosciences) at the University of Michigan Flow Cytometry Core Facility.
Fluorescence microscopy
For MDP-labeled uptake experiments, cultured macrophages were incubated with medium alone or medium containing CPZ or polyinosinic acid for 30 min, and consequently incubated with MDP- Rhodamine (20 μg/ml) for 3 h. In all experiments, nuclei were stained with nucleic acid dye 4', 6'-diamino-2-phenylindole (DAPI; Molecular Probes) and the cells were fixed with 2.5% paraformaldehyde. Samples were imaged using an Olympus Fluoview-500 Confocal laser scanning microscope at the University of Michigan Microscopy and Image Analysis Facility.
Statistical analysis
Statistical significance between groups was determined by two tailed Student's t test. Differences were considered significant when p < 0.05.
RESULTS
The PepT1 transporter is dispensable for intracellular internalization of fluorescent-labeled MDP and MDP-induced signaling in mouse macrophages
It has been reported that the plasma membrane transporter, PepT1, can translocate MDP into the cytosol of epithelial cells (17, 18). PepT1 is expressed primarily at the apical membrane of intestinal epithelial cells, but its expression is also detected in human monocytes (20). To determine the requirement of PepT1 for MDP uptake and signaling, we used macrophages from genetically modified mice in which PepT1 has been disrupted by homologous recombination. In wild-type mice, PepT1 was expressed in bone marrow-derived macrophages although at lower levels than in the small intestine (Fig. 1A). As expected, the expression of PepT1 was undetectable in macrophages from PepT1-null mice (Fig. 1A). 3 hrs after incubation, Rhodamine-labeled MDP was found intracellularly in a punctate granular pattern in macrophages which is in line with previous results (22). Notably, the intracellular localization of MDP was unimpaired in macrophages deficient in PepT1 (Fig. 1B). Furthermore, NF-κB activation as well as phosphorylation of p38, JNK and ERK in response to MDP was comparable in wild-type and PepT1-null macrophages (Fig. 1C). Finally, enhancement of LPS-induced IL-6 and TNF-α production by MDP was unimpaired in PepT1 mutant macrophages (Fig. 1D and E). These results indicate that PepT1 is not required for intracellular internalization of MDP and MDP-induced signaling in macrophages.
Figure 1. PepT1 is not required for fluorescent-labeled MDP uptake or MDP-induced signaling in mouse macrophages.
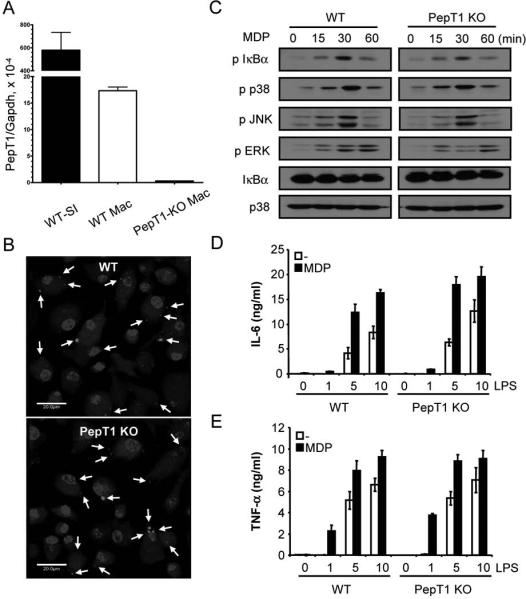
(A) Expression of PepT1 mRNA isolated from bone marrow-derived macrophages (Mac) from WT and PepT1 KO mice by real-time PCR. Results were normalized to GAPDH levels. Expression of PepT1 in small intestine (SI) of WT mouse is shown for comparison. (B) Macrophages derived from WT and PepT1 KO mice were incubated for 3 h with MDP-Rhodamine (20 μg/ml, red), the nuclei were counterstained with DAPI (blue) and cells were fixed and imaged by confocal microscopy. Arrows indicate intracellular MDPRhodamine (C) Macrophages from WT and PepT1 KO were stimulated with MDP (10 μg/ml) for the indicated periods. Cell lysates were prepared and blotted with indicated antibodies. Results are from one representative experiment of three independent experiments. p, phosphorylated. (D, E) Macrophages from WT and PepT1 KO mice were stimulated with the indicated amount of LPS (ng/ml) in absence or presence of MDP (10 μg/ml). Cells supernatants were collected 24 h after stimulation and IL-6 and TNF-α were measured by ELISA. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of two independent experiments.
Uptake of fluorescent-labeled MDP is inhibited by chlorpromazine in mouse macrophages
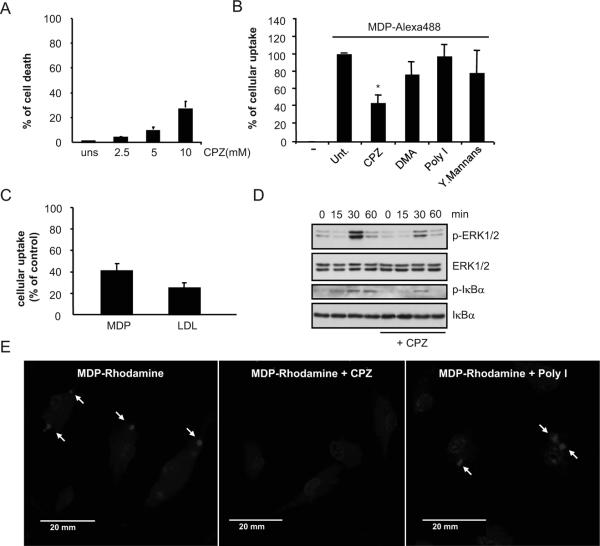
To study the mechanism by which MDP is internalized, macrophages were pre-treated with compounds that target several processes of cellular entry followed by incubation with Alexa-488-labeled MDP. We first assessed CPZ, a drug that disrupts clathrin-mediated endocytosis. Pilot studies showed that CPZ at concentration of 5 μM or lower induced less than 10% toxicity in mouse macrophages when evaluated 3.5 hrs after addition of the drug (Fig. 2A). Incubation of macrophages with 5 μM of CPZ inhibited MDP uptake by ~ 50-60% as assessed by flow cytometric analysis (Fig. 2B). In contrast, incubation with dimethylamiloride, an inhibitor of pinocytosis, polyinosinic acid or mannan, specific inhibitors of scavenger receptors or entry via mannose receptors, respectively, did not affect MDP uptake (Fig. 2B). The inhibition of MDP uptake by CPZ was similar to that of fluorescent LDL, a molecule known to be internalized by clathrin-mediated endocytosis (Fig. 2C). Importantly, incubation of macrophages with MDP induced NF-κB and MAPK activation as determined by immunoblotting with antibodies recognizing phosphorylated forms of IκBα and Erk and this response was attenuated in macrophages pre-treated with 5 μM of CPZ (Fig. 2D). Consistently, intracellular localization of MDP-Rhodamine was inhibited in macrophages treated with CPZ, but not polyinosinic acid (Fig. 2E). These studies suggest that MDP uptake is regulated by a clathrin-dependent endocytosis pathway and inhibition of this pathway attenuates signaling induced by MDP in macrophages.
Figure 2. Effect of inhibitors of different cellular uptake pathways on the internalization of fluorescent-labeled MDP by mouse macrophages.
(A) Bone-marrow derived macrophages were treated with the indicated doses of CPZ for 3. 5 hrs and the induction of cell death was evaluated by LDH release. Values represent mean ± SD of triplicate cultures. (B) Percentage of MDP-labeled macrophages after incubation for 15 min with medium alone (-), medium containing MDP-Alexa488 alone (untreated, unt.) or in the presence of CPZ (5 μM), dimethylamiloride (DMA), polyinosinic acid (Poly I) or mannans from Sacharomyces cerevesiae (Mannans). Results are representative of two experiments. (C) Bone-marrow derived macrophages were pretreated or not with CPZ (5 μM) for 30 minutes and then incubated with MDP-Alexa488 or LDL-BODIPY for 60 minutes. The uptake was analyzed by FACS and plotted as percent of uptake inhibition of the untreated cells. Results are presented as the mean of triplicate wells ± SD. Results are representative of two experiments (D) Bone-marrow derived macrophages were pretreated or not with CPZ (5 μM) and stimulated with MDP (10 μg/ml) for the indicated periods of time. Cell lysates were prepared and blotted with indicated antibodies. Results are from one representative experiment of three independent experiments. p, phosphorylated (E) Fluorescence confocal images of mouse macrophages incubated for 3 h with MDP-Rhodamine (red), alone or in the presence of CPZ or Poly I. In all cases, the nuclei were counterstained with DAPI (blue), and cells were fixed and imaged by fluorescence confocal microscopy. Arrows indicate intracellular MDP-Rhodamine *, denotes p < 0.05 between untreated and CPZ-treated cultures. Results are representative of three experiments.
Clathrin-dependent endocytosis pathway regulates MDP-induced responses in HEK293 epithelial cells
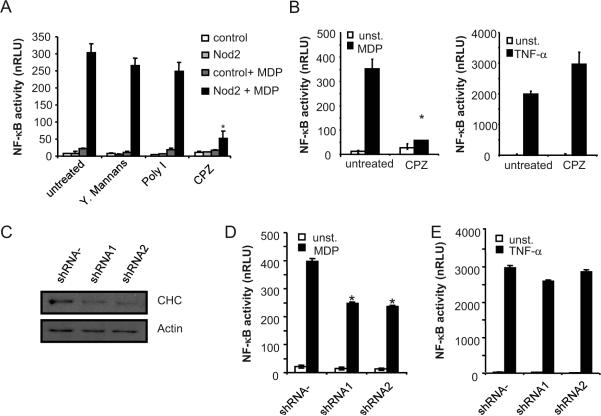
We next tested if CPZ affects NF-κB activation in HEK293 cells, an epithelial cell line that responds to MDP stimulation when NOD2 is ectopically expressed (31). Expression of NOD2, but not control plasmid, induced NF-κB activation by MDP in HEK293 cells as measured by reporter luciferase assay (Fig. 3A). Treatment with CPZ, but not with mannan or polyinosinic acid, inhibited MDP-induced NF-κB activation (Fig. 3A). The effect of CPZ was specific in that NF-κB activation induced by TNF-α was unimpaired in CPZ-treated HEK293 cells when compared to that induced with MDP (Fig. 3B). To further assess the role of clathrin-dependent endocytosis in MDP-induced signaling, HEK293 cells were transfected with two short hairpin RNA (shRNA) constructs that target the clathrin heavy chain gene or control shRNA construct. Immunobloting analysis revealed downregulation of clathrin heavy chain levels by the two different shRNAs when compared to control shRNA construct (Fig. 3C). Functional assays revealed that both shRNAs against the clathrin heavy chain gene attenuated MDP-induced NF-κB activation, but were ineffective in inhibiting that induced by TNF-α stimulation (Fig. 3D and E). These results suggest that clathrin-dependent endocytosis pathway regulates MDP-induced signaling in HEK293 cells.
Figure 3. Clathrin-mediated endocytosis regulates MDP-induced NOD2-dependent NF-κ B activation in HEK293T cells.
(A) HEK293T cells were transiently transfected with a NF-κB luciferase reporter plasmid along with a control β-galactosidase plasmid (control), and NOD2 expression plasmid when indicated (NOD2), in triplicate. The cells were treated with medium alone or with medium containing 100 ng/ml MDP (MDP) for 16 h, in the absence (untreated) or presence of mannans, Poly I or CPZ. Luciferase and β-galactosidase activity was measured in cell lysates and values normalized for transfection efficiency (nRLU). (B) Luciferase reporter gene assays were performed as in (A). Transfected cells were treated with media alone (unst.) or with media containing either 100 ng/ml MDP or 10 ng/ml human TNF-α for 16 h, alone (untreated) or in the presence of CPZ. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.01 between untreated and CPZ-treated samples (Nod2+MDP). (C) HEK293T cells were transfected with a control plasmid (shRNA -) or two shRNA plasmids targeting the clathrin heavy chain gene (CHC) (shRNA1 and shRNA2). Cell lysates were prepared and blotted with indicated antibodies. (D, E) HEK293T cells were transiently transfected with a control plasmid (shRNA -) or with shRNA plasmids (shRNA1 and shRNA2). Luciferase reporter gene assays were performed as in A. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05 between control shRNA- plasmid and shRNA1 or shRNA2 constructs in MDP stimulated cultures.
Dynasore inhibits MDP uptake and MDP-induced caspase-1 activation
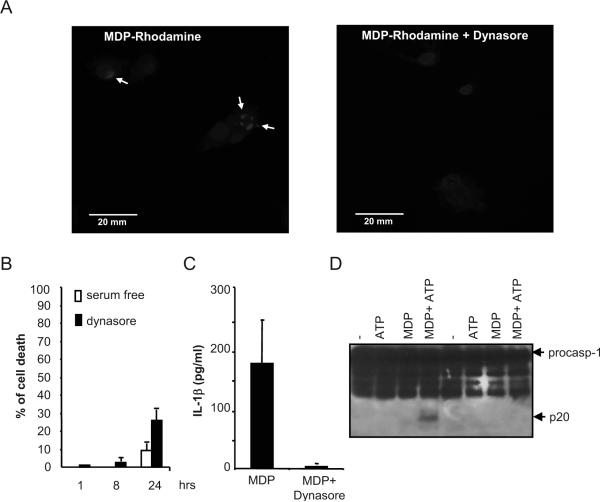
Dynamin proteins are GTPases that are essential for budding of clathrin vesicles from the plasma membrane (32). To further assess the role of dynamin in MDP internalization and signaling, macrophages were treated with dynasore, a cell-permeable inhibitor of dynamin (25). Intracellular uptake of Rhodamine-labeled MDP was inhibited by pre-treatment of macrophages with dynasore (Fig. 4A). Macrophage cell death after incubation with 8 hrs with dynasore at 80 μM in serum-free mediun was less than 5% and increased to ~25% by 24 hrs of incubation (Fig. 4B). Notably, stimulation of human monocytes with MDP induced IL-1β secretion which was abrogated by treatment with dynasore (Fig. 4C). Furthermore in mouse macrophages dynasore inhibited caspase-1 activation induced by MDP and ATP (Fig. 4D). These results suggest that MDP is internalized by a dynamin-dependent endocytosis pathway which is critical for MDP-induced caspase-1 activation and IL-1β secretion.
Figure 4. Dynasore impairs MDP-uptake and caspase-1 activation.
(A) Fluorescence confocal images of mouse macrophages incubated for 3 h with MDP-Rhodamine (red), alone or in the presence of dynasore. In all cases, the nuclei were counterstained with DAPI (blue), and cells were fixed and imaged by fluorescence confocal microscopy. Arrows denote intracellular MDP-Rhodamine (B) Bone-marrow derived macrophages were treated with dynosore for the indicated time and the induction of cell death was evaluated by the release of LDH. Values represent mean ± SD of triplicate cultures. (C) Human monocytes were stimulated with 1 μg/ml MDP, in the absence (MDP) or in the presence of dynasore (MDP + Dynasore) for 16 hrs and the levels of IL-1β were measured in cell supernatants. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05, statistically significant differences in the cytokines levels between untreated cells or dynasore-treated cells. (D) Bone-marrow derived macrophages were pretreated or not with dynasore and stimulated with MDP, ATP or MDP + ATP. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 (procasp-1) and its processed p20 subunit. Results are representative of three independent experiment.
Functional dynamin is required for MDP-induced signaling
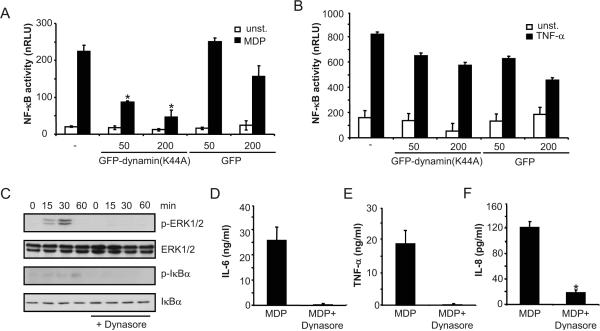
We next investigated whether MDP internalization and signaling was dependent on dynamin using construct that expresses dominant negative dynamin II (K44A) (33). HEK293 cells were co-transfected with NOD2 or control plasmid in the presence or absence of the dynamin (K44A) mutant construct. Stimulation of the cells with MDP induced NF-κB activation in a NOD2-dependent manner which was greatly inhibited by the dominant interfering dynamin construct, but little or not at all by control plasmid (Fig. 5A). In contrast, expression of dynamin (K44A) did not affect NF-κB activation induced by TNF-α when compared to that observed with control plasmid (Fig. 5B). Importantly, ERK and Iκ-Bα phosporylation induced by MDP in mouse macrophages was impaired by treatment with dynasore (Fig. 5C). In line with these results, secretion of IL-6 and TNF-α by mouse macrophages or IL-8 by human monocytes in response to MDP was reduced in cells treated with dynasore (Fig. 5D-F). Similar uptake of labeled-MDP was observed in mouse macrophages and human monocytes as determined by flow cytometric analysis (data not shown). These results suggest that the dynamin-dependent endocytosis pathway is critical for MDP-induced NOD2-dependent activation and cytokine responses.
Figure 5. Fucntional dynamin is required for MDP-induced NOD2-mediated NF-kB activation.
(A, B) Luciferase reporter gene assays were performed as in Figure 4. HEK293T cells transiently transfected in triplicate with a NF-κB luciferase reporter plasmid, along with a β-galactosidase transfection plasmid and a NOD2 expression plasmid (-), and together with 50 ng or 200 ng of a plasmid producing Dynamin (K44A), or with a control plasmid. The cells were treated with media alone (unst.), or media containing 100 ng/ml MDP, or 10 ng/ml human TNF-α for 16 h. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05 between cells transfected with NOD2 plasmid and NOD2 plasmid + Dynamin (K44A) construct in MDP-stimulated cultures (C) Bone-marrow derived macrophages were pretreated or not with dynasore and stimulated with MDP (10 μg/ml) for the indicated periods of time. Cell lysates were prepared and blotted with indicated antibodies. p, phosphorylated. Results are from one representative experiment of three independent experiments. (D,E) Mouse macrophages were pretreated, or not, with dynasore and then stimulated, or not, with 10 μg/ml of MDP in the presence of 5 ng/ml of LPS. Cells supernatants were collected 24 h after stimulation and IL-6 and TNF-α were measured by ELISA. (F) Human monocytes were stimulated with 1 μg/ml MDP, in the absence (MDP) or in the presence of dynasore (MDP + Dynasore) for 16 hrs and the levels of IL-8 were measured in cell supernatants. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05, statistically significant differences in the cytokines levels between untreated cells or dynasore-treated cells.
Vacuolar maturation is important for MDP-induced signaling in macrophages
Rhodamine-labeled MDP is first internalized into small intracellular structures that coalesce into larger acidified vesicles (22). To test whether vacuolar maturation is important for MDP-induced signaling, macrophages were treated with bafilomycin A, a specific inhibitor of the vacuolar ATPase proton pump (34). Bafilomycin A inhibits vacuolar acification and blocks maturation of endosomal compartments (35). Consistently, bafilomycin A did not inhibit early MDP uptake but inhibited the subsequent localization of MDP in large vesicles (Fig. 6A and B) that were shown to co-localize with markers of acidified endosomal vesicles (22). Importantly, secretion of IL-6 and TNF-α by mouse macrophages or IL-8 by human monocytes was inhibited by bafilomycin A (Fig. 6D-F). These results suggest that vacuolar maturation and/or acidification is important for MDP-induced cytokine responses.
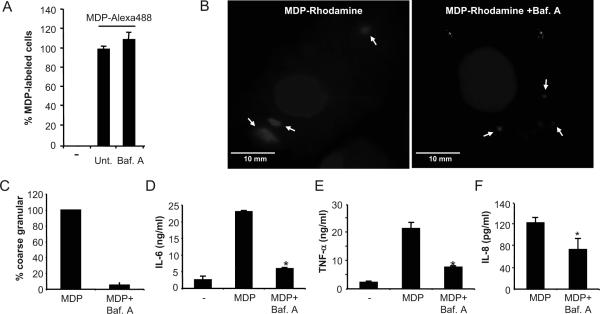
figure 6. Bafilomycin A blocks MDP-induced NF-κB activation in mouse macrophages and human monocytes.
(A) Percentage of MDP-labeled macrophages after incubation for 15 min. with media alone (-), media containing MDP-Alexa488 (untreated, unt.) or MDP-Alexa488 in the presence of bafilomycin A (Baf. A). (B) Fluorescence confocal images of mouse macrophages incubated for 3 h with MDP-Rhodamine (red), alone or in the presence of Bafilomycin A. In all cases, the nuclei were counterstained with DAPI (blue), and cells were fixed and imaged by fluorescence confocal microscopy. Notice difference in size of intracellular MDP-Rhodamine structures between untreated and bafilomycin A-treated cells (C) Percentage of MDP-labeled cells that displayed granular coarse (large) pattern in the absence (MDP) or presence of bafilomycin A (MDP + Baf. A). The bars represent the mean of 15-20 optical sections ± SD and are representative of at least three different experiments. (D, E) Mouse macrophages were stimulated with 5 ng/ml of LPS alone (-) or together with 10 μg/ml of MDP, in the absence (MDP), or presence of bafilomycin A (MDP + Baf. A). Cells supernatants were collected 24 h after stimulation and IL-6 and TNF-α were measured by ELISA. (F) Human monocytes were stimulated with 1 μg/ml of MDP alone (MDP) or in the presence of bafilomycin A (MDP + Baf. A) for 16 hrs and levels of IL-8 were measured in cell supernatants. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05 between MDP and MDP+bafilomycin A-treated cells.
DISCUSSION
Recent studies have provided critical insight into the signaling components and regulatory steps that are induced upon NOD2 activation in response to MDP stimulation. However, the cellular events that act upstream of NOD2 to induce MDP-mediated signaling have remained poorly understood. Stimulation of phagocytic and epithelial cells with MDP can result in NF-κB and MAPK activation, although delivery of MDP to the cytosol by transfection is thought to enhance MDP-induced signaling (3). These results suggest that both macrophages and epithelial cells possess the machinery to internalize MDP. In this study, we have identified an endocytic pathway that mediates MDP uptake and induces NOD2-dependent signaling. Studies with both pharmacological inhibitors and RNAi interference revealed that MDP internalization is mediated by a clathrin-dependent endocytosis pathway. This endocytosis pathway was also dependent on dynamin, a GTPase that is required for budding of the clathrin-coated pits from the plasma membrane (32, 36). Clathrin-dependent endocytosis constitutes a major route for the uptake of nutrients, pathogens, growth factors and transmembrane proteins in mammalian cells (37-41). Similarly, LPS is endocytosed by an endocytic pathway that is dependent on clathrin and dynamin (42). The mechanism by which MDP is targeted to clathrin-coated pits for endocytosis remains unclear. Because endocytosis is typically mediated through transmembrane proteins that are selectively recruited to coated pits by interaction to clathrin adaptors, the findings suggest that MDP might be recognized by a plasma membrane receptor that mediates its delivery to clathrin-coated pits. Our initial studies revealed that MDP uptake is unimpaired by treatment with an inhibitor of scavenger receptors or mannan, a molecule that interacts with the mannose receptor. These results appear to rule out the latter receptors, although other surface receptors might be involved in promoting MDP internalization. Many pathogens that express NOD2-stimulatory activity are internalized into host cells by clathrin-dependent endocytosis (41, 43). Thus, both MDP and bacteria appear to utilize the same route to trigger NOD2 activation and signaling. Further studies are needed to determine the mechanism whereby extracellular MDP istargeted to the clathrin-dependent endocytosis pathway.
Recent studies have shown that MDP induces caspase-1 activation through the Nlrp3 inflammasome, an event that requires exogenous ATP to deliver MDP from acidified vesicles into the cytosol via the pannexin-1 pore (22). In contrast, other authors have concluded that MDP-mediated caspase-1 activation induced by an inflammasome complex containing Nlrp1 and NOD2 (46). Regardless of the mechanism involved, our results indicate that MDP internalization via dynamin-mediated endocytosis is required for caspase-1 activation. Unlike the activation of caspase-1, NF-κB and MAPK activation induced by MDP is mediated solely via NOD2 and does not require exogenous ATP (3, 22, 44). These results suggest that after internalization via clathrin-dependent endocytosis, MDP relies on different mechanisms to activate Nlrp3 and NOD2. One possibility is that NOD2 activation by MDP is mediated via a surface receptor also present in endocytic vesicles or by another mechanism independent of cytosolic recognition. Alternatively, after internalization in acidified vesicles, MDP may leak or be actively transported into the cytosol where is sensed by NOD2. Our results rule out the peptide transporter PepT1, although other transporter systems operating in endosomes/lysosomes may be involved. Furthermore, the studies with bafilomycin A suggest that vacuolar acidification and/or maturation is important for MDP-induced cytokine responses. These results are in line with recent findings showing that bafilomycin A blocks activation of NOD2 in macrophages infected with a Listeria mutant that cannot escape the phago-lysosome (45). Consistent with our findings, localization of the Listeria mutant in the phago-lysosome, which leads to the degradation of the bacterial cell wall and release of peptidoglycan fragments, was required for NOD2-induced signaling (45). Further studies are needed to understand the mechanism by which MDP and peptidoglycan fragments derived from bacteria activate NOD2.
ACKNOWLEDGEMENTS
The authors are grateful to Joel Whitfield from the Cellular Immunology Core Facility of the University of Michigan Cancer Center for ELISA assays, Tom Kirchhausen for generous supply of dynasore and Theo Ross for plasmids.
This work was supported by NIH grants DK61707 (to G. N.) and GM035498 (to D. E. S.). Luigi Franchi was supported by a Fellowship from the Arthritis Foundation and Yun-Gi Kim by a Fellowship from the University of Michigan Comprehensive Cancer Center. MDP derived compounds synthesis was supported by grant GM065248 (to G.-J. B.).
ABBREVIATIONS
- CPZ
chlorpromazine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- KO
knockout
- MDP
muramyl dipeptide
- WT
wild-type
REFERENCES
- 1.Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 3.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 4.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. NOD2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 5.Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 7.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and NOD2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008;283:137–144. doi: 10.1074/jbc.M704746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. Embo J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 12.Hugot J-P, Chamaillard M, Zouali H, Lesage S, Cézard J-P, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel J-F, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 13.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat. Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 14.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, Nakajima M, Kobayashi H, Fujiwara L, Tsutsumi H, Utani A, Nishigori C, Heike T, Nakahata T, Miyachi Y. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-B activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, Ghosh P, Moran A, Predergast MM, Tromp G, Williams CJ, Inohara N, Nunez G. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. Embo J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol. 2006;84:1313–1319. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 19.Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun. 1996;220:848–852. doi: 10.1006/bbrc.1996.0493. [DOI] [PubMed] [Google Scholar]
- 20.Charrier L, Driss A, Yan Y, Nduati V, Klapproth JM, Sitaraman SV, Merlin D. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab Invest. 2006;86:490–503. doi: 10.1038/labinvest.3700413. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Smith DE, Ma K, Jappar D, Thomas W, Hillgren KM. Targeted Disruption of Peptide Transporter Pept1 Gene in Mice Significantly Reduces Dipeptide Absorption in Intestine. Mol Pharm. 2008 doi: 10.1021/mp8001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of NOD2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 23.Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 1984;160:55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. Embo J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-kB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 2000;275:27823–27831. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- 27.Long G, Pan X, Kormelink R, Vlak JM. Functional entry of baculovirus into insect and mammalian cells is dependent on clathrin-mediated endocytosis. J Virol. 2006;80:8830–8833. doi: 10.1128/JVI.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 30.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168:2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 31.McDonald C, Chen FF, Ollendorff V, Ogura Y, Marchetto S, Lecine P, Borg JP, Nunez G. A role for Erbin in the regulation of NOD2-dependent NF-kappaB signaling. J Biol Chem. 2005;280:40301–40309. doi: 10.1074/jbc.M508538200. [DOI] [PubMed] [Google Scholar]
- 32.Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci U S A. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 36.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 38.Roth TF, Porter KR. Yolk Protein Uptake in the Oocyte of the Mosquito Aedes Aegypti. L. J Cell Biol. 1964;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 40.Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 41.Veiga E, Cossart P. The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol. 2006;16:499–504. doi: 10.1016/j.tcb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. Embo J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veiga E, Guttman JA, Bonazzi M, Boucrot E, Toledo-Arana A, Lin AE, Enninga J, Pizarro-Cerda J, Finlay BB, Kirchhausen T, Cossart P. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe. 2007;2:340–351. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. NOD2-Dependent Regulation of Innate and Adaptive Immunity in the Intestinal Tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 45.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]