Abstract
OBJECTIVE
22q11 deletion syndrome, a common human interstitial deletion syndrome (1:5000), is associated with a heterogeneous physical phenotype, including several factors that markedly increase the risk for olfactory disorder. Despite its potential consequences, pediatric studies of impaired olfaction are rare, and odor detection in children with 22q11 deletion syndrome has not yet been examined.
METHODS
The University of Pennsylvania Smell Identification Test was administered to 62 children, including 39 with 22q11 deletion syndrome and 23 neurotypical control siblings who ranged in age from 5.3 to 14.8 years. Lowered smell detection accuracy among affected children was predicted.
RESULTS
Substantially more children with 22q11 deletion syndrome (68%) as compared with neurotypical control subjects (13%) had University of Pennsylvania Smell Identification Test scores ≥2 SDs below the standardization sample mean. Frequency of impairment in younger versus older children did not differ. The score distributions of children with and without velopharyngeal insufficiency did not differ; however, markedly lower score variance among children with velopharyngeal insufficiency suggested its negative impact on olfaction. Posthoc error analyses revealed that affected children had special difficulty detecting smells that are associated with fumes and smoke.
CONCLUSIONS
Odor detection failures are ubiquitous among children with 22q11 deletion syndrome and are not associated with developmental delay or performance characteristics of younger affected children. Additional studies are needed to examine further the impact on olfaction of velopharyngeal insufficiency and compromised nasal airway patency. Children with 22q11 deletion syndrome should be evaluated routinely for olfactory disorder. When deficits are identified, caregivers should be warned of potential dangers that are associated with this type of sensory impairment.
Keywords: DiGeorge syndrome, velocardiofacial syndrome, nasal obstruction, dopamine, congenital abnormalities/anomalies
22Q11 DELETION SYNDROME (22q11DS), also referred to as DiGeorge or velocardiofacial syndrome, results from a meiotic deletion at the q11.2 site on chromosome 22. It is estimated to occur in at least 1:5000 live births1 and may be substantially more prevalent.2 22q11DS results in the loss of ~30 gene copies3 and a broadly heterogeneous physical phenotype, most typically including structural velopharyngeal anomalies, craniofacial or heart anomalies, and/or immunologic deficits secondary to thymic irregularities.4 Interest in the early neurologic and neurocognitive development of children with 22q11DS increased dramatically after reports of higher-than-expected rates of schizophrenia among affected adults.5
Clues regarding the source of neurocognitive differences in 22q11DS may reside in its genetic profile. Among the 30 deleted gene copies are 4 that have a direct and an indirect impact on production and release of glutamate, γ-aminobutyric acid (GABA), and dopa-mine, the 3 neurotransmitters that selectively subserve pathways that link basal ganglia and frontal/prefrontal cortex6 and that are implicated in the cause of schizophrenia.7 Neurocognitive and neurophysiologic evidence has suggested abnormalities in these pathways among children with 22q11DS.8,9 Identifying the full complement of early deficits in at-risk children may be essential for understanding why severe psychiatric disorder develops among a select few.
Olfaction is perhaps the most intriguing and least understood of the human senses. Olfaction works by chemical reaction and can function at long distances for continual monitoring of air quality, food and drink, and proximity to others. Aspects of olfaction are congenital, as suggested by studies of facial grimacing to foul smells in neonates10; however, odor discrimination also depends on exposure and learning.11 Glutamate, GABA, and dopamine are the primary neurotransmitters of ol-faction,12–14 and, perhaps not surprising, olfactory disorder has been recognized as one of the earliest signs of idiopathic Parkinson’s disease and thus primary basal ganglia pathology.15 Other evidence has suggested that some olfactory disorders result from abnormalities in networks of the lateral orbitofrontal cortex,16 and studies have suggested that olfactory disorders are overrepresented among patients with schizophrenia.17–19
There are many other sources of olfactory disorder in adults and children, the most common of which include upper respiratory infection, sinonasal disease, and head trauma.20 Any condition that alters upper airway patency might be expected to impair olfaction; however, empirical reports of olfactory disorder in pediatric samples are rare. One study included 65 children who were aged 5 to 15 and suggested that nasal obstruction secondary to adenoid hypertrophy, chronic rhinitis, and/or acute upper respiratory infection was associated with diminished olfaction.21
Olfaction has not been examined previously in children with 22q11DS. Conditions that are common to this population and that might be implicated in possible ol-factory disorder as a result of compromised upper airway patency could include velopharyngeal insufficiency (VPI) and history of nasal regurgitation/nasogastric tube feeding. No studies yet have examined the association between VPI and olfaction. One study examined the association between early nasal regurgitation/nasogastric tube feeding on diminished olfaction in children with the genetic syndrome of coloboma, congenital heart disease, choanal atresia, mental and growth retardation, genital anomalies, and ear malformations and hearing loss22; unexpectedly, no associations were found. This study awaits replication. Given congenital anomalies that could alter upper airway patency, as well as chronic irregularities in glutamate/GABA/dopamine systems, a majority of children with 22q11DS might be at high risk for olfactory disorder.
Pediatric studies of olfaction have broad clinical as well as etiologic significance. When food smells are not perceived accurately, nutrition is endangered, and spoiled or inedible substances may not be detected accurately. When odors such as smoke or fumes are not identified, safety is jeopardized. Odor perception differences must be characterized in at-risk children before caregivers can be forewarned.
As part of a longitudinal study of neurocognitive development in school-aged children with 22q11DS, we administered the University of Pennsylvania Smell Identification Test (UPSIT) to 62 children, including 39 with 22q11DS and 23 neurotypical control siblings who ranged in age from 5.3 to 14.8 years. We predicted lowered smell detection accuracy among affected children as compared with sibling control subjects. In secondary analyses, we explored possible sources of olfac-tory differences. Because olfactory discrimination is influenced by learning11 and because younger children with 22q11DS have been observed to be both generally delayed and impulsive,23 we examined possible differences in odor detection accuracy between younger and older children with 22q11DS. We also compared subgroups of affected children with and without a history of VPI and with and without a history of nasal regurgitation/nasogastric tube feeding.
METHODS
Participants
Sixty-two children were included in these analyses, 39 (20 girls) with the 22q11DS and 23 neurotypical control siblings (16 girls). Racial composition was 95% white. Affected children ranged in age from 5.9 to 14.8 years, with a mean age of 9.7 (±2.3). Siblings ranged in age from 5.3 to 14.7 years, with a mean age of 9.8 (±2.9).
The institutional review board of The Rockefeller University approved this project before data collection and at regular 1-year intervals. Parents learned of our project through Web site postings and brochures that were sent to genetic counselors, doctors’ offices, speech and language specialists, and parent support groups. All children were confirmed positive for the 22q11 deletion via florescence in situ hybridization assay before enrollment in the study. Fluency in English was required for participation of both parents and children. Consent forms were sent to parents 1 month before scheduled testing. The tests and testing procedures were explained to the parents by study staff and to child participants by their parents. Child verbal assent and parental informed consent were obtained on the morning of testing before the start of assessment procedures. Before the first visit, parents completed a lengthy study intake form regarding their child’s medical history, which included multiple questions regarding velopharyngeal status, chronic feeding problems, nasal regurgitation, and nasogastric tube feeding. In addition, medical charts were available for review by study staff.
Procedures
The UPSIT24 provides 40 scratch-and-sniff odorants embedded in 10- to 50-μm urea-formaldehyde polymer microencapsules fixed in a proprietary binder and presented in 0.5 × 1.5 inch scratch panels positioned at the bottom right corner of each booklet page. Stimuli are released by scratching a pencil tip in a standardized manner across each panel. The test is forced choice, with 4 response options printed above each odor strip [eg, “This odor smells most like a) gasoline b) pizza c) peanuts d) rose”]. The extensive multistaged development of this instrument was described previously.25 The third edition of the UPSIT used in this study provided normative data based on ~4000 male and female individuals who were aged 4 to 99 years, with valid gender-specific norms down to the fifth percentile in each 5-year age band.
The UPSIT is a simple task and was designed to be self-administered by children as young as 4 years. In the past decade, scratch-and-sniff technology has been used widely in children’s stickers, advertisements, and coloring books and now is very familiar to even the youngest children. Many children with 22q11DS, however, have weak pencil grip and poor graphomotor control.26 Because of early learning disabilities,27 they are inconsistent in their reading ability. To control for these possible confounders, testers administered each item of the UPSIT to all children. Testers scratched each smell patch, read each of the response options, handed the smell patch to the child, and answered possible questions about particular items. Overall, children’s responses tended to be quick and certain. Children were allowed to complete the task when they provided cogent responses in a timely manner. As is standard professional practice, when a child seemed to be unengaged, unable, or unwilling to participate for any reason, testing did not proceed. All control siblings completed the UPSIT testing with no difficulty. UPSIT testing was discontinued for 3 affected children (ages 6.2, 6.5, and 7.1). Two additional children came for testing with head colds and were not administered the UPSIT.
The UPSIT was given as part of a larger battery that spanned 2 consecutive mornings. Testing was conducted by a licensed clinical psychologist or by 1 of 4 specially trained testers with a minimum of 2 years of child testing and/or treatment experience at the doctoral level. A licensed clinical psychologist supervised testers. All testing was completed by 1 PM to control for circadian effects. Parents waited immediately outside the testing room for their children and were available to the children as needed. For all administrations, the order of the booklets (1-4) was maintained. In some cases, UPSIT smell test booklets were administered between other subtests or on consecutive mornings. During testing, some children asked for clarification of particular stimulus items, and testers provided analogous response options. Response options that were queried most frequently included turpentine, “like paint cleaner”; menthol or wintergreen, “like some cough drops”; cedar, “like a Christmas tree”; and leather, “like new shoes.” No UPSIT items included response options that belonged to similar odor classes (eg, 2 types of mint), so these analogies were enough to trigger recognition while not invalidating particular items. All children who were included in these analyses were healthy, without upper respiratory infection, and unmedicated at the time of testing.
Scoring and Database and Data Analysis
Testers marked children’s responses to all items to ensure coding accuracy. A specially trained research worker (S.M.) scored each test booklet with scoring templates provided in the UPSIT manual. All accuracy scores were retallied to ensure accuracy. All individual item responses were entered, total accuracy scores were calculated with a simple algorithm, and the age-corrected percentile equivalences for total raw accuracy scores (heretofore referred to as UPSIT scores) were obtained from the appropriate gender-specific, age-corrected norms table in the UPSIT manual. VPI status and history of chronic nasal regurgitation/nasogastric tube feeding were scored dichotomously on the basis of information in children’s medical charts and medical history forms that were completed by parents before their first visit. All data were entered and maintained in a Statview database and analyzed using Statview Version 3.0 for PC or SAS Version 6.0 for PC.
Unpaired t tests were used to examine possible mean age differences between groups. Box plots were used to examine distributional characteristics of UPSIT scores, and F tests were used to examine variance equality between groups. Score distributions and variances differed broadly. For analyses that compared affected children and control siblings, χ2 tests were calculated to examine group differences in the frequency of children with scores in the impaired range (≥2 SDs below the standardization sample mean).
For secondary analyses that examined differences among affected children, UPSIT score distributions by age group, history of VPI, and history of nasal regurgitation/nasogastric tube feeding were examined. For all subgroups, distributions were similarly skewed (positive), suggesting low percentile score clusters for all groups. Variances of age groups and by history of VPI differed markedly. UPSIT scores of groups with and without a history of nasal regurgitation/nasogastric tube feeding did not differ with regard to variance. UPSIT score group differences were examined with Mann-Whitney U tests that compared subgroups of younger and older affected children, children with and without a history of VPI, and children with and without a history of nasal regurgitation/nasogastric tube feeding.
In additional exploratory analyses, error frequency differences of affected children and control siblings were compared for specific odors using χ2 analysis, with probability set to .01 to correct for multiple comparisons. Logistic regression was used to examine the likelihood of potentially critical odor detection failures in children with 22q11DS.
RESULTS
Mean age of affected children and control siblings did not differ (mean difference: 0.04, degree of freedom [df] = 60, t = 0.06). Initial review of UPSIT scores suggested that the distributions of group scores and group variances differed broadly (Fig 1). UPSIT scores of affected children were clustered tightly at the low end of the scale (positively skewed), and an F test of variances revealed a large difference (variance ratio: 2.43, numerator df = 22, denominator df = 38, P = .02). Group medians and interquartile ranges (IQRs) for UPSIT scores differed broadly (22q11 median: 4.0 [IQR: 6.75]; sibling median: 24.0 [IQR: 18.0]).
FIGURE 1.
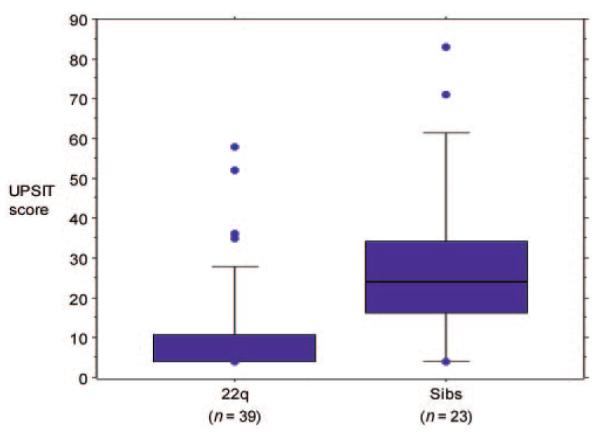
Comparison of UPSIT scores of children with 22q11DS and control siblings.
Given these distribution characteristics, nonparametric tests were used for the primary group comparison. UPSIT scores were dichotomized (yes/no); scores ≥2 SDs below the standardization sample mean indicated impairment. χ2 analysis of the frequency of impairment by group suggested a large difference (χ2[1,N = 62] = 16.7, P < .001); 26 (68%) of 39 affected children, as compared with 3 (13%) of 23 control siblings, had UPSIT scores in the impaired range.
Secondary analyses were conducted to explore possible factors that contributed to these differences among affected children. To examine age effects on performance in affected children (n = 39), younger and older subgroups were created using the sample median age (9.4; younger n = 19, older n = 20). The distribution and variance characteristics of age subgroups were compared. Both distributions were positively skewed, and variances differed (variance ratio: 0.23, numerator df = 18, denominator df = 19, P = .003). Mann-Whitney U test suggested that mean ranks for younger (19.6) and older (20.4) children did not differ (U = 182.5, z =−0.2, P = .83). There was no evidence of an association between age and lowered UPSIT scores among children with 22q11DS.
We also considered the scores of affected children with a history of VPI. No sibling control subjects had a history of VPI. Among the 39 children with 22q11DS, 17 had a history of VPI, 9 of whom had undergone corrective (flap) surgery. Table 1 shows the descriptive statistics of UPSIT scores for these children. Given the distribution characteristics and broad variance differences between groups (variance ratio = 12.4, numerator df = 12, denominator df = 16, P < .001), Mann-Whitney U test was calculated to compare distributions. Group mean ranks did not differ (22q without VPI mean rank: 20.7; 22q with VPI mean rank: 19.1; U = 172.5, z = −0.41, P = .68). Although the mean score of children without VPI seemed to be twice that of children with VPI (Table 1), subgroup medians in these skewed distributions were equivalent, indicating that the larger group mean among children without a VPI history was attributable to sporadic high scores.
TABLE 1.
Descriptive Statistics Comparing UPSIT Scores for Children With and Without VPI
| n | Mean (SD) | Median | Variance | Skew | |
|---|---|---|---|---|---|
| No VPI | 22 | 13.4 (16.4) | 4.0 | 269.5 | 1.7a |
| VPI | 17 | 7.0 (4.7) | 4.0 | 21.8 | 1.2a |
| VPI no surgical correction | 9 | 6.4 (4.2) | 5.0 | 17.8 | 1.6a |
| VPI with surgical correction | 8 | 7.6 (5.3) | 4.0 | 28.6 | .86 |
Benchmark for significant skewness≥1.
To corroborate that group differences between affected children and siblings were not dependent on the UPSIT scores of affected children with a history of VPI, we calculated a second χ2 to compare the frequencies of UPSIT scores in the impaired range in affected children without a history of VPI (n = 22) and control siblings (n = 23). Differences between groups (excluding affected children with a history of VPI) were significant (χ2 = 12.2, df = 1, P < .001).
We also examined the possible influence of early feeding problems on olfaction. Thirteen (33%) affected children in this sample had an early history of chronic nasal regurgitation, 5 of whom required nasogastric tube feeding for periods of up to 6 weeks. Mann-Whitney U test suggested no difference in the mean ranks of UPSIT scores for these 2 groups of affected children (U = 159, z =−0.3, P = .77). In contrast to VPI status results, variances between groups did not differ.
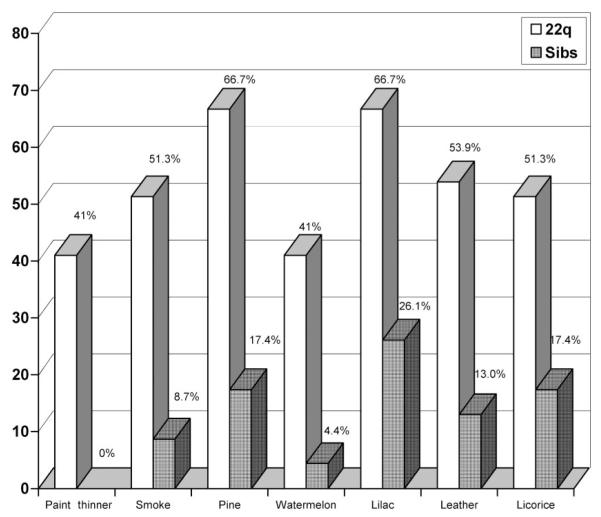
For clinical purposes, we conducted additional exploratory analyses to compare the frequency of specific smell errors in affected children and control siblings. To control for repeated tests, significance for these analyses was P ≤ .01. Children with 22q11DS were found to be especially inaccurate in detecting 7 of 40 odors (χ2 ≥ 6.5, P < .01). In the magnitude of error differences from greatest to least, these included paint thinner, smoke, pine, watermelon, lilac, leather, and licorice (Fig 2). Differences in detection accuracy for 3 additional odors (cherry, clove, and menthol) approached significance (χ2 ≥ 6.1, P ≤ .02). No control siblings failed to detect paint thinner as compared with 40% of affected children (logistic regression could not be used to compare group likelihood risk with 1 group = 0). Logistic regression suggested that compared with control siblings, children with 22q11DS were 2.4 times less likely to detect smoke (r = 0.40, P = .003). As an example of failure to detect pleasurable odors, children with 22q11DS were found to be 2.8 times less likely to perceive watermelon accurately (r = 0.40, P = .011).
FIGURE 2.
Error rates for selected odors in children with 22q11DS and sibling controls
DISCUSSION
Studies of olfaction in pediatric samples are rare, and olfactory disorder in children with 22q11DS has not been reported previously. The UPSIT, standardized for children who are 4 years and older, was administered to 62 school-aged children who ranged in age from 5.3 to 14.8 years, including 39 with the 22q11DS and 23 neurotypical control siblings. UPSIT scores for the affected group were nonnormally distributed, and group medians differed markedly. χ2 analyses suggested that a substantially larger proportion of children with 22q11DS had scores in the impaired range (≥2 SDs below the standardization sample mean). During testing, virtually all children exhibited frequent spontaneous facial reactions, and the reactions of some affected children seemed to indicate marked hypersensitivity. For this reason, we propose that children with 22q11DS manifest dysosmia (distorted sense of smell) rather than anosmia (loss of smell). This requires additional investigation.
In secondary analyses that examined possible sources of lowered UPSIT scores among affected children, chronological age was considered. UPSIT norms are age corrected. If a given population is globally delayed with early impulsivity, then younger as compared with older affected children might be expected to perform especially poorly as compared with same-age peers, and group differences might be attributable to markedly lowered scores among only younger affected children. No age effects were found, however. Olfactory disorder in children with 22q11DS seems to be equally prevalent in younger and older children and therefore is not likely to reflect general developmental delay or impulsive responding among younger children.
Other medical conditions also might contribute to olfactory disorder in children with 22q11DS, perhaps the most common of which is VPI. Medical charts indicated that 49% of affected children in this sample were positive for VPI. Given the relatively small sample size, the VPI group included children with and without a history of corrective flap surgery (a surgically created flap does not resolve altered airway patency). No differences were found in the UPSIT scores of affected children with and without a history of VPI. However, sample distribution characteristics importantly inform these findings. The variance of UPSIT scores in affected children with VPI was significantly lower, and, interestingly, lowest among those without a history of surgical correction (Table 1), perhaps indirectly indicating a consistent negative impact of (uncorrected) VPI on olfaction in children with 22q11DS. At the same time, an additional analysis that compared only affected children without VPI and siblings substantiated a large group difference in odor detection. Olfactory disorder in this population seems to have multiple determinants. These findings require replication and additional study.
Other factors that might affect olfaction in children with 22q11DS also were considered. Affected children with a history of nasal regurgitation/nasogastric tube feeding were compared with those without. Consistent with previous findings in children with the genetic syndrome of coloboma, congenital heart disease, choanal atresia, mental and growth retardation, genital anomalies, and ear malformations and hearing loss,22 no differences were found. Unlike the variances among children with and without VPI, there were no variance differences in children with and without a history of early feeding difficulties.
History of upper airway surgeries among children with 22q11DS also might be expected to influence odor detection. In this sample, however, only 4 affected children (and no control siblings) had a history of adenoidectomy and/or tonsillectomy. This was not a sufficient number for statistical comparison (the percentile scores for these 4 cases were broadly distributed: 4, 10, 16, and 52). Mouth-breathing also might be expected to alter odor detection; however, only 3 children in this sample had this diagnosis, and their percentile scores were varied (4, 14, and 16). Generally speaking, research that examines the influence of VPI, early feeding problems, and mouth-breathing in pediatric populations is rare. With regard to 22q11DS, its extremely broad physical phenotype will require replications with larger samples of affected children.
Overall, the results suggested that olfactory disorder among children with 22q11DS is ubiquitous across age bands and has multiple determinants, one of which may be VPI. A majority of affected children have olfactory disorder, suggesting its centrality in the phenotypic characterization of 22q11DS. Exploratory analyses that were conducted for clinical purposes revealed that children with 22q11DS are particularly inaccurate in detecting potentially toxic fumes and smoke, as well as pleasurable smells, which may reduce their quality of life. The probability of odor detection impairment with serious safety consequences should be conveyed to teachers, professionals, parents, and caregivers of children with 22q11DS. Many more investigations of olfactory disorder in this and other at-risk pediatric populations are needed. Comparisons of children with 22q11DS to nonsyndromic children with histories of nasal airway difficulties would be especially valuable.
Limitations
The UPSIT was given as part of a larger battery that focused on neurocognitive development in children with 22q11DS. VPI status for secondary analyses was determined by medical chart review and parent report at intake. Independent evaluation of velopharyngeal structure was not conducted. For future studies of olfaction in children with 22q11DS, direct measurement of the degree of nasal obstruction should be made by rhinometry.
The extent to which this sample represents larger groups of children with 22q11DS cannot be determined at this time. The number of publications that report findings from unique populations of children with 22q11DS remains small. It is possible that the type of study described here attracts the participation of children with a lower prevalence of serious medical conditions. This awaits additional consideration.
The administration method of the UPSIT was adapted to suit the needs of this particular child population. All items were read to all children, and all children were provided with odor label analogies as needed. In some cases, UPSIT booklets were administered between other subtests or on consecutive mornings. These methodologic deviations were not ideal but were deemed necessary to ensure the highest possible quality of participant responses. Furthermore, the UPSIT does not provide a standardized means to query qualitative perceptions; however, this is a very important area for future study. Reports have suggested that independent brain pathways govern detection of odor valence (pleasant/unpleasant) versus detection accuracy.28 Determining which types of errors are most typical among affected children could reveal selective brain pathway vulnerabilities. This sample was predominantly white. Additional studies are needed to determine the relevance of these findings to other races.
ACKNOWLEDGMENTS
This research was supported by a grant from the Child Health and Human Development Branch of the National Institutes of Health (K08-HD040321, to C.S.) and also by a General Clinical Research Center grant (M01-RR00102) from the National Center for Research Resources, National Institutes of Health.
We thank Maude Blundell, MS, for contributions to the early recruitment phase of this study and Kawame Anyane-Yeboa, MD, for participant referrals.
Abbreviations
- 22q11DS
22q11 deletion syndrome
- GABA
γ-aminobutyric acid
- VPI
velopharyngeal insufficiency
- UPSIT
University of Pennsylvania Smell Identification Test
- df
degree of freedom
- IQR
interquartile range
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Botto LD, May K, Fernhoff PM, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(pt 1):101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KC, Owen MJ. Velo-cardio-facial syndrome: a model for understanding the genetics and pathogenesis of schizophrenia. Br J Psychiatry. 2001;179:397–402. doi: 10.1192/bjp.179.5.397. [DOI] [PubMed] [Google Scholar]
- 3.Yagi H, Furutani Y, Hamada H, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AK, Goodship JA, Wilson DI, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulver AE, Nestadt G, Goldberg R, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Martin JH. Neuroanatomy: Text and Atlas. Appleton & Lange; Norwalk, CT: 1989. The basal ganglia; pp. 267–289. [Google Scholar]
- 7.van Elst LT, Valerius G, Buchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobin C, Kiley-Brabeck K, Karayiorgou M. Associations between prepulse inhibition and executive visual attention in children with the 22q11 deletion syndrome. Mol Psychiatry. 2005;10:553–562. doi: 10.1038/sj.mp.4001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacFarlane A. Olfaction in the Development of Social Preferences in the Human Neonate. Elsevier; New York, NY: 1975. [DOI] [PubMed] [Google Scholar]
- 11.Engen T, Lipsitt LP. Decrement and recovery of responses to olfactory stimuli in the human neonate. J Comp Physiol Psychol. 1965;59:312–316. doi: 10.1037/h0021820. [DOI] [PubMed] [Google Scholar]
- 12.Berkowicz DA, Trombley PQ, Shepherd GM. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J Neurophysiol. 1994;71:2557–2561. doi: 10.1152/jn.1994.71.6.2557. [DOI] [PubMed] [Google Scholar]
- 13.Kratskin IL, Belluzzi O. Anatomy and neurochemistry of the olfactory bulb. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2nd ed Marcel Dekker; New York, NY: 2003. pp. 139–164. [Google Scholar]
- 14.Berkowicz DA, Trombley PQ. Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 2000;855:90–99. doi: 10.1016/s0006-8993(99)02342-2. [DOI] [PubMed] [Google Scholar]
- 15.Hawkes CH, Shephard BC, Daniel SE. Is Parkinson’s disease a primary olfactory disorder? Q J Med. 1999;92:473–480. doi: 10.1093/qjmed/92.8.473. [DOI] [PubMed] [Google Scholar]
- 16.Malloy PF, Richardson E. Assessment of frontal lobe functions. In: Salloway SP, Malloy PF, Duffy JD, editors. The Frontal Lobes and Neuropsychiatric Illness. American Psychiatric Publishing, Inc; Washington, DC: 2001. pp. 125–137. [Google Scholar]
- 17.Rupp CI, Fleischhacker WW, Kemmler G, et al. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005;74:149–161. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Seckinger RA, Goudsmit N, Coleman E, et al. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res. 2004;69:55–65. doi: 10.1016/S0920-9964(03)00124-5. [DOI] [PubMed] [Google Scholar]
- 19.Minor KL, Wright BD, Park S. The Smell Identification Test as a measure of olfactory identification ability in schizophrenia and healthy populations: a Rasch psychometric study. J Abnorm Psychol. 2004;113:207–216. doi: 10.1037/0021-843X.113.2.207. [DOI] [PubMed] [Google Scholar]
- 20.Duncan HJ, Seiden AM. Long-term follow-up of olfactory loss secondary to head trauma and upper respiratory tract infection. Arch Otolaryngol Head Neck Surg. 1995;121:1183–1187. doi: 10.1001/archotol.1995.01890100087015. [DOI] [PubMed] [Google Scholar]
- 21.Ghorbanian SN, Paradise JL, Doty RL. Odor perception in children in relation to nasal obstruction. Pediatrics. 1983;72:510–516. [PubMed] [Google Scholar]
- 22.Chalouhi C, Faulcon P, Le Bihan C, Hertz-Pannier L, Bonfils P, Abadie V. Olfactory evaluation in children: application to the CHARGE syndrome. Pediatrics. 2005;116:81–88. doi: 10.1542/peds.2004-1970. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes M, Solot C, Wang PP, et al. Cognitive and behavior profile of preschool children with chromosome 22q11.2 deletion. Am J Med Genet. 1999;85:127–133. [PubMed] [Google Scholar]
- 24.Doty R. The University of Pennsylvania Smell Identification Test Administration Manual. Sensonics; Philadelphia, PA: 1983. [Google Scholar]
- 25.Doty RL. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 26.Sobin C, Monk SH, Kiley-Brabeck K, Khuri J, Karayiorgou M. Neuromotor defects in children with the 22q11 deletion syndrome. Mov Disord. 2006 doi: 10.1002/mds.21103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy KC, Jones RG, Griffiths E, Thompson PW, Owen MJ. Chromosome 22qII deletions. An under-recognised cause of idiopathic learning disability. Br J Psychiatry. 1998;172:180–183. doi: 10.1192/bjp.172.2.180. [DOI] [PubMed] [Google Scholar]
- 28.Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:195–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]