Abstract
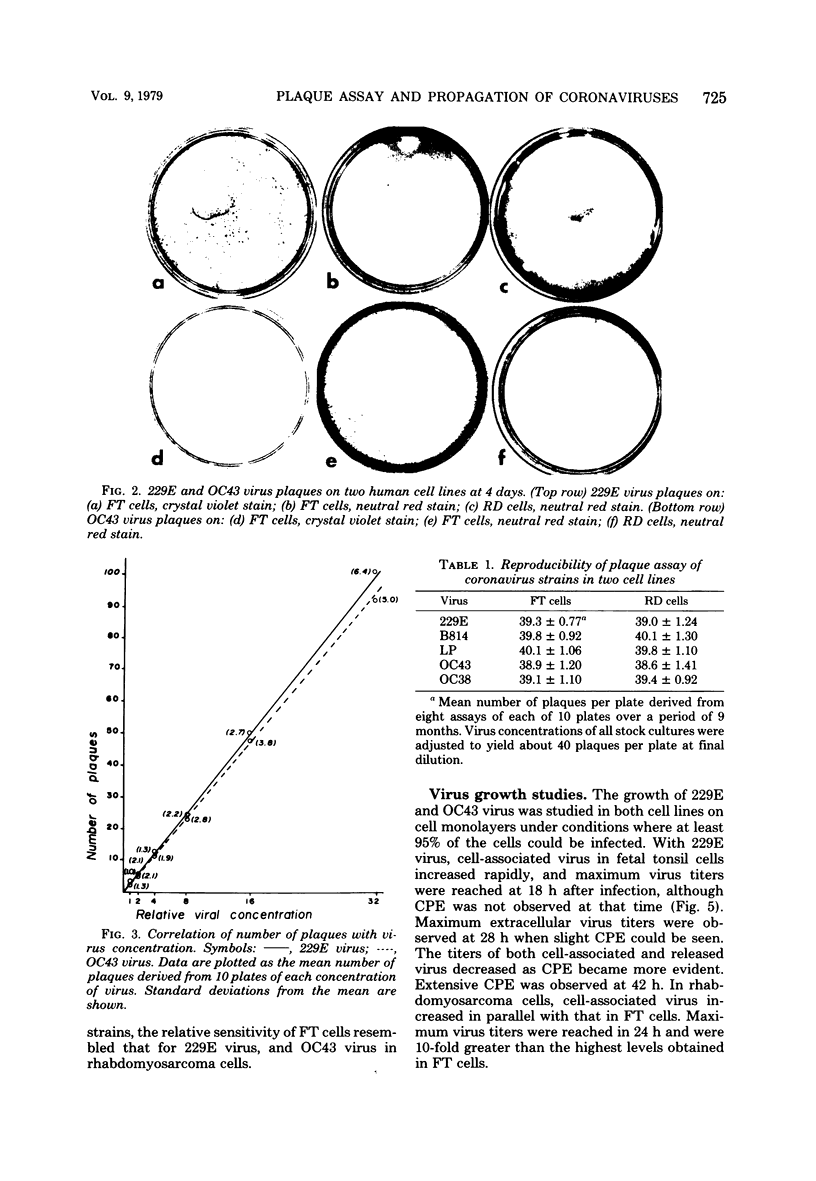
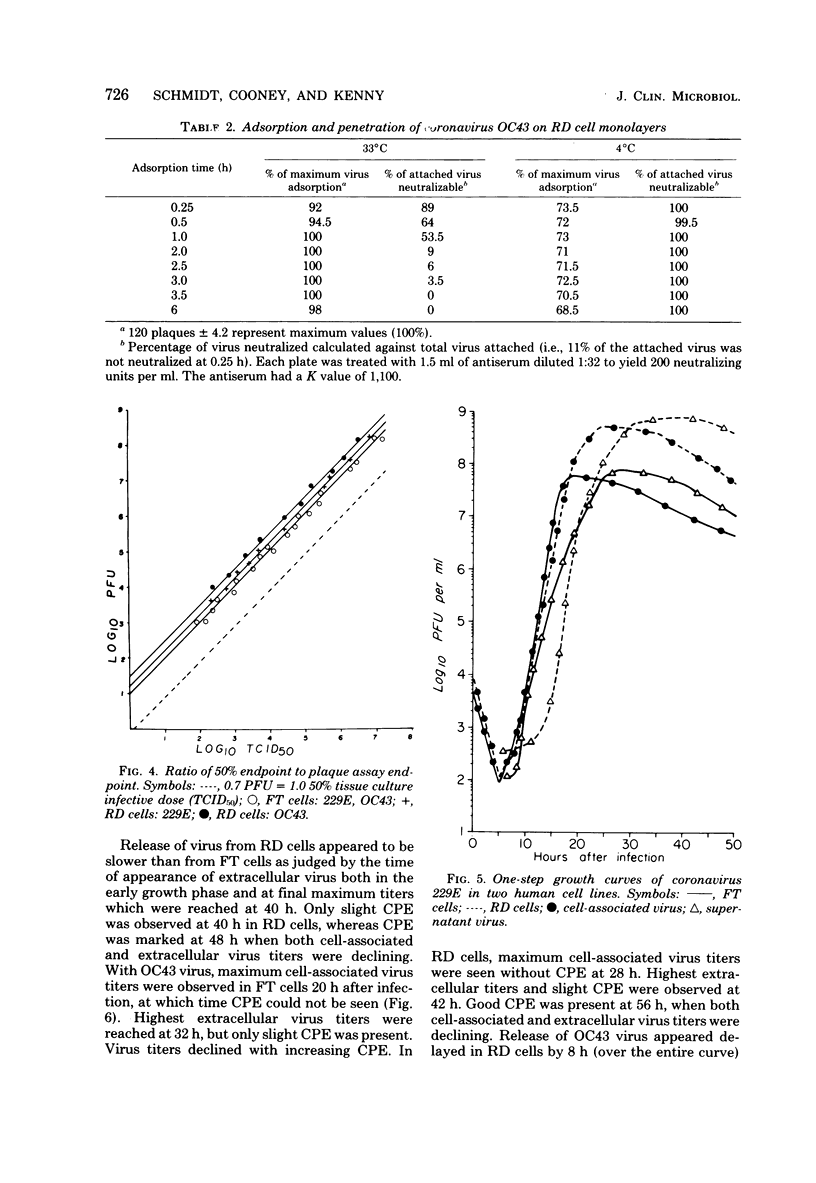
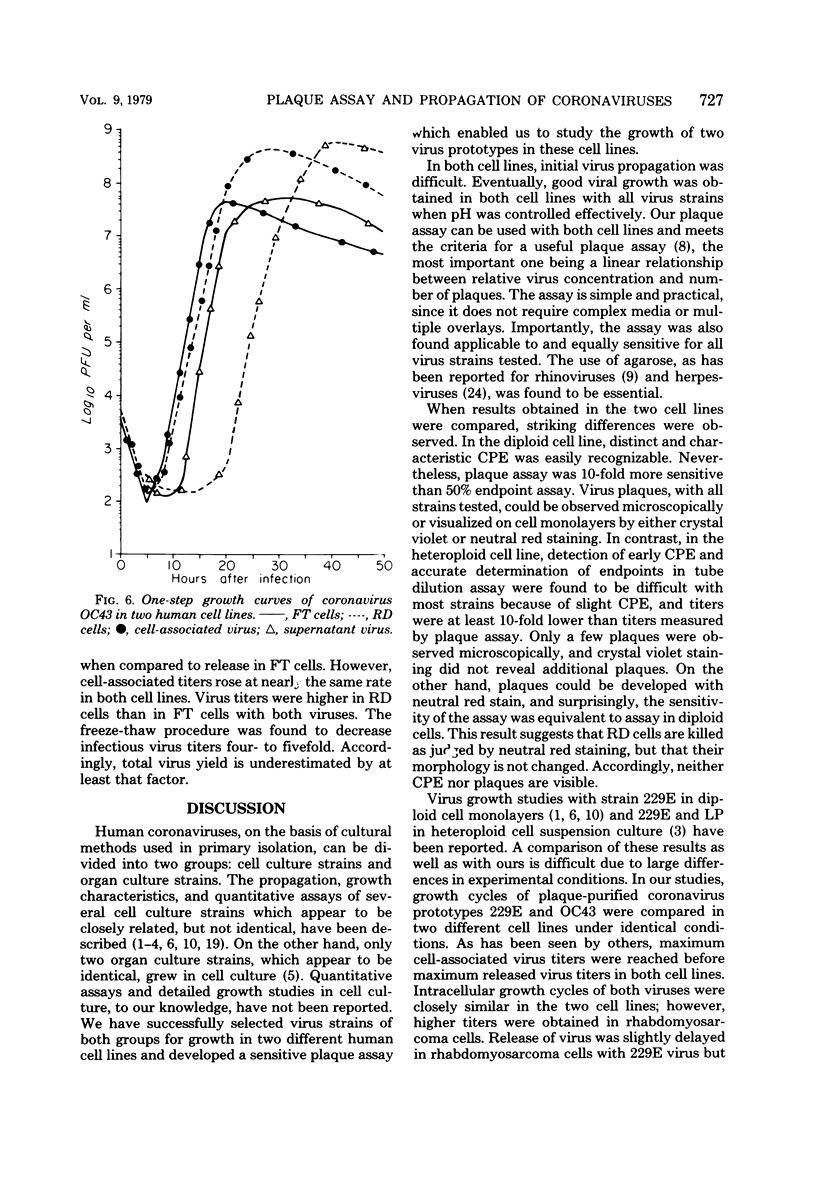
Propagation and plaque assay of human coronavirus prototypes were studied in two human cell lines: a diploid fetal tonsil (FT) and a heteroploid rhabdomyosarcoma (RD) cell lines. Plaques, observed within 2 to 3 days on FT cell monolayers with both 229E and OC43 viruses, appeared as colorless areas after staining with neutral red or crystal violet, whereas neutral red staining was required for visualization of plaques on RD cells. The plating efficiencies were approximately equal between the two cell lines, but virus assay by plaque formation was 15- to 30-fold more efficient than tube dilution assay with 50% endpoints. The discrepancy between 50% endpoint and plaque-forming unit values was striking and appeared to result from the fact that killing of cells (particularly RD cells) by coronaviruses was not accompanied by visible changes in the cells but killing was detected by the failure of infected cells to stain with a vital dye. The latent phase in one-step growth curves was 5 to 6 h for both viruses in either cell line, but the maximum yield of intracellular virus was reached in 18 to 20 h for FT cells and 24 to 28 h for RD cells. Virus release also differed between the two cell lines: in FT cells, the maximum yield of extracellular virus was reached 2 to 3 h later than that of intracellular virus, whereas in RD cells, the difference was 5 h for 229E virus and 10 h for OC43 virus. Although both cell lines appear equally useful for plaque assay, RD cells would be preferred for mass virus propagation because yields (5 X 10(8) plaque-forming units per ml) were 10-fold higher than in FT cells, a finding true for both virus prototypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. B., McIntosh K., Dees J. H., Chanock R. M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E). J Virol. 1967 Oct;1(5):1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A. F. An investigation of the replication of coronaviruses in suspension cultures of L132 cells. Arch Gesamte Virusforsch. 1972;37(4):297–307. doi: 10.1007/BF01241452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A. F. Antigenic relationships amongst coronaviruses. Arch Gesamte Virusforsch. 1970;31(3):352–364. doi: 10.1007/BF01253769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburne A. F., Tyrrell D. A. The propagation of "coronaviruses" in tissue-culture. Arch Gesamte Virusforsch. 1969;28(2):133–150. doi: 10.1007/BF01249379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucková M., McIntosh K., Kapikian A. Z., Chanock R. M. The adaptation of two human coronavirus strains (OC38 and OC43) to growth in cell monolayers. Proc Soc Exp Biol Med. 1970 Nov;135(2):431–435. doi: 10.3181/00379727-135-35068. [DOI] [PubMed] [Google Scholar]
- Bucknall R. A., King L. M., Kapikian A. Z., Chanock R. M. Studies with human coronaviruses. II. Some properties of strains 229E and OC43. Proc Soc Exp Biol Med. 1972 Mar;139(3):722–727. doi: 10.3181/00379727-139-36224. [DOI] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E. Immunogenicity of rhinoviruses. Proc Soc Exp Biol Med. 1970 Feb;133(2):645–650. doi: 10.3181/00379727-133-34536. [DOI] [PubMed] [Google Scholar]
- Fiala M., Kenny G. E. Enhancement of rhinovirus plaque formation in human heteroploid cell cultures by magnesium and calcium. J Bacteriol. 1966 Dec;92(6):1710–1715. doi: 10.1128/jb.92.6.1710-1715.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Kindig D. A., Mann J. Growth and intracellular development of a new respiratory virus. J Virol. 1967 Aug;1(4):810–816. doi: 10.1128/jvi.1.4.810-816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Procknow J. J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966 Jan;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Henigst W. Vorkommen von Antikörpern gegen Coronavirus (OC 43) in der gesunden Bevölkerung und bei Respirationstraktkranken. Zentralbl Bakteriol Orig A. 1974;229(2):150–158. [PubMed] [Google Scholar]
- Kapikian A. Z., James H. D., Jr, Kelly S. J., Dees J. H., Turner H. C., McIntosh K., Kim H. W., Parrott R. H., Vincent M. M., Chanock R. M. Isolation from man of "avian infectious bronchitis virus-like" viruses (coronaviruses) similar to 229E virus, with some epidemiological observations. J Infect Dis. 1969 Mar;119(3):282–290. doi: 10.1093/infdis/119.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Becker W. B., Chanock R. M. Growth in suckling-mouse brain of "IBV-like" viruses from patients with upper respiratory tract disease. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2268–2273. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Dees J. H., Becker W. B., Kapikian A. Z., Chanock R. M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967 Apr;57(4):933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Kapikian A. Z., Hardison K. A., Hartley J. W., Chanock R. M. Antigenic relationships among the coronaviruses of man and between human and animal coronaviruses. J Immunol. 1969 May;102(5):1109–1118. [PubMed] [Google Scholar]
- McIntosh K., Kapikian A. Z., Turner H. C., Hartley J. W., Parrott R. H., Chanock R. M. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970 Jun;91(6):585–592. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYRRELL D. A., BYNOE M. L. CULTIVATION OF A NOVEL TYPE OF COMMON-COLD VIRUS IN ORGAN CULTURES. Br Med J. 1965 Jun 5;1(5448):1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Bynoe M. L., Hoorn B. Cultivation of "difficult" viruses from patients with common colds. Br Med J. 1968 Mar 9;1(5592):606–610. doi: 10.1136/bmj.1.5592.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of Herpesvirus hominis on human embryonic fibroblasts. Proc Soc Exp Biol Med. 1969 Jun;131(2):588–592. doi: 10.3181/00379727-131-33932. [DOI] [PubMed] [Google Scholar]