Abstract
Alterations in immunity that occur with aging likely contribute to the development of infection, malignancy and inflammatory diseases. Naturally occurring CD4+ regulatory T cells (Treg) expressing high levels of CD25 and forkhead box P3 (FOXP3) are essential for regulating immune responses. Here we investigated the effect of aging on the number, phenotypes and function of CD4+ Treg in humans. The frequency and phenotypic characteristics of CD4+,FOXP3+ T cells as well as their capacity to suppress inflammatory cytokine production and proliferation of CD4+,CD25− T cells (target cells) were comparable in young (age ≤ 40) and elderly (age ≥ 65) individuals. However, when CD4+,FOXP3+ Treg and CD4+,CD25− T cells were co-cultured at a ratio of 1:1, the production of anti-inflammatory cytokine IL-10 from CD4+,CD25− T cells was more potently suppressed in the elderly than in the young. This finding was not due to changes in CTLA-4 expression or apoptosis of CD4+,FOXP3+ Treg and CD4+,CD25− T cells. Taken together, our observations suggest that aging may affect the capacity of CD4+,FOXP3+ T cells in regulating IL-10 production from target CD4+ T cells in humans although their other cellular characteristics remain unchanged.
Keywords: human, regulatory T cells, aging, IL-10 and FOXP3
1. Introduction
Alterations in the immune system that occur with aging (Effros et al., 2003; Frasca et al., 2005; Goronzy and Weyand, 2005; Linton and Dorshkind, 2004; Miller, 1999) likely contribute to increased risk of infection and malignancy in the elderly (Effros et al., 2003; Goronzy and Weyand, 2005; Linton and Dorshkind, 2004; Miller, 1999). Although the exact cause for such findings is yet to be determined, it is conceivable that aging may cause diminished immunity against microorganisms and malignant cells via altering the mechanisms involved in generating immune responses. The latter response is inevitably accompanied by some degrees of inflammation, which should be kept in balance. The increased frequency of infection and tumors in the elderly suggests the possibility of reduced inflammatory responses with aging (Effros et al., 2003; Goronzy and Weyand, 2005; Linton and Dorshkind, 2004; Miller, 1999). However, aging appears to be associated with dysregulated inflammation as suggested by increased plasma levels of inflammatory cytokines IL-6 and TNF-α that predict an increased mortality risk independently of other risk factors in the elderly (Huang et al., 2005; Krabbe et al., 2004). Furthermore, some chronic inflammatory diseases such as polymyalgia rheumatica and giant cell arteritis are more commonly found in the elderly (Hasler and Zouali, 2005; Hunder, 2000), and metabolic disorders like atherosclerosis and type II diabetes that develop with aging are now viewed as chronic inflammatory disorders driven in part by an imbalance between pro- and anti-inflammatory cytokines (Huang et al., 2005; Vasto et al., 2007). However, it is largely unknown about the mechanisms underlying dysregulated inflammation with aging in humans.
A population of naturally occurring CD4+,CD25+ T cells with immune regulatory function exists in humans and mice (Sakaguchi et al., 1995; von Boehmer, 2005; Wing et al., 2006). However, CD25 as a single marker for CD4+ Treg has been challenged since T cells stimulated by T cell receptor (TCR) triggering can up-regulate the expression of this molecule. Recent studies demonstrate that the forkhead family transcriptional factor FOXP3 is expressed in CD4+,CD25+ Treg and that transfection of the same molecule to CD4+,CD25− T cells, which do not have regulatory function, confers the immune regulatory property (reviewed in (Ziegler, 2006)) (Fontenot et al., 2003; Hori et al., 2003; Khattri et al., 2003). Furthermore, mutations in the Foxp3 gene have been found in scurfy mice with X-linked lymphoproliferative disease as well as in humans with immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX) (Bennett et al., 2001; Brunkow et al., 2001). Although the mechanism(s) of immune regulation by CD4+,CD25+ Treg is not fully understood, such regulation is dependent on cell contact rather than on soluble factors like cytokines (reviewed in (von Boehmer, 2005; Wing et al., 2006)). The target cells for suppression appear to be both T cells and antigen presenting cells (APC) (von Boehmer, 2005; Wing et al., 2006).
In the current study, we investigated whether aging affects the number, phenotype and function of human CD4+ Treg, defined by their expression of CD25 and FOXP3. In particular, we focused on the direct inhibitory effect of Treg on conventional CD4+ T cells in the absence of APC since the latter cells can affect T cell function. The results of our study showed that the frequency, phenotypic characteristics and anti-proliferative function of CD4+,FOXP3+ Treg were comparable in the young (age ≤ 40) and the elderly (age ≥ 65). However, when CD4+,CD25− T cells were stimulated in the presence of the same number of CD4+,FOXP3+ Treg, the production of anti-inflammatory cytokine IL-10 from the former cells was more potently suppressed in the elderly than in the young. These findings suggest that aging may affect the capacity of CD4+,FOXP3+ Treg in regulating IL-10 production from CD4+,CD25− T cells in humans although other cellular characteristics of CD4+,FOXP3+ T cells remain unchanged with aging.
2. Experimental Procedures
2.1. Human subjects
Healthy elderly (age ≥ 65, n = 32) and young subjects (age ≤ 40, n = 29) were recruited for this study (mean age ± SD, 77.1 ± 7.8 and 30.5 ± 5.9). There was no gender difference between the two groups (P = 0.427 and 0.576 by Fisher’s exact tests for phenotypic and functional studies, respectively). Individuals who were taking immunosuppressive drugs or who had any disease potentially affecting the immune system including autoimmune diseases, infectious diseases, malignancy, diabetes, and asthma were excluded (Hong et al., 2004; Kang et al., 2004). Informed consent was obtained from all subjects. This work was approved by the institutional review committees of Yale University and the Veterans Administration New England Health Care System West Haven Campus.
2.2. Flow cytometry and cell sorting
Peripheral blood mononuclear cells (PBMCs) were stained with mouse anti-human CD4 and CD25 antibodies (Abs) (BD Pharmingen, San Jose, CA) followed by washing, permeabilization with permeabilizing buffer and staining with mouse anti-FOXP3 Abs (clone PCH101, eBioscience, San Diego, CA). Some cells were stained additionally with Abs to CD45RA, CTLA-4, CCR7, CCR4, CCR5, CXCR3, IL-15Rα, IL-2/15Rβ, IL-7Rα (the first six Abs from BD Pharmingen and the latter three Abs from R&D Systems, Minneapolis, MN), or appropriate isotype Abs. Stained cells were analyzed on a FACSCalibur® (BD Immunocytometry, San Jose, CA). For cell sorting, PBMCs were stained with mouse anti-human CD4 and CD25 Abs and sorted into CD4+,CD25bright and CD4+,CD25− T cells using a FACSAria® (BD Immunocytometry). Collected flow cytometry data were analyzed using FlowJo® software (Tree Star, Ashland, OR).
2.3. In vitro proliferation and cytokine assays
Sorted CD4+,CD25− T cells (target cells) were labeled with carboxyfluorescein diacetate (CFSE, Molecular Probe, Eugene, OR) as described previously (Glimm and Eaves, 1999). CD4+,CD25bright T cells (see Figure 1A, Treg) and target cells (CD4+,CD25−) were mixed at different ratios of Treg to target cells (0:1, 0.01:1, 0.1:1 and 1:1) and incubated for 7 days in a 96-well round bottom tissue culture plate in the presence of polystyrene latex microspheres (diameter 6 μm, Polyscience Inc, Warrington, PA) coated with anti-CD3 and of anti-CD28 Abs (beads to cell ratio 2:1) as previously described (Lowin-Kropf et al., 1998); (Kim et al., 2007). Cells were analyzed on a FACSCalibur® following incubation. Collected flow cytometry data were analyzed using FlowJo® software (Tree Star, Ashland, OR). Inhibitory index for cell proliferation was calculated for each sample as follows: (proliferation of target cells alone –proliferation of target cells in the presence of Treg)/proliferation of target cells alone × 100. Some sorted target cells were incubated for 7 days in a 96-well flat bottom tissue culture plate coated with anti-CD3/-CD28 Abs (4 and 2 μg/ml, respectively) in the presence or absence of CD4+,CD25bright T cells. Supernatants were harvested and analyzed using a commercially available multiplex cytokine assay kit according to the manufacturer’s instruction (Bio-Rad Laboratories, Hercules, USA). Inhibitory index for cytokine production was calculated as follows: (cytokine production from target cells alone – cytokine production from target cells in the presence of Treg)/cytokine production from target cells alone × 100. In some experiments, the frequency of live cells was determined by staining with annexin V.
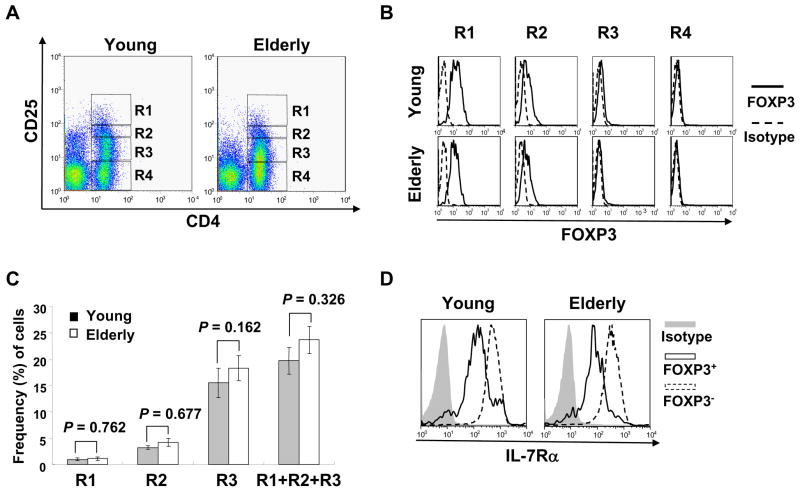
Fig. 1.
Correlation of CD25, FOXP3 and IL-7Rα expression by CD4+ T cells in young and elderly humans. Purified peripheral blood mononuclear cells (PBMCs) from young (n = 10) and elderly (n = 10) individuals were stained with Abs to CD4 and CD25, followed by permeabilization and staining with Abs to FOXP3 or isotype Abs. Some cells were additionally stained with Abs to IL-7Rα or isotype Abs. (A) Representative dot plots showing CD4+ T cell subsets expressing CD25 at different levels. (B) Representative histograms demonstrating the relationship of CD25 and FOXP3 expression by CD4+ T cells. (C) The frequency of CD4+ T cell subsets expressing CD25 differentially in young and elderly individuals. Error bars indicate standard error of the mean (SEM). (D) Representative histograms showing the expression of IL-7Rα on CD4+,FOXP3+ and FOXP3− T cells. P values were obtained by the Mann-Whitney U test.
2.4. Statistical analysis
The Mann-Whitney U test was used to compare measurable variables between the young and the elderly. P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 12.0 (SPSS, Chicago, IL).
3. Results
3.1. The relationships of CD25, FOXP3 and IL-7 receptor α chain (IL-7Rα) expression by CD4+ T cells are similar between young and elderly individuals
CD25 has been used as a marker for identifying naturally occurring CD4+ Treg with immune regulatory function (Sakaguchi and Sakaguchi, 2005; Wang et al., 2006; Yamaguchi and Sakaguchi, 2006). However, the expression of CD25, which can be up-regulated upon T cell activation, on CD4+ T cells is heterogeneous, and there is no established limit for the minimal level of CD25 expression that defines a homogeneous Treg population. Now FOXP3 is considered the best marker for CD4+ Treg although cell sorting using this marker is impossible because of the necessity of cell permeabilization for FOXP3 staining (Fontenot et al., 2003; Hori et al., 2003; Khattri et al., 2003; Ziegler, 2006). Thus, we first determined the relationship of CD25 expression with FOXP3 expression in CD4+ T cells from young (age ≤ 40) and elderly (age ≥ 65) individuals. Based on CD25 expression and isotype staining, CD4+ T cells were divided into four groups: CD25bright, CD25medium, CD25dim and CD25− (Figure 1A, R1–R4, respectively) as previously described (Miyara et al., 2006; Seddiki et al., 2006b; Sugiyama et al., 2005). In both young and elderly individuals, most CD4+,CD25bright T cells were FOXP3+ although some CD4+,CD25medium cells also contained FOXP3 expressing cells (Fig. 1B). In contrast, FOXP3 expressing cells were hardly found in CD25dim and CD25− cells irrespective of age (Fig. 1B). In analyzing the frequency of the different subsets of CD25 expressing CD4+ T cells, young and elderly groups had similar frequencies of CD25bright, CD25medium and CD25dim CD4+ T cells (Fig. 1C).
Several studies suggested a potential role for IL-7Rα(CD127) as a marker for identifying CD4+,FOXP3+ Treg (Liu et al., 2006; Seddiki et al., 2006a). Of interest, we recently observed decreased expression of IL-7Rα on effector memory CD8+ T cells in the elderly (Kim et al., 2006), suggesting the effect of aging on the expression of this molecule. Therefore, we investigated the relationship of this molecule with CD4+,FOXP3+ Treg in the young and the elderly. A large fraction of CD4+,FOXP3+ Treg had decreased levels of IL-7Rα expression compared to CD4+,FOXP3− T cells in both groups (Fig. 1D). There was no significant difference in expressing IL-7Rα on CD4+,FOXP3+ Treg cells between the young and the elderly (mean fluorescent intensity (MFI) of IL-7Rα expression ± standard deviation (SD), 23.9 ± 33.1 vs. 18.3 ± 6.3, P = 0.272, n = 10 for each group). Considerable numbers of CD4+,FOXP3− T cells in the young and the elderly also expressed low levels of IL-7Rα (Fig. 1D, mean frequency (%) ± standard deviation (SD), 15.5 ± 3.34 vs. 12.7 ±4.11, P = 0.647, n = 10 for each group), which indicates that IL-7Rα is not superior to CD25 in identifying CD4+,FOXP3+ T cells. Therefore, we identified a pure population of CD4+,FOXP3+ Treg cells for functional studies by gating on CD4+,CD25bright T cells (R1 in Fig. 1A, Treg cells), which was typically less than 1.5% of total CD4+ T cells. In fact, the frequency of FOXP3+ cells in CD4+,CD25bright T cells was not different between the young and the elderly (mean frequency (%) ± S.D., 89.3 ± 6.4 vs 90.2 ± 4.5, P = 0.597, n = 10 for each group).
3.2. The elderly have a higher frequency of CD4+,FOXP3+ T cells with a memory phenotype (CD45RA−) compared to the young, although both groups have comparable frequencies of CD4+,FOXP3+ T cells in peripheral blood
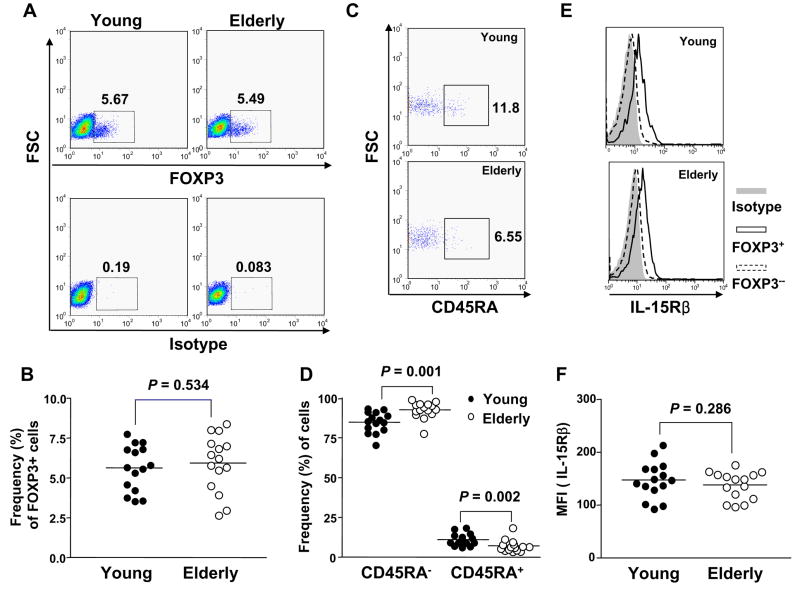
We next analyzed the frequency of CD4+,FOXP3+ T cells in the peripheral blood from young and elderly individuals. The frequency of CD4+,FOXP3+ T cells was not different between the two groups (young vs elderly, mean frequency (%) ± SD, 5.28 ± 0.54 vs 5.45 ± 0.62, P = 0.880) (Fig. 2A and B). In addition, the frequency of the total CD4+ T cells among lymphocytes was not different between the young and the elderly (mean frequency (%) ± SD, 44.8 ± 2.21 vs 44.97 ± 3.95, P = 0.970, n = 15 for each group). These findings indicate that aging does not affect the frequency of CD4+,FOXP3+ Treg in humans. Based on the expression of CD45RA, CD4+ T cells can be divided into naïve (CD45RA+) and memory (CD45RA−) cells, and studies indicate that most CD4+,CD25+ Treg have the memory phenotype (Baecher-Allan et al., 2001; Seddiki et al., 2006b). Of interest, aging is associated with a decreased frequency of naïve CD4+ T cells and an increased frequency of memory CD4+ T cells (Linton and Dorshkind, 2004). Thus, we determined the frequency of naïve (CD45RA+) and memory (CD45RA−) cells in CD4+,FOXP3+ T cells in young and elderly individuals. Consistent with previous studies (Baecher-Allan et al., 2001; Seddiki et al., 2006b), most CD4+,FOXP3+ T cells were CD45RA− in both young and elderly individuals (Fig. 2C). The frequency of CD4+,FOXP3+ T cells with the naïve phenotype was lower in the elderly than in the young (mean frequency (%) ± SD, 3.49 ± 0.846 vs 8.13 ± 0.987 P = 0.002) whereas the frequency of CD4+,FOXP3+ T cells with the memory phenotype was higher in the elderly than in the young (mean frequency (%) ± SD, 94.35 ± 1.14 vs 86.74 ± 1.39, P = 0.001) (Fig. 2D). A similar finding was noticed in CD4+,FOXP3− T cells (data not shown), which indicates that the age-associated alteration in the proportion of naïve and memory subsets occurs in both CD4+,FOXP3+ and FOXP3− T cell populations.
Fig. 2.
Comparing the frequency of CD4+,FOXP3+ T cells and their naïve (CD45RA+) and memory (CD45RA−) phenotypes between young and elderly humans. PBMCs from young (n = 15) and elderly (n = 15) individuals were stained with Abs to CD4, CD45RA and IL-2/15Rβ, followed by permeabilization and staining with Abs to FOXP3 or isotype Abs. (A) Representative dot plots showing identification of FOXP3+ T cells in CD4+ T cells. Numbers in the plots indicate the frequency of FOXP3+ or isotype stained cells. (B) The frequency of FOXP3+ T cells in CD4+ T cells from young and elderly individuals. (C) Representative dot plots showing CD4+,FOXP3+ T cells with and without expressing CD45RA. Numbers in the plots indicate the frequency of CD45RA+ cells. (D) The frequency of CD4+,CD45RA−,FOXP3+ T cells and CD4+,CD45RA+,FOXP3+ T cells. (E) Representative histograms showing the expression of IL-2/-15Rβ on CD4+,FOXP3+ and FOXP3− T cells. (F) MFI of IL-15Rβ expression on CD4+,FOXP3 in young and elderly individuals. P values were obtained by the Mann-Whitney U test.
It is largely unknown how CD4+,FOXP3+ Treg cells are generated and maintained although studies suggest the unique differential lineage of such cells in the thymus (Fontenot et al., 2003; Itoh et al., 1999). This is an intriguing point since the elderly still have unaltered numbers of CD4+,FOXP3+ Treg cells despite the thymic atrophy that occurs with aging (Linton and Dorshkind, 2004). Of interest, recent studies reported the possibility of generating CD4+,FOXP3+ Treg cells in the periphery (Vukmanovic-Stejic et al., 2006). In order to address how CD4+,FOXP3+ T cells are maintained at similar numbers in the young and the elderly, we measured the expression of the IL-2/-15 receptor β chain (IL-2/15Rβ), a critical receptor chain for IL-2 and IL-15 signaling, on CD4+,FOXP3+ Treg. Although Treg cells are anergic to TCR triggering and have impaired proliferation and IL-2 production (Thornton and Shevach, 1998), these cells can be expanded significantly in response to a combination of TCR triggering and IL-2 or IL-15 in vitro (Clark and Kupper, 2006; Earle et al., 2005; Karakhanova et al., 2006). This suggests a role for these cytokines in maintaining CD4+,FOXP3+ Treg. In fact, this notion is supported by the fact that CD4+ Treg cells have high levels of CD25, the high affinity alpha chain of the IL-2R complex. In our study, CD4+,FOXP3+ Treg cells had higher levels of IL-2/15Rβ expression compared to CD4+,FOXP3− T cells in both young and elderly individuals (Fig. 2E). The two groups had no difference in expression of this cytokine receptor chain on CD4+,FOXP3+ Treg cells (Fig. 2F). These findings suggest that the expression of the IL-2/15Rβ chain which is essential for IL-2 and IL-15 signaling is properly maintained on CD4+,FOXP3+ in the elderly, likely contributing to the unaltered number of such cells with aging.
3.3. CD4+,CD25bright T cells in the young and the elderly have comparable levels of inhibitory function except for suppressing IL-10 production
Although we have not found an alteration in the number of CD25+,FOXP3+ T cells with aging, their immunosuppressive function could be different between the young and the elderly. In fact, this notion is supported by the findings of impaired immunosuppressive function of CD4+ Treg cells in patients with rheumatoid arthritis (Ehrenstein et al., 2004; Valencia et al., 2006). Thus, we next determined the inhibitory function of CD4+,CD25bright T cells (R1 in Fig. 1A, Treg) by measuring cytokine production and proliferation of autologous CD4+,CD25− T cells (R4 in Fig. 1A, target cells) in the presence and absence of the former cells in both young and elderly individuals. In this experiment CD25 was used as a surrogate marker for identifying CD4+,FOXP3+ T cells since staining for FOXP3 involves permeabilization of cells and CD4+,CD25bright T cells are mostly FOXP3+ T cells regardless of age (Fig. 1A and B).
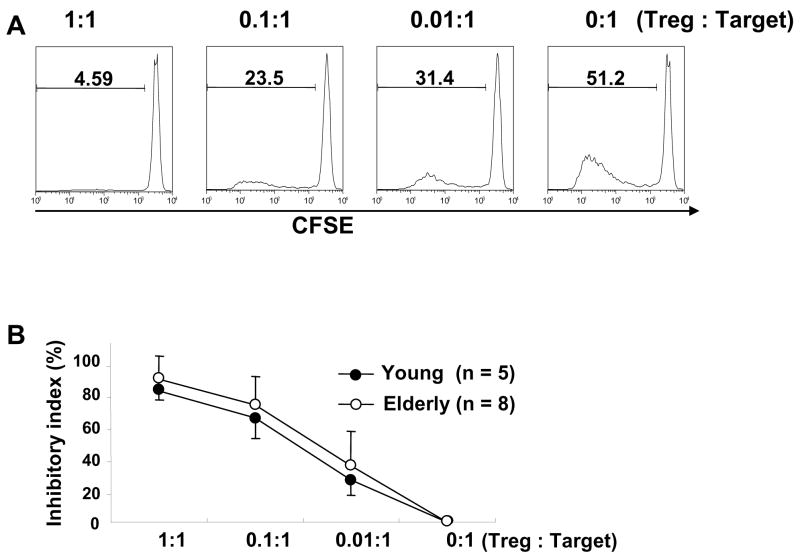
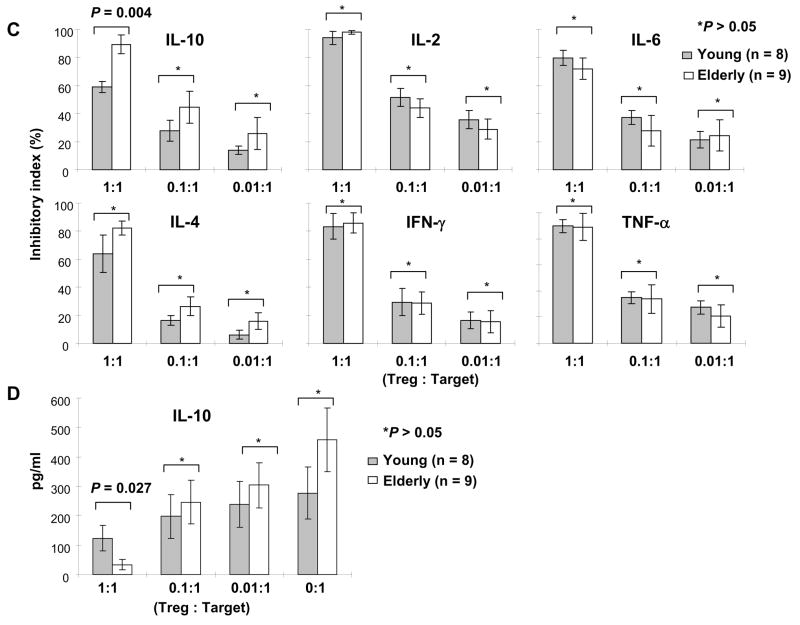
CD4+,CD25bright T cells (Treg) suppressed cytokine production and proliferation of CD4+,CD25− T cells in a cell-number dependent manner (Fig. 3). The higher the number of CD4+,CD25bright T cells, the stronger the inhibitory effect was. The young and the elderly had similar levels of the proliferation inhibitory index (Fig. 3B), which suggests that the capacity of CD4+,CD25bright T cells to suppress the proliferation of CD4+,CD25− T cells does not change with aging. Of interest, IL-10 production from CD4+,CD25− T cells was suppressed more in the elderly than in the young when CD4+,CD25bright T cells and CD4+,CD25− T cells were co-cultured in equal numbers (Treg to target ratio, 1:1) (Fig. 3C). However, in the same culture, the production of pro-inflammatory cytokines including IFN-γ, TNF-α, IL-6 and IL-2 from CD4+,CD25− T cells was suppressed at similar levels between the two groups (Fig. 3C). In fact, IL-10 levels in supernatant from the co-culture of CD4+,CD25bright and CD4+,CD25− T cells at a ratio of 1:1 were lower in the elderly than in the young (n = 9 and 8, respectively, mean (pg/ml) ± standard error of the mean (SEM), 33.0 ± 17.0 vs. 123.6 ± 43.2, P = 0.027) (Fig. 3D). IL-10 levels were not different between the two groups when CD4+,CD25− T were stimulated alone (mean (pg/ml) ± SEM, 457.9 ± 108.1vs. 277.6 ± 88.7, P = 0.229). IL-10 levels were either undetectable or lower than 10 pg/ml in all tested samples except one when CD4+,CD25bright T cells were stimulated alone. This indicates that the production of IL-10 was mostly from CD4+,CD25− T cells when the two cell populations were co-cultured. Also, there was a trend of stronger suppression of IL-4 production from CD4+,CD25− T cells in the elderly although it was not statistically significant. These findings suggest that aging affects the capacity of CD4+ Treg in regulating IL-10 production from CD4+,CD25− T cells. However, the effect of aging on such a capacity appears to be small since the suppression of IL-10 production was only significant in the presence of high numbers of CD4+,CD25bright T cells.
Fig. 3.
Comparing the capacity of CD4+,CD25bright T cells in suppressing the proliferation and cytokine production of CD4+,CD25− T cells between young and elderly humans. PBMCs from young and elderly individuals were stained with Abs to CD4 and CD25 and sorted into CD4+,CD25bright (R1 in Fig. 1A, Treg) and CD4+,CD25− (R4 in Fig. 1A, Target) T cells. (A) In some experiment, the latter cells were stained with CFSE and mixed with CD4+,CD25bright T cells at various ratios as indicated in the figure and incubated for 7 days in the presence of anti-CD3 and -CD28 Abs. Proliferating target cells were identified based on CFSE staining using flow cytometry. Numbers on histograms indicate the frequency of proliferating target cells. Representative data from a healthy elderly individual. (B) Proliferation inhibitory index was calculated as described in methods. Circles and error bars indicate the mean and standard deviation (SD), respectively. (C–D) Sorted CD4+,CD25bright and CD4+,CD25− T cells were co-cultured for 7 days at various ratios in the presence of anti-CD3 and CD28 Abs. Cytokine levels in tissue culture supernatant were measured using a multiplex cytokine assay kit. (C) Inhibitory index for cytokine production was calculated as described in methods. (D) IL-10 levels (pg/ml) in tissue culture supernatant. Bars and error bars indicate the mean and SEM, respectively. P values were obtained by the Mann-Whitney U test.
3.4. Age-associated alteration in the capacity of CD4+,CD25bright T cells to suppress IL-10 production from CD4+,CD25− T cells is not secondary to changes in CTLA-4 expression or apoptosis
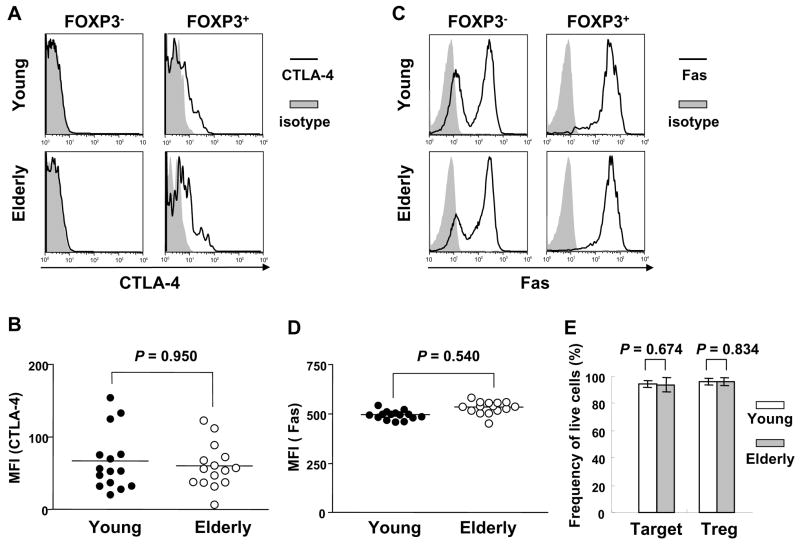
It is still largely unknown how CD4+ Treg suppress immune responses although cell to cell contact appears to be an important mechanism. One of the suggested cell-contact dependent mechanisms is immune regulation via CTLA-4 which provides an inhibitory signal in T cells (von Boehmer, 2005; Wing et al., 2006). However, in this case, the CTLA-4-mediated inhibitory mechanism is somewhat different from its conventional mechanism involving non-Treg and APCs. CD4+ Treg appear to provide an inhibitory signal to other T cells expressing CD80 via a mechanism called outside-in signaling where CTLA-4 expressed by CD4+ Treg binds to CD80 on activated non-Treg cells (Paust et al., 2004; Taylor et al., 2004; von Boehmer, 2005; Wing et al., 2006). In order to address why CD4+ Treg-mediated suppression of IL-10 production from CD4+,CD25− T cells was more potent in the elderly than in the young, we measured intracellular CTLA-4 expression in CD4+,FOXP3+ T cells. CTLA-4 was expressed only in CD4+,FOXP3+ T cells but not in CD4+,FOXP3− T cells in both young and elderly groups (Fig. 4A). The levels of CTLA-4 expression were not different between the two groups (Fig. 4B). This finding suggests that the age-associated enhancement in suppressing IL-10 production from CD4+,CD25− T cells by CD4+ Treg is not due to any alteration in CTLA-4 expression in CD4+ Treg.
Fig 4.
Measuring CTLA-4 and Fas expression by CD4+,FOXP3+ T cells as well as apoptosis of CD4+,CD25bright and CD25− T cells in young and elderly humans. (A – D) PBMCs from young (n = 15) and elderly (n = 15) individuals were stained with Abs to CD4, followed by permeabilization and staining with Abs to FOXP3, CTLA-4, Fas or isotype Ab. Representative histograms showing the expression of CTLA-4 (A) and Fas (C) by CD4+,FOXP3− and FOXP3+ T cells. MFI of CTLA-4 (B) and Fas (D) expression by CD4+,FOXP3+ T cells. (E) PBMCs from young (n = 8) and elderly (n = 9) individuals were stained with Abs to CD4 and CD25 and sorted into CD4+,CD25bright (Treg) and CD4+,CD25− T (Target) cells. The latter cells were stained with CFSE and incubated for 7 days with CD4+,CD25bright T cells at 1:1 ratio in the presence of anti-CD3/-CD28 Abs. The frequency of live Treg and target cells was measured using annexin V staining after incubation. P values were obtained by the Mann-Whitney U test.
We next studied whether the enhanced suppression of IL-10 production from CD4+,CD25− T cells in the presence of high numbers of CD4+,CD25bright Treg in the elderly was related to an alteration in activation-induced cell death (apoptosis). This is based on our observation that Fas, a member of the TNF receptor family mediating apoptosis, was highly expressed on CD4+,FOXP3+, even higher than on CD4+,FOXP3− (Fig 4C), suggesting their susceptibility to apoptosis. However, the young and the elderly had similar levels of Fas expression on CD4+,FOXP3+ T cells (Fig. 4D). We also measured the frequency of live CD4+,CD25bright (Treg) and CD4+,CD25− T cells (Target) after incubating them (1:1 ratio) in the presence of anti-CD3/-CD28 Abs. The frequency of live CD4+,CD25bright and CD4+,CD25− T cells was similar between the two groups as measured by annexin V staining (Fig. 4E). This finding indicates that apoptosis is not responsible for the age-associated enhancement in suppressing IL-10 production from CD4+,CD25− T cells in the presence of high numbers of CD4+,CD25bright Treg.
3.5. The expression of chemokine receptors on CD4+,FOXP3+ T cells remains unchanged with aging
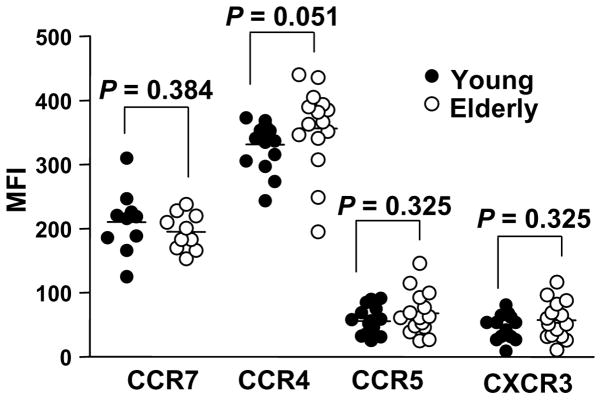
In order for CD4+ Treg to execute their immune regulatory function via cell contact, these cells need to migrate and localize to inflamed or infected sites where they can interact with other immune cells. This process is regulated by interactions between chemokines in the tissues and chemokine receptors expressed on T cells. Of interest, alterations in chemokine receptor expression on T cells have been noticed in aged humans and mice (Mo et al., 2003; Yung and Mo, 2003), raising the possibility of aging affecting the same receptors on CD4+ Treg. However, in our study, the expression levels of chemokine receptors CCR7, CCR4, CCR5 and CXCR3 on CD4+,FOXP3+ T cells were similar between young and elderly individuals (Fig. 5), suggesting their cell trafficking dependent on these receptors likely remains unchanged with aging.
Fig. 5.
Chemokine receptor expression on CD4+,FOXP3+ T cells in young and elderly humans. PBMCs from young (n = 15) and elderly (n = 15) individuals were stained with Abs to CD4, CCR7, CCR4, CCR5, CXCR3 or isotype Abs, followed by permeabilization and staining with Abs to FOXP3. MFI of CCR7, CCR4, CCR5 and CXCR3 expression on CD4+,FOXP3+ T cells was calculated. P values were obtained by the Mann-Whitney U test.
4. Discussion
Alterations in the immune system that occurs with aging likely contribute to the development of infection, malignancy and inflammatory diseases in the elderly (Effros et al., 2003; Goronzy and Weyand, 2005; Hasler and Zouali, 2005; Huang et al., 2005; Krabbe et al., 2004; Linton and Dorshkind, 2004; Miller, 1999). Although the exact cause for such alterations is yet to be determined, changes in regulating inflammatory immune responses may account in part for developing such conditions with aging. CD4+ T cells that express high levels of CD25 and FOXP3 have been recognized as naturally occurring Treg that have a critical role in regulating inflammatory immune responses. In the current study, we investigated whether the number and function of human CD4+ Treg is altered with aging. The frequency and phenotypic characteristics of CD4+,FOXP3+ T cells as well as their capacity to suppress inflammatory cytokine production and proliferation of CD4+,CD25− T cells were comparable in young and elderly individuals. However, when CD4+,FOXP3+ Treg and CD4+,CD25− T cells were co-cultured at a ratio of 1:1, the production of the anti-inflammatory cytokine IL-10 from CD4+,CD25− T cells were more potently suppressed in the elderly than in the young. This finding was not due to changes in CTLA-4 expression or apoptosis of CD4+,FOXP3+ Treg and CD4+,CD25− T cells. Taken together, our findings suggest that aging may affect the capacity of CD4+,FOXP3+ T cells to regulate IL-10 production from CD4+,CD25− T cells in humans although other cellular characteristics of CD4+,FOXP3+ T cells remain unchanged with aging.
CD25 was used as a single marker for CD4+ Treg in most studies investigating the effect of aging on human CD4+ Treg (Gregg et al., 2005; Tsaknaridis et al., 2003), although this molecule can also be up-regulated on T cells. In our study, in conjunction with CD25, we used FOXP3 which is considered as an essential and sufficient marker to identify CD4+ Treg (Ziegler, 2006). As previously reported (Ehrenstein et al., 2004), the expression of FOXP3 correlated with the expression of CD25 although only CD4+,CD25bright T cells were mostly FOXP3+ (R1 in Fig. 1A and B). In our study the frequency of CD4+,FOXP3+ T cells was not different between young (age ≤ 40) and elderly (age ≥ 65) individuals (mean (%) ± SD, 5.28 ± 0.54 vs 5.45 ± 0.62, Fig. 2B). Of interest, a recent study reported a moderately increased frequency of CD4+,FOXP3+ T cells in elderly individuals of age 70 or older compared to young individuals of age 30 or younger (5.8% vs 4.4%) (Lages et al., 2008). Although the cause for such a discrepancy is not clear, it could be related to the difference in defining young and elderly groups between the two studies.
We observed no difference in suppressing the proliferation of CD4+,CD25− T cells in the presence of CD4+,CD25bright Treg between the young and the elderly, which is consistent with the results of a mouse study (Nishioka et al., 2006). Of interest, two previous human studies on aging and CD4+ Treg reported inconsistent results (Gregg et al., 2005; Tsaknaridis et al., 2003). One study found no difference in suppressing target T cell proliferation in small numbers of young and elderly human subjects (3 for each group) (Gregg et al., 2005) whereas the other study reported decreased anti-proliferative capacity of CD4+,CD25+ T cells in the elderly (Tsaknaridis et al., 2003). However, in the latter study, CD4+,CD25+ T cells, including FOXP3+ and FOXP3− cells, were isolated using magnetic beads and no statistical analysis was done (Tsaknaridis et al., 2003). The results of our study clarify this issue by demonstrating no age-associated change in CD4+ Treg-mediated suppression of CD4+,CD25− T cell proliferation. A recent study reported suppression of cytotoxic activity of CD8+ T cells and NK cells by CD4+,CD25+ T cells in humans (Trzonkowski et al., 2006). In this study, the elderly had increased numbers of CD4+,CD25+ T cells that correlated with impaired cytotoxicity of CD8+ T cells and NK cells as well as reduced IL-2 production by CD4+,CD25− T cells (Trzonkowski et al., 2006). However, when magnetic bead-sorted CD4+,CD25+ T cells were added to target CD8+ T cells, NK cells and CD4+,CD25− T cells in young and elderly subjects, the suppression of cytotoxic activity and IL-2 production of the target cells appeared to be similar between the two groups, which supports our findings. Of note, in this study, the frequency of CD4+,CD25+ T cells was higher in the elderly compared to the young, suggesting that the overall capacity of Treg could be increased with aging. However, this interpretation has a limitation since the expression of FOXP3, the best marker for Treg, was not measured in CD4+ T cells.
In our study, we noticed that IL-10 production from CD4+,CD25− T cells was suppressed in the presence of CD4+,CD25bright Treg with FOXP3 expression. This finding is different from a previous study reporting increased production of IL-10 from the former cells in the presence of CD4+,CD25+ T cells (Dieckmann et al., 2002). Although the origin of such a discrepancy is unclear, in our study the suppression of IL-10 production from CD4+,CD25− T cells (target) in the presence of high numbers of CD4+,CD25bright Treg (Treg to target ration, 1:1) was more profound in the elderly than in the young while the suppression of other cytokine production was similar between the two groups (Fig. 3C). The exact mechanism for this phenomenon still remains unknown although we explored several possibilities. At least, this phenomenon appears not to be stemming from alterations in CTLA-4 expression or apoptosis of CD4+ Treg and CD4+,CD25− target cells (Fig. 4). Also, this finding is unlikely originating from a defect in CD4+,CD25− T cells since IL-10 production from CD4+,CD25− T cells in the absence of CD4+ Treg was not different between the young and the elderly. We recognize that there is individual variation in IL-10 production from CD4+ T cells as with other cytokines. This was the reason for us to obtain the inhibitory index for IL-10 to avoid the influence of the individual variation (Figure 3C). Indeed, total IL-10 levels in culture supernatants (Figure 3D) and the IL-10 inhibitory index were lower in the elderly when Treg and target cells were co-cultured at 1:1 ratio.
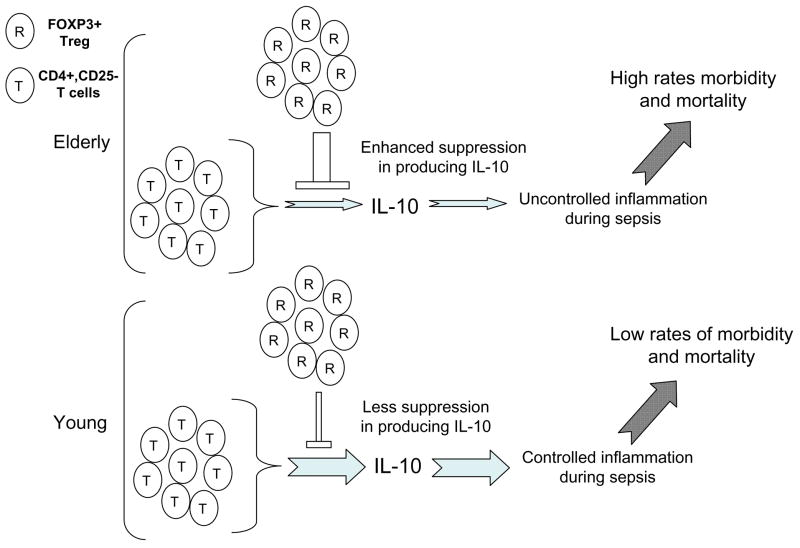
Our study found the enhanced suppression of IL-10 production from CD4+,CD25− T cells in the presence of high numbers of CD4+,FOXP3+ Treg in the elderly. It is arguable that our finding may not have any biological significance since the proportion of CD4+,FOXP3+ Treg is about 5% of the total CD4+ T cells in circulation. However, a mouse study reported an age-associated increase in the frequency of CD4+,FOXP3+ Treg in the secondary lymphoid tissues including lymph nodes and spleen, which was about 30% of the total CD4+ T cells (Lages et al., 2008). In addition, infiltration of CD4+,FOXP3+ Treg was noticed in tissues with tumors or inflammation such as sarcoidosis and rheumatoid arthritis (Jokinen et al., 1994; Miyara et al., 2006; Yamaguchi and Sakaguchi, 2006). These findings suggest that large numbers of CD4+,FOXP3+ T cells can be recruited to inflamed sites as a counter regulatory force. Thus, the age-associated increase in suppressing IL-10 production from CD4+,CD25− T cells in the presence of high numbers of CD4+,FOXP3+ Treg could be detrimental in controlling inflammation at the tissue level since this cytokine has broad anti-inflammatory properties (O’Garra and Vieira, 2007). In fact, IL-10 has an important role in limiting damage to host tissue during infection via regulating the immune response against microorganisms (reviewed in (Moore et al., 2001)). For instance, IL-10 protected mice from various forms of sepsis models including septic peritonitis (Cusumano et al., 1996; Florquin et al., 1994; Standiford et al., 1995). Also, IL-10 pretreatment reduced fever and the release of pro-inflammatory cytokines, including TNF-α, IL-6 and IL-8, in humans experimentally received endotoxin (Pajkrt et al., 1997). The incidence and mortality of sepsis increases with aging (Angus et al., 2001). Such findings could be secondary in part to uncontrolled inflammatory responses to microorganisms with augmented production of pro-inflammatory cytokines (Chorinchath et al., 1996; Turnbull et al., 2003). Therefore, it is conceivable that the enhanced suppression of anti-inflammatory cytokine IL-10 production from CD4+,CD25− T cells by large numbers of CD4+ Treg in the elderly accounts in part for their increased mortality and morbidity from sepsis with augmented inflammatory cytokine production (Fig. 6). Of interest, healthy elderly people had higher levels of serum IL-10 compared to frail elderly people (Uyemura et al., 2002) although serum levels of this cytokine were not different between healthy young and elderly people in a study involving small numbers of human subjects (Peterson et al., 1994).
Fig. 6.
A conceptual model showing the potential role for FOXP3+ Treg in interfering IL-10 production from CD4+,CD25− T cells in the elderly that leads to increased morbidity and mortality of sepsis.
In summary, we found that the frequency, phenotypic characteristics and anti-proliferative function of CD4+ Treg were comparable in the young (age ≤ 40) and the elderly (age ≥ 65). Of interest, IL-10 production from CD4+,CD25− T cells was suppressed more in the elderly than in the young in the presence of high numbers of CD4+ Treg although the inhibition of inflammatory cytokine production was similar between the two groups. These findings suggest the possibility that aging affects the capacity of CD4+ Treg to regulate IL-10 production from CD4+,CD25− T cells in humans. The results of our study introduce a possible explanation for dysregulated inflammation with aging that may contribute to the development of unwanted inflammatory pathologies in the elderly.
Acknowledgments
We thank Dr. Alexia Belperron for critical review of this manuscript and Lynne Iannone, Barbara Foster and Yale Center for Clinical Investigation for assisting the recruitment of human subjects. This work was supported in part by grants from the National Institute of Health (K08 AR49444, R01 AG028069, R21 AG030834), the Arthritis Foundation, the American Foundation for Aging Research and Claude D. Pepper Older Americans Independence Center (P30AG21342 NIH/NIA). Insoo Kang is a recipient of the World Class University Program of Republic of Korea. The authors have no conflicting financial interests in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2006 doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusumano V, Genovese F, Mancuso G, Carbone M, Fera MT, Teti G. Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect Immun. 1996;64:2850–2852. doi: 10.1128/iai.64.7.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, Bluestone JA. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florquin S, Amraoui Z, Abramowicz D, Goldman M. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J Immunol. 1994;153:2618–2623. [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17:378–384. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Glimm H, Eaves CJ. Direct evidence for multiple self-renewal divisions of human in vivo repopulating hematopoietic cells in short-term culture. Blood. 1999;94:2161–2168. [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler P, Zouali M. Immune receptor signaling, aging, and autoimmunity. Cell Immunol. 2005;233:102–108. doi: 10.1016/j.cellimm.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–618. doi: 10.1016/j.mad.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Huang H, Patel DD, Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front Biosci. 2005;10:192–215. doi: 10.2741/1521. [DOI] [PubMed] [Google Scholar]
- Hunder GG. Clinical features of GCA/PMR. Clin Exp Rheumatol. 2000;18:S6–8. [PubMed] [Google Scholar]
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Jokinen EI, Mottonen TT, Hannonen PJ, Makela M, Arvilommi HS. Prediction of severe rheumatoid arthritis using Epstein-Barr virus. Br J Rheumatol. 1994;33:917–922. doi: 10.1093/rheumatology/33.10.917. [DOI] [PubMed] [Google Scholar]
- Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- Karakhanova S, Munder M, Schneider M, Bonyhadi M, Ho AD, Goerner M. Highly efficient expansion of human CD4+CD25+ regulatory T cells for cellular immunotherapy in patients with graft-versus-host disease. J Immunother. 2006;29:336–349. doi: 10.1097/01.cji.0000203080.43235.9e. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7R{alpha} expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Hwang KA, Kang I. Dual Roles of IL-15 in Maintaining IL-7R{alpha}lowCCR7 Memory CD8+ T Cells in Humans via Recovering the Phosphatidylinositol 3-Kinase/AKT Pathway. J Immunol. 2007;179:6734–6740. doi: 10.4049/jimmunol.179.10.6734. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin-Kropf B, Shapiro VS, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–871. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. Aging and Immune Function. In: Paul WE, editor. Fundamental Immunology. Lippincott-Raven; Philadelphia: 1999. pp. 947–966. [Google Scholar]
- Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Kambouchner M, Valeyre D, Chapelon-Abric C, Debre P, Piette JC, Gorochov G. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, Hanash S, Yung RL. T cell chemokine receptor expression in aging. J Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- Pajkrt D, Camoglio L, Tiel-van Buul MC, de Bruin K, Cutler DL, Affrime MB, Rikken G, van der Poll T, ten Cate JW, van Deventer SJ. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J Immunol. 1997;158:3971–3977. [PubMed] [Google Scholar]
- Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Chao CC, Carson P, Hu S, Nichol K, Janoff EN. Levels of tumor necrosis factor alpha, interleukin 6, interleukin 10, and transforming growth factor beta are normal in the serum of the healthy elderly. Clin Infect Dis. 1994;19:1158–1159. doi: 10.1093/clinids/19.6.1158. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int Rev Immunol. 2005;24:211–226. doi: 10.1080/08830180590934976. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006a;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, de Saint Groth BF. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006b;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol. 1995;155:2222–2229. [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections] J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans-impact of immunosenescence. Clin Immunol. 2006;119:307–316. doi: 10.1016/j.clim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, Whitham RH, Bakke A, Jones RE, Offner H, Bourdette DN, Vandenbark AA. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- Uyemura K, Castle SC, Makinodan T. The frail elderly: role of dendritic cells in the susceptibility of infection. Mech Ageing Dev. 2002;123:955–962. doi: 10.1016/s0047-6374(02)00033-7. [DOI] [PubMed] [Google Scholar]
- Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Peng G, Wang HY. Regulatory T cells and Toll-like receptors in tumor immunity. Semin Immunol. 2006;18:136–142. doi: 10.1016/j.smim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Wing K, Fehervari Z, Sakaguchi S. Emerging possibilities in the development and function of regulatory T cells. Int Immunol. 2006;18:991–1000. doi: 10.1093/intimm/dxl044. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Yung RL, Mo R. Aging is associated with increased human T cell CC chemokine receptor gene expression. J Interferon Cytokine Res. 2003;23:575–582. doi: 10.1089/107999003322485071. [DOI] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]