Abstract
Myeloid cell leukemia-1 (Mcl-1) is an anti-apoptotic member of the Bcl-2 protein family. It interacts with pro-apoptotic Bcl-2 family members, thereby inhibiting mitochondrial activation and induction of apoptosis. Mcl-1 is essential for embryonal development and the maintenance of B, T and hematopoietic stem cells. We have recently shown that induction of Mcl-1 by growth factors rescues primary human hepatocytes from CD95-mediated apoptosis. This prompted us to further analyze the relevance of Mcl-1 for hepatocellular homeostasis. Therefore, we generated a hepatocyte-specific Mcl-1 knock-out mouse (Mcl-1flox/flox-AlbCre). Deletion of Mcl-1 in hepatocytes results in liver cell damage caused by spontaneous induction of apoptosis. Livers of Mcl-1flox/flox-AlbCre mice are smaller compared to control littermates, due to higher apoptosis rates. As a compensatory mechanism, proliferation of hepatocytes is enhanced in the absence of Mcl-1. Importantly, hepatic pericellular fibrosis occurs in Mcl-1 negative livers in response to chronic liver damage. Furthermore, Mcl-1flox/flox-AlbCre mice are more susceptible towards hepatocellular damage induced by agonistic anti-CD95 antibodies or concanavalin A.
Conclusion
The present study provides in vivo evidence that Mcl-1 is a crucial anti-apoptotic factor for the liver, contributing to hepatocellular homeostasis and protecting hepatocytes from apoptosis induction.
Keywords: Mcl-1, Bcl-xL, CD95, fibrosis, proliferation
Apoptosis, or programmed cell death, regulates tissue development and homeostasis in multi-cellular organisms. Extrinsic or intrinsic death signals activate pro-apoptotic pathways, resulting in the activation of caspases and finally in cell death. An important event during apoptosis process is the permeabilization of the outer mitochondrial membrane (OMM). Integrity of the OMM is regulated by the Bcl-2 protein family, which is divided into three groups: anti-apoptotic members Bcl-2, Bcl-xL and myeloid cell leukemia-1 (Mcl-1), pro-apoptotic multidomain members Bax and Bak, and pro-apoptotic BH3-only proteins. Mitochondrial activation is regulated by selective interactions of Bcl-2 proteins via their Bcl-2 homology (BH) domains (1–4).
Expression of Mcl-1 has been found to be induced in cells at various stages of differentiation, in response to specific growth, differentiation, and survival factors. The importance of Mcl-1 during differentiation has been pointed out in Mcl-1 knock-out mice, which die at an early stage of embryogenesis (5). In conditional Mcl-1 knockout models, hematopoietic stem cells as well as early-stage B or T cells die due to apoptosis induction (6, 7). Due to its anti-apoptotic properties, Mcl-1 is a potential proto-oncogene. Mcl-1 transgenic mice show an increased incidence of B cell lymphomas (8). In addition, enhanced expression of Mcl-1 is observed in a wide range of tumors, including hepatocellular carcinoma (HCC) (9, 10).
To date, little is known about the role of Mcl-1 in non-transformed cells. Mcl-1 expression is indispensable for the survival of hematopoietic stem cells, early stage B and T cells (6, 7), and macrophages (11). We have recently shown that induction of Mcl-1 by hepatocyte growth factor (HGF) protects primary human hepatocytes from CD95 (APO-1/Fas)-induced apoptosis (12). This observation is in line with studies which have shown that Mcl-1 transgenic mice are rescued from fulminant hepatic failure induced by CD95 triggering (13). Therefore, we assume that Mcl-1 is an important anti-apoptotic factor for the liver.
In this study, we generated hepatocyte-specific Mcl-1 knock-out mice. In the absence of Mcl-1, liver homeostasis is critically affected. Spontaneous induction of apoptosis is increased in Mcl-1 negative hepatocytes, resulting in profound liver damage and hepatic fibrosis. Furthermore, hepatocytes lacking Mcl-1 expression are more sensitive towards pro-apoptotic stimuli. Therefore, we conclude that Mcl-1 is a central anti-apoptotic factor for hepatocytes.
Materials and Methods
Generation of conditional Mcl-1 knock-out mice
Mcl-1flox/flox mice (7) were bred to heterozygous Albumin-Cre mice (14) (both C57BL/6 background). Male Mcl-1flox/wt-AlbCre offspring was bred to female Mcl-1flox/flox mice. Mcl-1flox/flox-AlbCre offspring (referred to as Mcl-1−/− mice) was compared to their control littermates with the genotypes Mcl-1flox/wt-AlbCre+/−, Mcl-1flox/flox-AlbCre−/−, or Mcl-1flox/wt-AlbCre−/− (referred to as control or Mcl-1+/+ mice). A scheme of the different genotypes can be found in (7), Fig. 1A.
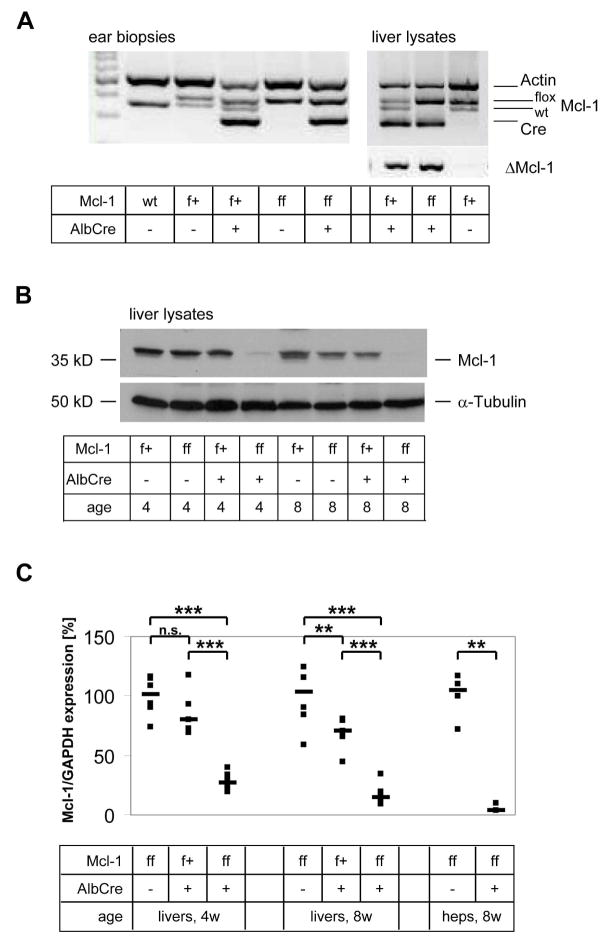
Figure 1.
Deletion of Mcl-1 in hepatocytes of Mcl-1flox/flox-AlbCre mice. (A) DNA from ear biopsies (left) or liver specimens (right panel) was isolated. PCR was performed using primers for wildtype (wt), floxed, or deleted (Δ) Mcl-1, Cre, and actin as internal control. (B) Liver lysates of Mcl-1flox/+, Mcl-1flox/flox, Mcl-1flox/+-AlbCre and Mcl-1flox/flox-AlbCre mice at the age of 4 and 8 weeks, were analyzed by western blot for Mcl-1 expression and for α–Tubulin as loading control. (C) mRNA from total livers or from isolated hepatocytes of Mcl-1flox/flox-AlbCre and control mice at the age of 4 or 8 weeks was isolated and transcribed into cDNA. Mcl-1 and GAPDH expression were analyzed by RealTime-PCR, and Mcl-1/GAPDH ratio was calculated. Both single (squares) and median values (bars) are presented. **: p<0.01; ***: p<0.001; n.s.: not significant.
All animals were bred at the animal facility of the University of Mainz, had free access to water and food under standard conditions with a 12h dark/light cycle, and received humane care. All experiments were done in accordance with the Federal law and were approved by the Local Committee for Experimental Animal Research.
Animal genotyping
For genotyping, ear biopsies were digested overnight at 56°C with proteinase K (Calbiochem, Schwalbach, Germany) in buffer containing 100mM Tris/HCl pH7.6, 50mM EDTA pH8.0, 0.5% SDS. Afterwards, proteinase K was heat-inactivated at 96°C for 3 min, and the solution was diluted with 10vol. of water. 1μl of the solution was used for PCR-based genotyping. PCR was performed using the following primers: Mcl-1 flox: 5′-CTGAGAGTTGTACCGGACAA-3′ and 5′-GCAGTACAGGTTCAAGCCGATG-3′; Mcl-1 deleted (Δ): 5′-CTGAGAGTTGTACCGGACAA-3′ and 5′-ACGCTCTTTAAGTGTTTGGCC-3′; Cre: 5′-GCACTGATTTCGACCAGGTT-3′ and 5′-CCCGGCAAAACAGGTAGTTA-3′; Actin (internal control for Cre PCR): 5′-TGTTACCAACTGGGACGACA-3′ and 5′-GACATGCAAGGAGTGCAAGA-3′. PCR was performed in a standard thermocycler and analyzed on 2% agarose gels.
Transaminase levels
About 100μl of blood was collected from the tail vein. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured in the Institute of Clinical and Laboratory Medicine at the University Hospital Mainz by standard procedures.
Acute liver damage
Mice were injected with 0.5mg/kg of an anti-mouse CD95 monoclonal antibody intraperitonally (Jo2; BD Pharmingen, Heidelberg, Germany) (15) or with 25mg/kg concanavalin A (ConA, Sigma) into the tail vein. After the indicated time points, mice were sacrificed, blood was collected, and livers were shock-frozen in liquid nitrogen or transferred into 4% PBS-buffered formalin (55mM Na2HPO4, 12mM NaH2PO4, 4% Formalin, pH7.4) until further analysis.
Isolation of hepatocytes
Hepatocytes were isolated by a two-step perfusion technique (16). Briefly, mice were anesthetized with 2.5% Avertin (2,2,2-Tribromoethanol; Sigma) and livers were perfused in situ with about 100ml of buffer I containing 140mM NaCl, 7mM KCl, and 10 mM HEPES pH7.4. Livers were then perfused with 50ml of buffer II containing 70mM NaCl, 7mM KCl, 5mM CaCl2, 100mM HEPES pH7.6, and 0.5mg/ml collagenase (Serva, Heidelberg, Germany). Thereafter, livers were mechanically disrupted in buffer I, and cells were suspended and washed twice with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Karlsruhe, Germany).
Tissue lysis and western blotting
About 20mg of shock-frozen liver tissue were minced, transferred into ice-cold lysis buffer containing 20mM Tris/HCl pH8.0, 5mM EDTA, 0.5% Triton-X 100, and 1 × protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), and incubated on ice for 15min. Cell debris was removed by centrifugation (10,000g, 4°C). Protein concentration was determined by Dc Protein Assay (Bio-Rad, Munich, Germany), and equal amounts of protein were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. Immunodetection was performed using the following primary antibodies: Mcl-1 (Rockland, Gilbertsville, PA, USA), Bcl-xL (H-62, Santa Cruz Biotechnology, Heidelberg, Germany), Bak (BD Pharmingen), Bax (Cell Signaling, Frankfurt, Germany), Bid (Cell Signaling), and α-Tubulin (Sigma). Peroxidase-conjugated species-specific secondary antibodies (Santa Cruz Biotechnology) were used at a dilution of 1:10,000. Bound antibody was visualized using chemiluminescent substrate (Perkin Elmer, Zaventem, Belgium) and exposure to Fuji Medical X-Ray film. All western blots were performed for at least two male and two female mice at each age indicated.
Caspase assay
Caspase-3 activity was measured as previously described (17).
DNA and RNA isolation
For DNA isolation, about 20mg of shock-frozen liver tissue were lysed overnight with proteinase K (see above). For isolation of total RNA, about 20mg of shock-frozen liver tissue were homogenized in 1ml TRI-Reagent (Sigma), and further isolated according to the manufacturer’s instructions. RNA concentration was measured in a NanoDrop photometer (Peqlab, Erlangen, Germany), and 1μg of total RNA was subjected to reverse transcription using Oligo-dT primers. Isolated DNA and cDNA were used for PCR (see above) and RealTime-PCR approaches, respectively.
Real-Time Quantitative Polymerase Chain Reaction (RT-QPCR)
Relative target mRNA expression was analyzed by RT-QPCR using the QuantiTect SYBR Green PCR Kit and QuantiTect primers (Qiagen, Hilden, Germany) for murine Mcl-1, IL6, TNF, collagen-1, and GAPDH. The relative increase in reporter fluorescent dye emission was monitored. The level of target mRNA, relative to GAPDH, was calculated using the formula: Relative target mRNA/GAPDH mRNA expression = 1/2^[ct target-ct GAPDH] *100, where ct is defined as the number of the cycle in which emission exceeds threshold levels.
Histology and Immunohistochemistry
Liver specimen were fixed in 4% PBS-buffered formalin, embedded in paraffin, sectioned, and stained with haematoxylin and eosin as well as Sirius red using standard histological techniques. In addition, slides were immunostained for activated caspase-3 (rabbit polyclonal antibody; Cell Signaling Technology, 1:300 dilution) and Ki67 (monoclonal rabbit clone SP6; NeoMarkers, 1:100 dilution) using the Ventana Discovery® automated staining system with an iView™ DAB kit (Ventana, Tucson, Arizona, USA), replacing the secondary antibody with a donkey anti-Rabbit biotinylated ab (Jackson 711-065-152, 1:80 dilution). All sections were counterstained with haematoxylin. The whole section was evaluated for the number of positive hepatocytes, and pictures were taken from representative high-power fields (at 200× magnification).
Detection of cell proliferation
Mice were exposed to 0.8mg/ml BrdU (Roche) in drinking water for 60h. Mice were sacrificed and livers were shock-frozen in liquid nitrogen. 5μm tissue sections were prepared and BrdU-positive cells were stained with the In Situ Cell Proliferation Kit (Roche) according to the manufacturer’s instructions. Afterwards, nuclei were counterstained with 2μg/ml DAPI (Molecular Probes, Karlsruhe, Germany) for 15min at room temperature. Sections were analyzed for FITC and DAPI staining with a fluorescence microscope using corresponding filters. From each mouse, two independent sections were stained and 7 microscopic fields from each section were analyzed by counting FITC- and DAPI-positive nuclei.
Detection of apoptosis
Mice were sacrificed and livers were shock-frozen in liquid nitrogen. 5μm tissue sections were prepared and transferred into 4% PBS-buffered formalin. Apoptosis was detected by staining cell nuclei with DNA strand breaks (TUNEL technology) using the In Situ Cell Death Detection Kit, Fluorescein (Roche), according to the manufacturer’s instructions. Counterstaining and further procedure was performed as described above.
Data Analysis
All graphs show both single and median values from at least three independent experiments. Histological images show representative results. Data were analyzed by Mann-Whitney U test using SPSS software with p<0.05 considered significant.
Results
Deletion of Mcl-1 in hepatocytes of Mcl-1flox/flox-AlbCre mice
After breeding Mcl-1flox/flox mice to Mcl-1flox/wt-AlbCre mice, offspring was screened for deletion of Mcl-1 and expression of Cre (Fig. 1A). All animals positive for Cre and for a floxed Mcl-1 allele were also positive for the deleted Mcl-1 fragment in liver lysates. We analyzed Mcl-1 expression in liver lysates of 4 and 8 week old mice. Mcl-1 protein expression was significantly reduced in liver lysates of Mcl-1flox/flox-AlbCre mice compared to Mcl-1flox/wt mice, but was only slightly reduced in Mcl-1flox/wt-AlbCre mice (Fig. 1B). RealTime-PCR also showed a significant reduction in Mcl-1 mRNA expression in total liver lysates of Mcl-1flox/flox-AlbCre mice (4 weeks: 27%; 8 weeks: 15%; n=8, p<0.001), and a slight reduction in Mcl-1 mRNA expression in Mcl-1flox/wt-AlbCre mice (4 weeks: 81%, n.s.; 8 weeks: 71%; n=5, p<0.05) compared to Mcl-1flox/flox mice (n=6; Fig. 1C). To analyze if the remaining Mcl-1 expression observed was originating from hepatocytes or from non-parenchymal cells, we isolated hepatocytes from Mcl-1flox/flox-AlbCre mice and found that Mcl-1 mRNA expression was reduced to 4% of that of control hepatocytes (n=4, p<0.05; Fig. 1C). Deletion of Mcl-1 expression was equally efficient both in male and in female mice (data not shown).
Next, we asked whether other Bcl-2 family members might be regulated in hepatocytes to compensate for the loss of Mcl-1 expression. Expression of Bcl-xL, Bid, Bax and Bak, however, were not altered in liver lysates of Mcl-1flox/flox-AlbCre mice (Fig. S1).
Basal liver damage and hepatic fibrosis in Mcl-1flox/flox-AlbCre mice
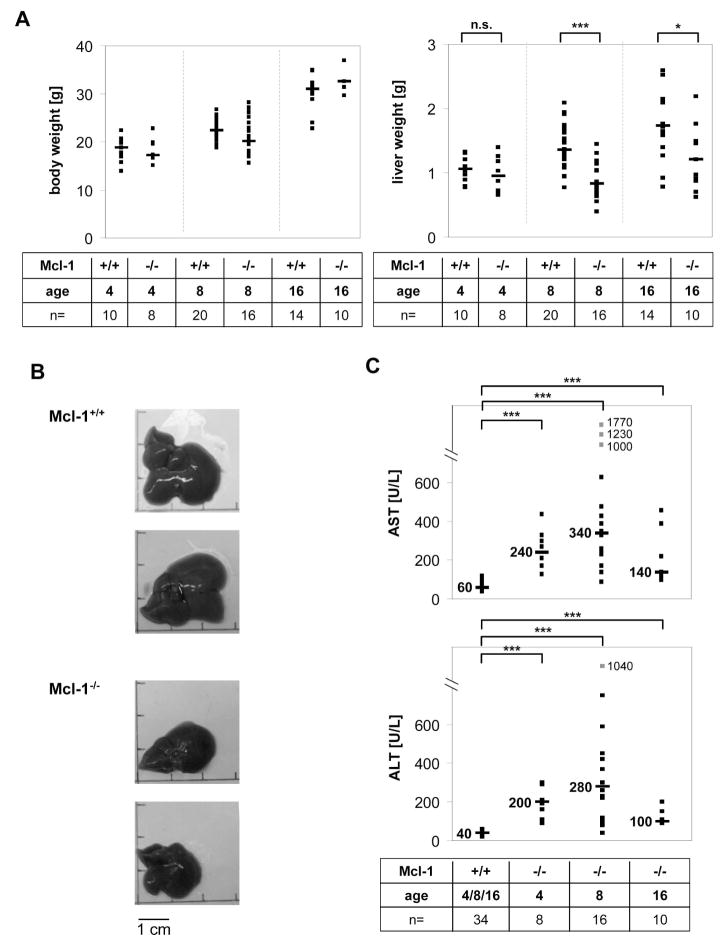
We analyzed the effect of a loss of Mcl-1 expression for liver homeostasis. Interestingly, livers of Mcl-1flox/flox-AlbCre mice appeared much smaller, and liver weight was profoundly reduced (64–78% compared to control littermates, p<0.001; Fig. 2A/B). Body weight, however, was only slightly decreased (Fig. 2A). Furthermore, aspartate and alanine aminotransferase (AST and ALT) values, which are indicators of hepatocyte injury, were highly increased in these mice. 8 week old Mcl-1flox/flox-AlbCre mice show a 7.6-fold increase in serum ALT levels (median: 280 U/L vs. 40 U/L in control; p<0.001) and a 5.7-fold increase in serum AST levels (median: 340 U/L vs. 80 U/L in control; p<0.001; Fig. 2C). We also analyzed 4 week old animals and again found a significant increase in serum transaminase levels (Fig. 2C). Interestingly, AST and ALT levels were only slightly, yet still significantly, enhanced in 4 month old animals (Fig. 2C). In addition, we determined serum bilirubin values. There was no increase in bilirubin values in Mcl-1flox/flox-AlbCre mice in the age of 8 weeks to 4 month, demonstrating that liver function was not impaired (data not shown).
Figure 2.
Liver damage in Mcl-1flox/flox-AlbCre mice. (A) 4 to 16 week old Mcl-1flox/flox-AlbCre (Mcl-1−/−) or control (Mcl-1+/+) mice were analyzed for body weight and liver weight. (B) Macroscopic appearance of representative livers from 8 week old female Mcl-1+/+ and Mcl-1−/− mice. (C) Serum of Mcl-1+/+ and Mcl-1−/− mice between 4 and 16 weeks of age was collected. Serum AST and ALT levels were measured. Both single (squares) and median values (bars) are presented. Values beyond scaling are marked separately. *: p<0.05; ***: p<0.001; n.s: not significant.
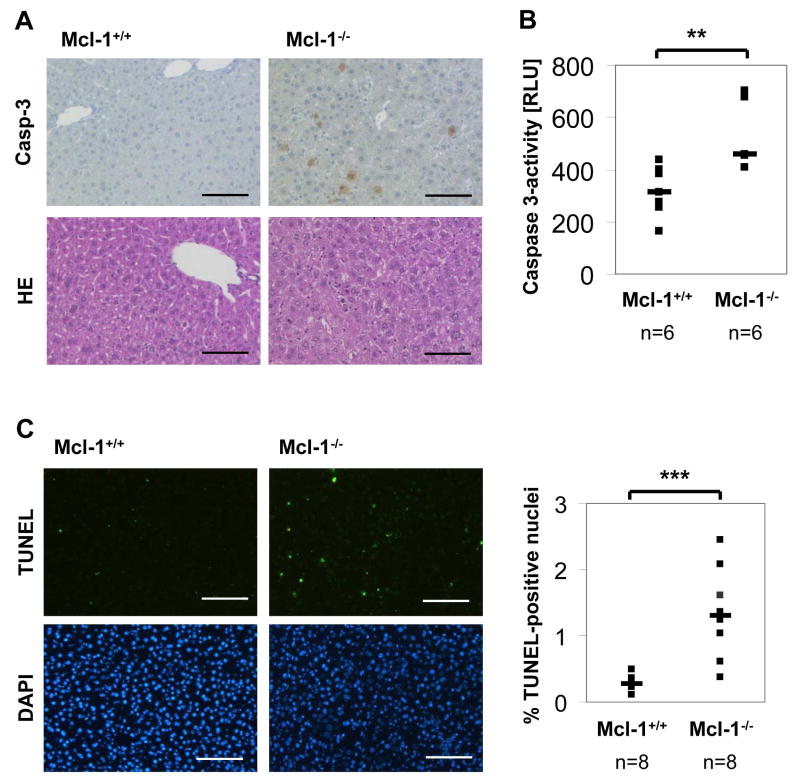
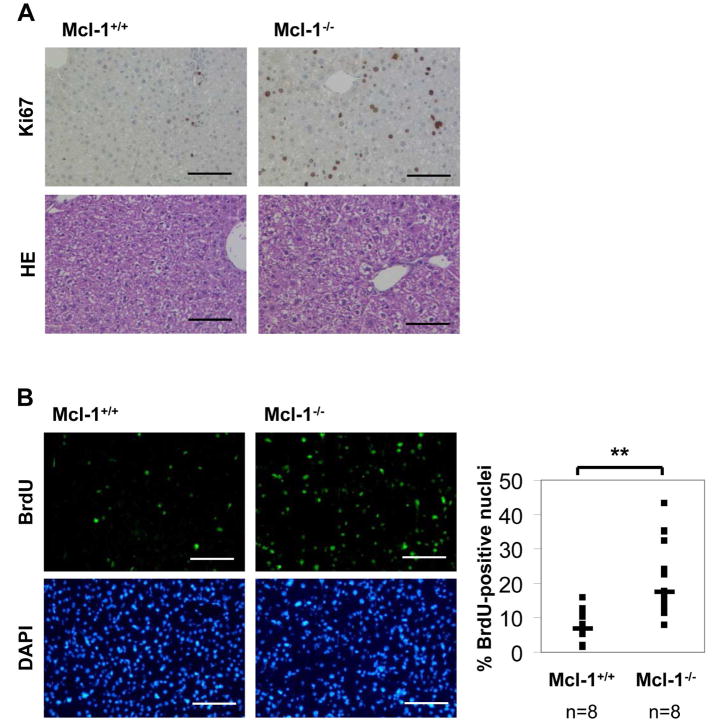
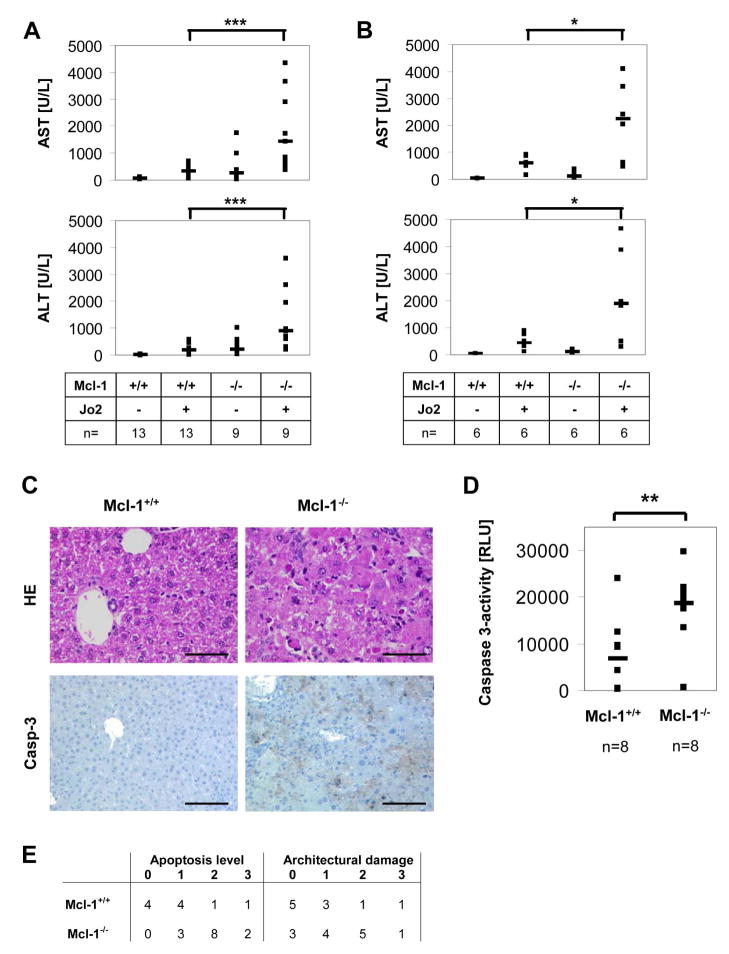
As Mcl-1flox/flox-AlbCre mice reveal a basal liver damage, we asked whether this was due to spontaneous induction of apoptosis in hepatocytes. Therefore, we analyzed liver histology and stained liver sections of 4 week old mice for active caspase-3 as a marker for the execution phase of apoptosis. Among Mcl-1−/− hepatocytes, 1–3% of cells were stained positive for active caspase-3. In contrast, control liver sections revealed hardly any or no caspase-3 positive hepatocytes (Fig. 3A). To further validate this result, we also determined caspase-3 activity fluorometrically in liver lysates. Again, we found that caspase-3 activity was significantly enhanced in Mcl-1−/− hepatocytes (Fig. 3B). Furthermore, we analyzed DNA strand breaks to determine apoptosis rates. TUNEL assays revealed that 5-fold more hepatocytes were positive for DNA strand breaks in Mcl-1−/− livers compared to controls (p<0.001; Fig. 3C). Next, we tested whether reduced liver mass in Mcl-1flox/flox-AlbCre mice is due to increased apoptosis rates or could also be caused by reduced proliferation of hepatocytes. To determine proliferation of Mcl-1−/− hepatocytes, 4 week old mice received BrdU in the drinking water for 60h. The amount of BrdU-positive nuclei was about 2.5-fold increased in Mcl-1−/− hepatocytes compared to controls (17.5 vs. 7%, p<0.01; Fig. 4B). Furthermore, we analyzed Ki67 expression, another marker for cell proliferation. Again, expression was profoundly increased in Mcl-1−/− hepatocytes compared to controls (Fig. 4A). Therefore, we conclude that the proliferation rate of Mcl-1−/− hepatocytes is enhanced, presumably to partially compensate for the loss of hepatocytes due to apoptosis.
Figure 3.
Livers of Mcl-1flox/flox-AlbCre mice show higher apoptosis rates. (A) Liver sections of 4 week old Mcl-1+/+ and Mcl-1−/− mice were stained for active caspase-3 and with eosin/hematoxylin. (B) Total liver lysates were analyzed for active caspase-3 activity using fluorometric substrates. (C) Liver sections were stained for DNA strand breaks using TUNEL-technology, and cell nuclei were counterstained using DAPI. The ratio of TUNEL-positive nuclei was calculated. Both single (squares) and median values (bars) are presented. The bar corresponds to 100μm. ** p<0.01, *** p<0.001. RLU: relative light units.
Figure 4.
Livers of Mcl-1flox/flox-AlbCre mice show higher proliferation rates. (A) Liver sections of 4 week old Mcl-1+/+ and Mcl-1−/− mice were stained for Ki67 and with eosin/hematoxylin. (B) Mcl-1+/+ and Mcl-1−/− mice received BrdU (0.8mg/ml; 60h) in their drinking water. Liver sections were stained for BrdU incorporation, and cell nuclei were counterstained using DAPI. The ratio of BrdU-positive nuclei was calculated. Both single (squares) and median values (bars) are presented. The bar corresponds to 100μm. ** p<0.01.
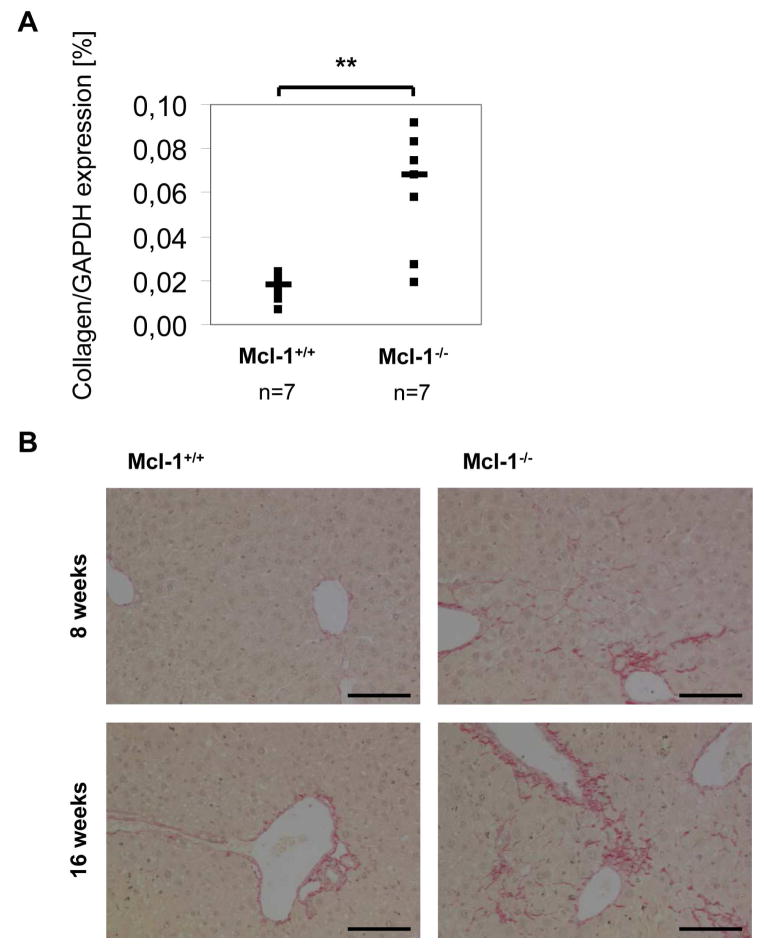
Next, we analyzed IL6 and TNF-expression in liver lysates of 16 week old mice, to determine if the increase in apoptosis induction in Mcl-1−/− hepatocytes leads to an inflammatory response. We could, however, observe no difference in IL6 or TNF mRNA-expression (data not shown). Next, we asked whether hepatic fibrosis occurs as a response to chronically increased apoptosis rates in Mcl-1−/− livers. Therefore, we analyzed mRNA expression of collagen-1. Interestingly, collagen-1 expression was significantly enhanced in Mcl-1−/− negative livers, indicating fibrosis induction (p<0.01; Fig. 5A). In addition, we stained Mcl-1−/− liver sections with Sirus Red. Mcl-1−/− mice revealed a mild degree of pericellular fibrosis at the age of 8 weeks, and even more at the age of 16 weeks, which was not observable in control littermates (Fig. 5B).
Figure 5.
Fibrosis induction in Mcl-1flox/flox-AlbCre mice. (A) mRNA from total liver lysates of 16 week old Mcl-1+/+ and Mcl-1−/− mice was isolated and transcribed into cDNA. Collagen-1 and GAPDH expression were analyzed by RealTime-PCR, and collagen/GAPDH ratio was calculated. Both single (squares) and median values (bars) are presented. **: p<0.01; n.s.: not significant. (B) Liver sections of 8 or 16 week old Mcl-1+/+ and Mcl-1−/− mice were stained with Sirius red for collagen deposition. The bar corresponds to 100μm.
Effect of Mcl-1 deletion on CD95- or T cell-mediated liver damage
CD95-mediated apoptosis of hepatoctytes contributes to the pathophysiology of various liver diseases (18). Hepatocytes constitutively express CD95, and the liver is highly susceptible towards CD95-induced apoptosis (18, 19). To analyze the effect of Mcl-1 deletion on CD95 triggering on hepatocytes, we treated mice with the agonistic CD95-antibody Jo2. After Jo2-administration, 8 week old Mcl-1−/− mice had significantly elevated serum AST and ALT levels compared to control (AST: 4-fold, ALT: 9-fold, p<0.001; Fig. 6A). Histology, immunohistochemical staining for active caspase-3, and analysis of caspase-3 activity in total liver lysates, revealed that Mcl-1−/− livers had both enhanced apoptosis levels and more severe architectural damage after Jo2-treatment (Fig. 6C/D/E). Additionally, we treated older animals, which had only slightly enhanced basal transaminase levels (Fig. 2C), with Jo2. Again, Mcl-1flox/flox-AlbCre mice had significantly increased serum transaminase levels after Jo2-administration compared to control (p<0.05; Fig. 6B).
Figure 6.
Mcl-1flox/flox-AlbCre mice are more susceptible towards CD95-mediated liver damage. 8 (A, C–E) or 24 week old (B) Mcl-1+/+ and Mcl-1−/− mice were treated with Jo2 (0.5mg/kg) i.p. After 3h, mice were sacrificed. (A, B) Blood was collected and serum AST and ALT levels were determined. Both single (squares) and median values (bars) are presented. (C) Liver sections were stained with eosin/hematoxylin and for active caspase-3. The bar corresponds to 100μm. (D) Total liver lysates were analyzed for caspase-3 activity. (E) Apoptosis rate and architectural damage were analyzed within H/E-stained sections and were clustered into 4 groups (0: no, 1: low, 2: moderate, 3: high apoptosis rate/architectural damage). * p<0.05; **: p<0.01; *** p<0.001. RLU: relative light units.
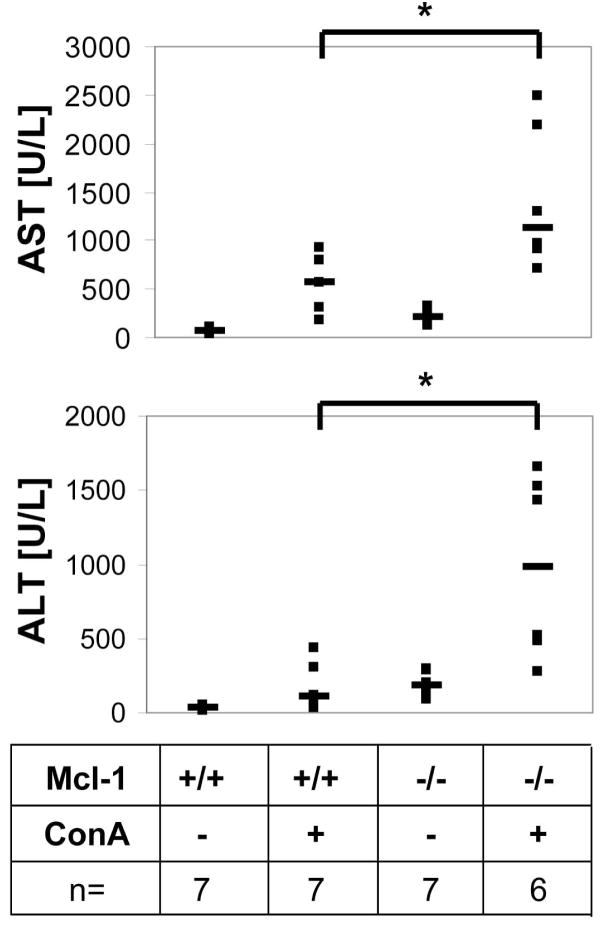
Concanavalin A (ConA)-induced hepatitis is frequently used as a model for autoimmune liver disease and is dependent on T cell activity as well as CD95-signaling (20, 21). We tested whether Mcl-1 deletion in hepatocytes influences T cell-mediated liver injury in vivo. 8 week old mice were injected with 25mg/kg ConA into the tail vein. Mcl-1−/− mice showed an increased liver damage after ConA-treatment compared to control (AST: 3-fold, ALT: 6-fold increase; p<0.05; Fig. 7). We conclude that Mcl-1 deletion renders hepatocytes more susceptible towards apoptosis stimuli, e.g. death receptor ligands and activated T cells.
Figure 7.
Mcl-1flox/flox-AlbCre mice are more susceptible towards T cell-mediated liver damage. 8 week old Mcl-1+/+ and Mcl-1−/− mice were treated with ConA (25mg/kg) intravenously. After 4h, mice were sacrificed. Blood was collected and serum AST and ALT levels were determined. Both single (squares) and median values (bars) are presented. *: p<0.05.
Discussion
The aim of this study was to investigate the role of Mcl-1 for liver cell homeostasis and susceptibility of hepatocytes towards apoptosis stimuli. Our results clearly demonstrate that Mcl-1 is an important anti-apoptotic factor in the liver and contributes to hepatocyte survival, e.g. after death receptor activation. Mcl-1 was first described in the human myeloid leukemia cell line ML-1, where it is induced early during phorbol ester-induced differentiation (22). Further studies revealed that Mcl-1 is promoting viability rather than proliferation. In a vast number of cell types, transformed or untransformed, and after diverse stress or growth signals, Mcl-1 expression is rapidly induced and rescues cells from apoptosis induction. By contrast, Mcl-1 degradation is a prerequisite for cells to die (23). Therefore, Mcl-1 expression is crucial in developing and differentiating cells, and in cells facing constant stress signals (like cancer cells). Mouse models revealed that Mcl-1 expression is crucial for embryonal development (5), and for the viability of hematopoietic (stem) cells (6, 7, 11). So far, little is known about the importance of Mcl-1 in fully differentiated liver cells. We have recently shown that Mcl-1 is induced by growth factors in primary human hepatocytes (PHH) and contributes to the survival of PHH and HCC cells in vitro (9, 12, 17). Thus, we hypothesized that Mcl-1 might also be a crucial factor for liver cell homeostasis in vivo. Therefore, we generated a hepatocyte-specific Mcl-1 knock-out mouse. By mating Mcl-1flox/flox mice with mice expressing the Cre-recombinase under the control of an albumin promoter, we obtained mice which still express Mcl-1 in hepatocytes at the time of birth. Deletion of the floxed DNA in this system is hepatocyte-specific (24). However, a complete recombination of the floxed alleles takes up to 8 weeks (14). In our study, Mcl-1 expression was drastically reduced, yet not completely absent, in liver lysates of Mcl-1flox/flox-AlbCre mice at the age of 4 and 8 weeks on protein and mRNA level.
We postulate that the remaining level of Mcl-1 expression was originating from non-parenchymal cells. Isolated hepatocytes of 8 week old animals, with only a minor ratio of non-parenchymal cells, showed a residual Mcl-1 expression of less than 5%. Interestingly, deletion of one Mcl-1 allele had only a minor influence on Mcl-1 mRNA, but not on Mcl-1 protein expression. In contrast, MEFs lacking one Mcl-1 allele already showed a significant decrease in Mcl-1 protein expression (7).
Loss of Mcl-1 expression in hepatocytes of Mcl-1flox/flox-AlbCre mice was not compensated by an increase of Bcl-xL expression. Bcl-xL is another anti-apoptotic protein of the Bcl-2 family prominently expressed in the liver. A conditional knock-out of Bcl-xL in hepatocytes results in spontaneous hepatocyte apoptosis and development of liver fibrosis (25). These studies have also shown that a deletion of Bcl-xL does not lead to an induction of Mcl-1. Therefore, despite of similar functions and sequence homology, a loss of one of these proteins is not compensated by the induction of the other. In addition, expression of the pro-apoptotic Bcl-2 family proteins Bak and Bax was also not altered in hepatocytes lacking Mcl-1. In our model, deletion of Mcl-1 expression in hepatocytes caused a profound increase in hepatocyte apoptosis rate, and a decrease in liver size. Under physiologic conditions, liver size is highly regulated. Following a two-thirds hepatectomy in wild type mice, liver mass normalizes after about 10 days (26). In Mcl-1flox/flox-AlbCre mice, we observed an enhanced proliferation rate of hepatocytes. However, this was not sufficient to compensate for apoptosis induction in the liver. As a marker of liver cell damage, AST and ALT serum levels were also drastically increased in Mcl-1flox/flox-AlbCre mice. Interestingly, transaminase levels were higher in younger Mcl-1flox/flox-AlbCre animals. In 16 week old animals, transaminase levels were still higher than in control mice, yet not as high as in 4 to 8 week old mice.
Under physiologic conditions, enhanced apoptosis induction in the liver can cause an inflammatory responses (27). However, in our model, no increase of IL6 and TNF expression was observed in Mcl-1−/− livers. Progressive hepatic fibrosis is the main cause of organ failure in chronic liver diseases of different etiology. In Mcl-1−/− livers, pericellular collagen deposition as a marker of fibrogenesis was observed, already at the age of 8 weeks. In parallel, bilirubin levels remained normal indicating that liver function was not impaired to a significant extent, at least not within the time frame analyzed.
Our data support the finding that Mcl-1 expression is a prerequisite for cell viability, but not for proliferation (28). The proliferation rate of Mcl-1−/− hepatocytes was enhanced. In contrary, hepatocyte viability in Mcl-1−/− livers was decreased due to apoptosis induction.
Besides its importance for cell viability during growth and differentiation, Mcl-1 is also important for cell viability after stress or apoptosis signals. To demonstrate this, we analyzed the effect of Mcl-1 deletion on hepatocyte apoptosis after death receptor triggering. The death receptor CD95 is constitutively expressed on hepatocytes. Therefore, the liver is highly susceptible towards activation of CD95 (19). Mice injected with agonistic CD95-antibodies die within a few hours upon injection due to liver failure (29). Importantly, hepatocellular CD95 expression is increased in different acute and chronic liver diseases (18). For example, CD95-induced apoptosis is a key mechanism in the pathophysiology of fulminant hepatic failure (30). In our study, a deletion of Mcl-1 caused an increase in hepatocellular damage after administration of the agonistic CD95-antibody Jo2. This was reflected by increased AST and ALT levels, enhanced caspase-3 activation, and increased architectural damage in liver tissue. Higher susceptibility of Mcl-1flox/flox-AlbCre mice towards CD95-triggering could also be observed in older mice, which show only slightly enhanced basal transaminase levels compared to controls. Therefore, we conclude that this effect was actually dependent on Mcl-1 deletion and not or only in part due to basal liver damage in these animals. Our results are in line with studies which have shown that Mcl-1 transgenic mice are more resistant towards fulminant hepatic failure induced by Jo2 (13). Furthermore, HGF, an inducer of Mcl-1 expression in hepatocytes (12), protects mice from Jo2-induced liver damage (31). In contrast, a knock-out of Bcl-xL in hepatocytes does not reduce CD95-mediated apoptosis (25). Furthermore, Mcl-1flox/flox-AlbCre mice were more susceptible towards T cell-mediated liver damage. Administration of ConA is a well established in vivo model for T cell-mediated hepatitis (21). Since activation of CD95 is also involved in T cell-mediated hepatitis, our results may be at least in part explained by the increased susceptibility of Mcl-1−/− hepatocytes towards CD95 triggering.
In contrary, Mcl-1 deletion in hepatocytes did not influence the degree of liver damage after carbon tetrachloride (CCl4) administration (supplementary material). CCl4 is hepatotoxic, leading to a chronic liver injury and fibrogenesis (32). In our study, the extent of fibrosis after CCl4 exposure was not different in Mcl-1−/− animals compared to control littermates. This might be explained by the limited time of CCl4 exposure (5 weeks).
Interestingly, in previous studies we did not observe any effect of postnatally induced Mcl-1 deletion on liver morphology (6). Crossing of Mcl-1flox/null mice with MxCre mice results in a rapid deletion of Mcl-1 in bone marrow cells and hepatocytes after i.p. administration of polyinocinic-polycytidylic acid in adult mice. In these mice, no liver damage was histologically detectable within 14 days after induction of Mcl-1 deletion. In the present model, recombination of the floxed Mcl-1 alleles starts already during embryogenesis and liver morphology is significantly altered. Thus, we conclude that expression of Mcl-1 is very important for proliferating hepatocytes in the liver of younger animals. This is in line with previous studies showing that Mcl-1 is important during differentiation (33). In the adult mouse, when the liver has only minor renewing activity, Mcl-1 expression is of minor importance as long as hepatocytes are not exposed to apoptosis stimuli.
In summary, the present study shows that Mcl-1 expression is crucial for hepatocyte survival in vivo. A deletion of Mcl-1 in hepatocytes causes activation of apoptosis, resulting in chronic liver damage and fibrogenesis. Furthermore, Mcl-1 deletion renders hepatocytes more susceptible towards death receptor-induced apoptosis. Therefore, we conclude that Mcl-1 is an important survival factor for hepatocytes.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants of the Deutsche Forschungsgemeinschaft (DFG SCHU 1443/3-1) and by an intramural fund (MAIFOR) of the University of Mainz to HSB.
We thank the Dana Farber Cancer Institute, Boston, MA, USA, for providing Mcl-1flox/flox mice. In addition, we thank Ari Waisman for helpful discussions, Sandra Heine and Silvia Behnke for excellent technical assistance, and Cornelius Fritsch for critically reading the manuscript.
List of Abbreviations
- Mcl-1
Myeloid cell leukemia-1
- HCC
hepatocellular carcinoma
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ConA
concanavalin A
- BrdU
5-bromo-2-deoxyuridine
Contributor Information
Binje Vick, Email: binje.vick@gmail.com.
Achim Weber, Email: Achim.Weber@usz.ch.
Toni Urbanik, Email: toni.urbanik@gmail.com.
Thorsten Maass, Email: maass_thorsten@web.de.
Andreas Teufel, Email: teufel@uni-mainz.de.
Peter H. Krammer, Email: p.krammer@dkfz.de.
Joseph T. Opferman, Email: Joseph.Opferman@stjude.org.
Marcus Schuchmann, Email: schuchm@mail.uni-mainz.de.
Peter R. Galle, Email: galle@mail.uni-mainz.de.
Henning Schulze-Bergkamen, Email: bergkam@uni-mainz.de.
References
- 1.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin-Bey ES, Gores GJ. Death by association: BH3 domain-only proteins and liver injury. Am J Physiol Gastrointest Liver Physiol. 2005;289:G987–990. doi: 10.1152/ajpgi.00371.2005. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 6.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 7.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, Craig RW. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226–3239. [PubMed] [Google Scholar]
- 9.Fleischer B, Schulze-Bergkamen H, Schuchmann M, Weber A, Biesterfeld S, Muller M, Krammer PH, et al. Mcl-1 is an anti-apoptotic factor for human hepatocellular carcinoma. Int J Oncol. 2006;28:25–32. [PubMed] [Google Scholar]
- 10.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, Bodingbauer M, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, et al. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology. 2004;39:645–654. doi: 10.1002/hep.20138. [DOI] [PubMed] [Google Scholar]
- 13.Baskin-Bey ES, Huang W, Ishimura N, Isomoto H, Bronk SF, Braley K, Craig RW, et al. Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology. 2006;44:252–262. doi: 10.1002/hep.21236. [DOI] [PubMed] [Google Scholar]
- 14.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun L, Mead JE, Panzica M, Mikumo R, Bell GI, Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988;85:1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze-Bergkamen H, Untergasser A, Dax A, Vogel H, Buchler P, Klar E, Lehnert T, et al. Primary human hepatocytes--a valuable tool for investigation of apoptosis and hepatitis B virus infection. J Hepatol. 2003;38:736–744. doi: 10.1016/s0168-8278(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 20.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 21.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG., Jr Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging. 2003;2:297–302. doi: 10.1162/15353500200303154. [DOI] [PubMed] [Google Scholar]
- 25.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, Miyagi T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Schuchmann M, Ruckert F, Garcia-Lazaro JF, Karg A, Burg J, Knorr N, Siebler J, et al. MORT1/FADD is involved in liver regeneration. World J Gastroenterol. 2005;11:7248–7253. doi: 10.3748/wjg.v11.i46.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze-Bergkamen H, Schuchmann M, Fleischer B, Galle PR. The role of apoptosis versus oncotic necrosis in liver injury: facts or faith? J Hepatol. 2006;44:984–993. doi: 10.1016/j.jhep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Yang T, Buchan HL, Townsend KJ, Craig RW. MCL-1, a member of the BLC-2 family, is induced rapidly in response to signals for cell differentiation or death, but not to signals for cell proliferation. J Cell Physiol. 1996;166:523–536. doi: 10.1002/(SICI)1097-4652(199603)166:3<523::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 30.Ryo K, Kamogawa Y, Ikeda I, Yamauchi K, Yonehara S, Nagata S, Hayashi N. Significance of Fas antigen-mediated apoptosis in human fulminant hepatic failure. Am J Gastroenterol. 2000;95:2047–2055. doi: 10.1111/j.1572-0241.2000.02268.x. [DOI] [PubMed] [Google Scholar]
- 31.Kosai K, Matsumoto K, Nagata S, Tsujimoto Y, Nakamura T. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem Biophys Res Commun. 1998;244:683–690. doi: 10.1006/bbrc.1998.8293. [DOI] [PubMed] [Google Scholar]
- 32.Streetz KL, Tacke F, Leifeld L, Wustefeld T, Graw A, Klein C, Kamino K, et al. Interleukin 6/gp130-dependent pathways are protective during chronic liver diseases. Hepatology. 2003;38:218–229. doi: 10.1053/jhep.2003.50268. [DOI] [PubMed] [Google Scholar]
- 33.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002;16:444–454. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.