Abstract
Objective
The endogenous role of the VEGF family member vascular endothelial growth factor-B (VEGF-B) in pathological angiogenesis remains unclear.
Methods and Results
We studied the role of VEGF-B in various models of pathological angiogenesis using mice lacking VEGF-B (VEGF-B−/−) or overexpressing VEGF-B167. After occlusion of the left coronary artery, VEGF-B deficiency impaired vessel growth in the ischemic myocardium whereas, in wild-type mice, VEGF-B167 overexpression enhanced revascularization of the infarct and ischemic border zone. By contrast, VEGF-B deficiency did not affect vessel growth in the wounded skin, hypoxic lung, ischemic retina, or ischemic limb. Moreover, VEGF-B167 overexpression failed to enhance vascular growth in the skin or ischemic limb.
Conclusion
VEGF-B appears to have a relatively restricted angiogenic activity in the ischemic heart. These insights might offer novel therapeutic opportunities.
Keywords: VEGF-B, angiogenesis, arteriogenesis, collateral growth, cardiac ischemia, limb ischemia
Vascular endothelial grow factor (VEGF) is a key regulator of angiogenesis in health and disease by binding to VEGF receptor-2 (VEGFR-2),1 but the angiogenic activity of its homologue VEGF-B, which binds VEGFR-1 (Flt-1), is less defined.2,3 By contrast, genetic and inhibition studies revealed that Flt-1, and its other specific ligand PlGF, stimulate pathological angiogenesis only.4,5
VEGF-B is produced as 2 different isoforms: a heparin-binding VEGF-B167 and diffusible VEGF-B186 isoform.3,6,7 VEGF-B is widely expressed in many tissues and cell types, including cardiac and skeletal myocytes and endothelial and mural cells.3,6,7 In vitro, VEGF-B stimulates endothelial cell growth and proliferation.8 By contrast, loss-of-function studies failed to reveal a consistent role for VEGF-B in pathological angiogenesis. Indeed, in 2 lines, loss of VEGF-B did not cause vessel defects in the embryo or healthy adult mouse, whereas recovery of coronary flow was impaired after transient occlusion ex vivo but, apparently, not because of reduced coronary vessel growth.9,10 Moreover, loss of VEGF-B failed to reduce angiogenesis in the cornea9 or ischemic retina.11 One study proposed a role for VEGF-B in pulmonary hypertension,12 but this finding was contested by others.13 The effects of VEGF-B deficiency on revascularization of the infarcted myocardium in vivo remain unknown. Gain-of-function studies also showed inconsistent effects. Indeed, overexpression of VEGF-B stimulates angiogenesis in skin wounds and ischemic limbs14–16 or promotes hypertrophy of remote myocardium after myocardial infarction,17 whereas VEGF-B had negligible effects after adenoviral gene transfer in rabbit carotid arteries or normoxic hindlimbs.18,19 The therapeutic potential of VEGF-B (VEGF-B167) to promote revascularization of ischemic myocardium remains unknown. Overall, the precise role of VEGF-B in pathological angiogenesis remains to be established.
As these controversies might be attributable to differences in genetic background or phenotyping methodology, we characterized the angiogenic role of VEGF-B in different mouse models of disease. By using VEGF-B−/− mice on a pure C57BL/6 inbred background, and various strategies to overexpress VEGF-B, we found that VEGF-B promotes vessel growth in the ischemic heart but not in other organs.
Methods
For detailed Methods, please see the supplemental materials (available online at http://atvb.ahajournals.org).
Animals, Models, Histology, Immunohistochemistry, and Morphometric Analyses
VEGF-B−/− mice9 were back-crossed onto a C57BL/6 background for 8 generations. C57BL/6 and NMRI nu/nu mice (all 8 to 12 weeks old) were obtained from Charles River Laboratories (Les Ocins, France) and adult SCID mice were from Taconic M&B Europe (Ry, Denmark). LacZ-tagged Flt1 mice, expressing βgalactosidase (β-Gal) under the control of the Flt-1 promotor were kindly provided by J. Rossant (Toronto, Ontario, Canada).20 Animal experiments were approved by local committees. All experimental approaches are described in detail in the supplemental methods.
Production and Administration of VEGF-B Protein, Plasmid, and Adenovirus
Recombinant human VEGF-B167 (rhVEGF-B167) protein was obtained from Amrad Corporation and delivered systemically via osmotic minipumps. Adenoviruses, constructed by cloning the murine PlGF-2, or the human VEGF-B167 or VEGF-B186 cDNA into the pACCMVpLpA plasmid, were intradermally or intravenously injected. A plasmid expressing murine VEGF-B167 (pcDNA3.mVEGF-B167) or an empty pcDNA3 plasmid was administered via muscle electroporation. These experimental methods are described in more detail in the supplemental methods.
Statistics
Data (mean±SEM) were analyzed using 2-tailed Student t test, with P<0.05 considered statistically significant.
Results
Loss of VEGF-B Impairs Revascularization After Myocardial Infarction
To study the potential of VEGF-B to stimulate revascularization of the ischemic heart (infarct and border zone), we used a previously established model of acute myocardial infarction (MI) in wild-type (WT) and VEGF-B−/− mice.21 At 7 days after MI, the density of both thrombomodulin positive (TM+) capillaries and smooth muscle α-actin positive (SMA+) covered vessels in the infarct area of VEGF-B−/− mice was only 65% of that of WT mice (n= 14; P<0.05 in all groups; Figure 1A and 1B). Revascularization of the ischemic border zone was also impaired in VEGF-B−/− mice (TM+ vessels: 340±39 vessels/mm2 in WT versus 234±38 vessels/mm2 in VEGF-B−/− mice; n=5; P<0.05). The number of macrophages, infiltrating into the infarct area, was normal (Mac3+ area/infarct area: 0.22±0.06% in VEGF-B−/− mice versus 0.22±0.05% in WT mice; n=12; P=NS). Thus, loss of VEGF-B impairs angiogenesis and vessel maturation in the ischemic heart.
Figure 1.
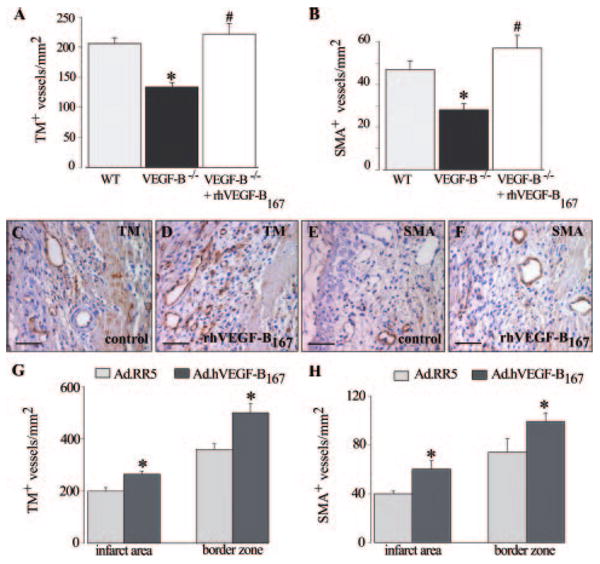
A and B, Morphometric analysis revealed a reduced number of TM+ and SMA+ vessels in the infarcted area of VEGF-B−/− mice, as compared to WT animals (*P<0.05) or to VEGF-B−/− mice, treated with rhVEGF-B167 (#P<0.05 vs no treatment). C–F, Immunostaining showed increased TM+ and SMA+ vessels after delivery of rhVEGF-B167 to WT mice. G and H, Systemic injection of Ad.hVEGF-B167 stimulates the growth of TM+ and SMA+ vessels in the infarct area and border zone of WT mice (*P<0.05). Scale bars: 50 μm.
We next analyzed whether administration of recombinant human (rh) VEGF-B167 protein rescued the impaired myocardial revascularization in VEGF-B−/− mice, and therefore administered rhVEGF-B167, the predominant isoform in cardiac and skeletal muscle.7 Continuous systemic delivery of a daily dose of 1.5 μg rhVEGF-B167 for 1 week to VEGF-B−/− mice normalized the impaired revascularization of the infarct (n=5; P<0.05; Figure 1A and 1B), and increased the growth of TM+ vessels in the ischemic border zone by 1.5-fold (P<0.05, n=5).
VEGF-B167 Therapy Enhances Ischemic Myocardial Revascularization
We then used 3 different techniques to investigate whether delivery of VEGF-B stimulated revascularization of ischemic hearts in WT mice. First, we administered VEGF-B167 protein. Pilot studies revealed that delivery of rVEGF167 via osmotic minipumps significantly increased the VEGF-B167 blood plasma levels (Note I, please see supplemental materials). VEGF-B167 protein therapy indeed increased the density of capillaries and arterioles in the infarct area (TM+ vessels/mm2: 206±10 after vehicle versus 285±33 after rhVEGF-B167; SMA+ vessels/mm2: 47±4 after vehicle versus 62±11 after rhVEGF-B167; n=5; P<0.05; Figure 1C through 1F), and stimulated vessel growth in the ischemic border by 1.5-fold and 1.3-fold, respectively (P<0.05; n=5).
Second, similar results were obtained after intravenous injection of an adenoviral vector encoding hVEGF-B167 (Ad.hVEGF-B167), known to transduce hepatocytes, which then release the transgene product into the circulation for up to 21 days (Note II, please see supplemental materials). Compared to control Ad.RR5 virus, VEGF-B167 gene transfer increased the densities of TM+ and SMA+ vessels in the infarct and in the ischemic border (n=9; P<0.05 versus Ad.RR5; Figure 1G and 1H) at 7 days after MI. VEGF-B167 gene transfer did not stimulate macrophage recruitment (Mac3+ area/infarct area: 0.20±0.05% after Ad.hVEGF-B167 versus 0.19±0.06% after Ad.RR5; n=9; P=NS).
Third, implantation of VEGF-B167-expressing myoblasts, but not control LacZ+ myoblasts, in the ischemic border zone induced vessel growth in the ischemic myocardium (supplemental Figure I). Hence, using different techniques (delivery of protein or gene transfer) and routes of administration (locally or systemically), VEGF-B167 therapy enhanced vessel growth in ischemic hearts of WT mice.
VEGF-B Does Not Affect Vessel Growth in Skin, Lung, or Retina
To further study the role of VEGF-B in pathological angiogenesis, we analyzed skin wound healing, using a linear skin incision model. Daily measurements revealed no defects in the rate or extent of skin wound healing in VEGF-B−/− mice (Figure 2A through 2C). Consistent herewith, loss of VEGF-B failed to affect the number of endothelial cell-lined and mural cell–covered vessels in the granulation tissue at 5 days after wounding (CD31+ vessels/mm2: 270±16 in WT mice versus 290±20 in VEGF-B−/− mice; SMA+ vessels/mm2: 67±9 in WT mice versus 62±7 in VEGF-B−/− mice; n=5; P=NS). Macrophage infiltration was also normal (F4/80+ area as % of total granulation tissue area: 4.59±0.25 in WT mice versus 4.55±0.92 in VEGF-B−/− mice; n=5; P=NS). Additional studies using adenoviral vectors to locally overexpress VEGFB167 or VEGF-B186 in the skin confirmed that VEGF-B does not affect the skin vasculature (supplemental Figure II).
Figure 2.
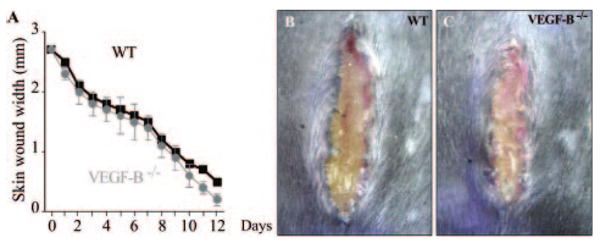
A–C, Skin wound healing is comparable in WT and VEGF-B−/− mice as analyzed by measurement of the wound width (A), and illustrated by macroscopic inspection (B and C).
Furthermore, loss of VEGF-B failed to affect mural cell recruitment and vessel remodeling in hypoxic lungs (supplemental Figure IIIA through IIIC) or neovascularization in ischemic retinas (supplemental Figure IIIE through IIIF). Together, VEGF-B plays a negligible role in vessel growth, maturation, and remodeling in normal or wounded skin, in hypoxic lungs, or in ischemic retinas.
Loss of VEGF-B Does Not Affect Revascularization of Ischemic Limbs
We next analyzed whether VEGF-B affects revascularization of ischemic limbs.21 In an established mouse model of hind-limb ischemia, the ischemic gastrocnemius muscle is revascularized by capillary angiogenesis, whereas collateral vessel growth occurs in the adductor muscle.21 In the ischemic gastrocnemius muscle, vessel densities in the regenerating areas were comparable in VEGF-B−/− mice at 7 days after ischemia (Table). In addition, laser Doppler perfusion analysis and endurance/graded treadmill exercise tests confirmed normal revascularization of ischemic limbs in VEGF-B−/− mice after ischemia (Table; supplemental Figure IVA and IVB). Moreover, to exclude the possibility that VEGF-B would only act as a modifier of PlGF, we analyzed mice lacking both VEGF-B and PlGF. However, compared to WT or VEGF-B−/− mice, limb reperfusion was comparably reduced in mice lacking PlGF alone or in mice lacking both VEGF-B and PlGF (supplemental Figure IVC and IVD; Table), indicating that VEGF-B is redundant for limb reperfusion even in conditions of genetically crippled limb revascularization in PlGF−/− mice.22 When analyzing in the adductor muscle the number and lumen size of preexisting collateral vessels, we found, again, no genotypic differences at 7 days after ischemia (Table; supplemental Figure IVE and IVF). Macrophage recruitment around the collaterals (supplemental Figure IVG through IVI) as well as in vitro macrophage activation were also unaffected by, respectively, loss or addition of VEGF-B (supplemental Figure IVG through IVI). Thus, endogenous VEGF-B is redundant for the revascularization of ischemic limbs.
Table. Negligible Role of VEGF-B in Revascularization After Limb Ischemia.
| WT Mice | ||||||||
|---|---|---|---|---|---|---|---|---|
| WT Mice | VEGF-B−/− Mice | Empty Plasmid | pmVEGF-B167 | Ad.RR5 | Ad.hVEGF-B167 | |||
| Angiogenesis | ||||||||
| Capillary-to-myocyte ratio | 0.96±0.06 | 0.97±0.06 | ND | ND | 1.16±0.06 | 1.19±0.10 | ||
| Total limb perfusion | ||||||||
| Laser Doppler (% of non-ligated) | 90±5 | 90±11 | 86±9 | 82±6 | 92±5 | 94±4 | ||
| Treadmill exercise test | ||||||||
| Endurance (% of baseline) | 56±6 | 50±7 | ND | ND | ND | ND | ||
| Grade exercise (% of baseline) | 57±4 | 52±6 | ND | ND | ND | ND | ||
| Collateral growth | ||||||||
| Lumen area, μm2 | ||||||||
| Main collateral artery | 2810±300 | 2480±150 | 2890±190 | 2630±280 | 2294±425 | 2415±511 | ||
| 2nd collateral branch | 740±75 | 790±50 | 760±60 | 790±65 | 704±190 | 688±163 | ||
| 3rd collateral branch | 120±10 | 140±7 | 96±3 | 98±6 | 105±13 | 92±13 | ||
| Collateral side branches (n/mm2) | ||||||||
| 2nd collateral branches | 3±0.8 | 3±0.7 | 2±0.3 | 3±0.4 | 4±1.8 | 3±1.4 | ||
| 3rd collateral branches | 5±1.0 | 6±1.5 | 8±1.2 | 9±1.6 | 7±1.6 | 10±3.6 | ||
| Total perfusion area, μm2/mm2 | 2730±700 | 3000±800 | 3140±360 | 3460±670 | 3098±569 | 3020±524 | ||
Analysis was performed as described in methods. ND indicates not determined; P=NS for WT/control mice vs VEGF-B−/− /VEGF-B–treated animals.
VEGF-B167 Therapy Does Not Enhance Revascularization in Ischemic Limbs
As our findings above do not exclude the possibility that overexpression of VEGF-B167 might enhance collateral growth or capillary angiogenesis in ischemic limbs, as suggested by others,15,16 we overexpressed VEGF-B167 in ischemic hindlimbs of WT mice using various established methods. To analyze the effect of VEGF-B167 overexpression on both collateral growth and capillary angiogenesis in the ischemic limb, we intravenously injected the adenoviral vector Ad.hVEGF-B167, similar as done for our MI experiments and resulting in increased VEGF-B plasma levels (Note II, please see supplemental materials). At 7 days after ligation, Ad.hVEGF-B167, gene transfer did not improve angiogenesis in the gastrocnemius muscle (Table; Figure 3A and 3B) and failed to increase the number and size of preexisting collateral vessels in the adductor muscle (Table). VEGF-B167 therapy did not stimulate infiltration of macrophages (numbers around the main collateral vessel: 14±2 after Ad.RR5 versus 12±1 after Ad.hVEGF-B167 gene transfer, n=5; P=NS), and failed to improve limb perfusion (Table). Also, VEGF-B167 therapy failed to stimulate ischemic limb revascularization at later time points, thus excluding a delayed effect (Note III, please see supplemental materials).
Figure 3.
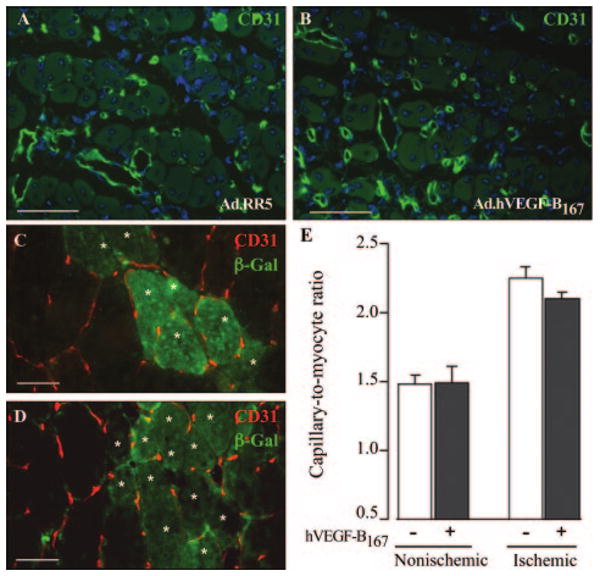
A and B, Immunolabeling for CD31 showed comparable vessel densities in regenerating gastrocnemius muscles of Ad.RR5 or Ad.hVEGF-B167 injected mice. C and D, Double staining for β-Gal (green cells, marked by an asterisk) and CD31 revealed an identical vascularization pattern in gastrocnemius muscles, injected with control or VEGF-B167 overexpressing myoblasts. E, Capillary-to-myocyte ratio is comparable in normal or ischemic limbs, injected with control or VEGF-B167 over-expressing myoblasts. Scale bars: 50 μm.
To further assess any possible effect of VEGF-B on ischemic limb revascularization (in particular capillary angiogenesis), we implanted VEGF-B167–expressing mouse myoblasts into the anterior tibialis muscles (supplemental Figure I). In healthy (nonligated) muscle, no vessel growth was induced at the engraftment site of VEGF-B167–expressing myoblasts, as compared to control LacZ+ myoblasts (Figure 3E). To assess whether VEGF-B167 affects vessel growth in ischemic skeletal muscle, myoblasts were implanted immediately after ligation of the femoral artery. Capillary density was increased in the ischemic anterior tibialis muscle at 28 days postligation, but implantation of VEGF-B 167–expressing myoblasts did not further enhance angiogenesis (Figure 3C through 3E). To exclude a transient effect on vessel growth, legs were also harvested 7 days after ischemia induction and myoblast implantation, again not resulting in increased vessel densities (not shown).
In addition, in vivo electroporation of a plasmid expressing murine VEGF-B167 (pmVEGF-B167) in the adductor muscle (to analyze collateral growth) was ineffective (Note IV, please see supplemental materials; Table). Overall, using various established strategies, VEGF-B167 therapy failed to stimulate revascularization of ischemic limbs.
Upregulation of VEGF-B167, but not of Flt-1, in the Ischemic Myocardium
In an effort to obtain some insight in the cardio-restricted properties of VEGF-B, we analyzed, by ELISA, the levels of VEGF-B in cardiac and skeletal muscle of WT mice. Compared to skeletal muscle, the heart expressed higher levels of VEGF-B in baseline conditions (pg/mg protein: 272±8 in heart versus 174±6 in skeletal muscle; n=6; P<0.05). At 4 days after MI, levels of VEGF-B were increased by 1.8±0.3 -fold and 2.0±0.3-fold in the infarct and infarct border zone, respectively (n=6; P<0.05). At 7 days, the fold upregulation of VEGF-B was 1.4±0.1 and 1.5±0.1, in the respective areas (n=3; P=NS). By contrast, at 4 and 7 days after limb ischemia, VEGF-B protein expression did not increase in the ischemic skeletal muscle (fold increase: 0.9±0.1 at day 4 and 0.8±0.1 at day 7; n=3 to 6; P=NS). This cardio-restricted upregulation of VEGF-B expression was specific, as the PlGF levels were increased in both ischemic myocardium and limb (not shown). Thus, VEGF-B expression is upregulated in ischemic hearts only, consistent with the cardio-restricted phenotype of VEGF-B−/− mice (for discussion, see below).
To analyze the expression of Flt-1, we performed RT-PCR using specific primers and found more abundant Flt-1 transcripts in the heart than skeletal muscle in baseline conditions (trancript levels of Flt-1 per 1000 copies of GADPH: 1.29±0.11 in the myocardium versus 0.40±0.04 in skeletal muscle, n=5; P<0.05). However, Flt-1 mRNA expression did not increase in the heart or skeletal muscle at 4 and 7 days after induction of ischemia (not shown). To identify the cell types expressing Flt-1 in vivo, we subjected LacZ-tagged Flt-1 reporter mice to MI or limb ischemia. Immunostaining for β-Gal revealed a similar labeling pattern for Flt-1 in both heart and skeletal muscle in ischemic as well as in nonischemic conditions. Indeed, double immuno-labeling studies showed expression of Flt-1 in SMA+ SMCs and most CD31+ ECs of nonischemic (Figure 4A, 4C, 4E, and 4G) and ischemic heart or limb tissue at 4 (Figure 4B, 4D, 4F, and 4H) and 7 days (not shown) after induction of ischemia. In the ischemic heart and skeletal muscle, Flt-1 expression also colocalized with some of the CD45+ infiltrating leukocytes (not shown). Thus, the cardio-restricted activity of VEGF-B is unlikely explained by differences in Flt-1 expression.
Figure 4.
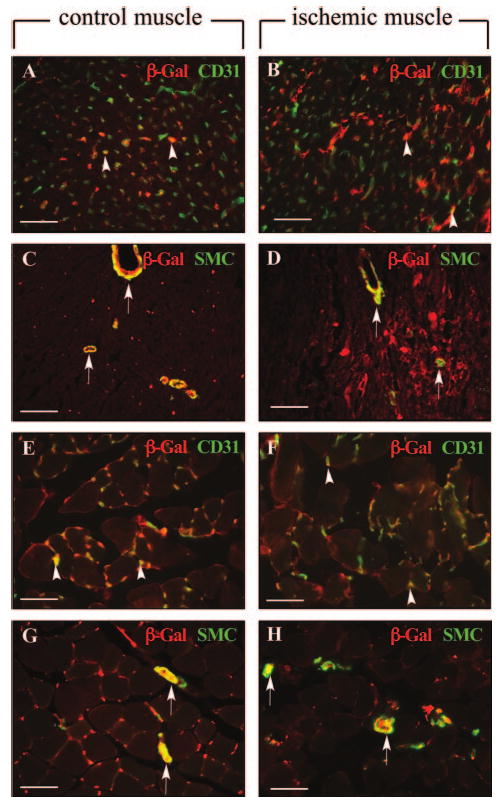
A-H, Double immunostaining of β-Gal and CD31 (A, B, E, F) or SMA (C, D, G, H) in normal and ischemic heart (A–D) or skeletal muscle (E–H) sections of LacZ-tagged Flt-1 mice revealed labeling of Flt-1 expressing cells in most CD31+ cells (arrowheads) and in all SMA+ cells (arrows). Scale bars: 50 μm.
Discussion
We studied loss- and gain-of-function of VEGF-B in pathological angiogenesis by using various genetic and experimental approaches. The principal finding is that VEGF-B has a relatively restricted role in pathological angiogenesis and, even more remarkably, predominantly in the ischemic myocardium.
The precise role and therapeutic potential of VEGF-B in the revascularization of the ischemic myocardium in vivo has not been studied thus far. By stimulating the ingrowth of new vessels in the ischemic infarct borders, VEGF-B resembles other angiogenic factors such as VEGF and PlGF (and others), which have, quantitatively and qualitatively, similar effects in rodent models of MI (reviewed in4). VEGF-B differs, however, from these agents in its selectivity to stimulate angiogenesis primarily in ischemic myocardium and not in other tissues. These loss- and gain-of-function findings in the mouse, together with findings that VEGF-B promotes compensatory hypertrophy of the remote myocardium after myocardial infarction,17 warrant further consideration of the therapeutic potential of VEGF-B for promoting functional recovery of MI.
In contrast to its role in the ischemic heart, various types of under- and overexpression studies indicate that VEGF-B is dispensable for vessel growth in the skin, lung, retina, and particularly the ischemic limb. Indeed, although others reported a minor role of VEGF-B in skin, retina, or lung,11–14 the negligible role of VEGF-B in ischemic limbs was unexpected, given that VEGF-B stimulates revascularization of the ischemic heart, another type of muscle. To confirm that the lack of an effect of VEGF-B was not attributable to a particular experimental condition, we used different complementary, nonoverlapping strategies. First, loss of VEGF-B did not impair revascularization of ischemic limbs, neither did it aggravate the revascularization defect in PlGF−/− mice, indicating that VEGF-B was ineffective alone and as a modifier of its homologue PlGF. Gain-of-function of VEGF-B, achieved via various strategies, also failed to stimulate the revascularization of ischemic limbs. This failure was not attributable to insufficient expression, as injection of the same dose of a VEGF-B expressing adenovirus or implantation of the same engineered VEGF-B myoblasts sufficed to stimulate vessel growth in the ischemic heart. Also, each of these strategies have previously been used in ischemic limbs to demonstrate the angiogenic activity of other well-known angiogenic factors, such as VEGF and PlGF (21,23; unpublished Tjwa M and Carmeliet P, 2008). In contrast to our findings, others reported that VEGF-B167 promotes ischemic limb revascularization.15,16 The precise explanation for this discrepancy remains unknown, and might be attributable to experimental differences in gene transfer method, ischemic limb model or methods of analysis.
A remarkable observation is that VEGF-B has angiogenic properties, which substantially differ from those of its homologues VEGF and PlGF. Of all angiogenic members of the VEGF family, VEGF is the most widely active, affecting angiogenesis in health and disease.1,4 PlGF, by contrast, is redundant for embryonic vascularization and vessel maintenance in healthy conditions, but involved in the angiogenic switch in disease conditions.21,22 VEGF-B is not only dispensable in development and health, but also in most conditions of pathological angiogenesis, except for the ischemic heart. The selectivity and specificity of each of these VEGF family members is remarkable, when comparing it, for instance, to the largely redundant and overlapping angiogenic activity of the 24 FGF family members.24 Though beyond the scope of the present study, it remains outstanding why VEGF-B has such a restricted angiogenic activity in the ischemic heart. The lack of its angiogenic activity in noncardiac tissues cannot be simply attributed to absent expression of VEGF-B or its receptor Flt-1, because both are constitutively expressed in these tissues, including in skeletal muscle.7,21,22 Interestingly, however, VEGF-B levels were up-regulated by ischemia in the ischemic heart but not in the ischemic muscle, which might possibly explain, at least in part, why angiogenesis was impaired in VEGF-B−/− mice in the ischemic myocardium, but not in the ischemic limb. Another possible mechanism might be that the endothelial cells in the heart have distinct tissue-specific characteristics as compared to endothelial cells in other tissues, similar to the distinct differentiation properties of endothelial cells in the nervous system, endocrine organs, lymphoid tissue, etc.25 It is indeed known that coronary endothelial cells are derived from unique progenitors, ie, the epicardium-derived progenitor cells, and require distinct angiogenic signals (such as thymosin-β4).26 Perhaps, also, the intracellular signaling pathway, induced by VEGF-B, is distinct in these coronary endothelial cells. Because VEGF and PlGF transmit specific angiogenic signals through Flt-1,5 VEGF-B could also have a different angiogenic activity profile. Further hypothetical explanations for the selective angiogenic activity of VEGF-B in the heart might include differences in the composition of extracellular matrix proteins (which modulate the activity of VEGF-B27), or in a role for Neuropilin-1, another VEGF-B receptor (reviewed in6).
Regardless of the mechanisms, few other tissue-selective angiogenic molecules have been identified thus far, including EG-VEGF, BDNF, and others.1 Such tissue-specific angiogenic signals are medically relevant, as they offer attractive therapeutic opportunities. Indeed, the potent activity of VEGF and its associated risk of adverse effects (bleeding, leakage, hypotension, malignancy, etc), for instance, has precluded systemic administration of this angiogenic agent for the revascularization of ischemic tissues.1,4 In contrast, a molecule such as VEGF-B, which would more selectively stimulate vessel growth in the heart, and primarily in ischemic conditions, would be expected to stimulate vessels in the specific target organ without causing adverse effects. Our observations that VEGF-B did not cause any noticeable side effects of general toxicity, hemorrhage, edema, or hypotension (not shown) are consistent with such a model. Overall, these mouse genetic findings support a—therapeutically attractive—model whereby VEGF-B selectively promotes angiogenesis in the ischemic myocardium.
Supplementary Material
Table I: Negligible role of VEGF-B in pulmonary vessel remodeling after hypoxia
Figure I: As an alternative method to deliver VEGF-B to cardiomyocytes, we implanted in the myocardial wall of the infarct border, mouse myoblasts, transduced ex vivo with a retrovirus, to constitutively produce mouse VEGF-B167, since a similar strategy using VEGF-A-expressing myoblasts was previously shown to stimulate vessel growth in muscle 6-8. A,B, Immunofluorescent staining for VEGF-B (green) confirmed the absence of VEGF-B in control myoblasts expressing only LacZ (A) and production of mVEGF-B167 in myoblasts expressing both VEGF-B and LacZ (B). Nuclei are counterstained with Dapi (blue). The production of mVEGF-B167 by engineered myoblasts in vivo was further confirmed by determining mVEGF-B expression levels in cell lysates and muscle extracts. Indeed, myoblasts, transduced with a retrovirus encoding mVEGF-B167, secreted 26 ± 2 ng/106 cells/24h of VEGF-B, while control LacZ-expressing myoblasts failed to express detectable amounts of VEGF-B. At 3 days after implantation of myoblasts into the gastrocnemius muscle, increased amounts of VEGF-B protein were found in the muscles implanted with the VEGF-B167-expressing myoblasts (299 ± 67 pg/mg protein versus 22 ± 2 pg/mg protein in control muscles; N=6; P<0.05). Further in vitro experiments revealed that the secreted VEGF-B protein was bio-active (not shown). C,D, Double immunofluorescent staining for β-galactosidase (site of myoblast engraftment) and CD31 (blood vessels) in the ischemic myocardium revealed no signs of vessel growth around the implantation site of control myoblasts (C), but a robust angiogenic induction around the site of VEGF-B167-expressing myoblast implantation (D). Thus, intramyocardial implantation of VEGF-B167-expressing myoblasts enhanced vessel growth in the ischemic myocardium at 28 days post-MI, while control LacZ+ myoblasts failed to promote angiogenesis. Scale bars: 50 μm.
Figure II: We showed previously that adenoviral gene transfer of PlGF into the skin of ears enlarged pre-existing vessels with subsequent stabilization by acquisition of a pericyte coat 9. We therefore injected adenoviruses, expressing hVEGF-B167 (Ad.hVEGF-B167), hVEGF-B186 (Ad.hVEGF-B186) or mPlGF-2 (Ad.mPlGF) intradermally into the ear skin. A-I, Ears were whole-mount immunostained for CD31 (endothelial cells; brown) in panels A,D,G, for CD31 (green) and SMA (smooth muscle cells; red) in panels B,E,H, and for F4/80 (macrophages, green) and SMA (red) in panels C,F,I. As expected, gene transfer of mPlGF-2 increased the density, tortuosity and size of pre-existing vessels, and stimulated their coverage by mural cells (A,B). In contrast, Ad.hVEGF-B167 or Ad.hVEGF-B186 gene transfer minimally enlarged the pre-existing vessels without increase in number, tortuosity or mural cell coverage (D,E,G,H). Consistent herewith, we found that many F4/80+ macrophages had infiltrated after Ad.mPlGF gene transfer, while only few macrophages were present after Ad.hVEGF-B167 or Ad.hVEGF-B186 gene transfer (C,F,I). A control virus (Ad.CMV) failed to affect any of these parameters (not shown). Scale bars: 100 μm in panels A,B,D,E,G,H and 50 μm in C,F,I.
Figure III: A-D, To further analyze the effects of VEGF-B on vessel remodeling and recruitment of mural cells, we examined in VEGF-B−/− mice the remodeling of pulmonary vessels in response to chronic hypoxia 10. Hart's elastin staining revealed no genotypic differences between WT and VEGF-B−/− mice in pulmonary vessel structure in normoxia (A,B; see also Table S1). In WT mice, continuous hypoxic conditions for 4 weeks increased the number of thick-walled muscularized vessels by ∼4.6-fold (C; Table S1). A comparable 4.7-fold increase in the number of thick-walled muscularized vessels was observed in VEGF-B−/− mice (D; Table S1), suggesting that loss of VEGF-B did not impair mural cell recruitment. Similar results were obtained when hypoxic vessel remodeling was analyzed by immunostaining for SMA (Table S1). Consistent herewith, no genotypic differences were observed in the development of right ventricle (RV) hypertrophy, which normally is caused by pulmonary hypertension (Table S1). Compared to WT mice, VEGF-B−/− mice were as sensitive to chronic hypoxia, as evidenced by the similar increase in hematocrit levels (Table S1). The lack of an effect of VEGF-B on pulmonary hypertension is consistent with earlier findings by Louzier et al. 11. E,F, To further analyze the effects of VEGF-B on angiogenesis, we studied in VEGF-B−/− mice retinal neovascularization in response to ischemia, using an established model 10. Neovascularization of the ischemic retina, analyzed by H&E staining on cross-sections, was comparable in WT (E) and VEGF-B−/− mice (F; arrows indicate neovessels). Indeed, compared to WT mice, loss of VEGF-B failed to reduce the number of endothelial cells, forming new intravitreal vessel sprouts (per retinal cross-section: 102 ± 13 in WT mice versus 118 ± 10 in VEGF-B−/− mice; N=5; P=NS) or the number of neovascular tufts (per retinal cross-section: 42 ± 4 in WT mice versus 50 ± 8 in VEGF-B−/− mice; N=5; P=NS; Figure S3E,F). These findings are consistent with findings by Reichelt et al. 12. Scale bars: 50 μm in panels E,F and 25 μm in panels A-D.
Figure IV: A-D, At 7 days after ligation, laser Doppler perfusion analysis on ischemic limbs also failed to show any genotypic differences (A,B; see also Table 1). Ischemic limb perfusion was also measured in mice lacking both VEGF-B and PlGF – the rationale for this experiment being that limb perfusion was reduced in PlGF−/− mice 10 and that, possibly, the consequences of VEGF-B deficiency might be more apparent when limb revascularization was already impaired by prior loss of PlGF. However, at 7 days after ischemia, laser Doppler imaging revealed that, compared to WT or VEGF-B−/− mice (Table 1; P<0.05), limb perfusion was comparably reduced in mice lacking PlGF alone or in mice lacking both VEGF-B and PlGF (C,D; % perfusion of non-ligated limb: 47 ± 5% in PlGF−/− mice versus 55 ± 9% in VEGF-B−/−:PlGF−/− mice; N=5; P=NS). Please note the color scale: from blue (low perfusion) to red (high perfusion). E,F, H&E staining of transverse sections through the adductor muscles revealed a comparable size of the main collateral vessel in WT (E) and VEGF-B−/− mice (F). G-I, Morphometric analysis revealed a similar amount of F4/80+ macrophages around the collaterals in WT and VEGF-B−/− adductor muscles (G), as also illustrated by microscopic pictures (H,I). In addition, VEGF-B failed to stimulate the production by cultured macrophages of TNF-alpha, previously implicated in collateral growth 9 (data not shown). Please note that the black staining inside the vessels results from bismuth-gelatin filling of the vessels, which enables a better macroscopic visualization of the collaterals. Scale bars: 50 μm.
Acknowledgments
Sources of Funding: This work was supported by grants to PC from Bristol-Myers-Squibb, FWO (#G0125.00 & #G.0121.02), EU (QLRT-2001-01955), Leducq foundation, Concerted Research Activities (#GOA2001/09), and IAP-P5/02, and IAP-P6/30, and by NIH (HL65572) to H.M.B. and M.L.S., and by Swedish Research Council, Novo Nordisk Foundation, Wallenbergs Foundation, and Karolinska Institute. to U.E. G.v.D. (postdoc DE 740/1-1); M.T. (postdoc IWT and FWO).
Footnotes
Disclosures: None.
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Grimmond S, Lagercrantz J, Drinkwater C, Silins G, Townson S, Pollock P, Gotley D, Carson E, Rakar S, Nordenskjold M, Ward L, Hayward NK, Weber G. Cloning and characterization of a novel human gene related to vascular endothelial growth factor. Genome Research. 1996;6:124–131. doi: 10.1101/gr.6.2.124. [DOI] [PubMed] [Google Scholar]
- 3.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson R, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Tjwa M, Luttun A, Autiero M, Carmeliet P. VEGF and PlGF: two pleiotropic growth factors with distinct roles in development and homeostasis. Cell Tissue Res. 2003;314:5–14. doi: 10.1007/s00441-003-0776-3. [DOI] [PubMed] [Google Scholar]
- 6.Nash AD, Baca M, Wright C, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006;19:61–69. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Aase K, Li H, von Euler G, Eriksson U. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors. 2001;19:49–59. doi: 10.3109/08977190109001075. [DOI] [PubMed] [Google Scholar]
- 8.Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, Alitalo K, Betsholtz C, Eriksson U. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM, Townson S, Wells C, Little M, Cummings MC, Hayward NK, Kay GF. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 11.Reichelt M, Shi S, Hayes M, Kay G, Batch J, Gole GA, Browning J. Vascular endothelial growth factor-B and retinal vascular development in the mouse. Clin Experiment Ophthalmol. 2003;31:61–65. doi: 10.1046/j.1442-9071.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Wanstall JC, Gambino A, Jeffery TK, Cahill MM, Bellomo D, Hayward NK, Kay GF. Vascular endothelial growth factor-B-deficient mice show impaired development of hypoxic pulmonary hypertension. Cardiovasc Res. 2002;55:361–368. doi: 10.1016/s0008-6363(02)00440-6. [DOI] [PubMed] [Google Scholar]
- 13.Louzier V, Raffestin B, Leroux A, Branellec D, Caillaud JM, Levame M, Eddahibi S, Adnot S. Role of VEGF-B in the lung during development of chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003;284:L926–L937. doi: 10.1152/ajplung.00247.2002. [DOI] [PubMed] [Google Scholar]
- 14.Mould AW, Greco SA, Cahill MM, Tonks ID, Bellomo D, Patterson C, Zournazi A, Nash A, Scotney P, Hayward NK, Kay GF. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res. 2005;97:e60–e70. doi: 10.1161/01.RES.0000182631.33638.77. [DOI] [PubMed] [Google Scholar]
- 15.Wright CE. Effects of vascular endothelial growth factor (VEGF)A and VEGFB gene transfer on vascular reserve in a conscious rabbit hindlimb ischaemia model. Clin Exp Pharmacol Physiol. 2002;29:1035–1039. doi: 10.1046/j.1440-1681.2002.03773.x. [DOI] [PubMed] [Google Scholar]
- 16.Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, Levy BI. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res. 2003;93:114–123. doi: 10.1161/01.RES.0000081594.21764.44. [DOI] [PubMed] [Google Scholar]
- 17.Tirziu D, Chorianopoulos E, Moodie KL, Palac RT, Zhuang ZW, Tjwa M, Roncal C, Eriksson U, Fu Q, Elfenbein A, Hall AE, Carmeliet P, Moons L, Simons M. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest. 2007;117:3188–3197. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, Achen MG, Stacker SA, Hedman M, Alitalo K, Yla-Herttuala S. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther. 2003;14:1451–1462. doi: 10.1089/104303403769211664. [DOI] [PubMed] [Google Scholar]
- 19.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 20.Fong GH, Klingensmith J, Wood CR, Rossant J, Breitman ML. Regulation of flt-1 expression during mouse embryogenesis suggests a role in the establishment of vascular endothelium. Dev Dyn. 1996;207:1–10. doi: 10.1002/(SICI)1097-0177(199609)207:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 23.von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, Merchant MJ, Cooke JP, Blau HM. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. Faseb J. 2006;20:2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- 24.Rusnati M, Presta M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr Pharm Des. 2007;13:2025–2044. doi: 10.2174/138161207781039689. [DOI] [PubMed] [Google Scholar]
- 25.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 26.Smart N, Rossdeutsch A, Riley PR. Thymosin beta4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007;10:229–241. doi: 10.1007/s10456-007-9077-x. [DOI] [PubMed] [Google Scholar]
- 27.Ikuta T, Ariga H, Matsumoto K. Extracellular matrix tenascin-X in combination with vascular endothelial growth factor B enhances endothelial cell proliferation. Genes Cells. 2000;5:913–927. doi: 10.1046/j.1365-2443.2000.00376.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table I: Negligible role of VEGF-B in pulmonary vessel remodeling after hypoxia
Figure I: As an alternative method to deliver VEGF-B to cardiomyocytes, we implanted in the myocardial wall of the infarct border, mouse myoblasts, transduced ex vivo with a retrovirus, to constitutively produce mouse VEGF-B167, since a similar strategy using VEGF-A-expressing myoblasts was previously shown to stimulate vessel growth in muscle 6-8. A,B, Immunofluorescent staining for VEGF-B (green) confirmed the absence of VEGF-B in control myoblasts expressing only LacZ (A) and production of mVEGF-B167 in myoblasts expressing both VEGF-B and LacZ (B). Nuclei are counterstained with Dapi (blue). The production of mVEGF-B167 by engineered myoblasts in vivo was further confirmed by determining mVEGF-B expression levels in cell lysates and muscle extracts. Indeed, myoblasts, transduced with a retrovirus encoding mVEGF-B167, secreted 26 ± 2 ng/106 cells/24h of VEGF-B, while control LacZ-expressing myoblasts failed to express detectable amounts of VEGF-B. At 3 days after implantation of myoblasts into the gastrocnemius muscle, increased amounts of VEGF-B protein were found in the muscles implanted with the VEGF-B167-expressing myoblasts (299 ± 67 pg/mg protein versus 22 ± 2 pg/mg protein in control muscles; N=6; P<0.05). Further in vitro experiments revealed that the secreted VEGF-B protein was bio-active (not shown). C,D, Double immunofluorescent staining for β-galactosidase (site of myoblast engraftment) and CD31 (blood vessels) in the ischemic myocardium revealed no signs of vessel growth around the implantation site of control myoblasts (C), but a robust angiogenic induction around the site of VEGF-B167-expressing myoblast implantation (D). Thus, intramyocardial implantation of VEGF-B167-expressing myoblasts enhanced vessel growth in the ischemic myocardium at 28 days post-MI, while control LacZ+ myoblasts failed to promote angiogenesis. Scale bars: 50 μm.
Figure II: We showed previously that adenoviral gene transfer of PlGF into the skin of ears enlarged pre-existing vessels with subsequent stabilization by acquisition of a pericyte coat 9. We therefore injected adenoviruses, expressing hVEGF-B167 (Ad.hVEGF-B167), hVEGF-B186 (Ad.hVEGF-B186) or mPlGF-2 (Ad.mPlGF) intradermally into the ear skin. A-I, Ears were whole-mount immunostained for CD31 (endothelial cells; brown) in panels A,D,G, for CD31 (green) and SMA (smooth muscle cells; red) in panels B,E,H, and for F4/80 (macrophages, green) and SMA (red) in panels C,F,I. As expected, gene transfer of mPlGF-2 increased the density, tortuosity and size of pre-existing vessels, and stimulated their coverage by mural cells (A,B). In contrast, Ad.hVEGF-B167 or Ad.hVEGF-B186 gene transfer minimally enlarged the pre-existing vessels without increase in number, tortuosity or mural cell coverage (D,E,G,H). Consistent herewith, we found that many F4/80+ macrophages had infiltrated after Ad.mPlGF gene transfer, while only few macrophages were present after Ad.hVEGF-B167 or Ad.hVEGF-B186 gene transfer (C,F,I). A control virus (Ad.CMV) failed to affect any of these parameters (not shown). Scale bars: 100 μm in panels A,B,D,E,G,H and 50 μm in C,F,I.
Figure III: A-D, To further analyze the effects of VEGF-B on vessel remodeling and recruitment of mural cells, we examined in VEGF-B−/− mice the remodeling of pulmonary vessels in response to chronic hypoxia 10. Hart's elastin staining revealed no genotypic differences between WT and VEGF-B−/− mice in pulmonary vessel structure in normoxia (A,B; see also Table S1). In WT mice, continuous hypoxic conditions for 4 weeks increased the number of thick-walled muscularized vessels by ∼4.6-fold (C; Table S1). A comparable 4.7-fold increase in the number of thick-walled muscularized vessels was observed in VEGF-B−/− mice (D; Table S1), suggesting that loss of VEGF-B did not impair mural cell recruitment. Similar results were obtained when hypoxic vessel remodeling was analyzed by immunostaining for SMA (Table S1). Consistent herewith, no genotypic differences were observed in the development of right ventricle (RV) hypertrophy, which normally is caused by pulmonary hypertension (Table S1). Compared to WT mice, VEGF-B−/− mice were as sensitive to chronic hypoxia, as evidenced by the similar increase in hematocrit levels (Table S1). The lack of an effect of VEGF-B on pulmonary hypertension is consistent with earlier findings by Louzier et al. 11. E,F, To further analyze the effects of VEGF-B on angiogenesis, we studied in VEGF-B−/− mice retinal neovascularization in response to ischemia, using an established model 10. Neovascularization of the ischemic retina, analyzed by H&E staining on cross-sections, was comparable in WT (E) and VEGF-B−/− mice (F; arrows indicate neovessels). Indeed, compared to WT mice, loss of VEGF-B failed to reduce the number of endothelial cells, forming new intravitreal vessel sprouts (per retinal cross-section: 102 ± 13 in WT mice versus 118 ± 10 in VEGF-B−/− mice; N=5; P=NS) or the number of neovascular tufts (per retinal cross-section: 42 ± 4 in WT mice versus 50 ± 8 in VEGF-B−/− mice; N=5; P=NS; Figure S3E,F). These findings are consistent with findings by Reichelt et al. 12. Scale bars: 50 μm in panels E,F and 25 μm in panels A-D.
Figure IV: A-D, At 7 days after ligation, laser Doppler perfusion analysis on ischemic limbs also failed to show any genotypic differences (A,B; see also Table 1). Ischemic limb perfusion was also measured in mice lacking both VEGF-B and PlGF – the rationale for this experiment being that limb perfusion was reduced in PlGF−/− mice 10 and that, possibly, the consequences of VEGF-B deficiency might be more apparent when limb revascularization was already impaired by prior loss of PlGF. However, at 7 days after ischemia, laser Doppler imaging revealed that, compared to WT or VEGF-B−/− mice (Table 1; P<0.05), limb perfusion was comparably reduced in mice lacking PlGF alone or in mice lacking both VEGF-B and PlGF (C,D; % perfusion of non-ligated limb: 47 ± 5% in PlGF−/− mice versus 55 ± 9% in VEGF-B−/−:PlGF−/− mice; N=5; P=NS). Please note the color scale: from blue (low perfusion) to red (high perfusion). E,F, H&E staining of transverse sections through the adductor muscles revealed a comparable size of the main collateral vessel in WT (E) and VEGF-B−/− mice (F). G-I, Morphometric analysis revealed a similar amount of F4/80+ macrophages around the collaterals in WT and VEGF-B−/− adductor muscles (G), as also illustrated by microscopic pictures (H,I). In addition, VEGF-B failed to stimulate the production by cultured macrophages of TNF-alpha, previously implicated in collateral growth 9 (data not shown). Please note that the black staining inside the vessels results from bismuth-gelatin filling of the vessels, which enables a better macroscopic visualization of the collaterals. Scale bars: 50 μm.


