Abstract
Aspergillus fumigatus is an environmental fungus that causes life-threatening infections in neutropenic patients. In the absence of intact innate immunity, inhaled A. fumigatus spores (conidia) germinate in the lung, forming hyphae that invade blood vessels and disseminate to other tissues. Although macrophages and neutrophils are postulated to provide defense against invasive fungal infection, animal models and human studies suggest that circulating monocytes also contribute to antifungal immunity. Although human monocyte subsets, defined as either CD14+CD16− or CD14+ CD16+, have been extensively characterized, their respective roles during fungal infection remain undefined. We isolated CD14+CD16− and CD14+CD16+ monocytes from healthy allogeneic hematopoietic stem cell transplantation donors and compared their ability to phagocytose and inhibit A. fumigatus conidia. Both monocyte subsets efficiently phagocytose conidia, but only CD14+CD16− monocytes inhibit conidial germination yet secrete little TNF. In contrast CD14+CD16+ do not inhibit conidial germination and secrete large amounts of TNF. Although CD14+CD16− and CD14+CD16+ monocytes differ in their response to dormant conidia, responses are similar if conidia are already germinated at the time of monocyte uptake. Our study demonstrates that functional CD14+CD16− and CD14+CD16+ monocytes can be isolated from allogeneic hematopoietic stem cell transplantation donors and that these subsets differ in their response to A. fumigatus conidia.
Monocytes are bone marrow-derived cells that circulate in the blood and migrate into tissues where they differentiate into macrophages and dendritic cells. Circulating human monocytes are divided into two major subsets based on expression of CD14 (the LPS coreceptor), CD16 (the FcγRIII low affinity IgG receptor) (1, 2), and the CCR2, CCR5, and CX3CR1 chemokine receptors (3, 4). Approximately 90% of circulating monocytes in humans are “classical” CD14+CD16− cells. In contrast, CD14+CD16+ monocytes, first described by Ziegler-Heitbrock et al. (5, 6), represent ~10% of all monocytes and express variable levels of CD14. Compared with CD14+ CD16− monocytes, circulating CD14+CD16+ monocytes are more differentiated and express higher levels of HLA-DR (7). Because frequencies of CD14+CD16+ monocytes increase under inflammatory conditions (1), it has been suggested that they may contribute to antimicrobial defense.
Murine models of bacterial and fungal infection demonstrate that circulating monocytes are recruited to sites of infection and mediate microbial killing (8). It is not known, however, whether human monocyte subsets play similar roles during infection. Direct comparison of TLR-induced cytokine secretion by human monocytes revealed that CD14+CD16+ monocytes are the predominant source of TNF (9), suggesting that these cells make unique contributions to inflammatory responses. The relative ability of CD14+ CD16− and CD14+CD16+ monocytes to provide defense against live pathogens, however, has not been determined. Given the defensive role of murine monocytes against infection, it is reasonable to postulate that a paucity of monocytes following treatment-induced leukocytopenia increases the risk of bacterial and fungal infections.
Among the microbial pathogens causing lethal infections following treatment-induced neutropenia, A. fumigatus remains one of the most common and devastating (10). Infection occurs following inhalation of airborne conidia, which, in immunocompetent hosts, are rapidly inactivated by pulmonary innate immune defenses. However, in immunocompromised hosts, such as patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT),3 inhaled conidia can germinate and form tissue invasive hyphae with potentially lethal consequences (11). Conidia germination is a multistage process involving changes in both volume and size. Initially, swelling occurs with approximate doubling in conidia volume. Subsequently, budding is initiated with the appearance of surface protuberances and formation of germ tubes. Germ tubes continue polar growth and become hyphae, which further extend and branch (12). Conidia of any of these germination stages are termed “germinated conidia” herein. In contrast to dormant conidia, germinated A. fumigatus conidia express β-glucans on their surfaces and stimulate tissue macrophages to secrete inflammatory cytokines and chemokines (13). It has been postulated that conidial clearance is mediated by alveolar macrophages while destruction of hyphae requires neutrophils (11). A. fumigatus infections remain a significant cause of morbidity and mortality in leukemia patients and patients undergoing stem cell transplantation patients and treatment can be difficult. Although granulocyte transfusion from donors has been tried as a therapy for invasive fungal infections, success has been sporadic and limited (14). In a murine model of hematopoietic stem cell transplantation, infusion of bone marrow myeloid progenitors led to enhanced survival following lethal challenge with A. fumigatus and Pseudomonas aeruginosa (15). Human monocyte subsets express β-glucan receptor, dectin-1 (16). Whether adoptive transfer of human monocytes can enhance clearance of invasive fungal infections in allo-HSCT patients remains unstudied, largely because the ability of human monocyte subsets to respond to fungal pathogens remains undefined.
Herein, we investigated the ability of human monocyte subsets to respond to A. fumigatus conidia. CD14+CD16− and CD14+ CD16+ monocyte subsets had surprisingly distinct responses to A. fumigatus conidia. Although CD14+CD16− monocytes were efficient at restricting conidia germination and did not secrete TNF following infection, CD14+CD16+ cells failed to suppress germination of conidia yet produced high levels of inflammatory cytokines. Our results demonstrate that monocyte subsets make distinct contributions to defense against A. fumigatus infection, with CD14+CD16− monocytes inhibiting fungal germination, while CD14+CD16+ cells enhance inflammatory responses.
Materials and Methods
Human research ethics
Cells were obtained from healthy donors giving steady-state leukocyte concentrates or already undergoing G-CSF primed leukopheresis collection for allogeneic transplantation. All donors had signed informed consents using protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, Memorial Sloan-Kettering Cancer Center.
Cells
G-CSF mobilization of stem cells and collection by leukopheresis was followed by positive immunomagnetic selection for CD34+ hematopoietic stem cells. The ISOLEX 300i immunomagnetic system (Baxter Health-care) was used to perform a combined positive CD34+ cell selection and negative selection of T cells. Samples were obtained from leukopheresed white blood cells before CD34 separation and from the flow-through fraction immediately after CD34 enrichment. Where indicated, peripheral blood cells were additionally obtained from healthy untreated donors. Mononuclear cell fractions were obtained by centrifugation over Ficoll-Paque PLUS (endotoxin free, GE Healthcare) from whole blood diluted 1:1 with RPMI 1640 medium (Life Technologies). Ficoll-purified cell fractions were resuspended at a final concentration of 5 × 107 cells/ml in RPMI 1640 medium containing human serum albumin and DMSO. Cells were frozen in a methanol bath at −80°C overnight before transfer to liquid nitrogen.
Flow cytometry
The following Abs were purchased from BD Pharmingen: anti-CD14-PerCP-Cy5.5 (M5E2), anti-CD16-FITC (3G8), anti-CD16-PE (3G8), anti-CD163-PE (3G8), anti-CCR2-Alexa Fluor 647 (48607), anti-CD86-PE (2331), and anti-HLA DR-PE (L243). For analysis of monocyte populations, a large gate was drawn to include lymphocyte/monocyte populations, and cells were further gated on CD14 and CD16 where indicated.
Isolation and culture of monocyte subsets
For monocyte subset purification, cryopreserved mononuclear cell fractions were thawed in a 37°C water bath, washed once and purified using MACS cell separation reagents. CD14+ cells were purified by magnetic separation using CD14 MicroBeads and comprised both CD14+CD16− and CD14+CD16+ subsets. CD14+CD16+ subsets were purified using a CD16 monocyte isolation kit (Miltenyi Biotec) according to manufacturer’s protocol. In brief, donor PBMCs were depleted of granulocytes and NK cells with anti-CD15 and anti-CD56 microbeads. CD16+ monocytes were positively enriched using anti-CD16 microbeads. Finally, CD14+CD16− monocytes were positively purified from flow-through cells using anti-CD14 microbeads. Following separation, purity of the resultant CD14+, CD14+CD16−, and CD14+CD16+ populations was 92–95% based on flow cytometric analysis. Purified cells were plated at 2–4 × 105/well in 96-well flat-bottom plates in RPMI 1640 medium supplemented with 10% FBS. Where indicated, cells were also cultured in parallel in the presence of 5% human serum. Recombinant GM-CSF (sargramostim, Leukine; Berlex) and IFN-γ (R&D Systems) were added at 1000 U/ml. For stimulation, heat killed Listeria monocytogenes (HKLM) was added at a final concentration of 108/ml and LPS (Sigma-Aldrich) at 1 ng/ml. Supernatants were collected for cytokine analysis 16 h after stimulation, and human TNF-α was quantified using OptEIA kit from BD Pharmingen according to the manufacturer’s instructions. Cells were spun onto glass slides for morphological assessment using a cytocentrifuge (Shandon) and stained using Diff-Quik Satin Set (Dade Behring). Images of cultured live cells were obtained on Zeiss Axioplan 2 Imaging microscopes.
Infection with Aspergillus fumigatus
A. fumigatus strain 293 was grown on Sabouraud dextrose agar slants at 37°C for 6–10 days. Resting conidia were harvested from slants, resuspended in PBS containing 0.025% (w/v) Tween 20, and counted. Conidia were adjusted to the desired concentration in culture medium and added to cells. Where indicated, voriconazole (Pfizer) was added to monocyte/conidia cultures at 0.5 μg/ml. For preparation of heat-killed conidia, resting conidia were heat-killed at 100°C for 30 min in a heating block. For preparation of germinated conidia, conidia were incubated at 37°C in RPMI 1640 for 6 h before monocyte infection. Preparations incubated for 6 h contained swollen conidia and budding conidia with hyphal extensions. For conidia killing experiments, infected monocytes were lysed with PBS/Tween 20 and colony forming units were estimated by plating serial dilutions of cell lysates on Sabouraud dextrose agar.
Phagocytosis of Aspergillus fumigatus
Dormant or germinated conidia were labeled using 10 mg/ml Alexa Fluor 633 ester dye (Invitrogen) in sodium bicarbonate buffer for 14–18 h at 4°C. As a control, conidia were incubated in buffer alone. The incubation did not adversely affect conidia viability and ability to form hyphae. Labeled conidia were collected, washed extensively with PBS, counted and used to infect cells. Infected cells were cultured for 4 h at 37°C or on ice and phagocytosis was examined by flow cytometry. Conidia-associated label was visualized in the allophycocyanin channel.
Results
Characterization of circulating monocyte populations in G-CSF-primed allo-HSCT donors
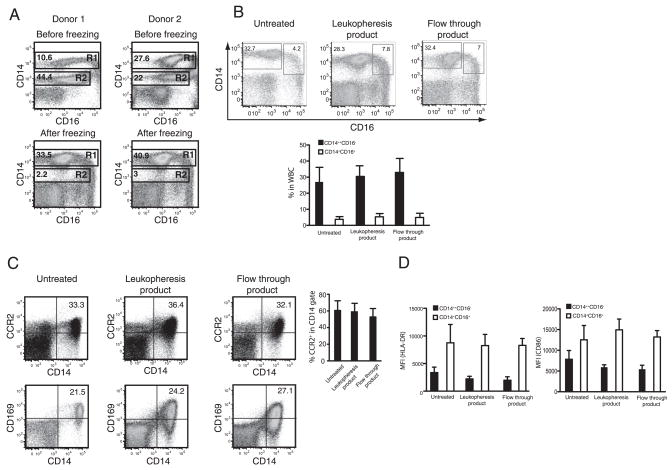
Allo-HSCT donors receive G-CSF to mobilize bone marrow progenitor and stem cells before leukopheresis and enrichment of CD34+ stem cells. We reasoned that G-CSF-mobilized peripheral blood may also be a significant source of monocytes. Although G-CSF administration increases the total number of circulating leukocytes, the effect on circulating monocytes is less clear. We therefore obtained PBMC aliquots from G-CSF mobilized allo-HSCT donors and analyzed them before CD34 enrichment. Ficoll separation of mononuclear cells from G-CSF primed collections routinely yielded 2–4 × 108 total leukocytes/ml, with ~65–80% viable cells after freezing and thawing. Although freshly harvested G-CSF-PBMC contained 25–45% neutrophils (CD14lowCD16low), this population was selectively lost during freezing/thawing (Fig. 1A, gate R2). In contrast, CD14+ monocytes were unaffected by freezing and thawing and could therefore be stored indefinitely following stem cell harvesting (Fig. 1A, gate R1).
FIGURE 1.
Normal monocyte composition in G-CSF-mobilized blood. A, Monocyte and neutrophil populations in G-CSF mobilized leukopheresis products were analyzed by flow cytometry before and after freezing. Gate R1 represents monocytes and gate R2 represents neutrophils. Dot plots of representative donors are shown from a total of four donors analyzed before freezing and eleven donors analyzed postfreezing. B, Expression of CD14 and CD16 in leukocytes collected by gradient centrifugation from untreated and G-CSF-treated donors before and after purification of CD34+ stem cells was analyzed by flow cytometry. Representative dot plots are shown and total of 12 normal donors and 19 G-CSF-mobilized donors were analyzed. Leukopheresis product corresponds to 8 donors and flow through product corresponds to 11 donors. Error bars represent SD (SD). C, Expression of CD163 and CCR2 on leukocytes purified from peripheral blood of normal and G-CSF-mobilized donors before and after purification of CD34+ stem cells were examined by flow cytometry. Representative dot plots are shown. Bar graph, Leukocytes from indicated fractions were gated on CD14 and CCR2 expression in the gate was analyzed. Error bars represent SD and same number of donors was analyzed as in (B). D, Monocytes were gated on CD14 and CD16 (as depicted in B) and expression of HLA-DR and CD86 in the indicated gates was analyzed. Bar graphs represent mean fluorescent intensity of each marker and same number of donors was analyzed as in B. Error bars represent SD.
We next investigated monocyte subsets in allo-HSCT donor leukopheresis products and compared them to steady-state peripheral blood monocytes obtained from healthy donors. The proportion of CD14+CD16− and CD14+CD16+ monocytes in G-CSF-PBMC was similar in multiple donors and was also similar to the monocyte composition of peripheral blood from an untreated donor (Fig. 1B, compare untreated and leukopheresis product). As previously reported for classical CD14+CD16− monocytes in peripheral blood, the majority of CD14+CD16− monocytes in mobilized leukopheresis products expressed CD163 and CCR2 and the proportion of CCR2-expressing CD14+CD16− monocytes was similar in untreated and G-CSF-mobilized donors (Fig. 1C). These CD14+CD16− monocytes were also phenotypically immature and expressed low levels of HLA-DR, CD80, and CD86 (Fig. 1D and data not shown). In contrast, CD14+CD16+ cells expressed higher levels of costimulatory molecules and HLA-DR (Fig. 1D). Because surface expression of activation markers on monocytes isolated from G-CSF mobilized or untreated donors was similar, our findings suggest that G-CSF mobilization increases the frequency of circulating monocyte populations without inducing their activation.
CD34+ stem cell enrichment does not alter monocyte viability or phenotype
During stem cell purification, mobilized leukopheresis product is subjected to immunomagnetic separation of CD34+ cells and CD34− leukocytes are collected as a flow-through fraction, suggesting that the flow-through fraction must be an abundant source of both monocyte subsets. We next investigated the monocyte populations in the CD34− flow through fraction to determine whether this process affected monocyte survival or differentiation. A total of eight flow-through products and eleven unfractionated leukopheresis products were analyzed. Processing of 25 ml of flow through product yielded 2–5 × 109 total leukocytes after Ficoll separation. Immunomagnetic fractionation did not adversely affect the relative proportions of monocyte subsets (Fig. 1, B and C) suggesting that viability of these subsets was not adversely affected by purification steps. Thus, CD34+ stem cell enrichment did not lead to a specific loss of either monocyte population and allowed for isolation of large numbers of monocytes. Additionally, flow cytometric analysis indicated that CD34+ stem cell enrichment did not affect monocyte surface expression of CD163, CCR2, HLA-DR, CD80, CD86, or CD11c (Fig. 1, C and D and data not shown). Overall, our results demonstrate that monocytes in the flow-through fraction after positive CD34 collection from G-CSF primed leukopheresis product remain viable and do not undergo detectable differentiation. Because large numbers of monocytes can be obtained from this fraction, we used flow through monocytes for all subsequent functional studies.
Cytokine production by monocyte subsets in response to A. fumigatus conidia
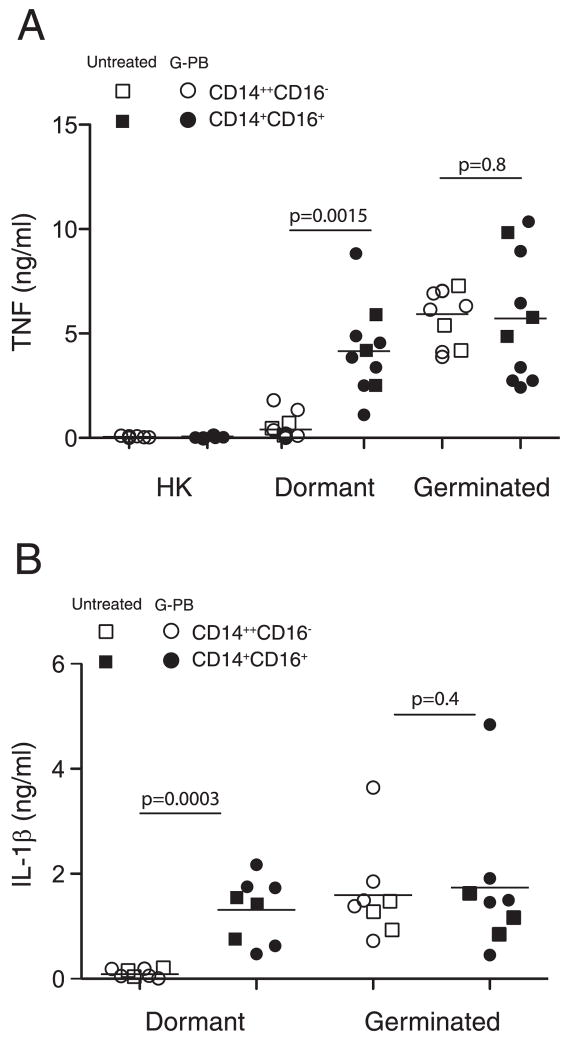
It is unknown whether CD14+CD16− and CD14+CD16+ monocytes differ in their response to A. fumigatus conidia. Murine macrophages respond robustly to germinated but not dormant A. fumigatus conidia (11), a difference that is in part attributable to increased β-glucan exposure during germination. Therefore, we measured TNF-α secretion after incubation of CD14+CD16− and CD14+CD16+ monocytes with live dormant conidia, heat killed dormant conidia, or conidia that had undergone germination for 6 h. Both monocyte subsets readily secreted TNF following stimulation with germinated conidia (Fig. 2A). Dormant conidia, however, stimulated TNF-α production by CD14+CD16+ but not CD14+CD16− monocytes (Fig. 2A). In contrast, heat killed dormant conidia did not induce TNF production by either monocyte subset, suggesting that conidial germination is required to trigger monocyte recognition. Monocyte populations from multiple donors were tested and the relative responses to killed, live and germinated conidia were similar although the amounts of secreted TNF-α varied between donors. This pattern of cytokine secretion was similar when cells were cultured in the presence of human serum (data not shown). We next extended our analysis and examined secretion of IL-1, IL-12, and IL-10 by infected monocytes. We observed a similar pattern of IL-1 secretion by monocytes in response to dormant and germinated conidia (Fig. 2B). Monocytes did not secrete IL-12 or IL-10 following stimulation with conidia (data not shown). To confirm that G-CSF mobilization did not adversely affect monocyte function, cytokine secretion by monocyte subsets purified from blood of untreated donors was also examined. Our data indicate that cytokine responses of CD14+CD16− and CD14+CD16+ monocytes derived from untreated PBMCs were similar to that of G-CSF mobilized cells (Fig. 2, A and B).
FIGURE 2.
Cytokine secretion by monocyte subsets in response to resting and germinated conidia. A, CD14+CD16− and CD14+CD16+ monocytes were purified from untreated donors or flow through fractions of G-CSF-mobilized donors (G-PB) and infected with heat-killed conidia, dormant conidia and conidia germinated for 6 h (germinated) at 1:1 conidia:cell ratio. Supernatants were collected 16 h later and TNF (A) and IL-1β (B) secretion was analyzed by ELISA. Each data point corresponds to a value obtained for an individual donor with three untreated and five to seven G-CSF treated donors analyzed. Shown are mean values. The unpaired Student t test was used to compare groups. Statistical analysis was performed with Prizm 5. A value of p = 0.05 was considered significant.
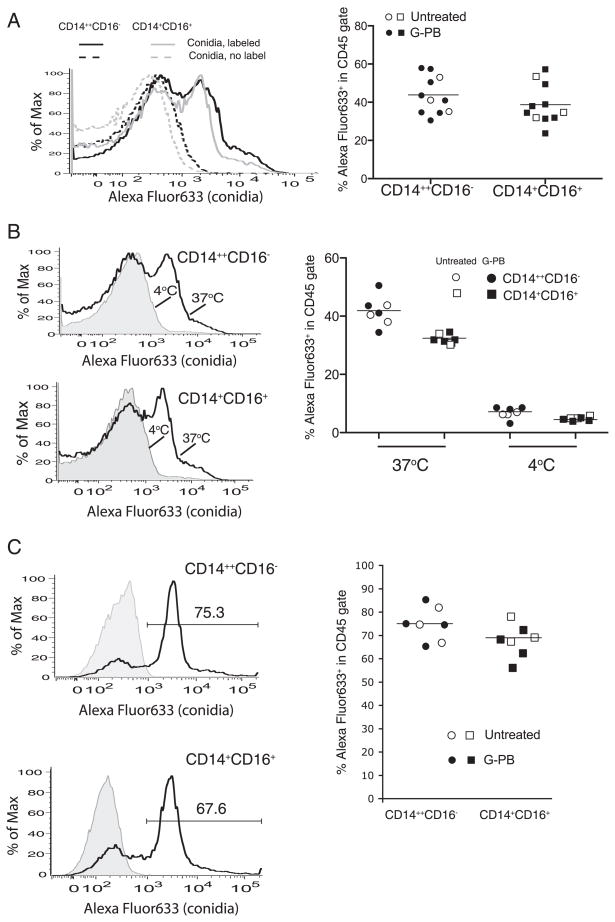
The distinct cytokine responses of CD14+CD16− and CD14+ CD16+ monocytes may be due to differences in conidial uptake. We next examined ability of monocyte subsets to phagocytose Alexa Fluor 633-labeled conidia. We found that resting conidia were phagocytosed with similar efficiency by CD14+CD16− and CD14+CD16+ monocytes (Fig. 3A). Conidia uptake by monocytes isolated from normal PBMC and G-CSF-mobilized peripheral blood was comparable (Fig. 3A), indicating that G-CSF moblization does not adversely affect the phagocytic function of monocytes. Acquisition of label by both monocyte subsets was dramatically reduced when cells were incubated at 4°C, confirming that conidia were phagocytosed (Fig. 3B). In addition, germinated conidia were phagocytosed by CD14+CD16− and CD14+CD16+ monocytes with equal efficiency and no differences were observed between uptake of germinated conidia by monocytes purified from untreated PBMCs and G-CSF-mobilized PBMCs (Fig. 3C). These data indicate that different cytokine responses of monocyte subsets cannot be attributed to differences in their rate of phagocytosis.
FIGURE 3.
Phagocytosis of resting and germinated conidia by monocyte subsets. CD14+CD16− and CD14+CD16+ monocytes were purified from PBMC of untreated donors (untreated) and flow through fractions of G-CSF-mobilized donors (G-PB) and infected with Alexa Fluor 633-labeled dormant (A and B) conidia and conidia germinated for 6 h (C) at 1:1 conidia:cell ratio at 37°C (A–C) or on ice (B). At the end of infection, cells were stained for CD45 and acquisition of Alexa Fluor 633 label in CD45 gate was analyzed by flow cytometry. Each data point corresponds to an individual donor. Infection with dormant conidia was analyzed in cells from 8 G-CSF-mobilized donors and three untreated donors and infection with germinated conidia was analyzed in cells from four G-CSF-mobilized donors and three untreated donors.
GM-CSF does not enhance TNF secretion by conidia stimulated CD14+CD16− monocytes
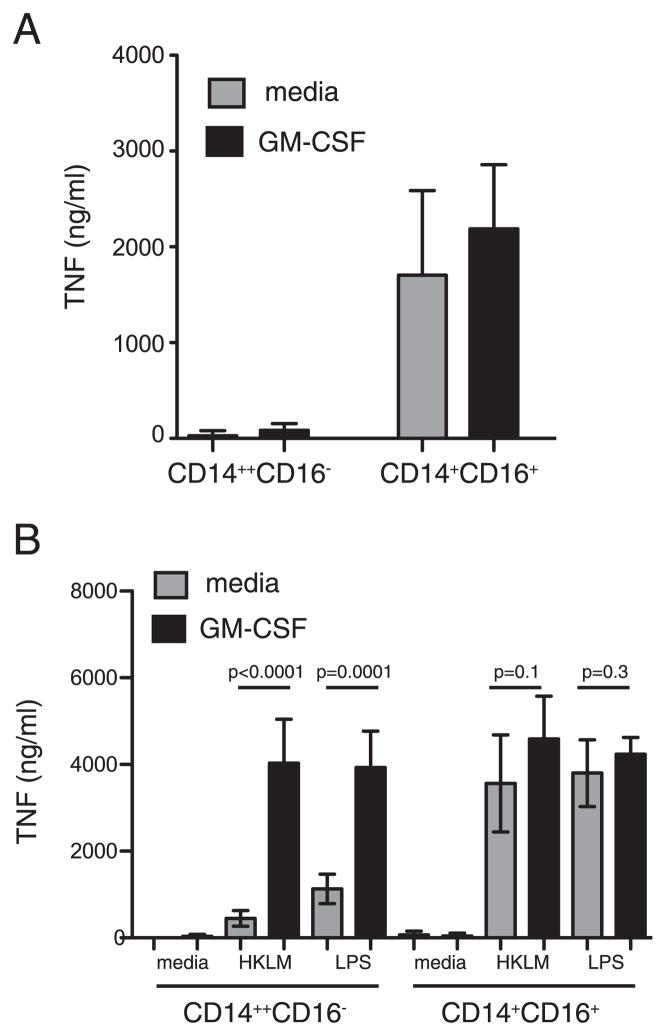
GM-CSF primes monocytes for enhanced TNF-α secretion in response to endotoxin (17, 18). Thus, it is possible that CD14+CD16− monocytes may require GM-CSF stimulation to respond to A. fumigatus conidia. To address this issue, CD14+CD16− and CD14+CD16+ monocytes were purified and cultured for 12 h in the presence of recombinant human GM-CSF, washed, and resuspended in GM-CSF-free medium before addition of dormant conidia. TNF-α secretion was assayed 12 h later. CD14+CD16+ monocytes readily secreted TNF-α in response to resting conidia, and TNF-α secretion was further augmented by GM-CSF (Fig. 4A). However, CD14+CD16− monocytes cultured in the presence of growth factor failed to secrete TNF-α following stimulation with conidia (Fig. 4A). In contrast, TNF-α secretion by CD14+CD16− monocytes in response to HKLM or LPS was significantly enhanced following GM-CSF culture and was comparable to TNF-α secretion by CD14+CD16+ monocytes (Fig. 4B). These results demonstrate that human monocyte subsets, regardless of GM-CSF stimulation, differ in their response to A. fumigatus conidia.
FIGURE 4.
Effect of GM-CSF priming on TNF secretion by monocytes following microbial stimulation. CD14+CD16− and CD14+CD16+ monocytes were purified from flow through fractions of 4–5 donors and cultured with GM-CSF or medium alone for 16 h. At the end of culture, GM-CSF was removed, fresh medium was added, and cells were infected with underminated conidia (A) or stimulated with HKLM or LPS (B). Supernatants were removed 16 h later, and TNF-α secretion was analyzed by ELISA. Shown are mean values (error bars, SD). The unpaired Student t test was used to compare groups. Statistical analysis was performed with Prizm 5. A value of p = 0.05 was considered significant.
Inhibition of hyphae formation by CD14+ monocytes
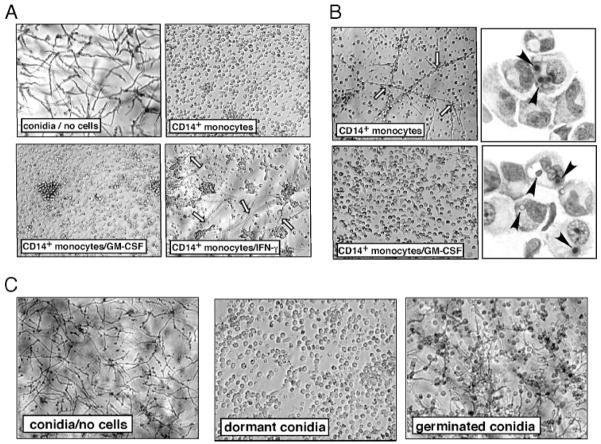
Macrophages are believed to protect against invasive aspergillosis by inhibiting germination of inhaled conidia (19, 20). To determine whether human monocytes inhibit conidial germination and the formation of hyphae, unfractionated human CD14+ monocytes from G-CSF-mobilized allo-HSCT donors (i.e., containing both CD14+CD16− and CD14+CD16+ subsets) were thawed and cultured overnight in medium alone or medium containing GM-CSF or IFN-γ. Live dormant conidia were then added to monocytes and fungal growth was monitored by microscopy for 24 to 48 h. After 24 h in the absence of monocytes, conidia germinated and formed extensive networks or mats of hyphae (Fig. 5A). In contrast, conidial germination and hyphal growth were markedly diminished in the presence of untreated or GM-CSF treated monocytes. Addition of cytochalasin D to inhibit conidial phagocytosis reversed the inhibitory effect of monocytes (data not shown), suggesting that internalization of conidia is required for monocyte-mediated inhibition of germination. Addition of IFN-γ partially suppressed the inhibition of germination by monocytes (Fig. 5A). Because IFN-γ and not GM-CSF pretreatment significantly enhanced killing of intracellular L. monocytogenes (data not shown), we conclude that distinct mechanisms are involved in anti-bacterial and anti-fungal monocyte defenses.
FIGURE 5.
Inhibition of conidia germination by CD14+monocytes. CD14+monocytes were purified from flow through fractions and cultured with GM-CSF, IFN-γ, or medium alone for 16 h. At the end of culture, fresh medium was added, and cells were infected with dormant conidia (A–C) or conidia allowed to germinate for 6 h (C) at 1:1 conidia:cell ratio. Infection was allowed to progress for 24 h (A and C) or 48 h (B) and the appearance of extracellular hyphae in the wells was monitored. B, After 48 h, cells primed with GM-CSF or medium alone were harvested, cytospins were prepared, and germination status of conidia was examined by light microscopy. Cells from each donor were plated in triplicate wells, and experiments were repeated for six additional donors. Shown are representative pictures from one well, with similar pictures obtained for each well of the triplicate. Results shown were obtained with monocytes cultured in the presence of FCS. Black arrowheads indicate conidia, white arrows indicate hyphae. Culture of cells in the presence of human serum yielded similar results.
In wells containing unfractionated, untreated monocytes, some hyphae formation was observed by 48 h postinfection (Fig. 5B, top left). Examination of monocyte cytospin preparations revealed that many monocytes contained swollen conidia (Fig. 5B, top right). Hyphal formation, however, was not observed in wells containing GM-CSF treated monocytes and most intracellular conidia had not germinated in these cells 48 h postinfection (Fig. 5B, bottom). These results indicate that GM-CSF treatment enhances the duration of monocyte-mediated inhibition of conidial germination, perhaps by enhancing monocyte survival under in vitro culture conditions.
We next tested whether unfractionated monocytes could inhibit formation of hyphae by germinated conidia. Conidia were allowed to germinate for 6 h before addition to CD14+ monocytes and overnight culture. In parallel, freshly harvested resting conidia were added to separate monocyte cultures. As shown in Fig. 5C, monocytes did not restrict hyphal formation by germinated conidia.
CD14+CD16+ monocytes have reduced ability to restrict germination of A. fumigatus conidia
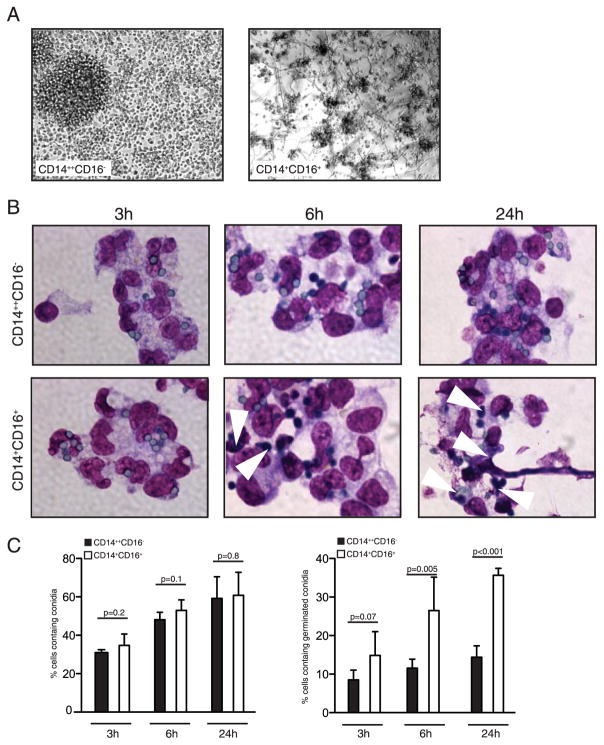
We next purified CD14+CD16− and CD14+CD16+ monocyte subsets and examined the ability of each subset to inhibit germination of conidia. Although CD14+CD16− monocytes from multiple donors inhibited conidial germination and formation of hyphae, we consistently observed reduced ability of CD14+CD16+ monocytes to suppress fungal growth (Fig. 6A). Priming of CD14+CD16+ monocytes with GM-CSF did not enhance fungal inhibition (data not shown). Survival of CD14+CD16+ monocytes after 24 h of culture was also significantly reduced compared with CD14+CD16− subset (Fig. 6A and data not shown).
FIGURE 6.
CD14+CD16− and not CD14+CD16+ monocytes suppress germination of conidia. Monocyte subsets were purified from flow through fractions and cultured with GM-CSF or medium alone for 16 h. At the end of culture, GM-CSF was removed and cells were infected with dormant conidia for various length of time. A, Appearance of hyphae was observed 24 h later in cultures of CD16+ monocytes but not CD14+CD16− monocytes. Shown are representative pictures from one well, with similar pictures obtained for each well of the triplicate. Experiment was repeated for seven donors. B, Monocytes were harvested 3, 6, and 24 h following infection, and cytospins were prepared. Budding cells are indicated by arrows. C, Numbers of cells containing conidia in cytospin preparations were counted. Each bar graph represents 400–500 counted cells of an individual donor. Total of five donors were analyzed. Shown are mean values (error bars, SD). The unpaired Student t test was used to compare groups. Statistical analysis was performed with Prizm 5. A value of p = 0.05 was considered significant.
The fate of conidia following phagocytosis by CD14+CD16− and CD14+CD16+ monocytes was evaluated next. Cytospins from monocytes cultured with conidia for 3, 6, and 24 h were examined using light microscopy and revealed that both monocyte subsets phagocytosed conidia at similar rates. We found that 50–60% of monocytes contained conidia after 6 h and numerous monocytes contained multiple conidia (Fig. 6, B and C). Swelling of intracellular conidia was detected in both monocyte subsets 6 h after uptake of dormant conidia (Fig. 6B). We next assessed the extent of conidia germination in both monocyte subsets. Conidia that reached the stage of visible polar protuberances, germ-tubes (protuberances of approximately the diameter of swollen conidia) and sporelings (germ tubes with length exceeding that of swollen conidia) were collectively counted as “germinated.” Germ-tube formation (germination) was restricted to CD14+CD16+ monocytes, however (Fig. 6C). By 24 h, CD14+CD16− monocytes contained few swollen conidia with rare germ tubes. In contrast, CD14+CD16+ monocytes contained swollen conidia with germ tube formation, and hyphal growth was readily apparent (Fig. 6, B and C). Overall, our results indicate that CD14+CD16+ monocytes have a significantly reduced ability to restrict conidial germination and hyphal formation as compared with CD14+CD16− monocytes. Replacement of FCS with human serum in the cell culture medium did not improve the ability of CD14+CD16+ monocytes to inhibit germination (data not shown).
Because monocytes were routinely frozen before in vitro studies, concern remained that failure of CD14+CD16+ monocytes to restrict conidia germination was due to damaging effects of the freeze/thaw process. To confirm that CD14+CD16+ monocytes are intrinsically unable to prevent germination process, we examined responses of monocyte subsets freshly purified from peripheral blood of healthy donors. Monocyte subsets were purified from mononuclear cell fractions immediately after Ficoll-Paque gradient and cultured in the presence of GM-CSF overnight. Next day, cells were infected with live dormant conidia and extent of phagocytosis and conidia germination were examined 6 and 24 h following infection. Our results demonstrate that conidia were taken up with equal efficiency by freshly harvested CD14+CD16− and CD14+CD16+ monocytes (Supplemental Fig. 1A).4 However, in contrast to CD14+CD16− cells, CD14+CD16+ monocytes had significantly reduced ability to restrict germination of conidia at both 6 and 24 h (Supplemental Fig. 1, B and C). In addition, infection with dormant conidia led to copious TNF-α production by freshly harvested CD14+CD16+ but not CD14+CD16− monocytes (supplemental Fig. 1D). Thus, CD14+CD16+ monocytes are intrinsically incapable of restricting germination of A. fumigatus conidia.
Monocytes inhibit germination without killing A. fumigatus conidia
Restriction of germination by human monocytes may result from conidial killing after phagocytosis. Alternatively, monocytes may inhibit spore maturation without exerting fungicidal effect. To investigate this further, unfractionated CD14+ monocytes were purified and cultured overnight in medium alone or in the presence of GM-CSF and then infected with resting conidia. Cells were lysed after 1, 24, and 48 h postinfection and numbers of live conidia in the wells were quantified. Although unfractionated monocytes inhibited formation of hyphae in the cultures (data not shown), killing of conidia was not observed at time points examined (Fig. 7A). Additionally, pretreatment with GM-CSF did not enhance monocyte-mediated fungal killing (Fig. 7A).
FIGURE 7.
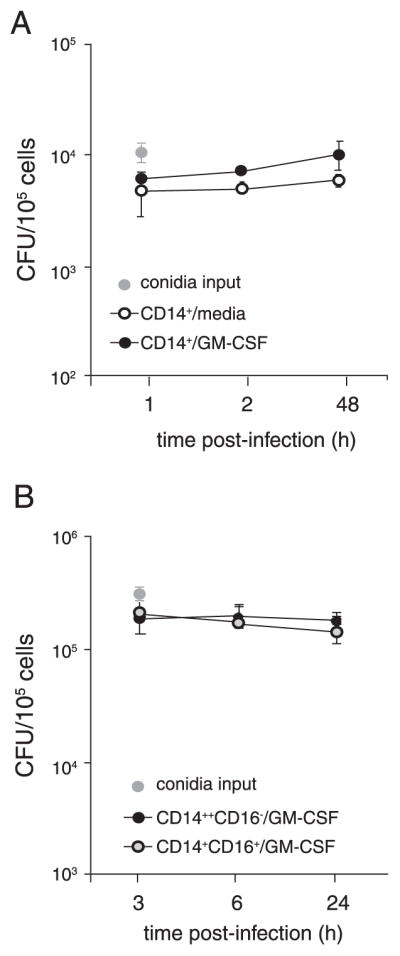
Monocytes do not kill intracellular conidia. CD14+ monocytes (A) or CD14+CD16− and CD14+CD16+ subsets (B) were purified from flow through fractions and cultured with GM-CSF or medium alone for 16 h. Cells were infected with dormant conidia at 1:1 conidia:cell ratio. At the indicated times following infection, medium was removed, cells were lysed, and lysates were plated for estimation of fungal colony forming units. Experiments were repeated for five donors and similar results were obtained. Each bar represents values in triplicate wells (error bars, SD).
We next examined ability of purified monocyte subsets to kill conidia. We were specifically interested in determining whether TNF secretion by CD14+CD16+ monocytes in response to germinated conidia enhances their fungicidal capacity. Because unrestricted germination of conidia following uptake by CD14+CD16+ monocytes in vitro led to reduced cell survival, the antifungal agent voriconazole was added to the culture medium. Plating of conidia at 3 and 6 h postinfection confirmed our finding that both monocyte subsets phagocytose conidia at similar rates. In the presence of voriconazole, CD14+CD16− and CD14+CD16+ monocytes had similar survival rates after 24 h of culture; with recovery of ~75% live cells compared with input number. As shown in Fig. 7B, neither monocyte subset killed phagocytosed conidia. Although CD14+ monocytes cultured in the presence of FCS restricted germination of conidia to the same degree as monocytes cultured with human serum, we asked whether components of human serum are required to induce fungicidal mechanisms. We observed that addition of human serum to the culture medium did not induce killing of conidia by monocytes (data not shown).
Discussion
In the present report, responses of CD14+CD16− and CD14+ CD16+ human monocyte subsets to stimulation with A. fumigatus conidia were directly compared. Our results suggest that monocyte subsets have nonredundant functions during aspergillosis. CD14+ CD16+ monocytes produce cytokines in response to conidia but do not prevent germination or the generation of fungal hyphae, while CD14+CD16− monocytes efficiently prevent conidial germination. Our results show that neither subset can kill conidia, which contrasts with previously published studies showing that unfractionated circulating leukocytes are fungicidal. To our knowledge, we provide the first comparison of antimicrobial responses of CD14+CD16− and CD14+CD16+ monocytes and our results suggest that these subsets may play distinct roles.
Migration of murine monocytes to the sites of inflammation is required for protection against a number of microbial pathogens (8). Studies in mouse models of infection have demonstrated that CCR2-expressing monocytes are recruited to the tissues during innate immune responses, where they produce inflammatory cytokines and directly kill microbes (8). The roles of human monocyte subsets during antimicrobial responses are not well characterized. Antimicrobial activity of human monocytes against intracellular bacteria has been demonstrated in vitro (21, 22), but distinct monocyte subsets have not been evaluated. Thus, it remains to be established whether CCR2+CD14+CD16− monocytes function similarly to their murine counterparts, and whether human CD14+ CD16− and CD14+CD16+ subsets have distinct roles during microbial infections. Our study demonstrates that CD14+CD16− and CD14+CD16+ cells respond differently to an important fungal pathogen of immunocompromised patients. Although both subsets phagocytose conidia efficiently, only CD14+CD16− monocytes inhibit germination of resting conidia. These results are surprising because CD14+CD16+ cells are thought to be more mature and share features with tissue macrophages (9) and, thus, might be expected to have superior antimicrobial functions.
Our results indicate that different mechanisms are involved in monocyte-mediated inhibition of conidia germination and killing of intracellular bacteria. Although priming with IFN-γ rendered monocytes highly bactericidal, it reduced the ability of CD14+CD16− monocytes to inhibit germination of conidia. In contrast, brief culture with GM-CSF was required to optimally inhibit hyphae formation. Mice deficient in GM-CSF develop features of pulmonary alveolar proteinosis (PAP) and have heightened susceptibility to fungal and bacterial pathogens (23). PAP in humans has been reported and is characterized by high levels of anti-GM-CSF autoantibodies (24). Patients with PAP are at high risk of developing secondary opportunistic infections, including disseminated aspergillosis (25). Pathways involved in GM-CSF-mediated enhancement of anti-fungal functions of CD14+CD16− monocytes are not known. GM-CSF enhances cytokine production by and microbicidal functions of monocytes and macrophages (26, 27). Stimulation of human monocytes with GM-CSF enhances respiratory burst activity (28, 29), and production of reactive oxygen species may at least partially mediate antifungal activity. Our results suggest that GM-CSF therapy may benefit patients with invasive fungal infections. It is of interest that GM-CSF stimulation did not enhance the ability of CD14+CD16+ monocytes to inhibit germination. One possible explanation is that monocyte subsets have different potential to express anti-microbial compounds. Alternatively, conidia may be allocated to distinct compartments in different monocyte subsets following phagocytosis and may not be accessible to antifungal effectors in CD14+CD16+ monocytes.
Monocyte-mediated killing of conidia has been previously reported (30). Our results differ from that work in that monocytes had fungistatic and not fungicidal effect. This discrepancy might be explained by the fact that only brief monocyte culture was used in our experiments, and monocytes retained an undifferentiated immature phenotype at the time of infection with conidia. In contrast, monocytes were cultured for several days in the study by Roilides et al. (30) and, thus, may have differentiated. Our results suggest that, despite monocyte ability to inhibit germination of conidia and formation of hyphae, immune components other than monocytes are required for complete eradication of A. fumigatus conidia.
The function of CD16-expressing monocytes during microbial infections is not known. The frequency of CD14+CD16+ cells in the peripheral circulation is elevated in patients with sepsis, patients on hemodialysis with acute and chronic infections, and patients with cancer (31–36), but the functional consequences of these increases remain unclear. CD16+ cells secrete higher levels of TNF-α in response to TLR ligands (9), raising the possibility that these cells contribute to the development of inflammation and pathology in response to infection. However, it appears that cytokine responses to fungal pathogens differ from those induced by bacterial ligands. Although we found that CD14+CD16+ monocytes secrete TNF after stimulation with resting conidia, both monocyte subsets secrete TNF-α following exposure to germinated conidia. TNF-α production by CD14+CD16+ cells responding to dormant conidia most likely results from conidia germination during the course of incubation. Because multiple purification steps were conducted to achieve separation of CD14+CD16− and CD14+CD16+ monocytes, it is conceivable that purification may alter the functional responses of these cells. Belge et al. (9) examined cytokine secretion by individual monocyte subsets in unfractionated PBMCs using intracellular cytokine staining and found that ~3-fold more TNF was produced by CD14+CD16+ cells. Because our observations are similar to the results reported by Belge et al., we believe that TLR responsiveness and cytokine production may not have been significantly altered by immunomagnetic fractionation. We additionally observed that in vitro survival of CD14+CD16+ cells was significantly diminished 24 h following infection. A. fumigatus can produce number of mycotoxins, including abundant gliotoxin that has been shown to induce apoptosis of monocytes (37). A plausible explanation for the reduced survival of these cells is the production of gliotoxin by germinated conidia.
CD14+CD16+ monocytes become dendritic cells in an endothelial trafficking model (38) and develop into CD1b+ dendritic cells following TLR2 ligation (39), suggesting that they may contribute to Ag transfer to lymph nodes and initiation of T cell priming. Recently, CD14+CD16+ cells have been shown to preferentially harbor HIV-1 in vivo and were proposed to serve as a source of viral persistence during highly active antiretroviral therapy (40). Because CD14+CD16+ monocytes readily ingest A. fumigatus conidia, it would be of interest to determine whether these cells may contribute to dissemination of conidia to extra-pulmonary sites during infection. Indeed, studies in a murine model of aspergillosis showed that alveolar dendritic cells transport conidia to mediastinal lymph nodes during infection (41).
In patients with hematologic malignancies and those undergoing stem cell transplantation, the chemo- and radiotherapy treatments lead to a prolonged state of immunocompromise and heightened susceptibility to viral, bacterial, and fungal infections (42). Susceptibility to infection stems from neutropenia and monocytopenia induced by myeloablative treatments. Fungal infections following neutropenia can be life-threatening even when antifungal therapy is administered (10), and restoration of immune function is required to protect patients against infectious diseases. Transfusion of white blood cells has been shown to be protective against neutropenia-related infections (43). This approach has a number of limitations, however, possibly reflecting the inadequate numbers and short half-life of mature immune effector cells in each transfusion. Transfer of purified mature neutrophils has been tested as an alternative treatment but the efficacy of this approach is controversial and has a variable degree of success for the same reasons (14). Another explanation for the inconsistent results obtained with neutrophil transfers is that circulating monocytes contribute to defense against fungal infection. Indeed, several studies suggested that monocytopenia is an independent risk factor for infection-related mortality (44, 45). Circulating monocytes, in contrast to neutrophils, are relatively long-lived and can be maintained and further differentiated in vitro. We found that large numbers of monocytes can be collected from the ISOLEX flow through product after enrichment of CD34+ stem cells. In contrast to neutrophils, CD14+ cells are viable following freezing and thawing and had unimpaired functional responses. Although, neither monocyte subset directly kills A. fumigatus conidia, CD14+CD16− monocytes efficiently halt germination of conidia without inducing overt inflammatory responses. Given the abundance of CD14+CD16− monocytes in leukopheresis products following G-CSF stem cell mobilization, and their ability to inhibit conidial germination, adoptive transfer of monocytes in the settings of neutropenia and monocytopenia such as leukemia and allo HSCT may provide a therapeutic option against fungal infection.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health (K12 CA120121 to N.V.S.; AI67359 and AI39031 to E.G.P., M.C., and C.S.; CA23766 to E.G.P. and J.W.Y.).
Abbreviations used in this paper: allo-HSCT, allogeneic hematopoietic stem cell transplantation; HKLM, heat killed Listeria monocytogenes; PAP, pulmonary alveolar proteinosis.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukocyte Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 2.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukocyte Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 3.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 4.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte sub-populations. J Leukocyte Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 5.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 6.Ziegler-Heitbrock HW, Passlick B, Flieger D. The monoclonal antimonocyte antibody My4 stains B lymphocytes and two distinct monocyte subsets in human peripheral blood. Hybridoma. 1988;7:521–527. doi: 10.1089/hyb.1988.7.521. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–2058. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 8.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR+ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM. Fungal infections in bone marrow transplant patients. Curr Opin Infect Dis. 2004;17:347–352. doi: 10.1097/01.qco.0000136935.13662.af. [DOI] [PubMed] [Google Scholar]
- 11.Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryote Cell. 2007;6:1953–1963. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manavathu EK, Cutright J, Chandrasekar PH. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J Clin Microbiol. 1999;37:858–861. doi: 10.1128/jcm.37.3.858-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atallah E, Schiffer CA. Granulocyte transfusion. Curr Opin Hematol. 2006;13:45–49. doi: 10.1097/01.moh.0000190114.38650.b2. [DOI] [PubMed] [Google Scholar]
- 15.BitMansour A, Burns SM, Traver D, Akashi K, Contag CH, Weissman IL, Brown JM. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood. 2002;100:4660–4667. doi: 10.1182/blood-2002-05-1552. [DOI] [PubMed] [Google Scholar]
- 16.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human β-glucan receptor is widely expressed and functionally equivalent to murine dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MP, Zoon KC. Priming of human monocytes for enhanced lipopolysaccharide responses: expression of α interferon, interferon regulatory factors, and tumor necrosis factor. Infect Immun. 1993;61:3222–3227. doi: 10.1128/iai.61.8.3222-3227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisson SD, Dinarello CA. Production of interleukin-1 α, interleukin-1 β and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988;72:1368–1374. [PubMed] [Google Scholar]
- 19.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus: observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffner A, Douglas H, Braude AI, Davis CE. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983;42:1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leijh PC, van den Barselaar MT, van Furth R. Kinetics of phagocytosis and intracellular killing of Staphylococcus aureus and Escherichia coli by human monocytes. Scand J Immunol. 1981;13:159–174. doi: 10.1111/j.1365-3083.1981.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 22.Weemaes C, Leijh P, Blusse van Oud Alblas D, van der Meer J, van Furth R. Normal microbicidal function of monocytes in a girl with chronic granulomatous disease. Acta Paediatr Scand. 1981;70:421–425. doi: 10.1111/j.1651-2227.1981.tb16578.x. [DOI] [PubMed] [Google Scholar]
- 23.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–579. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 25.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 26.Jones TC. The effect of granulocyte-macrophage colony stimulating factor (rGM-CSF) on macrophage function in microbial disease. Med Oncol. 1996;13:141–147. doi: 10.1007/BF02990842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruef C, Coleman DL. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990;12:41–62. doi: 10.1093/clinids/12.1.41. [DOI] [PubMed] [Google Scholar]
- 28.Reed A, Herndon JB, Ersoz N, Fujikawa T, Schain D, Lipori P, Hemming A, Li Q, Shenkman E, Vogel B. Effect of prophylaxis on fungal infection and costs for high-risk liver transplant recipients. Liver Transpl. 2007;13:1743–1750. doi: 10.1002/lt.21331. [DOI] [PubMed] [Google Scholar]
- 29.Weisbart RH, Kwan L, Golde DW, Gasson JC. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987;69:18–21. [PubMed] [Google Scholar]
- 30.Roilides E, Dimitriadou A, Kadiltsoglou I, Sein T, Karpouzas J, Pizzo PA, Walsh TJ. IL-10 exerts suppressive and enhancing effects on anti-fungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–329. [PubMed] [Google Scholar]
- 31.Blumenstein M, Boekstegers P, Fraunberger P, Andreesen R, Ziegler-Heitbrock HW, Fingerle-Rowson G. Cytokine production precedes the expansion of CD14+CD16+ monocytes in human sepsis: a case report of a patient with self-induced septicemia. Shock. 1997;8:73–75. doi: 10.1097/00024382-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. [PubMed] [Google Scholar]
- 33.Horelt A, Belge KU, Steppich B, Prinz J, Ziegler-Heitbrock L. The CD14+CD16+ monocytes in erysipelas are expanded and show reduced cytokine production. Eur J Immunol. 2002;32:1319–1327. doi: 10.1002/1521-4141(200205)32:5<1319::AID-IMMU1319>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–2790. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleh MN, Goldman SJ, LoBuglio AF, Beall AC, Sabio H, McCord MC, Minasian L, Alpaugh RK, Weiner LM, Munn DH. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85:2910–2917. [PubMed] [Google Scholar]
- 36.Skrzeczynska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55:629–638. doi: 10.1046/j.1365-3083.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 37.Stanzani M, Orciuolo E, Lewis R, Kontoyiannis DP, Martins SL, St John LS, Komanduri KV. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood. 2005;105:2258–2265. doi: 10.1182/blood-2004-09-3421. [DOI] [PubMed] [Google Scholar]
- 38.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16+ (FcγRIII+) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 41.Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco P, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–1371. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- 42.Klastersky J, Aoun M. Opportunistic infections in patients with cancer. Ann Oncol. 2004;15(Suppl 4):iv329–335. doi: 10.1093/annonc/mdh947. [DOI] [PubMed] [Google Scholar]
- 43.Dignani MC, Anaissie EJ, Hester JP, O’Brien S, Vartivarian SE, Rex JH, Kantarjian H, Jendiroba DB, Lichtiger B, Andersson BS, Freireich EJ. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia. 1997;11:1621–1630. doi: 10.1038/sj.leu.2400811. [DOI] [PubMed] [Google Scholar]
- 44.Cordonnier C, Ribaud P, Herbrecht R, Milpied N, Valteau-Couanet D, Morgan C, Wade A. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006;42:955–963. doi: 10.1086/500934. [DOI] [PubMed] [Google Scholar]
- 45.Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, Blazquez C, Fernandez-Aviles F, Carreras E, Salavert M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.