Abstract
The contribution of endothelin-1 (ET-1), acting via endothelin-A receptors (ETA), on post-incisional pain was examined in a rat model of incision through the hairy skin of the lumbar dorsum. Post-incisional mechanical hyperesthesia was evaluated by cutaneous trunci muscle reflexes (CTMR) of subcutaneous muscles responding to stimulation with von Frey filaments near the wound (primary responses) and at a distance, especially on the contralateral dorsum (secondary responses, involving spinal circuits). The role of ETA was determined by pre-incisional, subcutaneous injection of the selective receptor antagonist BQ-123 at the incision site, 15 min or 24 hr before surgery. Control incisions showed both primary tactile allodynia and hyperalgesia, and a weaker secondary hyperesthesia, peaking 3-4 h after surgery and lasting at least 24h. Primary allodynia, but not hyperalgesia, was dose-dependently suppressed by 15 min pre-incisional BQ-123. In contrast, both secondary allodynia and hyperalgesia were inhibited by local BQ-123. The suppression of primary allodynia by local antagonist disappeared in 24 h, but that of secondary hyperesthesia remained strong for at least 24 h. Systemically delivered BQ-123 was without effect on any post-incisional hyperesthesia, and if the antagonist was locally injected 24 h before surgery there was no difference on hyperesthesia compared to vehicle injected at that time. We conclude that ET-1, released from skin by incision, activates nociceptors to cause primary allodynia and to sensitize spinal circuits through central sensitization. Blockade of ETA in the immediate peri-operative period prevents the later development of central sensitization.
Keywords: ET-1, BQ-123, ETA, tactile allodynia, hyperalgesia, nociception, incision
1. Introduction
Endothelin-1 (ET-1) is an endogenous peptide of 21 amino acids whose effects are mediated by two G-protein-coupled receptors, ETA and ETB (DeNucci et al., 1988; Lipa et al., 1999; Luscher and Barton, 2000). ET-1, ETA and ETB receptors (ETAR and ETBR, respectively) all occur in the epidermis, dermis and subcutaneous vasculature (Ahn et al., 1998). Subcutaneous injection of ET-1 causes pain via ETAR and induces spontaneous impulses in nociceptive cutaneous C- and Aδ-fibers (Gokin and Strichartz, 2001). ET-1 injections also cause abdominal constrictions in mice and incapacitation of movement of the dog’s knee joint (Ferreira et al., 1989; Raffa and Jacoby, 1991). Furthermore, overproduction of ET-1 is a characteristic of injured skin (Imokawa et al., 1992; Kadono et al., 2001), peaking 1-2 days after U.V. damage and rising after local administration of TNF-α or IL-1α (Ahn et al., 1998; Imokawa et al., 1992; Steinhoff et al., 2003).
Both C- and Aδ-fibers nociceptors amplify inflammation by releasing neuropeptides to target cells, while other cells involved directly in inflammatory processes release cytokines and other mediators (Pastore et al., 2005; Steinhoff et al., 2003) which themselves often have receptors on nociceptive neurons. Together these complex, inter-related processes constitute multiple positive feedback pathways for the elevation of cytokines and of ET-1 at sites of inflammation, setting the stage for a robust and rapid response when local injury occurs. The painful culmination of these changes is a robust activation of primary afferent nociceptors in the skin (Steinhoff et al., 2003) that far outlasts the incision-induced injury discharge, and probably spreads well beyond the region of cutaneous injury, and most probably effects central sensitization in spinal cord.
Incision of the skin is very likely to activate these injury-induced pathways too. Not only is there an obvious local, transient inflammation response at the wound, but there is generally secondary hyperalgesia in the surrounding area. The complex reactions to incision are likely to involve responses to local injury, including those that trigger wound healing, recruit immune cells, release cytokines and other cellular activators, and also trigger changes in the expression of genes in the sensory neurons (DRG) and activate second order sensory neurons and various types of glia in the spinal cord (Ji and Strichartz, 2004; Raghavendra et al., 2004; Tanga et al., 2004; Wieseler-Frank et al., 2005).
The skin injury used in the present paper is localized unilaterally on the thoraco-lumbar region of rats and tactile stimuli are applied next to the incision to assess “primary hyperesthesia”, and at a distance of several centimeters, contralaterally, to assess “secondary hyperesthesia”. The elevated local contractions of subcutaneous muscles at the site of stimulation that are used as nocifensive responses to touch are attenuated by systemic morphine, establishing their authenticity as indicators of pain.
Endogenous ET-1’s involvement in post-incisional pain is here investigated through the effects of an ETA receptor-selective antagonist, BQ-123, with a particular interest in the separation of primary and secondary tactile hyperesthesia following incision, and in the role of local versus systemic drug action.
2. Methods
2.1. Experimental Animals
All the procedures were approved by the Standing Committee on Animals of Harvard Medical School. Animals were treated in accordance with the guidelines for use of experimental animals of the International Association for the Study of Pain. The experiments were performed on adult male Sprague-Dawley rats, 250-300g (Charles River Laboratories, Cambridge, MA), which were kept in pairs inside a cage with soft bedding, on a 12 hour light-dark cycle with food and water provided ad libitum.
2.2. Handling of rats
All experiments were conducted at the room temperature of 23-25 °C. The rats were handled for a week before beginning experiments. Handling minimizes the stress, anxiety and agitation of naïve rats, by familiarization with the experimenter and the testing environment, and extinguishes the responses to innocuous tactile stimulation. The baseline behavioral responses were collected once a day during five to six days before the treatment day. Persistently restless rats were excluded from the study, as observation of responses to tactile stimulation necessitated at least periodical stillness.
2.3. Graded Response Assessment
Rats were clipped on the dorsum the day before the procedures in order to clearly observe the cutaneous truncii muscle reflex (CTMR) of the hairy skin in response to tactile stimulation. The CTMR is a response to tactile stimulation involving the local contraction of skeletal muscle beneath the skin with concurrent movement of the skin over the rat dorsum (Theriault and Diamond, 1988). The rats were probed with Von Frey hairs (VFHs) at distances of 0.5, 1 and 2cm ipsilateral and 2cm contralateral from the incision site. Each VFH was perpendicularly applied on the skin and lifted as soon as the nylon mono-filament bent. The stimulus duration and the interstimulus interval averaged 2 seconds for each of 4 applications of a single VFH. Von Frey hairs with forces ranging from 4-82mN were used to test for mechano-sensitivity. One baseline response “profile” per day was collected for five to six days preceding the treatment. Daily baseline responses were initially higher and progressively dropped to a steady level with handling. Each rat was poked 4 times with each force sequentially, at four locations: 0.5, 1 and 2cm ipsilateral and 2cm contralateral. Each distance defined hyperesthesia in relation to the incision site; CTMR at 0.5 and 1 cm corresponded to primary hyperesthesia whereas 2cm ipsilateral and 2cm contralateral corresponded to secondary hyperesthesia. Then, hyperesthesia was further defined as allodynia (responses after incision to forces that were ineffective in intact skin) or hyperalgesia (responses to forces that were present in intact skin but elevated after incision). The experimenter observed strong (larger area, short latency for response), weak (smaller area, longer latency and less forceful response) or no detectable CTMR to assign values of 1, 0.5 and 0, respectively. For each force, at each distance, the four CTMR values were summed.
2.4. Data Normalization and Analysis
To normalize the pre- and post-incisional graded responses, we divided the summed CTMR values by 4.0. For each force, at the specified distances, the normalized scores of all rats were averaged at each time point, beginning with the pre-treatment baseline taken over 5-6 days before incision (intact skin), and at 0.5, 1, 1.5, 2, 3, 4, 8 and 24 hours after treatment (Duarte et al., 2005). Post-incisional and dose-dependent BQ-123 effects were statistically evaluated with the Mann-Whitney U-Test (Statview, Inc., Cary, N.C.). A linear fit test for dose-dependence was used to justify the Bonferroni correction for Mann-Whitney comparison among groups. Areas-under-the-curve (AUC) were measured as deviations in graded response from the pre-incisional baseline response, integrated over 24h after surgery, and also were tested by Mann-Whitney, with correction for multiple groups.
2.5. Force versus Response Analysis
The increased sensitivity of skin to tactile stimulation by VFHs after surgery can be characterized by a leftward shift in the force vs response data; after incision weaker forces cause the same grade of response as stronger forces do before incision. By fitting the data of force vs response over a range of applied VFHs it is possible to interpolate a mid-point, the “ F0.5” value, equal to the force that would produce 0.5 of a full graded response (score = 1.0), which thus serves as a measure of cutaneous tactile sensitivity. In this paper we have fit force vs response curves to obtain F0.5 values and then compared these in intact skin to those at the time of peak response and at 24h after an incision that was preceded by local injection of ET-1 or of vehicle. Numerical values for F0.5 were determined from force-response curves by interpolation between the two response values just above and just below the mid-point, to find the force value on this line that intersected the horizontal line for response = 0.5. Ranges for these F0.5 values were determined by the same process, but using the response = 0.5 intercept for the lines connecting the two mean + SE values and the two mean — SE values above and below the response = 0.5 line (see Figure 3 for details).
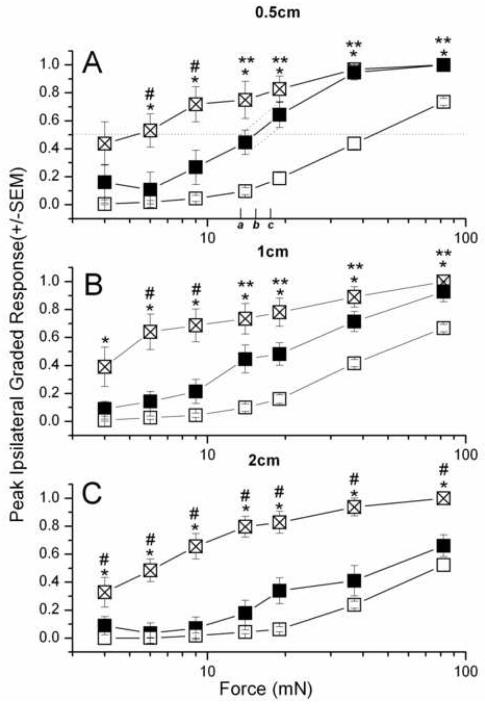
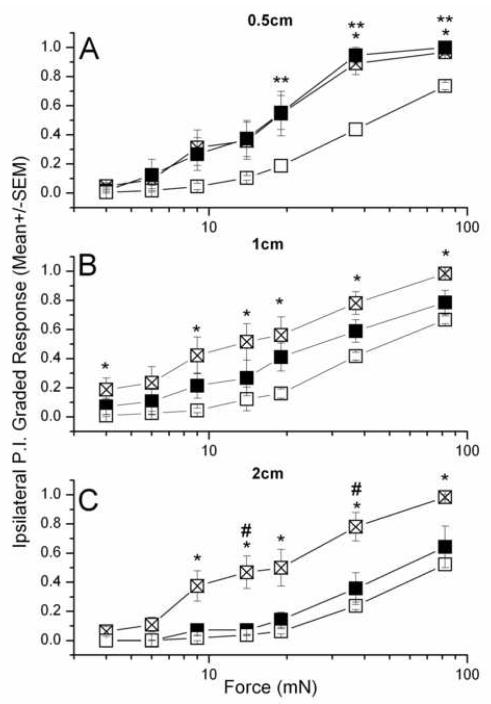
Figure 3. Forces vs peak responses for primary (A, B) and secondary (C) hyperesthesia in rats injected with 3mM BQ-123 (■, n=7) or vehicle (⊠, n=8) against intact skin (□, n=15).
Mann Whitney U-test: (BQ123 vs vehicle) - #, (Intact vs vehicle) - *, (Intact vs BQ-123) - **, P < 0.035. F0.5 values, listed in Table 1, were derived from interpolating between data points above and below the horizontal line for response =0.5, as shown in panel A. The solid line intersects at force b, the interpolated F0.5; and the range is set by the intersection of the dashed line connecting the two bounding mean + SE values (force a, lower range of F0.5) and the dashed line connecting the two bounding mean – SE values (force c, upper range of F0.5).
(The maximum neurobehavioral response is limited to the most robust CTMR (set equal to 1.0), which puts a ceiling on hyperalgesia but allows for a larger dynamic range over which allodynic responses can increase; this difference in the measurable increase of responses might account for the appearance of a leftward shift in nocifensive motor response, e.g. Figure 3, when a direct measure of the cutaneous sensitivity, such as afferent firing, to pain might have no such limit but be increased by the same factor for all forces. In this paper, while keeping this qualification in mind, we will treat the changes in CTMR empirically, without attempts at correction for limits of measure, since we have no data that would allow such a correction.)
2.6. Drugs and Surgery
The ETA receptor antagonist, BQ-123 was purchased from American Peptide (Sunnyvale, CA). BQ-123 was diluted in phosphate buffered saline (PBS, pH=6.8) to match the conditions we had described in an earlier description of this incision model (Duarte et al., 2005). To minimize local skin irritations by the time of surgery, all rats were clipped on the day preceding the treatment. Just prior to injection, the skin was swiped with alcohol. All injections were administered to rats, anesthetized by Sevoflurane (Abbot Laboratories, North Chicago, IL). Fifteen minutes prior to incision, all rats were injected subcutaneously with 0.4 mL of either BQ-123 (0.3 and 3 mM), or vehicle (PBS). The raised region resulting from the injection, of diameter about 1.2 cm, was circled with an indelible marker and circles of radii 1 and 2 cm were drawn around the injection site before returning the animals to their cage for recovery. Fifteen minutes later, the rats were re-anesthetized with Sevoflurane to receive an incision in the center of the wheal. At 1 cm from the spine, the rat’s right dorsum received a 1 cm-long lateral (para-spinal) incision with a no.10 surgical blade (Harvard Apparatus, Holliston, MA). The incision was closed within 1-2 minutes with a non-absorbable, 3-0 silk thread using a half-circled 18 mm needle (Harvard Apparatus, Holliston, MA). To guard against infections, all sutured incision sites were smeared with Povidone Iodine (The Clinipad Corporation, Rocky Hill, CT).
The following drug regimens were used for the designated procedures:
2.6.1. Local action of BQ-123 in intact skin
The rats were subcutaneously injected with 0.4 mL of 3mM BQ-123, on the dorsum at the location where the incision would have been made in the incision procedure.
2.6.2. Effects of local BQ-123 injection on post-incisional hyperesthesia
Rats were subcutaneously injected 15 minutes pre-operatively at the incision site with 0.4mL of 0.3 or 3 mM BQ-123, or with PBS vehicle, and then tested with VFH at 0.5, 1, 1.5, 2, 3, 4, 8 and 24 hours, and occasionally at 2-7 days, after surgery.
2.6.3. Effects of systemic BQ-123 on post-incisional hyperesthesia
Rats were subcutaneously injected 15 minutes pre-operatively with 0.4mL of either 0.3 or 3 mM BQ-123 at the nuchal midline, approximately 8 cm from the incision site, and tested as in Procedure 2, above.
3. Results
3.1. Tactile sensitivity and post-incisional hyperesthesia of hairy skin vary among different anatomical locations
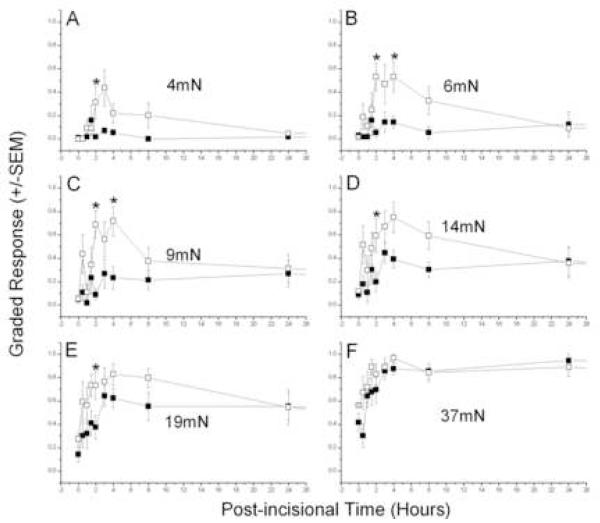
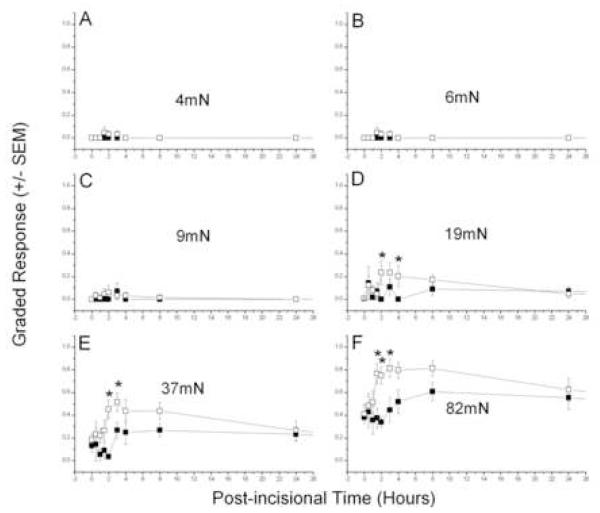
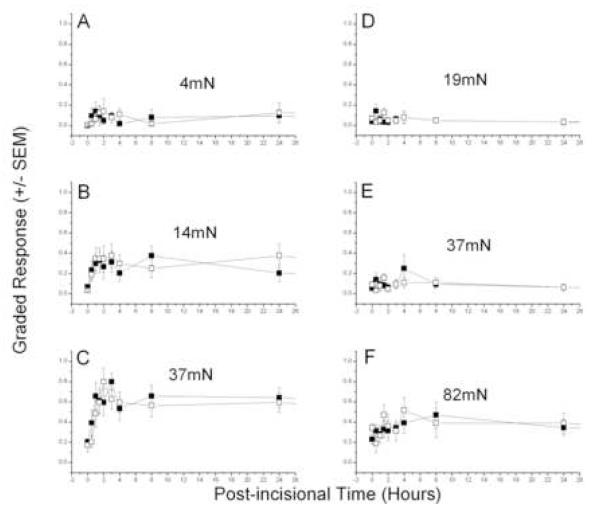
Incisions on the back increased the local responses to tactile punctate stimulation with von Frey filaments, as reported previously (Duarte et al., 2005). Primary and secondary hyperesthesia were apparent at the first testing, 0.5 hour after incision, (Figs. 1 and 2). Primary allodynia (Fig. 1A-C), elicited by relatively weak forces applied next to the incision, peaked between 2 and 4 hours and were returning to baseline levels by 24 hours, while primary hyperalgesia (Fig. 1D-F), tested by stronger forces, also peaked at about 4 hours but declined much more slowly. Post-incisional responses were largest next to the midline, nearer the incision, and fell off with distance from this location. Responses on the contralateral side indicated a smaller, transient, secondary allodynia, with an apparently longer latency for appearance (Fig. 2A-D), but the secondary hyperalgesia, which developed sooner, also lasted longer, like that for primary hyperalgesia (Fig. 2 E-F). Although the absolute magnitude of peak post-incisional responses were smaller on the contralateral side (Fig. 2) compared to the ipsilateral side (Fig. 1), the baseline sensitivity is also lower at these more lateral locations used to test for secondary hyperesthesia. This fact emphasizes the importance of the test location in hairy skin for quantitating post-incisional pain, and is dealt with directly in the next section. These results of incision are similar to those reported previously, except for the shorter duration of allodynia in the current paper, a difference that is caused by a change in the testing procedure (see Discussion).
Figure 1. Selective suppression of primary post-incisional allodynia by ETA receptor blockade.
Testing at 0.5cm ipsilateral to a 1cm-long incision of rats injected with pre-incisional 3mM BQ-123 (■, n=7) or vehicle (□, n=8). Mann Whitney U-test (Vehicle vs 3mM BQ-123) - *, P < 0.05.
Figure 2. Suppression of secondary post-incisional hyperesthesia by ETA receptor blockade.
Testing at 2cm contralateral to a 1cm-long incision of rats injected with pre-incisional 3mM BQ-123 (■, n=7) or vehicle (□, n=8). Mann Whitney U-test (Vehicle vs 3mM BQ-123) - *, P < 0.05.
Post-incisional changes in CTMR are reported here relative to the baseline, pre-incisional response levels. This takes into account the variation over the back in the nocifensive sensitivity of intact skin to punctate mechanical stimulation, as indicated by the baseline, pre-incisional force-response curves ( cf. Fig. 3, open squares). A relatively robust parameter to fit these data is the force for half-maximum response, F0.5, which depends on values for responses to several forces in the most sensitive, steepest part of the force-response function. The F0.5 parameter indicates the position of the curve on the force axis, a measure of the sensitivity of the skin to punctate tactile stimulation, and is analogous to the potency (ED50) of an agonist (force) to produce an effect (CTMR).
3.2. Incision shifts the Response vs Force relation to the left
Curves fit to the peak graded response vs force after the incision show a leftward shift relative to the curves for intact skin (Fig. 3), as has been previously reported in inflamed skin (Andrew and Greenspan, 1999) (but see Methods for cautions). The mid-force values (interpolated value, with range from interpolation between S.E.s, see Methods) of the intact skin near the midline are comparable to each other :46 (40-49) mN at 0.5 cm, and 47 (42-52) mN at 1 cm ipsilateral, whereas those for skin located more laterally are larger: 73 (66-81) mN at 2cm ipsilateral and 94 (88-104) mN at 2cm contralateral. All F0.5 values are listed in Table 1.
Table 1.
Tactile Sensitivity of Skin After Incision: F0.5 Values from Response vs Force Analysis. (interpolated values, with range in parentheses)
| Distance from Skin | Conditions | |||
|---|---|---|---|---|
| pre— incision |
p.i. - vehicle |
p.i. — 3mM BQ123 |
p.i. — 0.3mM BQ123 |
|
| 0.5 cm. ipsilateral |
46(40- 49) |
5.2(2.3- 7.8) |
13(10.8-18) | 11(8-14.5) |
| 1 cm. ipsilateral | 47(42- 52) |
4.8(3.8- 5.9) |
19.5(15-23) | 12(7.7-16) |
| 2 cm. ipsilateral | 73(66- 81) |
6.1(4.9- 7.4) |
48(34-51) | 19(16.5-25) |
| 2cm.contralateral | 94(88- 104) |
33(29- 41) |
61(50-76) | 41(30.5-51) |
See Methods 2.5 and Figure 3 legend for description of analysis.
The leftward shifts of these curves after incision, showing increased tactile sensitivity, are quantitated by the ratio of F 0.5 values before and after incision (Table 1). For the primary sensitivity, around the incision site, F0.5 changed from 46 mN to 5.2 (2.7-7.3) mN, an 8-9-fold increase in sensitivity. At 1 cm distance on the side ispsilateral to the incision F0.5 = 4.8 (3.8-5.8) mN at peak response time, there was a 10-fold sensitivity increase over the 47mN of intact skin. For the secondary responses, measured at 2cm ipsilateral, F0.5 changed by 12-fold, from 73mN in intact skin to 6.1(4.9-7.4)mN after incision, while at 2 cm contralateral the shift was only about 3-fold, from 94 mN to 33 (29-41) mN.
3.3. Inhibition of ETA receptors by BQ-123 reduces post-incisional pain
Local subcutaneous injection of 3 mM BQ-123, 15 min. before the incision, strongly suppressed the primary allodynia that follows incision (Fig. 1). The degree of inhibition of the peak response was greatest with the weakest forces and decreased with increasing VFHs, such that the peak hyperalgesia tested by the 37 mN filament was unaffected by BQ-123 (Fig. 1F). Similar effects of 3 mM BQ-123 on secondary allodynia were detected on the contralateral side (Fig. 2). In intact skin, BQ-123 at 3 mM had no effect on tactile sensitivity at the injection site or contralateral to it, measured over the 24 h after the inhibitor’s subcutaneous injection (data not shown).
Analyses of force—response curves show the effect of BQ-123 in limiting the increase in acute sensitivity from incision (cf. Table 1). The 8-10-fold reduction in F0.5 for primary hyperesthesia from incision in vehicle-injected skin was limited to a 3-4 fold reduction in skin pre-injected with 3mM BQ-123 (Fig. 3A). In other words, the incision-induced increase in primary sensitivity with 3mM BQ-123 was only about 0.4 of that with vehicle . When skin was tested at 1cm from the incision on the ipsilateral side, the 10-fold increased sensitivity from incision was reduced to a 2-3-fold change by 3mM BQ-123, a reduction of sensitivity increase to about 0.25 of that with vehicle. This trend of increasing pharmacological effect with distance from the incision continued for responses measured 2cm ipsilateral from the incision, where the approximately 12-fold increase in sensitivity after incision was reduced by 3mM BQ-123 to a 1.5 fold change, 0.12 of that with vehicle. However, on the contralateral side, where there is no direct neuronal connection between the incision and the test site (Theriault E and Diamond J, 1988), the effect of 3 mM BQ-123 was much less, with the 3-fold increase in sensitivity from incision being merely halved by the inhibitor.
These anti-allodynic actions of BQ-123 are concentration dependent (Fig. 4). The suppression by pre-incisional 0.3mM BQ-123 paralleled the anatomic variance seen with the higher concentration, i.e. the most robust effects occurred at the larger ipsilateral distance. (Although approximately the same degree of suppression by BQ-123 was measured at a distance of 2cm on the contralateral side, the larger variance in peak response precluded this from reaching statistical significance; Fig. 4D.)
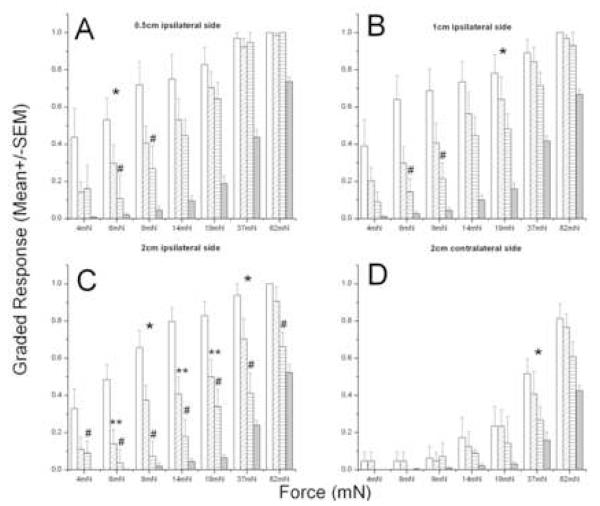
Figure 4. BQ-123′s dose-dependent suppression of peak post-incisional responses.
Vehicle(□, n=8), 0.3 mM BQ-123( , n=8), 3mM BQ-123(
, n=8), 3mM BQ-123( , n=7) and intact skin(■, n=15). Linear Fit (vehicle, 0.3 and 3mM BQ-123 groups) - *, P < 0.05. Mann Whitney U-test with Bonferroni correction : (BQ123 vs vehicle) - #, (Intact vs vehicle) - *, (Intact vs BQ-123) - **, P < 0.035.
, n=7) and intact skin(■, n=15). Linear Fit (vehicle, 0.3 and 3mM BQ-123 groups) - *, P < 0.05. Mann Whitney U-test with Bonferroni correction : (BQ123 vs vehicle) - #, (Intact vs vehicle) - *, (Intact vs BQ-123) - **, P < 0.035.
Shifts in the force-response curves for acute changes after incision can be used to quantitate the relative effectiveness of the two doses at different test locations. The sensitivity change at 0.5 cm ipsilateral from the wound was reduced by 60% by 3mM BQ-123 and by 52% by 0.3 mM BQ-123. Tests at 1cm show that 3mM reduced post-incisional sensitivity by 75% and 0.3 mM reduced it by 60%, and at 2cm ipsilateral the respective effects of these concentrations on reducing sensitivity are 88% and 68%. Thus, when tested at increasing ipsilateral distance from the incision, and from the site of drug injection, the absolute suppression of post-incisional sensitivity increases and the relative effect of the higher concentration over the lower is also greater. However, when assessed on the contralateral side the inhibition of acute sensitivity is again smaller, 47% with 3mM and 18% with 0.3 mM BQ-123. These complex relationships between effect, dose and distance can be accounted for by a simple model presented in the Discussion (below).
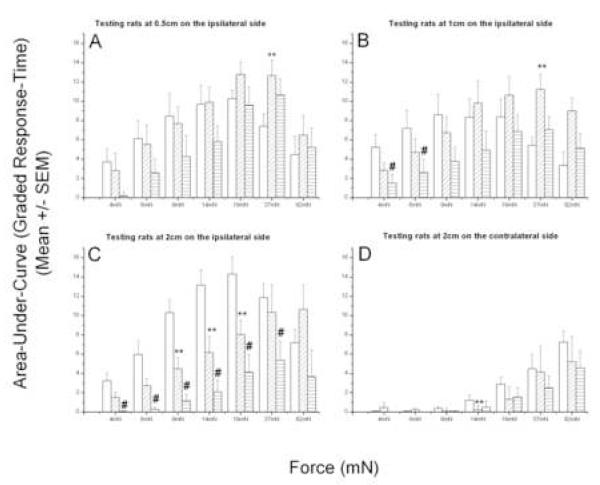
The integrated effect of BQ-123 was assessed by its reduction of the area-under-the-curve (AUC) for hyperesthesia after the incision (see Methods for description). Areas were calculated as the elevation of the graded response above the pre-incisional baseline level, integrated over the 24 h immediately following surgery. Review of the time-course of post-incisional primary and secondary hyperesthesia shows that the major increase in allodynia is transient and occurs over the first 4-8 h, whereas the hyperalgesic responses, to stronger forces, are longer lasting, remaining elevated for 24h or longer (Figs. 1 & 2). (Due to the increasing baseline values at stronger forces, the absolute area-under-curve declines for the higher forces, e.g. 37 and 82 mN in panels A-C, Figure 5. The anomalous effect of a larger AUC at high forces for BQ-123 treated rats,e.g. at 37 mN in Figure 5A & B, results from a difference in the baseline values between vehicle and drug-treated groups, see Figure 1.) The AUC in the allodynic range was reduced by both 3 and 0.3 mM BQ-123, for both primary (Fig. 5 A-B) and secondary ipsilateral (Fig. 5C) and contralateral (Fig. 5D) allodynia. As with the peak amplitude of hyperalgesia, the AUC for hyperalgesic forces tested near the incision (primary locus) was largely unaffected by BQ-123. Also, as with the peak amplitude of response, the AUC was most robustly affected at the largest ipsilateral distance, 2cm (Fig. 5, compare A with C).
Figure 5. BQ-123′s dose-dependent reduction of post-incisional area-under-the-curve for hyperesthesia (0-24h).
Vehicle(□, n=8), 0.3 mM BQ-123 ( , n=8), 3mM BQ-123(
, n=8), 3mM BQ-123( , n=7). Mann Whitney U-test with Bonferroni correction: (3mM BQ123 vs vehicle) - #, (0.3mMBQ-123 vs vehicle) - **, P < 0.05.
, n=7). Mann Whitney U-test with Bonferroni correction: (3mM BQ123 vs vehicle) - #, (0.3mMBQ-123 vs vehicle) - **, P < 0.05.
3.4. Reduction of Allodynia is Due to Local, Not Systemic Effects of Inhibitor
Secondary hyperesthesia is generally recognized as requiring sensitization of the central nervous system (CNS), such as occurs from repeated afferent discharge of primary nociceptors. Since the contralateral skin on the back receives no direct axonal innervation from the incised side (Theriault and Diamond, 1988), elevated responses measured there must involve changes in the CNS, such as at the spinal cord (Treede et al., 1992), in regions that could be modified by systemically circulating drugs. The possible actions of systemically distributed BQ-123 were tested by delivering the high dose (3mM, 0.4 mL) subcutaneously at the nuchal midline, a locus from which other drugs are known to suppress secondary allodynia. There was, however, no suppression from thus delivered systemic inhibitor, of either primary (Fig. 6A-C) or secondary hyperesthesia (Fig. 6 D-F), and no effect at 2cm ipsilateral from the incision (data not shown), the locus where locally delivered BQ-123 was most effective.
Figure 6. Effects of systemic BQ-123 on primary and secondary post-incisional hyperesthesia.
Nuchal midline injection of pre-incisional 3mM BQ-123 (■, n=8) or vehicle (□, n=8). Testing at 0.5cm ipsilateral (A-C) or 2cm contralateral (D-F) to a 1cm-long incision.
3.5. Effects of 3mM BQ-123 on Secondary Hyperesthesia are Persistent
At 24 hours after the incision much of the transient primary allodynia had subsided (Fig. 1) and the effects of BQ-123, selectively suppressing allodynia at this primary site (Fig. 3A), were effectively absent by this time; and the skin near the incision that had been pre-treated with BQ-123 was as mechanically sensitive as that treated with vehicle (Fig. 7A). However, at the more distant sites, where BQ-123 inhibited hyperalgesia as well as allodynia, the suppression of hyperesthesia at 24h post-incision was as or more effective than in the acute phase 3-4 h after incision (Fig. 7 B,C; compare to Fig. 3 B,C). Inhibition of secondary analgesia thus persisted long after the drug was delivered.
Figure 7. 24 hours post-incision responses of rats injected with pre-incisional 3mM BQ-123 (■, n=7) or vehicle (⊠ , n=8) against intact (□, n=15).
Mann Whitney U-test with Bonferroni correction : (BQ123 vs vehicle) - #, (Intact vs vehicle) - *, (Intact vs BQ-123) - **, P < 0.035.
In order to see if this persistent effect might be due to residual BQ-123, present long after its injection, 3mM of this agent was delivered subcutaneously at the incision site 24 h before surgery, and then post-incisional tactile sensitivity was tested from 0.5 to 24 h, as in tests for effects of the local administration just before surgery. There were no significant effects of this very early delivery on either primary or secondary hyperesthesia, however (data not shown), showing that the persistent suppression of secondary post-incisional pain was due to actions of the drug in the peri-operative period and not to residual drug in the rat.
4. Discussion
In this study post-incisional hyperesthesia, from a 1cm long incision through the hairy skin on the back closed by a single suture, was reduced by the ETAR antagonist BQ-123 in a concentration dependent manner. Primary allodynia to punctate mechanical stimulation was almost abolished by 3mM BQ-123 , injected locally under the surgical site 15 min before the incision. Primary hyperalgesia, the response to more forceful punctate stimuli near the wound, was less affected by BQ-123, but both allodynia and hyperalgesia at distant sites on the back skin, showing secondary hyperesthesia, were lessened by this drug. Inhibition of primary allodynia was significant for about 4-8 h after the incision, whereas the suppression of secondary responses by BQ-123 remained strong at 24 h after surgery. There was no residual effect of BQ-123 when it was locally injected 24h before the incision, and systemic BQ-123 had no effect on post-incisional pain from an acute operation.
Although the concentrations of subcutaneously injected BQ-123 that were effective against post-incisional allodynia (0.3 – 3 mM) are 5-6 orders of magnitude greater than the Ki values reported for actions against ETA receptors in isolated cells (Sakamoto et al., 1993), this agent still appears to retain selectivity for this target. Both ET-1-induced pain and its activation of impulses in cutaneous nociceptors are blocked by subcutaneous 3.2 mM BQ-123 (Gokin et al., 2001) although the antagonist has no effect on mechanosensitivity of intact skin (see Results, above). Isolated sensory neurons show ET-1-induced changes that are prevented by BQ-123 (at 1 μM), but the antagonist causes no changes in unmodified Na currents in these cells, belying any local anesthetic-type effect. Although there is a hypothetical possibility that such high concentrations of BQ-123, if achieved at the site of action within the dermal layers (see Khodorova et al., 2003), could also inhibit ETB receptors, the actions of selective ETB receptor antagonists on ET-1-induced pain are opposite those of BQ-123. Finally, in preliminary experiments we have found no consistently significant effects of equally high concentrations of these antagonists of ETB receptors on the onset of post-incisional pain. The actions of BQ-123 in this setting, we conclude, selectively antagonize ETA receptors.
These results show that ETARs contribute to both primary and secondary hyperesthesia. It is likely that incision releases ET-1 into the skin, initially from keratinocytes, from where it acts on cutaneous nociceptors to both lower the threshold for tactile stimulation (Balonov et al., 2006) and to induce spontaneous impulses, leading to the central sensitization that underlies secondary hyperesthesia (LaMotte, 1992; Treede et al., 1992). Activation of cutaneous terminals of nociceptors also results in local release of glutamate and neuropeptides Substance P and CGRP (deGroot et al., 2000; Jin et al., 2006; McGovern et al., 1995), which can further stimulate keratinocytes (Viac et al., 1996) and lead to further release of ET-1.
Different types of cells are potential sources of ET-1. Following U.V.-induced skin inflammation in mice, keratinocytes secrete increased levels of ET-1 (Ahn et al., 1998). Keratinocytes also produce pro-inflammatory cytokines such as TNF-α, TGF-β and IL-1β that in turn, can trigger ET-1 synthesis and secretion from endothelial and epithelial cells (Ahn et al., 1998; Ansel et al., 1990; Kock et al., 1990). Human polymorphonuclear neutrophils (PMNs) also synthesize ET-1, providing an additional source to accompany inflammatory cytokine release in the early stages of inflammation. Intravenous cytokine administration also increases plasma levels of ET-1 in rats (Battistini et al., 1996; Klemm et al., 1995; Miyamori et al., 1991; Vemulapalli et al., 1994; Vemulapalli et al., 1991). IL-1α transcriptionally and translationally elevates the activity of endothelin-converting-enzymes-1α (ECE-1α) in human keratinocytes, an enzyme whose action yields biologically active ET-1 by cleavage of its inert precursor. Inhibition of ECE-1 predictably suppresses ET-1 levels, whereas both ECE-1 transcripts and ECE-1α protein secretion are increased by U.V.-inducible IL-1α (Hachiya et al., 2002). A five-fold increase in endogenous ET-1 is caused by TNF-α, the most rapid inducer of ET-1 (Klemm et al., 1995). IL-1β and IL-1α are known to increase sensory nerve fiber excitability and produce hyperalgesia (Ferreira et al., 1988; Meyer et al., 2006), and these changes may be linked by ET-1 (Khodorova et al., 2002). Therefore, sites of tissue injury have elevated cytokines related to inflammation and these can contribute to endogenous ET-1 release and, by this pathway, to the development of local allodynia.
Primary hyperalgesia, however, might be mediated by mechanisms independent of ET-1, since it was virtually unaffected by BQ-123, in contrast to primary allodynia, which was strongly suppressed by BQ-123. There appear to be no basal level of ET-1 that tonically modulates mechano-sensitivity of intact skin, via ETA receptors, since there were no effects of BQ-123 on responses to von Frey filaments in intact skin. Previous work from our laboratory showed that exogenous ET-1 injected into the rat’s footpad caused pain-like behavior, via ETARs, that was not blocked by tetrodotoxin (TTX) applied to the proximal sciatic nerve, although the same delivery of TTX did block mechano-nociception of that paw (Houck et al., 2004). Tetrodotoxin-resistant axons in the rat sciatic nerve account for a very small fraction of all afferent fibers; these are among the slowest conducting C-fibers (c.v. 0.5-0.8 m.sec-1) and are unresponsive to noxious thermal or mechanical stimuli applied to a cutaneous receptive field identified by electrical stimulation (Gokin and Strichartz, 2001). We surmise that the back is also innervated by similar nociceptive fibers that can be activated by ET-1e released by incision. Nociceptive fibers might become hyper-excitable and fire a larger number of impulses because of ET-1’s activation facilitating effects on TTX-resistant Na+ channels, which occurs via ETARs (Zhou et al., 2002). Other mechanisms could include NMDA and CGRP receptors, which we have shown contribute to the initiation of ET-1induced tactile allodynia in intact paw (Khodorova et al., 2006). Although the later stages of this ET-1-induced allodynia also involve TRPV-1 receptors (Balonov et al., 2006), this pathway does not appear to contribute to incision-induced mechanosensitivity (Pogatzki-Zahn et al., 2005), suggesting that ET-1’s interactions in intact skin may differ from those after an incision.
Evidence that mechanical hyperalgesia stems from acidosis at the incision site might explain our inability to suppress hyperalgesia as effectively as allodynia, with the ETAR antagonist. Following incision of the plantar skin, gastrocnemius muscle or cutaneous paraspinal region, a localized tissue acidosis with concurrent pain behavior was identified, with the degree of post-incisional pain paralleling local tissue acidosis (Steen et al., 1995; Woo et al., 2004). We postulate that the development of post-incisional primary mechanical hyperalgesia involves H+ sensitive channels, such as ASICS (Julius and McCleskey, 2006), but no substantial contribution from ETA receptors.
In contrast to the selective inhibition of allodynia in the primary region near the incision, ETAR antagonism suppressed both allodynia and hyperalgesia in the secondary skin regions, and for at least 24 h after the incision, an effect not due to systemically circulating BQ-123. Indeed, the plasma half-life of BQ-123 is only 4 min, and within an hour almost all the compound has been removed by the liver and excreted into the bile (Fukami et al., 1996; Kato et al., 1999; Nakamura et al., 1996). When rats were incised 24 hours instead of 15 minutes after BQ-123’s injection, no significant suppression of primary or secondary hyperesthesias occurred. Nonetheless, secondary post-incisional pain suppression induced by acutely applied BQ-123 remained effective a day after the procedure. We interpret this result as showing that the local blockade of activation of ETAR-containing fibers prevented the generation of afferent impulses that are essential for central sensitization. It is noteworthy that the most effective and long-lasting inhibition of hyperesthesia by BQ-123 was at the most distal site from the incision, the secondary region several centimeters removed from the injection site on the contralateral side. This heightened effect shows the critical role of ET-1-activated fibers for driving central sensitization.
We and others have previously shown that central sensitization appears after incision in rat hairy skin, as evidenced by the actions of systemic local anesthetics on post-incisional pain (Duarte et al., 2005) and by recordings from high threshold and low threshold afferents as well as wide dynamic range (WDR) spinal cord neurons (Kawamata et al., 2005). During incision and suturation an increase in activity was recorded in all three classes of spinal neurons, but spontaneous activity and that evoked by light tactile stimulation, as is used to assess allodynia, for several hours after surgery was only prominent in the WDR neurons (Kawamata et al., 2005). Both primary and secondary tactile allodynia were apparent from 30 min to 3-4 days after surgery, in agreement with our own findings in rat hairy skin after an almost identical incision.
In summary, our findings show that endogenous ET-1, acting via ETA receptors, elicits post-incisional local allodynia and contributes critically to the activation of afferent fibers that drive central sensitization and accompanying secondary hyperesthesia. Since the intensity of acute post-operative pain is a predictor of the likelihood of chronic post-operative pain (Perkins and Kehlet, 2000), inhibition of these ETAR —linked pathways may be an effective strategy to minimize both acute and prolonged pain following surgery.
Acknowledgments
The authors would like to thank Dr. Alla Khodorova for her thoughtful advice. Part of the work was reported at the 2004 Society for Neuroscience Annual Meeting, San Diego, CA. This study was supported by USPHS grant from NIH/NCI, CA080153.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn GY, Butt KI, Jindo T, Yaguchi H, Tsuboi R, Ogawa H. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J Dermatol. 1998;25:78–84. doi: 10.1111/j.1346-8138.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Ansel J, Perry P, Brown J, Damm D, Phan T, Hart C, Luger T, Hefeneider S. Cytokine modulation of keratinocyte cytokines. J Invest Dermatol. 1990;94:101S–107S. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRP-V1 receptors. Exptl Biol Med. 2006;231:1165–70. [PubMed] [Google Scholar]
- Battistini B, Forget MA, Laight D. Potential roles for endothelins in systemic inflammatory response syndrome with a particular relationship to cytokines. Shock. 1996;5:167–83. doi: 10.1097/00024382-199603000-00002. [DOI] [PubMed] [Google Scholar]
- deGroot J, Zhou SS, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- DeNucci G, Thomas R, D’Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz G. Reduction of post-incisional allodynia by subcutaneous bupivacaine. Anesthesiology. 2005;103:113–25. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Romitelli M, deNucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13:S220–2. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- Fukami T, Niiyama K, Amano Y, Hisaka A, Fujino N, Sawasaki Y, Ihara M, Ishikawa K. Cyclic pentapeptide endothelin A receptor antagonists with attenuated in vivo clearance. Chem Pharm Bull. 1996;44:609–614. doi: 10.1248/cpb.44.609. [DOI] [PubMed] [Google Scholar]
- Gokin AP, Fareed MU, Pan H-L, Hans G, Strichartz GR, Davar G. Local injection of Endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–66. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokin AP, Strichartz G. Differential activity-dependence and TTX-sensitivity of physiologically characterized rat sciatic C-fibers recorded in vivo. Soc Neurosci Ann Mtg Abstracts. 2001;928:13. [Google Scholar]
- Hachiya A, Kobayashi T, Takema Y, Imokawa G. Biochemical characterization of endothelin-converting enzyme-1alpha in cultured skin-derived cells and its postulated role in the stimulation of melanogenesis in human epidermis. J Biol Chem. 2002;277:5395–403. doi: 10.1074/jbc.M105874200. [DOI] [PubMed] [Google Scholar]
- Houck CS, Khodorova A, Reale A, Strichartz GR, Davar G. Sensory fibers resistant to the actions of tetrodotoxin mediate nocifensive responses to local administration of endothelin-1 in rats. Pain. 2004;110:719–726. doi: 10.1016/j.pain.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;252:reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- Jin YH, Nishioka H, Wakabayashi K, Fujita T, Yonehara N. Effect of morphine on the release of excitatory amino acids in the rat hind instep: Pain is modulated by the interaction between the peripheral opioid and glutamate systems. Neuroscience. 2006;138:1329–1339. doi: 10.1016/j.neuroscience.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Julius D, McCleskey EW. Cellular and molecular properties of primary afferent neurons. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Elsevier/Churchhill Livingstone; Philadelphia: 2006. pp. 35–47. [Google Scholar]
- Kadono S, Manaka I, Kawashima M, Kobayashi T, Imokawa G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116:571–7. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- Kato Y, Akhteruzzaman S, Hisaka A, Sugiyama Y. Hepatobiliary transport governs overall elimination of peptidic endothelin antagonists in rats. J Pharmacol Exp Ther. 1999;288:568–574. [PubMed] [Google Scholar]
- Kawamata M, Koshizaki M, Shimada SG, Narimatsu E, Kozuka Y, Takahashi T, Namiki A, Collins JG. Changes in response properties and receptive fields of spinal dorsal horn neurons in rats after surgical incision in hairy skin. Anesthesiology. 2005;102:141–51. doi: 10.1097/00000542-200501000-00023. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci. 2002;22:7788–7796. doi: 10.1523/JNEUROSCI.22-17-07788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorova A, Navarro B, Jouaville LS, Murphy J-E, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nature Med. 2003;9:1055–61. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Vasko MR, Richter JA, Strichartz GR. NMDA receptors favor tactile allodynia induced by injection of low concentrations of endothelin-1 into the rat’s hindpaw. Abstarct of Ann Mtg of Soc for Neurosci. 2006;245:23. [Google Scholar]
- Klemm P, Warner TD, Hohlfeld T, Corder R, Vane JR. Endothelin 1 mediates ex vivo coronary vasoconstriction caused by exogenous and endogenous cytokines. Proc Natl Acad Sci USA. 1995;92:2691–5. doi: 10.1073/pnas.92.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, Luger TA. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–14. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH. Neurophysiological mechanisms of cutaneous secondary hyperalgesia in primate. In: Willis W, editor. Hyperalgesia and Allodynia. Raven Press; New York: 1992. pp. 175–185. [Google Scholar]
- Lipa JE, Neligan PC, Perreault TM, Baribeau J, Levine RH, Knowlton RJ, Pang CY. Vasoconstrictor effect of endothelin-1 in human skin: role of ETA and ETB receptors. Am J Physiol. 1999;276:H359–67. doi: 10.1152/ajpheart.1999.276.2.H359. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- McGovern UB, Jones KT, Sharpe GR. Intracellular calcium as a second messenger following growth stimulation of human keratinocytes. Br J Dermatol. 1995;132:892–896. doi: 10.1111/j.1365-2133.1995.tb16944.x. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Elsevier/Churchill Livingstone; Philadelphia: 2006. pp. 16–20. [Google Scholar]
- Miyamori I, Takeda Y, Yoneda T, Iki K, Takeda R. Interleukin-2 enhances the release of endothelin-1 from the rat mesentric artery. Life Sci. 1991;49:1295–300. doi: 10.1016/0024-3205(91)90193-f. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hisaka A, Sawasaki Y, Suzuki Y, Fukami T, Ishikawa K, Yano M, Sugiyama Y. Carrier-mediated active transport of BQ-123, a peptidic endothelin antagonist, into rat hepatocytes. J Pharmacol Exp Ther. 1996;278:564–572. [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174:5047–56. doi: 10.4049/jimmunol.174.8.5047. [DOI] [PubMed] [Google Scholar]
- Perkins FM, Kehlet H. Chronic pain as an outcome of surgery: a review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Jacoby HI. Endothelin-1, -2 and -3 directly and big-endothelin-1 indirectly elicit an abdominal constriction response in mice. Life Sci. 1991;48:PL85–90. doi: 10.1016/0024-3205(91)90130-4. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Yanagisawa M, Sawamura T, Enoki T, Ohtani T, Sakurai T, Nakao K, Toyo-oka T, Masaki T. Distinct subdomains of human endothelin receptors determine their selectivity to endothelinA-selective antagonists and endothelinB-selective agonists. J Biol Chem. 1993;268:8547–53. [PubMed] [Google Scholar]
- Steen KH, Steen AE, Reeh PW. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci. 1995;15:3982–9. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Theriault E, Diamond J. Nociceptive cutaneous stimuli evoked localized contractions in a skeletal muscle. J Neurophysiol. 1988;60:446–62. doi: 10.1152/jn.1988.60.2.446. [DOI] [PubMed] [Google Scholar]
- Theriault E, Diamond J. Nociceptive cutaneous stimuli evoked localized contractions in a skeletal muscle. J Neurophysiol. 1988;60:446–62. doi: 10.1152/jn.1988.60.2.446. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Vemulapalli S, Chiu PJ, Griscti K, Brown A, Kurowski S, Sybertz EJ. Phosphoramidon does not inhibit endogenous endothelin-1 release stimulated by hemorrhage, cytokines and hypoxia in rats. Eur J Pharmacol. 1994;257:95–102. doi: 10.1016/0014-2999(94)90699-8. [DOI] [PubMed] [Google Scholar]
- Vemulapalli S, Chiu PJ, Rivelli M, Foster CJ, Sybertz EJ. Modulation of circulating endothelin levels in hypertension and endotoxemia in rats. J Cardiovasc Pharmacol. 1991;18:895–903. doi: 10.1097/00005344-199112000-00017. [DOI] [PubMed] [Google Scholar]
- Viac J, Gueniche A, Doutremepuich JD, Reichert U, Claudy A, Schmitt D. Substance P and keratinocyte activation markers: an in vitro approach. Arch Dermatol Res. 1996;288:85–90. doi: 10.1007/BF02505049. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14:166–74. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–75. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Davar G, Strichartz G. Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: A mechanism for the pain-inducing actions of ET-1. J Neurosci. 2002;22:6325–6330. doi: 10.1523/JNEUROSCI.22-15-06325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]