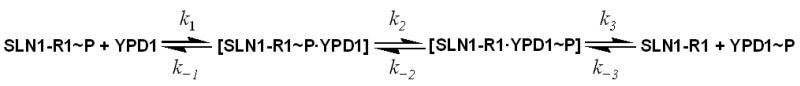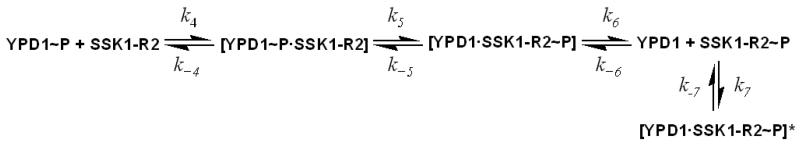Abstract
The multi-step His-Asp phosphorelay system in Saccharomyces cerevisiae allows cells to adapt to osmotic, oxidative and other environmental stresses. The pathway consists of a hybrid histidine kinase SLN1, a histidine-containing phosphotransfer (HPt) protein YPD1 and two response regulator proteins, SSK1 and SKN7. Under non-osmotic stress conditions, the SLN1 sensor kinase is active and phosphoryl groups are shuttled through YPD1 to SSK1, therefore maintaining the response regulator protein in a constitutively phosphorylated state. The cellular response to hyperosmotic stress involves rapid efflux of water and changes in intracellular ion and osmolyte concentration. In this study, we examined the individual and combined effects of NaCl and glycerol on phosphotransfer rates within the SLN1-YPD1-SSK1 phosphorelay. The results show that the combined effects of glycerol and NaCl on the phosphotransfer reaction rates are different from the individual effects of glycerol and NaCl. The combinatory effect is likely more representative of the in vivo changes that occur during hyperosmotic stress. In addition, the effect of osmolyte concentration on the half-life of the phosphorylated SSK1 receiver domain in the presence/absence of YPD1 was evaluated. Our findings demonstrate that increasing osmolyte concentrations negatively affects the YPD1•SSK1∼P interaction thereby facilitating dephosphorylation of SSK1 and activating the HOG1 MAP kinase cascade. In contrast, at the highest osmolyte concentrations, reflective of the osmoadaptation phase of the signaling pathway, the kinetics of the phosphorelay favor production of SSK1∼P and inhibition of the HOG1 pathway.
Two-component signal transduction systems in prokaryotes and eukaryotes regulate cellular responses to a variety of environmental stresses (2). Canonical two-component systems contain a membrane-bound sensor histidine kinase (HK)1 and a cytoplasmic response regulator (RR). Upon stimulation, the HK autophosphorylates a conserved histidine residue located within the cytoplasmic kinase domain. The phosphoryl group is then transferred to a conserved aspartate residue within the receiver domain of the RR protein. Typically, phosphorylated RRs modulate downstream gene expression (e.g. many bacterial response regulators are transcription factors). Multi-step phosphorelay systems are more complex and employ additional signaling components, such as a histidine-containing phosphotransfer (HPt) protein and multiple HKs or RRs (3-6).
In Saccharomyces cerevisiae, a multi-step phosphorelay system is responsible for adaptation to osmotic, oxidative and other environmental stresses (7, 8). The branched pathway consists of a sensor histidine kinase SLN1, a histidine-containing phosphotransfer (HPt) protein YPD1, and two independent response regulator proteins, SSK1 and SKN7. The SLN1 gene encodes a hybrid histidine kinase with two membrane spanning regions and an extracellular sensing domain (9). The HPt protein, YPD1, serves as a non-enzymatic but essential mediator between SLN1 and two downstream RR proteins SSK1 and SKN7 (10-12). In vitro data suggest that YPD1 serves a regulatory function as well by forming a stable complex with the phosphorylated SSK1 response regulator domain under non-osmotic stress conditions thereby shielding the phosphoryl group from hydrolysis (13). Phosphorylation and dephosphorylation of SSK1 therefore functions as an on/off switch in controlling the activity of the downstream HOG1 mitogen-activated protein (MAP) kinase cascade (14, 15). Under hyperosmotic conditions, we propose that dissociation of the YPD1•SSK1∼P complex must occur, followed by dephosphorylation of SSK1. The unphosphorylated form of SSK1 directly interacts with and activates SSK2/SSK22 (MAPKKK), which leads to the activation of PBS2 (MAPKK) and the HOG1 MAP kinase (14, 15).
In a previous study, we used rapid-quench kinetics to characterize the individual phosphotransfer reactions between YPD1 and the response regulator domains associated with SLN1, SSK1 and SKN7 (referred to as SLN1-R1, SSK1-R2, and SKN7-R3, respectively) (16). For the SLN1-R1∼P to YPD1 reaction, a maximum forward rate constant of 29 s−1 was determined with a Kd of 1.4 μM for the SLN1-R1∼P·YPD1 complex. A very rapid phosphotransfer rate of 160 s−1 was measured for the subsequent reaction between YPD1∼P to SSK1-R2 and the reaction is strongly favored over phosphotransfer to SKN7-R3. Phosphotransfer reactions between YPD1 and SLN1-R1 or SKN7-R3 were reversible; while reverse transfer from SSK1-R2∼P to YPD1 was not observed under the conditions tested. These parameters are in good agreement with the concept that SSK1 is constitutively phosphorylated under normal osmotic conditions (14-16).
When S. cerevisiae is exposed to hyperosmotic stress and water leaves the cell by passive diffusion, SLN1 kinase activity diminishes (8, 17-21). This allows for accumulation of unphosphorylated SSK1 and its activation of the downstream HOG1 MAP kinase cascade. Activated phospho-HOG1 translocates into the nucleus (22) and activates gene expression of multiple genes including the GPD1 (glycerol-phosphate dehydrogenase) and GPP2 (glycerol-3-phosphate phosphatase) genes which leads to increased glycerol synthesis (8, 23-26). Accumulation of intracellular glycerol, a chemically inert osmolyte, allows yeast cells to adapt to hyperosmotic stress conditions.
A number of different osmolytes are found inside yeast and other fungi including monovalent salts, amino acids, polyols and carbohydrates (8, 18, 27). S. cerevisiae, S. pombe, C. albicans, and D. hansenii almost exclusively employ glycerol as an osmolyte in osmoregulation (8, 17, 28-30). Osmoadaptation is achieved through a series of cellular responses that are temporally regulated. For instance, closure of the osmotically sensitive glycerol channel (Fps1) upon hyperosmotic shock provides an additional route to increase the glycerol concentration inside the cell almost immediately to compensate for water efflux (31, 32). HOG1 phosphorylation occurs in the cell during the first 1-3 min after exposure to osmotic shock (33, 34). Both GPD1 and glycerol concentrations are at half-maximal level within 20 min (35). An increase in intracellular glycerol concentration increases the turgor pressure which is mediated by the elasticity of the plasma membrane and cell wall upon water exchange (35).
Elucidating the molecular events that trigger the cellular responses to hyperosmotic stress including the rapid efflux of water, changes in intracellular ion and osmolyte concentration, phosphorylation/dephosphorylation of SSK1-R2 and the kinetics of the SLN1-YPD1-SSK1 phosphorelay is important for fully understanding the overall regulation of this signaling pathway. Previous results indicated that YPD1 can form a stable complex with SSK1-R2∼P and significantly extend the phosphorylated lifetime of the RR domain under non-osmotic stress conditions (13). Upon hyperosmotic stress, however, we hypothesize that changes in intracellular ion/solute concentrations disrupts the YPD1•SSK1-R2∼P complex, thus facilitating dephosphorylation of SSK1 and subsequent HOG1 MAP kinase cascade activation. We therefore examined the effect of osmolyte concentrations on the half-life of the phosphorylated SSK1-R2 in the presence and absence of YPD1 and the kinetics of the individual phosphorelay reactions. This study provides new insights into the mechanisms that underlie the osmoregulatory pathway in S. cerevisiae and the specific effects of osmolytes in regulating the pathway.
Materials and Methods
Materials
All chemicals and biochemicals were of ultrapure grade. Glutathione-Sepharose 4B resin was purchased from Amersham. [γ-32P] ATP (3000 Ci/mmol) was purchased from Perkin-Elmer. The SLN1-R1, YPD1, SSK1-R2, GST-HK and GST-HK-R1 proteins were purified as described previously (10, 29, 30). Ficoll 400, D-(+)-trehalose, proline and betaine were purchased from Sigma-Alrdich. NaCl and glycerol were from Mallinckrodt Chemicals and Pharmco-Aaper, respectively.
SLN1-R1 Phosphorylation
The SLN1-R1 domain was phosphorylated via incubation with the SLN1-HK domain as follows. GST-tagged SLN1-HK (7 μM) bound to glutathione-Sepharose 4B resin was incubated with 7 μM [γ-32P] ATP for 30 min. Unincorporated [γ-32P] ATP was washed from phospho-SLN1-HK with 50 mM Tris-HCl, pH 8.0, 100 mM KCl, 15 mM MgCl2, 2 mM DTT, and 20% glycerol by 3 consecutive centrifugations (1 min at 1000 × g). The SLN1-R1 protein (18.6 μM) was then added in the same buffer and incubated for 10 min at room temperature in a total volume of 300 μL. Phospho-SLN1-R1 was recovered in the supernatant after gently pelleting the GST-SLN1-HK bound to the resin. EDTA was added to the supernatant to a final concentration of 30 mM to prevent autodephosphorylation (16).
YPD1 Phosphorylation
The YPD1 protein was phosphorylated similarly to that of SLN1-R1 with the following modifications. Incubation of GST-tagged SLN1-HK-R1 (7 μM) and [γ-32P] ATP (7 μM) was for 60 min (16). YPD1 protein (18.6 μM) then was added in the reaction mixture for phosphorylation.
Rapid-Quench Experiments
Experiments were carried out to obtain rate data for the individual phosphotransfer steps using a rapid-quench kinetics instrument SFM-Q/4 Quench-Flow instrument (BioLogic). Phosphotransfer reactions were monitored in the millisecond timescale for acquisition of pre-steady state experimental data. The phosphodonor protein was diluted to 0.45 μM in 50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM DTT and glycerol (0.3-1.2 M), or NaCl (0.2-1.0 M). The diluted phosphorylated protein (60 μL) was then mixed with 60 μL of phospho-accepting protein partner (0.45 μM-20 μM) in 50 mM Tris-HCl, pH 8.0, 20 mM MgCl2, 1 mM DTT and 0.3-1.2 M glycerol, or 0.2-1 M NaCl, or glycerol/NaCl combinations (0.3/0.2 M, 0.55/0.4 M and 0.75/0.6 M). The reactions were quenched with 60 μL of the stop buffer (8% SDS, 80 mM EDTA) after a specified time. To analyze the results, 30 μL of the quenched reaction was mixed with 10 μL of 4× SDS-PAGE loading buffer (200 mM Tris pH 6.8, 400 mM DTT or β-mercaptoethanol, 8% SDS, 0.4 % bromophenol blue, and 40% glycerol) , and then 30 μL samples were loaded onto 15% SDS-PAGE gels. After gel electrophoresis, wet gels were wrapped in plastic wrap and analyzed using a phosphorimager (Molecular Dynamics, Storm 840). The phosphotransfer reaction kinetic parameters were quantified based on the disappearance of the 32P-label from the phospho-donor protein or the appearance of 32P-label in the phospho-accepting protein. The data were analyzed using the least squares fitting of Excel (Microsoft Office v. 10.1.12) and Enzfitter (version 2.04, Biosoft, Cambridge, U.K.). Individual datasets were analyzed using eq 1 for experiments with NaCl, glycerol, Ficoll 400 and NaCl-glycerol combinations,
| (1) |
where [S] is the concentration of the phospho-accepting protein, kobs is the observed first-order rate constant for the phosphotransfer reaction at a particular [S], kfwd is the maximal forward net rate constant for phosphoryl transfer from the phosphorylated protein to the phospho-acceptor protein, krev is the corresponding maximum reverse net rate constant for the reaction between phospho-donor and phospho-acceptor proteins, and Kd is the dissociation constant of the phospho-donor-acceptor complex. The concentration of Ficoll 400 used in the control experiment was 0.75 M.
Phosphorylated life-time experiments of SSK1-R2 in the presence of osmolytes and presence/absence of YPD1
Phosphorylation of the response regulator domain SSK1-R2 was achieved similarly to that of SLN1-R1 with the following modifications. SSK1-R2 was added to the reaction mixture (40 μL) with GST-HK (7 μM) and [γ-32P] ATP (7 μM) and incubated for 30 min. Phospho-SSK1-R2 was recovered in the supernatant (30 μL) and was then added to a reaction mixture containing 3 μM YPD1 and indicated osmolyte concentrations (NaCl, trehalose, glycerol, proline, or betaine) in a total reaction volume of 120 μL. For the reaction in the absence of YPD1, the isolated SSK1-R2 (12 μM) in 50 mM Tris-HCl (pH 8.0), 100 mM KCl, 10 mM MgCl2, 2 mM DTT was added to the indicated osmolyte concentrations in a total reaction volume of 75 μl. Aliquots (15 μL) were removed at indicated time points for both reactions, mixed with 5 μL of 4X stop buffer (0.25 M Tris-HCl pH 8.0, 8% SDS, 60 mM EDTA, 40% glycerol, 0.008% bromophenol blue) to terminate the reaction, and kept at 20 °C until gel analysis. Dephosphorylation of phospho-SSK1-R2 followed first-order rate kinetics and the half-life of phospho-SSK1-R2 was determined according to the formula t1/2 = ln2/k, where k is the rate constant for the dephosphorylation reaction.
Results
The phosphorylated life-time of SSK1-R2 in the presence of osmolytes
A value of 13 ± 3 min was previously measured for the half-life of phosphorylated SSK1-R2 in the absence of YPD1 (13). However, in the presence of YPD1, the phosphorylated SSK1-R2 half-life increased to 38 ± 4 hrs (13). Thus, it was proposed that the HPt protein YPD1 forms a complex with SSK1 and sterically shields the phosphorylated aspartate residue from hydrolysis. However, the mechanism of rapid dephosphorylation of SSK1-R2∼P under conditions of hyperosmotic stress remains unclear.
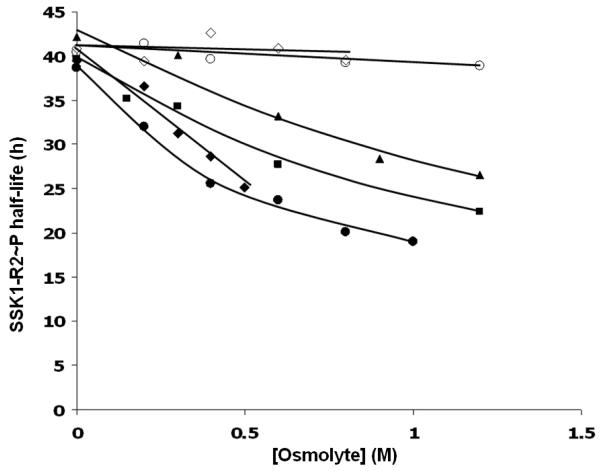
In order to assess the stability of the phosphorylated form of SSK1-R2 under conditions of hyperosmotic stress, the half-life of SSK1-R2∼P was measured as a function of osmolyte concentration in the presence and absence of YPD1. Osmolytes used were glycerol, betaine, proline, sodium chloride, and trehalose. Ficoll 400 was also tested as a control for viscosity. Through the course of the experiment, no trace of phosphorylated YPD1 was detectable, suggesting that there is no observable reverse phosphotransfer reaction between the two proteins. Our results demonstrate an approximately 2-fold decrease in the life-time of phosphorylated SSK1-R2 in the presence of YPD1 and osmolytes (with the exception of proline) (Fig. 1). In contrast, experiments conducted in the absence of YPD1 or the presence of Ficoll 400 showed no effect of osmolytes on the intrinsic stability of SSK1-R2∼P (data not shown).
Fig. 1.
Effects of osmolytes on the half-life of SSK1-R2∼P in the presence of YPD1. Curves are drawn by hand for (○) proline, (◇) Ficoll 400, (▲) glycerol, (■) betaine, (◆) trehalose, and (●) NaCl.
Effect of osmolytes on the phosphotransfer reaction between SLN1-R1 and YPD1
Rapid mixing of SLN1-R1∼P and YPD1 followed by rapid quench allowed measurement of first order rate constants (Scheme 1) by concurrently monitoring the disappearance of SLN1-R1∼P and the appearance of YPD1∼P.
Scheme 1.
All data were obtained in triplicate and were analyzed using eq 1 (in the methods section) to calculate the values shown in Tables 1-3. In the absence of osmolytes, the maximum forward rate constant, kfwd, was 27.0 ± 2.6 s−1, while the Kd for the SLN1-R1∼P:YPD1 complex was 2.8 ± 0.8 μM, in good agreement with previously obtained data in the absence of osmolytes (16).
Table 1.
Kinetic constants a for phosphotransfer reactions in the absence and presence of NaCl.
| Reaction | SLN1-R1∼P + YPD1 | YPD1∼P + SSK1-R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| NaCl | 0 M | 0.2 M | 0.6 M | 1 Ma | 0 M | 0.2 M | 0.6 M | 1 Mb |
| kfwd (s−1) | 27.0 ± 2.6 | 32.9 ± 1.8 | 24.6 ± 0.8 | 14.7 ± 0.8 | 110 ± 8 | 77.0 ± 0.2 | 41.0 ± 0.5 | 32.0 ± 3.2 |
| krev (s−1) | 9.2 ± 3.3 | 0.21 ± 2.1c | 10.0 ± 0.9 | 20.7 ± 0.4 | 4.1 ± 1.4 | 0.8 ± 0.3 | 2.2 ± 0.7 | 6.1 ± 2.9 |
| Kd (μM) | 2.8 ± 0.8 | 2.3 ± 0.6 | 2.6 ± 0.4 | 6.2 ± 1.6 | 3.8 ± 1.5 | 2.2 ± 0.3 | 1.4 ± 0.1 | 3.0 ± 1.6 |
| kfwd/Kd (μM−1s−1) | 9.6 ± 2.9 | 14.3 ± 3.8 | 9.5 ± 1.5 | 2.4 ± 0.6 | 29 ± 4 | 35.0 ± 4.8 | 28.6 ± 2.0 | 10.6 ± 5.8 |
Table 3.
Kinetic constantsa for phosphotransfer reactions in the absence and presence of glycerol and NaCl.
| Reaction | SLN1-R1∼P + YPD1 | YPD1∼P + SSK1-R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| [Glycerol]M/[NaCl]M | 0/0 | 0.3/0.2 | 0.55/0.4 | 0.75/0.6 | 0/0 | 0.3/0.2 | 0.55/0.4 | 0.75/0.6 |
| kfwd (s−1) | 27.0 ± 2.6 | 53.8 ± 1.3 | 57.5 ± 0.4 | 66.0 ± 0.8 | 110 ± 8.0 | 136 ± 1.0 | 141 ± 2.1 | 160 ± 3.0 |
| krev (s−1) | 9.2 ± 3.3 | 5.4 ± 1.4 | 6.4 ± 0.5 | 7.2 ± 0.9 | 4.1 ± 1.4 | 3.6 ± 0.5 | 6.9 ± 0.9 | 10.2 ± 2.8 |
| Kd (μM) | 2.8 ± 0.8 | 3.2 ± 0.4 | 2.7 ± 0.1 | 2.3 ± 0.1 | 3.8 ± 1.5 | 4.4 ± 0.1 | 4.3 ± 0.2 | 4.3 ± 1.4 |
| kfwd/Kd (μM−1s−1) | 9.6 ± 2.9 | 16.8 ± 2.1 | 21.3 ± 0.8 | 28.7 ± 1.3 | 28.6 ± 4.3 | 30.9 ± 0.7 | 32.8 ± 1.6 | 37.2 ± 2.2 |
Subsequent phosphotransfer reactions between SLN1-R1∼P and YPD1 were performed in the presence of different NaCl concentrations (as shown in Table 1). The value of kfwd decreases from 27.0 ± 2.6 s−1 with no NaCl present to a value of 14.7 ± 0.8 s−1 at 1 M NaCl, while krev increases from 9.2 ± 3.3 s−1 to 20.7 ± 0.4 s−1 at 1 M NaCl. No change in the dissociation constant was observed up to 0.6 M NaCl. A slight increase was observed at 1 M salt. For the second order rate constant (kfwd/Kd), the limit of eq 1 when [S] tends to zero and krev = 0, remained unchanged from 0 to 0.6 M NaCl. The highest concentration of NaCl (1 M) was not considered physiologically relevant (see discussion).
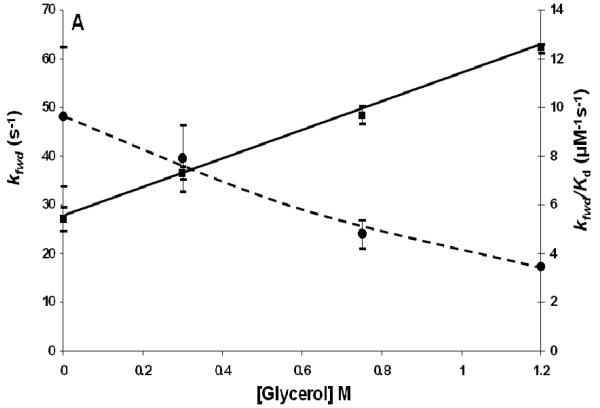
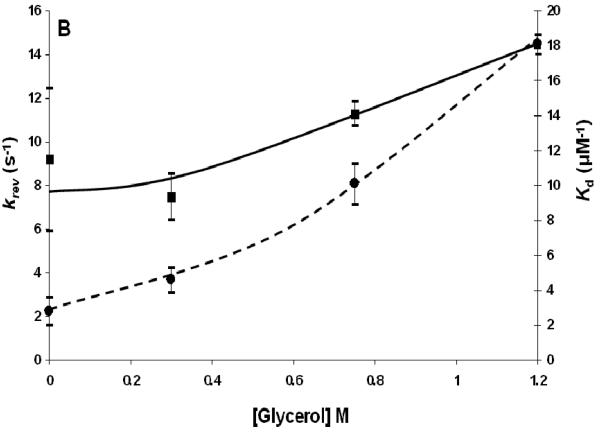
The phosphotransfer reactions between SLN1-R1∼P and YPD1 in the presence of glycerol were done in a manner analogous to those in the presence of NaCl with concentrations of glycerol as shown in Fig. 2A, Table 2. The concentrations of glycerol chosen reflect the range of intracellular levels observed during hyperosmotic stress (35, 36). The value of kfwd increases from 27.0 ± 2.6 s−1 with no glycerol present to a value of 62.3 ± 1.0 s−1 at 1.2 M glycerol and the value of krev increases from 9.2 ± 3.3 s−1 to 14.5 ± 0.1 s−1, at 1.2 M glycerol. The dissociation constant increases approximately 6-fold (Fig. 2B) and the second order rate constant decreases approximately 3-fold in the presence of 1.2 M glycerol.
Fig. 2.
A) kfwd and kfwd/Kd for the SLN-R1∼P·YPD1 phosphotransfer reaction at different concentrations of glycerol. Curves are drawn by hand for kfwd (■) and kfwd/Kd(●). B) krev and Kd for the SLN-R1∼P·YPD1 phosphotransfer reaction at different concentrations of glycerol. Lines are drawn by hand for krev (■) and Kd (●).
Table 2.
Kinetic constantsa for phosphotransfer reactions in the absence and presence of glycerol.
| Reaction | SLN1-R1∼P + YPD1 | YPD1∼P + SSK1-R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Glycerol | 0 M | 0.3 M | 0.75 M | 1.2 M | 0 M | 0.3 M | 0.75 M | 1.2 M |
| kfwd (s−1) | 27.0 ± 2.6 | 36.5 ±1.4 | 48.3 ± 2.1 | 62.3 ± 1.0 | 110 ± 8.0 | 107 ± 2 | 141 ± 1.0 | 185 ± 4.0 |
| krev (s−1) | 9.2 ± 3.3 | 7.5 ± 1.1 | 11.3 ± 0.6 | 14.5 ± 0.1 | 4.1 ± 1.4 | 3.8 ± 0.9 | 4.4 ± 0.6 | 9.5 ± 1.4 |
| Kd (μM) | 2.8 ± 0.8 | 4.6 ± 0.8 | 10 ± 1 | 18 ± 1 | 3.8 ± 1.5 | 2.1 ± 0.3 | 4.0 ± 0.1 | 11.5 ± 2.7 |
| kfwd/Kd (μM−1s−1) | 9.6 ± 2.9 | 7.9 ± 1.4 | 4.8 ± 0.6 | 3.4 ± 0.1 | 28.6 ± 4.3 | 28.2 ± 7.2 | 51.0 ± 9.2 | 35.2 ± 3.2 |
Rate constants were also measured in the presence of both NaCl and glycerol (Table 3). The maximum forward rate constant increased by about 2-fold at the highest concentration of both osmolytes (0.75 M glycerol, 0.6 M NaCl). The reverse rate constant and the Kd values remained unchanged. The combinatory effect of both osmolytes at the highest concentrations tested caused about a 3-fold increase in the second-order rate constant.
Effect of osmolytes on the phosphotransfer reaction between YPD1 and SSK1-R2
To observe the effect of sodium chloride and glycerol on the phosphotransfer rates between YPD1∼P and SSK1-R2 (Scheme 2), experiments similar to those described above were carried out. In this case, YPD1∼P was mixed with SSK1-R2 and the data were collected by following the appearance of SSK1-R2∼P and disappearance of YPD1∼P.
Scheme 2.
In the absence of osmolytes, the maximum forward rate constant, kfwd, was 110 ± 8.0 s−1. The reverse rate constant, krev, and Kd for the reaction were 4.1 ± 1.4 s−1 and 3.8 ± 1.5 μM, respectively, in good agreement with previously obtained data (16). The maximum forward (kfwd) and reverse rate constants (krev) in the presence of NaCl are shown in Table 1. The value of kfwd decreases from 110 ± 8.0 s−1 with no NaCl present to a value of 32.0 ± 3.2 s−1 at 1 M NaCl, while krev remained essentially the same. The dissociation constant in these experiments did not change significantly. However, there is a deviation on krev and Kd in going from 0 to 0.2 M, which might be due to systematic error or some unknown phenomenon. The second order rate constant remained unchanged from 0 to 0.6 M NaCl. The highest concentration of NaCl (1 M) was not considered physiologically relevant (see discussion).
In contrast to the effect of glycerol on the phosphotransfer reaction between SLN1-R1∼P and YPD1, the effect of glycerol on the phosphotransfer reactions between YPD1∼P and SSK1-R2 was different. All experiments were conducted in a manner analogous to those obtained for SLN1-R1∼P/YPD1. The forward (kfwd) and the reverse rate constants (krev) change in the same direction; that is, their values increase with increasing glycerol concentration. Specifically, the value of kfwd increases from 110 ± 8.0 s−1 with no glycerol present to a value of 185.0 ± 4.0 s−1 at 1.2 M glycerol and the value of krev increases from 4.1 ± 1.4 s−1 to 9.5 ± 1.4 s−1, at 1.2 M glycerol. The dissociation constant increases approximately 3-fold and the second order rate constant remains approximately the same in the presence of 1.2 M glycerol. The data are summarized in Table 2.
Experiments carried out to determine the combined effect of NaCl and glycerol on the YPD1∼P and SSK1-R2 phosphotransfer reaction were conducted in the presence of varying concentrations of both NaCl and glycerol. As osmolyte concentrations increased, the maximum forward and reverse rate constants increased, while no change was observed in the second order rate constant or Kd.
To differentiate between a true glycerol effect and an effect of viscosity on the phosphotransfer reactions, a control experiment was performed in the presence of Ficoll 400. Ficoll 400, as a high molecular weight polymer (macroviscosogen) is able to vary viscosity of the reaction mixture; however it has no effect on the rates of diffusion of the phosphotransfer reactions (37). Glycerol (a microviscosogen), on the other hand, is capable of affecting both parameters. Kinetic data obtained in the presence of Ficoll 400 were similar to the data obtained in the absence of osmolytes (data not shown) suggesting that the effects on the phosphotransfer reactions observed in the presence of glycerol are viscosity-independent.
Discussion
The nature of the interaction between SSK1-R2 and YPD1 is important due to the regulatory function of SSK1-R2 and its phosphorylation state that leads to activation or inactivation of the HOG1 MAP kinase cascade. Previous in vitro studies have suggested a protective role for YPD1 in shielding the phosphoryl group of SSK1-R2 from hydrolysis (13, 38). A protein-protein interaction such as this would help prevent activation of HOG1 under non-osmotic stress conditions. Increasing osmolyte concentrations caused a reduction in the half-life of SSK1-R2∼P in the presence of YPD1 by approximately 2-fold (Fig. 1). In contrast, in the absence of YPD1, osmolytes had no effect on the dephosphorylation rate of SSK1-R2. Our data suggest that osmolytes negatively affect the stability of the SSK1-R2∼P·YPD1 complex in a concentration-dependent manner (Fig. 1) and, as a consequence, the rate of phosphate hydrolysis increases. The underlying mechanism for complex destabilization can be explained as the ability of osmolytes to create extensive hydrogen bonding and or ionic interactions with surface residues of the proteins involved in the complex as well as conformational changes. Proline was an exception in this case, and we suggest that its structural rigidity and higher hydrophobicity compared to other osmolytes, renders it incapable of influencing complex stability. We suggest that this modest 1.5- to 2-fold decrease observed for the SSK1-R2∼P half-life in the presence of osmolytes is only one of potentially multiple contributing factors that lead to dephosphorylation of SSK1 and subsequent activation of the HOG1 MAPK cascade. The existence of an SSK1-specific aspartyl phosphatase, for example, cannot be ruled out.
Osmotic shock can dramatically alter the intracellular concentrations of osmolytes. However, cells can adapt by modulating intracellular osmolyte concentrations in order to balance the external osmolarity of the cell. When homeostasis is restored, the osmotic pressure and the production of osmolytes are reduced and the activity of the osmoregulation pathway components returns to its default or prestimulus state.
In order to better understand the overall physiology of the cell response to hyperosmotic shock, detailed kinetic studies are important. Although many two-component systems have been identified in prokaryotes, only a small number of bacterial and eukaryotic phosphotransfer systems have been kinetically characterized (16, 39-42). As shown in Scheme 1, for phosphotransfer between SLN1-R1 and YPD1, the ratio k1/k−1 is defined as the Kd (the dissociation constant of the SLN1-R1∼P•YPD1 complex). The forward and reverse net rate constants for the phosphotransfer reaction are kfwd and krev, respectively. Transfer of phosphoryl groups from YPD1∼P to SSK1-R2, as depicted in Scheme 2, involves formation of the YPD1∼P•SSK1-R2 complex; the ratio k4/k−4 is defined as the Kd (the dissociation constant of the YPD1∼P•SSK1-R2 complex). k7 is the rate constant for the formation of the dead-end complex. In eq. 1 above, the observed rate constant is given in terms of a forward and reverse rate constant, kfwd and krev. However, these rate constants are net rate constants, not microscopic rate constants. kfwd is defined as in eq. 2.
| (2) |
and if k−1 is not greater than k2, krev will be defined as in eq. 3
| (3) |
This is likewise true for Scheme 2 where kfwd and krev are given by eqs. 4 and 5
| (4) |
| (5) |
The treatment of eq. 2-5 assumes a rapid pre-equilibrium (reflected in the Kd) of the initial encounter complex compared to the chemical steps and k−3 and k−6 are negligible under the conditions tested. If dissociation of the product complex, SLN1-R1·YPD1∼P, is fast compared to the phosphotransfer step, k3>k2, then kfwd = k2. This is the case for the kinetics of the reverse reaction, transfer of phosphate from YPD1 to SLN1-R1 in the absence of osmolytes (16).
The kinetic data obtained in this study showed that in the presence of osmolytes, the rates of phosphoryl transfer were saturable at high YPD1 concentration suggesting the formation of a YPD1∼P·SSK1-R2 complex as observed previously (16). Phosphoryl transfer between SLN1-R1∼P and YPD1 gives a significant krev, thus k3 ≤ k2. This is also true for phosphoryl transfer between YPD1∼P and SSK1-R2; krev is finite and k6 ≤ k5. However, phosphoryl transfer from SSK-R2∼P to YPD1 is not observed, and thus either the YPD1·SSK1-R2 complex is not formed or is non-productive. However, attempts to demonstrate phosphoryl transfer from SSK1-R2∼P to YPD1 were unsuccessful and thus a dead-end complex must form between SSK1-R2∼P and YPD1. Scheme 2 shows the formation and dissociation of the dead-end complex with rate constant k7 and k−7. Thus, SSK1-R2∼P and YPD1 can form either a [SSK1-R2∼P·YPD1] or [SSK1-R2∼P·YPD1]* complex, but the latter is more stable. The measurement of the observed rate constants (kfwd and krev) and binding affinity (Kd) on the basis of eq 1 provides quantitative data for comparison of the phosphotransfer reactions between SLN1-R1 and YPD1, and YPD1 and SSK1-R2 in the absence and presence of osmolytes.
The maximum rate, kfwd, will only be observed if the concentrations of the response regulator molecules (SLN1-R1 or SSK1-R2) are significantly greater than the Kd for the YPD1•RR complex in vivo. However, the physiologic concentrations of these molecules are much lower than the Kd. As reported in the Saccharomyces Genome Database (www.yeastgenome.org), the in vivo concentrations of SLN1-R1, YPD1, and SSK1-R2 are estimated to be 0.03, 0.29, and 0.05 μM, respectively2. Given a Kd of 2.8 μM for the SLN1-R1∼P·YPD1 complex, the concentration of the complex formed is estimated to be 0.005 μM, 16 % of the limiting component, SLN1-R1. As a result, it is the second order rate constant, kfwd/Kd that will be physiologically important. This is equally true of the YPD1∼P·SSK1-R2 complex. At limiting concentrations of the components in the reaction, the second order rate constant applies and includes all processes from the initial binding of the components to give a productive complex through the dissociation of the product complex. For Schemes 1 and 2, rate constants k1 through k3 and k4 through k6 will be included in kfwd/Kd for the SLN1-R1∼P to YPD1 and YPD1∼P to SSK1-R2 reactions, respectively. The rate constant for reformation of the SLN1-R1·YPD1∼P complex from SLN1-R1 and YPD1∼P (k−3) or the YPD1·SSK1-R2∼P complex from YPD1∼P and SSK1-R2 (k−6) are not included in the second order rate constant. Thus, any changes in the Kd values of the reactant complex, i.e., the SLN1-R1∼P·YPD1 and YPD1∼P·SLN1-R2 complexes, are accounted for in the second order rate constant. This would include a change in the half-life for the reactant complexes in the presence of viscosogen.
For both phosphotransfer reactions, SLN1-R1∼P to YPD1 and YPD1∼P to SSK1-R2 in the presence of NaCl, no significant change in kfwd/Kd or Kd is observed up to 0.6 M NaCl. Thus, NaCl exhibits no significant effect on the SLN1-R1∼P and YPD1 or YPD1∼P and SSK1-R2 rates of phosphotransfer.
The concentration of NaCl at 1 M was not considered physiologically relevant for these experiments. Upon osmotic shock, the yeast cell volume can decrease by as much as 50-60%, which accordingly would increase the internal osmolyte concentration by only two- to three-fold (43). Under normal conditions, a basal internal concentration of NaCl and KCl within S. cerevisiae is approximately 0.2 M (44), consequently upon hyperosmotic shock this concentration will raise to approximately 0.6 M.
The effect of glycerol on phosphotransfer kinetics is in general different than the effect of NaCl. For the SLN-R1∼P·YPD1 complex, the Kd increases by about 6-fold as the glycerol concentration increases to 1.2 M (Table 2), suggesting less initial complex is present at the same SLN1-R1∼P and YPD1 concentrations, compared to the absence of glycerol. In addition, the second-order rate constant for the forward reaction (kfwd/Kd), Fig. 2A, decreases with an increase in glycerol concentration, and thus glycerol alone cannot contribute to an increase in phosphorylation of SSK1-R2 in the osmoadaptation phase, once osmotic pressure has been re-established.
In the case of the YPD1∼P·SSK1-R2 complex, the Kd does not change as glycerol concentration increases to 1.2 M. Although, kfwd and krev increase slightly, the second-order rate constant (kfwd/Kd) remains relatively constant. Therefore, the net effect of glycerol on the YPD1∼P·SSK1-R2 complex parallels the effect of glycerol on SLN1-R1∼P·YPD1 complex, and glycerol alone does not give a net increase in phospho-SSK1-R2.
The combinatory effects of NaCl and glycerol on the pathway, however, are more revealing. As shown in Table 3, in the case of SLN1-R1∼P·YPD1 complex, the second-order rate constant increases three-fold, as the concentration of glycerol and NaCl increase to 0.75 M and 0.6 M, respectively. A synergistic effect is observed that is different from the effect of glycerol or NaCl alone. For the YPD1∼P to SSK1-R2 phosphotransfer reaction, kfwd/Kd also increases, although by only 30%. The combined effect of NaCl and glycerol is thus to increase the level of phosphorylated SSK1-R2 and return the pathway to its prestimulus state, i.e. the effect of osmotic stress has been attenuated.
The subtle effects of NaCl and glycerol on the SLN1-YPD1-SSK1 phosphorelay system reveal some important regulatory aspects of the cell response to osmotic shock. In the early stages of the cell's response to hyperosmotic stress, immediate water loss can lead to a modest increase in intracellular ion/solute concentrations. The half-life studies in the presence of osmolytes suggest a reduction in SSK1-R2∼P·YPD1 complex stability; thereby leading to a higher rate of SSK1-R2 dephosphorylation. This likely represents one contributing factor that leads to subsequent HOG1 pathway activation. However, in the osmoadaptation phase, after HOG1-dependent transcriptional targets such as GPD1 have been upregulated and intracellular glycerol levels approach molar concentrations, our results indicate that the combinatory effect of high levels of NaCl and glycerol on rates of phosphotransfer favors phosphorylation of SSK1 and signal attenuation. Thus, the studies on the phosphorelay kinetics in the presence of osmolytes provide insight into the post-hyperosmotic shock events, restoration of intracellular homeostasis and cessation of glycerol production. These studies explain for the first time the effects of osmolytes on the SLN1-YPD1-SSK1 phosphorelay and elucidate some basic aspects of the osmoregulation pathway including the presence of a feedback-like control mechanism and the combinatory effect of NaCl and glycerol.
Acknowledgement
We would like to thank Dr. Fabiola Janiak-Spens for superb technical advice regarding quench flow experiments and experimental procedures.
Footnotes
This work was supported by a U.S. Public Health Service grant (GM 59311) to A.H.W. from the National Institutes of Health, and a grant from the Oklahoma Center for Advancement of Science and Technology (OCAST) to A.H.W. (HR 06-123), and the Grayce B. Kerr endowment to the University of Oklahoma to support the research of Dr. P.F. Cook.
Abbreviations: ATP, adenosine-5′-triphosphate; DTT, dithiothreitol; EDTA, ethylenediaminetetraacetic acid; GST, glutathione-Stransferase; HK, histidine kinase; HPt, histidine-containing phosphotransfer; IPTG, isopropyl β-D-thiogalactopyranoside; PAGE, polyacrylamide gel electrophoresis; PCR, polymerase chain reaction; PMSF, phenylmethanesulfonyl fluoride; RR, response regulator; MAP, mitogen-activated protein; MAPK, MAP kinase; MAPKK, MAP kinase kinase; MAPKKK, MAP kinase kinase kinase; SDS, sodium dodecyl sulfate.
An average cell volume of 37 μm3 (1) was used to calculate the protein concentrations inside the cell using number of molecules/cell given in the Saccharomyces Genome Database website (www.yeastgenome.org).
References
- 1.Tyson CB, Lord PG, Wheals AE. Dependency of size of Saccharomyces cerevisiae cells on growth rate. J. Bacteriol. 1979;138:92–98. doi: 10.1128/jb.138.1.92-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 3.West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001;26:369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 4.Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 5.Morgan BA, Bouquin N, Johnston LH. Two-component signal-transduction systems in budding yeast MAP a different pathway? Trends Cell Biol. 1995;5:453–457. doi: 10.1016/s0962-8924(00)89114-x. [DOI] [PubMed] [Google Scholar]
- 6.Wurgler-Murphy SM, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem. Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]
- 7.Saito H. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem. Rev. 2001;101:2497–2509. doi: 10.1021/cr000243+. [DOI] [PubMed] [Google Scholar]
- 8.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ota IM, Varshavsky A. A yeast protein similar to bacterial two-component regulators. Science. 1993;262:566–569. doi: 10.1126/science.8211183. [DOI] [PubMed] [Google Scholar]
- 10.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 11.Ketela T, Brown JL, Stewart RC, Bussey H. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG1 pathway. Mol. Gen. Genet. 1998;259:372–378. doi: 10.1007/s004380050824. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Ault A, Malone CL, Raitt D, Dean S, Johnston LH, Deschenes RJ, Fassler JS. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 1998;17:6952–6962. doi: 10.1093/emboj/17.23.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janiak-Spens F, Sparling DP, West AH. Novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain. J. Bacteriol. 2000;182:6673–6678. doi: 10.1128/jb.182.23.6673-6678.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie T, Tatebayashi K, Yamada R, Saito H. Phosphorylated Ssk1 prevents unphosphorylated Ssk1 from activating the Ssk2 MAP kinase kinase kinase in the yeast HOG osmoregulatory pathway. Mol. Cell. Biol. 2008;28:5172–5183. doi: 10.1128/MCB.00589-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janiak-Spens F, Cook PF, West AH. Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system. Biochemistry. 2005;44:377–386. doi: 10.1021/bi048433s. [DOI] [PubMed] [Google Scholar]
- 17.Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 2008;283:7309–7313. doi: 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blomberg A, Adler L. Physiology of osmotolerance in fungi. Adv. Micro. Physiol. 1992;33:145–212. doi: 10.1016/s0065-2911(08)60217-9. [DOI] [PubMed] [Google Scholar]
- 19.Reiser V, Raitt DC, Saito H. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J. Cell Biol. 2003;161:1035–1040. doi: 10.1083/jcb.200301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao W, Deschenes RJ, Fassler JS. Intracellular glycerol levels modulate the activity of Sln1p, a Saccharomyces cerevisiae two-component regulator. J. Biol. Chem. 1999;274:360–367. doi: 10.1074/jbc.274.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrander DB, Gorman JA. The extracellular domain of the Saccharomyces cerevisiae Sln1p membrane osmolarity sensor is necessary for kinase activity. J. Bacteriol. 1999;181:2527–2534. doi: 10.1128/jb.181.8.2527-2534.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albertyn J, Hohmann S, Thevelein JM, Prior BA. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1994;14:4135–4144. doi: 10.1128/mcb.14.6.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albertyn J, Hohmann S, Prior BA. Characterization of the osmotic-stress response in Saccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr. Genet. 1994;25:12–18. doi: 10.1007/BF00712960. [DOI] [PubMed] [Google Scholar]
- 25.Blomberg A, Adler L. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J. Bacteriol. 1989;171:1087–1092. doi: 10.1128/jb.171.2.1087-1092.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rep M, Krantz M, Thevelein JM, Hohmann S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. J. Biol. Chem. 2000;275:8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- 27.Millar JB. Stress-activated MAP kinase (mitogen-activated protein kinase) pathways of budding and fission yeast. Biochem. Soc. Symp. 1999;64:49–62. [PubMed] [Google Scholar]
- 28.Aiba H, Yamada H, Ohmiya R, Mizuno T. The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 1995;376:199–201. doi: 10.1016/0014-5793(95)01277-4. [DOI] [PubMed] [Google Scholar]
- 29.Thome PE, Trench RK. Osmoregulation and the genetic induction of glycerol-3-phosphate dehydrogenase by NaCl and the euryhaline yeast Debaryomyces hansenii. Mar. Biotechnol. 1999;1:230–238. doi: 10.1007/pl00011772. [DOI] [PubMed] [Google Scholar]
- 30.Fan J, Whiteway M, Shen S-H. Disruption of a gene encoding glycerol 3-phosphatase from Candida albicans impairs intracellular glycerol accumulation-mediated salt-tolerance. FEMS Microbiol. Letts. 2005;245:107–116. doi: 10.1016/j.femsle.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Shankarnarayan S, Malone CL, Deschenes RJ, Fassler JS. Modulation of yeast Sln1 kinase activity by the CCW12 cell wall protein. J. Biol. Chem. 2008;283:1962–1973. doi: 10.1074/jbc.M706877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995;14:1360–1371. doi: 10.1002/j.1460-2075.1995.tb07122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 34.Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 35.Klipp E, Nordlander B, Krüger R, Gennemark P, Hohmann S. Integrative model of the response of yeast to osmotic shock. Nature Biotech. 2005;23:975–982. doi: 10.1038/nbt1114. [DOI] [PubMed] [Google Scholar]
- 36.Hohmann S. Shaping up: The response of yeast to osmotic stress. In: Hohmann S, Mager WH, editors. Yeast Stress Responses. Chapman & Hall; New York: 1997. pp. 101–145. [Google Scholar]
- 37.Blacklow SC, Raines RT, Lim WA, Zamore PD, Knowles JR. Triosephosphate isomerase catalysis is diffusion controlled. Biochemistry. 1988;27:1158–1167. doi: 10.1021/bi00404a013. [DOI] [PubMed] [Google Scholar]
- 38.Janiak-Spens F, Sparling JM, Gurfinkel M, West AH. Differential stabilities of phosphorylated response regulator domains reflect functional roles of the yeast osmoregulatory SLN1 and SSK1 proteins. J. Bacteriol. 1999;181:411–417. doi: 10.1128/jb.181.2.411-417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimshaw CE, Huang S, Hanstein CG, Strauch MA, Burbulys D, Wang L, Hoch JA, Whiteley JM. Synergistic kinetic interactions between components of the phosphorelay controlling sporulation in Bacillus subtilis. Biochemistry. 1998;37:1365–1375. doi: 10.1021/bi971917m. [DOI] [PubMed] [Google Scholar]
- 40.Stewart RC. Kinetic characterization of phosphotransfer between CheA and CheY in the bacterial chemotaxis signal transduction pathway. Biochemistry. 1997;36:2030–2040. doi: 10.1021/bi962261k. [DOI] [PubMed] [Google Scholar]
- 41.Fisher SL, Kim S-K, Wanner BL, Walsh CT. Kinetic comparisons of the specificity of the vancomycin resistance kinase VanS for two response regulators, VanR and PhoB. Biochemistry. 1996;35:4732–4740. doi: 10.1021/bi9525435. [DOI] [PubMed] [Google Scholar]
- 42.Mayover TL, Halkides CJ, Stewart RC. Kinetic characterization of CheY phosphorylation reactions: Comparison of P-CheA and small-molecule phosphodonors. Biochemistry. 1999;38:2259–2271. doi: 10.1021/bi981707p. [DOI] [PubMed] [Google Scholar]
- 43.Morris GJ, Winters L, Coulson GE, Clarke KJ. Effect of osmotic stress on the ultrastructure and viability of the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 1983;129:2023–2034. doi: 10.1099/00221287-132-7-2023. [DOI] [PubMed] [Google Scholar]
- 44.Ferrando A, Kron SJ, Rios G, Fink GR, Serrano R. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell. Biol. 1995;15:5470–5481. doi: 10.1128/mcb.15.10.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]