Abstract
Sialic acids are key structural determinants and contribute to the functionality of a number of immune cell receptors. Previously, we demonstrated that differentiation of human dendritic cells (DCs) is accompanied by an increased expression of sialylated cell surface structures, putatively through the activity of the ST3Gal.I and ST6Gal.I sialyltransferases. Furthermore, DC endocytosis was reduced upon removal of the cell surface sialic acid residues by neuraminidase. In the present work, we evaluate the contribution of the sialic acid modifications in DC maturation. We demonstrate that neuraminidase-treated human DCs have increased expression of major histocompatibility complex (MHC) and costimulatory molecules, increased gene expression of specific cytokines and induce a higher proliferative response of T lymphocytes. Together, the data suggest that clearance of cell surface sialic acids contributes to the development of a T helper type 1 proinflammatory response. This postulate is supported by mouse models, where elevated MHC class II and increased maturation of specific DC subsets were observed in DCs harvested from ST3Gal.I−/− and ST6Gal.I−/− mice. Moreover, important qualitative differences, particularly in the extent of reduced endocytosis and in the peripheral distribution of DC subsets, existed between the ST3Gal.I−/− and ST6Gal.I−/− strains. Together, the data strongly suggest not only a role of cell surface sialic acid modifications in maturation and functionality of DCs, but also that the sialic acid linkages created by different sialyltransferases are functionally distinct. Consequently, with particular relevance to DC-based therapies, cell surface sialylation, mediated by individual sialyltransferases, can influence the immunogenicity of DCs upon antigen loading.
Keywords: cell maturation, dendritic cell, major histocompatibility complex, sialic acid, tumour immunity
Introduction
Sialic acids are typically found as terminal monosaccharides of glycans carried by cell surface glycoproteins. Over 20 different sialyltransferases are known to mediate the sialylation and generate sialic acid diversity.1,2 As a result of their terminal position, sialic acids are key structural determinants for a number of cell surface receptors involved in the immune response, such as the Siglecs and selectins (reviewed in refs 3 and 4). Sialic acid moieties are also ligands for a diverse array of exogenous lectins, from other host cells and invasive pathogens.5 Deficiencies in specific sialylation result in immunological abnormalities in animal experiments, such as aberrant T-lymphocyte counts in the mice deficient for ST3Gal.I,6 and altered B-lymphocyte proliferation in mice deficient for ST6Gal.I.7
Dendritic cells (DCs) are special antigen-presenting cells that uptake, process and present antigens found in peripheral blood and tissues to lymphocytes and regulate cellular and humoral immune responses.8 Antigen uptake, as well as inflammatory stimuli, induces DC maturation, enabling DC interaction with T lymphocytes and the initiation of adaptive immune responses.9 Maturation of DC is characterized by phenotypic and physiological changes, i.e. the antigen-uptake machinery is down-regulated10 and the expression of surface major histocompatibilty complex class II (MHC II), class I (MHC I) and costimulatory molecules, such as CD80 and CD86, is up-regulated.11 Ultimately, the synthesis of DC-specific cytokines is changed12 contributing to the polarization towards T helper type 1 (Th1) or type 2 (Th2),13 with the type and context of stimuli and the consequent activation state of DC affecting its Th1- or Th2-promoting effector function.13
As a result of their role in initiating the specific immune response, monocyte-derived DCs (moDCs) are currently used in immune adoptive vaccine protocols to treat cancer patients.14 However under some circumstances (such as lack of or inappropriate maturation), DCs can also induce and maintain antigen tolerance,15,16 a situation counterproductive to the therapeutic value of DC therapy.
Currently, there is great interest in altering the DC immunogenicity towards Th1-promoting cells, and subsequent use in immunotherapy against cancer, because most tumour antigens are self antigens and therefore poor immunogens. The identification of mechanisms for DC activation is critical to more successful exploitation of the DC modality in the prevention and treatment of cancer.
Evidence from different groups suggests that the state of sialylation of DCs may influence their response.17,18 We have previously studied the surface sialylation of human moDCs and we observed a significantly increased expression of sialylated structures during the differentiation of monocytes into moDCs, most probably as the result of the activity of ST3Gal.I and ST6Gal.I sialyltransferases.19 In addition, we have also observed that the removal of the sialylated structures by neuraminidase treatment diminished the moDC capacity for endocytosis,19 suggesting a triggering of DC maturation. Boog et al.17, showed that removal of sialic acid restored specific immune responses to mice non-responder DCs, leading to the proposal that reduction of sialic acid contributed to DC activation. In the present study we analysed the consequences of reduced sialylation, in human DCs, to evaluate its contribution to the triggering of cell maturation.
Our data demonstrated that neuraminidase treatment results in increased expression of MHC and costimulatory molecules, and affects some aspects of the functionality of human DCs. Namely, neuraminidase-treated DCs show increased gene transcription of proinflammatory and Th1-promoting cytokines and induce greater proliferative responses of T lymphocytes. To correlate a specific sialylation deficiency with the triggering of DC maturation, we also analysed DCs from ST3Gal.I−/− and ST6Gal.I−/− mice. The expression of specific maturation markers were examined in ex vivo DCs obtained from blood, lymph nodes and spleen. The results show an increased expression of MHC II molecules in ST6Gal.I−/− DCs, mainly in the B220+ DC subset. The DCs differentiated in vitro from bone marrow progenitors (BMDCs) of both deficient mouse strains presented increased expression of MHC II molecules and a reduced capacity for endocytosis, when compared with wild-type (WT) mice with normal sialyltransferase expression profiles. Overall, these data suggest a novel role for the sialylated glycans; in particular, those generated by ST3Gal.I and ST6Gal.I sialyltransferases, in the modulation of the DC maturation state. Finally, we suggest that the immunogenicity of antigen-loaded DCs used in cell vaccines may depend on its sialylation content.
Materials and methods
Media and reagents
RPMI-1640 supplemented with 10% fetal calf serum from Sigma (St Louis, MO, USA), 2 mm l-glutamine, 1% non-essential amino acids, 1% pyruvate, 100 μg/ml penicillin/streptomycin and 50 μm 2-mercaptoetanol, all from Gibco-Invitrogen (Paisley, UK) was used throughout this study for cell culture. Human and mouse recombinant interleukin-4 (IL-4) and granulocyte–macrophage colony-stimulating factor (GM-CSF) were purchased from R&D Systems (Minneapolis, MN). Neuraminidase from Clostridium perfringens and collagenase D from Clostridium histolyticum were from Roche Diagonostics (Basel, Switzerland). Tetanus toxoid (TT) and mitomycin C were purchased from Sigma. Fluorescein isothiocyanate (FITC) -conjugated ovalbumin and carboxyfluorescein diacetate succinimidyl ester (CFSE) were purchased from Molecular Probes (Leiden, the Netherlands). Phytohaemagglutinin was obtained from Fluka (Buchs, Switzerland). Anti-human, anti-mouse and isotype control monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Diego, CA), except when otherwise stated.
Human cell isolation and generation of DCs
Human moDCs were generated from monocytes isolated, as described previosuly19, by positive selection using CD14 antibody-coated magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany), from peripheral blood mononuclear cells (PBMCs) of healthy volunteers provided and ethically approved by the Portuguese Blood Institute. Monocytes were cultured in complete RPMI-1640 medium supplemented with 1000 U/ml of recombinant human IL-4 (rhIL-4) and rhGM-CSF, for 6 days, to give rise to immature moDC.
For T-lymphocyte proliferation assays, moDCs were generated, as described above, from monocytes isolated, after informed consent, from PBMCs of healthy volunteers who had been vaccinated with TT within the previous 12 months. In these cases, the negative fractions (CD14− PBMCs), obtained after monocyte isolation, were maintained in culture until mixed lymphocyte cultures with autologous moDCs. On day 5, moDCs were pulsed with or without 5 μg/ml TT and, 18 hr later, moDCs were inactivated with 0·05 mg/ml mitomycin C for 30 min and washed three times with 10 ml RPMI-1640 medium. These conditions were previously optimized, by testing different parameters described in the literature.20,21
Neuraminidase treatment
Cells were resuspended in RPMI-1640 (5 × 106/ml) and treated with 200 mU/ml neuraminidase in serum-free RPMI for 90 min at 37°. In parallel, identical samples were mock-treated in the same conditions with heat-inactivated neuraminidase (20 min at 100°).
Phenotypical analysis of human moDC maturation
For the analysis of the moDC maturation phenotype, 2 × 105 cells were first incubated with 0·1 mg/ml of FITC-conjugated ovalbumin, in RPMI-1640 + 10% fetal calf serum, for 1 hr, at 4° or 37°. Following incubation, moDCs were treated or mock-treated with neuraminidase as described above. Half of the cells were reserved for RNA extraction and real-time polymerase chain reaction (PCR). The remaining cells were washed with FACS Flow (BD Biosciences, Franklin Lakes, NJ) and stained with either phycoerythrin (PE)-labelled anti-human leucocyte antigen (HLA)-DR (L243), anti-HLA-ABC (W6/32) (Dako Glostrup, Denmark), anti-CD80 (L307.4) or PE-Cy5-labelled anti-CD86 (IT2.2) and analysed by flow cytometry. All samples were analysed using a FacsCalibur Flow cytometer and Cell Quest® (BD Biosciences) and Infinicyt® software (Cytognos, Spain).
Real-time PCR
The RNA was extracted from 1 × 106 moDCs using the RNeasy Mini Kit (Qiagen, Chatsworth, CA) and genomic DNA was eliminated with the RNase-Free DNase Set (Qiagen), as described by the manufacturer. The concentrations of the RNA samples were determined spectrophotometrically. The RNA was reverse transcribed with random primers using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA).
Real-time PCR was performed in a 7500 Fast Real-Time PCR System (Applied Biosystems) using TaqMan® Fast Universal PCR Master Mix, TaqMan® probes and primers provided by Applied Biosystems. The assay ID provided by the manufacturer are the following: CD1a (Hs00233332_m1); CD1b (Hs00233507_m1), CD1c (Hs00233509_m1); IL-6 (Hs00174131_m1); IL-12α (Hs00168405_m1); IL-1β (Hs00174097_m1); tumour necrosis factor-α (TNF-α; Hs00174128_m1); IL-10 (Hs00174086_m1); β-actin (4352935E); glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 4333764F). Each reaction was performed in duplicate. Thermal cycling conditions were 95° for 20 seconds followed by 40 cycles of 95° for 3 seconds and 60° for 30 seconds.
The messenger RNA (mRNA) expression was normalized using the geometric mean of the expression of the β-actin and GAPDH genes as a reference. The relative expression for each gene was calculated according to the 2−ΔΔCt method described by Livak and Schmittgen.22 The efficiency of the amplification reaction for each primer/probe is above 95% (as determined by the manufacturer).
T-lymphocyte proliferation assays
CD14-negative PBMCs, obtained as described above, were labelled with 5·56 μg/ml CFSE, for 15 min at 37°, according to the manufacturer’s instructions. The labelled cells were then incubated with the autologous, TT-loaded moDCs (treated or mock-treated with neuraminidase), in the proportion of 8 : 1, in a 96-well round-bottom plate, for 7 days. As a positive control for T-lymphocyte proliferation, PBMCs were stimulated with 2 μg/ml phytohaemagglutinin, for 3 days. Evaluation of the T-lymphocyte proliferation was performed by flow cytometry, after staining the samples with allophycocyanin-labelled anti-CD3 (clone 33-2A3) (Immunostep, Salamanca, Spain). The number of proliferating T lymphocytes was calculated as the percentage of CD3+ cells, with decreased CFSE fluorescence.
Isolation and generation of mice DCs
ST3Gal.I−/− and ST6Gal.I−/− mice were obtained from the Consortium For Functional Glycomics and originally provided by Priatel et al.6 and Hennet et al.7, respectively. Mice were used at 10–12 weeks of age and C57BL/6 WT animals were used as controls. All handling of animals was previously approved by the Ethics Committee of the Medical Sciences Faculty of Lisbon, under the supervision of officially recognized animal handlers.
ST3Gal.I−/−, ST6Gal.I−/− and C57BL/6 mice were killed by asphyxiation with CO2; their spleen and axillar lymph nodes were removed and their blood was collected by cardiac puncture into tubes containing ethylenediaminetetraacetic acid. Cells from spleens and the axillar lymph nodes were obtained by flushing with culture medium and mechanical disruption. Spleens were also incubated for 20 min at 37° with collagenase D (1 mg/ml) and submitted to red blood cell lysis (Tris–HCl 0·17 m, NH4Cl 0·16 m) for 5 min at room temperature.
The BMDC were obtained mainly as described previously.23 Briefly, the bone marrow was flushed from tibiae and femurs with complete medium. The cell suspension was filtered and exposed to red blood cell lysis as described above. Bone marrow cells were cultured at 5 × 105 cells/ml on six-well plates, in culture medium supplemented with GM-CSF and IL-4, for 6 days. The resulting DCs were then magnetically separated by anti-CD11c antibody-coated-magnetic beads (Miltenyi) and confirmed by anti-CD11c staining. The average separation efficiency was at least 85%.
Endocytosis and phenotypic analysis of BMDCs
The endocytosis assays with mouse BMDCs were performed by minor modifications of the above described moDCs endocytosis assay. Briefly, 3 × 105 cells/ml of BMDC were incubated, for 30 min, with FITC-conjugated ovalbumin at a final concentration of 0·05 mg/ml, at 4° or 37°. After stopping the reaction, the cells were stained with PE-labelled anti-MHC II (I-Ab) (AF6-120·1). To neutralize fluorescent ovalbumin linkage at the cell surface, trypan blue was added and cells were washed twice before acquisition by flow cytometry.
Flow cytometry analysis of mouse DC subsets
Blood, lymph node and splenic cells were stained with allophycocyanin-labelled anti-CD11c (HL3), peridinin chlorophyll protein-labelled anti-CD8α (53-6.7) or FITC-labelled anti-B220(RA3-6B2) in combination with anti-CD11b (NS-1), anti-CD86 (GL1) or anti-MHC II (I-Ab) (AF6-120.1) PE-labelled mAbs. Samples were analysed as described above. The DCs were considered CD11c- and I-Ab-positive cells. The cut-off point for positive staining was above the level of the control isotype mAbs.
Statistical analysis
Statistical comparison between two different groups was performed using Student’s t-test (GraphPad, GraphPad Software, La Jolla, CA). Statistical significance was defined as P< 0·05.
Results
Clearance of cell surface sialic acids by neuraminidase treatment enhances cell surface expression of MHC I, MHC II, CD80 and CD86
In our previous work, we have shown that human moDCs, after treatment with neuraminidase, had a lower endocytosis capacity (only 75% uptake of ovalbumin).19 A possible explanation for this observation is that neuraminidase treatment triggered DC maturation, a process known to be accompanied by diminished antigen uptake. DC activation was unlikely to be the result of heat stable factors in the neuraminidase preparation, such as bacterial endotoxins, because control DCs treated in parallel with heat-inactivated neuraminidase did not show decreased endocytosis. To assess maturation status of the DCs after neuraminidase treatment, we have analysed, by flow cytometry, the expression of MHC I and MHC II and the costimulatory molecules, CD80 and CD86, by moDCs during endocytosis and when treated with neuraminidase. The efficiency of the neuraminidase treatment was evaluated by appropriate lectin binding assays as described elsewhere19 (data not shown).
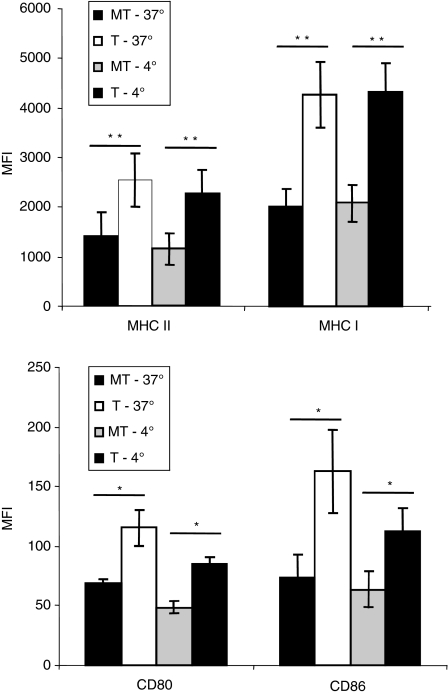
At the end of endocytosis, the expression level of MHC I, MHC II, CD80 and CD86 molecules was significantly higher in the moDCs treated with neuraminidase compared with mock-treated cells (Fig. 1). To further investigate whether this increased expression was mainly the result of the neuraminidase treatment itself, we have also performed control assays with moDCs which had not undergone endocytosis (assays performed at 4°). According to our results, the expression level of these markers was also significantly elevated in the cells treated with neuraminidase, although, except for MHC I, with less extent (Fig. 1). Control assays with the moDCs incubated in the absence of the endocytic agent, were comparable to the assays carried out at 4° in the presence of the agent (data not shown). The neuraminidase treatment causes a significant increase of MHC I and MHC II expression in moDCs, as well as the expression of costimulatory molecules, CD80 and CD86, and this is even more eminent when followed by endocytosis.
Figure 1.
Increased expression of major histocompatibility complex class II (MHC II), class I MHC I, CD80, CD86 in human monocyte-derived dentritic cells (moDCs) after neuraminidase treatment. Neuraminidase treated (T) or mock-treated (i.e. inactivated neuraminidase) (MT) moDCs were pulsed with the endocytic agent ovalbumin (0·05 mg/ml) and incubated for 1 hr at 37° or 4° (control). After the endocytosis assay, the expression of MHC II (HLA DR), MHC I (HLA ABC) and the costimulatory molecules CD80 and CD86 were evaluated by flow cytometry. The results are the mean fluorescence intensity (MFI) of at least three individual experiments ± SEM. Significantly different values (*P< 0·05 or **P< 0·01 paired Student’s t-test) were observed for all the maturation markers employed, comparing neuraminidase-treated with mock-treated human moDCs, either in cells that had internalized ovalbumin (assay at 37°) or in the control cells (assay at 4°).
Besides MHC molecules, human DCs prominently express other antigen-presenting molecules, the CD1 family (CD1a, -b and -c) that mediate the presentation of lipid and glycolipid antigens to T lymphocytes.24 The trafficking of such molecules to the plasma membrane is generally independent of the DC maturation stage,25 except in the case of strict stimulus.26,27 In the case of neuraminidase treatment of moDCs, we have addressed, in parallel, the CD1a, CD1b and CD1c gene expression, but no significant differences were found (data not shown), suggesting that the neuraminidase treatment does not alter the expression of CD1 on the cell surface in moDCs.
Altered expression of cytokines in neuraminidase-treated DCs
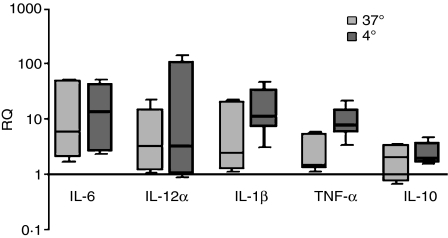
To investigate the functional consequence of the exposure of human moDCs to neuraminidase, the expression of IL-6, IL-12α, IL-1β, TNF-α and IL-10 mRNAs were analysed by quantitative real-time PCR. Although there was some sample-to-sample variation, at the end of endocytosis, all neuraminidase-treated moDC samples had increased levels of IL-6 (around 2 to 50-fold) compared with the corresponding mock-treated moDCs (Fig. 2). The mRNAs for IL-12α, IL-1β and TNF-α were also augmented by the neuraminidase treatment in the majority of the samples, although in one out of five samples this increase was almost insignificant (Fig. 2). Regarding the IL-10 expression, even though a number of moDC samples presented ± twofold increased expression, after endocytosis and neuraminidase treatment, two out of five samples showed a slight IL-10 down-regulation (Fig. 2). As above, we also investigated the effect of neuraminidase itself, by analysing the expression of these cytokines in the moDCs which had not undergone endocytosis. Our results showed that the neuraminidase treatment per se led to a similar increased expression of IL-12α and IL-6 (Fig. 2). For the IL-10, IL-1β and TNF-α, there was an up-regulation slightly higher than the neuraminidase-treated moDCs which had undergone endocytosis (Fig. 2). Therefore, removing the sialic acid in moDCs led to an increased expression of IL-6, IL-1β, IL-12α and TNF-α genes. However, the range of up-regulation seemed to depend upon the individual donor and to be slightly lessened during endocytosis.
Figure 2.
Expression of cytokines is altered in neuraminidase-treated human monocyte-derived dendritic cells (moDCs). Neuraminidase treated and mock-treated (i.e. inactivated neuraminidase) moDCs were pulsed with ovalbumin, as described in the Materials and methods. After endocytosis, total RNA was used to generate complementary DNA that was analysed by real-time polymerase chain reaction. Boxes represent minimum and maximum of the mean relative expression, RQ, (± SEM) of interleukin-6 (IL 6), IL-12α, IL-1β, tumour necrosis factor-α (TNF-α) and IL-10 genes. The results (median with interquartile range) represent the expression of a given gene in five different neuraminidase-treated moDCs compared with the expression in the respective mock-treated moDCs. Values above or below one indicate, respectively, over-expression or down-regulation of a specific gene after neuraminidase treatment of moDCs, either in cells that have internalized ovalbumin (assay at 37°) or in the control cells (assay at 4°).
Increased T-lymphocyte priming induced by neuraminidase-treated human DCs
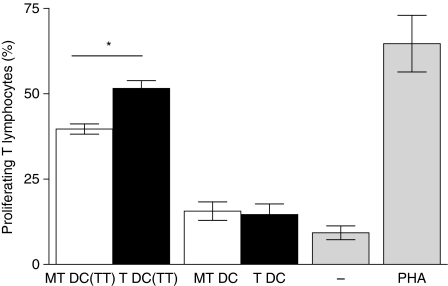
To further investigate the functional effect of neuraminidase treatment on human DCs, we evaluated the ability of either neuraminidase-treated or mock-treated moDC to induce autologous T-lymphocyte proliferation. We have performed mixed lymphocyte cultures with moDCs loaded with TT, treated or mock-treated with neuraminidase, and autologous CFSE-labelled T lymphocytes. According to our results, the number of proliferating T lymphocytes is significantly increased when antigen-loaded moDCs lose their surface sialic acid (Fig. 3), as the mean value for mixed lymphocyte cultures with moDCs loaded with TT, treated or mock-treated with neuraminidase were 51% and 39% of CD3+ cells, respectively (P= 0·03). It should be noted that the immunogenicity of ovalbumin-pulsed DCs and TT-pulsed DCs may not be the same. Nevertheless, we chose TT as antigen and not ovalbumin because of the specific need for antigen-specific human lymphocyte responders for the mixed lymphocyte culture.
Figure 3.
Increased T-lymphocyte proliferation in mixed leucocyte reactions with neuraminidase-treated dendritic cells (DCs). Human monocyte-derived DCs (moDCs) were stimulated with tetanus toxoid (TT; 5 μg/ml), inactivated with mitomycin C and treated [T DC(TT)] or mock-treated (i.e. inactivated neuraminidase) [MT DC(TT)] with neuraminidase, as described in the Materials and methods. Control assays with unstimulated moDCs, treated (T DC) or mock-treated (MT DC) with neuraminidase, were also performed in parallel. The moDCs were then cultured with autologous, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled CD14− peripheral blood mononuclear cells (PBMCs; 1 : 8 ratio), for 7 days. As a control, CD14− PBMCs were cultured alone, in the absence or presence of phytohaemagglutinin. Cells were stained with allophycocyanin-labelled anti-CD3 and analysed by flow cytometry. Graph represents the percentage of proliferating T lymphocytes, of five individual experiments ± SEM, estimated by the percentage of CFSE-diluted, CD3+ cells. Significantly different values were observed for the percentage of T-lymphocyte proliferation induced by T DC(TT) (*P< 0·05 paired Student’s t-test), when compared with the one induced by MT DC(TT).
DCs ex vivo from ST6Gal.I−/− mice show an augmented cell surface expression of MHC II
In our previous work, we studied the sialyltransferases involved in human moDC sialylation and highlighted the significant contribution of ST3Gal.I and ST6Gal.I.19 Having observed that neuraminidase treatment induced DC maturation we wished to further inspect the type of sialylation, whose shortage was involved in this process, by focusing on ST3Gal.I-deficient and ST6Gal.I-deficient DCs. Therefore, the following question was whether ex vivo DCs from mice deficient in one of these two sialyltransferases presented a more mature phenotype. To address this question we analysed the expression of MHC II and CD86 in DCs present in their blood, lymph nodes and spleens.
The MHC II expression was higher in ST6Gal.I−/− DCs when compared with WT mice, but no apparent differences were observed regarding CD86 expression (Table 1). ST3Gal.I-deficient mice presented insignificant variation of the MHC II and CD86 expression (Table 1).
Table 1.
Expression of major histocompatibility complex class II and CD86 in ST3Gal.I−/− and ST6Gal.I−/− mouse dendritic cells compared with wild-type control
| N | MHC II1 | MHC II (B220+)2 | CD861 | ||
|---|---|---|---|---|---|
| Blood | WT | 8 | 157·0 ± 40·7 | 117·3 ± 20·6 | 22·78 ± 0·55 |
| ST3Gal.I−/− | 6 | 236·0 ± 59·5 | 166·0 ± 60·2 | 20·19 ± 1·79 | |
| ST6Gal.I−/− | 10 | 280·4 ± 37·9 *P= 0·046 | 272·2 ± 26·9 ***P= 0·0005 | 26·55 ± 2·01 | |
| Spleen | WT | 6 | 575·9 ± 61·4 | 784·8 ± 94·0 | 66·55 ± 5·37 |
| ST3Gal.I−/− | 7 | 554·0 ± 140·5 | 654·2 ± 92·5 | 65·65 ± 5·23 | |
| ST6Gal.I−/− | 11 | 903·1 ± 91·0 **P= 0·009 | 1047 ± 93·8 | 62·75 ± 7·28 | |
| Lymph nodes | WT | 7 | 975·6 ± 105·8 | 864·3 ± 153 | 209·4 ± 47·6 |
| ST3Gal.I−/− | 6 | 1465 ± 366·8 | 1197 ± 171 | 198·0 ± 14·4 | |
| ST6Gal.I−/− | 9 | 1498 ± 74·5 **P= 0·007 | 1810 ± 177 **P= 0·003 | 245·0 ± 44·5 |
DCs, dendritic cells; MHC II, major histocompatibility complex class II; WT, wild-type.
DCs were considered CD11c and MHC II positive cells.
N indicates the number of mice used for each determination.
Values are the mean ± SEM of mean fluorescence intensity (MFI).
Values are related to total DCs.
Values related to plasmacytoid (B220+) DCs.
To determine whether the increased expression of MHC II in ST6Gal.I−/− DCs could be linked to one particular DC subset, we focused our analysis on the lymphoid, plasmacytoid and conventional DC subsets, based on the DC expression of CD8α, B220 and CD11b markers, respectively (Table 1). When examining each of these subsets for the expression of MHC II and CD86, we noted that the increased MHC II expression, detected in the total ST6Gal.I−/− DCs, was mainly present in the plamacytoid (B220+) DC subset (Table 1). These results suggest that the more mature phenotype found in DCs from ST6Gal.I−/− mice, was mainly related to the plasmacytoid DC subset.
Surprisingly, when we studied the distribution of lymphoid, plasmacytoid and conventional DC subsets, we found differences in the ST3Gal.I-deficient mice, which presented not only an increased percentage of total DCs, but also a significant inferior percentage of CD8α+ DCs and, conversely, increased number of B220+ DCs in the three analysed sources (Table 2). The surface expression of each subset marker was also evaluated based on the overall intensity values, but no significant differences were found (data not shown).
Table 2.
Distribution of dendritic cell subsets in ST3Gal.I−/− and ST6Gal.I−/− mice compared with wild-type control mice
| N | % DCs1 | % Lymphoid DCs (CD8α+)2 | % Plasmacytoid DCs (B220+)2 | % Conventional DCs (CD11b+)2 | ||
|---|---|---|---|---|---|---|
| Blood | WT | 8 | 0·938 ± 0·08 | 4·400 ± 0·88 | 23·94 ± 2·16 | 7·353 ± 0·79 |
| ST3Gal.I−/− | 6 | 1·208 ± 0·09 *P= 0·044 | 0·7183 ± 0·32 **P= 0·002 | 43·03 ± 3·62 **P= 0·002 | 8·500 ± 2·26 | |
| ST6Gal.I−/− | 10 | 0·797 ± 0·06 | 4·435 ± 0·51 | 17·98 ± 1·20 | 5·535 ± 0·69 | |
| Spleen | WT | 6 | 6·426 ± 0·89 | 11·12 ± 1·09 | 25·19 ± 2·13 | 25·65 ± 4·94 |
| ST3Gal.I−/− | 7 | 11·82 ± 1·04 **P= 0·004 | 7·300 ± 1·30 *P= 0·048 | 40·56 ± 5·56 *P= 0·048 | 36·44 ± 2·28 | |
| ST6Gal.I−/− | 11 | 5·074 ± 0·72 | 8·071 ± 0·89 | 32·10 ± 1·52 *P= 0·025 | 30·13 ± 3·66 | |
| Lymph nodes | WT | 7 | 3·042 ± 0·47 | 10·19 ± 1·61 | 35·79 ± 3·45 | 28·18 ± 6·82 |
| ST3Gal.I−/− | 6 | 7·220 ± 0·44 ***P= 0·0002 | 3·720 ± 1·81 *P= 0·032 | 54·80 ± 5·10 *P= 0·015 | 42·09 ± 6·65 | |
| ST6Gal.I−/− | 9 | 3·690 ± 0·75 | 9·929 ± 1·98 | 41·11 ± 1·97 | 34·16 ± 6·24 |
DCs, dendritic cells; MHC II, major histocompatibility complex class II; WT, wild-type.
DCs were considered CD11c and MHC II positive cells.
N indicates the number of mice used for each determination.
Percentage related to total leucocytes.
Percentage related to total DCs.
ST3Gal.I−/− and ST6Gal.I−/− BMDCs present lower endocytosis capacity and increased cell surface expression of MHC II
Since we demonstrated that at least ex vivo ST6Gal.I−/− DCs presented increased expression of MHC II, we then asked whether ST6Gal.I−/− or ST3Gal.I−/− DCs had impaired endocytic capacity. To answer this, we chose DCs differentiated in vitro from bone marrow, because the in vitro generation of DCs from their precursors produces a percentage of DCs much higher than those obtained by isolation from ex vivo sources.
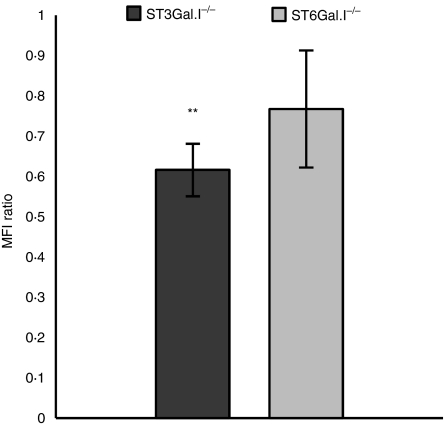
We performed FITC-conjugated ovalbumin endocytosis assays with ST3Gal.I−/− and ST6Gal.I−/− BMDCs. For comparison similar assays with WT BMDCs were always run in parallel. The percentage of BMDCs that endocytosed ovalbumin was roughly identical to that of WT BMDCs (± 62%). However, the fluorescence intensity of FITC-labelled ovalbumin was reduced in BMDCs from ST3Gal.I−/− and ST6Gal.I−/− mice [61% (P= 0·0078) and 76% of fluorescence intensity relative to WT, respectively] (Fig. 4). These observations demonstrate that ST3Gal.I−/− and, to a lesser extent, ST6Gal.I−/− BMDCs endocytose less ovalbumin than WT BMDCs.
Figure 4.
Decreased endocytic ability of ST3Gal.I−/− and ST6Gal.I−/− bone marrow-derived dendritic cells (BMDCs). The ST3Gal.I−/− or ST6Gal.I−/− BMDCs were incubated, for 30 min, with 0·05 mg/ml fluorescein isothiocyanate-conjugated ovalbumin, as described in the Materials and methods. Control assays were performed in parallel with wild-type (WT) BMDCs. The mean fluorescence intensity (MFI) values obtained at 4° were subtracted from the 37° values. The results are expressed as the ratio of the MFI ± SEM of ST3Gal.I−/− BMDCs (six mice) or ST6Gal.I−/− BMDCs (seven mice) related to WT BMDC. Significantly different values were observed for the endocytic capacity of ST3Gal.I−/− BMDC (**P< 0·01).
We also monitored the expression of MHC II in ST3Gal.I−/− and ST6Gal.I−/− BMDCs after undergoing endocytosis. As expected from the ex vivo DC analysis, ST6Gal.I−/− BMDCs showed a higher expression of MHC II than WT (Fig. 5). Unexpectedly, ST3Gal.I−/− BMDCs showed a significant and even higher expression of MHC II (Fig. 5). To investigate whether the increased MHC II expression was exclusively derived from the endocytosis or if it was present, originally, in the ST6Gal.I−/− and ST3Gal.I−/− BMDCs, we have analysed the MHC II expression in the control BMDCs which had not undergone endocytosis. Although not statistically significant, the intensity of MHC II fluorescence was also higher in both control ST6Gal.I−/− and ST3Gal.I−/− BMDCs than control WT BMDCs (Fig. 5).
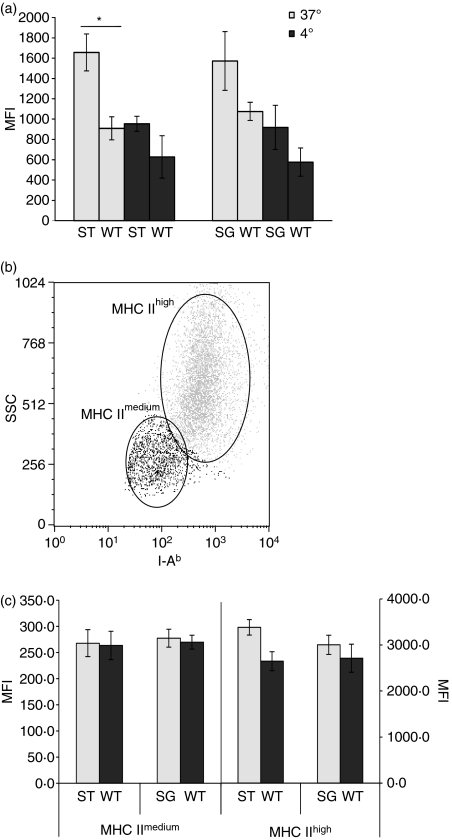
Figure 5.
Major histocompatibility complex (MHC) expression is increased in ST3Gal.I−/− and ST6Gal.I−/− bone marrow-derived dendritic cells (BMDCs). The MHC II expression of ST3Gal.I−/− (ST) and ST6Gal.I−/− (SG) BMDCs was analysed by staining with phycoerythrin-conjugated anti I-Ab, after exposure to fluorescein isothiocyanate-conjugated ovalbumin and incubation at 37° or 4°, as described in the Materials and methods. Control assays were performed in parallel with wild-type (WT) BMDCs. (a) The results are expressed as the mean fluorescence intensity (MFI) ± SEM of ST3Gal.I−/− BMDCs (six mice) and ST6Gal.I−/− BMDCs (seven mice). Significantly different values, related to WT BMDC, were observed at 37°, for the ST3Gal.I−/− BMDCs (*P< 0·05). (b) Dot-plot showing that, after endocytosis (at 37°), BMDCs express different levels of MHC II, defining a MHC IImedium and a MHC IIhigh subpopulation. The data shown correspond to analysis of one representative population of BMDCs (either from WT, ST3Gal.I−/− or ST6Gal.I−/− mice) acquired by flow cytometry. (c) MHC IImedium subpopulations of ST3Gal.I−/− and ST6Gal.I−/− BMDCs are no different from WT BMDCs, while MHC IIhigh subpopulations showed increased MHC II expression. The results are the means ± SEM of the MFI for MHC II for the MHC IImedium and MHC IIhigh subpopulations, gated as described above. The left axis corresponds to the MHC IImedium subpopulation and the right axis corresponds to the MHC IIhigh population.
Considering the strikingly elevated MHC II expression following endocytosis, we decided to examine this parameter in detail. In particular, we wished to address whether the increased intensity of MHC II was applied to all the BMDCs or to a particular subpopulation. After endocytosis, either WT, ST3Gal.I−/− or ST6Gal.I−/− BMDCs presented two distinguishable BMDC subpopulations based on the mean fluorescence intensity of MHC II (MHC IImedium and MHC IIhigh) (Fig. 5). When gating both subpopulations and comparing the intensity of MHC II fluorescence, the MHC IImedium subpopulation was approximately identical, in the three analysed BMDCs. However, the MHC IIhigh population of both ST6Gal.I−/− and ST3Gal.I−/− BMDCs presented a slightly increased intensity of MHC II fluorescence (Fig. 5). The percentage of MHC IIhigh BMDCs compared to the MHC IImedium subpopulation was about 10% and 5% higher in ST6Gal.I−/− and ST3Gal.I−/− BMDCs, than in WT BMDCs, respectively, but the differences were not statistically significant (data not shown). Accordingly, the fluorescence intensity of FITC-labelled ovalbumin of the MHC IImedium subpopulation was approximately identical, in the three analysed BMDCs, whereas the MHC IIhigh population of both ST3Gal.I−/− and ST6Gal.I−/− BMDCs presented a decreased intensity [49% (P= 0·0094) and 78% of fluorescence intensity relative to WT, respectively] (data not shown).
Collectively, these data indicate that BMDCs deficient for ST6Gal.I or ST3Gal.I, upon endocytosis, show an augmented number of fully mature BMDCs subpopulation with decreased endocytosis capacity.
Discussion
With the recent progress of glycobiology it is becoming evident that sialic acid is relevant to the immune responses and the evaluation of sialylation-modification at the leucocyte surface contributes to a better understanding of the immune functions. We have previously demonstrated that the expression of surface sialylated structures was modulated during moDC differentiation and maturation, and we suggested that changes in sialylation are related to specific DC functions.19 Nevertheless, because of the characteristic complexity of DCs, the identification of the specific nature and role of these structures is still difficult to predict.
Here, we show that the sialylation shortage, generated by neuraminidase treatment, induces an increased expression of MHC molecules and costimulatory molecules at the cell surface of human moDCs. This increase is considerably higher than that induced solely by the uptake of ovalbumin (Fig. 1) or other endocytic agents, such as lucifer yellow and dextran (data not shown). Additionally, according to our experiments investigating the functional consequences of sialic acid loss in DCs, neuraminidase treatment alters the gene expression of specific cytokines and increases the ability of DCs to prime T lymphocytes. Notably, sialic acid shortage leads to an up-regulation of IL-1α, IL-6 and TNF-α proinflammatory cytokines,28,29 and of IL-12, which drives Th1 lymphocyte differentiation and the cell-mediated immune response.30 In contrast, the expression of IL-10, which plays a major role inducing tolerance in naïve T lymphocytes31 and in biasing their development into Th2 lymphocytes,32 remained basically unchanged. Increased expression of signals, such as antigen presentation and costimulatory molecules and IL-12, driving the development of naive precursors into Th1 lymphocytes33 are key determinants for DC immunogenicity. To evaluate the cytokine profile, the expression of mRNA, but not of protein, was analysed and it is relevant to note that a discrepancy between mRNA and protein expression is possible. Notwithstanding, our strategy is well suited to elucidate the Th1- or Th2-skewed DC activation profile. Our results suggest that neuraminidase creates an overall proinflammatory effect on moDCs, increasing their immunogenicity.
The idea of using neuraminidase treatment to alter DC immunogenicity, that is to say the activation towards Th1, needs further clarification. Neuraminidase treatment removes non-specifically α-2,3-linked and α-2,6-linked sialic acid from all cell surface sialylated constituents. Our current data cannot predict the precise sialylated cell surface structure that, upon desialylation, leads to Th1-skewed DC activation. To obtain a better insight, we studied DCs that were deficient for ST3Gal.I and ST6Gal.I, because these two sialyltransferases have a good correlation between their mRNA expression levels and the differentiation and maturation process of human DCs and are probably the most relevant for moDCs sialylation.19 Absence of the sialic acid linkages specified by these sialyltransferases may both lead to Th1-skewed DC activation. In addition, the data derived from these mouse models with ST6Gal.I or ST3Gal.I deficiencies suggest that the overall extent of cell surface sialylation, and not of specific sialylated glycoprotein entities, may be the important contributing factor. On the other hand, it is important to note that differences in DC maturation and distribution were noted between ST6Gal.I-deficient compared to ST3Gal.I-deficient and WT animals (see Table 2). These differences may indicate functional specializations for sialic acid linkages specified by the two sialyltransferases, which, in their turn, may be dependent on the presence of several cell surface molecules. Indeed, human moDCs express several receptors, such as Siglecs, that recognize sialic-acid-containing glycans, and may have preferences for either the Sia(α2,3) linked to Gal(β1,3)GalNAc or the Sia(α2,6) to Gal(β1,4)GlcNAc structures synthesized by ST3Gal.I or ST6Gal.I, respectively.34 Siglecs have the potential to interact not only in trans with sialylated ligands found in other cells, but also in cis with ligands found at the same cell surface.34 The physiological functions of the majority of Siglecs are still unknown. However, because they present immunoreceptor tyrosine-based inhibitory motifs, they are expected to have a major role in controlling immunity. Furthermore, additional glycan-recognizing receptors exist, and removal of the sialic acid residues normally residing as the outermost residue of glycans may uncover ligands for other immunologically relevant carbohydrate-binding molecules such as galectins. Some members of this family of lectins that recognize β-galactosyl-containing glycoconjugates, have been implicated in both innate and adaptive immune responses.35 So far it is known that Gal-3 expression in DCs is pivotal to control the magnitude of T-lymphocyte priming36 but the biological functions of the galectins expressed by DCs are probably complex and are not well understood. In this scenario, it is difficult to anticipate the extent of molecules and mechanisms that are engaged when DCs are treated with neuraminidase.
One point that should be addressed is the fact that the observed DC maturation could be the result of the engagement of the innate receptors because the neuraminidase, used throughout this study, was of bacterial origin and could be contaminated with microbial products, particularly endotoxins. Nevertheless, to rule out that possibility, control assays were performed with a neuraminidase inactivated by heat which should not destroy the endotoxins.
Considering the fact that differential sialylation can influence the accessibility of MHC epitopes to antibodies that recognize them,17,37 it would be possible that the increased antibody binding to MHC molecules that is observed in neuraminidase-treated DCs, was the result of an enhanced antibody affinity rather than of augmentation of the number of binding sites of such molecules. However, it should be noted that the increased immunogenicity of neuraminidase-treated DCs is confirmed not only by the increased binding of MHC antibodies, as shown by flow cytometry, but also by the transcription of specific cytokines and by proliferation assays in mixed lymphocyte cultures. In addition, qualitative differences in the extent of antibody binding to MHC II molecules were also observed among ST6Gal.I−/− and ST3Gal.I−/− DC subsets, corroborating the idea that clearance of cell surface sialic acids is indeed involved in an elevated expression of MHC II at the cell surface.
There is evidence correlating reduced sialylated structures with the activation of other leucocytes such as neutrophils and T lymphocytes.38,39 In the case of DCs, there is one report describing how murine DCs treated with neuraminidase induced specific T-lymphocyte responses more efficiently.17 Interestingly, some sialyltransferases, like ST6Gal.I, are known to be down-regulated after DC maturation,18,19 suggesting that maturation, on its own, leads to the reduction of specific sialylation.
Lymphocytes and neutrophils, from ST3Gal.I−/− and ST6Gal.I−/− mice have been the object of several studies,6,7,40 but, as far as we know this is the first reported characterization of their DCs. Therefore we initially studied the distribution of lymphoid, plasmacytoid and conventional DC subsets in blood, lymph nodes and spleen, based on the expression of specific markers. We have found that ST3Gal.I-deficient mice presented a significantly lower percentage of CD8α+ DCs and, conversely, increased number of B220+ DCs in the periphery (Table. 1). It is relevant to note that ST3Gal.I−/− lymphocytes have been described as having a similar CD8/B220 disproportion and the dramatic defect for CD8+ T lymphocytes in ST3Gal.I−/− mice has been relatively well documented.6 The observed alteration in the DC population, suggests a higher content of plasmacytoid (B220+) DCs and may be related to the fact that both CD8 and B220 molecules are the substrate of ST3Gal.I.6 However, further analysis is required to validate the relationship of ST3Gal.I expression and plasmacytoid DC abundance.
Our findings strongly suggest that sialic acid linkages mediated by different sialyltransferases are functionally distinct in DCs and have roles in DC maturation. Sialic acid deficiency, either in ST3Gal.I−/− and ST6Gal.I−/− BMDCs or in human neuraminidase treated moDCs, triggers DC maturation. In contrast to classical maturation stimuli,10 desialylation-triggered maturation does not dramatically abrogate endocytosis, and human or mouse DCs with reduced sialic acid retain around 70% of their endocytic capacity. In addition, the MHC II expression of desialylated DCs is intensified upon antigen loading, which accords with the increased capacity to induce T-lymphocyte proliferation.
Further functional studies are still required to understand the competence and immune modulatory capacities of desialylated DCs. Nevertheless, because surface sialylation influences the immunogenicity of DCs upon antigen loading, our findings should have a particular relevance to DC-based therapies and they propose that sialylation is an issue to be considered when refining the immunological parameters of vaccination with DCs.
Acknowledgments
We thank Manuela Correia for her exceptional technical assistance and Joana Ribeiro and David Lopes for their assistance in obtaining sorted moDC and murine DC subsets, respectively. The studies were supported by the Immunology Department, FCM-UNL, by the Fundação para a Ciência e Tecnologia, Portugal (SFRH/BPD/21619/2005) to M.G.C. and by the National Institutes of Health (AI-056082) to J.T.Y.L.
Glossary
Abbreviations:
- BMDC
bone marrow-derived dendritic cell
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cell
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL
interleukin
- mAb
monoclonal antibody
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
- moDC
monocyte-derived dendritic cell
- PCR
polymerase chain reaction
- PE
phycoerythrin
- Th1
T helper type 1
- TNF
tumour necrosis factor
- TT
tetanus toxoid
- WT
wild-type
Conflict of interest
There are no financial or commercial conflicts of interest with this work.
Disclosure
The authors have no financial conflict of interest.
References
- 1.Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P. The human sialyltransferase family. Biochimie. 2001;83:727–37. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 2.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851–7. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 4.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–89. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 5.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–55. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 6.Priatel JJ, Chui D, Hiraoka N, et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12:273–83. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- 7.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–9. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 13.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 14.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 15.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–14. [PubMed] [Google Scholar]
- 17.Boog CJ, Neefjes JJ, Boes J, Ploegh HL, Melief CJ. Specific immune responses restored by alteration in carbohydrate chains of surface molecules on antigen-presenting cells. Eur J Immunol. 1989;19:537–42. doi: 10.1002/eji.1830190319. [DOI] [PubMed] [Google Scholar]
- 18.Jenner J, Kerst G, Handgretinger R, Muller I. Increased alpha2,6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp Hematol. 2006;34:1212–8. doi: 10.1016/j.exphem.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Videira PA, Amado IF, Crespo HJ, Alguero MC, Dall’Olio F, Cabral MG, Trindade H. Surface alpha 2-3- and alpha 2-6-sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconj J. 2008;25:259–68. doi: 10.1007/s10719-007-9092-6. [DOI] [PubMed] [Google Scholar]
- 20.Verkade MA, van Druningen CJ, Op de Hoek CT, Weimar W, Betjes MG. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clin Exp Med. 2007;7:65–71. doi: 10.1007/s10238-007-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajana S, Herrera-Gonzalez N, Narvaez J, Torres-Aguilar H, Rivas-Carvalho A, Aguilar SR, Sanchez-Torres C. Differential CD4(+) T-cell memory responses induced by two subsets of human monocyte-derived dendritic cells. Immunology. 2007;122:381–93. doi: 10.1111/j.1365-2567.2007.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔC(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Porcelli SA, Segelke BW, Sugita M, Wilson IA, Brenner MB. The CD1 family of lipid antigen-presenting molecules. Immunol Today. 1998;19:362–8. doi: 10.1016/s0167-5699(98)01289-4. [DOI] [PubMed] [Google Scholar]
- 25.van der Wel NN, Sugita M, Fluitsma DM, Cao X, Schreibelt G, Brenner MB, Peters PJ. CD1 and major histocompatibility complex II molecules follow a different course during dendritic cell maturation. Mol Biol Cell. 2003;14:3378–88. doi: 10.1091/mbc.E02-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martino A, Volpe E, Auricchio G, Colizzi V, Baldini PM. Influence of pertussis toxin on CD1a isoform expression in human dendritic cells. J Clin Immunol. 2006;26:153–9. doi: 10.1007/s10875-006-9009-3. [DOI] [PubMed] [Google Scholar]
- 27.Rigano R, Buttari B, Profumo E, et al. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect Immun. 2007;75:1667–78. doi: 10.1128/IAI.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–65. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 29.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 30.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 31.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–8. [PubMed] [Google Scholar]
- 32.Liu L, Rich BE, Inobe J, Chen W, Weiner HL. Induction of Th2 cell differentiation in the primary immune response: dendritic cells isolated from adherent cell culture treated with IL-10 prime naive CD4+ T cells to secrete IL-4. Int Immunol. 1998;10:1017–26. doi: 10.1093/intimm/10.8.1017. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 34.Varki A, Angata T. Siglecs – the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 35.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 36.Breuilh L, Vanhoutte F, Fontaine J, et al. Galectin-3 modulates immune and inflammatory responses during helminthic infection: impact of galectin-3 deficiency on the functions of dendritic cells. Infect Immun. 2007;75:5148–57. doi: 10.1128/IAI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberti PA, Hackett CJ, Askonas BA. Influenza virus infection of mouse lymphoblasts alters the binding affinity of anti-H-2 antibody: requirement for viral neuraminidase. Eur J Immunol. 1979;9:751–7. doi: 10.1002/eji.1830091003. [DOI] [PubMed] [Google Scholar]
- 38.Razi N, Varki A. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology. 1999;9:1225–34. doi: 10.1093/glycob/9.11.1225. [DOI] [PubMed] [Google Scholar]
- 39.Sadighi Akha AA, Berger SB, Miller RA. Enhancement of CD8 T-cell function through modifying surface glycoproteins in young and old mice. Immunology. 2006;119:187–94. doi: 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasirikenari M, Segal BH, Ostberg JR, Urbasic A, Lau JT. Altered granulopoietic profile and exaggerated acute neutrophilic inflammation in mice with targeted deficiency in the sialyltransferase ST6Gal I. Blood. 2006;108:3397–405. doi: 10.1182/blood-2006-04-014779. [DOI] [PMC free article] [PubMed] [Google Scholar]