Abstract
A rapid antiviral immune response may be related to viral interaction with the host cell leading to activation of macrophages via pattern recognition receptors (PPRs) or specific viral receptors. Carcinoembryonic cell adhesion antigen 1a (CEACAM1a) is the specific receptor for the mouse hepatitis virus (MHV), a coronavirus known to induce acute viral hepatitis in mice. The objective of this study was to understand the mechanisms responsible for the secretion of high-pathogenic MHV3-induced inflammatory cytokines. We report that the induction of the pro-inflammatory cytokines interleukin (IL)-6 and tumour necrosis factor (TNF)-α in peritoneal macrophages does not depend on CEACAM1a, as demonstrated in cells isolated from Ceacam1a−/− mice. The induction of IL-6 and TNF-α production was related rather to the fixation of the spike (S) protein of MHV3 on Toll-like receptor 2 (TLR2) in regions enriched in heparan sulphate and did not rely on viral replication, as demonstrated with denatured S protein and UV-inactivated virus. High levels of IL-6 and TNF-α were produced in livers from infected C57BL/6 mice but not in livers from Tlr2−/− mice. The histopathological observations were correlated with the levels of those inflammatory cytokines. Depending on mouse strain, the viral fixation to heparan sulfate/TLR2 stimulated differently the p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB in the induction of IL-6 and TNF-α. These results suggest that TLR2 and heparan sulphate receptors can act as new viral PPRs involved in inflammatory responses.
Keywords: carcinoembryonic cell adhesion antigen 1a (CEACAM1a), heparan sulphate, inflammation, mouse hepatitis virus, Toll-like receptor 2
Introduction
The ability of innate immune mechanisms to respond to viral infection plays a major role in the survival of the host. The first step of a viral infection is the fixation of virus to its specific receptor, exposed on target cells. However, many viruses can use, as specific receptors, surface molecules located on macrophages or lymphocytes that are involved in innate defence mechanisms, thus modifying the antiviral functions of these cells.1–3
The rapid antiviral immune response may be related to viral interactions with host cells, leading to activation of susceptible cells via pattern recognition receptors (PPRs).4 Although many PPRs have been identified for bacteria, few PPRs are known for viruses on macrophages. Toll-like receptors (TLRs) and the helicases retinoid-inducible gene (RIG), melanoma differentiation-associated gene-5 (MDA5) and RNA-dependent protein kinase (PKR) are known to be engaged following some viral infections and to initiate the production of pro-inflammatory cytokines, chemokines and interferon α/β.5
The occurrence of a new respiratory disease, the severe acute respiratory syndrome (SARS), in which over-exuberant pulmonary inflammation is induced, has raised the possibility that some viral components may stimulate the production of inflammatory cytokines by macrophages. Wang et al. demonstrated that a surface viral protein from the SARS virus directly stimulated the production of tumour necrosis factor (TNF)-α and interleukin (IL)-6.6,7 The induction of macrophage inflammatory cytokines by viral surface proteins has been occasionally observed with other viruses, such as feline infectious peritonitis virus and Epstein–Barr virus.8,9
The mechanisms triggered by coronaviruses in the induction of the inflammatory response by macrophages are not known. Mouse hepatitis virus type 3 (MHV3), a coronavirus, induces a rapid acute hepatitis characterized by the death of susceptible C57BL/6 mice within 3–5 days post-infection.10–12 Histopathological studies revealed an extensive necrosis with inflammatory perivascular foci and impairment of immunosuppressive cytokines such as IL-4, IL-10, prostaglandin E2 (PGE2) and transforming growth factor (TGF)-β.13,14
Carcinoembryonic antigen cell adhesion molecules 1 (CEACAM1), previously known as biliary glycoproteins, are members of the immunoglobulin superfamily.15 Murine CEACAM1 proteins are mostly present in the liver and intestine and have been reported to act as receptors for different bacterial and viral pathogens, including MHV.16,17 These observations were supported by the fact that C57BL/6 mice knocked out for the Ceacam1a gene are completely resistant to MHV-A59 infection.18
CEACAM1a promotes various functions, such as cellular adhesion, signalling, tumour suppression and angiogenesis.15 Activation of carcinoembryonic antigen (CEA) induces the secretion of IL-1α, IL-6 and TNF-α by Kupffer cells and then promotes the establishment of hepatic metastasis.19 Furthermore, it was demonstrated that production of IL-1α and TNF-α by Kupffer cells, via the activation of CEA, involved tyrosine phosphorylation.20 In addition, the signalling pathway implicated in CEA-activated cells seemed to be different from the pathway involved in lipopolysaccharide (LPS)-activated Kupffer cells.20 It was reported that stimulation of CEACAM1a on PC12 cells by antibodies induced a rapid and transient CEACAM1a tyrosine dephosphorylation, which was involved in the downstream activation of extracellular signal-related kinase (ERK)-1 and ERK-2 but not Jun N-terminal kinase (JNK) or p38 mitogen-activated protein kinase (MAPK).21 In contrast, the replication of MHV3 in peritoneal macrophages involved the activation of the p38 and the ERK-1/2 MAPK in less than 30 min, suggesting that cellular activation was a result of the fixation of the virus to its receptor.22
However, de Haan et al. demonstrated that some murine coronavirus variants generated by in vitro cultures expressed an extended host range and also used heparan sulphate binding sites as an entry receptor instead of using the specific CEACAM1a receptor.23 Recently, Watanabe et al. have observed that another serotype, the MHV-JHM virus, can interact with heparan sulphate, but this receptor cannot allow the internalization of this virus.24
It has been demonstrated that a few viral proteins, such as the capsid of the hepatitis B virus (HBV), the core of the hepatitis C virus (HCV), or herpes simplex virus (HSV) glycoproteins, may induce pro-inflammatory cytokines via the activation of TLR2 associated or not with membranes enriched in heparan sulphate.25–28 The recognition by TLRs of pathogen-associated molecule patterns (PAMPs) triggers the toll/interleukin-1 receptor-like (TIR)-domain-dependent association with adapters (such as myeloid differentiation primary response gene 88 (MyD88) and toll-interleukin-1 receptor domain-containing adapter protein (TIRAP)) that recruit IL-1 receptor-associated kinase (IRAK) and, following their phosphorylation, activates other factors involved in the activation of the transcription factor nuclear factor (NF)-κB, resulting in the expression of inflammatory cytokines such as IL-1β, IL-6 and TNF-α.29 Moreover, during chronic HCV infections, the serum IL-6 levels correlate with the viral load and the length of the infection,30 whereas TNF-α levels increase hepatic cellular apoptosis.31,32 The in vivo relevance of this observation may be related to the expression of TLRs in liver injury and the inflammatory state of chronically HCV-infected patients,33 but the mechanisms involved have not yet been elucidated.
The respective roles of CEACAM1a, the coronavirus receptor, TLR2, heparan sulphate binding sites, and other unspecific viral PPRs in the induction of the inflammatory responses seen in human or murine acute coronavirus infection have not been yet determined.
In this study, we demonstrate that the pro-inflammatory cytokines IL-6 and TNF-α, produced by MHV3-infected peritoneal macrophages, were induced by the fixation of protein S of MHV3 on TLR2 associated with regions enriched in heparan sulphate instead of CEACAM1a. Furthermore, the release of IL-6 and TNF-α was mostly dependent on the activation of the ERK-1/2 MAPK and JNK pathways and, to a lesser extent, the p38 MAPK and NF-κB pathways. The pathology induced by L2-MHV3 is related to the release of intrahepatic IL-6 and TNF-α via the TLR2 receptor, as demonstrated in Tlr2−/− mice and by histopathological observations.
Materials and methods
Mice
C57BL/6 (Ceacam1a+/+) and SJL (Ceacam1b+/+) mice were purchased from Charles River Laboratories (St-Constant, QC, Canada), whereas Tlr2 knock-out mice (B6·129-Tlr2tm1Kir/J) were purchased from Jackson Laboratories (Bar Harbor, MA). C57BL/6 Ceacam1a knock-out (Ceacam1a−/−) mice were generated by Dr N. Beauchemin, as previously described.34 The animals, certified as MHV3-free by the manufacturer, were housed in an environment with high efficiency particulate air (HEPA)-filtered air (Forma Scientific, Marietta, OH). Female mice between 8 and 12 weeks of age were used in all experiments. The study was conducted in compliance with the regulations of the Animal Committee of the University of Quebec in Montreal (UQAM).
Viruses
The pathogenic L2-MHV3 is a cloned substrain isolated from the liver of infected DBA2 mice and was produced in L2 cells as previously described.35 The pathogenic properties of L2-MHV3 were assessed regularly.
In vivo viral infection
Groups of four C57BL/6 and Tlr2−/− mice were infected by the intraperitoneal (i.p.) route with 1000 TCID50 (tissue culture infective dose 50%) of L2-MHV3. Mock-infected mice received a similar volume of RPMI-1640 (Gibco Laboratories, Grand Island, NY). At 96 hr post-infection (p.i.), the mice were anaesthetized by i.p. injection using ketamine hydrochloride (200 mg/kg) (Vetrepharm Canada Inc., Belleville, ON, Canada) and xylazine (10 mg/kg) (Bayer Inc., Toronto, ON, Canada). Mice were bled by section of the portal vein and aortic artery, as described by Watanabe et al.36 The liver was harvested following exsanguination as previously described.37 Briefly, the livers were pressed through a 70-μm cell strainer (Falcon Scientific Co., Montreal, QC, Canada) which was then washed with 10 ml of RPMI-1640 supplemented with L-glutamine (2 mm), antibiotics (penicillin 100 U/ml and streptomycin 100 mg/ml) (Gibco Laboratories) and 20% fetal calf serum (FCS) (Gemini Bio-Products, Woodland, CA). The liver extracts were then deposited on 7 ml of FCS to allow debris sedimentation. The top layer was centrifuged for 10 min at 1000 g and the supernatant was collected for virus titration and cytokine quantification by enzyme-linked immunosorbent assay (ELISA) after being passed through a 0·45-μm filter (Sarstedt Inc., Montreal, QC, Canada).
Histopathological analysis
Livers obtained from mock-infected and L2-MHV3-infected C57BL/6 and Tlr2−/− mice were prepared for histopathology and stained with haematoxylin-eosin.
Virus titration
The supernatants of liver extracts from C57BL/6 and Tlr2−/− mice were used as the viral suspension. They were serially diluted in 10-fold steps using RPMI-1640 and tested on L2 cells cultured in 96-well plates. Cytopathic effects, characterized by syncytia formation and cell lysis, were recorded at 72 hr p.i. and virus titers are expressed as log10 TCID50.
Cells
The continuous mouse fibroblast L2 cell line was grown in RPMI-1640 supplemented with 5% FCS. L2 cells were used for viral production.
Resident peritoneal macrophages from C57BL/6, Ceacam1a−/−, SJL, and Tlr2−/− mice were obtained by peritoneal washings using RPMI-1640 supplemented with 20% FCS and 2-mercaptoethanol (Gibco Laboratories) and enriched by adherence to plastic. Peritoneal exudate cells (106) were allowed to adhere for 2 hr and non-adherent cells were then washed away. Cell viability, ranging from 90 to 100%, was assayed by a trypan blue exclusion test (Sigma-Aldrich, Montreal, QC, Canada).
In vitro viral infection
Resident peritoneal macrophages were seeded in 24-well plates at a concentration of 106 cells/ml in RPMI-1640 supplemented with 20% FCS. In some experiments, AgB10 (a monoclonal murine anti-CEACAM1a antibody [immunoglobulin G (IgG)] produced in rats and affinity-purified on a HiTrap protein G column, (Pharmacia, Upsala, Sweden) provided by Dr N. Beauchemin) (2 μg/106 cells), functional grade TLR2 monoclonal antibody (mAb) (1 μg/106 cells; which does not inhibit completely monocyte functions) (eBioscience, San Diego, CA) and heparin (400 U) (Sigma-Aldrich) were added for 2 hr. In other experiments, inhibitors specific for SH2 domain-containing protein (SHP)-1 [sodium stibogluconate (SS), 10 μm, for 15 min]38 (Sigma-Aldrich), p38 MAPK (SB203580, 10 μg/ml, for 2 hr), ERK-1/2 MAPK (U0126, 10 μg/ml, for 2 hr), NF-κB (SN50, 18 μm, for 2 hr) or JNK (SP600125, 25 μm, for 2 hr) (Calbiochem, San Diego, CA) were also added prior to infection. Cells were then infected with 0·1–1·0 multiplicity of infection (MOI) of infectious L2-MHV3, L2-MHV3 virus treated for 1 hr in phosphate buffer (Gibco Laboratories) at pH 8·0 and 37°, or L2-MHV3 treated for 1 hr with UV. Cells were then incubated at 37°, under 5% CO2, for 24 hr. Supernatants were collected for cytokine quantification by ELISA.
Cytokine determination by ELISA
Determination of IL-6 and TNF-α levels in liver extracts from mock- or L2-MHV3-infected C57BL/6 and Tlr2−/− mice, and in supernatants from in vitro infections, was performed using either Mouse IL-6 or Mouse TNF-α BD OptEIA ELISA Sets (BD Biosciences, Mississauga, ON, Canada).
Statistical analysis
For in vivo studies, statistical analyses were performed using an analysis of variance (anova) test. For in vitro studies, statistical analyses were performed using a Student’s t-test. All statistical analyses were calculated with the prism 4·03 software (GraphPad Software, La Jolla, CA). Error bars represent standard errors and a value of P ≤ 0·05 was considered significant.
Results
Secretion of macrophage IL-6 and TNF-α induced by MHV3 does not depend on activation of CEACAM1a
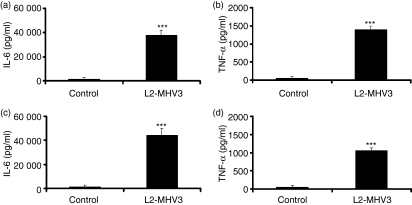
Macrophages can produce pro-inflammatory cytokines such as IL-6 and TNF-α during viral or bacterial infections.19,39,40 In some murine strains, they also express CEACAM1a, rendering them permissive to MHV infection. We postulated that these cells produced inflammatory cytokines when infected by MHV3 through engagement of CEACAM1a, the MHV receptor. To verify this hypothesis, resident peritoneal macrophages were isolated from uninfected C57BL/6 and Ceacam1a−/− mice and infected in vitro with L2-MHV3. IL-6 and TNF-α released in supernatants were then quantified 24 hr p.i. by ELISA. Results showed that infection of C57BL/6 peritoneal macrophages with L2-MHV3 induced the production of pro-inflammatory cytokines IL-6 (P < 0·001) and TNF-α (P < 0·001) (Fig. 1a,b). Surprisingly, infection of Ceacam1a−/− peritoneal macrophages also induced the production of IL-6 (P < 0·001) and TNF-α (P < 0·001) (Fig. 1c,d). Furthermore, SJL peritoneal macrophages, which express CEACAM1b,41 induced lower levels of IL-6 and TNF-α than C57BL/6 mice when infected with L2-MHV3 (data not shown). These observations suggest that CEACAM1a is not triggered by MHV3 fixation to induce secretion of IL-6 and TNF-α by peritoneal macrophages.
Figure 1.
Production of interleukin (IL)-6 (a and c) and tumour necrosis factor (TNF)-α (b and d) by in vitro uninfected and L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 (a and b) and carcinoembryonic cell adhesion antigen 1a-deficient (Ceacam1a−/−) (c and d) peritoneal macrophages at 24 hr post-infection (p.i.). The cells were infected with 0·1–1·0 multiplicity of infection (MOI) of L2-MHV3, and IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (***P < 0·001).
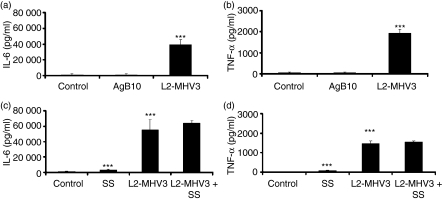
To confirm that CEACAM1a is not implicated in the production of MHV3-induced IL-6 and TNF-α in peritoneal macrophages, these cells were isolated from C57BL/6 mice and treated or not for 2 hr with a specific anti-CEACAM1a monoclonal antibody (AgB10), which is known to be a potent activator on murine dendritic cells.42 The isolated cells were then in vitro infected with L2-MHV3 for 24 hr and released cytokines were quantified. Although an increase of IL-6 and TNF-α was observed in MHV3-infected peritoneal macrophages (P < 0·001 for both cytokines), binding of AgB10 to CEACAM1a did not trigger the secretion of IL-6 and TNF-α nor inhibit the secretion of these cytokines compared with MHV3-infected cells (Fig. 2a,b). Preliminary results showed no difference in IL-6 and TNF-α levels with 1, 2, 3 or 5 μg of AgB10/106 cells. Moreover, the isotype control mAb did not induce production of IL-6 and TNF-α in resident peritoneal macrophages (data not shown).
Figure 2.
Production of interleukin (IL)-6 (a and c) and tumour necrosis factor (TNF)-α (b and d) by in vitro L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 peritoneal macrophages in the presence of an anti-carcinoembryonic cell adhesion antigen 1a (CEACAM1a) antibody or a SH2 domain-containing protein (SHP)-1 inhibitor. Cells were treated or not with a monoclonal anti-CEACAM1a monoclonal antibody (mAb) (AgB10 at 2 μg/106 cells) 2 hr before infection (a and b) or with a SHP-1 inhibitor [sodium stibogluconate (SS) at 10 μm] 15 min before infection (c and d) and infected further with 0·1–1·0 multiplicity of infection (MOI) of L2-MHV3 for 24 hr. The IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (***P < 0·001).
However, CEACAM1a may exhibit a regulatory role in association with immunoreceptor tyrosine-based inhibition motifs (ITIMs) and SHP-1 molecules.43 To verify whether signals are induced by viral fixation to CEACAM1a leading to IL-6 and TNF-α production, resident peritoneal macrophages purified from uninfected C57BL/6 mice were treated for 15 min with a SHP-1 inhibitor (SS), and thereafter infected with L2-MHV3. Although a slight increase of IL-6 and TNF-α was noted in SS-treated macrophages compared with untreated cells (P < 0·001 for both cytokines), addition of the SHP-1 inhibitor did not alter the secretion of IL-6 and TNF-α triggered by MHV3 in macrophages (Fig. 2c,d). These results suggest that CEACAM1 is not implicated in the production of the pro-inflammatory cytokines by MHV3-infected macrophages.
Membrane regions enriched in heparan sulphate are implicated in the secretion of IL-6 and TNF-α by macrophages in the presence of MHV3
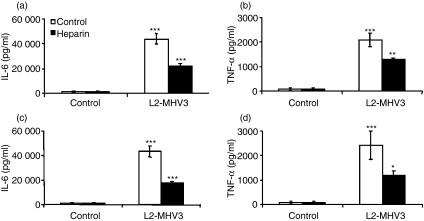
It has been reported that some isolates of coronavirus may use heparan sulphate binding sites instead of CEACAM1a as an entry receptor with the involvement of the S1 and S2 subunits of the spike protein of MHV.23,44 To determine whether that the pro-inflammatory cytokines induced by L2-MHV3 in macrophages depend on viral fixation to regions enriched in heparan sulphate, resident peritoneal macrophages from C57BL/6 and Ceacam1a−/− mice were treated in vitro with heparin and infected further with L2-MHV3. The IL-6 and TNF-α production was then quantified in supernatant at 24 hr p.i. Production of IL-6 by MHV3-infected C57BL/6 (Fig. 3a) and Ceacam1a−/− (Fig. 3c) peritoneal macrophages was impaired when cells from both mouse strains were treated with heparin (P < 0·001 for both mouse strains). The TNF-α levels were slightly reduced in peritoneal macrophages from both mouse strains (P < 0·01 for C57BL/6 mice; P < 0·05 for Ceacam1a−/− mice) (Fig. 3b,d).
Figure 3.
Production of interleukin (IL)-6 (a and c) and tumour necrosis factor (TNF)-α (b and d) by in vitro L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 (a and b) and carcinoembryonic cell adhesion antigen 1a-deficient (Ceacam1a−/−) (c and d) peritoneal macrophages in the presence of heparin. Cells were treated or not with heparin (400 U) 2 hr before infection and infected further with 0·1–1·0 multiplicity of infection (MOI) of L2-MHV3 for 24 hr. The IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (*P < 0·05; **P < 0·01; ***P < 0·001).
MHV3 uses TLR2 to induce the production of IL-6 and TNF-α
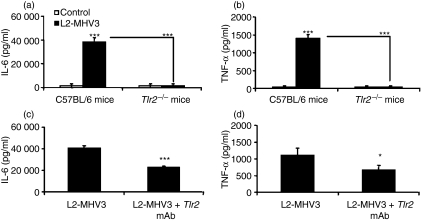
Some viruses, such as HBV, HCV and herpesviruses, have been reported to promote production of the pro-inflammatory cytokines IL-6, IL-12 and TNF-α by their fixation to TLR2 in regions enriched in heparan sulphate.25–27 We investigated whether TLR2 is also implicated in the establishment of an inflammatory state during the MHV infection. Resident peritoneal macrophages were purified from uninfected C57BL/6 and Tlr2−/− mice and infected further in vitro with L2-MHV3 for 24 hr, and pro-inflammatory cytokines were then quantified. Results indicated that the production of IL-6 and TNF-α was not induced in Tlr2−/− peritoneal macrophages infected with L2-MHV3 compared with C57BL/6 infected cells (P < 0·001 for both cytokines) (Fig. 4a,b). The addition of a functional grade TLR2 mAb partially impaired IL-6 (P < 0·001) and TNF-α (P < 0·05) production by resident C57BL/6 peritoneal macrophages (Fig. 4c,d). The partial effect was attributable to the intrinsic properties of the TLR2 mAb, as specified by eBioscience. These results thus confirmed that TLR2 molecules are involved in the induction of a pro-inflammatory response during an MHV3 infection.
Figure 4.
Production of interleukin (IL)-6 (a) and tumour necrosis factor (TNF)-α (b) by in vitro uninfected and L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 and Toll-like receptor 2-deficient (Tlr2−/−) peritoneal macrophages at 24 hr post-infection (p.i.). Production of IL-6 (c) and TNF-α (d) by in vitro L2-MHV3-infected peritoneal macrophages from C57BL/6 mice in the presence of a functional grade TLR2 monoclonal antibody (mAb) at 24 hr p.i. The cells were treated with 1 μg/106 cells of the TLR2 mAb 2 hr before infection. The cells were infected with 0·1–1·0 multiplicity of infection (MOI) of L2-MHV3 and IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (***P < 0·001).
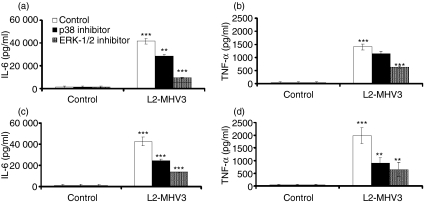
MAPKs are implicated in the TLR2/heparan sulphate region-dependent production of IL-6 and TNF-α induced by MHV3 in macrophages
To identify the intracellular signalling pathways involved in the TLR2/heparan sulphate region-dependent production of IL-6 and TNF-α, peritoneal macrophages from C57BL/6 and Ceacam1a−/− mice were treated with p38 (SB203580) and ERK-1/2 (U0126) MAPK inhibitors and infected in vitro with L2-MHV3. As shown in Figures 5a,b, inhibition of both p38 and ERK-1/2 MAPK impaired IL-6 production by MHV3-infected C57BL/6 peritoneal macrophages (P < 0·01 for the p38 inhibitor; P < 0·001 for the ERK-1/2 inhibitor), whereas TNF-α production was partially impaired by the ERK-1/2 inhibitor only (P < 0·001). Addition of either p38 or ERK-1/2 MAPK inhibitors to MHV3-treated Ceacam1a−/− macrophages also impaired the production of IL-6 (P < 0·001 for both inhibitors) and TNF-α (P < 0·01 for both inhibitors) (Fig. 5c,d), indicating that CEACAM1 may modulated the TNF-α pathway via the p38 MAPK molecule. Interestingly, the ERK-1/2 MAPK inhibitor induced a greater reduction in IL-6 than the p38 MAPK inhibitor in both C57BL/6 and Ceacam1a−/− cells.
Figure 5.
Roles of p38 and extracellular signal-related kinase (ERK)-1/2 mitogen-activated protein kinase (MAPK) in the production of interleukin (IL)-6 (a and c) and tumour necrosis factor (TNF)-α (b and d) by in vitro L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 (a and b) and carcinoembryonic cell adhesion antigen 1a-deficient (Ceacam1a−/−) (c and d) peritoneal macrophages. The cells were treated with p38 MAPK (SB203580 at 10 μg/ml) or ERK-1/2 (U0126 at 10 μg/ml) inhibitors 2 hr before infection and infected further with 0·1–1·0 multiplicity of infection (MOI) of L2-MHV3 for 24 hr. IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (**P < 0·01, ***P < 0·001).
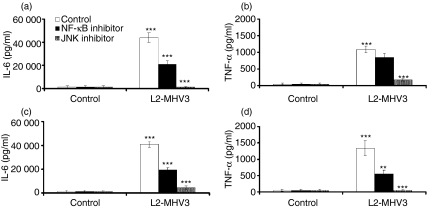
Pro-inflammatory cytokines such as IL-1, IL-6, IL-12 or TNF-α may also be induced via the activation of NF-κB and JNK/AP-1.45 To verify that JNK and NF-κB are involved in the production of IL-6 and TNF-α from MHV3-infected peritoneal macrophages, these cells were isolated from C57BL/6 and Ceacam1a−/− mice and treated with NF-κB (SN50) or JNK (SP600125) inhibitors. Afterwards, the cells were infected in vitro with L2-MHV3 for 24 hr and IL-6 and TNF-α levels were quantified. Inhibition of NF-κB decreased levels of IL-6 (P < 0·001) but not TNF-α in L2-MHV3-infected C57BL/6 peritoneal macrophages (Fig. 6a,b). However, addition of NF-κB inhibitor to MHV3-treated Ceacam1a−/− cells impaired the production of both IL-6 (P < 0·001) and TNF-α (P < 0·01) (Fig. 6c,d), indicating that CEACAM1a may also modulate TNF-α production via the NF-κB molecule. In addition, inhibition of the JNK pathway greatly impaired the production of both IL-6 and TNF-α by MHV3-infected C57BL/6 and Ceacam1a−/− peritoneal macrophages (P < 0·001 for IL-6 and TNF-α, in both mouse strains) (Fig. 6a–d). No cytotoxicity was observed in peritoneal macrophages from either mouse strain when they were treated with all of the above-mentioned inhibitors. Moreover, despite the fact that a slight impairment in TNF-α production was noted with the p38 MAPK and NF-κB inhibitors in C57BL/6 macrophages, the results obtained remained non-significant in several experiences.
Figure 6.
Roles of nuclear factor (NF)-κB and Jun N-terminal kinase (JNK) in the production of interleukin (IL)-6 (a and c) and tumour necrosis factor (TNF)-α (b and d) by in vitro L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 (a and b) and carcinoembryonic cell adhesion antigen 1a-deficient (Ceacam1a−/−) (c and d) peritoneal macrophages. The cells were treated with NF-κB (SN50 at 18 μm) or JNK (SP600125 at 25 μm) inhibitors 2 hr before infection and infected further with 0·1–1·0 multiplicity of infection (MOI) of L2-MHV3 for 24 hr. IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (**P < 0·01, ***P < 0·001).
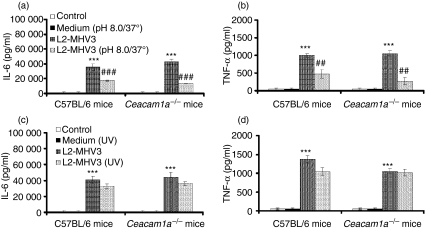
The MHV3 S protein, but not viral replication, induces the pro-inflammatory cytokines IL-6 and TNF-α
Murine coronavirus spike (S) proteins can bind to CEACAM1a, the specific viral receptor, to infect susceptible cells.16 S1 and S2 subunits may also be involved in heparan sulphate binding sites.44 Cytokine induction may depend on the fixation of viral proteins to the TLR2/heparan sulphate region at the cell surface. To test this hypothesis, peritoneal macrophages were isolated from both C57BL/6 and Ceacam1a−/− mice and infected in vitro with infectious MHV3 or MHV3 treated at pH 8·0 and 37° to denature the S glycoprotein.46 IL-6 and TNF-α levels were then quantified at 24 hr p.i. Production of IL-6 and TNF-α was decreased in both C57BL/6 (P < 0·001 for IL-6; P < 0·01 for TNF-α) and Ceacam1a−/− (P < 0·001 for IL-6; P < 0·01 for TNF-α) peritoneal macrophages in the presence of pH 8·0/37°-treated MHV3 compared with the levels of cytokines in the presence of infectious MHV3 (Fig. 7a,b).
Figure 7.
Roles of viral spike (S) protein and viral replication in the production of interleukin (IL)-6 (a and c) and tumour necrosis factor (TNF)-α (b and d) by in vitro L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 (a and b) and carcinoembryonic cell adhesion antigen 1a-deficient (Ceacam1a−/−) (c and d) peritoneal macrophages. The cells were infected for 24 hr with 0·1–1·0 multiplicity of infection (MOI) of infectious L2-MHV3, L2-MHV3 treated at pH 8·0 and 37° (a and b) or L2-MHV3 inactivated with UV (c and d). IL-6 and TNF-α levels were evaluated by enzyme-linked immunosorbent assay (ELISA). Results are representative of three experiments (***P < 0·001 compared with control cells; ##P < 0·01 and ###P < 0·001 compared with MHV3-infected cells).
To determine whether intracellular viral replication may be also involved in the induction of cytokines, a similar experiment was conducted with UV-inactivated viruses. As shown in Fig. 7c,d, the inactivation of the virus with UV did not inhibit the production of IL-6 and TNF-α in MHV3-treated C57BL/6 and Ceacam1a−/− macrophages. No viral replication was observed in the cell cultures, as confirmed by MHV3 immunolabelling.
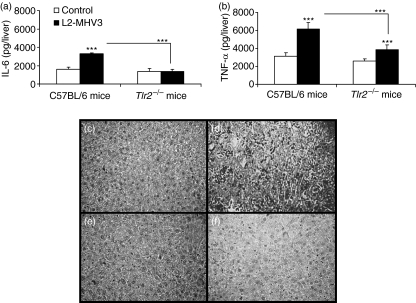
Production of IL-6 and TNF-α in the liver of MHV3-infected C57BL/6 and Tlr2−/− mice
Human viral hepatitis is strongly associated with a strong inflammatory response in the liver.33 It has also been demonstrated that the pathogenic L2-MHV3 induces an acute hepatitis in susceptible C57BL/6 mice with inflammatory foci in intrahepatic tissues.13 To determine whether IL-6 and TNF-α induced by fixation of S viral protein to TLR2/heparan sulphate receptors are involved in the histopathological disorders, the levels of these cytokines were quantified in total liver extracts from MHV3-infected C57BL/6 and Tlr2−/− mice at 96 hr p.i. As shown in Figure 8(a), intrahepatic IL-6 levels increased in MHV3-infected C57BL/6 mice (P < 0·001) compared with mock-infected mice, but not in MHV3-infected Tlr2−/− mice (P < 0·001 compared with infected C57BL/6 mice). Despite the fact that intrahepatic TNF-α was induced in MHV3-infected C57BL/6 and Tlr2−/− mice (P < 0·001 for both mice), the levels remained lower in the livers from Tlr2−/− mice than in those from wild-type mice (P < 0·001 compared with infected C57BL/6 mice) (Fig. 8b). Histological analysis of livers revealed an extensive necrosis with inflammatory foci only in surviving MHV3-infected C57BL/6 mice (Figs 8c,d), whereas low numbers of inflammatory foci were detected in livers from Tlr2−/− mice (Fig. 8e,f). The viral titres found in the livers of MHV3-infected C57BL/6 and Tlr2−/− mice were, respectively, 109·0 ± 100·1 and 107·6 ± 100·8 viruses/liver. Thus, the levels of IL-6 and TNF-α and the viral load may be related to the extensive necrosis observed in the livers of C57BL/6 mice infected with the pathogenic L2-MHV3.
Figure 8.
Intrahepatic interleukin (IL)-6 (a) and tumour necrosis factor (TNF)-α (b) levels in liver extracts from mock-infected and L2-mouse hepatitis virus 3 (MHV3)-infected C57BL/6 and Toll-like receptor 2-deficient (Tlr2−/−) mice at 96 hr post-infection (p.i.). Four mice were used in each experimental group and infected with 1000 TCID50 (tissue culture infective dose 50%) of L2-MHV3 (***P < 0·001). Histopathological observations of livers from control (c and e) and L2-MHV3-infected (d and f) C57BL/6 (c and d) and Tlr2−/− (e and f) mice at 96 hr p.i.
Discussion
In this study, we have demonstrated that MHV3 protein S used TLR2 and heparan sulphate regions, instead of CEACAM1a, the specific viral receptor, on the cell surface of peritoneal macrophages to promote the secretion of IL-6 and TNF-α. Viral fixation to TLR2/heparan sulphate triggers the activation of the p38 MAPK, ERK-1/2 MAPK, NF-κB and JNK pathways. These results suggest a new mechanism by which surface viral proteins may induce an inflammatory response by their fixation to non-specific cell surface receptors. In addition, our results revealed a new modulating role for CEACAM1a on macrophages in the secretion of TNF-α via the p38 MAPK and NF-κB pathways.
Our results also suggest that TLR2 associated with heparan sulphate can be considered as a new viral PPR as its engagement by viral S protein initiates production of inflammatory cytokines, as already described for TLRs and helicases RIG-1, MDA5 and PKR.5
We have demonstrated here that the induction of pro-inflammatory cytokines did not result from activation of CEACAM1a, even when CEACAM1a was used as a specific receptor for MHV on target cells in susceptible C57BL/6 mice.16 In fact, Ceacam1a−/− mice are completely resistant to MHV-A59 infections.18 The irrelevance of CEACAM1a engagement in the production of viral-induced inflammatory cytokine is supported by the comparable secretion of IL-6 and TNF-α by macrophages from both C57BL/6 and Ceacam1a−/− mice, and by experiments conducted with specific anti-CEACAM1a mAb and SHP-1 inhibitors. Pretreatment of uninfected or MHV3-infected peritoneal macrophages from C57BL/6 mice with the AgB10 mAb did not induce or inhibit the production of IL-6 and TNF-α. Furthermore, addition of an inhibitor to SHP-1, implicated in the CEACAM1a signalling pathway,43 did not impair cytokine production by C57BL/6 macrophages. These results demonstrated that CEACAM1a is not involved in the induction of the production of inflammatory cytokines by MHV3-infected macrophages. However, this conclusion does not totally exclude the possibility that cytokine production may be associated with intracellular pathways related to CEACAM1a, as demonstrated by a slight increase of IL-6 and TNF-α in macrophages from C57BL/6 mice treated with the SHP-1 inhibitor. The production of IL-6 and TNF-α in SJL-derived macrophages also indicates that CEACAM1b is not also involved. However, it has been reported that incubation of dendritic cells with a specific CEACAM1a mAb (AgB10) promotes the release of various chemokines and cytokines, such as macrophage inflammatory protein (MIP)-1α, MIP-2, IL-6 and IL-12, and increases the expression of the costimulatory molecules CD40, CD54, CD80 and CD86 on these cells.42 CEACAM1a pro-inflammatory properties may be differently regulated in different cell types.
We have demonstrated that production of IL-6 and TNF-α depends rather on heparan sulphate, as treatment of peritoneal macrophages from C57BL/6 and Ceacam1a−/− mice with heparin impaired IL-6 and TNF-α production. This observation suggests that MHV3 may use heparan sulphate or a closely related receptor to induce the production of cytokines by macrophages, rather than the CEACAM1a receptor. In fact, the production of IL-6 and TNF-α by MHV3-infected peritoneal macrophages is induced by viral fixation to a receptor located in regions enriched in heparan sulphate but not by viral replication, as demonstrated by experiments using viruses with denaturated S protein or UV-inactivated viruses. Thus, denaturation of viral S protein by incubation at pH 8·0 and 37° inhibited the production of IL-6 and TNF-α, whereas UV inactivation of MHV3, which does not alter viral fixation to the cell surface but inhibits efficient viral replication (confirmed by MHV3 immunolabelling), did not impair the production of IL-6 and TNF-α in C57BL/6 or Ceacam1a−/− peritoneal macrophages. Therefore, these results revealed that viral replication is not essential for induction of TNF-α and IL-6 and that the presence of the viral S protein is sufficient to stimulate production of cytokines by macrophages.
It has been previously reported that murine coronavirus showing extended host range used heparan sulphate as an entry receptor. However, the change from a restricted CEACAM1 tropism to an extended host range has been associated with only a few mutations in the S1 subunit, which are generally obtained from in vitro persistently infected cells.44 It was also reported that treatment of susceptible cells with heparin inhibits infection with an extended-host range MHV-A59 variant.23 The MHV3 used in this work is not a host-range variant because it was produced in vivo in the liver from susceptible mice before two or three in vitro passages into L2 cells, indicating that the ability of MHV3 to bind heparan sulphate receptors is a general property of this virus.
We observed that IL-6 and TNF-α production in MHV3-infected C57BL/6 peritoneal macrophages depended on ERK-1/2 MAPK and JNK, whereas p38 MAPK and NF-κB pathways were only involved in IL-6 production. It was reported that the p38 and ERK-1/2 MAPK pathways were activated early in MHV3-infected Swiss-Webster peritoneal macrophages,22 but receptors involved in this activation were not identified. The respective roles of the p38 MAPK, ERK MAPK and JNK pathways in MHV viral replication have not yet been determined. However, the p38 MAPK and JNK pathways, but not the ERK-1/2 pathway, were activated in MHV-A59-infected J774.1 cells. In addition, UV-irradiated MHV-A59 did not activate the MAPKs.47
Nevertheless, the downstream activation of CEACAM1 seems to involve the ERK-1/2 pathway in particular, whereas expression of the CEACAM1 signalling pathway may be regulated through the NF-κB pathway.21,48,49 However, we have demonstrated that viral fixation to the CEACAM1a receptor is not directly involved in the secretion of IL-6 and TNF-α by MHV3-infected peritoneal macrophages as these cytokines were also induced in cells from Ceacam1a−/− mice and regulated by the MAPK pathways. However, Scheffrahn et al. proposed that CEACAM1a could either inhibit or stimulate the activation of the ERK-1/2 MAPK pathway depending on the history of the cells, i.e. according to the cell type and cell state.50
Activation of TLRs leads to the secretion of pro-inflammatory cytokines through the activation of NF-κB. Indeed, inhibition of the NF-κB pathway partially inhibits IL-6 and TNF-α production by macrophages isolated from Ceacam1a−/− mice. Furthermore, the p38 and ERK-1/2 MAPK pathways can be induced by peptidoglycan/TLR2 activation in different cell types such as Kupffer cells and eosinophils.51,52 Nevertheless, no information is available on the regulation of CEACAM-mediated intracellular signalling activation by TLRs. Our results suggest that CEACAM1a may regulate the secretion of TNF-α via the p38 MAPK and NF-κB pathways.
The results obtained with MHV3-infected Tlr2−/− mice suggest that TLR2 molecules are crucial in the production of IL-6 and TNF-α and in determining the levels of infectious viruses during the induction of histopathological disorders. These results also suggest that viruses that possess glycoproteins and an envelope, such as coronaviruses, may induce a strong inflammatory response directly by their fixation to TLRs and membranes enriched in heparan sulphate via the S protein or by their release of free S proteins, without viral replication. The PPR role of the TLRs and the heparan sulphate receptor in the presence of infectious viruses demonstrated a new viral mechanism for amplification of the inflammatory response, thus explaining the severity of diseases induced by human or mouse coronaviruses. Cytokine regulation in coronavirus infections has been poorly explored. Our results indicate that MHV3 is a powerful model for such studies. We have demonstrated that the murine coronavirus MHV3 can stimulate the production of IL-6 and TNF-α by activating TLR2/heparan sulphate regions at the cell surface of macrophages. In addition, these results suggest an interesting mechanism by which a virus and its viral surface proteins may regulate the immune response in addition to viral replication in macrophages.
In contrast, the surface TLR4 and intracellular TLR9 are induced during a SARS-coronavirus infection and correlate with the production of IL-6 and IFN-γ.53 Preliminary results have shown a slight impairment of IL-6 and TNF-α production when Ceacam1a−/− resident peritoneal macrophages infected with MHV3 were treated with a functional grade TLR4 mAb (eBioscience) (data not shown). It was recently demonstrated that MHV-A59 and SARS-CoV produce IFN-α via the activation of intracellular TLR7 in plasmacytoid dendritic cells, thus controlling viral infection.54 However, MHV-A59 and MHV-JHM were not able to induce interferon (IFN)-β via the activation of intracellular TLR3, indicating that the double-stranded viral RNA is not accessible to cellular PPRs.55
However, the interaction between CEACAM1a and other TLR molecules needs to be further investigated. The identification of these new PPRs should allow coronavirus infections such as SARS to be more effectively controlled or, in some cases, exploited. In other viral infections, it was reported that the interaction between HSV-1 and TLR2 contributed to lethal encephalitis, whereas human cytomegalovirus promotes the secretion of pro-inflammatory cytokines through its fixation to TLR2 and CD14.27,28 Thus, TLRs seemed to be primordial during viral infections in the establishment of innate immunity, but a stronger inflammatory response can also aggravate the disease without an increase in viral replication.
Further work is in progress to elucidate the different roles of CEACAM, TLRs, heparan sulphate and viral constituents in the production of inflammatory and immunosuppressive cytokines by hepatic cells infected with attenuated MHV3 variants.
Acknowledgments
This work was supported by a grant from NSERC-Canada. AJ was supported by a Canadian NSERC studentship.
References
- 1.Kelly J, Beddall MH, Yu D, Iyer SR, Marsh JW, Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–12. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotta S, Stilla A, Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paludan SR, Melchjorsen J, Malmgaard L, Mogensen SC. Expression of genes for cytokines and cytokine-related functions in leukocytes infected with Herpes simplex virus: comparison between resistant and susceptible mouse strains. Eur Cytokine Netw. 2002;13:306–16. [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 5.Unterholzner L, Bowie AG. The interplay between viruses and innate immune signaling: recent insights and therapeutic opportunities. Biochem Pharmacol. 2008;75:589–602. doi: 10.1016/j.bcp.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Ye L, Ye L, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128:1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng CT, Perrone LA, Zhu H, Makino S, Peters CJ. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol. 2005;174:7977–85. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 8.Takano T, Hohdatsu T, Toda A, Tanabe M, Koyama H. TNF-alpha, produced by feline infectious peritonitis virus (FIPV)-infected macrophages, upregulates expression of type II FIPV receptor feline aminopeptidase N in feline macrophages. Virology. 2007;364:64–72. doi: 10.1016/j.virol.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Addario M, Ahmad A, Morgan A, Menezes J. Binding of the Epstein-Barr virus major envelope glycoprotein gp350 results in the upregulation of the TNF-alpha gene expression in monocytic cells via NF-kappaB involving PKC, PI3-K and tyrosine kinases. J Mol Biol. 2000;298:765–78. doi: 10.1006/jmbi.2000.3717. [DOI] [PubMed] [Google Scholar]
- 10.Levy-Leblond E, Oth O, Dupuy JM. Genetic study of mouse sensivity to MHV3 infection: influence of the H-2 complex. J Immunol. 1979;112:1359–62. [PubMed] [Google Scholar]
- 11.Le Prevost C, Levy-Leblond E, Virelizier JL, Dupuy JM. Immunopathology of mouse hepatitis virus type 3 infection. I. Role of humoral and cell-mediated immunity in resistance mechanism. J Immunol. 1975;114:221–5. [PubMed] [Google Scholar]
- 12.Dupuy JM, Levy-Leblond E, Le Prevost C. Immunopathology of mouse hepatitis virus type 3 infection. II. Effect of immunosuppression in resistant mice. J Immunol. 1975;114:226–30. [PubMed] [Google Scholar]
- 13.Martin JP, Chen W, Koehren F, Pereira CA. The virulence of mouse hepatitis virus 3, as evidence by permissivity of cultured hepatic cells toward escaped mutants. Res Virol. 1994;145:297–302. doi: 10.1016/S0923-2516(07)80034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacques A, Bleau C, Martin JP, Lamontagne L. Intrahepatic endothelial and Kupffer cells involved in immunosuppressive cytokines and natural killer (NK)/NK-T cell disorders in viral acute hepatitis. Clin Exp Immunol. 2008;152:298–310. doi: 10.1111/j.1365-2249.2008.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beauchemin N, Draber P, Dveksler G, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–9. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 16.Dveksler GS, Pensiero MN, Cardellichio CB, Williams RK, Jiang GS, Holmes KV, Dieffenbach CW. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–91. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leusch HG, Drzeniek Z, Markos-Pusztai Z, Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic -glycosides of mannose. Infect Immun. 1991;59:2051–7. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmila E, Turbide C, Olson M, Jothy S, Holmes KV, Beauchemin N. Ceacam1a−/− mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J Virol. 2004;78:10156–65. doi: 10.1128/JVI.78.18.10156-10165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmiston KH, Gangopadhyay A, Shoji Y, Nachman AP, Thomas P, Jessup JM. In vivo induction of murine cytokine production by carcinoembryonic antigen. Cancer Res. 1997;57:4432–6. [PubMed] [Google Scholar]
- 20.Gangopadhyay A, Lazure DA, Thomas P. Carcinoembryonic antigen induces signal transduction in Kupffer cells. Cancer Lett. 1997;118:1–6. doi: 10.1016/s0304-3835(97)00216-4. [DOI] [PubMed] [Google Scholar]
- 21.Budt M, Cichocka I, Reutter W, Lucka L. Clustering-induced signaling of CEACAM1 in PC12 cells. Biol Chem. 2002;383:803–12. doi: 10.1515/BC.2002.084. [DOI] [PubMed] [Google Scholar]
- 22.McGilvray ID, Lu Z, Wei AC, Dackiw AP, Marshall JC, Kapus A, Levy G, Rotstein OD. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J Biol Chem. 1998;273:32222–9. doi: 10.1074/jbc.273.48.32222. [DOI] [PubMed] [Google Scholar]
- 23.de Haan CAM, Li Z, te Lintelo E, Bosch BJ, Hijema BJ, Rottier JM. Murine coronavirus with an extended host range uses heparan sulphate as an entry receptor. J Virol. 2005;79:14451–6. doi: 10.1128/JVI.79.22.14451-14456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe R, Sawicki SG, Taguchi F. Heparan sulfate is a binding molecule but not a receptor for CEACAM1-independent infection of murine coronavirus. Virology. 2007;366:16–22. doi: 10.1016/j.virol.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper A, Tal G, Lider O, Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–76. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 26.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–24. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 27.Kurt-Jones EA, Chan M, Zhou S, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101:1315–20. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–96. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–44. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Oyanagi Y, Takahashi T, Matsui S, et al. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19:464–72. doi: 10.1111/j.1478-3231.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 31.Vidigal P, Germer JJ, Zein NN. Polymorphisms in the interleukin-10, tumor necrosis factor-alpha, and transforming growth factor-beta1 genes in chronic hepatitis C patients treated with interferon and ribavirin. J Hepatol. 2002;36:271–7. doi: 10.1016/s0168-8278(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 32.Lio D, Caruso C, Di Stefano R, et al. IL-10 and TNF-alpha polymorphisms and the recovery from HCV infection. Hum Immunol. 2003;64:674–80. doi: 10.1016/s0198-8859(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 33.Riordan SM, Skinner NA, Kurtovic J, Locarnini S, McIver CJ, Williams R, Visvanathan K. Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. Inflamm Res. 2006;55:279–85. doi: 10.1007/s00011-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 34.Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 2006;25:5527–36. doi: 10.1038/sj.onc.1209541. [DOI] [PubMed] [Google Scholar]
- 35.Dupuy JM, Rodrigue D. Heterogeneity in evolutive patterns of inbred mice infected with a cloned substrain of mouse hepatitis virus type 3. Intervirology. 1981;16:114–7. doi: 10.1159/000149255. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H, Ohtsuka K, Kimura M, et al. Details of an isolation method for hepatic lymphocytes in mice. J Immunol. 1992;146:145–54. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- 37.Lamontagne L, Massicotte E, Page C. Mouse hepatitis viral infection induces an extrathymic differentiation of the specific intrahepatic alpha beta-TCRintermediate LFA-1high T-cell population. Immunology. 1997;90:402–10. doi: 10.1111/j.1365-2567.1997.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–7. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 39.Nunez A, Gomez-Villamandos JC, Sanchez-Cordon PJ, Fernandez de Marco M, Pedrera M, Salguero FJ, Carrasco L. Expression of proinflammatory cytokines by hepatic macrophages in acute classical swine fever. J Comp Pathol. 2005;133:23–32. doi: 10.1016/j.jcpa.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Xie GQ, Jiang JX, Chen YH, Liu DW, Zhu PF, Wang ZG. Induction of acute hepatic injury by endotoxin in mice. Hepatobiliary Pancreat Dis Int. 2002;1:558–64. [PubMed] [Google Scholar]
- 41.Barthold SW, Smith AL. Response of genetically susceptible and resistant mice to intranasal inoculation with mouse hepatitis virus JHM. Virus Res. 1987;7:225–39. doi: 10.1016/0168-1702(87)90030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kammerer R, Stober D, Singer BB, Obrink B, Reimann J. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J Immunol. 2001;166:6537–44. doi: 10.4049/jimmunol.166.11.6537. [DOI] [PubMed] [Google Scholar]
- 43.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, Mandelboim O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 44.de Haan CAM, te Lintelo E, Li Z, Raaben M, Wurdinger T, Bosch BJ, Rottier PJM. Cooperative involvement of the S1 and S2 subunits of the murine coronavirus spike protein in receptor binding and extended host range. J Virol. 2006;80:10909–18. doi: 10.1128/JVI.00950-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 46.Zelus BD, Schickli JH, Blau DM, Weiss SR, Holmes KV. Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37 degrees C either by soluble murine CEACAM1 receptors or by pH 8. J Virol. 2003;77:830–40. doi: 10.1128/JVI.77.2.830-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee S, Narayanan K, Mizutani T, Makino S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J Virol. 2002;76:5937–48. doi: 10.1128/JVI.76.12.5937-5948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muenzner P, Billker O, Meyer TF, Naumann M. Nuclear factor-kappa B directs carcinoembryonic antigen-related cellular adhesion molecule 1 receptor expression in Neisseria gonorrhoeae-infected epithelial cells. J Biol Chem. 2002;277:7438–46. doi: 10.1074/jbc.M108135200. [DOI] [PubMed] [Google Scholar]
- 49.Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Obrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol. 2002;168:5139–46. doi: 10.4049/jimmunol.168.10.5139. [DOI] [PubMed] [Google Scholar]
- 50.Scheffrahn I, Singer BB, Sigmundsson K, Lucka L, Obrink B. Control of density-dependent, cell state-specific signal transduction by the cell adhesion molecule CEACAM1, and its influence on cell cycle regulation. Exp Cell Res. 2005;307:427–35. doi: 10.1016/j.yexcr.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell Toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J Cell Physiol. 2007;210:667–75. doi: 10.1002/jcp.20860. [DOI] [PubMed] [Google Scholar]
- 52.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating Toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 53.Okabayashi T, Kariwa H, Yokota S, et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–24. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cervantes-Barragan L, Zust R, Weber F, et al. Control of coronavirus infection through plasmacytoid-cell-derived type 1 interferon. Blood. 2008;109:1131–7. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou H, Perlman S. Mouse hepatitis virus does not induce beta interferon synthesis and does not inhibit its induction by double-stranded RNA. J Virol. 2007;81:568–74. doi: 10.1128/JVI.01512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]