Abstract
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is an important molecule in the down-regulation of T-cell activation. A study was undertaken to evaluate the association of the CTLA-4 gene polymorphisms −319C/T, +49A/G, (AT)n, CT60A/G and Jo31G/T with the levels of membrane CTLA-4 (mCTLA-4) and cytoplasmic CTLA-4 (cCTLA-4) in CD4+ T lymphocytes from patients with multiple sclerosis (MS) and with susceptibility to MS, and the course of the disease. It was found that the Jo31GG and CT60GG genotypes were associated with decreased mean fluorescence intensity (MFI) of total CTLA-4 (mCTLA-4 + cCTLA-4) molecules in CD4+ T cells from both relapsing-remitting (RR) and secondary progressive (SP) MS patients compared with others. Consequently, possessing the Jo31G allele and/or the CT60G allele were associated with susceptibility to MS. The percentages of cells expressing mCTLA-4 and cCTLA-4 in RR patients were higher in carriers of the alleles non-predisposing to MS (namely CT60A and Jo31T), but the percentages of corresponding cells were unexpectedly significantly lower in SP patients than in RR patients. Increased risk of paresthesia and pyramidal signs as a first manifestation of disease, and earlier transition to the SP form in those patients, was also noted. It is hypothesized that the decreasing frequencies of cells expressing immunosuppressive mCTLA-4 and cCTLA-4 in carriers of alleles non-predisposing to MS (i.e. CT60A and Jo31T) may lead to inadequate down-regulation of ongoing T-cell responses in these patients and, as a consequence, earlier progression of disease from the RR form to the SP form.
Keywords: costimulatory molecule cytotoxic T-lymphocyte antigen-4 expression, CTLA-4 gene polymorphism, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) that leads to inflammatory demyelination and axonal neurodegeneration.1,2 Studies of experimental allergic encephalomyelitis (EAE), which is an animal model for MS, have demonstrated that the activation of self-reactive CD4+ T cells in response to myelin antigens is a crucial event during disease induction.3,4 Therefore, aberrant immune surveillance seems to be involved in the pathogenesis of this disease.
Recently, the down-regulatory molecule cytotoxic T-lymphocyte antigen-4 (CTLA-4) has been suggested to have a considerable impact on the biology of T-cell responses in MS.5–9 In fact, CTLA-4 is critical for the induction of peripheral tolerance and for the deletion of self-reactive T cells.10–12 Many mechanistic models have been postulated for CTLA-4 function, which involves competition with costimulatory CD28 by more effective ligation of CTLA-4 to their common B.7 ligands, inhibition of downstream T-cell receptor (TCR) signalling by the SH2 domain of phosphatases, inhibition of lipid-raft and microcluster formation, and negative regulation of the immune response by extrinsic components such as transforming growth factor-β (TGF-β) and the tryptophan-degrading enzyme, indoleamine 2,3-dioxygenase (IDO).13
After rapid transcription of the CTLA-4 gene following TCR/CD3 and costimulatory signalling, the majority of the CTLA-4 protein is found to be located primarily in the cytoplasmic compartment: in the trans-Golgi network (TGN), in endosomes, in secretory granules and in lysosomal vesicles, and only a small portion is transiently expressed on the T-cell surface during activation. According to a recently proposed mechanism of CTLA-4 trafficking, newly synthesized CTLA-4 molecules are located in the TGN, where they associate with the chaperone molecule TCR-interacting molecule (TRIM) or, alternatively, bind to the clathrin adaptor protein, AP-1, which mediates the translocation of CTLA-4 to endosomal or lysosomal vesicles. After T-cell stimulation, CTLA-4 molecules are translocated and released to the cell surface, a process that is strongly promoted by the presence of TRIM. Phosphorylation of tyrosine Y201 in the cytoplasmic tail stabilizes surface-exposed CTLA-4 and enables CTLA-4-mediated negative signalling. Dephosphorylation of Y201 induces the internalization and transport of CTLA-4 to endosomal or lysosomal compartments via a clathrin adapter complex (AP-2). Internalized CTLA-4 is degraded or recycled to the cell surface by secretory lysosymes.14
The regulation of CTLA-4 expression is pivotal as its concentration on the cell membrane determines the strength of down-regulatory signals for T cells. Thus, maintenance of an optimal surface expression level of CTLA-4 seems to be crucial for the regulation of T-cell responses and peripheral tolerance, and for preventing autoimmunity. As CTLA-4 synthesis depends on the rate of gene transcription and/or translation, polymorphisms in the corresponding genes might result in abnormal expression and function, as well as dysregulated trafficking of CTLA-4 within cellular compartments.
The CTLA-4 gene has been described as a susceptibility locus for many autoimmune diseases,15–18 but the results for MS are inconsistent. In some studies, CTLA-4 gene polymorphisms were found to be associated with MS, either as single markers19–22 or as part of a haplotype.21,23,24,26 In addition, some reports indicated a role of CTLA-4 gene polymorphisms as potential modifiers of disease,21,25,26 although other studies did not support a possible role for the CTLA-4 locus as a susceptibility gene for MS.27–32
Our attention has focused on five polymorphic sites in the CTLA-4 gene [one in the promoter region, one in the first exon and three in the 3′ untranslated region (UTR): −319C/T (rs5742909); +49A/G (rs231775); (AT)n; CT60A/G (rs3087243); and Jo31G/T (rs11571302). Each of these polymorphic sites has been reported to be associated with altered immune responses or susceptibility to autoimmune disease. At position −319C/T in the promoter region, the T allele has been found to be associated with greater promoter activity than the C allele.33,34 and with significantly increased expression of both CTLA-4 mRNA in unstimulated cells and cell-surface CTLA-4 on activated cells.35 The +49A/G transition causes a Thr/Ala substitution in the leader peptide.36 This polymorphism affects the inhibitory function of CTLA-4.37 It has been suggested that the +49A/G gene polymorphism in the leader sequence may influence the rates of endocytosis or surface trafficking.37 Additionally, Anjos et al.38 found that +49A/G polymorphisms influence the glycosylation of CTLA-4 and intracellular/surface partitioning. The presence of a dinucleotide short repeat, (AT)8, at position 642 in the 3′-UTR region seems to be associated with a higher level of mRNA transcription than longer repeats.39 The CT60A/G polymorphism was shown to be a susceptibility marker for Graves’ disease,16,40 and is associated with variations in the mRNA level of soluble CTLA-4.12 The functional role of the Jo31G/T polymorphism has not been established, but some recent studies indicate a role for this polymorphism in susceptibility to autoimmune disease.40–42
In our recently published study,9 we found abnormal expression of membrane and intracellular CTLA-4 in freshly drawn T cells from peripheral blood, as well as dysregulated responses to ex vivo stimulation in MS patients compared with healthy individuals. Therefore, we wanted to determine whether these abnormalities in CTLA-4 protein expression could be related to CTLA-4 genotypes. We also attempted to verify the possible association between the polymorphisms −319C/T, +49A/G (AT)n repeat, CT60A/G and Jo31G/T in the CTLA-4 gene and susceptibility to MS. We evaluated, in addition, the Jo31G/T gene polymorphism, which, to the best of our knowledge, has not yet been studied in MS patients, although some recent studies indicated a role for this polymorphism in susceptibility to autoimmune disease.40–42 The clinical relevance of these gene polymorphisms to gender, age at disease onset, clinical data presented at the onset of MS, interattack interval and time to secondary progressive (SP) transition was also investigated.
Materials and methods
Genotyping studies
Patients
Two-hundred and thirty unrelated MS patients (147 women and 83 men), with clinically definite MS according to the Poser Committee43 classification, were enrolled in this study. All patients were from the MS Clinic, Department of Neurology, Wroclaw Medical University. All medical documentation was available from the records of the MS Clinic. A neurological examination, together with an evaluation of severity using Kurtzke’s expanded disability status score (EDSS),44 was performed on the day that blood samples were drawn for the genetic studies. The median age [± standard deviation (SD)] of the MS patients examined at the time of blood sampling was 39 ± 10·00 years. The mean age at disease onset in this group was 30 ± 7·60 years (median ± SD), and the mean duration of the disease (± SD) was 6 ± 4·38 years. Disease severity according to the EDSS scale ranged between 1 and 8 [(median ± SD): 3 ± 2]. As a first symptom of disease, optic neuritis occurred in 48 cases, diplopia in 54 cases, ataxia/vertigo in 43 cases, parenthesis in 31 cases and pyramidal signs in 67 cases. The median value for interattack intervals (first two relapses) was 19 months. One hundred and seventy-three patients (75·2%) presented a relapsing-remitting (RR) form of MS, while 57 patients (24·8%) presented a secondary progressive (SP) form of MS. The secondary progressive form of MS was defined when continual worsening of symptoms and signs was evident for a period of at least 6 months without superimposed relapses. This resulted in a change of score on the EDSS scale of at least 0·5 points. In the later observation, periods of stability could be recorded but disease was characterized by continuous progression.45 The median time of progression from the RR form of MS to the SP form of MS was 6 years. The control group consisted of 380 gender- and age-matched healthy subjects. Genetic homogenity of the Polish population was observed, as reflected by virtually identical frequencies of H-Y polymorphisms in different regions of Poland.46 Informed consent was obtained from each individual to participate in the study.
Determination of polymorphisms
Genomic DNA was isolated from whole frozen blood using the NucleoSpin® Blood kit (MARCHEREY-NAGEL, Duren, Germany). The single-nucleotide gene polymorphisms CTLA-4−319C/T, +49A/G, CT60A/G and Jo31G/T were genotyped using polymerase chain reaction (PCR), followed by single-nucleotide primer-extension reactions with dideoxy-NTPs labelled with different fluorochromes corresponding to each allele (ABI PRISM SNaPshot ddNTP Primer Extension Reaction Kit; Applied Biosystems, Warrington, UK). Primer sequences used for the first PCR and the extension reaction, as well as the annealing temperatures used for the PCRs, are listed in Table 1 (Bionovo, Legnica, Poland).
Table 1.
Description of the primer sequences and annealing temperature used for cytotoxic T-lymphocyte antigen-4 (CTLA-4) genotyping
| Polymorphic site | Type of reaction | Primer sequence | Product size (bp) | Annealing temperature (°) |
|---|---|---|---|---|
| −319C/T | PCR | F: 5′-TGG TTA AGG ATG CCC AGA AGA TTG-3′ | 247 | 58 |
| R: 5′-TGG TTT TAC GAG AAA GGA AGC CGT-3′ | ||||
| SNaPshot | F: 5′-CAC TTA GTT ATC CAG ATC CT-3′ | 50 | ||
| R: 5′-TGA AGC TTC ATG TTC ACT TT-3′ | ||||
| CT60A/G | PCR | F: 5′-CTT CAC CAC TAT TTG GGA TAT AAC-3′ | 290 | 56 |
| R: 5′-AGC AAC ATA GGA CCA CAG GT-3′ | ||||
| SNaPshot | F: 5′-GAC TGC TAT GTC TGT GTT AAC CCA-3′ | 50 | ||
| Jo31G/T | PCR | F: 5′-AAT AAA CAG TCT GTC AGC AAA GCC-3′ | 214 | 59 |
| R: 5′-ATT CCT CTC AGA GGA AGC TGC TTC-3′ | ||||
| SNaPshot | F: 5′-ATA GGA GCT TTC TCA GTG TAC TGC-3′ | 50 | ||
| +49A/G | PCR | F: 5′-TTG CCT TGG ATT TCA GCG GCA CAA-3′ | 142 | 58 |
| R: 5′-CAC CTC CTC CAT CTT CAT GCT CC-3′ | ||||
| SNaPshot | F: 5′-GGC TCA GCT GAA CCT GGC T-3′ | 50 | ||
| R: 5′-AGT GCA GGG CCA GGT CCT GG-3′ | ||||
| (AT)n | PCR | (JOE) F: 5′-GCC AGT GAT GCT AAA GGT TG-3′ | 82–132 | 55 |
| R: 5′-AAC ATA CGT GGC TGT ATG CA-3′ |
F, forward; PCR, polymerase chain reaction; R, reverse.
The CTLA-4 3′-UTR containing an (AT)n repeat was amplified using the primer pairs (listed in Table 1), for which the 5′ end of the forward primers were labeled with JOE (Bionovo). The PCR mixture and conditions were the same as described previously.47 The primers were designed according to the complete CTLA-4 gene sequence derived from the NCBI Sequence Viewer (http://www.ncbi.nlm.nih.gov/).
Functional studies
Patients
Forty-eight patients (28 women and 20 men), 45 ± 10 years of age (median ± SD) and with clinically definite MS according to the Poser’s Committee classification, were enrolled in the functional study.43 The patients had not progressed on the EDSS scale, had not exhibited relapses within the 12 months before examination and had not received treatment with immunomodulatory drugs or corticosteroids during the 1 year preceding entry into the study. The MS patients were then subdivided into two groups according to the clinical course of the disease.45 Twenty-eight patients (36 ± 9 years, median ± SD) presented the RR form of MS, whereas 20 patients (48 ± 5 years, median ± SD) presented the SP form of MS. In the RR group, the median duration of the disease (± SD) was 6 ± 3 years and the EDSS ranged from 1 to 4·5 (median ± SD: 3 ± 1). In the SP group of patients, the median total duration of the disease was 17 ± 6 years (± SD), and the median duration of the SP form of MS was 7 ± 4 years (± SD). In this group, the EDSS ranged from 4·5 to 6·5 (median ± SD: 6 ± 0·5). Disease severity, as judged by the EDSS, was assessed on the day of blood collection.
The Local Ethics Committee approved this study and all patients and controls gave their informed consent for the study procedures.
Cytofluorometry study
Peripheral blood mononuclear cells (PBMCs) were separated and labelled as described previously by Kosmaczewska et al.9 Data were analyzed using cell quest software. The results are expressed as the proportion of CD4+ T cells co-expressing the CTLA-4 molecule, and as mean fluorescence intensity (MFI) value, expressed in arbitrary units (AU). At least 10 000 events per sample were analysed.
Statistical analyses
Evaluation of the Hardy–Weinberg equilibrium (HWE) was performed for all study markers by comparing the observed and expected frequencies of the genotypes using chi-square analysis. The chi-square test was used to compare the categorical data between patients with MS and controls. Differences were considered to be statistically significant if the P-value was < 0·05. An unpaired t-test was used to evaluate the association between the age at diagnosis and gene polymorphisms. The interattack interval and time to transition from the RR form of MS to the SP form of MS were estimated using the Kaplan–Meier method. The log-rank test was used to compare the interattack interval and time to transition from the RR form of MS to the SP form of MS in the two groups. Haplotype estimation analysis was made using software http://analysis.bio-x.cn/myAnalysis.php The linkage disequilibrium (LD) coefficients D′ = D/Dmax and r2– values for the pair of the most common alleles at each locus – were estimated using the software program (http://202.120.7.14/analysis/myAnalysis.php). As a result of multiple comparisons of haplotype frequencies, Bonferroni multiple adjustments were made to the level of significance.
The cytofluorometric variables analyzed (MFI and proportion of positive cells) were not normally distributed. As a consequence, we used a non-parametric test to analyze the results. The results of expression of both membrane CTLA-4 (mCTLA4) and cytoplasmic CTLA-4 (cCTLA-4) in the RR and SP groups were compared using the Mann–Whitney U-test. The summary statistics are presented as the median and interquartile range. The level of statistical significance was set at P ≤ 0·05.
Results
No polymorphism data for the cases and controls demonstrated deviation from HWE.
Analysis of associations between CTLA-4 gene polymorphisms and susceptibility to MS
We found that among the five polymorphisms studied, two (CT60A/G and Jo31G/T) were associated with susceptibility to MS. A higher frequency of MS patients carried the CT60G and Jo31G alleles compared with controls (0·89 versus 0·82, P = 0·03; and 0·87 versus 0·82, P = 0·04, respectively) (Table 2). For the other polymorphisms [CTLA-4−319C/T, +49G/A and the (AT)n repeat], no significant differences were found in allele and genotype frequencies between cases and controls (data not shown).
Table 2.
Jo31G/T and CT60A/G genotype frequencies in patients with multiple sclerosis (MS) and in controls
| Frequency (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Patients with MS | Controls | P-value | Odds ratio | 95% confidence interval | |||
| Jo31G/T | Genotype | G/G | 85 (37·1) | 119 (31·6) | |||
| G/T | 118 (51·5) | 189 (50·1) | |||||
| T/T | 26 (11·4) | 69 (18·3) | |||||
| G+ carriers G− carriers | GT+GG | 203 (88·6) | 308 (81·7) | 0·03 | 1·75 | 1·08–2·84 | |
| TT | 26 (11·4) | 69 (18·3) | |||||
| CT60A/G | Genotype | A/A | 29 (12·6) | 66 (17·6) | |||
| A/G | 110 (47·8) | 180 (48·1) | |||||
| G/G | 91 (39·6) | 128 (34·2) | |||||
| G+ carriers G− carriers | GA+GG | 201 (87·4) | 308 (82·4) | 0·04 | 1·66 | 1·02–2·70 | |
| AA | 29 (12·6) | 66 (17·6) | |||||
LD and haplotype analysis
The polymorphisms investigated in the 3′-UTR were in strong LD with each other, but the strongest LD was between Jo31G/T and CT60A/G [(AT)n:CT60A/G D’0·72, r2 = 0·4; (AT)n:Jo31G/T D’0·65, r2 = 0·3; Jo31G/T:CT60A/G D’0·89, r2 = 0·75]. Moreover, either CT60A/G or Jo31G/T were in LD with +49A/G (+49A/G:CT60A/G D’0·76, r2 = 0·3; +49A/G:Jo31G/T D’0·76, r2 = 0·3). A haplotype evaluation of all polymorphic sites was performed and the estimated frequencies of the haplotypes were different in the patients and in the controls (the global P-value after the Bonferroni correction was 0·0001). The haplotype +49A/−319C/(AT)8/CT60G/Jo31G was significantly more frequently observed in MS patients and increased the risk of MS by 10-fold [P = 0·00005; odds ratio (OR): 10·06; 95% confidence interval (CI): 2·55–39·60].
Analysis of polymorphisms in the CTLA-4 gene and clinical MS data
The five polymorphic markers −319C/T, +49A/G, CT60G/A, Jo31G/T and (AT)n repeats in the CTLA-4 gene were subjected to analysis for correlations with clinical data regarding gender, age at disease onset, initial manifestation of disease (optic neuritis, ataxia and/or vertigo, diplopia, pyramidal signs, paresthesia), first interattack interval and transition into progression from the RR form of MS to the SP form of MS.
We found that the +49A/G, CT60A/G and Jo31G/T polymorphisms correlated with the first manifestation of disease (Table 3): we noticed that the presence of either the +49A allele or the CT60A allele decreased the risk of occurrence of diplopia as a first manifestation of the disease; patients possessing the CT60A allele were more prone to paresthesia than those who did not possess the CT60A allele (Table 3); and the presence of CT60A and Jo31T alleles were associated with an increased risk of pyramidal signs as a first manifestation of disease (Table 3). None of the polymorphic markers examined correlated with gender or age at disease onset.
Table 3.
Association between the cytotoxic T-lymphocyte antigen-4 (CTLA-4) gene polymorphisms +49A/G, CT60A/G and Jo31G/T, and the initial manifestation of multiple sclerosis (MS)
| Dipopia |
Paresthesia |
Pyramidal signs |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTLA-4 gene SNP | With n = 54 | Without n = 176 | P | OR | CI | With n = 31 | Without n = 199 | P | OR | CI | With n = 67 | Without n = 163 | P | OR | CI |
| +49A carriers | 0·71 | 0·84 | 0·05 | 0·49 | 0·24–0·99 | 0·90 | 0·79 | 0·22 | 2·45 | 0·71–8·47 | 0·81 | 0·81 | 0·98 | 0·99 | 0·48–2·04 |
| CT60A carriers | 0·48 | 0·64 | 0·03 | 0·52 | 0·28–0·96 | 0·81 | 0·57 | 0·01 | 3·11 | 1·22–7·91 | 0·70 | 0·56 | 0·05 | 1·81 | 0·99–3·31 |
| J031T carriers | 0·54 | 0·66 | 0·1 | 0·61 | 0·33–1·12 | 0·74 | 0·61 | 0·16 | 1·83 | 0·78–4·30 | 0·76 | 0·58 | 0·01 | 2·29 | 1·29–4·36 |
CI, confidence interval; OR, odds ratio; SNP, single nucleotide polymorphism.
Kaplan–Meier analysis of CTLA-4 gene polymorphisms showed no correlation with the first interattack interval, but demonstrated a significant correlation with time to transition from the RR form of MS to the SP form of MS. Median time to transition to the SP form of MS was 10 years for patients possessing the Jo31T allele compared with 16 years for Jo31GG patients (P = 0·007) (Fig. 1a). Similarly, disease progression was earlier for patients carrying the +49A allele compared with patients carrying the +49GG allele (10 years versus 16 years, P = 0·014) (Fig. 1b). Moreover, patients possessing the shortest alleles – (AT)8 or CT60A alleles – showed a strong trend towards earlier transition to the SP form of MS (median time: 11 years versus 16 years, P = 0·07, and 11 years versus 15 years, P = 0·09, respectively.) (Fig. 1c,d).
Figure 1.
Influence of the cytotoxic T-lymphocyte antigen-4 (CTLA-4) gene polymorphism on time to transition from the relapsing-remitting (RR) form of multiple sclerosis (MS) to the secondary progressive (SP) form of MS. Kaplan–Meyer estimate of time to transition from the RR form of MS to the SP form of MS according to: (a) the Jo31G/T polymorphism, (b) the +49A/G polymorphism, (c) the (AT)n polymorphism and (d) the CT60A/G polymorphism.
Functional study
The expression of CTLA-4 in CD4+ T cells regarding gene polymorphisms
MFI
In both RR and SP forms of MS, stronger expression of mCTLA-4 and cCTLA-4 was observed relative to the single cell (as shown by MFI results) in the CD4+ T-cell subset of patients carrying Jo31T and CT60A alleles than in patients carrying the CT60 or Jo31GG genotype (P = non-significant) (Table 4). Total CTLA-4 levels (mCTLA-4 + cCTLA-4) in MS patients (RR + SP) possessing Jo31T and CT60A alleles were significantly higher than in Jo31GG and CT60GG patients, as shown in Table 4. Analysis performed for dinucleotide (AT)n polymorphisms showed no significant differences for mCTLA-4, cCTLA-4 and total CTLA-4, in patients with either RR or SP forms of MS, or for all MS patients (Table 4.).
Table 4.
Mean fluorescence intensity (MFI) of CD4+ T cells expressing membrane cytotoxic T-lymphocyte antigen-4 (mCTLA-4) or cytoplasmic cytotoxic T-lymphocyte antigen-4 (cCTLA-4) in patients with relapsing-remitting (RR) and secondary progressive (SP) multiple sclerosis (MS), in relation to Jo31, CT60 and (AT)n polymorphisms
| MFI | RR | SP | RR + SP | ||||
|---|---|---|---|---|---|---|---|
| Jo31G/T | T+ carriers (n = 16) | T− carriers (n = 12) | T+ carriers (n = 15) | T− carriers (n = 5) | T+ carriers (n = 31) | T− carriers (n = 17) | P |
| mCTLA-4 | 15·8 + 12·58 | 13·3 + 14·05 | 17·0 + 8·15 | 14·8 + 4·18 | 16·7 + 10·53 | 13·9 + 11·87 | 0·10 |
| cCTLA4 | 18·2 + 18·88 | 15·05 + 10·67 | 23·85 + 16·08 | 19·3 + 9·90 | 21·0 + 17·29 | 15·7 + 10·14 | 0·03 |
| Total | 38·8 + 25·82 | 29·4 + 20·66 | 43·55 + 21·63 | 38·0 + 13·52 | 39·6 + 23·46 | 29·6 + 18·46 | 0·03 |
| CT60A/T | A+ carriers (n = 16) | A− carriers (n = 12) | A+ carriers (n = 14) | A− carriers (n = 6) | A+ carriers (n = 30) | A− carriers (n = 18) | |
| mCTLA-4 | 15·8 + 12·54 | 13·7 + 14·24 | 17·65 + 8·28 | 14·10 + 3·9 | 16·6 + 10·59 | 13·7 + 10·31 | 0·13 |
| cCTLA4 | 18·1 + 18·69 | 15·7 + 10·92 | 24·7 + 16·56 | 20·15 + 8·97 | 19·6 + 17·54 | 18·0 + 9·54 | 0·09 |
| Total | 38·15 + 25·45 | 29·60 + 21·48 | 47·40 + 22·10 | 35·90 + 12·10 | 39·6 + 23·63 | 33·8 + 16·72 | 0·05 |
| (AT)n | (AT)8+ carriers (n = 19) | (AT)8− carriers (n = 8) | (AT)8+carriers (n = 14) | (AT)8− carriers (n = 4) | (AT)8+carriers (n = 23) | (AT)8− carriers (n = 12) | |
| mCTLA-4 | 15·7 + 11·98 | 14·95 + 6·95 | 17·65 + 0·42 | 17·05 + 4·63 | 16·50 + 10·47 | 15·60 + 13·84 | 0·44 |
| cCTLA4 | 17·7 + 10·97 | 14·70 + 3·02 | 24·40 + 47·77 | 21·25 + 9·39 | 19·60 + 33·67 | 17·50 + 11·49 | 0·21 |
| Total | 35·7 + 19·93 | 29·40 + 24·74 | 49·6 + 49·15 | 38·30 + 13·48 | 38·15 + 36·68 | 33·80 + 21·01 | 0·31 |
The results are expressed as median ± standard deviation (SD).
Proportion of CD4+ T cells co-expressing CTLA-4
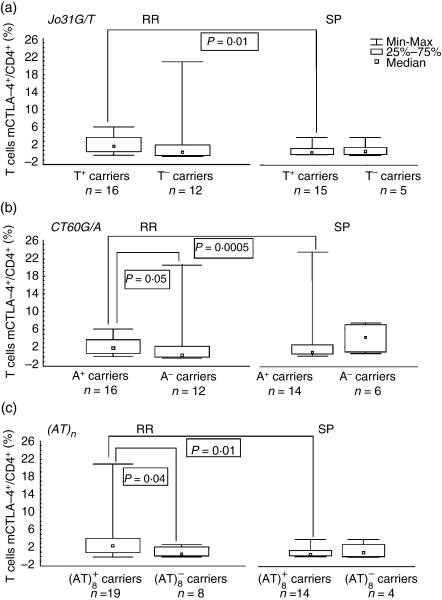
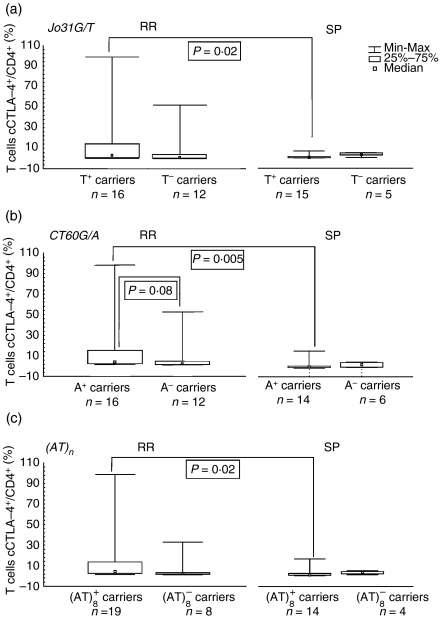
The percentage of cells expressing mCTLA-4 and cCTLA-4 in RR patients was higher in individuals possessing alleles (CT60A and Jo31T) not predisposing to MS (mCTLA-4: 2·25 versus 0·6, P = 0·05, and 2·20 versus 0·95, P = non-significant, respectively; cCTLA-4: 3·0 versus 0·9, P = non-significant, and 3·0 versus 1·1, P = non-significant, respectively), but for patients with the SP form of MS possesing CT60A and Jo31T alleles, we observed a tendency for lower proportions of mCTLA-4+/CD4+ T cells and cCTLA-4/CD4+ T cells compared with carriers of the CT60GG and Jo31GG genotypes. However, this reduction reached statistical significance only in comparison with corresponding RR patients [for mCTLA-4: 0·55 ± 0·57% versus 2·25 ± 1·83%, P = 0·0005, and 0·6 ± 1·0% versus 2·20 ± 1·93% (median ± SD), P = 0·01, respectively (Fig. 2); for cCTLA-4: 0·55 ± 4·43 versus 3·00 ± 32·61, P = 0·005, and 0·60 ± 4·29 versus 3·00 ± 32·83 (median ± SD), P = 0·02, respectively) (Fig. 3)].
Figure 2.
Proportion of CD4+ T cells expressing membrane cytotoxic T-lymphocyte antigen-4 (mCTLA-4) in patients with relapsing-remitting (RR) and secondary progressive (SP) multiple sclerosis (MS) with regard to: (a) the Jo31G/T polymorphism, (b) the CT60A/G polymorphism and (c) the (AT)n polymorphism.
Figure 3.
Proportion of CD4+ T cells expressing cytoplasmic cytotoxic T-lymphocyte antigen-4 (cCTLA-4) in patients with relapsing-remitting (RR) and secondary progressive (SP) multiple sclerosis (MS) with regard to: (a) the Jo31G/T polymorphism, (b) the CT60A/G polymorphism and (c) the (AT)n polymorphism.
The same pattern was observed for patients carrying the (AT)8 allele. For patients possessing the shortest allele (eight repeats), the proportions of CD4+ T cells expressing mCTLA-4 or cCTLA-4 were markedly higher only in patients with the RR form of MS (2·5 ± 4·46% versus 0·6 ± 1·1%, P = 0·04, and 2·90 ± 24·6% versus 0·5 ± 4·43%, P = 0·15), while in patients with the SP form of MS, the proportions of CD4+ T cells expressing mCTLA-4 or cCTLA-4 were non-significantly lower compared with patients lacking the (AT)8 allele (0·6 ± 1·01% versus 1·0 ± 1·85%, and 0·5 ± 4·43% versus 2·9 ± 1·89%), and significantly decreased in relation to carriers of the (AT)8+ alleles and who had the RR form of MS (P = 0·01 and P = 0·02).
Analyses of MFI and of the proportion of CD4+ T cells co-expressing CTLA-4 could not be performed for the +49A/G and −319C/T polymorphisms because of the small number of patients (especially in the SP group), possesing +49 GG genotype allele and possessing the −319T allele.
Discussion
In the present study we analyzed the association of five polymorphic sites in the CTLA-4 gene (one in the promoter region, one in first exon and three in the 3′-UTR region) with the susceptibility and clinical course of MS, as well as with the level of both membrane and cytoplasmic CTLA-4 molecules in freshly isolated peripheral blood CD4+ T lymphocytes from RR and SP patients. For the first time Jo31G/T polymorphisms were studied in patients with MS. We report an association between Jo31G/T and CT60A/G polymorphisms and susceptibility to MS in the Polish population. In particular, we found that the presence of Jo31G and CT60G alleles predispose to MS. Our results are in line with those obtained for other autoimmune diseases, for example Graves’ disease,16,40 and allergy and asthma,41 in which a significant prevalence of the Jo31G and CT60G alleles was observed. In contrast to our findings, other studies26,29,31 found no association between the CT60A/G polymorphism and susceptibility to MS; however, significant over-transmission of the +49G-CT60G haplotype24 or a trend towards under-transmission of the CT60G allele30 was detected in MS families. Performed by us, haplotype analysis showed that CT60G, and Jo31G as a part of haplotype +49A/−319C/(AT)8/CT60G/Jo31G increased the risk of MS by 10-fold.
We did not find any association between the −319C/T, +49A/G and (AT)n polymorphisms and susceptibility to MS in univariate analysis. The data in the literature regarding this point have not been consistent. Ligers et al.19 found that the CTLA-4−319T allele is less common among MS patients in comparison with controls, while many others have demonstrated that the CTLA-4−319C/T polymorphism does not contribute to disease susceptibility.20,32,48,49 Some studies have described the +49A/G polymorphism as a susceptibility locus for MS,20,24,26,50 although others showed no association.27–29,32 Polymorphism data for dinucleotide (AT)n, obtained by Roxburgh et al.30 and Kantarci et al.,22 showed only a trend towards increased transmission of the eight-repeat allele in MS families, suggesting a weak association of this polymorphism with MS susceptibility. Recently, Heggarty et al.26 demonstrated no association of this marker with the RR form of MS.
We next assessed whether the polymorphisms examined in this study influenced the clinical course of the disease. It is believed that some demographic data and early characteristics are of predictive value for the course of MS in the long term. Younger age of onset and female gender are believed to be associated with better prognosis.51,52 Clinical manifestations, such as optic neuritis, diplopia, or sensory disturbances at the time of onset, seem to be favorable initial manifestations, while cerebellar or pyramidal signs indicate poor prognosis.51,53,54 Additionally, a short interattack interval between the first relapse and the second relapse indicates unfavourable outcome.54 Moreover, it is well accepted that transition from the RR form to the SP form of MS indicates an unfavorable prognosis, demonstrating the irreversibility of the disability.55,56 To the best of our knowledge, this is the first detailed analysis of the association between the CTLA-4 gene polymorphisms studied and the initial manifestations of disease. We found that the presence of either the +49A allele or the CT60A allele decreased the risk of occurrence of diplopia as a first manifestation of disease. Furthermore, patients possessing the CT60A allele were more prone to paresthesia than those without these markers. The presence of CT60A and Jo31T alleles was also associated with the occurrence of pyramidal signs as a first manifestation of disease, described as a predictor of poor clinical outcome. Of note, the results from the Kaplan–Meier analysis demonstrated that the transition time to the SP form of MS was shorter for patients carrying the Jo31T and CT60A alleles compared with patients lacking these alleles. Similarly, disease progression occurred sooner in patients carrying the +49A allele than in homozygous +49GG individuals. Our findings, showing a relationship between alleles non-predisposing to MS and a more aggressive course of the disease were in agreement with the results of a previous study performed by Fukuzawa et al.,57 indicating an association between the non-predisposing genotype +49AA and severe clinical disability. Consistent with other studies,22,30,48,57 we did not observe any correlation between gender, age at onset and remission period, and the studied polymorphisms.
In order to explain whether the severity of MS depends on the genetic determinants of the CTLA-4 gene polymorphisms studied, we performed functional studies. We analyzed our previous data on CTLA-4 protein expression in CD4+ T cells freshly obtained from patients with MS in the context of the CTLA-4 gene polymorphisms determined. We found that Jo31T and CT60A alleles seem to be truly non-predisposing to MS, as individuals carrying these alleles had significantly higher levels of total CTLA-4 expression, assessed by MFI values, in comparison to patients with GG genotypes, suggesting higher inhibitory activity in MS patients carrying Jo31T or CT60A alleles. It is also noteworthy, however, that rapid progression of MS from the RR clinical form to the SP clinical form, in patients with Jo31T and CT60A alleles, was accompanied by a progressively diminishing population of CTLA-4+/CD4+ T cells possessing the potential to terminate the ongoing immune response. From our results it seems that alleles CT60A and Jo31T, despite being non-predisposing to MS, predispose to a more aggressive course of the disease. These unexpected observations indicate that the dynamics of MS depend on the magnitude of the CD4+ T-cell population co-expressing the immunosuppressive CTLA-4 molecule rather than its density in a single cell. Similarly, the shortest allele with eight AT repeats, indicated by our group as predisposing patients to earlier SP progression, was, similarly to Jo31T and CT60A, associated with lower proportions of mCTLA-4+/CD4+ and cCTLA-4+/CD4+cells, but with no differences in MFI levels. It might suggest that differences in MFI, caused by polymorphic features, are associated with predisposition to disease, while variations in the number of cells are associated with progression of disease.
We and others42 found very strong LD between CT60A/G and Jo31G/T polymorphisms; therefore, it seems impossible to dissect which genetic marker is actually responsible for the results observed.
In summary, our findings suggest that the dysregulated CTLA-4 expression in MS patients and T-cell responses could result, at least in part, from variations at the genetic level. We clearly show, for the first time, that 3′-UTR polymorphisms of the CTLA-4 gene influence both membrane and cytoplasmic CTLA-4 levels, as measured by MFI, and in this way they have impact on disease susceptibility. During the progression of disease (assessed by transition to the SP form of MS), 3′-UTR polymorphisms affect the population of cells expressing CTLA-4. This may lead to inadequate down-regulation of T-cell responses in patients with the SP form of MS, at least in those carrying non-predisposing alleles, and, as a consequence, to earlier disease progression. At this stage, the exact nature of our findings is difficult to explain and requires further investigation.
Acknowledgments
This work was supported by Wroclaw Medical University, Poland (grant no. 1290) and the State Committee for Scientific Research, Poland (KBN, grant no. 2 PO5B 049 26).
Disclosure
The authors have no financial conflict of interest.
References
- 1.Kutzelnigg A, Lucchinetti CF, Stadelmann Ch, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 2.De Stefano N, Filippi M, Miller D, et al. Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology. 2007;69:1942–52. doi: 10.1212/01.wnl.0000291557.62706.d3. [DOI] [PubMed] [Google Scholar]
- 3.Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1 + 2- T lymphocytes. J Immunol. 1981;127:1420–3. [PubMed] [Google Scholar]
- 4.Zamvil S, Nelson P, Trotter J, et al. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and clinical demyelination. Nature. 1985;317:355–8. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz AA, Sullivan TJ, Krummel MF, Sobel RA, Allison JP. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 6.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–6. [PubMed] [Google Scholar]
- 7.Mena E, Rohowsky-Kochan C. Expression of costimulatory molecules on peripheral blood mononuclear cells in multiple sclerosis. Acta Neurol Scand. 1999;100:92–6. doi: 10.1111/j.1600-0404.1999.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira EM, Bar-Or A, Waliszewska AI, Cai G, Anderson DE, Krieger JI, Hafler DA. CTLA-4 dysregulation in the activation of myelin basic protein reactive T cells may distinguish patients with multiple sclerosis from healthy controls. J Autoimmun. 2003;20:71–81. doi: 10.1016/s0896-8411(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 9.Kosmaczewska A, Bilinska M, Ciszak L, et al. Different patterns of activation markers expression and CD4+ T-cell responses to ex vivo stimulation in patients with clinically quiescent multiple sclerosis (MS) J Neuroimmunol. 2007;189:137–46. doi: 10.1016/j.jneuroim.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 11.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 12.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–8. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 13.Rudd Ch. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–60. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 14.Valk E, Rudd CE, Schneider H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008;29:272–9. doi: 10.1016/j.it.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases-a general susceptibility gene to autoimmunity? Genes Immun. 2000;1:170–84. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 16.Ueda H, Howson JM, Esposito L, et al. Association study of the T-cell regulatory gene CTLA-4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 17.Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–15. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 18.Kavvoura FK, Ioannidis JP. CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE Review and meta-analysis. Am J Epidemiol. 2005;162:3–16. doi: 10.1093/aje/kwi165. [DOI] [PubMed] [Google Scholar]
- 19.Ligers A, Xu Ch, Saarinen S, Hillert J, Olerup O. The CTLA4 gene is associated with multiple sclerosis. J Neuroimmunol. 1999;97:182–90. doi: 10.1016/s0165-5728(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 20.Harbo HF, Celius EG, Vartdal F, Spurkland A. CTLA4 promoter and exon 1 dimorphisms in multiple sclerosis. Tissue Antigens. 1999;53:106–10. doi: 10.1034/j.1399-0039.1999.530112.x. [DOI] [PubMed] [Google Scholar]
- 21.Mäurer M, Ponath A, Kruse N, Rieckmann P. CTLA4 exon 1 dimorphism is associated with primary progressive multiple sclerosis. J Neuroimmunol. 2002;131:213–15. doi: 10.1016/s0165-5728(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 22.Kantarci OH, Hebrink DD, Achenbach SJ, et al. CTLA4 is associated with susceptibility to multiple sclerosis. J Neuroimmunol. 2003;134:133–41. doi: 10.1016/s0165-5728(02)00395-8. [DOI] [PubMed] [Google Scholar]
- 23.Malferrari G, Stella A, Monferini E, et al. Ctla-4 and multiple sclerosis in the Italian population. Exp Mol Pathol. 2005;78:55–7. doi: 10.1016/j.yexmp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Suppiah V, Alloza I, Heggarty S, Goris A, Dubois B, Carton H, Vandenbroeck K. The CTLA4 + 49 A/G*G-CT60*G haplotype is associated with susceptibility to multiple sclerosis in Flanders. J Neuroimmunol. 2005;164:148–53. doi: 10.1016/j.jneuroim.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Bilinska M, Frydecka I, Noga L, Dobosz T, Zoledziewska M, Suwalska K, Tutak A, Pokryszko-Dragan A. Progression of multiple sclerosis is associated with exon 1 CTLA-4 gene polymorphism. Acta Neurol Scand. 2004;110:67–71. doi: 10.1111/j.1600-0404.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 26.Heggarty S, Suppiah V, Silversides J, et al. CTLA4 gene polymorphisms and multiple sclerosis in Northern Ireland. J Neuroimmunol. 2007;187:187–91. doi: 10.1016/j.jneuroim.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Dyment DA, Steckley JL, Willer CJ, Armstrong H, Sadovnick AD, Risch N, Ebers GC. No evidence to support CTLA-4 as a susceptibility gene in MS families: the Canadian Collaborative Study. J Neuroimmunol. 2002;123:193–8. doi: 10.1016/s0165-5728(01)00493-3. [DOI] [PubMed] [Google Scholar]
- 28.Teutsch SM, Booth DR, Bennetts BH, Heard RN, Stewart GJ. Association of common T cell activation gene polymorphisms with multiple sclerosis in Australian patients. J Neuroimmunol. 2004;148:218–30. doi: 10.1016/j.jneuroim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Lorentzen AR, Celius EG, Ekstrom PO, et al. Lack of association with the CD28/CTLA4/ICOS gene region among Norwegian multiple sclerosis patients. J Neuroimmunol. 2005;166:197–201. doi: 10.1016/j.jneuroim.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Roxburgh RH, Sawcer S, Maranian M, et al. No evidence of a significant role for CTLA-4 in multiple sclerosis. J Neuroimmunol. 2006;171:193–7. doi: 10.1016/j.jneuroim.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Greve B, Simonenko R, Illes Z, et al. Multiple sclerosis and the CTLA-4 autoimmunity polymorphism CT60: no association in patients from Germany, Hungary and Poland. Mult Scler. 2007;17:153–8. doi: 10.1177/1352458507082357. [DOI] [PubMed] [Google Scholar]
- 32.Bagos PG, Karnaouri AC, Nikolopoulos GK, Hamodrakas SJ. No evidence for association of CTLA-4 gene polymorphisms with the risk of developing multiple sclerosis: a meta-analysis. Mult Scler. 2007;13:156–68. doi: 10.1177/1352458507078059. [DOI] [PubMed] [Google Scholar]
- 33.Wang XB, Zhao X, Giscombe R, Lefvert AK. A CTLA-4 gene polymorphism at position -319 in the promoter region affects the expression of protein. Genes Immun. 2002;3:233–4. doi: 10.1038/sj.gene.6363869. [DOI] [PubMed] [Google Scholar]
- 34.Chistiakov DA, Savost’anov KV, Turakulov RI, Efremov IA, Demurov LM. Genetic analysis and functional evaluation of the C/T(-319) and A/G(-1661) polymorphisms of the CTLA-4 gene in patients affected with Graves’ disease. Clin Immunol. 2006;118:233–42. doi: 10.1016/j.clim.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Ligers A, Teleshova N, Masterman T, Huang WX, Hillert J. CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun. 2001;2:145–52. doi: 10.1038/sj.gene.6363752. [DOI] [PubMed] [Google Scholar]
- 36.Nistico L, Buzzetti R, Pritchard LE, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet. 1996;5:1075–80. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 37.Kouki T, Sawai Y, Gardine CA, Fisfalen ME, Alegre ML, DeGroot LJ. CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves’ disease. J Immunol. 2000;165:6606–11. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 38.Anjos S, Nguyen A, Ounissi-Benkalha H, Tessier MC, Polychronakos C. A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J Biol Chem. 2002;277:46478–86. doi: 10.1074/jbc.M206894200. [DOI] [PubMed] [Google Scholar]
- 39.Wang XB, Kakoulidou M, Giscombe R, et al. Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: effect of an AT-rich gene sequence. J Neuroimmunol. 2002;130:224–32. doi: 10.1016/s0165-5728(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 40.Kavvoura FK, Akamizu T, Awata T, et al. Cytotoxic T-lymphocyte associated antigen 4 gene polymorphisms and autoimmune thyroid disease: a meta-analysis. J Clin Endocrinol Metab. 2007;92:3162–70. doi: 10.1210/jc.2007-0147. [DOI] [PubMed] [Google Scholar]
- 41.Munthe-Kaas MC, Carlsen KH, Helms PJ, et al. CTLA-4 polymorphisms in allergy and asthma and the TH1/TH2 paradigm. J Allergy Clin Immunol. 2004;114:280–7. doi: 10.1016/j.jaci.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 42.Mayans S, Lackovic K, Nyholm C, et al. CT60 genotype does not affect CTLA-4 isoform expression despite association to T1D and AITD in northern Sweden. BMC Med Genet. 2007;6:8–13. doi: 10.1186/1471-2350-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 44.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status score (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 45.Lublin FD, Reingold SC. Defining in the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 46.Ploski R, Wozniak M, Pawlowski R, et al. Homogeneity and distinctiveness of Polish paternal lineages revealed by Y chromosome microsatellite haplotype analysis. Hum Genet. 2002;110:592–9. doi: 10.1007/s00439-002-0728-0. [DOI] [PubMed] [Google Scholar]
- 47.Suwalska K, Pawlak E, Karabon L, et al. Association studies of CTLA-4, CD28, and ICOS gene polymorphisms with B-cell chronic lymphocytic leukemia in the Polish population. Hum Immunol. 2008;69:193–201. doi: 10.1016/j.humimm.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 48.van Veen T, Crusius JB, van Winsen L, et al. CTLA-4 and CD28 gene polymorphisms in susceptibility, clinical course and progression of multiple sclerosis. J Neuroimmunol. 2003;140:188–93. doi: 10.1016/s0165-5728(03)00184-x. [DOI] [PubMed] [Google Scholar]
- 49.Rasmussen HB, Kelly MA, Francis DA, Clausen J. CTLA4 in multiple sclerosis. Lack of genetic association in a European Caucasian population but evident of interaction with HLA-DR2 among Shanghai Chinese. J Neurol Sci. 2001;184:143–7. doi: 10.1016/s0022-510x(00)00502-5. [DOI] [PubMed] [Google Scholar]
- 50.Masterman T, Ligers A, Zhang Z, et al. CTLA4 dimorphisms and the multiple sclerosis phenotype. J Neuroimmunol. 2002;131:208–12. doi: 10.1016/s0165-5728(02)00274-6. [DOI] [PubMed] [Google Scholar]
- 51.Midgard R, Alberktsen G, Riise T, Kvale G, Nyland H. Prognostic factors for survival in multiple sclerosis: a longitudinal, population based study in More and Romsdal, Norway. J Neurol Neurosurg Psychiatry. 1995;58:417–21. doi: 10.1136/jnnp.58.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trojano M, Avolio C, Manzari C, Calo A, De Robertis F, Serio G, et al. Multivariate analysis of predictive factors of multiple sclerosis course with validated method to assess clinical results. J Neurol Neurosurg Psychiatry. 1995;58:300–6. doi: 10.1136/jnnp.58.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins SA, McDonnell GV. Benign multiple sclerosis? Clinical course long term follow up, and assessment of prognostic factors. J Neurol Neurosurg Psychiatry. 1999;67:148–52. doi: 10.1136/jnnp.67.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 3 Multivariate analysis of predictive factors and models of outcome. Brain. 1991;114:1045–56. doi: 10.1093/brain/114.2.1045. [DOI] [PubMed] [Google Scholar]
- 55.Minderhoud JM, van der Hoevem JH, Prange AJA. Course and prognosis of chronic progressive multiple sclerosis. Results of an epidemiological study. Acta Neurol Scand. 1988;78:10–15. doi: 10.1111/j.1600-0404.1988.tb03611.x. [DOI] [PubMed] [Google Scholar]
- 56.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. NEJM. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 57.Fukazawa T, Yanagawa T, Kikuchi S, et al. CTLA-4 gene polymorphism may modulate disease in Japanese multiple sclerosis patients. J Neurol Sci. 1999;171:49–55. doi: 10.1016/s0022-510x(99)00251-8. [DOI] [PubMed] [Google Scholar]