Abstract
Major histocompatibility complex class II (MHCII) antigen expression is directly correlated with immunogenicity, and inversely correlated with tumorigenicity, in clones of the L1210 murine B lymphoma. Moreover, loss of MHCII expression on human diffuse large B-cell lymphoma is associated with dramatic decreases in patient survival. Thus, the role that MHCII antigens play in the progression of B-cell lymphomas is clinically important. In this study, we investigated the basis for the immunogenicity of MHCII+ L1210 clones. Immunogenic, but not tumorigenic L1210 clones stimulated the proliferation of naïve T cells and their interleukin (IL)-2 production, which indicates that the immunogenic clones can function as antigen-presenting cells (APCs). However, subclonal variants of the immunogenic L1210 clones, which form tumours slowly in mice, could not activate T cells. The costimulatory molecules B7-1, B7-2 and CD40 were expressed on the immunogenic L1210 clones, but not the tumorigenic clones. Importantly, the tumour-forming subclonal variants expressed MHCII and B7-1, but lacked B7-2 and CD40. These results suggest that MHCII and B7-1 expression on L1210 cells is insufficient to activate naïve T cells, and, furthermore, loss of B7-2 and/or CD40 expression contributes to the decreased immunogenicity of L1210 subclones. Blocking B7-1 or B7-2 function on immunogenic L1210 cells reduced their capacity to activate naïve T cells. Furthermore, incubation of immunogenic L1210 cells with CD40 antibodies significantly enhanced APC function. Therefore, the immunogenicity of L1210 cells directly correlates (i) with their ability to stimulate naïve T cells, and (ii) with the concomitant expression of MHCII, B7-1, B7-2, and CD40.
Keywords: antigen presentation, costimulatory molecules, lymphoma, MHC class II antigens, tumour immunogenicity
Introduction
Major histocompatibility complex class II (MHCII) molecules, which are constitutively expressed on professional antigen-presenting cells (APCs) such as B cells and dendritic cells (DCs), initiate adaptive, antigen (Ag)-specific immune responses by presenting antigenic peptides to CD4+ T cells. While MHCII expression is the initial critical component for T-cell activation, costimulatory signals resulting from CD40–CD40L and B7–CD28 interactions are essential for the generation of effective T cell-mediated immune responses.1 CD40 signalling up-regulates B7 surface expression on B cells and DCs, rendering them better equipped for Ag presentation and costimulation.2,3 Moreover, CD40 signalling is critical for maturation of DCs, and their resulting maximal capacity to present Ag, produce interleukin (IL)-12, and stimulate potent T-cell activation.4 Once activated, CD4+ T cells proliferate, produce cytokines such as IL-2, provide help for CD8+ T cells, and recruit additional effector cells to further augment the immune response.5
MHCII expression on tumours has been demonstrated to play a role in generating antitumour immunity. Multiple different tumour types, including sarcoma, breast carcinoma, and leukaemia, have been converted into immunogenic targets by stable transfection with expression vectors for MHCII molecules.6,7 These MHCII+ tumour cells generated protective immunity against challenge with the MHCII-deficient parental tumours.6,7 In addition, MHCII+ breast carcinoma cells were capable of activating CD4+ T cells by functioning directly as APCs.8,9 However, co-expression of B7-1 (also known as CD80) and/or B7-2 (CD86) costimulatory molecules on MHCII+ sarcoma cells was necessary to generate optimal T-cell activation and antitumour immunity.10,11
In the clinical setting, MHCII expression on diffuse large B-cell lymphoma (DLBCL) cells is tightly correlated with increased numbers of tumour-infiltrating lymphocytes (CD4+ and CD8+ T cells) and significantly better overall patient survival.12–15 Importantly, this correlation has been observed in several different cohorts of DLBCL patients, including those with primary mediastinal lymphoma, and those treated with the MACOP-B (methotrexate, leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin) regimen of chemotherapy.13,14,16 Moreover, loss of MHC expression on other human B-cell malignancies is associated with aggressive disease.17 These studies provide compelling evidence that MHCII expression plays a critical role in the progression of B-cell lymphomas. While MHCII expression on DLBCL tumours has been hypothesized to play a role in the generation of tumour immunity, the underlying basis for the relationship between patient survival and MHCII expression on human B-cell lymphomas remains undefined.
MHCII antigen expression is inversely correlated with tumorigenicity, and directly correlated with immunogenicity, in clones of the L1210 murine B-cell lymphoma.18 The clones of L1210 were originally derived by limiting dilution in vitro, and subsequently analysed for the ability to form tumours in mice.18 L1210 clones (2 and 7) were defined as immunogenic because they did not form tumours in mice, but induced protective immunity to challenge by parental L1210 cells or tumorigenic clone 3-3.18 Moreover, the immunogenic clones stimulated significantly higher CD8+ cytotoxic T lymphocyte (CTL) responses ex vivo, despite the fact that the levels of MHCI antigens were comparable on the immunogenic versus tumorigenic clones.18 Subclones isolated by limiting dilution from a primary immunogenic L1210 clone (clone 7) also displayed heterogeneity in tumour formation.18 The characteristics of L1210 subclone 7-15·6 were similar to those of the parental clone 7 from which it was derived. In contrast, injection of subclone 7-23 or 7-41 into syngeneic mice ultimately resulted in tumour growth, although these tumours formed with significantly slower kinetics than the tumorigenic L1210 clones 3-3, 4, 5 and 6.18 Specifically, mice injected with tumorigenic L1210 clone 3-3, 4, 5 or 6 had a mean survival time (MST) of 12–16 days, whereas mice injected with clone 7-23 or 7-41 had MSTs of 34 and 68 days, respectively.18 The disparity in tumour-forming kinetics could not be attributed to differences in growth rate (H. Fuji and H. Iribe, unpublished data). However, the molecular and immunological characteristics responsible for the variations in immunogenicity among the L1210 clones were not investigated further.
In this study, we examined the L1210 lymphoma clones for: (i) the ability to function as APCs for naïve and primed T cells and (ii) the expression of MHC and costimulatory molecules. Using mixed lymphocyte reactions (MLRs), we demonstrated that immunogenic, but not tumorigenic clones were capable of stimulating allogeneic and syngeneic primary T-cell proliferation. Furthermore, immunogenic L1210 clones were shown to function as potent APCs in studies using antigen-specific T cells (DO11·10) which produce IL-2 in response to a specific ovalbumin peptide (pOVA). Lastly, flow cytometric analysis demonstrated that the immunogenic L1210 clones 2 and 7-15·6, but not the tumorigenic clones 3-3, 4, 5 and 6, expressed MHCII, B7-1, B7-2 and CD40, while the tumour-forming subclones 7-23 and 7-41 retained expression of MHCII and B7-1, but lacked B7-2 and CD40. Blocking B7-1 or B7-2 function on immunogenic L1210 clone 7-15·6 cells reduced their capacity to activate naïve T cells. In addition, incubation of clone 7-15·6 with CD40 antibodies (Abs) resulted in the up-regulation of MHCII, B7-1 and B7-2 expression, and an increased capacity to induce IL-2 production by naïve T cells. Our collective results demonstrate that the immunogenicity of L1210 clones correlates with their ability to directly activate naïve T cells ex vivo. Thus, the immunogenicity and APC function of L1210 cells are directly correlated with concomitant expression of MHCII and the costimulatory molecules B7-1, B7-2 and CD40.
Materials and methods
Animals
DBA/2 (syngeneic) mice were purchased from Taconic (Germantown, NY). C57BL/6 (allogeneic) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were kept under pathogen-free conditions according to institutional guidelines.
Cell culture
BALB/c-derived A20 and DBA/2-derived L1210 are H-2d-expressing murine B-cell lymphomas. The L1210 clones (2, 3-3, 4, 5 and 6) and subclones (7-15·6, 7-23 and 7-41) utilized in these studies were isolated previously by limiting dilution from parental L1210 and immunogenic clone 7, respectively.18 300-18 is a pre-B-cell line, and DO11·10 is a T-cell hybridoma that produces IL-2 in response to the ovalbumin peptide323–339 (pOVA) presented in the context of I-Ad. All cells were maintained in RPMI-1640 (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 50 U/ml penicillin/streptomycin (Invitrogen), 1 mm sodium pyruvate (Invitrogen), and 50 μm 2-mercaptoethanol (Invitrogen) as previously described.19
Primary cells
Bone marrow-derived dendritic cells (BMDCs) were isolated from DBA/2 mice, cultured for 7 days in granulocyte–macrophage colony-stimulating factor (GM-CSF), and matured with lipopolysaccharide (LPS) prior to use. For allogeneic and syngeneic MLRs, primary T cells were freshly isolated from C57BL/6 and DBA/2 mice, respectively. The laboratory of Dr Deb Fowell (University of Rochester, Rochester, NY) graciously provided primary DO11·10 T cells. Briefly, lymph nodes and spleens were harvested from DO11·10 transgenic mice. Single-cell suspensions were generated and combined with an antibody cocktail containing monoclonal antibodies specific for CD8α (clone 3·155), CD24 (clone J11D), and MHCII (clone BP107). Guinea pig complement was added and T cells were subsequently purified using Ficoll columns (GE-Healthcare, Piscataway, NJ).
RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was isolated using TRIzol (Invitrogen) as specified by the manufacturer. Reverse transcriptase (RT) reactions were performed on 2 μg of total RNA using Superscript II RT (Invitrogen) as described previously.20 Standard semiquantitative RT-PCR was performed as previously described,21 using the indicated cycle numbers: class II transactivator (CIITA) (30), MHCII (IA-α), B7-1, B7-2 and CD40 (28–30), and actin (20). The primers utilized in RT-PCR were described previously as follows: CIITA, IAα and actin;22 B7-1 and B7-2;23 and CD40.24
Antibodies
Phycoerythrin (PE)-conjugated anti-mouse Abs specific for MHCI (Kb/Dd), MHCII (IA/IE), B7-1, B7-2, CD40, B220, CD11b, CD5 and rat immunoglobulin G2 (IgG2) isotype control were obtained from Biolegend (San Diego, CA), as was unconjugated CD16/CD32 (Fc receptor (FcR) block) Ab. low endotoxin azide-free (LEAF)-purified anti-B7-1 (clone 16-10A1), anti-B7-2 (clone GL-1) and anti-CD40 (clone 1C10) Abs and isotype-matched rat anti-IgG2a (clone RTK2758) and Armenian hamster anti-Ig (clone HTK888) Abs were purchased from Biolegend for use in MLR experiments.
Antigens
Chicken albumin (ovalbumin) and bovine serum albumin (BSA) were purchased from Sigma. The ovalbumin peptide323–339 (ISQAVHAAHAEINEAGR) was purchased from Anaspec (San Jose, CA). Antigens were reconstituted in phosphate-buffered saline (PBS), sterile filtered, aliquotted, and stored at −20° prior to use.
Flow cytometry
Cells (1 × 106) were stained for 60 min at 4° in fluorescence-activated cell sorting (FACS) wash buffer (1× PBS, 2% BSA and 1% sodium azide) containing anti-CD16/CD32 and PE-conjugated Abs at concentrations suggested by the manufacturer. Background staining was determined using PE-conjugated rat IgG2a isotype control. Cells were subsequently washed with FACS wash buffer and fixed in 2% paraformaldehyde (Sigma, St Louis, MO). FACS analysis was performed using a FACScan (Becton Dickinson, San Jose, CA) with cellquest (BD, Franklin Lakes, NJ) and winmdi software (Scripps Research Institute, La Jolla, CA).
Mixed lymphocyte reactions (MLRs)
Target cells were pretreated with mitomycin C (Sigma) for 30 min at 37°, and washed with PBS prior to use. Primary T cells (1 × 105) were cultured with target cells at effector-to-target (E:T) ratios of 50 : 1, 20 : 1, and 5 : 1 for 3 days at 37° in 96-well plates. T cells were also cultured independently with phorbol 12-myristate 13-acetate and ionomycin (PMA/I). Wells were subsequently pulsed with [3H]-thymidine overnight and T-cell proliferation was measured by [3H]-thymidine incorporation using a Tomtec Harvester (Tomtec, Hamden, CT).
In Ag-specific MLRs, target cells were pulsed overnight with 5 or 50 μm ovalbumin (OVA) or BSA, or 0·2 μm OVA peptide (pOVA) in 24-well plates, and washed prior to use. BMDCs were supplemented with antigen 3 hr prior to the addition of LPS. Primary or hybridoma DO11·10 T cells (1 × 105) were cultured with target cells (at E:T ratios of 1 : 1 and 10 : 1) and antigen for 24 hr at 37° in 24-well plates. T cells were also cultured independently with PMA/I. Supernatants collected from each well were centrifuged, transferred to fresh Eppendorf tubes and stored at −20°. To quantify T-cell activation, supernatants were assayed for IL-2 protein using the Mouse IL-2 ELISA Ready-SET-Go! kit from eBioscience (San Diego, CA) according to the manufacturer’s instructions. In Ag-specific MLRs with anti-B7 or anti-CD40 Abs, cells were treated with 10 μg/ml specific or isotype Ab for 1–2 hr prior to being combined with primary DO11·10 T cells for 24 hr.
Statistical analysis
prism 4·0 software (GraphPad software, LaJolla, CA) and the paired Student’s t-test in Microsoft Office Excel 2003 were used for statistical analysis of the data.
Results
Immunogenic L1210 clones function as efficient APCs
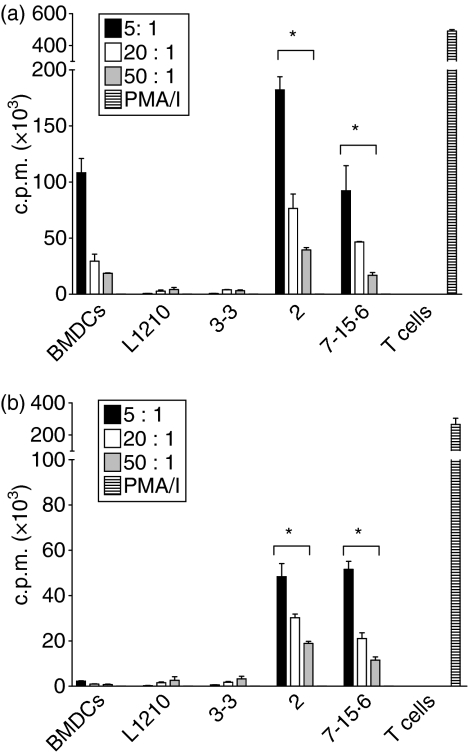
To determine whether the immunogenicity of L1210 clones correlates with their ability to activate T cells, MLRs were performed in which primary T cells isolated from C57BL/6 (H-2b) or DBA/2 (H-2d) mice were cultured with immunogenic (clones 2 and 7-15·6) or tumorigenic (parental and clone 3-3) L1210 cells (all H-2d). T-cell proliferation was used as the readout for T-cell activation, which was measured by [3H]thymidine uptake. As shown in Fig. 1a, significant allogeneic T-cell proliferation was stimulated by L1210 clones 2 and 7-15·6, but not L1210 parental cells or clone 3-3. The allogeneic proliferative response to L1210 clone 7-15·6 was comparable to that elicited by allogeneic (H-2d) BMDCs, while L1210 clone 2 stimulated a twofold greater response than BMDCs. Interestingly, L1210 clones 2 and 7-15·6 also stimulated significant levels of syngeneic T-cell proliferation, although the degree of proliferation induced was 2–4-fold lower than that observed in the allogeneic setting (Fig. 1b). Parental L1210 cells and clone 3-3 did not elicit any detectable syngeneic T-cell proliferation (Fig. 1b).
Figure 1.
Stimulation of T-cell proliferation by L1210 clones. Parental L1210 cells, tumorigenic clone 3-3, and immunogenic clones 2 and 7-15·6 were treated with mitomycin C and combined with (a) allogeneic (C57BL/6, H-2b) or (b) syngeneic (DBA/2, H-2d) T cells at different effector-to-target (E:T) ratios. The counts per minute (c.p.m.) of [3H]thymidine incorporation indicates the T-cell proliferative response against the target cells. DBA/2 bone marrow-derived dendritic cells (BMDCs) were used as positive control target cells, and T cells treated with phorbol 12-myristate 13-acetate and ionomycin (PMA/I) were included as positive controls for T-cell proliferation. Bars represent the means (± standard deviation) for triplicate wells with background levels subtracted out. Each graph shown is one representative experiment of four independent experiments performed; *, P< 0·05, compared with tumorigenic L1210 cells.
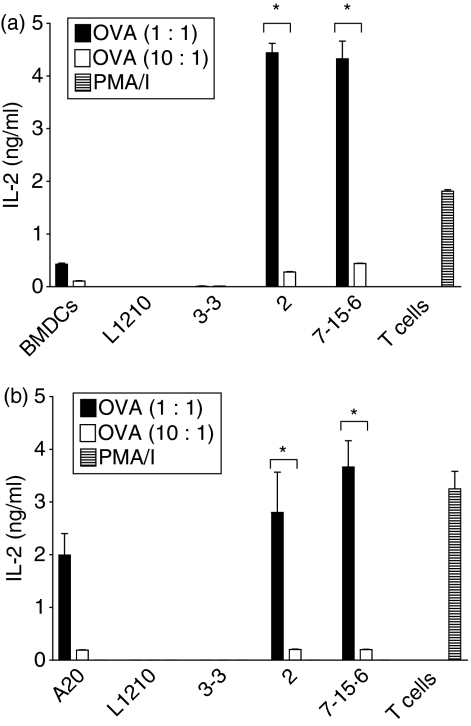
To examine the capacity of different L1210 clones to process exogenous Ag and stimulate T cells in an Ag-specific fashion, L1210 parental cells and clones were first pre-incubated with whole ovalbumin (OVA) or BSA (control) and then tested for the ability to stimulate IL-2 production from DO11·10 T cells bearing a transgenic T-cell receptor (TCR) specific for an OVA-derived peptide (pOVA; residues 323–339) presented in the context of MHCII (I-Ad). As shown in Fig. 2, immunogenic L1210 clones 2 and 7-15·6 pre-incubated with OVA stimulated IL-2 production by both ‘primed’ hybridoma DO11·10 T cells (Fig. 2a; 4·5 ng/ml IL-2) and ‘naïve’ primary DO11·10 T cells (Fig. 2b; 3–4 ng/ml IL-2). Similar results were obtained when clones 2 and 7-15·6 were first pre-incubated with pOVA, which does not require any additional processing (data not shown). In marked contrast, parental L1210 and clone 3-3 cells pre-incubated with either whole OVA (Fig. 2) or the pOVA both failed to stimulate DO11·10 responder T cells to secrete IL-2 (data not shown). MLR cultures supplemented with BSA, as well as non-supplemented cultures, resulted in no IL-2 production from either primed or naïve DO11·10 T cells (data not shown). In addition, IL-2 production was not detected for any cell line cultured individually or in the presence of Ag alone (data not shown). These collective studies demonstrate that the immunogenicity of MHCII+ L1210 clones is directly correlated with their ability to: (i) process and present exogenous antigen, and (ii) stimulate naïve T-cell proliferation and IL-2 production. Thus, the immunogenic MHCII+ L1210 clones 2 and 7-15·6 have the capacity to directly activate naïve T cells by functioning as APCs.
Figure 2.
DO11·10 T cells produce interleukin (IL)-2 in response to immunogenic L1210 clones. L1210 parental cells, tumorigenic clone 3-3, and immunogenic clones 2 and 7-15·6 were pulsed overnight with whole ovalbumin (OVA; 50 μm), and then combined with the (a) DO11·10 hybridoma or (b) primary DO11·10 T cells for 24 hr at effector-to-target (E:T) ratios of 1 : 1 or 10 : 1. Supernatants were collected and assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA). Bars represent the means (± standard deviation) for triplicate wells and each graph shown is one representative experiment of three independent experiments performed; *, P< 0·01, compared to tumorigenic L1210 cells. PMA/I, phorbol 12-myristate 13-acetate and ionomycin.
Subclones of an immunogenic L1210 clone differ in their capacity to stimulate T cells
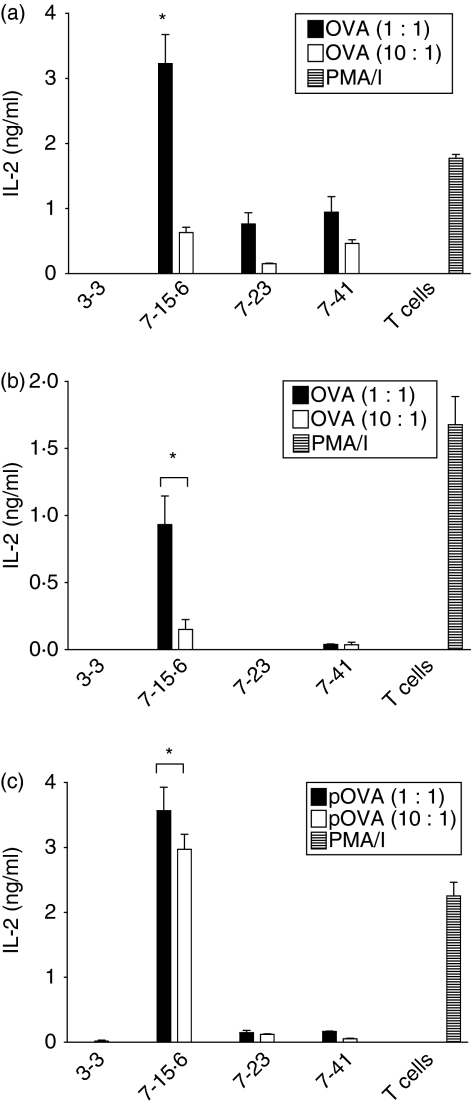
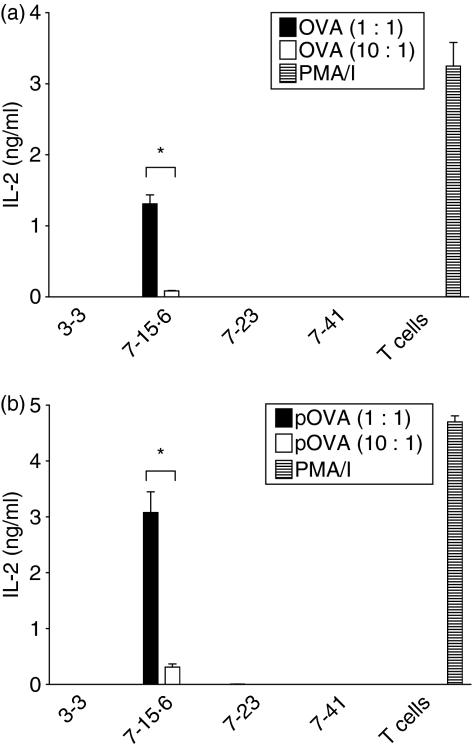
Previous studies identified subclones of immunogenic L1210 clone 7 (7-23 and 7-41) that formed tumours after injection into immunocompetent mice with slower kinetics than the original tumorigenic L1210 clones 3-3, 4, 5 and 6.18 To determine whether the differences in the rate of tumour formation correlate with variation in the ability to activate T cells, Ag-specific MLRs were performed using primed and naïve DO11·10 T cells. When L1210 clones 7-15·6, 7-23 and 7-41 were pre-incubated with high concentrations of whole OVA (50 μm), each was capable of stimulating detectable IL-2 production from primed DO11·10 T cells (Fig. 3a). However, clone 7-15·6 stimulated threefold to fourfold more IL-2 production than either clone 7-23 or clone 7-41 when incubated with primed responder T cells at a ratio of 1 : 1. When pre-incubated with lower OVA concentrations (5 μm), L1210 clone 7-15·6 still stimulated significant levels of IL-2 production from primed T cells, whereas clones 7-23 and 7-41 induced little to no detectable IL-2 production (Fig. 3b). Furthermore, clone 7-15·6 pulsed with pOVA stimulated significantly higher levels (> 20-fold) of IL-2 from the primed T-cell hybridoma, relative to variants 7-23 and 7-41 (Fig. 3c). Importantly, only clone 7-15·6 was capable of stimulating any detectable IL-2 production from naïve, primary DO11·10 T cells (Fig. 4). The slower kinetics of tumour formation exhibited by clones 7-23 and 7-41 may therefore reflect a limited capacity to stimulate an endogenous T-cell response.
Figure 3.
Interleukin (IL)-2 production by the DO11·10 T-cell hybridoma in response to L1210 subclones. Tumorigenic clone 3-3, tumour-forming subclones 7-23 and 7-41, and immunogenic clone 7-15·6 were supplemented with whole ovalbumin (OVA) or ovalbumin peptide (pOVA) and combined with the DO11·10 hybridoma for 24 hr at different effector-to-target (E:T) ratios. Supernatants were collected and assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA). Bars represent the means (± standard deviation) for triplicate wells, and each graph shown is one representative experiment of multiple independent experiments performed; (a) 50 μm OVA; (b) 5 μm OVA; (c) 0·2 μm pOVA. *, P< 0·005, compared with L1210 tumour-forming subclones. PMA/I, phorbol 12-myristate 13-acetate and ionomycin.
Figure 4.
Interleukin (IL)-2 production by primary DO11·10 T cells in response to L1210 subclones. Tumorigenic clone 3-3, tumour-forming subclones 7-23 and 7-41, and immunogenic clone 7-15·6 were pulsed overnight with whole ovalbumin (OVA) or ovalbumin peptide (pOVA) and then combined with primary DO11·10 T cells for 24 hr at different effector-to-target (E:T) ratios. Supernatants were collected and assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA). Bars represent the means (± standard deviation) for triplicate wells and each graph shown is one representative experiment of four independent experiments performed; (a) 5 μm OVA; (b) 0·2 μm pOVA. *, P< 0·01, compared with L1210 tumour-forming subclones. PMA/I, phorbol 12-myristate 13-acetate and ionomycin.
Costimulatory molecules are differentially expressed on L1210 clones that vary in immunogenicity
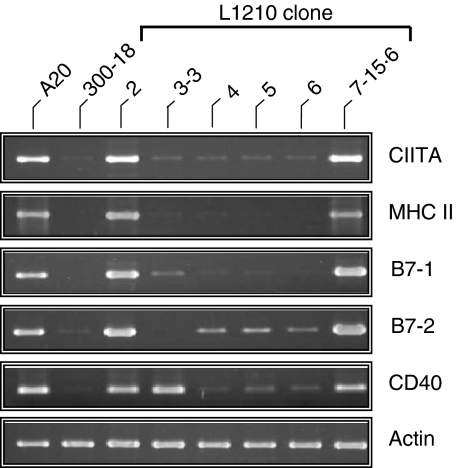
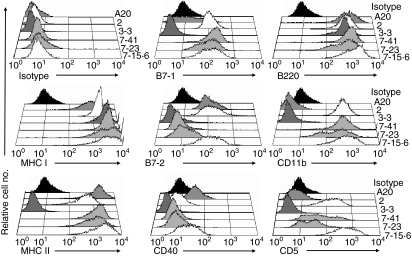
While MHCII expression was previously correlated with the immunogenicity of the L1210 clones, comparable levels of MHCI expression were observed on all of the L1210 clones.18 However, costimulatory signals are also critical for APCs to effectively activate naïve T cells. We therefore hypothesized that differential expression of one or more of the conventional costimulatory molecules (B7-1, B7-2, and CD40) may contribute to the immunogenicity of the MHCII+ L1210 clones. Consistent with this hypothesis, immunogenic L1210 clones 2 and 7-15·6, which express the CIITA and MHCII molecules,19 also expressed mRNA for the costimulatory molecules B7-1, B7-2 and CD40 (Fig. 5). Conversely, tumorigenic L1210 clones 3-3, 4, 5 and 6 expressed little to no mRNA for these costimulatory genes, with the exception of CD40 mRNA in clone 3-3 (Fig. 5). Flow cytometric analysis demonstrated that, in addition to MHCII Ag expression, L1210 clones 2 and 7-15·6 expressed the costimulatory molecules B7-1, B7-2 and CD40 on the cell surface (Fig. 6 and Table 1). In contrast, tumorigenic clones 3-3, 4, 5 and 6, and parental L1210 cells lacked surface expression of these costimulatory molecules (Fig. 6, Table 1 and data not shown). Although CD40 transcripts were detected in tumorigenic clone 3-3 (Fig. 5), the CD40 protein was not detected on these cells (Fig. 6). In addition, all of the L1210 clones expressed high levels of the B-cell marker B220 (Fig. 6), but completely lacked expression of the DC markers CD8α, CD11c and CD83 (data not shown). Interestingly, MHCII+ L1210 clones 2 and 7-15·6, but not 3-3, 4, 5 or 6, also expressed CD5 (Ly-1) and CD11b (Mac-1) (Fig. 6 and data not shown).
Figure 5.
Differential gene expression in tumorigenic and immunogenic L1210 clones. Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis for the class II transactivator (CIITA), major histocompatibility complex class II (MHCII; IAα), B7-1, B7-2 and CD40 was performed on RNA isolated from tumorigenic (3-3, 4, 5 and 6) and immunogenic (2 and 7-15·6) L1210 clones. A20 and 300-18 cells were included as positive and negative controls, respectively. Actin was included as a control for mRNA integrity and quality of the cDNA reactions.
Figure 6.
Phenotypic analysis of immune response genes on L1210 clonal variants. Flow cytometric analysis was performed for major histocompatibility complex class I (MHCI), MHCII (IA/IE), B7-1, B7-2, CD40, B220, CD11b and CD5 expression on tumorigenic L1210 clone 3-3 (dark grey histograms), tumour-forming subclones 7-23 and 7-41 (light grey histograms), and immunogenic clones 2 and 7-15·6 (white histograms). A20 was used as a control for positive staining (dark grey histograms). Rat immunoglobulin G2a (IgG2a) isotype staining of variant 7-23 was used to represent maximum isotype control staining (black histograms) in each histogram.
Table 1.
Summary of the immunological properties of L1210 lymphoma clones
| Cell line | Immunogenicity | Isotype | MHCI | MHCII | B7-1 | B7-2 | CD40 | Stimulated naïve T cells | Stimulated primed T cells |
|---|---|---|---|---|---|---|---|---|---|
| L1210 | − | 3 | nd | 3 | 3 | 3 | 3 | − | − |
| 2 | +++ | 4 | 1476 | 316 | 111 | 136 | 15 | +++ | +++ |
| 3-3 | − | 3 | 2594 | 4 | 3 | 4 | 4 | − | − |
| 7-15·6 | +++ | 8 | 1863 | 1365 | 109 | 116 | 22 | +++ | +++ |
| 7-23 | +/− | 9 | 2258 | 765 | 90 | 9 | 9 | − | + |
| 7-41 | +/− | 7 | 2849 | 1176 | 152 | 8 | 6 | − | + |
Numbers for the isotype control antibodies, major histocompatibility complex (MHC) and costimulatory molecules represent the geometric mean fluorescence intensity.
For stimulation of naïve and primed T cells: −, none; +, low; ++, moderate; +++, high level; nd, not done.
Expression of the MHC and costimulatory molecules was also examined on L1210 subclones 7-23 and 7-41. Importantly, high levels of MHCII Ag expression were retained on the tumour-forming clones 7-23 and 7-41 (Fig. 6 and Table 1). These results indicate that the decreased immunogenicity of these subclones, and their inability to stimulate Ag-specific naïve T cells (Fig. 4), are not attributable to alterations of MHC expression. Moreover, this reinforces the conclusion that MHCII expression alone is not sufficient for the immunogenicity of L1210 cells. While comparable levels of expression of MHCI, B7-1, CD5 and CD11b were observed on all of the MHCII+ L1210 clones, expression of the costimulatory molecules B7-2 and CD40 was not detected on tumour-forming clones 7-23 and 7-41 (Fig. 6 and Table 1). Therefore, loss of B7-2 and/or CD40 expression could contribute to the decreased immunogenicity of L1210 clones 7-23 and 7-41. Taken together, these results strongly suggest that MHCII and B7-1 expression is insufficient for L1210 cells to activate naïve T cells, and for the generation of tumour-eradicating immunity against this lymphoma.
Blocking B7 expression on immunogenic MHCII+ L1210 cells reduces the T-cell stimulatory capacity
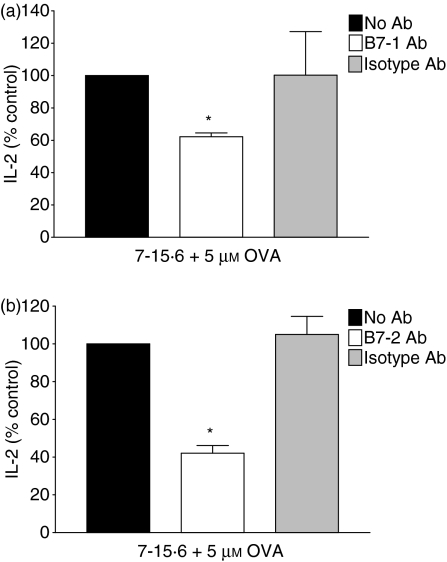
To directly examine whether B7 expression on immunogenic L1210 cells plays an essential role in activating naïve T cells, Ag-specific MLRs were performed in the presence of neutralizing antibodies to B7-1 and B7-2 molecules, and IL-2 production was measured. Blocking B7-1 function on L1210 clone 7-15·6 pulsed with OVA (5 μm) reduced T-cell stimulation by ∼40% when naïve DO11·10 T cells were used as responders (Fig. 7a). Furthermore, blocking B7-2 function on L1210 clone 7-15·6 decreased IL-2 production from naïve T cells by 55% (Fig. 7b). Comparable results were observed in previous studies using these specific Abs to test the effects of blocking B7-1 and B7-2 function on autoreactive T-cell proliferation.25 Similar decreases in IL-2 production from naïve T cells were observed whether L1210 clone 7-15·6 was pre-incubated with pOVA, or high concentrations (50 μm) of whole OVA (data not shown). IL-2 was not detected from clone 7-15·6 or naïve T cells cultured individually in the presence or absence of Abs (data not shown). These results suggest that both B7-1 expression and B7-2 expression play a functional role in the ability of MHCII+ immunogenic L1210 cells to activate naïve T cells.
Figure 7.
Blocking B7 expression on immunogenic L1210 cells reduces T-cell stimulatory capacity. Immunogenic L1210 clone 7-15·6 was pulsed overnight with whole ovalbumin (OVA; 5 μm), incubated either in the absence of antibody (Ab) (black bars), with 10 μg/ml anti-B7 Ab (white bars), or with isotype control Ab (grey bars), and combined with primary DO11·10 T cells at an effector-to-target (E:T) ratio of 1 : 1. Supernatants were collected and subsequently assayed for interleukin (IL)-2 by enzyme-linked immunosorbent assay (ELISA). IL-2 stimulated by cells in the absence of Ab is represented as 100%. Data represent the percentage of IL-2 (mean± standard deviation) stimulated in three independent experiments performed; (a) anti-B7-1 Ab; (b) anti-B7-2 Ab; *, P< 0·05, compared with isotype-treated controls.
Incubation with CD40 antibodies increases the capacity of immunogenic L1210 clone 7-15·6 to stimulate IL-2 production from naïve T cells
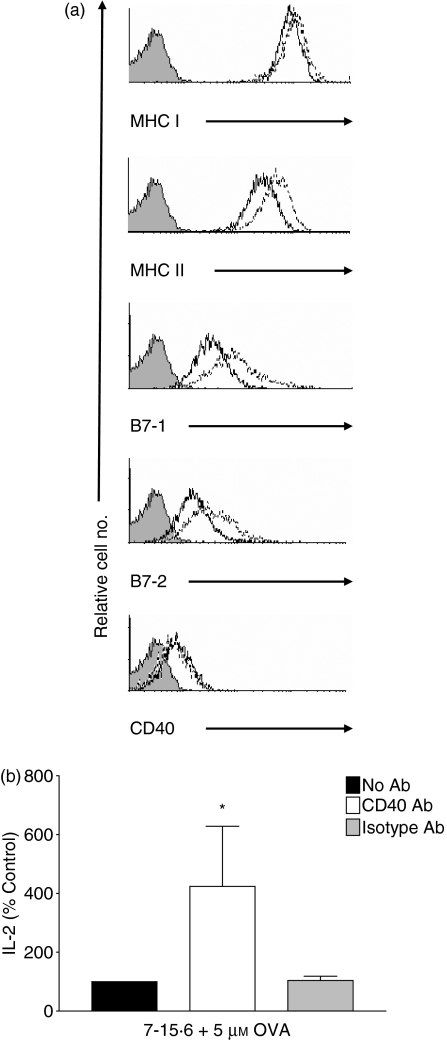
CD40 signalling has been reported to render APCs more competent via the consequential up-regulation of B7 costimulatory molecule expression.3 To evaluate the role of CD40 in the immunogenicity of L1210 cells, we used (i) flow cytometry to examine whether incubation of clone 7-15·6 with anti-CD40 Abs induced changes in the surface phenotype, and (ii) OVA-specific MLR assays to assess the capacity of anti-CD40 Ab-treated clone 7-15·6 to stimulate IL-2 production by naïve DO11·10 T cells. MHCI, MHCII, B7-1 and B7-2 expression was up-regulated on L1210 clone 7-15·6 within 3 hr of exposure to anti-CD40 Abs (data not shown) but there was no effect on CD40 surface expression. Incubation of clone 7-15·6 with anti-CD40 Abs for 24 hr substantially increased MHCII, B7-1 and B7-2 surface expression (Fig. 8a). Importantly, incubation of L1210 clone 7-15·6 cells with anti-CD40 Abs resulted in a fourfold increase in their ability to stimulate IL-2 production by naïve DO11·10 T cells in MLRs using low concentrations of OVA (5 μm) or pOVA (Fig. 8b and data not shown). When high concentrations of OVA (50 μm) were used in MLRs, L1210 clone 7-15·6 induced 40% more IL-2 production from naïve primary DO11·10 T cells when incubated with CD40 Abs, versus isotype control Abs (data not shown). Thus, ligation of L1210 clone 7-15·6 with anti-CD40 Abs resulted in enhanced expression of MHC and costimulatory molecules, and a significantly increased ability to function as APCs and stimulate IL-2 production from naïve T cells.
Figure 8.
Incubation of immunogenic L1210 clones with anti-CD40 antibodies (Abs) increases antigen-presenting cell (APC) function. (a) Immunogenic L1210 clone 7-15·6 was incubated for 24 hr with 10 μg/ml anti-CD40 Ab and the surface expression of major histocompatibility complex class I (MHCI), MHCII, B7-1, B7-2 and CD40 was examined by flow cytometry. Shaded histograms represent isotype control staining, solid lines indicate surface expression in untreated 7-15·6 cells, and dotted lines represent surface expression after anti-CD40 Ab treatment. Histograms are representative of two independent experiments performed. (b) Clone 7-15·6 was pulsed overnight with whole ovalbumin (OVA; 5 μm) and incubated either in the absence of Ab (black bars), with 10 μg/ml anti-CD40 Ab (white bars), or with isotype-matched control Ab (grey bars), and combined with primary DO11·10 T cells at an effector-to-target (E:T) ratio of 1 : 1. Supernatants were collected and subsequently assayed for interleukin (IL)-2 by enzyme-linked immunosorbent assay (ELISA). IL-2 stimulated by cells in the absence of Ab is represented as 100%. Data represent the percentage of IL-2 (mean± standard deviation) stimulated in three independent experiments performed; *, P< 0·05, compared with isotype-treated controls.
Discussion
Tumour immunogenicity can be defined by the expression of tumour-specific Ags that facilitate tumour-specific immunity and tumour rejection.26 Defining the characteristics that contribute to tumour immunogenicity is essential for the development of tumour-eradicating immunotherapies. In contrast to genetically altered tumour cells, the L1210 B-cell lymphoma model allows the examination of immunogenic and tumorigenic L1210 clones that arose spontaneously within the heterogeneous population. MHCII expression on clones of the L1210 B-cell lymphoma was previously shown to directly correlate with immunogenicity.18 In this study, we sought to further characterize the factors that are responsible for the enhanced immunogenicity of MHCII+ L1210 lymphoma cells. Our studies, summarized in Table 1, demonstrate that immunogenic, MHCII+ L1210 clones have the capacity to function as APCs and stimulate naïve T cells to proliferate and produce IL-2. In contrast, tumorigenic, MHCII-deficient L1210 clones and parental L1210 cells were completely incapable of activating T cells. L1210 subclones 7-23 and 7-41, which exhibited decreased immunogenicity in mice,18 were also not capable of stimulating naïve T cells. Moreover, these subclones stimulated significantly lower levels of IL-2 from ‘primed’ hybridoma T cells, relative to the highly immunogenic L1210 clones 2 and 7-15·6.
The ability of immunogenic L1210 clones to function as APCs correlates with concomitant expression of MHCII and the costimulatory molecules B7-1, B7-2 and CD40. MHCI expression was previously shown to be comparable among immunogenic and tumorigenic L1210 clones.18 Importantly, high levels of MHCI and MHCII expression were also detected on the tumour-forming L1210 subclones 7-23 and 7-41. While all of the MHCII+ L1210 clones expressed comparable levels of B7-1, clones 7-23 and 7-41 lacked expression of CD40 and B7-2. Since L1210 clones 7-23 and 7-41 could not stimulate naïve T cells ex vivo, and ultimately formed tumours in mice,18 these results strongly suggest that MHCII and B7-1 expression on L1210 lymphoma cells is not sufficient for the activation of T cell-mediated tumour-eradicating immunity. Moreover, blocking B7-1 or B7-2 expression on immunogenic MHCII+ L1210 cells diminished their capacity to stimulate IL-2 production from naïve T cells, demonstrating that both B7-1 expression and B7-2 expression contribute to the potent immunogenicity exhibited by immunogenic L1210 cells. As B7-1 costimulation alone is not sufficient for naïve T-cell activation by MHCII+ L1210 subclones, loss of B7-2 expression may directly contribute to their decreased immunogenicity.
Our studies strongly suggest that CD40 expression is also a critical costimulatory molecule for the immunogenicity of MHCII+ L1210 cells. CD40 is the first costimulatory molecule to engage following MHC:TCR binding, and CD40 ligation subsequently activates the APC and up-regulates B7 surface expression on normal and malignant B cells.3,27,28 Moreover, blocking of CD40 signalling in allogeneic B cells induced T-cell tolerance in MLRs.29 In our study, incubation of immunogenic L1210 clones with anti-CD40 Abs resulted in enhanced MHCI, MHCII, B7-1 and B7-2 expression. Importantly, these CD40-stimulated L1210 cells activated significantly greater IL-2 production from naïve T cells. The increase in MHC and B7 expression on L1210 clone 7-15·6 following exposure to CD40 Abs is likely to play a role in the enhanced capacity to activate T cells. However, CD40 signalling in L1210 cells may also up-regulate other cell surface molecules that enhance APC capacity, such as intercellular adhesion molecule 1 (ICAM-1; CD54). Indeed, CD40:CD40 ligand (CD40L) interactions increased ICAM-1 expression on B cells and samples from non-Hodgkin’s lymphoma patients,27,28,30 and ICAM-1 compensated for costimulation in the absence of B7:CD28 interactions.30 Whether ICAM-1 or other cell surface molecules participate in the increased APC functions of CD40-stimulated L1210 cells requires further investigation.
We previously demonstrated that parental L1210 cells are derived from an early stage of B-cell ontogeny.19 As early-stage murine B cells express low to undetectable levels of MHCII Ags31–33 and lack costimulatory molecules, our current studies suggest that the immunogenic clones represent a more mature stage of B-cell ontogeny. Alternatively, under certain conditions mouse pro-B cells can differentiate into B/macrophage cells34 or DCs.35 The ability of the immunogenic clones to function as potent APCs and activate naïve T cells is consistent with a DC-like phenotype. However, all of the L1210 clones expressed high levels of the B-cell marker B220, but completely lacked expression of the DC markers CD8α, CD11c and CD83 (data not shown). Moreover, the MHCII+ L1210 clones 2, 7-15·6, 7-23 and 7-41 expressed CD5 (Ly-1) and CD11b (Mac-1). This immunophenotype is consistent with both B-1a36,37 and B/macrophage cells.34 Thus, immunogenic L1210 clones may represent B-1a cells or B/macrophages, but over time variants arose that lost expression of B7-2 and CD40.
Studies in mice demonstrate that the immunogenicity of multiple different types of tumour can be enhanced by stable transfection with expression vectors for MHCII molecules.6,7,10,38–41 Mice injected with these MHCII+ tumour cells generated protective immunity against subsequent challenge with the non-immunogenic parental tumours.6,7 In some instances, the MHCII+ tumour cells had the capacity to function as APCs and activate CD4+ T cells.8,9 Interestingly, the requirement for costimulatory molecules on the MHCII+ tumours to activate immune responses appears to vary among different tumour model systems. For example, B7-1 and B7-2 costimulatory molecules were equally efficient in activating tumour immunity against Sa1 sarcoma cells that were cotransfected with MHCII expression vectors.10,11 In contrast, MHCII+ TS/A breast adenocarcinoma cells were capable of efficiently activating T cells despite lacking expression of B7-1 or B7-2.8 Thus, the mechanisms underlying generation of tumour immunity differ among these systems. Our collective studies strongly suggest that B7 and CD40 expression also plays an important role in the immunogenicity of MHCII+ L1210 clones.
Gene expression profiling analysis demonstrated that loss of MHCII expression on human DLBCL is tightly correlated with significant decreases in patient survival.12–15 A positive correlation between MHCII expression on tumour cells and improved prognosis has also been found for a variety of carcinomas.42,43 Importantly, MHCII expression on DLBCL is correlated with the numbers of tumour-infiltrating lymphocytes (TILs). Associations between lower numbers of TILs (primarily T cells) and poor patient outcome have been demonstrated in several types of B-cell lymphoma.44–47 Collectively, these results suggest that MHCII loss in B-cell lymphomas may constitute a strategy of tumour immunoevasion that ultimately results in decreased patient survival. However, it is currently unclear whether antigen presentation by these tumour cells plays a direct role in the putative immunosurveillance process. A recent report demonstrated that CD40 expression on DLBCL tumours correlates with improved prognosis.48 Furthermore, one study identified a correlation between loss of B7-2 expression and decreased TIL in aggressive human B-cell lymphomas,44 but the relationship between costimulatory molecule expression and prognosis for patients with MHCII+ B-cell malignancies has not been extensively investigated. Our current studies demonstrating that immunogenic MHCII+ L1210 cells expressing CD40 and B7 function as potent APCs to activate naïve T cells support the argument that MHCII and costimulatory molecule expression on B-cell lymphomas plays an important role in immunosurveillance.
Acknowledgments
We are extremely grateful to Drs Deb Fowell, Byron AuYeung, Theresa Sukiennicki, and Irina Bromberg at the University of Rochester, and Dr Sandra Gollnick at Roswell Park Cancer Institute for providing us with primary and hybridoma DO11·10 T cells, respectively. We thank Dr Nancy Luckashenak for her technical support and Drs Edith Lord and Lisa Rimsza for critical review of this manuscript. KAC was supported by National Cancer Institute Predoctoral Training Grant 55640201.
Glossary
Abbreviations:
- Abs
antibodies
- Ag
antigen
- APC
antigen-presenting cell
- BMDC
bone marrow-derived dendritic cell
- BSA
bovine serum albumin
- DLBCL
human diffuse large B-cell lymphoma
- MHCII
major histocompatibility complex class II
- MLR
mixed lymphocyte reaction
- MST
mean survival time
- OVA
ovalbumin
- PMA/I
phorbol 12-myristate 13-acetate and ionomycin
- RT-PCR
reverse transcriptase–polymerase chain reaction
- TIL
tumour-infiltrating lymphocyte
Disclosures
The authors have no financial conflict of interest with this work.
References
- 1.Chambers C. The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 2001;22:217–23. doi: 10.1016/s1471-4906(01)01868-3. [DOI] [PubMed] [Google Scholar]
- 2.Fujii S-I, Liu K, Smith C, Bonito A, Steinman R. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foy T, Aruffo A, Bajorath J, Buhlmann J, Noelle R. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 4.Celia M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrand-Rosenberg S, Thakur A, Clements V. Rejection of mouse sarcoma cells after transfection of MHC class II genes. J Immunol. 1990;144:4068–71. [PubMed] [Google Scholar]
- 7.James R, Edwards S, Hui K, Bassett P, Grosveld F. The effect of class II gene transfection on the tumorigenicity of the H-2K-negative mouse leukemia cell line K36.16. Immunology. 1991;72:213–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Meazza R, Comes A, Orengo A, Ferrini S, Accolla R. Tumor rejection by gene transfer of the MHC class II transactivator in murine mammary adenocarcinoma cells. Eur J Immunol. 2003;33:1183–92. doi: 10.1002/eji.200323712. [DOI] [PubMed] [Google Scholar]
- 9.Mortara L, Castellani P, Meazza R, et al. CIITA-induced MHC class II expression in mammary adenocarcinoma leads to a Th1 polarization of the tumor microenvironment, tumor rejection, and specific antitumor memory. Clin Cancer Res. 2006;12:3435–43. doi: 10.1158/1078-0432.CCR-06-0165. [DOI] [PubMed] [Google Scholar]
- 10.Baskar S, Clements VK, Glimcher LH, Nabavi N, Ostrand-Rosenberg S. Rejection of MHC class II-transfected tumor cells requires induction of tumor-encoded B7-1 and/or B7-2 costimulatory molecules. J Immunol. 1996;156:3821–7. [PubMed] [Google Scholar]
- 11.Thompson J, Dissanayake S, Ksander B, Knutson K, Disis M, Ostrand-Rosenberg S. Tumor cells transduced with the MHC class II transactivator and CD80 activate tumor-specific CD4+ T cells whether or not they are silenced for invariant chain. Cancer Res. 2006;66:1147–54. doi: 10.1158/0008-5472.CAN-05-2289. [DOI] [PubMed] [Google Scholar]
- 12.Miller T, Lippman S, Spier C, Slymen D, Grogan T. HLA-DR (Ia) immune phenotype predicts outcome for patients with diffuse large cell lymphoma. J Clin Invest. 1988;82:370–2. doi: 10.1172/JCI113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimsza L, Roberts R, Miller T, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the leukemia and lymphoma molecular profiling project. Blood. 2004;103:4251–8. doi: 10.1182/blood-2003-07-2365. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RA, Wright G, Rosenwald AR, et al. Loss of major histocompatibility class II gene and protein expression in primary mediastinal large B-cell lymphoma is highly coordinated and related to poor patient survival. Blood. 2006;108:311–8. doi: 10.1182/blood-2005-11-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE. CD4+ T-cell immune response to large B-cell non-hodgkin’s lymphoma predicts patient outcome. J Clin Oncol. 2001;19:720–6. doi: 10.1200/JCO.2001.19.3.720. [DOI] [PubMed] [Google Scholar]
- 16.Rimsza L, Farinha P, Fuchs D, Masoud H, Connors J, Gascoyne R. HLA-DR protein status predicts survival in patients with diffuse large B-cell lymphoma treated on the MACOP-B chemotherapy regimen. Leuk Lymphoma. 2007;48:542–6. doi: 10.1080/10428190601078605. [DOI] [PubMed] [Google Scholar]
- 17.Amiot L, Onno M, Lamy T, Dauriac C, Le Prise P-Y, Fauchet R, Drenou B. Loss of HLA molecules in B lymphomas is associated with an aggressive clinical course. Br J Haematol. 1998;100:655–63. doi: 10.1046/j.1365-2141.1998.00631.x. [DOI] [PubMed] [Google Scholar]
- 18.Fuji H, Iribe H. Clonal variation in tumorigenicity of L1210 lymphoma cells: nontumorigenic variants with an enhanced expression of tumor-associated antigen and Ia antigens. Cancer Res. 1986;46:5541–7. [PubMed] [Google Scholar]
- 19.Murphy SP, Holtz R, Lewandowski N, Tomasi TB, Fuji H. DNA alkylating agents alleviate silencing of CIITA gene expression in L1210 lymphoma cells. J Immunol. 2002;169:3085–93. doi: 10.4049/jimmunol.169.6.3085. [DOI] [PubMed] [Google Scholar]
- 20.Murphy SP, Tomasi TB. Absence of MHC class II antigen expression in trophoblast cells results from a lack of class II transactivator (CIITA) gene expression. Mol Reprod Dev. 1998;51:1–12. doi: 10.1002/(SICI)1098-2795(199809)51:1<1::AID-MRD1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Holtz R, Choi JC, Petroff MG, Piskurich JF, Murphy SP. Class II transactivator (CIITA) promoter methylation does not correlate with silencing of CIITA transcription in trophoblasts. Biol Reprod. 2003;69:915–24. doi: 10.1095/biolreprod.103.017103. [DOI] [PubMed] [Google Scholar]
- 22.Chang C-H, Fontes J, Peterlin M, Flavell R. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility class II genes. J Exp Med. 1994;180:1367–74. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikcevich K, Gordon K, Litjen Tan L, Hurst S, Kroepfl J, Gardinier M, Barrett T, Miller S. IFN-y-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158:614–21. [PubMed] [Google Scholar]
- 24.Williamson C, Dutton E, Abbott C, Beechey C, Ball S, Peters J. Thirteen genes (Cebpb, E2f1, Tcf4, Cyp24, Pck1, Acra4, Edn3, Kcnb1, Mc3r, Ntsr, Cd40, Plcg1 and Rcad) that probably lie in the distal imprinting region of mouse chromosome 2 are not monoallelically expressed. Genet Res. 1995;65:83–93. doi: 10.1017/s0016672300033103. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Sun D, Ke Y, Kaplan H, Shao H. The net effect of costimulatory blockers is dependent on the subset and activation status of the autoreactive T cells. J Immunol. 2007;178:474–9. doi: 10.4049/jimmunol.178.1.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, Hellstrom K. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523–32. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranheim E, Kipps T. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–35. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaperot L, Plumas J, Jacob M-C, Bost F, Molens J-P, Sotto J-J, Bensa J-C. Functional expression of CD80 and CD86 allows immunogenicity of malignant B cells from non-Hodgkin’s lymphomas. Exp Hematol. 1999;27:479–88. doi: 10.1016/s0301-472x(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 29.Buhlmann J, Fey T, Aruff A, et al. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995;2:645–53. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 30.Shinde S, Wu Y, Guo Y, Niu Q, Xu J, Grewal I, Flavell R, Liu Y. CD40L is important for induction of, but not response to, costimulatory activity; ICAM-1 as the second costimulatory molecule rapidly up-regulated by CD40L. J Immunol. 1996;157:2764–8. [PubMed] [Google Scholar]
- 31.Lala P, Johnson G, Battye F, Nossal G. Maturation of B lymphocytes: concurrent appearance of increasing Ig, Ia, and mitogen responsiveness. J Immunol. 1979;122:334–41. [PubMed] [Google Scholar]
- 32.Tarlinton D. Direct demonstration of MHC class II surface expression on murine pre-B cells. Int Immunol. 1994;5:1629–35. doi: 10.1093/intimm/5.12.1629. [DOI] [PubMed] [Google Scholar]
- 33.Miki N, Hatano M, Wakita K, Imoto S, Nishikawa S, Nishikawa S-I, Tokuhisa T. Role of I-A molecules in early stages of B cell maturation. J Immunol. 1992;149:801–7. [PubMed] [Google Scholar]
- 34.Borrello M, Phipps R. The B/Macrophage cell: an elusive link between CD5+ B cells and macrophages. Immunol Today. 1996;17:471–5. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- 35.Bjorck P, Kincade P. Cutting edge: CD19+ pro-B cells can give rise to dendritic cells in vitro. J Immunol. 1998;161:5795–9. [PubMed] [Google Scholar]
- 36.Montecino-Rodrigueza E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–33. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Berland R, Wortis H. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 38.Mongini C, Sanchez Lockhart M, Waldner C, Alvarez E, Gravisaco M, Roig M, Hajos S. Enhancement of anti-tumor immunity in syngeneic mice after MHC class II gene transfection. Br J Cancer. 1996;74:258–63. doi: 10.1038/bjc.1996.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong T, Clements V, Martin B, Ting J-Y, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA. 1997;94:6886–91. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armstrong TD, Clements VK, Ostrand-Rosenberg S. MHC class II-transfected tumor cells directly present antigen to tumor-specific CD4+ lymphocytes. J Immunol. 1998;160:661–6. [PubMed] [Google Scholar]
- 41.Dissanayake S, Thompson J, Bosch J, Clements V, Chen P, Ksander B, Ostrand-Rosenberg S. Activation of tumor-specific CD4+ T lymphocytes by major histocompatibility complex class II tumor cell vaccines: a novel cell-based immunotherapy. Cancer Res. 2004;64:1867–74. doi: 10.1158/0008-5472.can-03-2634. [DOI] [PubMed] [Google Scholar]
- 42.Altomonte M, Fonsatti E, Visintin A, Maio M. Targeted therapy of solid malignancies via HLA class II antigens: a new biotherapeutic approach? Oncogene. 2003;22:6564–9. doi: 10.1038/sj.onc.1206960. [DOI] [PubMed] [Google Scholar]
- 43.Seliger B, Maeurerb M, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–64. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 44.Stopeck AT, Gessner A, Miller TP, Hersh EM, Johnson CS, Cui H, Frutiger Y, Grogan TM. Loss of B7.2 (CD86) and intracellular adhesion molecule 1 (CD54) expression is associated with decreased tumor-infiltrating T lymphocytes in diffuse B-cell large-cell lymphoma. Clin Cancer Res. 2000;6:3904–9. [PubMed] [Google Scholar]
- 45.Lippman SM, Spier CM, Miller TP, Slymen DJ, Rybski JA, Grogan TM. Tumor-infiltrating T-lymphocytes in B-cell diffuse large cell lymphoma related to disease course. Mod Pathol. 1990;3:361–7. [PubMed] [Google Scholar]
- 46.List A, Spier C, Miller T, Grogan T. Deficient tumor-infiltrating T-lymphocyte response in malignant lymphoma: relationship to HLA expression and host immunocompetence. Leukemia. 1993;3:398–403. [PubMed] [Google Scholar]
- 47.Medeiros L, Picker L, Gelb A, Strickler J, Brain S, Weiss L, Horning S, Warnke R. Numbers of host “helper” T cells and proliferating cells predict survival in diffuse small-cell lymphoma. J Clin Oncol. 1989;7:1009–17. doi: 10.1200/JCO.1989.7.8.1009. [DOI] [PubMed] [Google Scholar]
- 48.Linderoth J, Ehinger M, Jerkeman M, et al. CD40 expression identifies a prognostically favourable subgroup of diffuse large B-cell lymphoma. Leuk Lymphoma. 2007;48:1774–9. doi: 10.1080/10428190701494520. [DOI] [PubMed] [Google Scholar]