Abstract
Cytokine messenger RNA (mRNA) expression was investigated in the spleen and lung digest cells of bacillus Calmette–Guérin (BCG)-vaccinated and non-vaccinated guinea pigs following low-dose, pulmonary exposure to virulent Mycobacterium tuberculosis. After purified protein derivative (PPD) stimulation, the levels of lung cell interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and spleen cell interleukin-12 (IL-12) p40 mRNAs were significantly increased in the non-vaccinated M. tuberculosis-infected guinea pigs compared to the BCG-vaccinated guinea pigs. In contrast, the expression of anti-inflammatory transforming growth factor-β and IL-10 mRNAs was significantly enhanced in the spleens of BCG-vaccinated animals. Despite the presence of protective cytokine mRNA expression, the non-vaccinated guinea pigs had significantly higher lung and spleen bacterial burdens. In contrast, BCG-vaccinated guinea pigs controlled the bacterial multiplication in their lungs and spleens, indicating that both protective as well as anti-inflammatory cytokine responses are associated with a reduction in bacteria. In addition, lung digest cells from non-vaccinated guinea pigs contained a significantly higher percentage of neutrophils, CD3+ and CD8+ T cells, while the percentage of macrophages was increased in the BCG-vaccinated animals. Total and purified lung digest T cells co-cultured with lung macrophages (LMøs) proliferated poorly after PPD stimulation in both non-vaccinated and BCG-vaccinated animals while robust proliferation to PPD was observed when T cells were co-cultured with peritoneal macrophages (PMøs). Macrophages within the lung compartment appear to regulate the response of T cells irrespective of the vaccination status in guinea pigs. Taken together, our results suggest that type I cytokine mRNA expression is not associated with vaccine-induced protection in the low-dose guinea pig model of tuberculosis.
Keywords: bacillus Calmette–Guérin, cytokine mRNA, guinea pig, lung digest cells, Mycobacterium tuberculosis, T-cell proliferation
Introduction
Tuberculosis remains a global health problem as one-third of the world’s population is infected with Mycobacterium tuberculosis,1∼ 10% of infected individuals develop active disease after M. tuberculosis infection, and two million patients die annually.2 The problem has been magnified by the association of tuberculosis with human immunodeficiency virus/acquired immune deficiency syndrome and by the emergence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) strains of M. tuberculosis.3,4Mycobacterium bovis bacillus Calmette–Guérin (BCG), one of the most widely used vaccines in the world, is the only vaccine available and is administered at birth in India and few other countries where tuberculosis is highly prevalent. Although BCG induces excellent protection in experimental models of tuberculosis5 and against severe, extrapulmonary tuberculosis in children,6 its efficacy against adult pulmonary tuberculosis is variable.7Mycobacterium tuberculosis is a successful respiratory pathogen8 because the bacilli can multiply within lung macrophages and eventually disseminate to extrapulmonary sites.9
Cellular immune responses play a pivotal role in controlling M. tuberculosis infection and involve several cell types including macrophages, dendritic cells and lymphocytes with the help of costimulatory molecules, cytokines and chemokines.10 Macrophages serve as the primary niche for M. tuberculosis as the bacilli can actively replicate within macrophages. Both CD4+ and CD8+ T cells contribute to protection against M. tuberculosis in addition to natural killer cells.11 Among the cytokines, both interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) have been identified as key players for anti-mycobacterial effector functions against mycobacteria, especially in the formation and maintenance of granulomas, which are a defensive reaction on the part of the host.12–14 Other cytokines, such as interleukin-12 (IL-12) and IL-23, also contribute to the host response to mycobacteria by enhancing the development of T helper type 1 immunity.15,16 However, recent reports from many laboratories indicated that IFN-γ levels may not predict vaccine efficacy.17,18 Similarly, studies in human newborns clearly demonstrated that measuring IFN-γ production alone may not be an optimal biomarker to assess the host cytokine response to BCG vaccination.19 On the other hand, the importance of IFN-γ has been well established in mice and humans as disruption of the mouse IFN-γ or the IFN-γ receptor gene resulted in an exacerbation of disease after M. tuberculosis or M.bovis infection.20,21 The role of TNF-α in anti-mycobacterial immunity has been reinforced by reports that the use of TNF-α neutralizing antibody in the treatment of chronic inflammatory diseases resulted in the reactivation of latent tuberculosis in humans.22
There is ample evidence to indicate that macrophages within the alveoli of the lung regulate pulmonary T-cell responses,23 as they suppressed the proliferation of T cells in vitro in humans and in rodents.24 For example, suppression of lung T-cell proliferation to purified protein derivative (PPD) occurred in co-cultures of lung macrophages from M. tuberculosis-infected mice.25 Previously, we have reported that alveolar macrophages (AMøs) as well as lung tissue macrophages (LMøs) obtained from BCG-vaccinated guinea pigs suppressed the proliferation of lung T cells to PPD while peritoneal macrophages (PMøs) induced robust proliferation.26 However, the effect of BCG vaccination on the responses of lung cells from M. tuberculosis-infected guinea pigs has not been studied. The purpose of the present study was to characterize the cytokine transcriptional changes in the spleen and lung digest cells following in vitro antigenic stimulation as well as the functional activity of lung digest cells in BCG-vaccinated and non-vaccinated guinea pigs challenged by the pulmonary route with virulent M. tuberculosis.
Materials and methods
Animals and BCG vaccination
This study was carried out using random-bred Hartley strain guinea pigs weighing 200–300 g obtained from Charles River Breeding Laboratories, Inc. (Wilmington, MA). Throughout the experiments, the animals were housed individually in polycarbonate cages in a temperature- and humidity-controlled environment; ambient lighting was automatically controlled to provide 12-h light 12-h dark cycles. Animals were given commercial chow (Ralston Purina, St Louis, MO) and tap water ad libitum. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee. Guinea pigs were vaccinated once intradermally with 1 × 103 colony-forming units (CFU) of M. bovis BCG (Danish 1331 strain, Statens Seruminstitut, Copenhagen, Denmark) in the left and right inguinal regions. For injection, the lyophilized vaccine was reconstituted with Sauton’s medium (Statens Seruminstitut).
Aerosol infection
Virulent M. tuberculosis H37Rv (ATCC 27294, American Type Culture Collection, Rockville, MD) grown in Dubos broth containing 0·05% Tween-80 for 8–10 days was harvested and a single cell suspension was prepared according to a published method.27 The CFU were determined by plating serial dilutions of the suspension on Middlebrook 7H10 agar (BVA Scientific Inc., San Antonio, TX) and counting the colonies 3 weeks later. Aliquots of bacteria were kept frozen at −80° until used for aerosol infection. Six weeks after BCG vaccination, vaccinated and non-vaccinated guinea pigs were infected by the pulmonary route in the ‘Madison’ aerosol chamber (University of Wisconsin Engineering Shops, Madison, WI), which is designed to deliver droplet nuclei directly to the alveolar spaces resulting in 10–20 primary, pulmonary lesions.28 After infection, the guinea pigs were housed individually in polycarbonate cages with microisolator bonnets in the BL3 containment facility at the Center for Comparative Medicine at Texas A&M University.
Preparation of lung digest cells and spleen cells
The lung digest cells were prepared using the protocols published earlier for mice and guinea pigs.26,29 The non-vaccinated and BCG-vaccinated guinea pigs were killed 5 weeks after virulent infection by the injection of 3 ml sodium pentobarbital (Sleepaway; Fort Dodge Laboratories Inc., Fort Dodge, IA). The finely cut lung tissue was incubated in RPMI-1640 medium (Irvine Scientific, Santa Ana, CA) supplemented with 2 μm glutamine (Irvine Scientific), 0·01 mm 2-mercaptoethanol (Sigma, St Louis, MO), 100 U/ml of penicillin, (Irvine Scientific), 100 μg/ml of streptomycin (Irvine Scientific), and 10% heat inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA) containing freshly made collagenase (150 U/ml, Sigma) and DNAse I (100 U/ml, Sigma) for 2 hr at 37°. Single cell suspensions were obtained by vigorous pipetting and passing the homogenate through a 100-μm and 40-μm nylon Falcon cell strainer (BD Biosciences, Bedford, MA). The cells were centrifuged at 440 g for 10 min, the pellet was resuspended in ACK lysis buffer [0·14 m NH4Cl, 1·0 mm KHCO3, 0·1 mm Na2EDTA (pH 7·2–7·4)], washed three times in RPMI-1640 medium by centrifuging for 10 min at 320 g, and the viable cells were counted by the trypan blue exclusion method. The viability of lung digest cells was more than 95% as determined by the trypan blue staining method. Spleen cells were prepared by homogenizing the tissue in a glass homogenizer, lysing the red blood cells using the ACK lysis buffer, washing in RPMI-1640 medium and counting the cells by the trypan blue staining method.30
Total RNA isolation and real-time polymerase chain reaction
Total lung digest cells and spleen cells were stimulated with Concanavalin A (Con A; 10 μg/ml), lipopolysaccharide (LPS; 1 μg/ml) or PPD (25 μg/ml) for 24 hr. At the end of the incubation period, the supernatant was removed by centrifugation, the cells were lysed with RLT buffer (Qiagen, Valencia, CA), and the lysates were kept frozen at −80° until RNA extraction. Total RNA was isolated using the RNeasy columns (Qiagen) and contaminating DNA was removed by RNAse-free DNAse treatment (Qiagen). Reverse transcription was performed with TaqMan Reverse Transcription reagents and the real-time polymerase chain reaction (PCR) using SYBR Green I double-stranded DNA binding dye (Applied Biosystems, Foster City, CA) and the ABI Prism 7700 sequence detector according to the protocols published from our laboratory.31 Real-time primers for guinea pig IFN-γ, TNF-α, transforming growth factor-β (TGF-β), IL-10, IL-12p40, inducible nitric oxide synthase (iNOS) and hypoxanthine phosphoribosyltransferase (HPRT) were designed with primerexpress software (Applied Biosystems) based upon sequences that we had published previously.31 Fold induction of mRNA was calculated from the threshold cycle values (Ct) normalized to HPRT Ct values and then to unstimulated cultures.
Flow cytometry
Lung digest cells were stained with monoclonal antibodies (mAbs) against guinea pig major histocompatibility complex (MHC) Class II, pan-T (CT5), CD4 (CT7), and CD8 T-cell (CT6) phenotypic markers (Biosource International, Camarillo, CA) using our previously published procedures.26,30 For each mAb or control, 5 × 105 to 10 × 105 cells were incubated with 10 μl of 1 mg/ml normal goat immunoglobulin G (IgG; Sigma) for 10 min to block FcR binding. This was followed by the addition of 50 μl of mouse anti-guinea pig MHC class II antibody (1 : 10), anti-guinea pig T-cell antibody (1 : 500), anti-CD4 (1 : 500), or anti-CD8 antibody (1 : 1000) and staining with the fluorescein isothiocyanate-conjugated AffiniPure goat anti-mouse IgG {H+L} (Jackson ImmunoResearch Laboratories, Inc. West Grove, CA). The proportions of positive cells were determined with a FACSCalibur flow cytometer and Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cytospin preparations of the lung digests were also performed and the cells were stained by the Diff-Quik staining method (American Scientific Products, McGraw Park, IL).
Harvesting of resident PMøs and LMøs
The resident PMøs were harvested using methods published earlier.26 Following death, the cells from the peritoneal cavity were harvested by flushing the cavity three times with 20 ml cold RPMI-1640 (Irvine Scientific). The ACK lysing buffer was used to deplete the erythrocytes. The cells were washed in RPMI-1640 medium and the viable cells were counted by the trypan blue exclusion method. The cells were suspended at 5 × 106 cells/ml in RPMI-1640 medium supplemented with glutamine, 2-mercaptoethanol, penicillin/streptomycin and 10% heat inactivated fetal bovine serum (Atlanta Biologicals). Peritoneal and lung digest cells (2 × 106/ml) were incubated in 96-well microtitre plates (Becton Dickinson Labware, Franklin Lakes, NJ) for 2–3 hr, and non-adherent cells were removed. The adherent cells comprised predominantly macrophages (> 95%) as determined by non-specific esterase staining as reported previously.26
Purification of T cells
The non-adherent cells were enriched for T lymphocytes on nylon wool columns (Polysciences, Inc. Warrington, PA) using our standard protocol.26,30 The column was loaded with 1 × 108 to 2 × 108 viable cells in a volume of 2 ml; the non-adherent cells were collected after incubation for 1 hr at 37° and the cells were counted by the trypan blue exclusion method. The purity of the cells ranged from 88 to 97% as determined by flow cytometry (unpublished data).
Proliferation of total lung digest cells and co-cultures of lung T cells and macrophages
The proliferation of lymphocytes to Con A and PPD was assessed in total lung digest cells, and in co-cultures of nylon-wool-purified lung T cells with LMøs or PMøs. The total lung digest cells (4 × 105/well) were plated onto 96-well plates with or without Con A (10 μg/ml, Sigma) or PPD (25 μg/ml; Statens Seruminstitut) and cultured for 4 days using our standard protocol.26 For the final 6 hr of culture, [3H]thymidine was added at a concentration of 1 μCi/well.26 The cells were harvested using a FilterMate harvester, and the [3H]thymidine uptake was measured in a scintillation counter (Beckman LS-1801). The stimulation index (SI) was calculated by dividing the counts per minute (c.p.m.) of stimulated cells by the c.p.m. of unstimulated cells.
For the co-culture experiments, nylon-wool-purified T cells were mixed at a ratio of 3 : 1 with PMøs or LMøs.26 The co-cultures of T cells (3 × 105) and macrophages (1 × 105) were plated onto 96-well plates and stimulated with either Con A or PPD for 96 hr as described above. At the initiation of the culture period, some cultures were treated with 200 ng/ml recombinant guinea pig (rgp) IFN-γ, a dose which has been shown to upregulate MHC class II expression, enhance H2O2 production, and reduce the intracellular growth of M. tuberculosis in guinea pig PMøs.32
Statistics
The data are expressed as means ± standard errors. The differences between non-vaccinated and BCG-vaccinated groups were analysed by Students t-test and values of P< 0·05 were considered statistically significant. The effects of treatments were assessed by analysis of variance (anova) and the differences between means were analysed by the Tukey post hoc test. There were five to ten guinea pigs per group and each experiment was performed at least three times.
Results
Effect of vaccination on lung and spleen bacillary loads
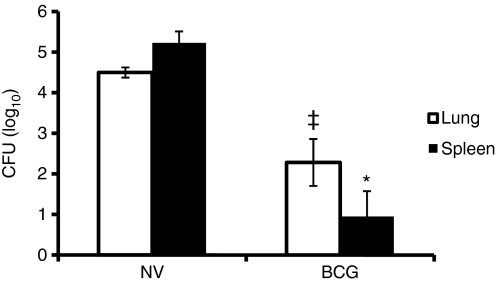
After aerosol infection, M. tuberculosis multiplied in the lung and disseminated to the spleen as evident from the high CFU in both these organs (Fig. 1). However, BCG vaccination caused a significant 100-fold reduction in the CFUs in lungs and a 10 000-fold reduction in spleens and, consequently, protected against infectious challenge with M. tuberculosis in guinea pigs.
Figure 1.
Effect of bacillus Calmette–Guérin (BCG) vaccination on lung and spleen colony-forming units (CFU). At 5 weeks after Mycobacterium tuberculosis infection, the spleen and the right lower lung lobe from non-vaccinated (NV) and BCG-vaccinated (BCG) guinea pigs were homogenized and appropriate dilutions were plated in duplicate onto Middlebrook 7H10 agar plates and incubated at 37° for 3 weeks. The number of CFU was counted and the results were calculated as mean log10 CFU per tissue. *P< 0·0007 and ‡P< 0·01 when compared with non-vaccinated group as determined by Student’s t-test.
Cytokine mRNA expression
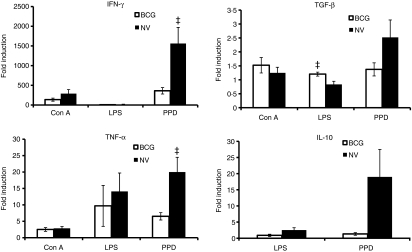
The unseparated lung or spleen cells were stimulated in vitro with Con A, LPS or PPD for 24 hr. The RNA was harvested and the mRNA expression for IFN-γ, TNF-α, TGF-β, IL-10, IL-12p40, iNOS and HPRT expression was examined by real-time reverse transcription-PCR. Figure 2 illustrates the mRNA levels for IFN-γ, TNF-α, TGF-β, and IL-10. Concanavalin-A-stimulated lung cells from both non-vaccinated and BCG-vaccinated animals did not express any IL-10 mRNA; however, stimulation with LPS caused the induction of IL-10 but not IFN-γ mRNA expression in lung cells (Fig. 2). In addition, LPS stimulation significantly increased the cytokine mRNA for TGF-β in the lung cells of BCG-vaccinated guinea pigs compared to the non-vaccinated guinea pigs. Incubation of lung digest cells with PPD induced a general increase in the mRNA expression for all the cytokines in both non-vaccinated and BCG-vaccinated animals. However, levels of IFN-γ as well as TNF-α mRNA expression were significantly increased in the lung digest cells of non-vaccinated, M. tuberculosis-infected guinea pigs compared to the BCG-vaccinated group. Neither IL-12p40 nor iNOS mRNA expression was detectable in the lung digest cells from either vaccinated or non-vaccinated guinea pigs under these stimulating conditions (data not shown).
Figure 2.
Cytokine messenger RNA (mRNA) expression in guinea pig lung digest cells. Total lung digest cells from non-vaccinated (NV) and bacillus Calmette–Guérin-vaccinated (BCG) guinea pigs 5 weeks following Mycobacterium tuberculosis infection were stimulated with Concanavalin A (Con A; 10 μg/ml), lipopolysaccharide (LPS; 1 μg/ml) or purified protein derivative (PPD; 25 μg/ml) for 24 hr. Interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) mRNA, transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) mRNA expression was quantified using real-time reverse transcription–polymerase chain reaction. Fold induction of mRNA was calculated from the threshold cycle values (Ct) normalized to HPRT Ct values and then to unstimulated cultures. The results are expressed as mean and the standard errors of means from five animals. The differences in the fold induction between cultures from non-vaccinated and BCG-vaccinated animals were determined by anova (‡P< 0·05).
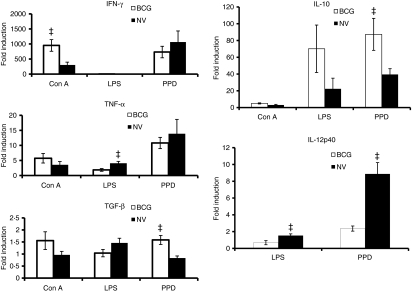
To assess the cytokine mRNA expression in an extrapulmonary site of M. tuberculosis infection, spleen cells from the same animals were examined after stimulation with Con A, LPS or PPD for 24 hr. As illustrated in Fig. 3, after Con A stimulation, the fold induction of IFN-γ mRNA expression was much higher in spleen cells than lung cells and the level was significantly increased in the BCG-vaccinated animals compared to the non-vaccinated group. The expression of TNF-α, TGF-β and IL-10 mRNA was comparable in the vaccinated and non-vaccinated groups after Con A stimulation. Spleen cells did not exhibit increases of IFN-γ cytokine mRNA in response to LPS stimulation, however, a significant increase in the TNF-α and IL-10 mRNA expression was observed in the LPS-treated spleen cells of non-vaccinated guinea pigs (Fig. 3). LPS also induced a significantly higher IL-12p40 mRNA level in spleen cells from non-vaccinated guinea pigs. After the stimulation of spleen cells with PPD, the mRNA expression for IFN-γ and TNF-α was similar in both vaccinated and non-vaccinated guinea pigs. However, the level of TGF-β (P< 0·01) as well as IL-10 mRNA (P< 0·05) was significantly increased in the spleen cells obtained from BCG-vaccinated guinea pigs compared to the non-vaccinated animals (Fig. 3). In fact, the level of IL-10 mRNA in the spleen cells was four times higher than that of lung cells after PPD stimulation. Surprisingly, mRNA expression for IL-12p40 was seen only in the spleen cells stimulated with LPS and PPD and the level was significantly increased in the non-vaccinated guinea pigs challenged with M. tuberculosis compared to the BCG-vaccinated animals (Fig. 3).
Figure 3.
Cytokine messenger RNA (mRNA) expression in guinea pig spleen cells. Total spleen cells from non-vaccinated (NV) and bacillus Calmette–Guérin-vaccinated (BCG) guinea pigs 5 weeks following Mycobacterium tuberculosis infection were stimulated with concanavalin A (Con A), lipopolysaccharide (LPS) or purified protein derivative (PPD) for 24 hr as described above. Interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), interleukin-10 (IL-10) and IL-12p40 mRNA expression was quantified using real-time reverse transcription–polymerase chain reaction. Fold induction of mRNA was calculated as described in Fig. 2. The results are expressed as mean and the standard errors of means from five animals. The differences in the fold induction between the cultures from non-vaccinated and BCG-vaccinated groups were determined by anova (‡P< −0·05).
Phenotypic analysis of lung digest cells
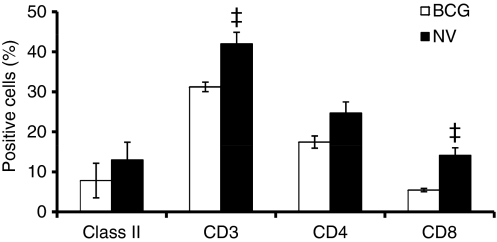
The phenotypic analysis of lung digest cells by flow cytometry is shown in Fig. 4. The proportions of MHC class II+ cells in the lung digests of non-vaccinated and BCG-vaccinated animals were similar after M. tuberculosis infection. In contrast, the proportions of CD3+ T cells were significantly (P< 0·01) increased in the non-vaccinated animals compared to the BCG-vaccinated group; there was a concomitant increase in the proportions of CD8+ T cells (P< 0·01) in the non-vaccinated group but no significant changes in the proportions of CD4+ T cells were apparent (Fig. 4).
Figure 4.
Phenotypic analysis of guinea pig lung digest cells. Lung cells obtained by enzymatic digestion from non-vaccinated (NV) and bacillus Calmette–Guérin-vaccinated (BCG) guinea pigs 5 weeks after infection with virulent Mycobacterium tuberculosis was characterized by flow cytometry. Proportions of major histocompatibility complex (MHC) class II+ cells, T cells and their subsets were determined by fluorescence-activated cell sorting analysis after staining the cells with monoclonal antibodies directed against the surface markers of MHC class II+ cells, T cells (CT5), CD4+ T cells (CT7), and CD8+ T cells (CT6). Results are expressed as % positive cells. ‡P< 0·01 compared with BCG-vaccinated group as determined by Student’s t-test.
The cytospin preparations of lung digests were stained using the Diff-Quik method and the differential counts revealed significant differences in the number of macrophages and neutrophils between the non-vaccinated and BCG-vaccinated groups (Table 1). The proportions of macrophages were significantly increased in the BCG-vaccinated guinea pigs (P< 0·01). In contrast, in the non-vaccinated guinea pigs, a significant increase in the percentage of neutrophils/eosinophils (P< 0·01) compared to that in the BCG-vaccinated animals was observed. Eosinophils and neutrophils were enumerated together because they are not clearly distinguishable from each other in the guinea pig.26 Similarly, Kurloff cells, mononuclear cells with a large cytoplasmic inclusion body that are uniquely present in guinea pig tissues, were numerous in the lung digests. No significant differences in the percentage of Kurloff cells and lymphocytes were apparent between the non-vaccinated and BCG-vaccinated guinea pigs.
Table 1.
Differential counts of guinea pig lung digest cells (mean % ± SEM)
| Groups | Lymphocytes | Macrophages | Neutrophils/ eosinophils | Kurloff cells |
|---|---|---|---|---|
| Non-vaccinated | 21·6 ± 2·14 | 26·5 ± 1·7 | 35·2 ± 3·3* | 16·9 ± 0·62 |
| BCG-vaccinated | 24·6 ± 0·96 | 37·4 ± 5·7* | 20·6 ± 1·4 | 20·2 ± 3·8 |
*P< 0·001 when compared between non-vaccinated and BCG-vaccinated group as determined by the Student’s t-test.
BCG, bacillus Calmette–Guérin.
Lymphoproliferation of lung digest cells
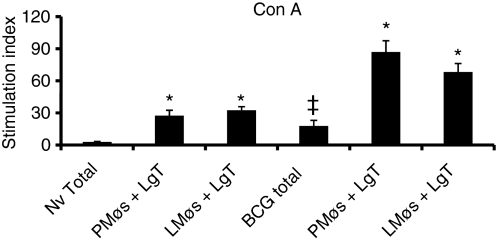
The proliferative ability of total lung digest cells or nylon-wool-purified T cells co-cultured with PMøs or LMøs was examined. The cultures were stimulated for 4 days in the presence of Con A or PPD and the proliferation was assessed from the tritiated thymidine uptake. Total lung digest cells proliferated poorly to Con A; however, the response was significantly higher (P< 0·05) in the BCG-vaccinated animals (Fig. 5). Proliferation of nylon-wool-purified T cells to Con A was significantly enhanced (P< 0·001) in the presence of PMøs or LMøs (Fig. 5) in both non-vaccinated and BCG-vaccinated groups when compared with the response in the total lung digest cells. However, the magnitude of the proliferative response was three to four times higher in the BCG-vaccinated, M. tuberculosis-infected guinea pigs compared to the non-vaccinated group (P< 0·05).
Figure 5.
Proliferation of total lung digest cells and purified T cells to concanavalin A (Con A). Proliferation of total (2 × 106/ml) lung digest cells (Total) and co-cultures of lung T (LgT) cells (3 × 105/well) and peritoneal (PMøs) or lung (LMøs) macrophages (1 × 105/well) from non-vaccinated (NV) and bacillus Calmette–Guérin-vaccinated (BCG) guinea pigs following Mycobacterium tuberculosis infection was assessed. Cells cultured for 4 days in the presence of Con A (10 μg/ml) were harvested after the addition of [3H]thymidine (1 μg/well) 6 hr earlier. The results are expressed as stimulation index calculated by dividing the counts per minute (c.p.m.) of stimulated cells by the c.p.m. of unstimulated cells. The results are the mean and the standard error of the mean from three experiments. There were five animals per group. *P< 0·001 in comparisons between total and co-cultures from non-vaccinated and BCG-vaccinated groups by anova. ‡P< 0·05 when compared to the response in total cells from non-vaccinated guinea pigs.
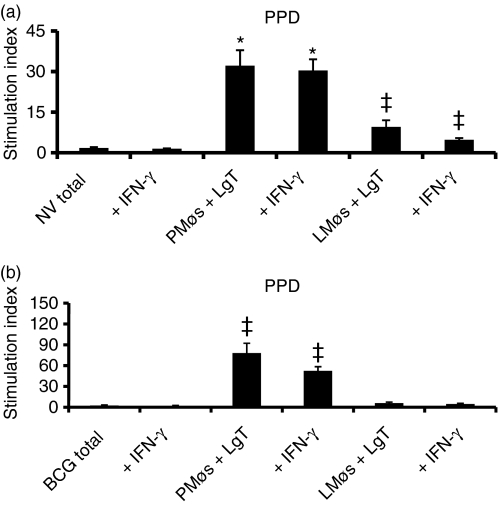
The ability of lung digest cells to proliferate in the presence of PPD was evaluated using both total lung digest cells and also co-cultures of nylon-wool-purified T cells and PMøs or LMøs. Total lung cells from non-vaccinated guinea pigs failed to proliferate in response to PPD (Fig. 6a). In contrast, T cells co-cultured in the presence of either LMøs or PMøs showed a significantly enhanced response to PPD although the degree of proliferation was much higher in co-cultures of T cells and PMøs (Fig. 6a). In the BCG-vaccinated guinea pigs, total lung cells or T cells co-cultured with LMøs did not proliferate to PPD at all (Fig. 6b). In contrast, proliferation of T cells to PPD was significantly enhanced by the addition of PMøs to the co-cultures. Again, BCG vaccination induced a higher magnitude of proliferative response to PPD in the co-cultures than was found for the non-vaccinated guinea pigs (P< 0·05). As is clear from Fig. 6(a, b), addition of 200 ng/ml rgpIFN-γ to cell cultures did not enhance the proliferative ability of T cells from either non-vaccinated or BCG-vaccinated guinea pigs.
Figure 6.
Proliferation of total lung digest cells and purified T cells to purified protein derivative (PPD). Total lung cells (Total) or co-cultures of purified T (LgT) cells and peritoneal (PMøs) or lung (LMøs) macrophages from non-vaccinated (NV, a) and BCG-vaccinated (BCG, b) guinea pigs 5 weeks following Mycobacterium tuberculosis infection were stimulated with PPD (25 μg/ml) for 4 days and the [3H]thymidine uptake was assessed. The cell populations were also treated with recombinant guinea pig interferon-γ (IFN-γ; 200 ng/ml) at the beginning of the culture period. The results represent mean ± SEM from 5–10 animals. Results are expressed as stimulation index. *P< 0·001 and ‡P< 0·05 when compared with total cells as determined by anova.
Discussion
There is very little published information on the cytokine or cellular events that occur within the lungs of vaccinated and non-vaccinated guinea pigs after M. tuberculosis infection.26,33 The cytokine responses in the spleen and lung digest cells from guinea pigs infected with M. tuberculosis were studied after stimulation with Con A, LPS or PPD. The non-vaccinated guinea pigs showed a predominant cytokine expression of IFN-γ, TNF-α and IL-12p40 mRNAs in their lungs and spleen, while IL-10 and TGF-β mRNA expression were dominant in the BCG-vaccinated guinea pigs after PPD stimulation (Figs 2 and 3). However, the lung and spleen CFUs were significantly higher in the non-vaccinated guinea pigs than in the vaccinated group despite the ability of these cells to upregulate the cytokine mRNAs crucial for anti-mycobacterial immunity. Previous reports from our laboratory had shown that PMøs, AMøs or spleen cells stimulated with PPD produced a significantly higher level of TNF-α protein if they were from the non-vaccinated, M. tuberculosis infected guinea pigs than if they were from the BCG-vaccinated guinea pigs.34 Taken together, these results indicate that despite the transcription of type I cytokines in the tissues of non-vaccinated guinea pigs, a reduction in the bacterial load was not evident. In contrast, a combination of both Type I as well as anti-inflammatory cytokine mRNA expression (IL-10, TGF-β) was apparent in the BCG-vaccinated guinea pigs and this was associated with a decrease in bacterial burden in both tissues. It is known that CD4+ CD25+ regulatory T cells that produce TGF-β and IL-10 are present in the lungs of mice infected with M. tuberculosis.35 In a recent study of cytokine mRNA levels in granulomas by laser capture microdissection, we demonstrated a similar predominance of TGF-β mRNA in the primary granulomas of BCG-vaccinated guinea pigs and the secondary granulomas of non-vaccinated animals at 6 weeks post-challenge.33,36 Both IFN-γ and TNF-α play pivotal roles in anti-mycobacterial immunity.12,13 Although IFN-γ was anticipated to serve as a correlate of BCG vaccine-induced protection against M. tuberculosis in our model, others have now reported that it does not correlate with vaccine efficacy in experimental animals17,18 or humans.19 Most of the IFN-γ mRNA is expected to result from activated T cells, however, it is clear that under certain culture conditions, activation of macrophages may occur in an autocrine fashion by responding to, as well as producing, IFN-γ.26,37,38
After M. tuberculosis infection, a significantly larger proportion of neutrophils appeared in the lungs of non-vaccinated guinea pigs, while BCG vaccination induced a significant influx of macrophages (Table 1). Mycobacterium tuberculosis infection is known to result in the recruitment of neutrophils and eosinophils to the granulomas of humans, mice and guinea pigs.39,40 Phenotypic analysis of lung digest cells by flow cytometry indicated that the proportions of CD3+ and CD8+ T cells were significantly increased in the non-vaccinated guinea pigs compared to the BCG-vaccinated animals after M. tuberculosis infection (Fig. 4). Previously, we have demonstrated that BCG vaccination alone caused a significant increase in CD3+ T cells in the lung digests in comparison with the naïve animals.26 While MHC class I-restricted CD8+ T cells have been shown to be protective against M. tuberculosis infection in mice,41,42 increased numbers of CD8+ T cells in the non-vaccinated guinea pigs was not associated with a reduction in the lung CFU (Fig. 1).
Total lung cells from non-vaccinated and BCG-vaccinated guinea pigs proliferated poorly to Con A or PPD (Figs 5 and 6). Proliferation of nylon-wool-purified lung T cells with LMøs resulted in a significant suppression of proliferation of lung T cells to PPD but not Con A, compared to co-cultures with PMøs (Figs 5 and 6). Our earlier studies also demonstrated that LMøs and AMøs suppressed lung T-cell proliferation in BCG-vaccinated, uninfected guinea pigs.26 Addition of gpIFN-γ to either total lung cells or to co-cultures of T cells and macrophages did not enhance proliferation. The suppression of T-cell proliferation by AMøs is thought to represent a protective immunoregulatory mechanism which maintains the balance between appropriately effective responses against infectious organisms and excessive responses to non-pathogenic stimuli.43 Although LMøs were lymphocytostatic, they restricted the intracellular replication of virulent M. tuberculosis when activated with rgpIFN-γ26 in our earlier studies suggesting that the suppression of T-cell proliferation by LMøs might also be an important immunoregulatory mechanism in controlling pulmonary inflammation and preserving lung function.
Taken together, these results indicate that BCG vaccination is associated with a greater influx of macrophages into the lungs, enhanced PPD-induced proliferation of T cells, an upregulation of anti-inflammatory cytokine (TGF-β and IL-10) mRNAs in the spleen, and a robust IFN-γ and TNF-α mRNA expression after PPD stimulation. In contrast, the significant upregulation of type 1 cytokines (IFN-γ, TNF-α and IL-12p40) in the guinea pigs was not associated with protection. These experimental approaches will significantly enhance our understanding of the mechanisms of vaccine-induced resistance, as well as the cellular and molecular events that occur at the site of M. tuberculosis infection. Future experiments will delineate the roles of these cytokines in host resistance, and establish biomarkers essential for assessing vaccine-induced protection.
Acknowledgments
This work was supported by USPHS, NIH grant RO1 AI 15495 to D.N.M. We are grateful to Jane Miller for her assistance with the FACS analysis.
References
- 1.Kaufmann S. Towards new leprosy and tuberculosis vaccines. Microbiol Sci. 1987;4:324–8. [PubMed] [Google Scholar]
- 2.Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 3.Young LS, Inderlied CB, Berlin OG, Gottlieb MS. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986;8:1024–33. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 5.Smith D, Harding G, Chan J, Edwards M, Hank J, Muller D, Sobhi F. Potency of 10 BCG vaccines as evaluated by their influence on the bacillemic phase of experimental airborne tuberculosis in guinea pigs. J Biol Stand. 1979;7:179–97. doi: 10.1016/s0092-1157(79)80021-9. [DOI] [PubMed] [Google Scholar]
- 6.Udani PM. BCG vaccination in India and tuberculosis in children: newer facets. Indian J Pediatr. 1994;61:451–62. doi: 10.1007/BF02751703. [DOI] [PubMed] [Google Scholar]
- 7.Roche PW, Triccas JA, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 8.Canetti G. The Tubercle Bacillus in the Pulmonary Lesion in Man. New York: Springer Publishing Co.; 1955. [Google Scholar]
- 9.Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1:97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990;145:149–54. [PubMed] [Google Scholar]
- 11.Bhatt K, Salgame P. Host immune response to Mycobacterium tuberculosis. J Clin Immunol. 2007;27:347–62. doi: 10.1007/s10875-007-9084-0. [DOI] [PubMed] [Google Scholar]
- 12.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AM, D’Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–20. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–66. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 16.Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majlessi L, Simsova M, Jarvis Z, et al. An increase in antimycobacterial Th1 cell response in prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74:2128–37. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittrucker H-W, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D. Poor correlation between BCG vaccine-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA. 2007;104:12434–9. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soares A, Scriba TJ, Joseph S, et al. Bacillus Calmette–Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180:3569–77. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–9. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemijo R, Le J, Shapiro D, Havell EA, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with bacillus Calmette–Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–40. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane J, Gershon S, Wise RP, Levens EM, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 23.Holt PG. Regulation of antigen presenting cell(s) in lung and airway tissues. Eur Respir J. 1993;6:120–9. [PubMed] [Google Scholar]
- 24.McCombs CC, Michalski JP, Westerfield BT, Light RW. Human alveolar macrophages suppress the proliferative response of peripheral blood lymphocytes. Chest. 1982;82:266–71. doi: 10.1378/chest.82.3.266. [DOI] [PubMed] [Google Scholar]
- 25.Apt AS, Kramnik IB, Moroz AM. Regulation of T-cell proliferative responses by cells from solid lung tissue of M. tuberculosis-infected mice. Immunology. 1991;73:173–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Jeevan A, Sawant K, Cho H, McMurray DN. Lung macrophages from bacille Calmette–Guérin-vaccinated guinea pigs suppress T cell proliferation but restrict intracellular growth of M. tuberculosis after recombinant guinea pig interferon-γ activation. Clin Exp Immunol. 2007;149:387–98. doi: 10.1111/j.1365-2249.2007.03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grover AA, Kim HK, Wiegeshaus EH, Smith DW. Host–parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J Bacteriol. 1967;94:832–40. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7:135–7. doi: 10.1016/s1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 29.Lyadova IV, Eruslanov EB, Khaidukov SV, et al. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. J Immunol. 2000;165:5921–31. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]
- 30.Jeevan A, Yoshimura T, Lee KE, McMurray DN. Differential expression of gamma interferon mRNA induced by attenuated and virulent Mycobacterium tuberculosis in guinea pig cells after Mycobacterium bovis BCG vaccination. Infect Immun. 2003;71:354–64. doi: 10.1128/IAI.71.1.354-364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen SS, McMurray DN. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun. 2003;71:4271–7. doi: 10.1128/IAI.71.8.4271-4277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeevan A, McFarland CT, Yoshimura T, Skwor T, Cho H, Lasco T, McMurray DN. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect Immun. 2006;74:213–24. doi: 10.1128/IAI.74.1.213-224.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ly LH, Russell MI, McMurray DN. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell Microbiol. 2007;9:1127–36. doi: 10.1111/j.1462-5822.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Lasco TL, Uchida K, et al. Mycobacterium bovis BCG vaccination modulates TNF-α production after pulmonary challenge with virulent Mycobacterium tuberculosis in guinea pigs. Tuberculosis. 2007;87:155–65. doi: 10.1016/j.tube.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Mason CM, Porretta E, Zhang P, Nelson S. CD4+ CD25+ transforming growth factor-β-producing T cells are present in the lung in murine tuberculosis and may regulate the host inflammatory response. Clin Exp Immunol. 2007;148:537–45. doi: 10.1111/j.1365-2249.2007.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ly LH, Russell MI, McMurray DN. Cytokine profiles in primary and secondary pulmonary granulomas of guinea pigs with tuberculosis. Am J Respir Cell Mol Biol. 2008;38:455–62. doi: 10.1165/rcmb.2007-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenton MJ, Vermeulen MW, Kim S, Burdick M, Strieter RM, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–56. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Invest. 1999;103:1023–9. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appleberg R, Silva MT. T-cell dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989;78:478–48. [PMC free article] [PubMed] [Google Scholar]
- 40.Lasco TM, Turner OC, Cassone L, Sugawara I, Yamada H, McMurray DN, Orme IM. Rapid accumulation of eosinophils in lung lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect Immun. 2004;72:1147–9. doi: 10.1128/IAI.72.2.1147-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flynn JL, Goldstein MM, Triebold KJ, Dalton DK, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med. 2004;200:1479–89. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upham JW, Strickland DH, Bilyk N, Robinson BW, Holt PG. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 1995;84:142–7. [PMC free article] [PubMed] [Google Scholar]