Abstract
Human T-lymphotropic virus 1 (HTLV-1) can cause HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The objective of this study was to gain insight into the pathogenesis of HAM/TSP by focusing on the CD8+ T-cell response. Twenty-three HTLV-1-seronegative controls (SC), 29 asymptomatic HTLV-1 carriers (AC) and 48 patients with HAM/TSP were enrolled in the study. We evaluated the production of interferon-γ (IFN-γ) in peripheral blood mononuclear cells stimulated with Tax overlapping peptides, the expression of genes related to the CD8+ cytotoxic T-cell response, the frequency of CD4+ Foxp3+ cells and of dendritic cells, and the HTLV-1 provirus load (PVL). The frequency of cells producing IFN-γ in response to Tax 161–233, but not to Tax 11–19, discriminated patients with HAM/TSP from AC. The increased pro-inflammatory response observed in patients with HAM/TSP was shared by AC with a high PVL, who also exhibited lower levels of granzyme H mRNA in unstimulated CD8+ T cells than AC with a low PVL. Patients with HAM/TSP showed higher frequencies of CD4+ Foxp3+ cells and lower frequencies of plasmacytoid dendritic cells (pDC) than AC. Our findings are consistent with a model in which HTLV-1, along with the host genetic background, drives quantitative and qualitative changes in pDC and CD4+ Foxp3+ cells that lead to a predominance of inflammatory responses over lytic responses in the CD8+ T-cell response of individuals predisposed to develop HAM/TSP.
Keywords: CD8+ T-cell response; human T-lymphotropic virus 1; paraparesis, tropical spastic; provirus load; T lymphocytes, regulatory
Introduction
The human T-lymphotropic virus 1 (HTLV-1) is a retrovirus discovered in the late 1970s, but that has had a long relationship with humankind.1 As a result of thousands of years of co-evolution, subtle mechanisms have developed that allow virus perpetuation, usually with no serious harm to the host. Nonetheless, these mechanisms are subverted in about 5–10% of HTLV-1-infected people, who over the years develop associated diseases such as adult T-cell lymphoma/leukaemia (ATLL) or HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). HAM/TSP is a severe disease characterized by chronic inflammation of the spinal cord, especially in the lower thoracic portion, which causes spastic paraparesis of the lower limbs.2
Previous studies have shown that patients with HAM/TSP have significantly higher provirus loads (PVLs) than asymptomatic HTLV-1 carriers (AC)3,4 and that certain HLA class I alleles are associated with lower PVLs and a diminished risk of the disease, at least in particular populations.5 In light of these findings, it was postulated that an impaired specific cytotoxic response by activated CD8+ T cells [cytolytic T lymphocytes (CTL)] of HAM/TSP patients would result in a greater increase of the PVL in response to a fixed increase in the expression of Tax.6 Goon et al.7 have shown that the viral protein Tax is the immunodominant antigen in the HTLV-1-specific CTL response, but that the frequency of Tax-specific CTL was not associated with the disease status of infected subjects. Thus, according to these authors, alterations in CTL function, rather than in CTL frequency, could lead to a deficient HTLV-1-specific CTL response in subjects who develop HAM/TSP.
The antigen-driven CTL response has two facets: the production and release of lytic factors such as perforin, granzymes, granulysin and natural killer group 2D (NKG2D), aimed at destroying the target cell; and the production of pro-inflammatory cytokines, mainly interferon-γ (IFN-γ), to inhibit viral replication, promote macrophage activation and induce expression of HLA class I molecules.8 It has been shown that HTLV-1-specific CTL from patients with HAM/TSP express fewer co-stimulatory molecules, which are critical for efficient lytic functions, and that a subpopulation of these CTL releases fewer lytic factors in response to Tax when compared with AC.9 The fact that this diminished cytotoxic response was independent of the PVL suggests that it may constitute an inherent trait of those individuals predisposed to develop HAM/TSP. It also implies that CTL efficiency per se is insufficient to determine the PVL and that other factors, such as Tax expression,10 the route of transmission and/or as yet unknown environmental factors (e.g. intercurrent disease), are likely to be involved.
IFN-γ production by HTLV-1-specific CTL has been detected in patients with HAM/TSP using both flow cytometry11 and enzyme-linked immunospot (ELISPOT) assays.7 Noteworthy, not much attention has been paid to this pro-inflammatory facet of the HTLV-1-specific CTL response in spite of well-documented evidence for exacerbated in vivo and in vitro production and release of pro-inflammatory cytokines by peripheral blood mononuclear cells (PBMC) from patients with HAM/TSP.12,13 In fact, a study reported higher frequencies of CD8+ IFN-γ+ T cells in PBMC from HAM/TSP patients compared with AC.11 These differences might be a consequence of alterations in antigen presentation by antigen-presenting cells (APC) and/or changes in regulation by regulatory T cells. In that regard, the putative role of dendritic cells (DC) as a vehicle for virus transmission,14 and the negative correlation between the frequency of cells with a T-regulatory-like phenotype (CD4+ Foxp3+) and CTL activity found in HTLV-1-infected subjects,15 open new avenues for further investigations on this matter.
In this study, we evaluated if patients with HAM/TSP can be distinguished from AC by their HTLV-1-specific pro-inflammatory CTL response. For that purpose, we envisaged a short-incubation ex vivo IFN-γ ELISPOT assay that measures the specific response to an array of peptide pools that cover the entire sequence of Tax. In contrast to evaluations based on the use of tetramers, this approach provides a broader picture of the Tax-specific CTL response, not limited by a particular HLA allele and a very limited set of known epitopes. The mRNA levels of lytic factors in ex vivo unstimulated CD8+ T cells, as well as the in vivo frequency of CD4+ Foxp3+ cells and of myeloid DC (mDC) and plasmacytoid DC (pDC), were also quantified.
Materials and methods
Subjects and cells
Twenty-nine AC and 48 patients with HAM/TSP from the clinical cohort at the Institute of Tropical Medicine Alexander von Humboldt in Lima, Peru, participated in the study, along with 23 HTLV-1-seronegative controls (SC). The study protocol was approved by the Institutional Ethics Committee of the Universidad Peruana Cayetano Heredia, and written informed consent was obtained from all participants. HTLV-1 infection was determined by at least one positive enzyme-linked immunosorbent assay (ELISA) result and at least one positive confirmatory result: either Western blot, Line Immuno-Assay (Innogenetics, Ghent, Belgium) or a detectable HTLV-1 PVL, as measured using our in-house assay.4 The disease status of HTLV-1-infected subjects was determined by trained physicians, and the diagnosis of HAM/TSP was made according to internationally accepted criteria.16,17 The ethnic background was defined as Andean if both parents, or four grandparents, were born in the Andean region.
PBMC were obtained as described previously.13 Cells were suspended in RPMI-1640 (Gibco, Paisley, UK), supplemented with 5% pooled human serum obtained from healthy donors, 100 IU/ml of penicillin and 100 μg/ml of streptomycin (Gibco), which is subsequently referred to as complete medium. For CTL gene-expression measurements, CD8+ T cells were positively selected using magnetic microbeads (Dynal, Invitrogen, Oslo, Norway).
Synthetic peptides
Eighty-six ‘overlapping’ 13-mer peptides, offset by four residues and covering the entire sequence of the Tax protein (strain ATK),18 were synthesized by Mimotopes (Clayton, Vic., Australia). The peptides were grouped into 10 pools of eight peptides each, and into one pool of six peptides. The pools were added to wells to reach a final concentration of 1 μg/ml for each individual peptide in the ELISPOT assays.
ELISPOT assays for IFN-γ
All assays were performed according to the manufacturer’s instructions (Diaclone, Cedex, France). Briefly, 96-well polyvinylidene difluoride (PVDF)-backed plates (Millipore, Watford, UK) were treated with 15 μl of 70% ethanol and washed three times with sterile phosphate-buffered saline (PBS). The wells were coated with 100 μl of IFN-γ capture antibody for 6 hr at 37°, washed three times with PBS and blocked for 1 hr at 37° with 100 μl of RPMI supplemented with 5% pooled human serum. Two-hundred and fifty thousand freshly isolated PBMC were added to each well and stimulated with Tax pools, Tax 11–19 peptide, or medium alone, for 6 hr at 37° in 5% CO2. In all assays, the CD28/CD49d costimulatory reagent (Becton Dickinson, San Diego, CA) was added at a final concentration of 0·5 μg/ml. After 6 hr of incubation, cells were discarded and the plates were incubated (for 10 min at 4°) with PBS containing 0·1% Tween-20 (PBS/0·1% Tween-20). The wells were washed three times with PBS/0·1% Tween-20 and then coated with 100 μl of biotinylated anti-(IFN-γ) at 1 μg/ml in PBS/1% bovine serum albumin (BSA). After a 3-hr incubation at 20°, the plates were washed three times with PBS/0·1% Tween-20, and 100 μl of streptavidin–alkaline phosphatase conjugate were added at a 1 : 1000 dilution in PBS/1% BSA. Following incubation for 1 hr at 20°, the plates were washed three times with PBS/0·1% Tween-20, and 100 μl of 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) substrate was added to each well. The number of spot-forming cells (SFC) was determined using the aid software (AID Elispot Reader, Strassberg, Germany). In 20 HTLV-1-infected subjects, we evaluated the frequencies of IFN-γ-producing cells in PBMC and in CD8+ T-cell-depleted PBMC. Because the frequencies were very low in CD8+ T-cell-depleted PBMC treated with Tax peptides (< 10% compared with PBMC), we decided to continue the experiments with PBMC.
Flow cytometry
In order to establish whether the donor was HLA-A*02 positive, 50 μl of whole blood was immunostained with saturating concentrations of anti-HLA-A*02–fluorescein isothiocyanate (FITC) (clone BB7.2; Becton Dickinson, Erembodegem, Belgium), according to the manufacturer’s instructions. The red cells were removed with lysing solution (Becton Dickinson, CA) and the white cells were fixed with 0·5% paraformaldehyde solution. Data from 10 000 cells were collected and analyzed.
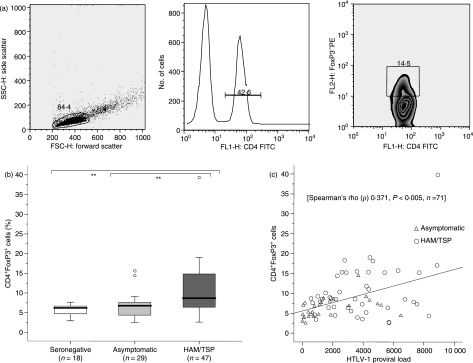
To determine the percentage of CD4+ Foxp3+ cells, freshly isolated PBMC (1 × 106 cells) were immunostained with saturating concentrations of anti-CD4–FITC (clone RPA-T4; Becton Dickinson, Belgium) for 30 min at 4°. Cells were then fixed and permeabilized using a commercial kit, following the manufacturer’s instructions (eBioscience, San Diego, CA). After a wash with PBS/1% BSA, the cells were stained intracellularly for 30 min at 4° with anti-Foxp3–phycoerythrin (PE) (clone PCH101) or rat IgG2a–PE (clone eBR2a) as the isotype control (eBioscience). Finally, the cells were washed with PBS/1% BSA and suspended in PBS/1% paraformaldehyde. The lymphocyte population was gated according to forward-scatter versus side-scatter parameters. The CD4+ T cells were selected from 30 000 lymphocyte events, and the percentage of Foxp3-expressing cells was determined within that CD4+ population (Fig. 1a).
Figure 1.
Frequency of CD4+ Foxp3+ cells and its correlation with provirus load (PVL). (a) A typical analysis of CD4+ Foxp3+ cells in a patient with human T-lymphotropic virus 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is shown. Lymphocytes were selected according to forward-scatter and side-scatter parameters, and then were gated on CD4+ T cells; the frequency of Foxp3+ cells was estimated as a percentage of the CD4+ T-cell population. (b) Data represent the percentage of Foxp3-expressing cells in the CD4+ population from freshly isolated peripheral blood mononuclear cells (PBMC). The Mann–Whitney U-test was used to evaluate differences among groups [HTLV-1-seronegative controls (SC), asymptomatic HTLV-1 carriers (AC) and patients with HAM/TSP]. Only P-values indicating significant differences are shown. Significance is indicated as follows: *P< 0·05, **P< 0·005, ***P< 0·001. (c) The Spearman test was used to evaluate the correlation between the frequency of CD4+ Foxp3+ cells and PVL in HTLV-1-infected subjects (AC + HAM/TSP). FITC, fluorescein isothiocyanate.
For detection of mDC or pDC, 0·5 × 106 freshly isolated PBMC were immunostained with anti-Lin1–FITC (clones SK7, 3G8, SJ25C1, L27, MϕP9 and NCAM16.2), anti-CD123–PE (clone 9F5), anti-HLA-DR–peridinin chlorophyll protein (PerCP) [clone L243 (G46-6)] and anti-CD11c–allophycocyanin (clone S-HCL-3) (Becton Dickinson, Belgium). Data from 50 000 cells were collected and the Lin1− HLA-DR+ population was selected from a Lin1 versus HLA-DR plot. The selected population was subsequently analyzed in an HLA-DR versus CD11c or CD123 plot to quantify the frequency of mDC (HLA-DRhigh CD11c+) or pDC (HLA-DRhigh CD123+). In all cases, data were acquired by means of the cellquest software (Becton Dickinson, CA) in a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson, CA) and analyzed using the flowjo program (Tree Star, Ashland, OR).
Quantification of lytic factor mRNA in CD8+ T cells
The expression of three genes related to the CD8+ T-cell lytic response (perforin, granzyme H and NKG2D) was estimated by quantifying mRNA levels using a SYBR-Green-based real-time quantitative polymerase chain reaction (qPCR) assay in an iCycler iQ instrument (Bio-Rad, Hercules, CA). Total RNA was extracted from 0·5–3 × 106 positively selected CD8+ T cells using the RNAqueos-Micro kit (Ambion, Austin, TX) according to the manufacturer’s instructions, and cDNA was synthesized using the RNA transcription system and Oligo (dT)15 primers (Promega, Madison, WI). cDNA obtained from 100 ng of total RNA was amplified with primers specific for perforin, granzyme H and NKG2D, described by Vine et al.19 In addition, the expression of two housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and beta-2-microglobulin (B2M) was evaluated in all assays. Primer sequences of GAPDH and B2M have been reported previously.20 qPCR reactions were performed in triplicate in a 20 μl reaction mixture consisting of 10 μl of 2× iQ SYBR-Green Supermix reagent (Bio-Rad) [final 1× concentrations: 50 mm KCl, 20 mm Tris–HCl (pH 8·4), 0·2 mm of each deoxyribonucleoside triphosphate (dNTP), 3 mm MgCl2, 0·3 U of iTaq hot-start DNA polymerase, 10 nm fluorescein and SYBR-green-containing buffer], 0·3 μm of NKG2D primers or 0·5 μm of perforin, granzyme H, GAPDH or B2M primers, and 2 μl of cDNA. In all cases, an initial denaturation step at 95° for 3 min was followed by 32 cycles at 95° for 30 s and 60° for 15 s. Data collection and analysis were performed using the icycler iq optical system software, version 3.0a (Bio-Rad).
The expression of each gene was normalized using the GENORM algorithm.20 Among the five genes evaluated (perforin, granzyme H, NKG2D, GAPDH and B2M), the GENORM program identified two genes (perforin, NKG2D) as the most stable genes. Therefore, these genes were used to calculate the normalization factor for each sample.
Quantification of HTLV-1 PVL
The HTLV-1 PVL was measured using qPCR, as previously described.4 Genomic DNA was extracted from 5 × 106 cells using the QIAampblood DNA mini kit (Qiagen, Hilden, Germany) and 40 ng was used for determining the copy number of the pX (tax/rex) region of the HTLV-1 proviral genome and the human endogenous retrovirus 3 (ERV-3) used as a normalizing gene. The PVL was calculated as follows: [(average copy number of pX)/(average copy number of ERV-3)] × (2 × 104), and expressed as the number of HTLV-1 copies per 104 PBMC.
Statistical analysis
For the comparison of demographic data, immune markers and PVL among AC, SC and patients with HAM/TSP, we used chi-square and Mann–Whitney U-tests. The correlation between the PVL and the frequency of CD4+ Foxp3+ cells, and between the PVL and the frequency of pDC, was evaluated using the Spearman test. In order to assess whether the production of Tax peptides induced by IFN-γ and/or the mRNA levels of lytic factors in CD8+ T cells were associated with the PVL in AC, we stratified this group into two subgroups according to the median PVL of all HTLV-1-infected subjects with data for peptide-induced IFN-γ production, mRNA levels of lytic factors and PVL: AC with a low PVL (< 2538 HTLV-1 copies/104 PBMC) and AC with a high PVL (≥ 2538 HTLV-1 copies/104 PBMC). Because of the exploratory nature of this study, we did not adjust the P-values for multiple comparisons.
Results
Characteristics of subjects
The demographic characteristics of the participants are shown in Table 1; 48 patients with HAM/TSP, 29 AC and 23 SC were included. Seventeen participants (10 patients with HAM/TSP and seven AC, from seven families) had first-degree family relationships among each other; the remaining 83 subjects were unrelated. The HTLV-1-infected subjects were significantly older (P< 0·001) and included more women (P< 0·05) than the SC, but there were no significant differences in age and gender between AC and patients with HAM/TSP. As for the ethnic background, the proportion of subjects of Andean origin was higher among patients with HAM/TSP than among AC (P< 0·05). Most participants (67 of 94, 71%) were HLA-A*02 positive; HLA-A*02-positive subjects were less frequent in the HAM/TSP group than among the AC (P< 0·05). There was no significant difference in HLA-A*02 frequency between AC with a high PVL and those with a low PVL (P = 0·7).
Table 1.
Demographic characteristics and immune markers in HTLV-1-seronegative controls, in asymptomatic human T-lymphotropic virus 1 (HTLV-1) carriers and in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)
| HTLV-1-seronegative controls |
Asymptomatic HTLV-1 carriers |
HAM/TSP patients |
||
|---|---|---|---|---|
| Median (IQR) (n= 23) | Median (IQR) (n= 29) | Median (IQR) (n= 48) | P-value* | |
| Age (years) | 27 (11) | 49 (21) | 50 (22) | 0·51 |
| Gender ratio (male/female) | 13/10 | 12/17 | 11/37 | 0·12 |
| Ethnic background (Andean/Mestizo) | 8/14 | 8/21 | 29/19 | 0·012 |
| Relatedness to a HAM/TSP patient (yes/no) | 14/15 | 17/31 | 0·42 | |
| HLA-A*02 (positive/negative) | 14/6 | 25/4 | 28/17 | 0·052 |
| CD4 (cells/μl) | 828 (416) | 971 (429) | 977 (584) | 0·91 |
| IFN-γ response to Tax pool 6 (SFCs/250 000 cells) | 0 (0) | 32 (83) | 64 (230) | 0·041 |
| IFN-γ response to Tax pool 7 (SFCs/250 000 cells) | 0 (0·8) | 33 (81) | 103 (157) | 0·0071 |
| IFN-γ response to Tax 11–19 (SFCs/250 000 cells) | 0 (3·8) | 205 (246) | 168 (261) | 0·41 |
| Perforin (relative expression) | 1·74 (0·73) | 1·97 (0·75) | 2·00 (0·75) | 0·81 |
| Granzyme H (relative expression) | 19·29 (31·75) | 23·55 (27·31) | 27·78 (63·01) | 0·61 |
| NKG2D (relative expression) | 2·63 (1·07) | 2·31 (0·81) | 2·27 (0·79) | 0·81 |
| CD4+ Foxp3+ (%) | 6·21 (2·06) | 6·76 (3·58) | 8·68 (8·85) | 0·0011 |
| Myeloid dendritic cells (%) | 7·08 (6·53) | 5·20 (5·75) | 4·13 (4·04) | 0·21 |
| Plasmacytoid dendritic cells (%) | 4·07 (1·58) | 5·52 (2·84) | 3·01 (1·54) | 0·0011 |
| Provirus load (HTLV-1 copy number/104 PBMC) | 1074 (2854) | 3476 (3672) | < 0·0011 |
Data were calculated on available results. The proportion of missing data was ≤ 27% for all variables with the exception of plasmacytoid dendritic cells (pDC) that were measured in the last phase only (14 patients with HAM/TSP and 13 asymptomatic HTLV-1 carriers). An additional group of HAM/TSP patients and asymptomatic HTLV-1 carriers was used to evaluate the frequency of CD4+ Foxp3+ cells.
IFN-γ, interferon-γ; IQR, interquartile range; NKG2D, natural killer group 2D; PBMC, peripheral blood mononuclear cells; SFC, spot-forming cells.
Mann–Whitney U-test.
Chi-square test (continuity correction).
P-value for the comparison between HAM/TSP patients and asymptomatic HTLV-1 carriers. Significant differences are indicated in bold.
IFN-γ secretion as a specific response to Tax pools
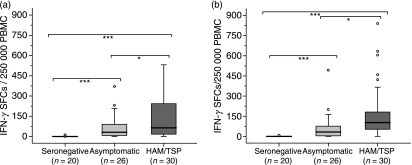
The PBMC of HTLV-1-infected subjects showed an IFN-γ response to the Tax 11–19 peptide. This response to Tax 11–19 discriminated HLA-A*02-positive subjects from HLA-A*02-negative subjects, but it did not discriminate patients with HAM/TSP from AC (Table 1). SC did not respond to any of the Tax pools or to the Tax 11–19 peptide. By contrast, the majority of the HTLV-1-infected subjects, particularly the patients with HAM/TSP, showed strong responses to several peptide pools. A univariate analysis showed differences in the specific response to pools 6 and 7 between patients with HAM/TSP and AC (P< 0·05), between patients with HAM/TSP and SC (P< 0·001), and between AC and SC (P< 0·001) (Fig. 2a,b). The discriminatory capacity of pools 6 and 7 held in HLA-A*02 subjects: significant differences in the specific response to pools 6 and 7 were observed between patients with HAM/TSP and AC (P< 0·05), between patients with HAM/TSP and SC (P< 0·001), and between AC and SC (P< 0·001) (data not shown). Taking all HTLV-1-infected subjects together, there was a significant correlation between the PVL and the frequency of cells producing IFN-γ in response to pool 6 [Spearman’s rho (ρ) 0·3; P < 0·05, n = 55].
Figure 2.
Frequency of interferon-γ (IFN-γ)-producing cells in response to Tax pools 6 or 7. Data represent the frequency of IFN-γ-producing cells, as measured using enzyme-linked immunospot (ELISPOT) assays, in freshly isolated peripheral blood mononuclear cells (PBMC) cultured for 6 hr and stimulated with Tax pools 6 or 7. (a) All subjects, pool 6; and (b) all subjects, pool 7. The Mann–Whitney U-test was used to evaluate differences among groups [HTLV-1-seronegative controls (SC), asymptomatic HTLV-1 carriers (AC), and patients with human T-lymphotropic virus 1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)]. Only P-values indicating significant differences are shown. Significance is indicated as follows: *P< 0·05, **P< 0·005, ***P< 0·001. SFC, spot-forming cells.
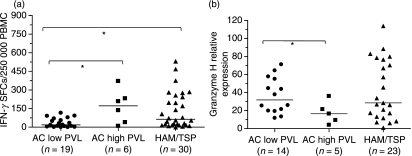
In addition, we investigated whether in AC the PVL somehow influences the Tax-induced release of IFN-γ by PBMC. In a univariate analysis, AC with low PVLs showed a diminished specific response to pool 6 compared to AC with high PVLs (P< 0·05) or to patients with HAM/TSP (P< 0·05) (Fig. 3a). As for pool 7, we could not find significant differences in the specific response.
Figure 3.
Frequency of interferon-γ (IFN-γ)-producing cells in response to Tax pool 6, and the relative expression of granzyme H in asymptomatic HTLV-1 carriers (AC) according to their provirus load (PVL). Data represent (a) the frequency of IFN-γ-producing cells, as measured using enzyme-linked immunospot (ELISPOT) assays, in peripheral blood mononuclear cells (PBMC) cultured for 6 hr and stimulated with Tax pools 6; and (b) the relative granzyme H mRNA levels in CD8+ T cells obtained by positive selection. The Mann–Whitney U-test was used to evaluate differences among groups (AC with a low PVL, AC with a high PVL and patients with human T-lymphotropic virus 1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)). Only P-values indicating significant differences are shown. Significance is indicated as follows: *P< 0·05, **P< 0·005, ***P< 0·001. SFC, spot-forming cells.
Gene expression related to CD8+ CTL response
As shown in Table 1, there were no differences at the mRNA level in the relative expression of perforin, granzyme H and NKG2D between AC and patients with HAM/TSP. However, when we evaluated the relative expression of those genes in AC considering their PVL, granzyme H discriminated AC with a low PVL from those with a high PVL (Mann–Whitney U-test: P< 0·05). Granzyme H expression did not distinguish AC with low or high PVLs from patients with HAM/TSP (Fig. 3b). We did not observe differences in the relative expression of perforin or NKG2D among the three groups.
CD4+ Foxp3+ cells, DC and PVL
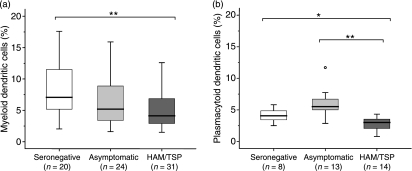
As shown in Figure 1(b), the frequency of CD4+ Foxp3+ cells was significantly increased in PBMC from patients with HAM/TSP when compared with that from either AC (P< 0·005) or SC (P< 0·005). No differences were found between AC and SC. In addition, a positive correlation between the frequency of CD4+ Foxp3+ cells and PVL was observed in HTLV-1-infected subjects [Spearman’s rho (ρ) 0·37, P< 0·01, n= 71] (Fig. 1c). When we compared the frequencies of mDC and pDC, we found a decrease of both types of DC in patients with HAM/TSP compared with SC (P< 0·005) (Fig. 4a,b). The frequency of pDC discriminated patients with HAM/TSP from AC (P< 0·005). AC showed slightly lower frequencies of mDC and higher frequencies of pDC than SC, yet these differences did not reach statistical significance. Finally, there was a negative correlation between the frequency of pDC and the PVL [Spearman’s rho (ρ) −0·42, P< 0·05, n =26] in HTLV-1-infected subjects.
Figure 4.
Frequency of myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC). Data represent (a) the percentage of mDC, or (b) the percentage of pDC, in a Lin− HLA-DR+ population from freshly isolated peripheral blood mononuclear cells (PBMC). The Mann–Whitney U-test was used to evaluate differences among groups [HTLV-1-seronegative controls (SC), asymptomatic HTLV-1 carriers (AC) and patients with human T-lymphotropic virus 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP)]. Only P-values indicating significant differences are shown. Significance is indicated as follows: *P< 0·05, **P< 0·005, ***P< 0·001.
Discussion
In this study we investigated the pro-inflammatory response of PBMC from a Peruvian population of patients with HAM/TSP and from AC by measuring the frequency of cells producing IFN-γ- in response to a battery of Tax peptides. The demographic characteristics of the patients with HAM/TSP in this study (more women than men, mean age 50 years, and predominantly of Andean ethnic background) are comparable with previous reports from our research group and from other research groups.4,13,21,22 The AC were similar to the patients with HAM/TSP except for ethnic background, where there was a higher proportion of Andean people among HAM/TSP patients.
Cells from patients with HAM/TSP showed a strong response to pools 6 and 7, which discriminated HAM/TSP from the asymptomatic condition, although the P-values would not survive correction for multiple comparisons. We consider that the IFN-γ production by PBMC is mainly attributable to HTLV-1-specific CD8+ T cells because: (i) Tax peptides are known as strong inducers of IFN-γ production by CD8+ T cells, but not by CD4+ T cells;7,23 and (ii) CD8+ T-cell-depleted PBMC treated with Tax peptides showed very low frequencies of IFN-γ-producing cells compared with PBMC (see the Materials and methods).
While the immunodominance of Tax for the CD8+ T-cell response has been extensively documented,7,24,25 quantification of the lytic response, and particularly the inflammatory response, elicited by specific epitopes has usually been limited to a few HLA class I backgrounds, mainly those associated with a lower risk of developing HAM/TSP, such as HLA-A*02. A study made available a detailed map of Tax epitopes in a diversity of HLA class I backgrounds and revealed previously unidentified regions of the protein capable of inducing a CD8+ T-cell response.26 Because we did not restrict the evaluation of the pro-inflammatory response to the presence of the HLA-A*02 allele, it is not surprising that Tax peptides other than Tax 11–19 stood out as important determinants of the production of IFN-γ by CTL. Moreover, pools 6 and 7 induced stronger responses in patients with HAM/TSP than the Tax 11–19-containing pool 1, even in an HLA-A*02 background. Nevertheless, it has to be pointed out that we observed an attenuating effect in the Tax 11–19-containing pool when comparing its strength with that of Tax 11–19 alone. Tax 11–19 did discriminate HLA-A*02-positive subjects from HLA-A*02-negative subjects, as expected (data not shown), but it could not discriminate patients with HAM/TSP from AC (Table 1). By contrast, pools 6 and 7 discriminated these two groups in both the total population and in the HLA-A*02-positive subjects. Therefore, our results show that Tax 11–19 is not necessarily the most informative Tax epitope when studying the activation of CD8+ T cells as part of a mechanism of HAM/TSP pathogenesis in an HLA class I-diverse population.
In our peptide array, pools 6 and 7 spanned Tax amino acids 161–233. This region encompasses several epitopes reported to induce CTL activity.26–28 It has been demonstrated that PBMC or CD8+ T cells from patients with HAM/TSP show higher frequencies of IFN-γ-producing cells and greater spontaneous release of this cytokine than PBMC or CD8+ T cells from AC after in vitro incubation,11–13 but few studies have addressed these aspects of the CD8+ T-cell function in a scenario of specific responses to HTLV-1 antigens. While Goon et al.7 found a strong response of CD8+ T cells to Tax with no discrimination between patients with HAM/TSP and AC by using a peptide library approach, another group reported increased frequencies of IFN-γ-producing CD8+ T cells in HLA-A*02-positive HAM/TSP subjects after stimulation with Tax 11–19 tetramers.29 Whether the discrepancy in our study, in which Tax 11–19 did not discriminate HAM/TSP from AC, was caused by the different methodologies utilized and/or the number and origin of the patients evaluated, is unclear at present. Yet, we consider that our approach, based on a peptide array, has the advantage of not limiting the analysis to a single HLA background. The epitopes relevant to our findings are currently being pinpointed by testing the PBMC response to the individual peptides included in pools 6 and 7 of our array.
Consistent with previous reports,3,4 the patients with HAM/TSP in this study had a significantly higher PVL than AC. Interestingly, when we divided the AC into two subgroups according to the PVL and quantified the frequency of IFN-γ-producing cells in response to pool 6, the subgroup of AC with a high PVL showed a higher pro-inflammatory response than those AC with a low PVL, and this response was not significantly different from that of patients with HAM/TSP. It is noteworthy that the same subgroup of AC with a high PVL showed significantly lower levels of granzyme H mRNA in unstimulated CD8+ T cells than those with a low PVL, suggesting a deficiency in lytic function. Granzyme H is produced by natural killer (NK) cells, as well as by CTL, as part of the mechanisms inducing lysis, and has been previously shown to be over-expressed in HTLV-1-infected subjects with a low PVL using microarray technology.19 A reduction of CD8+ T-cell lytic activity has also been described in patients with ATLL,30 although in that case perforin and granzyme B, rather than granzyme H, were found to be under-expressed. While it is possible that the mechanisms of immunosuppression differ between HAM/TSP and ATLL, the study on ATLL evaluated expression at the protein level and not at the mRNA level.
Taken together, our data are consistent with a model in which specific CD8+ T-cell activation in HTLV-1 carriers with a high PVL, who – according to limited evidence from longitudinal studies – might be at increased risk of developing HAM/TSP,31,32 is biased towards the production of pro-inflammatory cytokines with the loss of lytic capacity. Such a model would explain the apparent paradox that, in spite of having a high frequency of HTLV-1-specific CTL, patients with HAM/TSP usually have a higher PVL.24,28 Within this line, a study has recently shown that patients with HAM/TSP have a lower frequency, than AC, of CD8+ T cells expressing co-stimulatory molecules, as well as an impaired reactivity of HTLV-1-specific CTL towards an HLA-A*02/Tax 11–19 tetramer.9
The CD8+ T-cell response to a specific antigen is shaped through a complex mechanism that includes antigen presentation and T-cell activation by APC, as well as immunomodulation by regulatory T cells. In the present study we found an increased frequency of CD4+ Foxp3+ cells in HTLV-1-infected individuals, as reported previously,15 but, in contrast to these results, HAM/TSP patients in the present study had significantly higher frequencies than AC. Because a clear definition of regulatory T cells based on markers is still lacking, conflicting data have arisen in recent years, particularly in the HTLV-1 field. Human regulatory T cells have usually been characterized as CD4+ CD25+. However, CD25 expression is strongly induced by Tax protein,15 and therefore measuring the frequency of Foxp3+ cells in the CD4+ CD25+ population, as reported in other studies,33,34 is likely to underestimate the frequency of regulatory T cells in HTLV-1-infected subjects. Moreover, Foxp3 is also expressed in T-cell populations other than regulatory T cells.35 In spite of a recent effort to overcome these limitations by using various combinations of markers,36 an unambiguous identification of regulatory T cells is still not possible based on markers alone. So far, the function of the CD4+ Foxp3+ cells identified in this study has not been tested in the context of HTLV-1 infection, and it is therefore unclear whether they are directly involved in altering CTL activity by impairing the lytic response, as suggested previously,15 and/or by failing to suppress the antigen-specific pro-inflammatory response, as observed in our population.
The increase in CD4+ Foxp3+ cell frequency in patients with HAM/TSP is particularly interesting when considered along with the accompanying sharp decrease in the frequency of mature pDC, which is the DC subset that is preferentially involved in the control of viral infections. Fully mature DC are needed for appropriate antigen presentation and induction of an effective adaptive immune response.37 By contrast, immature/semimature DC might compromise the proper activation of specific CD4+ and CD8+ T cells, and have been reported to favour the generation and expansion of T cells with regulatory phenotypes.38 From this perspective, the high frequency of HLA-DRhigh-expressing pDC among AC in our study population suggests that, in these subjects, the adaptive immune response is properly induced, including an appropriate balance between CTL pro-inflammatory and lytic responses. Patients with HAM/TSP, however, with low frequencies of pDC, would have suboptimal antigen presentation, a skewed CTL activity (as observed in our population) and a tendency to generate cells with a regulatory-like phenotype. Consistent with this interpretation, we found a negative correlation between the frequency of mature pDC and the PVL. HTLV-1 can infect DC,39 and these cells can serve as vehicles for virus transmission to T cells.14 An altered synapse between these two cell types might affect T-cell activation. Impairment of maturation and interference with DC functions is a common strategy utilized by a diversity of viruses to evade the host defence responses.37 Interestingly, a previous study has demonstrated that the production of IFN-α by pDC from HTLV-1-infected individuals is reduced.40
In conclusion, our investigation represents the first study to provide evidence that an exacerbated pro-inflammatory response to a specific region (amino acids 161–233) of the Tax protein is associated with HAM/TSP pathogenesis in an HLA class I-diverse Peruvian population. Epitopes that are currently under investigation seem more informative than Tax 11–19 with regard to the mechanisms of disease. We also provide evidence of increased frequencies of CD4+ Foxp3+ cells accompanied by decreased frequencies of HLA-DRhigh pDC in patients with HAM/TSP.
Acknowledgments
We thank the staff at the Institute of Tropical Medicine Alexander von Humboldt (IMTAvH), in particular Viviana Quintana, Doris Agapito, Alfonso Silva-Santisteban, Erick Mayer, Fanny Ita, Jaime Zaldívar, Vanessa Adaui, Michael Talledo and Oscar Nolasco for their valuable assistance in the attention of HTLV-1-infected patients, HTLV-1 diagnosis and PCR assays. This work was supported by the Directorate-General for Development Cooperation of the Belgian Government through the Framework Agreement with the Institute of Tropical Medicine of Antwerp and through the Flemish Interuniversity Council (VLIR), and by the Peruvian National Board for Science and Technology (CONCYTEC).
Glossary
Abbreviations:
- AC
asymptomatic HTLV-1 carriers
- APC
antigen-presenting cell
- ATLL
adult T-cell lymphoma/leukaemia
- BSA
bovine serum albumin
- CTL
cytolytic T lymphocytes
- DC
dendritic cells
- ELISA
enzyme-linked immunosorbent assay
- ELISPOT
enzyme-linked immunospot
- FITC
fluorescein isothiocyanate
- HAM/TSP
HTLV-1-associated myelopathy/tropical spastic paraparesis
- HTLV-1
human T-lymphotropic virus 1
- IFN-γ
interferon-γ
- mDC
myeloid dendritic cells
- NKG2D
natural killer group 2D
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- pDC
plasmacytoid dendritic cells
- PE
phycoerythrin
- PVL
provirus load
- qPCR
quantitative PCR
- SC
HTLV-1-seronegative controls
- SFC
spot-forming cell
Disclosure
The authors declare no financial or commercial conflict of interest.
References
- 1.Van Dooren S, Salemi M, Vandamme AM. Dating the origin of the African human T-cell lymphotropic virus type-I (HTLV-I) subtypes. Mol Biol Evol. 2001;18:661–71. doi: 10.1093/oxfordjournals.molbev.a003846. [DOI] [PubMed] [Google Scholar]
- 2.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–81. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 3.Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–93. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 4.Adaui V, Verdonck K, Best I, et al. SYBR Green-based quantitation of human T-lymphotropic virus type 1 proviral load in Peruvian patients with neurological disease and asymptomatic carriers: influence of clinical status, sex, and familial relatedness. J Neurovirol. 2006;12:456–65. doi: 10.1080/13550280601039634. [DOI] [PubMed] [Google Scholar]
- 5.Jeffery KJ, Usuku K, Hall SE, et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci U S A. 1999;96:3848–53. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asquith B, Mosley AJ, Heaps A, Tanaka Y, Taylor GP, McLean AR, Bangham CR. Quantification of the virus/host interaction in human T lymphotropic virus I infection. Retrovirology. 2005;2:75–83. doi: 10.1186/1742-4690-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goon PK, Biancardi A, Fast N, et al. Human T cell lymphotropic virus (HTLV) type-1-specific CD8+ T cells: frequency and immunodominance hierarchy. J Infect Dis. 2004;189:2294–8. doi: 10.1086/420832. [DOI] [PubMed] [Google Scholar]
- 8.Brehm MA, Selin LK, Welsh RM. CD8 T cell responses to viral infections in sequence. Cell Microbiol. 2004;6:411–21. doi: 10.1111/j.1462-5822.2004.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabouri AH, Usuku K, Hayashi D, Izumo S, Ohara Y, Osame M, Saito M. Impaired function of human T-lymphotropic virus type 1 (HTLV-1)-specific CD8+ T cells in HTLV-1-associated neurologic disease. Blood. 2008;112:2411–20. doi: 10.1182/blood-2008-02-140335. [DOI] [PubMed] [Google Scholar]
- 10.Asquith B, Bangham CR. How does HTLV/I persist despite a strong cell/mediated immune response? Trends Immunol. 2007;29:4–11. doi: 10.1016/j.it.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J Immunol. 1998;161:482–8. [PubMed] [Google Scholar]
- 12.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best I, Adaui V, Verdonck K, Gonzalez E, Tipismana M, Clark D, Gotuzzo E, Vanham G. Proviral load and immune markers associated with human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Peru. Clin Exp Immunol. 2006;146:226–33. doi: 10.1111/j.1365-2249.2006.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–36. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 15.Toulza F, Heaps A, Tanaka Y, Taylor GP, Bangham CR. High frequency of CD4+FoxP3+ cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood. 2008;111:5047–53. doi: 10.1182/blood-2007-10-118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osame M. Review of WHO Kagoshima meeting and diagnostic guidelines for HAM/TSP. In: Blattner WA, editor. Human Retrovirology: HTLV. New York: Raven Press; 1990. pp. 191–7. [Google Scholar]
- 17.De Castro-Costa CM, Araujo AQ, Barreto MM, et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM) AIDS Res Hum Retroviruses. 2006;22:931–5. doi: 10.1089/aid.2006.22.931. [DOI] [PubMed] [Google Scholar]
- 18.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–22. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vine AM, Heaps AG, Kaftantzi L, et al. The role of CTLs in persistent viral infection: cytolytic gene expression in CD8+ lymphocytes distinguishes between individuals with a high or low proviral load of human T cell lymphotropic virus type 1. J Immunol. 2004;173:5121–9. doi: 10.4049/jimmunol.173.8.5121. [DOI] [PubMed] [Google Scholar]
- 20.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe N, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research 0034.1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotuzzo E, Cabrera J, Deza L, et al. Clinical characteristics of patients in Peru with human T cell lymphotropic virus type 1-associated tropical spastic paraparesis. Clin Infect Dis. 2004;39:939–44. doi: 10.1086/423957. [DOI] [PubMed] [Google Scholar]
- 22.Gastaldello R, Iñiguez AM, Otsuki K, et al. HTLV type 1 genetic types among native descendants in Argentina. AIDS Res Hum Retroviruses. 2008;24:1139–46. doi: 10.1089/aid.2007.0299. [DOI] [PubMed] [Google Scholar]
- 23.Goon PK, Igakura T, Hanon E, et al. Human T cell lymphotropic virus type I (HTLV-I)-specific CD4+ T cells: immunodominance hierarchy and preferential infection with HTLV-I. J Immunol. 2004;172:1735–43. doi: 10.4049/jimmunol.172.3.1735. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–8. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 25.Kannagi M, Harada S, Maruyama I, et al. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int Immunol. 1991;3:761–7. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- 26.Yashiki S, Fujiyoshi T, Arima N, et al. HLA-A*26, HLA-B*4002, HLA-B*4006, and HLA-B*4801 alleles predispose to adult T cell leukemia: the limited recognition of HTLV type 1 tax peptide anchor motifs and epitopes to generate anti-HTLV type 1 tax CD8(+) cytotoxic T lymphocytes. AIDS Res Hum Retroviruses. 2001;17:1047–61. doi: 10.1089/088922201300343735. [DOI] [PubMed] [Google Scholar]
- 27.Parker CE, Daenke S, Nightingale S, Bangham CR. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virol. 1992;188:628–36. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- 28.Parker CE, Nightingale S, Taylor GP, Weber J, Bangham CR. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–8. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota R, Nagai M, Kawanishi T, Osame M, Jacobson S. Increased HTLV type 1 tax specific CD8+ cells in HTLV type 1-asociated myelopathy/tropical spastic paraparesis: correlation with HTLV type 1 proviral load. AIDS Res Hum Retroviruses. 2000;16:1705–9. doi: 10.1089/08892220050193182. [DOI] [PubMed] [Google Scholar]
- 30.Kozako T, Arima N, Toji S, et al. Reduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patients. J Immunol. 2006;177:5718–26. doi: 10.4049/jimmunol.177.8.5718. [DOI] [PubMed] [Google Scholar]
- 31.Taylor GP, Tosswill JH, Matutes E, et al. Prospective study of HTLV-I infection in an initially asymptomatic cohort. J Acquir Immune Defic Syndr. 1999;22:92–100. doi: 10.1097/00042560-199909010-00012. [DOI] [PubMed] [Google Scholar]
- 32.Kwaan N, Lee T-H, Chafets DM, et al. Long-term variations in human T lymphotropic virus (HTLV)-I and HTLV-II proviral loads and association with clinical data. J Infect Dis. 2006;194:1557–64. doi: 10.1086/508899. [DOI] [PubMed] [Google Scholar]
- 33.Yamano Y, Takenouchi N, Li HC, Tomaru U, Yao K, Grant CW, Maric DA, Jacobson S. Virus-induced dysfunction of CD4+CD25+ T cells in patients with HTLV-I-associated neuroimmunological disease. J Clin Invest. 2005;115:1361–8. doi: 10.1172/JCI23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh U, Grant C, Griffith C, Fugo K, Takenouchi N, Jacobson S. Reduced Foxp3 protein expression is associated with inflammatory disease during human T lymphotropic virus type 1 infection. J Infect Dis. 2006;193:1557–66. doi: 10.1086/503874. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Tsuji-Takayama K, Suzuki M, et al. Comprehensive analysis of FOXP3 mRNA expression in leukemia and transformed cell lines. Leuk Res. 2008;32:651–8. doi: 10.1016/j.leukres.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Michaëlsson J, Barbosa HR, Jordan KA, et al. The frequency of CD127low expressing CD4+CD25high T regulatory cells is inversely correlated with human T lymphotrophic virus type-1 (HTLV-1) proviral load in HTLV-1-infection and HTLV-1-associated myelopathy/tropical spastic paraparesis. BMC Immunol. 2008;9:41–52. doi: 10.1186/1471-2172-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohl C, Shishkova J, Schneider-Schaulies S. Viruses and dendritic cells: enemy mine. Cell Microbiol. 2007;9:279–89. doi: 10.1111/j.1462-5822.2006.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills KH, McGuirk P. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 39.Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-I and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8:1699–706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 40.Hishizawa M, Imada K, Kitawaki T, Ueda M, Kadowaki N, Uchiyama T. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br J Haematol. 2004;125:568–75. doi: 10.1111/j.1365-2141.2004.04956.x. [DOI] [PubMed] [Google Scholar]