Abstract
The T helper type 2 (Th2) mediated expulsion of the gastrointestinal nematode Trichinella spiralis requires interleukin-4 receptor α (IL-4Rα) expression on both bone-marrow-derived and non-bone-marrow-derived cells. To more definitively investigate the role of IL-4/IL-13 responsiveness in the development of protective immunity to T. spiralis, cell-specific IL-4Rα signalling on CD4+ T cells (Lckcre IL-4Rα−/flox) and macrophages/neutrophils (LysMcre IL-4Rα−/flox) was analysed on the BALB/c background. Infection of wild-type and control IL-4Rα−/flox mice induced a Th2-type immune response with elevated IL-4 cytokine production, parasite-specific immunoglobulin G1 (IgG1), total IgE, intestinal mastocytosis and enteropathy. In contrast, global IL-4Rα-deficient BALB/c mice showed reduced worm expulsion, antibody production, intestinal mastocytosis and gut pathology. BALB/c mice generated with cell-specific deletion of IL-4Rα on CD4+ T lymphocytes or macrophages/neutrophils, controlled gastrointestinal helminth infection by eliciting a protective immune response comparable to that observed with wild-type and IL-4Rα−/flox controls. Together, this shows that the development of host protective Th2 responses accompanied by parasite loss is independent of IL-4Rα expression on CD4+ T cells and macrophages/neutrophils.
Keywords: cell-specific interleukin-4 receptor α transgenic mice, gastrointestinal nematodes, mastocytosis, T helper immune response, Trichinella spiralis
Introduction
Divergent evolutionary traits among gastrointestinal (GI) nematodes have afforded them the unique ability to occupy a variety of niches within the host intestine. As a consequence, each parasite elicits an array of effector responses but with no single dominant mechanism which brings about expulsion.1,2 However, CD4+ T cells, predominantly of the T helper type 2 (Th2) subset, play a central role in mediating the protective immune response to parasitic helminths.1,3–5 The Th2 cells induced by GI helminth infection produce a profile of cytokines, the most influential in regulating clearance of gut dwelling nematodes being interleukin-4 (IL-4) and IL-13. Binding of IL-4 and/or IL-13 to IL-4 receptor α (IL-4Rα) activates signal transducer and activator of transcription-6 (STAT6), a dependent pathway in protection against GI helminths including Trichinella spiralis and Nippostrongylus brasiliensis. However, the induction of a Th2-type immune response leading to the expulsion of worms is more complex than initially anticipated. Studies using IL-4Rα and STAT6-deficient mice have demonstrated that STAT6 signalling, which initiates the expulsion of N. brasiliensis is not associated with induction of Th2 responses, and intestinal mastocytosis is limited.6 In contrast, the absence of STAT6 activation during T. spiralis infection severely impairs both mast cell responses and cytokine responses that induce intestinal mastocytosis.7 The intricacy of IL-4 and IL-13 responsiveness can further be attributed to the wide range of cell types expressing IL-4Rα,8 hence the need to address signalling of the receptor on specific cell types and their role in helminth immunity.
Expulsion of adult T. spiralis has previously been shown to be dependent on IL-4Rα expression on both bone-marrow-derived and non-bone-marrow-derived cells.9 However, treatment with exogenous IL-4 eliminated the bone-marrow-derived dependence and emphasized the importance of IL-4/IL-13 responsiveness of non-immune cells, which may include intestinal epithelial cells, smooth muscle cells, fibroblasts and goblet cells. Furthermore, IL-4 treatment failed to induce a mast cell response or worm expulsion in T-cell-deficient, T. spiralis-infected mice7 suggesting that the IL-4Rα-independent contribution of T cells possibly includes the promotion of mast cell responses through the secretion of IL-310 and/or IL-13. The latter cytokine may act on dendritic cells or macrophages to inhibit the production of IL-12 and its subsequent suppression of mastocytosis.10–12 Alternatively, T-cell-secreted IL-13 as well as IL-4 may also directly stimulate intestinal cells.9
To investigate the role of IL-4/IL-4Rα signalling on CD4+ T cells and elucidate the role of IL-4/IL-13 responsiveness by macrophages and neutrophils in the expulsion of GI helminths, we infected transgene hemizygous Lckcre IL-4Rα−/flox and LysMcre IL-4Rα−/flox BALB/c mice with T. spiralis and analysed their immune responses. The Lckcre IL-4Rα−/flox mice have impaired IL-4-induced CD4+ T-cell proliferation and Th2 differentiation as a result of a null mutation of IL-4Rα specific to CD4+ T cells. Other T-cell populations (CD8+, γδ, natural killer T) demonstrated partial deletion of the receptor, while normal IL-4 and/or IL-13 responsiveness by non-T cells was maintained.13 In contrast, LysMcre IL-4Rα−/flox mice demonstrate selective impairment of IL-4Rα functioning only on macrophages and neutrophils while maintaining CD4+ T helper cell proliferative responses similar to the wild-type controls.14 In this study, we report that protective and pathological responses to T. spiralis are independent of IL-4 responsiveness on CD4+ T cells. Furthermore, we demonstrate that IL-4/IL-13 signalling on macrophages and neutrophils is not a prerequisite for the development of immunity to T. spiralis.
Material and methods
Generation and genotyping of conditional IL-4Rα-deficient mice
Gene targeting in BALB/c embryonic stem cells and Cre/loxP-specific site-specific recombination was performed to generate IL-4Rαflox/flox mice using previously described techniques.15 Lckcre mice16 and LysMcre mice17 were first backcrossed to BALB/c for nine generations and intercrossed with IL-4Rαflox/flox mice in specific pathogen-free conditions in individual ventilated cages. These mice were further mated with IL-4Rα-deficient (IL-4Rα−/−) mice to generate Lckcre IL-4Rα−/flox and LysMcre IL-4Rα−/flox mice, respectively. Transgene negative littermates (IL-4Rα−/flox) and BALB/c mice were used as wild-type controls where specified. Hemizygous IL-4Rα−/flox, Lckcre, LysMcre and global IL-4Rα−/−15 mice were originally obtained from Prof. F. Brombacher, University of Cape Town, South Africa. All mice were bred, maintained and housed in the University of Strathclyde animal facility under standard conditions with free access to food and water and all procedures were performed under Home Office regulations. All mice used in experiments were 8- to 10-week-old females.
Parasite infection
Trichinella spiralis larvae were maintained by serial passage in CD1, C57BL/6 and BALB/c mice and were recovered from infected mice as described previously.18 All experimental strains were infected orally with 400 T. spiralis larvae and killed at various times post-infection.
Histology
Intestinal pathology was assessed as described previously.19 First, small intestines were weighed and then samples of jejunum were taken 10 cm from the pylorus, opened longitudinally, and then fixed in Clarke’s fixative (25% acetic acid/75% ethanol). After 24 hr, the fixative was replaced with 70% ethanol and the gut sections were permeabilized using 1 m HCl at 60° for 7 min followed by staining with Schiff’s reagent (Sigma, Poole, UK). Sections were microdissected and villus and crypt lengths were measured using an eyepiece micrometer. Ten villi and crypt areas were measured for each sample and the mean length was determined for each. The mean number of mitotic figures in 10 randomly selected crypt areas was also determined.
Recovery of adult worms from small intestine
The remainder of the gut was opened longitudinally, wrapped in gauze squares and incubated in Hanks’ balanced salt solution at 37° for 3 hr to induce migration of worms from the gut epithelium into solution. Following incubation, the gauze squares containing the guts were agitated to release any trapped worms. Worms were counted using a scored Petri dish and an inverted dissecting microscope.
In vitro cytokine production
Spleen and mesenteric lymph nodes were removed under sterile conditions and single-cell suspensions were prepared by forcing the tissue through sterile Nitex membranes in RPMI-1640 (Gibco, Paisley, UK) supplemented with 25 mm HEPES, 10% fetal calf serum, 5 mm l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, 5·5 pg/ml amphotericin B and 0·05 mβ-mercaptoethanol (all Gibco). Viable cells were counted using the Trypan Blue exclusion assay and 1 × 105 cells/100 μl were incubated in sterile 96-well microtitre plates with or without 100 μg/ml T. spiralis antigen. Briefly, T. spiralis antigen was prepared by homogenizing T. spiralis larvae followed by several rounds of centrifugation at 9000 g for 5 min and rehomogenization of the pellet in phosphate-buffered saline. Following a 24 hr incubation at 37° with 5% CO2, the cells were centrifuged at 400 g. The cell supernatants were collected and frozen in fresh 96-well plates at −20° for future analysis.
Cytokine enzyme-linked immunosorbent assays
Cytokines present in tissue culture supernatants were detected using sandwich enzyme-linked immunosorbent assays (ELISAs) for IL-4 (Biosource International Inc, Camarillo, CA), IL-13 (BD Pharmingen, Abingdon, UK) and IFN-γ (Biosource International Inc).
Mast cell quantification
Consecutive samples of jejunum were sampled and fixed in Carnoy’s fixative, followed by processing using standard histological techniques. Sections were stained with 0·5% Toluidine Blue (Sigma) in 0·5 m HCl for mast cell visualization and counterstained with 0·5% Safranin O (Sigma) for 2 min.19 The number of mucosal mast cells was counted in 10 villus/crypt units and data were expressed as mean number of mucosal mast cells per vullus/crypt unit.
Measurement of antibody responses
Parasite-specific IgG1 and IgG2a and total IgE levels were determined as described previously, with minor modifications.19 Briefly, T. spiralis larval homogenate was used as a target antigen at 2 μg/ml. Sera were diluted one-third starting at one-tenth. Isotypes of IgG1 and IgG2a were detected using horseradish peroxidase-conjugated anti-mouse IgG1 and IgG2a at a 1/10 000 dilution (Southern Biotech, Cambridge, UK). Total IgE levels were measured using a sandwich ELISA as described previously.19 Absorbance was measured at 450 nm (reference 540 nm) using a Spectramax ELISA reader (Molecular Devices, Wokingham, UK).
Statistical analysis
Data are presented as means + SEM. The significant differences between means were determined using the Mann–Whitney U-test. A P-value of < 0·05 was considered significant.
Results
Expression of IL-4Rα on T cells is not required for expulsion of T. spiralis
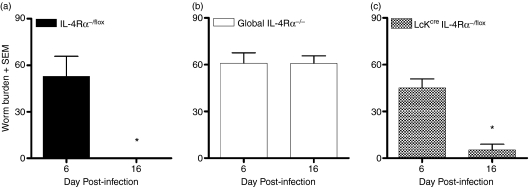
The dependency of IL-4Rα expression on CD4+ T cells and its role in the expulsion of GI nematodes remains controversial.9 Using recently generated Lckcre IL-4Rα−/flox mice,13 we investigated the role of IL-4/IL-4Rα signalling on CD4+ T cells during a T. spiralis infection. Phenotypic evaluation was assessed by the establishment (day 6 postinfection) and expulsion (day 16 postinfection) of the gut-dwelling nematode. No significant difference in parasite establishment was evident among the strains. However, wild-type IL-4Rα−/flox mice induced a resistant phenotype with complete expulsion of parasite load 16 days after infection (Fig. 1a). Global IL-4Rα−/− mice were unable to resolve their worm burden at this time-point and continued to harbour a significant number of adult worms (Fig. 1b). In contrast, Lckcre IL-4Rα−/flox mice expelled the adult worms with kinetics similar to that observed in the wild-type (Fig. 1c). These results show that IL-4Rα signalling on non-CD4+ T cells is sufficient to induce the protective responses to T. spiralis infection.
Figure 1.
Expulsion of Trichinella spiralis in mice deficient for interleukin-4 receptor α (IL-4Rα) expression on CD4+ T cells (Lckcre IL-4Rα−/flox). Establishment and expulsion of T. spiralis was measured in (a) IL-4Rα−/flox (BALB/c wild-type, bearing one floxed and one disrupted IL-4Rα allele), (b) global IL-4Rα−/− and (c) Lckcre IL-4Rα−/flox mice at days 6 and 16 postinfection. Adult T. spiralis worms were recovered from excised intestine to determine the total worm numbers per strain. Data are expressed as the mean worm burden ±SEM. Four mice were used per group. *P< 0·05, significantly different from day 6 postinfection.
Macrophage/neutrophil-specific IL-4Rα-deficient mice induce a protective phenotype to T. spiralis
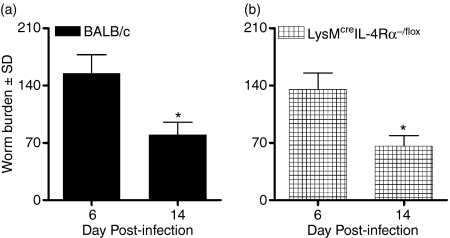
We have shown that IL-4Rα signalling on CD4+ T cells is not a prerequisite for the expulsion of T. spiralis. To determine whether a resistant phenotype is dependent on IL-4/IL-13 responsiveness of bone marrow-derived macrophages/neutrophils,14 we infected LysMcre IL-4Rα−/flox mice with 400 T. spiralis larvae and monitored their worm burden over time. Following a similar establishment of parasitic helminths at day 6 postinfection, both BALB/c wild-type (Fig. 2a) and LysMcre IL-4Rα−/flox (Fig. 2b) mice reduced their worm burdens to similar levels. Taken together, this suggests that the expression of IL-4Rα is not required for parasite expulsion from either CD4+ T cells or macrophages/neutrophils.
Figure 2.
Mice expel Trichinella spiralis in the absence of interleukin-4 (IL-4)/IL-13-responsive macrophages/neutrophils. (a) BALB/c wild-type and (b) LysMcre IL-4Rα−/flox mice were infected with 400 freshly isolated T. spiralis larvae and their worm burden was monitored at days 6 and 14 postinfection. Data are expressed as the mean worm burden ± SEM. Four mice were used per group. *P < 0·05, significantly different from day 6 postinfection.
Enteropathy induced by helminth infection is independent of IL-4Rα responsiveness on CD4+ T cells and macrophages/neutrophils
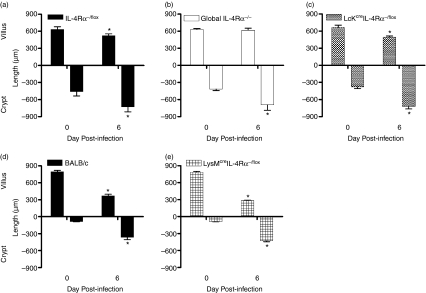
Interleukin-4 and IL-13 play a key role in the development of gut pathology following infection with T. spiralis.20–22 To investigate the role of CD4+ T-cell and macrophage/neutrophil-specific IL-4Rα expression in trichinellosis-induced enteropathy, we assessed the development of villus atrophy and crypt hyperplasia in Lckcre IL-4Rα−/− and LysMcre IL-4Rα−/− mice, respectively. Both cell-specific knock-outs induced a protective pathological response indicative of the wild-type control. Wild-type mice (BALB/c and IL-4Rα−/flox) developed significant changes to their intestinal architecture with villus atrophy and crypt hyperplasia noticeably associated with the establishment of GI helminths at 6 days after infection (Fig. 3a,d). Villus atrophy was ameliorated in global IL-4Rα−/− mice as described previously;20 however, the significant elevation of crypt hyperplasia in all strains suggests that this response is IL-4Rα-independent. The results here show that neither IL-4 signalling on Lckcre IL-4Rα−/flox nor IL-4/IL-13 signalling on macrophages/neutrophils is responsible for T. spiralis-induced intestinal pathology (Fig. 3b,c,e).
Figure 3.
The development of villus atrophy and crypt hyperplasia was measured in (a) interleukin-4 receptor α (IL-4Rα)−/flox, (b) IL-4Rα−/−, (c) Lckcre IL-4Rα−/flox, (d) BALB/c and (e) LysMcre IL-4Rα−/flox mice at days 0 and 6 postinfection. Data are expressed as mean length (in μm) + SEM. Three to five mice were used per group. *P < 0·05, significantly different from uninfected.
Mast cell hyperplasia is unaffected in both Lckcre IL-4Rα−/flox and LysMcre IL-4Rα−/flox mice
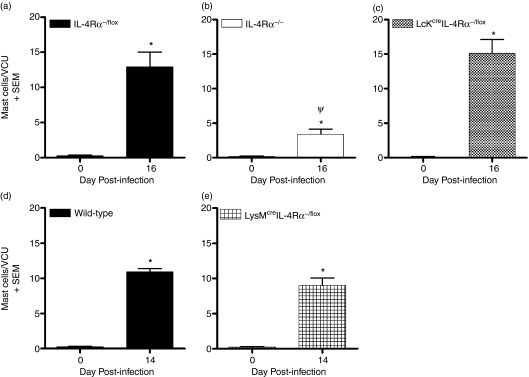
Mast cells contribute both to protective responses in T. spiralis infection and in the development of intestinal pathology.18,23,24 However, IL-4/IL-4Rα responsiveness is required for the generation of a mast cell response.20 Using mice selectively lacking IL-4Rα expression on CD4+ T cells, we investigated the intestinal mastocytosis in response to the GI nematode T. spiralis. Following infection, IL-4Rα-dependent mast cell hyperplasia was more profound within the IL-4Rα−/flox and Lckcre IL-4Rα−/flox mice with mast cell numbers significantly decreased in global IL-4Rα−/− mice (Fig. 4a–c).
Figure 4.
Deletion of interleukin-4 receptor α (IL-4Rα) on either CD4+ T cells or macrophages/neutrophils does not abrogate Trichinella spiralis-induced mastocytosis. Mast cell hyperplasia in response to the gastrointestinal nematode was measured in (a) IL-4Rα−/flox, (b) IL-4Rα−/−, (c) Lckcre IL-4Rα−/flox, (d) BALB/c and (e) LysMcre IL-4Rα−/flox mice. Carnoy’s-fixed jejuna from uninfected and infected (day 16 postinfection) mice were processed and stained with 0·5% toluidine blue and numbers of intestinal mast cells in 10 villus/crypt units (VCU) were counted. Data are expressed as the mean number of masts and/or villus/crypt units. Three to five mice were used per group. *P< 0·05, significantly different from uninfected; ψP < 0·05, significantly different from IL-4Rα−/flox and Lckcre IL-4Rα−/flox.
Interleukin-12 acting on macrophages is known to suppress mastocytosis.11,12 To evaluate whether the absence of IL-4/IL-13 responsiveness on macrophages exacerbates intestinal mastocytosis, we infected LysMcre IL-4Rα−/flox mice with T. spiralis. However, T. spiralis-induced intestinal mastocytosis in LysMcre IL-4Rα−/flox mice (Fig. 4d) resembled that observed in the BALB/c controls (Fig. 4e) with increasing numbers of the effector cell in the small intestine 14 days postinfection. Here, the data demonstrate that IL-4/IL-13 signalling via IL-4Rα on macrophages/neutrophils does not impair intestinal mastocytosis, which accompanies parasite infection.
Lckcre IL-4Rα−/− and LysMcre IL-4Rα−/− mice induce a Th2-type immune response
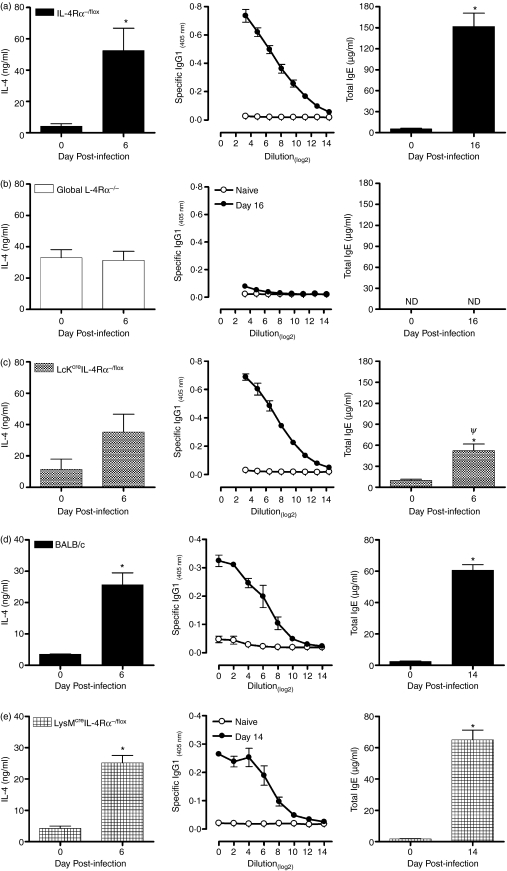
Interleukin-4, IL-13 and IL-4Rα play a central role in the generation of a protective Th2 response to T. spiralis.20 To determine whether the expression of IL-4Rα on CD4+ T cells or macrophages/neutrophils alters the T helper phenotype, cytokine and antibody responses were measured. Following infection, a Th2-type immune response characterized by an increase in parasite-induced IL-4 production from lymph node cells and parasite-specific serum IgG1 and total IgE levels was induced in wild-type mice (Fig. 5a). Infected global IL-4Rα-deficient mice constitutively produced significant levels of IL-4 although a slight antigen-specific response was induced, a parasite-specific IgG1 response was not elicited and no IgE could be detected (Fig. 5b). In contrast, some aspects of type 2 immunity induced in Lckcre IL-4Rα−/flox mice were similar to that observed within the control mice (Fig. 5c). Levels of parasite-induced IL-4 from lymph node cells and specific IgG1 were elevated, despite the absence of IL-4-responsive CD4+ T cells. Nevertheless, total IgE antibody responses were significantly reduced compared with control mice and although antigen-induced IL-4 levels in Lckcre IL-4Rα−/flox mice had increased by day 6 and were similar to control mice at this time, they were not significantly increased over values on day zero (Fig. 5b). Reduced levels of antigen-specific IgE have previously been reported in Lckcre IL-4Rα−/flox mice.13 The phenotypic characterization of Lckcre IL-4Rα−/flox mice demonstrated impaired proliferation and Th2 differentiation in response to IL-4.13 Consequently, the poor level of Th2 cells could explain the decreased total IgE antibody response because it is known that Th2 cells characteristically induce B cells to switch to produce IgG1 and IgE.25–27 However, unlike IgG1, IgE is strictly dependent on IL-4 signalling.28
Figure 5.
Intraepithelial invading helminths elicit a T helper type 2 (Th2) immune response independent of interleukin-4Rα (IL-4Rα) signalling on either CD4+ T cells or macrophages/neutrophils. Sera and mesenteric lymph nodes were removed from uninfected and infected (day 6 postinfection) (a) IL-4Rα−/flox, (b) global IL-4Rα−/−, (c) Lckcre IL-4Rα−/flox, (d) BALB/c and (e) LysMcre IL-4Rα−/flox mice and analysed for antibody and cytokine responses. Parasite-specific IL-4, immunoglobulin G1 (IgG1) and total IgE were measured and the data expressed as the mean value + SEM. Three to five mice were used per group. *P< 0·05, significantly different from naïve; ψP < 0·05, significantly different from BALB/c IL-4Rα−/flox and Lckcre IL-4Rα−/flox. ND, not detectable.
The absence of IL-4Rα expression on macrophages and neutrophils showed no influence on type 2 immunity following infections. As shown in Fig. 5, BALB/c (Fig. 5d) and LysMcre IL-4Rα−/flox (Fig. 5e) mice in response to T. spiralis provoked a significant IL-4 cytokine production day 6 postinfection. The response was followed by a marked elevation in type 2 antibody responses (Fig. 5d,e).
Neither Lckcre IL-4Rα nor LysMcre IL-4Rα-deficient mice demonstrated an increase in IL-13 and IFN-γ following infection (data not shown) compared with their wild-type counterparts.
Discussion
Expression of IL-4Rα on both bone-marrow-derived and non-bone-marrow-derived cells is required to induce expulsion of the GI nematode, T. spiralis.9 In this study, we provide evidence that the absence of IL-4Rα expression on CD4+ T cells and macrophages/neutrophils is not required for the development of protective immunity against T. spiralis. Mice specifically lacking IL-4/IL-4Rα signalling on CD4+ T cells and IL-4/IL-13 non-responsive macrophages/neutrophils induced a Th2-type immune response with intestinal mastocytosis and gut pathology reminiscent of resistant strains. The CD4+ T helper cells play a central role in protective immunity to the GI nematode T. spiralis.3–5,29 Following infection, these cells express Th2 cytokines in the mesenteric lymph node30 and are subsequently recruited to the gut mucosa.31 Functionally linked by their requirement for the IL-4Rα subunit,32 the Th2 cytokines IL-4 and IL-13 regulate protection against GI helminths within the mucosal habitat.1,33,34 Surprisingly, the absence of IL-4Rα expression on bone-marrow-derived T cells and mast cells is not required to contribute to IL-4-induced T. spiralis expulsion.7,34,35 Our findings specifically demonstrate that the abrogation of IL-4Rα signalling on CD4+ T cells is not a prerequisite for the induction of protective effector responses against this parasite. Recently generated Lckcre IL-4Rα−/flox mice have a null mutation of IL-4Rα on CD4+ T cells, an incomplete deletion on CD8+ T cells and other T-cell subpopulations, and normal expression on non-T cells.13 Nevertheless, Lckcre IL-4Rα−/flox mice in response to infection with T. spiralis, developed Th2-type immune responses, i.e. villus atrophy, crypt hyperplasia and intestinal mastocytosis, similar to those observed within the wild-type animals. Only T. spiralis-induced IgE production was substantially reduced in the absence of IL-4-responsive CD4+ T cells compared with wild-type animals. By comparison, no IgE production could be detected in infected global IL-4Rα−/− mice. Immunoglobulin E antibody production is strictly dependent on IL-4 signalling,28 highlighting the importance of IL-4Rα expression on B cells to induce antibody isotype class switching. Interestingly, Gurish et al.36 using IgE-deficient mice directly demonstrated that this antibody enhanced T. spiralis clearance and regulated mast cell responses in vivo. However, the level of jejunal mastocytosis was independent of IgE responses,36 which supports our findings that a significant reduction in IgE antibody does not influence intestinal mastocytosis.
Infection of global IL-4Rα-deficient mice induced an impaired Th2 immune response reminiscent of previous study findings.15,20 The absence of the IL-4Rα subunit was hallmarked by observations of delayed parasite expulsion, reduced parasite-specific IL-4 cytokine production, IgG1 secretion, intestinal mastocytosis and gut pathology. The complete absence of IL-4Rα responsiveness versus cell-specific deletion on CD4+ T cells suggests that non-CD4+ T-cell IL-4Rα-dependent responses are sufficient to induce protection against T. spiralis. The expression of IL-4Rα on non-haemopoietic cells is a general requirement for the expulsion of GI nematodes.9 Intestinal epithelial cells, smooth muscle cells37–39 and enteric nerve cells40 responding to IL-4 and/or IL-13 have previously been linked with the resolution of GI nematodes. However, because of the importance of these cytokines in the induction of protective responses, the individual contributions of non-bone-marrow-derived cells remain unknown. Recently, Horsnell et al., in response to N. brasiliensis,41 using a smooth muscle cell-specific IL-4Rα−/− mice (SM-MHCcre IL-4Rα−/lox mice), demonstrated that the absence of IL-4Rα signalling specifically on smooth muscle cells in response to N. brasiliensis led to dyscoordination of Th2 cytokine responses, goblet cell hyperplasia and acetylcholine responsiveness, which collectively drive smooth muscle contractions. Nippostrongylus brasiliensis-infected SM-MHCcre IL-4Rα−/lox mice elicited a delayed protective response and subsequent clearance of the gut dwelling nematode.41 That elegant study reiterates the importance of IL-4Rα expression on non-bone-marrow-derived cells and supports the growing evidence that no single non-bone-marrow-derived cell type is solely responsible for the expulsion of intestinal dwelling nematodes.2,42
Expulsion of T. spiralis, however, requires the additional dependence of IL-4Rα signalling on bone marrow-derived cells. The importance of IL-4Rα expression on T cells and mast cells has been excluded,9 while further investigation of receptor signalling on other BM cell types, such as natural killer cells and dendritic cells, have yet to be addressed. A role for T cells and mast cells remains pivotal because both cell types must be present to induce T. spiralis expulsion albeit in an IL-4-independent manner.7 Mice deficient for IL-4, which still produce IL-13, are effective at resolving T. spiralis infections7 yet neither T cells (CD4+ T cells) nor mast cells respond to IL-13 because both cell types lack the IL-13Rα1 subunit that is necessary to bind the cytokine.34,35
Interleukin-13 and mast cells are important for the development of protective responses and enteropathy to T. spiralis infection.20,43 However, IL-13 binding to IL-4Rα on either dendritic cells or macrophages has previously been proposed to inhibit IL-12 cytokine production, and suppression of mastocytosis.11,12 Nevertheless in this study, IL-4Rα-dependent protective immunity, in both wild-type and cell-specific IL-4Rα transgenic mice, was driven by the Th2 cytokine IL-4 while only residual levels of parasite-induced IL-13 were detected following infection. Both IL-4 and IL-13 are capable of inducing IL-4Rα-dependent immunity.7,20,44 The recent generation of LysMcre IL-4Rα−/flox mice has allowed us to address the contribution of IL-4Rα signalling specifically on macrophages and neutrophils while signalling on B and T lymphocytes as well as dendritic cells remains normal.14 Here, we have eliminated a disease-exacerbating role for IL-4/IL-13 responsiveness on macrophages and neutrophils by demonstrating that LysMcre IL-4Rα−/flox mice in response to T. spiralis infection develop a protective immune response. Expulsion of the GI nematode was accompanied by a robust Th2-type immune response and intestinal mastocytosis driven by IL-4. Similar resistance, independent of macrophage/neutrophil IL-4Rα expression has been demonstrated with N. brasiliensis.14 In response to N. brasiliensis, LysMcre IL-4Rα−/flox mice induce type 2 immunity with goblet cell hyperplasia equivalent to their littermate controls.14
Having eliminated the importance of IL-4Rα expression on T cells (CD4+ T cells), mast cells 34,35 and macrophages, we propose a role for receptor signalling on dendritic cells. Both IL-4 and IL-13 are known to regulate dendritic cell maturation in an IL-4Rα-dependent manner.45–47 This is particularly significant because dendritic cells control the Th1/Th2 balance of antigen-specific effector cells by responding to instructional signals from neighbouring cells at the site of infection.48 Mast cells, like dendritic cells, are strategically located at portals of pathogen entry and recent evidence identifying mast cell mediators responsible for modulating dendritic cell antigen-presenting and Th-polarizing functions49 provide a substantial argument that DCs are key to T. spiralis immunity. Furthermore, Feili-Hariri et al.50 demonstrated that bone-marrow-derived dendritic cells grown in the presence of granulocyte–macrophage colony-stimulating factor plus IL-4 strongly induce Th2 cells both in vitro and in vivo. However, in GI nematode infections the source of IL-4 inducing a protective phenotype has yet to be elucidated. Recently, we showed that mast-cell-derived IL-4 makes a major contribution to the generation and amplification of Th2 responses.50 An inter-relationship between mast cells and dendritic cells favourably supports previous hypotheses that mast cells produce the initial source of IL-4 for induction of a Th2 response.24 Given the constantly increasing list of biological substances that activate mast cells,51 it seems highly likely that GI helminths directly initiate the cascade of immune-mediated responses leading to their expulsion.
In summary, we have eliminated a role for IL-4Rα responsiveness on CD4+ T cells and macrophages/neutrophils in resolving T. spiralis infections. Furthermore, we demonstrated that neither IL-4/IL-4Rα signalling on CD4+ T cells nor IL-4/IL-13 responsiveness on macrophages/neutrophils are required to induce Th2-type immune responses to GI nematodes.
Acknowledgments
The authors would like to thank Dr W. Horsnell for his critical reading of the manuscript. This work was supported in part by the Royal Society of the United Kingdom and National Research Foundation of South Africa.
References
- 1.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence CE. Is there a common mechanism of gastrointestinal nematode expulsion? Parasite Immunol. 2003;25:271. doi: 10.1046/j.1365-3024.2003.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Grencis RK, Riedlinger J, Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985;56:213. [PMC free article] [PubMed] [Google Scholar]
- 4.Manson-Smith DF, Bruce RG, Parrott DM. Villous atrophy and expulsion of intestinal Trichinella spiralis are mediated by T cells. Cell Immunol. 1979;47:285. doi: 10.1016/0008-8749(79)90338-1. [DOI] [PubMed] [Google Scholar]
- 5.Garside P, Grencis RK, Mowat AM. T lymphocyte dependent enteropathy in murine Trichinella spiralis infection. Parasite Immunol. 1992;14:217. doi: 10.1111/j.1365-3024.1992.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 6.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 7.Urban JF, Jr, Schopf L, Morris SC, et al. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 8.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 9.Urban JF, Jr, Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol. 2001;167:6078. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]
- 10.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387. [PubMed] [Google Scholar]
- 11.Finkelman FD, Madden KB, Cheever AW, et al. Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370. [PubMed] [Google Scholar]
- 13.Radwanska M, Cutler AJ, Hoving JC, et al. Deletion of IL-4Ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 2007;3:e68. doi: 10.1371/journal.ppat.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 15.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302. [PubMed] [Google Scholar]
- 16.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 17.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 18.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence CE, Paterson JC, Higgins LM, MacDonald TT, Kennedy MW, Garside P. IL-4-regulated enteropathy in an intestinal nematode infection. Eur J Immunol. 1998;28:2672. doi: 10.1002/(SICI)1521-4141(199809)28:09<2672::AID-IMMU2672>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Scales HE, Ierna MX, Lawrence CE. The role of IL-4, IL-13 and IL-4Ralpha in the development of protective and pathological responses to Trichinella spiralis. Parasite Immunol. 2007;29:81. doi: 10.1111/j.1365-3024.2006.00920.x. [DOI] [PubMed] [Google Scholar]
- 21.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453. [PubMed] [Google Scholar]
- 22.Bancroft AJ, Artis D, Donaldson DD, Sypek JP, Grencis RK. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur J Immunol. 2000;30:2083. doi: 10.1002/1521-4141(200007)30:7<2083::AID-IMMU2083>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol. 1996;8:559. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Croft M, Swain S. B cell response to T helper cell subsets. II. Both the stage of T cell differentiation and the cytokines secreted determine the extent and nature of helper activity. J Immunol. 1991;147:3679. [PubMed] [Google Scholar]
- 26.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4) -producing cell in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4 producing cells. J Exp Med. 1990;172:921. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cell receptor transgenic mice. J Exp Med. 1992;176:1091. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 29.Urban JF, Jr, Katona IM, Finkelman FD. Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol. 1991;73:500. doi: 10.1016/0014-4894(91)90074-7. [DOI] [PubMed] [Google Scholar]
- 30.Grencis RK, Hultner L, Else KJ. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991;74:329. [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott JR, Grencis RK, Else KJ. Leucocyte recruitment during enteric nematode infection. Immunology. 2001;103:505. doi: 10.1046/j.1365-2567.2001.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 33.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 34.Finkelman FD, Wynn TA, Donaldson DD, Urban JF. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol. 1999;11:420. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Nakajima H, Watanabe N, Kagami S, Suto A, Saito Y, Saito T, Iwamoto I. Role of common cytokine receptor gamma chain (gamma(c))- and Jak3-dependent signaling in the proliferation and survival of murine mast cells. Blood. 2000;96:2172. [PubMed] [Google Scholar]
- 36.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 37.Castro GA, Badial-Aceves F, Smith JW, Dudrick SJ, Weisbrodt NW. Altered small bowel propulsion associated with parasitism. Gastroenterology. 1976;71:620. [PubMed] [Google Scholar]
- 38.Vallance BA, Blennerhassett PA, Collins M. Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am J Physiol. 1997;272:G321. doi: 10.1152/ajpgi.1997.272.2.G321. [DOI] [PubMed] [Google Scholar]
- 39.Vermillion DL, Collins M. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am J Physiol. 1988;254:G124. doi: 10.1152/ajpgi.1988.254.1.G124. [DOI] [PubMed] [Google Scholar]
- 40.Shea-Donohue T, Sullivan CA, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF., Jr The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol. 2001;167:2234. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 41.Horsnell WG, Cutler AJ, Hoving JC, et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Rα-deficient mice. PLoS Pathog. 2007;3:0046. doi: 10.1371/journal.ppat.0030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 43.McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol. 2005;175:3207. doi: 10.4049/jimmunol.175.5.3207. [DOI] [PubMed] [Google Scholar]
- 44.Garside P, Kennedy MW, Wakelin D, Lawrence CE. Immunopathology of intestinal helminth infection. Parasite Immunol. 2000;22:605. doi: 10.1046/j.1365-3024.2000.00344.x. [DOI] [PubMed] [Google Scholar]
- 45.Ahn JS, Agrawal B. IL-4 is more effective than IL-13 for in vitro differentiation of dendritic cells from peripheral blood mononuclear cells. Int Immunol. 2005;17:1337. doi: 10.1093/intimm/dxh312. [DOI] [PubMed] [Google Scholar]
- 46.Alters SE, Gadea JR, Holm B, Lebkowski J, Philip R. IL-13 can substitute for IL-4 in the generation of dendritic cells for the induction of cytotoxic T lymphocytes and gene therapy. J Immunother (1997) 1999;22:229. doi: 10.1097/00002371-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Lutz MB, Schnare M, Menges M, Rossner S, Rollinghoff M, Schuler G, Gessner A. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. J Immunol. 2002;169:3574. doi: 10.4049/jimmunol.169.7.3574. [DOI] [PubMed] [Google Scholar]
- 48.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 49.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J Immunol. 2006;177:3577. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 50.Feili-Hariri M, Falkner DH, Morel PA. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol. 2005;78:656. doi: 10.1189/jlb.1104631. [DOI] [PubMed] [Google Scholar]
- 51.Metz M, Maurer M. Mast cells – key effector cells in immune responses. Trends Immunol. 2007;28:234. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]