Abstract
Gene gun-mediated biolistic DNA vaccination with β-galactosidase (βGal)-encoding plasmid vectors efficiently modulated antigen-induced immune responses in an animal model of type I allergy, including the inhibition of immunoglobulin E (IgE) production. Here we show that CD4+ as well as CD8+ T cells from mice biolistically transfected with a plasmid encoding βGal under the control of the fascin promoter (pFascin-βGal) are capable of inhibiting βGal-specific IgE production after adoptive transfer into naïve recipients. Moreover, suppression of IgE production was dependent on interferon (IFN)-γ. To analyse the modalities of activation of CD4+ and CD8+ T cells regarding the localization of antigen synthesis following gene gun-mediated DNA immunization, we used the fascin promoter and the keratin 5 promoter (pK5-βGal) to direct βGal production mainly to dendritic cells (DCs) and to keratinocytes, respectively. Gene gun-mediated DNA immunization with each vector induced considerable activation of βGal-specific CD8+ cytotoxic T cells. Cytokine production by re-stimulated CD4+ T cells in draining lymph nodes and immunoglobulin isotype profiles in sera of immunized mice indicated that immunization with pFascin-βGal induced a T helper type 1 (Th1)-biased immune response, whereas immunization with pK5-βGal generated a mixed Th1/Th2 immune response. Nevertheless, DNA vaccination with pFascin-βGal and pK5-βGal, respectively, efficiently inhibited specific IgE production in the mouse model of type I allergy. In conclusion, our data show that uptake of exogenous antigen produced by keratinocytes and its presentation by untransfected DCs as well as the presentation of antigen synthesized endogenously in DCs represent equivalent pathways for efficient priming of cellular immune responses.
Keywords: dendritic cells, fascin promoter, gene gun-mediated DNA vaccination, keratin 5 promoter, keratinocytes, type I allergy
Introduction
Gene gun-mediated bombardment of the skin with naked antigen-encoding plasmid DNA adsorbed on gold microparticles – an in vivo gene transfer technique known as particle-mediated epidermal delivery (PMED) – has been established in various preclinical animal models as an efficient and reliable method of genetic vaccination to induce transgene-specific immune responses.1 Moreover, PMED was shown in clinical phase I studies to represent a DNA vaccine delivery method that was well tolerated and consistently induced significant humoral and cellular immune responses,2 whereas intramuscular and intradermal injection of DNA in saline as alternative routes of DNA immunization often proved insufficient to provide protective immunity in human trials and is therefore considered to be clinically ineffective.3
Numerous reports have substantiated the notion that dendritic cells (DCs) of the skin are crucial for the induction of immune responses in draining lymph nodes after cutaneous DNA immunization irrespective of the inoculation technique,4–7 although it has become a matter of discussion whether epidermal Langerhans cells or dermal DC populations are the principal antigen-presenting cells (APCs) under these circumstances.8–10
We obtained evidence supporting the notion of the pivotal role of DCs by focussing antigen production primarily to DCs after biolistic transfection of mouse skin using the promoter of the gene of the cytoskeletal actin-bundling protein fascin to drive transgene expression. During the differentiation process from immature DCs to primary stimulatory mature DCs, expression of the fascin gene was strongly upregulated.11,12 As a consequence, fascin, which is mandatory for the formation of dendrites,11 was synthesized in large amounts by mature DCs.11–13 In contrast, expression of the fascin gene in non-transformed cell types other than DCs could rarely be detected and was restricted to neuronal tissues, capillary endothelial cells and follicular DCs.11,14,15 In earlier studies we verified that the activity of the promoter of the fascin gene reflects the endogenous production of fascin, being high in mature DCs but negligible in immature DCs and in keratinocytes.16,17 Hence, gene gun immunization of mice with plasmid DNA carrying the fascin promoter as the regulatory element transcriptionally targeted transgene expression to skin-derived DCs.17 Subsequently, distinct type 1 immune responses were induced, including the potent activation of interferon (IFN)-γ-producing CD4+ T helper type 1 (Th1) cells as well as IFN-γ-producing CD8+ cytotoxic T lymphocytes (CTLs).17,18
These results are consistent with a number of publications which suggest that endogenous production of antigen by directly transfected DCs of the skin is sufficient to induce immune responses, in particular the generation of CD8+ CTLs.4,5,19 In contrast, several authors have concluded from their data that cross-presentation of antigen, produced and released by transfected cutaneous non-DCs, represents the main pathway of CD8+ T-cell priming following gene gun immunization.20–22 In an attempt to resolve this apparent discrepancy, we compared the immune responses initiated by biolistic transfection of skin with plasmids expressing the transgene β-galactosidase (βGal) under the control of the fascin promoter (pFascin), the keratinocyte-specific keratin 5 promoter (pK5) or the ubiquitously active cytomegalovirus immediate early promoter (pCMV). Employing this approach, we restrict βGal production selectively to DCs as APCs or to keratinocytes as non-APCs or to a rather broad variety of epidermal/dermal cells. In this paper, we show that presentation of antigen endogenously synthesized by directly transfected DCs and presentation of antigen by DCs that have taken up and processed exogenous protein are equivalent pathways for maximum stimulation of cellular immune responses. However, the quality of the Th cell immune response was different following DC-targeted and keratinocyte-specific biolistic transfection. Furthermore, we demonstrate that the inhibitory activity of biolistic transfection on the induction of an antigen-specific immunoglobulin E (IgE) response, which we show here to be dependent on IFN-γ production and exerted by CD4+ and CD8+ T cells, can be mediated by either of the two pathways of antigen presentation.
Materials and methods
Mice
BALB/c mice, C57BL/6 mice, interleukin (IL)-12p40−/− mice and IFN-γ−/− mice were bred and maintained in the Central Animal Facilities of the University of Mainz in specific pathogen-free conditions on a standard diet. The ‘Principles of laboratory animal care’ (NIH publication no. 85-23, revised 1985) were followed. The experiments were approved by the Ethics Commission according to the German Law on the Protection of Animals.
Plasmid vectors and DNA immunization protocol
The construction and purification of plasmid vectors encoding the transgene βGal under control of the fascin promoter (pFascin-βGal) and the cytomegalovirus (CMV) promoter (pCMV-βGal), respectively, have been described in detail previously.17,18
To obtain pK5-βGal and pK5-EGFP, parental vector pUC19-K523 was digested with SalI, which cuts immediately downstream of the bovine K5 promoter in this construct. Expression cassettes for βGal and enhanced green fluorescent protein (EGFP) encompassing the respective reporter gene and an SV40 polyadenylation signal were excised by restriction digest from pCMV-βGal (BamHI) and pCMV-EGFP (Acc65I/BamHI). 5′-protruding ends of linearized pUC19-K5 and of excised expression cassettes were filled in using Klenow enzyme, and expression cassettes were ligated with pUC19-K5. The functional activity of derived reporter expression constructs pK5-βGal and pK5-EGFP was validated by in vitro transfection.
Genetic immunization was performed by biolistic transfection with a total of 4 μg of plasmid DNA using the helium-driven Helios gene gun system (Bio-Rad, Munich, Germany) as described previously.18 Mice were immunized at weekly intervals three to five times as indicated.
Immunization protocol for the induction of IgE production
High and persistent βGal-specific IgE production was induced as previously described.24 Briefly, female BALB/c mice, 6–8 weeks of age at the beginning of the experiments, were sensitized by multiple intraperitoneal injections of 1 μg of recombinant βGal (Sigma-Aldrich, Deisenhofen, Germany) at intervals of 2 weeks. βGal protein was dissolved in 100 μl of phosphate-buffered saline (PBS) and adsorbed to an equal volume of Imject® Alum (Pierce, Rockford, IL) as adjuvant (βGal/alum) according to the manufacturer’s instructions.
Adoptive transfer of lymphocytes
BALB/c mice were immunized via the gene gun as described above by five consecutive applications of pFascin-βGal at weekly intervals. Two weeks after the last immunization spleen cells were prepared as described previously25 and pooled. CD4+ and CD8+ T cells in spleen cell suspensions from gene gun-immunized as well as from βGal/alum-injected control mice were enriched by magnetic separation as previously described.26 The purity of positively selected T-cell populations was verified by cytofluorometric analysis and was 83 ± 4% for CD4+ T cells and 89 ± 5% for CD8+ T cells; the frequency of the remaining T cells of the reciprocal subpopulation was below 2%. Unseparated spleen cells (107) or isolated CD4+ and CD8+ cells (2·5 × 106), respectively, were injected intravenously into naïve syngeneic mice. After 24 hr, recipients and control mice, which did not receive donor cells, were sensitized with βGal/alum as described above.
Measurement of serum antibodies
At the indicated time-points, immunized mice were bled by puncture of the retro-orbital plexus. Sera were collected and kept frozen at −20° until thawed for determination of βGal-specific IgG1, IgG2a and IgE by antigen capture enzyme-linked immunosorbent assay (ELISA) as reported previously.24 For comparison of samples obtained at different times in the course of immunization, IgG1, IgG2a and IgE contents in sera were standardized using high-titre reference sera, collected from mice repeatedly immunized with βGal/alum (IgE) or from mice initially vaccinated with pCMV-βGal and subsequently boosted with βGal/alum (IgG1, IgG2a). The antibody titre was defined as the reciprocal serum dilution yielding an absorbance reading of optical density (OD) = 0·2 after linear regression analysis. The titre of the reference serum was designated as 1 U/ml and the titres of the serum samples are presented as equivalent units. In experiments in which the relative contents of specific IgG1 and IgG2a in sera were directly compared, the titres were determined in ELISAs performed in parallel and the ratio of the two IgG isotypes was calculated as the quotient of their titres.
Measurement of CD8+ CTLs
The frequency of IFN-γ-producing CD8+ effector T cells recognizing the H-2Ld-binding βGal-derived nonamer peptide TPHPARIGL (βGal876–884; Sigma-Aldrich) in single-cell suspensions of spleen cells was determined by an enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) as previously described24 with the modification that 100 ng/ml βGal876–884 was used as the standard concentration to activate CD8+ T cells. Spots were counted and evaluated using the EliSpot Reader System (AID, Strasberg, Germany).
The cytotoxic activity of spleen cells, prepared from immunized mice, against LacZ-transfected P13·1 target cells and untransfected P815 control cells was determined using the JAM test as described previously.18 The difference between the two values was calculated and designated as specific lysis.
Determination of IL-5 and IFN-γ in culture supernatants
Draining lymph node cells (LNCs) of biolistically transfected mice (axillary and inguinal lymph nodes) and of mice immunized with βGal/alum (mesenterial lymph nodes) were prepared and incubated for 72 hr on 24-well tissue culture plates (5 × 106 LNCs/well) (Corning Costar, Bodenheim, Germany) in a volume of 1 ml of culture medium with or without recombinant βGal (25 μg/ml). Subsequently, contents of IL-5 and IFN-γ were quantified in culture supernatants using a sandwich ELISA following a standard procedure as described previously.18
Statistical analysis
Statistical evaluation of the experimental data was performed by Student’s t-test using SigmaStat software (SPSS Inc., Chicago, IL). Statistically significant differences (P < 0·05) between control groups and experimental groups are designated by symbols.
Results
Inhibition of specific IgE production by gene gun immunization is mediated by CD4+ as well as CD8+ T cells and depends on IFN-γ production
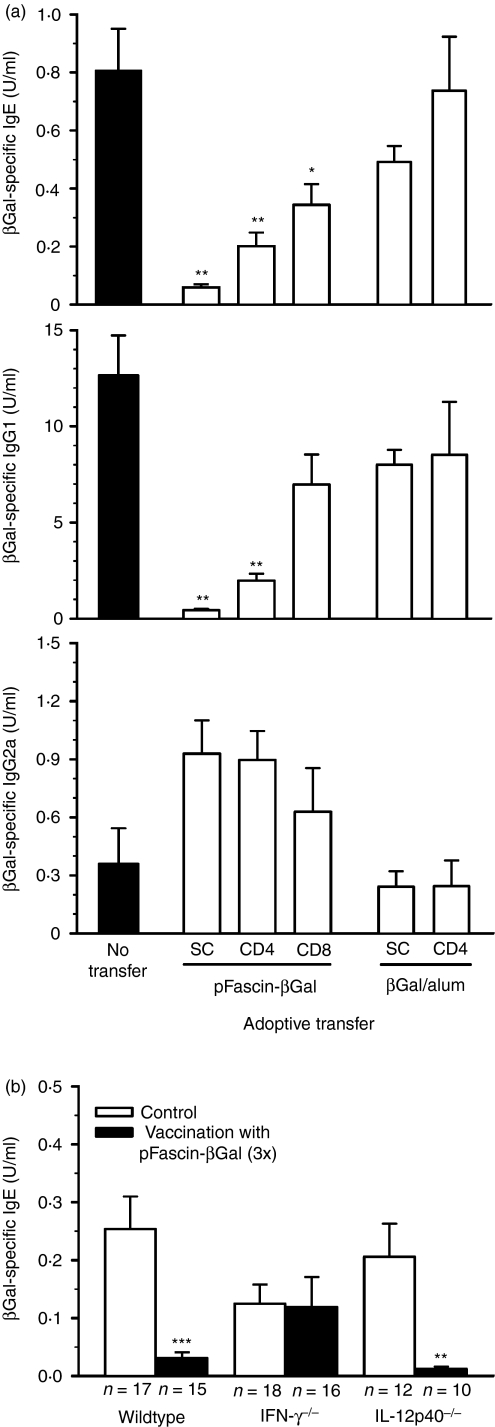
We previously showed that gene gun-mediated DNA vaccination with plasmid vectors encoding βGal under the control of the fascin promoter (pFascin-βGal) effectively inhibited subsequently induced specific IgE immune responses in a mouse model of type I allergy.27 The suppression of IgE production was associated with a shift towards a Th1-biased immune response as well as with the recruitment of large numbers of CD8+ CTLs.27 The inhibitory capacity can be adoptively transferred to naïve recipients using spleen cells from BALB/c mice previously immunized with pFascin-βGal (Fig. 1a). Despite sensitization with βGal/alum following adoptive transfer, the recipient mice produced strongly reduced levels of IgE and also IgG1 as compared with mice without cell transfer. Furthermore, IgG2a levels were substantially increased although the differences did not reach statistical significance. As a consequence, the ratio of βgal-specific IgG1 versus IgG2a titres dropped drastically (IgG1:IgG2a ratio: no transfer 76·7 ± 16·5 versus transfer of pFascin-βGal spleen cells 0·8 ± 0·2; P < 0·01). This finding is consistent with the situation prevailing in mice prophylactically vaccinated with pFascin-βGal and subsequently sensitized with βGal protein.27 In contrast, spleen cells collected from BALB/c mice sensitized with βGal/alum and thus exhibiting high titres of IgE in the sera (data not shown) were not able to significantly suppress the formation of IgE or to change the pattern of IgG isotypes (IgG1:IgG2a ratio: transfer of βGal/alum spleen cells 64·5 ± 29·2; P > 0·05 versus no transfer) upon adoptive transfer. In order to determine whether CD4+ or CD8+ T cells are the principal mediators of regulation, the two T-cell subpopulations were enriched by magnetic separation from spleen cells of mice biolistically transfected with pFascin-βGal and were subsequently transferred into naïve mice. Injection of either CD4+ or CD8+ T cells prior to sensitization with βGal/alum was sufficient to significantly reduce βGal-specific IgE production in the recipients (Fig. 1a). However, the inhibitory activity of each subpopulation was lower than that provided by total spleen cells, suggesting that both CD4+ and CD8+ T cells were required to efficiently down-modulate the IgE immune response. In contrast, modulation of IgG production towards a Th1-skewed isotype pattern could mainly be attributed to CD4+ T cells (IgG1:IgG2a ratio: transfer of pFascin-βGal CD4+ T cells 3·1 ± 0·3; P < 0·01 versus no transfer; transfer of pFascin-βGal CD8+ T cells 26·6 ± 10·2; P < 0·05 versus no transfer). CD4+ T cells isolated from mice immunized with βGal/alum did not show any potency in suppression of the IgE response and did not affect IgG formation (IgG1:IgG2a ratio: transfer of βGal/alum CD4+ T cells 90·2 ± 23·0; P > 0·05 versus no transfer) following adoptive transfer (Fig. 1a).
Figure 1.
Inhibition of specific immunoglobulin E (IgE) production by gene gun-mediated DNA vaccination is mediated by CD4+ and CD8+ T cells and depends on interferon (IFN)-γ production. (a) BALB/c mice were immunized five times at weekly intervals by biolistic transfection with pFascin-βGal or by intraperitoneal injection of β-galactosidase (βGal)/alum. Fourteen days after the last application, spleen cells (SC) and splenic CD4+ and CD8+ T cells were prepared and injected intravenously into naïve BALB/c mice. Recipients and control mice without transfer of cells (n = 4–5) were sensitized by intraperitoneal injection of βGal/alum every 2 weeks. Ten days after the fifth injection mice were bled and βGal-specific IgE, IgG1 and IgG2a titres were determined by enzyme-linked immunosorbent assay (ELISA). Data represent mean titres ± the standard error of the mean (SEM) for individual mice. Significant differences are indicated by asterisks (*P < 0·05, **P < 0·01 versus control mice). (b) C57BL/6 wild-type, IFN-γ−/− and interleukin (IL)-12p40−/− mice were vaccinated three times at weekly intervals by biolistic transfection with pFascin-βGal. Vaccinated mice and unvaccinated controls were sensitized with βGal/alum as described. Mice were bled after the fifth injection of βGal/alum and βGal-specific IgE titres in sera were determined by ELISA. Data represent mean titres ± SEM of the indicated numbers of individual sera obtained in three independent experiments. Significant differences are indicated by asterisks (**P < 0·01 and ***P < 0·001 versus unvaccinated mice).
CD4+ and CD8+ T cells from mice biolistically transfected with pFascin-βGal secrete large amounts of IFN-γ.18,27 To check whether the production of this cytokine is crucial for the inhibitory effect of gene gun vaccination with pFascin-βGal on βGal/alum-induced IgE production, C57BL/6 wild-type, IFN-γ−/− and IL-12p40−/− mice were biolistically vaccinated three times with pFascin-βGal, and the amount of IgE in the sera after the fifth subsequent immunization with βGal/alum was determined. Although C57BL/6 wild-type mice produced lower amounts of βGal-specific IgE compared with BALB/c mice (data not shown), they proved to be susceptible to the inhibitory effect of biolistic transfection with pFascin-βGal on the IgE immune response (Fig. 1b). Similarly, in gene gun-vaccinated IL-12p40−/− mice, which were not capable of producing bioactive IL-12, βGal-specific IgE levels in sera were significantly reduced. In contrast, suppression of IgE production was not observed in IFN-γ-deficient mice, indicating that this cytokine plays a crucial role in this regulation process.
Presentation of endogenous antigen and cross-presentation of exogenous antigen are equivalent pathways for induction of CD8+ CTL after gene gun immunization
Our previous data, obtained by gene gun-mediated DNA immunization with vectors encoding an antigen under the control of the fascin promoter, suggest that endogenous antigen production by directly transfected DCs of the skin is sufficient to induce potent CD4+ and CD8+ T-cell responses.18 However, these studies did not clarify whether this pathway was the only one or at least the principal one responsible for the priming of immune responses after biolistic transfection with pCMV, which instructs broad cutaneous antigen synthesis by DCs as well as by non-DCs. Thus the uptake of exogenous antigen released by transfected non-DCs and its processing and presentation by untransfected DCs might represent an alternative pathway to stimulate cellular immune responses. To analyse the modalities of activation of CD4+ and CD8+ T cells with inhibitory potential for IgE production with respect to the localization of antigen synthesis following gene gun-mediated DNA immunization, we sought to direct transgene expression selectively to non-DCs of the skin by constructing plasmids that encode βGal or EGFP under the control of the keratin 5 promoter (pK5-βGal and pK5-EGFP), which is specifically active in keratinocytes.23,28 After biolistic transfection of abdominal skin with these vectors we observed strong and extensive transgene expression in hair follicles and, at a lower frequency, weakly transgene-positive areas in the basal epidermis (data not shown). In contrast to application of particle bombardment with pFascin or pCMV18,25,27 we did not detect transgene-expressing cells in the draining lymph nodes (data not shown).
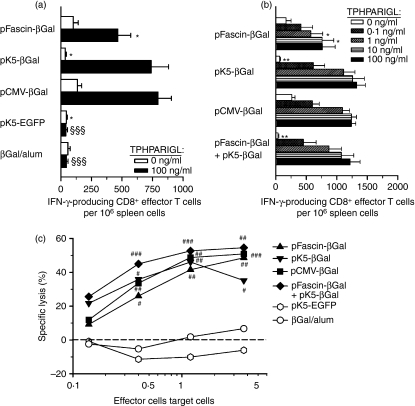
To measure the induction of βGal-specific CD8+ T cells in mice immunized via the gene gun with the different plasmid constructs, spleen cells were stimulated with the βGal-derived H-2Ld-restricted nonamer peptide TPHPARIGL. The secretion of IFN-γ by activated CD8+ T cells was determined by an ELISPOT. Immunization of mice with each of the three plasmids pFascin-βGal, pCMV-βGal and pK5-βGal induced substantial numbers of IFN-γ-producing CD8+ effector T cells, although in the case of pFascin-βGal their frequency tended to be somewhat lower (Fig. 2a). However, the difference in the number of CD8+ effector T cells arising from gene gun-mediated immunization with pFascin-βGal or pK5-βGal was not statistically significant (P = 0·137). In contrast, repeated injection of βGal/alum did not generate βGal-specific CD8+ T-cell responses, and neither did gene gun-mediated immunization with an EGFP-encoding control plasmid. Furthermore, in vitro activation of CD8+ T cells from mice primed in vivo by biolistic transfection with the three different βGal-encoding vectors, using decreasing peptide concentrations, resulted in comparably reduced numbers of IFN-γ-producing CD8+ effector T cells, suggesting similar avidity of these cells (Fig. 2b). Moreover, co-application of pFascin-βGal and pK5-βGal, resulting in presentation of endogenous antigen by directly transfected DCs and cross-presentation of exogenous antigen produced by non-DCs, did not lead to enhanced induction of IFN-γ-producing CD8+ effector T cells, indicating that each of the antigen presentation modalities led to maximum stimulation of the CD8+ T-cell response (Fig. 2b).
Figure 2.
Efficient priming of cytotoxic T lymphocyte (CTL) responses following gene gun-mediated DNA immunization targeting predominantly dendritic cells (DCs) or keratinocytes. BALB/c mice (n = 4) were immunized three times by biolistic transfection with the indicated plasmids or by intraperitoneal injection of β-galactosidase (βGal)/alum. (a, b) Frequencies of interferon (IFN)-γ-producing CD8+ effector T cells among pooled splenocytes were determined in triplicate by enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) 7–14 days after the last application by stimulation of the collected spleen cells for 22 hr with (closed and hatched bars) or without (open bars) the indicated concentrations of the H-2Ld-specific βGal peptide TPHPARIGL. Data represent the mean ± standard error of the mean (SEM) for cell numbers obtained in five (a) and two (b) independent experiments. Significant differences are indicated by symbols (*P < 0·05 and **P < 0·01 versus mice immunized with pCMV-βGal; §§§P < 0·001 versus mice immunized with βGal-encoding plasmids). (c) βGal-specific cytolytic activity of pooled spleen cells from immunized mice was determined in triplicate 7–14 days after the last application by JAM test. Data represent means of specific lysis obtained in three to six independent experiments. Significant differences are indicated by symbols (#P < 0·05, ##P < 0·01 and ###P < 0·001 versus mice immunized with pK5-EGFP). EGFP, enhanced green fluorescent protein; pCMV, plasmid vector harbouring the cytomegalovirus immediate early promoter.
In addition, the presence of cytolytically active CTLs among spleen cells of biolistically transfected mice was verified by determination of the βGal-specific cytotoxic potential of re-stimulated CD8+ T cells, expanded by cultivation of splenocytes with the cognate major histocompatibility complex (MHC) class I-restricted peptide. Consistent with the data obtained by the ELISPOT, spleen cells from mice immunized with the three βGal-encoding plasmids all displayed high specific cytolytic activity, whereas spleen cells from mice immunized with βGal/alum and with the control vector pK5-EGFP, respectively, did not mediate killing of βGal-expressing target cells (Fig. 2c). Specific lysis induced by spleen cells obtained after co-application of pFascin-βGal and pK5-βGal was slightly higher than the lysis induced by spleen cells from mice immunized with either of the two vectors; however, this difference did not reach statistical significance (Fig. 2c).
T-cell responses induced by gene gun-mediated DC-targeted and keratinocyte-targeted transgene expression, respectively, differ in their cytokine profile and antibody isotype pattern
We evaluated the quality of the antigen-specific T-cell response after biolistic DNA immunization with the three βGal-encoding plasmids by measuring the production of the cytokines IFN-γ and IL-5, representative for type 1 and type 2 responses, respectively, in cultures of draining LNCs activated in vitro by the addition of exogenous βGal protein. Because the isotype pattern of the humoral response serves as an indicator for the type of T-cell help supporting an immune reaction, we verified the cytokine data by determining the relative distribution of βGal-specific IgG subtypes and directly comparing IgG2a versus IgG1 titres in the sera of immunized animals.
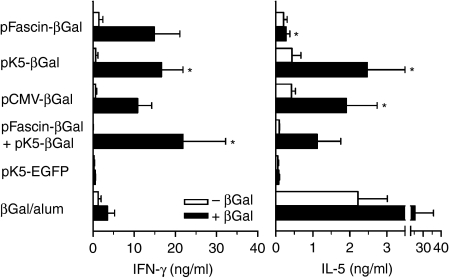
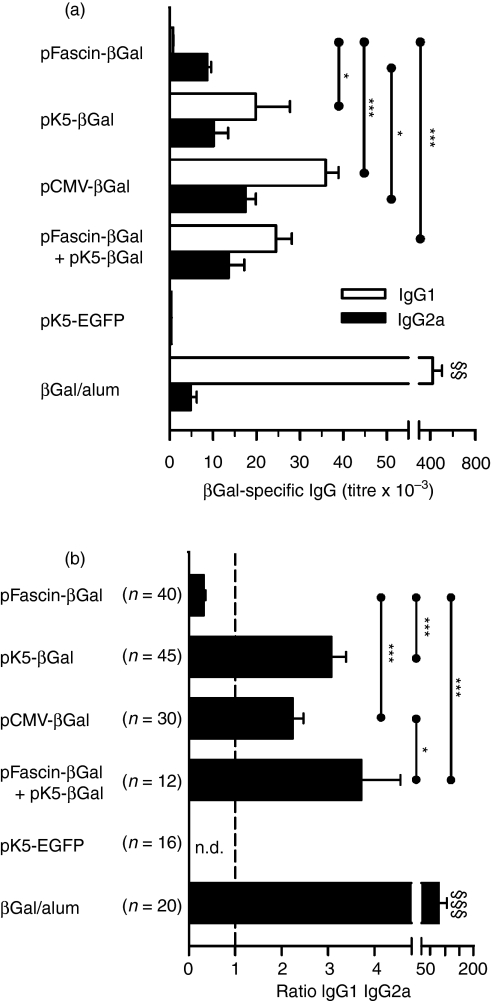
LNCs from axillary and inguinal lymph nodes of mice immunized with pFascin-βGal produced large amounts of IFN-γ but no IL-5 after re-stimulation with βGal (Fig. 3). In addition, specific antibody production in these mice was low and mainly of the IgG2a isotype (Fig. 4), which together are indicative of a distinct Th1 immune response. In contrast, LNCs from animals primed by biolistic transfection with pK5-βGal or pCMV-βGal in addition to IFN-γ secreted considerable amounts of IL-5 into culture supernatants, which points to a mixed Th1/Th2 immune response (Fig. 3). Similarly, co-application of pFascin-βGal and pK5-βGal resulted in synthesis of IFN-γ as well as IL-5 by the corresponding LNCs (Fig. 3). The notion of a mixed Th1/Th2 immune response in mice immunized with either pK5-βGal or pCMV-βGal or with pFascin-βGal and pK5-βGal at the same time was further supported by the isotype pattern determined in the sera of these mice. Comparable IgG2a titres concomitant with strongly increased levels of IgG1 were observed, pointing to a relative preponderance of the latter isotype (Fig. 4). However, intraperitoneal injection of βGal/alum predominantly recruited typical Th2 cells, which characteristically produced large quantities of IL-5, but failed to secrete IFN-γ upon re-stimulation (Fig. 3). Consistent with these data, a strongly Th2-polarized immune response of these mice was also indicated by a vast excess of IgG1 over IgG2a production (Fig. 4). Moreover, it is notable that the level of IgG1 production after immunization with βGal/alum is a multiple of the level of IgG1 production resulting from gene gun-mediated DNA immunization with βGal-encoding vectors.
Figure 3.
Cytokine profiles of lymph node cells (LNCs) after gene gun-mediated DNA immunization targeting predominantly dendritic cells (DCs) or keratinocytes are qualitatively different. BALB/c mice (n = 4) were immunized three times by biolistic transfection with the indicated plasmids or by intraperitoneal injection of β-galactosidase (βGal)/alum. Draining LNCs were prepared 7–14 days after the last application and cultured in vitro for 3 days with (closed bars) or without (open bars) βGal protein. Interferon (IFN)-γ and interleukin (IL)-5 in supernatants were quantified in triplicate using a sandwich enzyme-linked immunosorbent assay (ELISA). Data represent the mean ± standard error of the mean (SEM) for cytokine content determined in two to five independent experiments. Significant differences are indicated by asterisks (*P < 0·05 versus mice immunized with βGal/alum). EGFP, enhanced green fluorescent protein; pCMV, plasmid vector harbouring the cytomegalovirus immediate early promoter.
Figure 4.
Humoral immune responses after gene gun-mediated DNA immunization targeting predominantly dendritic cells (DCs) or keratinocytes are qualitatively different. BALB/c mice were immunized three times by biolistic transfection with the indicated plasmids or by intraperitoneal injection of β-galactosidase (βGal)/alum. Mice were bled 7–14 days after the last application and (a) βGal-specific immunoglobulin G1 (IgG1) titres (open bars) and IgG2a titres (closed bars) were determined by enzyme-linked immunosorbent assay (ELISA). Data represent the mean titre ± the standard error of the mean (SEM) for individual mice (n = 4). Data are representative of three to six independent experiments. The ratios of βGal-specific IgG1 and IgG2a titres, calculated for individual sera, are depicted in (b). Data represent the mean value ± SEM for the indicated numbers of mice. Significant differences are indicated by symbols (*P < 0·05 and ***P < 0·001 between mice immunized with βGal-encoding plasmids; §§P < 0·01 and §§§P < 0·001 versus mice immunized with βGal-encoding plasmids). EGFP, enhanced green fluorescent protein; pCMV, plasmid vector harbouring the cytomegalovirus immediate early promoter.
Specific IgE and IgG1 production is effectively suppressed by gene gun-mediated transgene expression targeted to DCs as well as to keratinocytes, whereas IgG2a secretion is enhanced
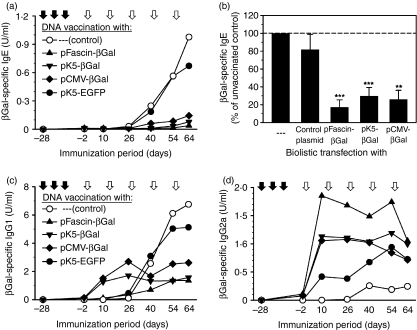
It was of interest to evaluate whether the immune response initiated by gene gun-mediated production of antigen by non-DCs is as effective in its impact on the induction of a subsequent antigen-specific IgE response as is DC-specific transgene expression. Therefore, BALB/c mice were vaccinated with the βGal-encoding vectors as indicated in Fig. 5 prior to sensitization by repeated intraperitoneal injection of βGal/alum. Sera were taken at distinct days throughout the sensitization period and contents of βGal-specific IgE as well as IgG1 and IgG2a in sera were designated as reference serum titre equivalents (U/ml), which allowed for proper comparison of samples obtained at different points in time and tested in multiple ELISAs. As depicted in Fig. 5a and 5b, production of βGal-specific IgE was suppressed by vaccination with each of the βGal-encoding plasmids as compared with the control plasmid (pK5-EGFP) and the untreated controls. The suppression was manifest already after the second injection of βGal/alum and was maintained throughout the sensitization period (Fig. 5a). Consequently, IgE titres after the fifth application of βGal/alum were significantly reduced in mice biolistically transfected with βGal-encoding plasmid DNA to 20–30% of IgE titres measured in unvaccinated control mice (Fig. 5b).
Figure 5.
Prophylactic gene gun-mediated DNA vaccination targeting predominantly dendritic cells (DCs) and keratinocytes, respectively, efficiently reduces immunoglobulin E (IgE) and IgG1 production, but enhances IgG2a production in a mouse model of type I allergy. BALB/c mice (n = 5) were vaccinated by three biolistic transfections (closed arrows) with the indicated plasmids or were left untreated as a control. Subsequently mice were sensitized by intraperitoneal injection of β-galactosidase (βGal)/alum every 2 weeks (open arrows). On various days of the immunization period βGal-specific IgE titres (a), IgG1 titres (c) and IgG2a titres (d) in sera were determined by enzyme-linked immunosorbent assay (ELISA). Data represent mean titres of individual mice. Data are representative of three to four independent experiments. (b) Inhibition of βGal-specific IgE production following biolistic transfection with βGal-encoding plasmids or the enhanced green fluorescent protein (EGFP)-encoding control plasmid was determined in comparison with unvaccinated mice, set to 100%, after the fifth injection of βGal/alum. Data represent the mean ± standard error of the mean (SEM) for inhibition determined in three to four independent experiments. Significant differences are indicated by asterisks (**P < 0·01 and ***P < 0·001 versus unvaccinated mice).
As already outlined, repeated injection of βGal/alum induced strong IgG1 production during the course of immunization, but only minor formation of IgG2a (Fig. 5c, d). Biolistic transfection with the EGFP-encoding control vector had no significant impact on the elicitation of βGal-specific IgG1 by subsequent sensitization with βGal/alum, but led to a modest increase in the production of specific IgG2a (days 10 and 54, P < 0·05 versus control mice) (Fig. 5c, d). In contrast, previous vaccination with βGal-encoding plasmids had profound modulatory effects on IgG subtype formation during the course of sensitization. Preceding vaccination with pK5-βGal similar to vaccination with pCMV-βGal led to a boost in IgG1 production immediately after the beginning of immunization with βGal/alum (pK5-βGal: days 10 and 26, P < 0·001 versus control mice; pCMV-βGal: day 10, P < 0·001, day 26, P < 0·01 versus control mice). However, in the long term IgG1 levels were considerably reduced (pK5-βGal: days 54 and 64, P < 0·01 versus control mice; pCMV-βGal: days 54 and 64, P < 0·05 versus control mice) (Fig. 5c), whereas production of IgG2a was substantially enhanced for the most part (pK5-βGal: days 10 and 40, P < 0·05, days 26, 54 and 64, P < 0·01 versus control mice; pCMV-βGal: days 10 and 26, P < 0·01, day 54, P < 0·05 versus control mice) (Fig. 5d). As compared with these groups the increase in IgG1 production soon after the beginning of the sensitization period was much less apparent after vaccination with pFascin-βGal (day 10, P < 0·001, day 26, P < 0·05 versus control mice; days 10 and 26, P < 0·01 versus mice vaccinated with pK5-βGal or pCMV-βGal), whereby IgG1 synthesis in the late sensitization phase was also significantly inhibited (days 54 and 64, P < 0·01 versus control mice) (Fig. 5c). The stimulatory effect on IgG2a production was more pronounced in mice vaccinated with pFascin-βGal (days 10, 26, 40, 54 and 64, P < 0·01 versus control mice), although the differences in IgG2a levels compared with mice vaccinated with pK5-βGal or pCMV-βGal did not yield statistical significance (Fig. 5d). The production of IgG1 and IgG2a in vaccinated mice 2 days before the first administration of βGal/alum was very low in relation to antibody production measured after subsequent immunization with βGal/alum, but the humoral response at that time (day –2 in Fig. 5) was absolutely consistent in magnitude as well as quality with the data shown in Fig. 4a.
Discussion
Allergen gene transfer using naked plasmid DNA represents a promising approach for the treatment of allergic diseases.29 We have previously shown in a mouse model of type I allergy that biolistic transfection of the skin using the gene gun device represents an alternative mode of DNA application which is suitable for interference with antigen-specific IgE production,25 although it is conceivable that intrinsic properties of allergens might modify the effectiveness of this therapy.30 Using the murine fascin promoter, which allows transcriptional targeting of transgene expression to DCs, for gene gun-mediated DNA vaccination we have improved this technique. We have demonstrated that this experimental approach, which was associated with the generation of potent Th1/T cytotoxic type 1 (Tc1) immune responses, was sufficient to exert inhibitory activity for IgE formation.27 In this report we have investigated the mechanisms underlying the suppression of the production of βGal-specific IgE antibodies, employing prophylactic biolistic transfection with the plasmid vector pFascin-βGal encoding βGal under control of the fascin promoter. CD8+ T cells from mice immunized with pFascin-βGal showed the capacity to suppress IgE production in transfer experiments. This finding is consistent with publications that identified this T-cell subpopulation as effector cells in the control of IgE production following intradermal or intramuscular injection of DNA.31–34 Furthermore, CD4+ T helper cells induced by biolistic transfection with pFascin-βGal substantially contribute to the suppression of IgE responses, probably by converting the allergen-specific Th2 response into a Th1-biased response, as was reported previously for successful genetic immunization using naked plasmid DNA.33,35–38 This assumption is supported by the observation that the IgG isotype pattern after transfer of CD4+ T cells from mice biolistically transfected with pFascin-βGal was Th1-biased in the recipients. As typical Th1 cells, these cells possess the potential to produce high levels of IFN-γ. It is therefore tempting to speculate that the inhibitory activity of the CD4+ T cells and probably also of the IFN-γ-producing CD8+ CTLs was mediated by this cytokine, either through directing the differentiation of newly activated CD4+ T cells into Th1 cells39 or through directly affecting the immunoglobulin class-switch and antibody synthesis in antigen-specific B cells.40 Consistent with this notion, negative regulation of IgE production by gene gun-mediated DNA immunization was not seen in IFN-γ−/− mice, confirming the central role of IFN-γ in the observed effect. Inhibition of IgE synthesis was not dependent on the presence of IL-12, because in IL-12p40−/− mice down-regulation of IgE production following treatment of mice with pFascin-βGal was marked.
In the present paper we show for the first time that the restriction of transgene expression to keratinocytes, i.e. to non-DCs after prophylactic DNA immunization, is effective in the prevention of antigen-specific IgE responses. To ensure that antigen production in the skin was strictly limited to keratinocytes, we used the plasmid construct pK5-βGal containing the bovine keratin 5 promoter to control transgene expression. Consistent with the transgene expression pattern we observed following biolistic transfection with pK5-βGal, in transgenic mice the keratin 5 promoter has previously been shown to be highly active in the skin, predominantly in the outer root sheath of hair follicles and also in basal epidermal keratinocytes,41–44 but not in cells of haematopoietic origin.42,45
The issue of whether the presentation of endogenous antigen produced by directly transfected DCs of the skin or the presentation of antigen produced and secreted by transfected non-DCs and then taken up and processed by untransfected DCs represents the main pathway for the induction of transgene-specific CTL responses following gene gun immunization is still a matter of controversy. Timares et al.19 used an inducible vector system to demonstrate, by adoptive transfer of migratory DCs derived from biolistically transfected skin, that the immune response generated in the recipients was attributable to directly transfected DCs. Porgador et al.5 showed, by depletion of directly transfected DCs from the LNC population of gene gun-immunized mice, that those cells are crucial for full functional capacity to stimulate CD8+ T cells in vitro. Cho et al.20 performed biolistic transfections with plasmids containing the APC-specific human CD11b promoter and mouse MHC II-Eα promoter, respectively, in order to transcriptionally target DCs in the skin, which resulted in poor induction of CD8+ T cells as compared with gene gun immunization using pCMV or control constructs. In contrast, our results show potent and long-lasting induction of CD8+ CTLs after biolistic transfection employing the fascin promoter. As the activities of the isolated human CD11b promoter and murine Eα promoter have been documented solely in macrophages/monocytes,20,46–49 and not in Langerhans cells or DCs, it cannot be ruled out that the efficiency of transgene expression following transfection of DCs of the skin has been low in the study of Cho et al.20 Hence, the discrepancy might be explained by the notion that the targeting of transgene expression mainly to macrophages led to suboptimal activation of naïve CD8+ T cells in the lymph nodes. Moreover, because biosynthesis of MHC class II molecules is down-regulated during DC maturation,50 promoter activity and concomitantly transgene expression were probably also down-regulated in the directly transfected DCs. Similarly, gene gun-mediated expression of ovalbumin (OVA) under the control of the CD11c promoter failed to induce efficient CD8+ T-cell responses, lending support to the interpretation that under those experimental conditions DCs as exclusive APCs were insufficient to trigger CTL responses.21,22 However, consistent with the data reported in the present study, we were recently able to detect significant numbers of OVA-specific CD8+ T cells by ELISPOT after gene gun-mediated transfection of mouse skin with pFascin-OVA (S. Sudowe, unpublished results). Thus, it appears that substantial differences in the activities of the two promoters employed might account for the differing results. Accordingly, the CD11c promoter is constitutively active at basal levels in immature DCs,51,52 but, because the expression of the CD11c molecule on the surface of mouse DCs after stimulation is not modulated,53–55 it is not likely that the activity of the promoter is enhanced after differentiation into mature DCs. In contrast, the activity of the fascin promoter strongly increases during maturation of DCs,16,17 assuring that upon transfection with pFascin endogenous production of the antigen is restricted to DCs with unique primary stimulatory activity, which then permits optimal priming of CTL responses to develop.
Cho et al.20 as well as Hon et al.21 reported that transgene expression limited to epidermal keratinocytes by performing biolistic transfection with vectors harbouring the keratin 14 (K14) promoter was sufficient to initiate considerable CTL responses. This led to the assumption that uptake and cross-presentation of antigen, released by or associated with apoptotic transfected cutaneous cells, represent the major mechanism of CD8+ T-cell priming following gene gun-mediated DNA immunization. The keratin proteins 5 and 14 are produced in the same epithelial cells and form dimers in keratin filaments. Therefore, co-expression of K5 and K14 genes is tightly regulated at the transcriptional level, implying that the activities of their promoters are congruent.28,42 The results of our experiments using the K5 promoter for in vivo transfection are consistent with the data obtained with the K14 promoter. Actually, the magnitude of the CTL response following DNA immunization with pK5-βGal was slightly, yet not significantly higher than that after bombardment with pFascin-βGal, suggesting that cross-presentation of exogenous antigen and the presentation of antigen synthesized endogenously represent equivalent pathways for CD8+ T-cell priming following biolistic transfection of the skin. We assume that the relative contributions of direct priming versus cross-priming to the elicitation of CD8+ T-cell responses following DNA immunization may vary, being dependent on several factors relating to the method of immunization as well as the identity of the antigen, in particular the type of transgene-expressing cells (DC versus non-DC), the form of antigen (cell-associated versus soluble)22,56,57 and the dose of available exogenous antigen (low versus high).58 Nevertheless, we are not able to determine which pathway is predominantly operative in the induction of CTL responses when skin DCs as well as non-DCs are transfected concomitantly, as is the case after gene gun-mediated DNA immunization with pCMV vectors that are ubiquitously active.
For the priming of CD4+ T cells it is, however, likely that the presentation of exogenous antigen dictates the quality of the resulting immune response because the pattern of cytokines secreted by in vitro stimulated CD4+ T cells as well as the immunoglobulin isotype profile detected in the sera of immunized mice showed similar polarization after biolistic transfection with pCMV-βGal and pK5-βGal, respectively. Similarly, co-application of pFascin-βGal and pK5-βGal changed the quality of the Th1-skewed immune response that is induced by pFascin-βGal, and biased the CD4+ T-cell response towards a mixed Th1/Th2 response as immunization with pK5-βGal alone did. Our finding that immunization of mice with pFascin-βGal and pK5-βGal induced specific IgG2a antibody titres to a similar extent is in contrast with the results of Cho et al.20 The authors reported that significant levels of specific IgG2a antibodies were induced by immunization of mice with plasmids encoding nucleoprotein under the control of the K14 promoter, but hardly any production of IgG2a was noted following immunization with plasmids harbouring the human CD11b or the mouse Eα promoter. This discrepancy strengthens the notion that the latter two promoters lacked optimal activity under the experimental conditions employed. The question of why gene gun-mediated immunization with pFascin-βGal and pK5-βGal, respectively, leads to such different outcomes in terms of the CD4+ T-cell response remains to be resolved. It is conceivable that processing of endogenous antigen with subsequent loading of antigenic peptides onto MHC class II molecules and their presentation by directly transfected DCs might generate a Th1-promoting signal. It was previously shown that DCs, which were transfected in vitro with antigen-encoding plasmid DNA, had the capacity to instruct in vitro59 or following adoptive transfer in vivo60,61 the differentiation of Th1 cells. However, the differential T helper cell polarization seen might be dependent on the amount of protein antigen released by or leaking from transfected non-DCs. With respect to the latter notion, our data are consistent with a report of Thompson,62 who demonstrated that the immune response is biased towards a Th1-dominated response by low doses of antigen whereas high antigen doses favour a Th2-orientated response. Thus, in the case of pFascin, a limited quantity of protein produced by only a small number of directly transfected DCs would be expected, while with pK5 extensive transgene expression in many transfected keratinocytes is likely to occur.
In conclusion, our data suggest that presentation of antigen endogenously synthesized by directly transfected cutaneous DCs and presentation of exogenous antigen, produced by non-DCs and ingested by untransfected DCs, represent equivalent pathways to efficiently induce transgene-specific immune responses following biolistic transfection of the skin. Both mechanisms are effective in inhibiting an antigen-specific IgE response induced in a mouse model of type I allergy.
Acknowledgments
The authors would like to thank Prof. Manfred Blessing (Center for Biotechnology and Biomedicine, Faculty of Veterinary Medicine, Leipzig University) for providing the keratin 5 promoter and Dr Franz Petry (Institute of Medical Microbiology and Hygiene, Johannes Gutenberg-University Mainz) for providing IFN-γ−/− and IL-12p40−/− mice. This work was supported by the Deutsche Forschungsgemeinschaft, SFB548 (Project B5).
Glossary
Abbreviations:
- alum
aluminiumhydroxide
- APC
antigen-presenting cell
- CMV
cytomegalovirus
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- EGFP
enhanced green fluorescent protein
- LNC
lymph node cell
- pCMV
plasmid vector harbouring the CMV immediate early promoter
- pFascin
plasmid vector harbouring the murine fascin promoter
- pK5
plasmid vector harbouring the bovine keratin 5 promoter
- PMED
particle-mediated epidermal delivery
- Th
T helper
- βGal
β-galactosidase
References
- 1.Chen D, Maa YF, Haynes JR. Needle-free epidermal powder immunization. Expert Rev Vaccines. 2002;1:265–76. doi: 10.1586/14760584.1.3.265. [DOI] [PubMed] [Google Scholar]
- 2.Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–22. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–8. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 5.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–82. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouloc A, Walker P, Grivel JC, Vogel JC, Katz SI. Immunization through dermal delivery of protein-encoding DNA: a role for migratory dendritic cells. Eur J Immunol. 1999;29:446–54. doi: 10.1002/(SICI)1521-4141(199902)29:02<446::AID-IMMU446>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Bot A, Stan AC, Inaba K, Steinman R, Bona C. Dendritic cells at a DNA vaccination site express the encoded influenza nucleoprotein and prime MHC class I-restricted cytolytic lymphocytes upon adoptive transfer. Int Immunol. 2000;12:825–32. doi: 10.1093/intimm/12.6.825. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Payne LG. Targeting epidermal Langerhans cells by epidermal powder immunization. Cell Res. 2002;12:97–104. doi: 10.1038/sj.cr.7290115. [DOI] [PubMed] [Google Scholar]
- 9.Garg S, Oran A, Wajchman J, Sasaki S, Maris CH, Kapp JA, Jacob J. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat Immunol. 2003;4:907–12. doi: 10.1038/ni962. [DOI] [PubMed] [Google Scholar]
- 10.Stoecklinger A, Grieshuber I, Scheiblhofer S, et al. Epidermal Langerhans cells are dispensable for humoral and cell-mediated immunity elicited by gene gun immunization. J Immunol. 2007;179:886–93. doi: 10.4049/jimmunol.179.2.886. [DOI] [PubMed] [Google Scholar]
- 11.Ross R, Ross XL, Schwing J, Längin T, Reske-Kunz AB. The actin-bundling protein fascin is involved in the formation of dendritic processes in maturing epidermal Langerhans cells. J Immunol. 1998;160:3776–82. [PubMed] [Google Scholar]
- 12.Ross R, Jonuleit H, Bros M, et al. Expression of the actin-bundling protein fascin in cultured human dendritic cells correlates with dendritic morphology and cell differentiation. J Invest Dermatol. 2000;115:658–63. doi: 10.1046/j.1523-1747.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosialos G, Birkenbach M, Ayehunie S, Matsumura F, Pinkus GS, Kieff E, Langhoff E. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol. 1996;148:593–600. [PMC free article] [PubMed] [Google Scholar]
- 14.Mosialos G, Yamashiro S, Baughman RW, Matsudaira P, Vara L, Matsumura F, Kieff E, Birkenbach M. Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J Virol. 1994;68:7320–8. doi: 10.1128/jvi.68.11.7320-7328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, Said JW. Fascin, a sensitive new marker for Reed-Sternberg cells of Hodgkin’s disease. Evidence for a dendritic or B cell derivation? Am J Pathol. 1997;150:543–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Bros M, Ross XL, Pautz A, Reske-Kunz AB, Ross R. The human fascin gene promoter is highly active in mature dendritic cells due to a stage-specific enhancer. J Immunol. 2003;171:1825–34. doi: 10.4049/jimmunol.171.4.1825. [DOI] [PubMed] [Google Scholar]
- 17.Ross R, Sudowe S, Beisner J, et al. Transcriptional targeting of dendritic cells for gene therapy using the promoter of the cytoskeletal protein fascin. Gene Ther. 2003;10:1035–40. doi: 10.1038/sj.gt.3301968. [DOI] [PubMed] [Google Scholar]
- 18.Sudowe S, Ludwig-Portugall I, Montermann E, Ross R, Reske-Kunz AB. Transcriptional targeting of dendritic cells in gene gun-mediated DNA immunization favors the induction of type 1 immune responses. Mol Ther. 2003;8:567–75. doi: 10.1016/s1525-0016(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 19.Timares L, Safer KM, Qu B, Takashima A, Johnston SA. Drug-inducible, dendritic cell-based genetic immunization. J Immunol. 2003;170:5483–90. doi: 10.4049/jimmunol.170.11.5483. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Youn JW, Sung YC. Cross-priming as a predominant mechanism for inducing CD8+ T cell responses in gene gun DNA immunization. J Immunol. 2001;167:5549–57. doi: 10.4049/jimmunol.167.10.5549. [DOI] [PubMed] [Google Scholar]
- 21.Hon H, Oran A, Brocker T, Jacob J. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol. 2005;174:5233–42. doi: 10.4049/jimmunol.174.9.5233. [DOI] [PubMed] [Google Scholar]
- 22.Lauterbach H, Gruber A, Ried C, Cheminay C, Brocker T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J Immunol. 2006;176:4600–7. doi: 10.4049/jimmunol.176.8.4600. [DOI] [PubMed] [Google Scholar]
- 23.Casatorres J, Navarro JM, Blessing M, Jorcano JL. Analysis of the control of expression and tissue specificity of the keratin 5 gene, characteristic of basal keratinocytes. Fundamental role of an AP-1 element. J Biol Chem. 1994;269:20489–96. [PubMed] [Google Scholar]
- 24.Sudowe S, Montermann E, Steitz J, Tüting T, Knop J, Reske-Kunz AB. Efficacy of recombinant adenovirus as vector for allergen gene therapy in a mouse model of type I allergy. Gene Ther. 2002;9:147–56. doi: 10.1038/sj.gt.3301625. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig-Portugall I, Montermann E, Kremer A, Reske-Kunz AB, Sudowe S. Prevention of long-term IgE antibody production by gene gun-mediated DNA vaccination. J Allergy Clin Immunol. 2004;114:951–7. doi: 10.1016/j.jaci.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Sudowe S, Arps V, Vogel T, Kölsch E. The role of interleukin-4 in the regulation of sequential isotype switch from immunoglobulin G1 to immunoglobulin E antibody production. Scand J Immunol. 2000;51:461–71. doi: 10.1046/j.1365-3083.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 27.Sudowe S, Ludwig-Portugall I, Montermann E, Ross R, Reske-Kunz AB. Prophylactic and therapeutic intervention in IgE responses by biolistic DNA vaccination primarily targeting dendritic cells. J Allergy Clin Immunol. 2006;117:196–203. doi: 10.1016/j.jaci.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Jiang CK, Epstein HS, Tomic M, Freedberg IM, Blumenberg M. Functional comparison of the upstream regulatory DNA sequences of four human epidermal keratin genes. J Invest Dermatol. 1991;96:162–7. doi: 10.1111/1523-1747.ep12460939. [DOI] [PubMed] [Google Scholar]
- 29.Weiss R, Scheiblhofer S, Gabler M, Ferreira F, Leitner WW, Thalhamer J. Is genetic vaccination against allergy possible? Int Arch Allergy Immunol. 2006;139:332–45. doi: 10.1159/000091946. [DOI] [PubMed] [Google Scholar]
- 30.Scheiblhofer S, Stoecklinger A, Gruber C, Hauser-Kronberger C, Alinger B, Hammerl P, Thalhamer J, Weiss R. Gene gun immunization with clinically relevant allergens aggravates allergen induced pathology and is contraindicated for allergen immunotherapy. Mol Immunol. 2007;44:1879–87. doi: 10.1016/j.molimm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CH, Chua KY, Tao MH, Huang SK, Hsieh KH. Inhibition of specific IgE response in vivo by allergen-gene transfer. Int Immunol. 1996;8:1405–11. doi: 10.1093/intimm/8.9.1405. [DOI] [PubMed] [Google Scholar]
- 32.Lee DJ, Tighe H, Corr M, Roman M, Carson DA, Spiegelberg HL, Raz E. Inhibition of IgE antibody formation by plasmid DNA immunization is mediated by both CD4+ and CD8+ T cells. Int Arch Allergy Immunol. 1997;113:227–30. doi: 10.1159/000237554. [DOI] [PubMed] [Google Scholar]
- 33.Maecker HT, Hansen G, Walter DM, DeKruyff RH, Levy S, Umetsu DT. Vaccination with allergen-IL-18 fusion DNA protects against, and reverses established, airway hyperreactivity in a murine asthma model. J Immunol. 2001;166:959–65. doi: 10.4049/jimmunol.166.2.959. [DOI] [PubMed] [Google Scholar]
- 34.Peng HJ, Su SN, Chang ZN, Chao PL, Kuo SW, Tsai LC. Induction of specific Th1 responses and suppression of IgE antibody formation by vaccination with plasmid DNA encoding Der f 11. Vaccine. 2002;20:1761–8. doi: 10.1016/s0264-410x(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 35.Adel-Patient K, Creminon C, Boquet D, Wal JM, Chatel JM. Genetic immunization with bovine β-lactoglobulin cDNA induces a preventive and persistent inhibition of specific anti-BLG IgE response in mice. Int Arch Allergy Immunol. 2001;126:59–67. doi: 10.1159/000049495. [DOI] [PubMed] [Google Scholar]
- 36.Toda M, Kasai M, Hosokawa H, et al. DNA vaccine using invariant chain gene for delivery of CD4+ T cell epitope peptide derived from Japanese cedar pollen allergen inhibits allergen-specific IgE response. Eur J Immunol. 2002;32:1631–9. doi: 10.1002/1521-4141(200206)32:6<1631::AID-IMMU1631>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Hochreiter R, Stepanoska T, Ferreira F, Valenta R, Vrtala S, Thalhamer J, Hartl A. Prevention of allergen-specific IgE production and suppression of an established Th2-type response by immunization with DNA encoding hypoallergenic allergen deivatives of Bet v 1, the major birch-pollen allergen. Eur J Immunol. 2003;33:1667–76. doi: 10.1002/eji.200323377. [DOI] [PubMed] [Google Scholar]
- 38.Gabler M, Scheiblhofer S, Kern K, et al. Immunization with a low-dose replicon DNA vaccine encoding Phl p 5 effectively prevents allergic sensitization. J Allergy Clin Immunol. 2006;118:734–41. doi: 10.1016/j.jaci.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 40.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 41.Missero C, Serra C, Stenn K, Dotto GP. Skin-specific expression of a truncated Ela oncoprotein binding to p105-Rb leads to abnormal hair follicle maturation without increased epidermal proliferation. J Cell Biol. 1993;121:1109–20. doi: 10.1083/jcb.121.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez A, Bravo A, Jorcano JL, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- 43.Amendt C, Schirmacher P, Weber H, Blessing M. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an accelaeration of carcinoma development. Oncogene. 1998;17:25–34. doi: 10.1038/sj.onc.1202161. [DOI] [PubMed] [Google Scholar]
- 44.Paulson QX, McArthur MJ, Johnson DG. E2F3a stimulates proliferation, p53-independent apoptosis and carcinogenesis in a transgenic mouse model. Cell Cycle. 2006;5:184–90. doi: 10.4161/cc.5.2.2307. [DOI] [PubMed] [Google Scholar]
- 45.Lomada D, Liu B, Coghlan L, Hu Y, Richie ER. Thymus medulla formation and central tolerance are restored in IKKα−/− mice that express an IKKα transgene in keratin 5+ thymic epithelial cells. J Immunol. 2007;178:829–37. doi: 10.4049/jimmunol.178.2.829. [DOI] [PubMed] [Google Scholar]
- 46.Shelley CS, Arnaout MA. The promoter of the CD11b gene directs myeloid-specific and developmentally regulated expression. Proc Natl Acad Sci USA. 1991;88:10525–9. doi: 10.1073/pnas.88.23.10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dziennis S, Van Etten RA, Pahl HL, Morris DL, Rothstein TL, Blosch CM, Perlmutter RM, Tenen DG. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood. 1995;85:319–29. [PubMed] [Google Scholar]
- 48.Kouskoff V, Fehling HJ, Lemeur M, Benoist C, Mathis D. A vector driving the expression of foreign cDNAs in the MHC class II-positive cells of transgenic mice. J Immunol Methods. 1993;166:287–91. doi: 10.1016/0022-1759(93)90370-m. [DOI] [PubMed] [Google Scholar]
- 49.Groux H, Cottrez F, Rouleau M, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–9. [PubMed] [Google Scholar]
- 50.Becker D, Reske-Kunz AB, Knop J, Reske K. Biochemical properties of MHC class II molecules endogenously synthesized and expressed by mouse Langehans cells. Eur J Immunol. 1991;21:1213–20. doi: 10.1002/eji.1830210518. [DOI] [PubMed] [Google Scholar]
- 51.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–50. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–6. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 53.Ban E, Dupré L, Hermann E, et al. CpG motifs induce Langerhans cell migration in vivo. Int Immunol. 2000;12:737–45. doi: 10.1093/intimm/12.6.737. [DOI] [PubMed] [Google Scholar]
- 54.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J Immunol. 2001;166:5688–94. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 55.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–24. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 56.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 57.Rush C, Mitchell T, Garside P. Efficient priming of CD4+ and CD8+ T cells by DNA vaccination depends on appropriate targeting of sufficient levels of immunologically relevant antigen to appropriate processing pathways. J Immunol. 2002;169:4951–60. doi: 10.4049/jimmunol.169.9.4951. [DOI] [PubMed] [Google Scholar]
- 58.Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–14. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klostermann B, Bellinghausen I, Böttcher I, Petersen A, Becker WM, Knop J, Saloga J. Modification of the human allergic immune response by allergen-DNA-transfected dendritic cells in vitro. J Allergy Clin Immunol. 2004;113:327–33. doi: 10.1016/j.jaci.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 60.Manickan E, Kanangat S, Rouse RJ, Yu Z, Rouse BT. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukoc Biol. 1997;61:125–32. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- 61.Timares L, Takashima A, Johnston SA. Quantitative analysis of the immunopotency of genetically transfected dendritic cells. Proc Natl Acad Sci USA. 1998;95:13147–52. doi: 10.1073/pnas.95.22.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]