Abstract
Enteric infections remain a major health problem causing millions of deaths in developing countries. The interplay among the host intestinal epithelium, the mucosa-associated immune system and microbiota performs an essential role in gut homeostasis and protection against infectious diseases. Dendritic cells (DCs) play a key role in orchestrating protective immunity and tolerance in the gut. The mechanisms by which DCs adapt their responses and discriminate between virulent microbes and trillions of innocuous bacteria remain ill-defined. Here we investigated the effect of cross-talk between commensal-related bacteria (CB) and Toll-like receptor (TLR) agonists on DC activation and the outcome of the in vitro T helper response. Human monocyte-derived DCs were exposed to eight different Gram-positive or Gram-negative CB strains prior to activation with five different TLR agonists. The key polarizing cytokines interleukin (IL)-12p70, IL-10, IL-1β and IL-6 were quantified and the fate of naïve T-cell differentiation was evaluated. We identified a unique combination of Lactobacillus casei and TLR3 signals that acted in synergy to selectively increase IL-12p70 secretion. Exposure to poly(I:C) converted L. casei-treated DCs into potent promoters of T helper type 1 (Th1) responses. We propose that DCs can integrate harmless and dangerous non-self signals delivered by viral products, to mount robust Th1 responses. Thus, in vivo DC targeting with selective probiotics may improve strategies for the management of enteric diseases.
Keywords: commensal bacteria, dendritic cells, interleukin-12, T helper type 1, Toll-like receptor
Introduction
In the gastrointestinal tract (GT), about 1014 commensal bacteria encompassing 300–500 different strains have developed a symbiotic relationship with mammalian hosts.1 The human microbiome encodes physiological components (for instance enzymes) that complement host capacities or deficiencies.2 The mucosal immune system needs to be tightly regulated to avoid collateral damage to self and food antigens as well as to innocuous bacteria of the commensal flora. Remarkably, it maintains the capacity to mount a powerful defence response against pathogens. In the gut mucosa, non-pathogenic bacteria are constantly in contact with epithelial cells and dendritic cells (DCs). DCs extend dendrites across the epithelial barrier and express molecules that participate in the formation of tight junctions.3 Intestinal bacteria can be recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) expressed by epithelial cells.4 The loss of gut epithelium integrity in MyD88-deficient mice after chemical exposure underscores the importance of this type of interaction.5
Such an equilibrium established by permanent contact with commensal flora enforces gut tolerance which is disturbed by the introduction of pathogens. Direct DC recognition of viral and microbial products [pathogen-associated molecular patterns (PAMPs)] by PRRs that include TLRs and NOD-like receptors (NLDs), together with inflammatory cytokine release, is mandatory to drive and sustain effector Th cell responses.6,7 DCs are at the interface between innate and adaptive immunity and it is now clear that they direct different classes of immune responses.8,9 DCs can be designated as tolerogenic or immunogenic because they can elicit tolerance or T-cell immunity. IL-12 and IL-10 produced by DCs dictate CD4+ T-cell fate, inducing differentiation into pro-inflammatory (Th1) and regulatory (Tr1) T cells, respectively. Transforming growth factor (TGF)-β is critical for the differentiation of adaptive forkhead box P3 (FoxP3) regulatory T cells (Tregs) but, together with IL-6, it promotes the generation of IL-17-producing cells in mice.10 In contrast, IL-1β or IL-21 promotes human Th17 development.11,12
Direct interaction between commensal-related bacteria (CB) and DCs may also occur in vivo,13 although the precise nature of the signals delivered remains ill-defined. Bifidobacteria prime human DCs via TLR2, while Lactobacilli do not signal through TLR1, TLR2, TLR3, TLR4, TLR6, TLR7 or TLR9.14,15 We16 and others17 have reported that Gram-positive and Gram-negative CB induce phenotypic and functional DC maturation. Yet, CB-treated mature DCs convert naïve CD4+ T cells into suppressor T cells in the absence of a TLR agonist signal.16 As microbe-associated molecular patterns are shared by commensals and pathogens, how the DCs sense harmless commensals and dangerous pathogens remains an important question to answer.
In the present study, we hypothesize that DCs may integrate signals received simultaneously or consecutively from CB and pathogens. Indeed, recent studies have validated the concept that synergy among multiple TLR signals sustains human Th1 responses.18 We thus postulate that CB impinge on gut tolerance at steady state by inducing suppressor CD4+ T cells and co-operate with selected pathogens to drive protective Th effector responses. We thus sought to investigate the effect of cross-talk between Gram-positive or Gram-negative CB and TLR agonists on monocyte-derived DC function and on the development of CD4+ T helper responses. Monocytes are the precursors of mucosal DCs in vivo; when recruited in large numbers at inflamed sites, they differentiate into DCs.19 Among eight different CB strains and five TLR agonists examined, we identified one combinatorial code, i.e. Lactobacillus casei and the TLR3 signal, which synergized to selectively increase IL-12p70 production by DCs and drive naïve CD4+ T-cell polarization towards a strong Th1 response without inducing Th17. We here propose a mechanism by which harmless non-self (i.e. CB) and dangerous (i.e. virus) signals may combine to shift DC function from tolerogenic to immunogenic and perhaps elicit protective immunity.
Materials and methods
Reagents
Eight gamma-irradiated CB strains (L. casei DN-114 001, L. casei no.2 DN-114 086, Lactobacillus rhamnosus DN-116 047, Lactobacillus plantarum DN-121 022, Bifidobacterium animalis DN-173 010, Bifidobacterium adolescentis DN-150 017, Streptococcus thermophilus DN-001 621 and Bacteroides thetaiotaomicron, a Gram-negative commensal-related species) were used in the cultures at a CB:DC ratio of 200 : 1 unless indicated otherwise. Bifidobacteria and Bacteroides are representative of the dominant species of human microflora, whereas Lactobacilli and Streptococci are representative of subdominant human flora. The irradiation process prevented bacterial overgrowth in cell cultures and maintained bacterial structural integrity. A similar DC cytokine profile (IL-12:IL-10 ratio) was obtained with live bacteria and 200 : 1 was determined to be the optimal CB:DC ratio for DC phenotypic maturation.16 Cytokines and chemokines were measured in culture supernatants (CSNs) using commercially available enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, IL-10, IL-12p70 and IL-17 (R&D Systems, Minneapolis, MN), interferon (IFN)-γ and IL-6 (BioSource, San Diego, CA), IL-23 (eBioscience, San Diego, CA) and chemokine (C-X-C motif) ligand 10 (CXCL10) (BD Biosciences, San Jose, CA). TLR agonists were used at the following concentrations: 100 μg/ml for peptidoglycan (PGN) (Sigma-Aldrich, St Louis, MO), 15 μg/ml for poly(I:C) (GE Health Sciences, Buckinghamshire, UK), 10 μg/ml for lipopolysaccharide (LPS) (Sigma-Aldrich), 20 μg/ml for flagellin, and 8 μg/ml for single-stranded RNA (ssRNA) (InvivoGen, San Diego, CA). Resveratrol was purchased from Sigma-Aldrich and used at a final concentration of 50 μm.
Cell preparation, purification and culture conditions
Human peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation of heparinized blood from health volunteers using Lymphoprep (Axis-Shield PoS AC, Dundee, UK). Permission to use human primary cells for the described experiments was obtained from the Ethic Research Committee of the Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CRCHUM). Monocyte-derived DCs were prepared from enriched monocytes as described by Demeure et al.20, following 5–6 days of culture in the presence of 25 ng/ml of IL-4 and 25 ng/ml of granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems). Naïve CD4+ T cells were isolated using the Human CD4+ Naïve T-cell Enrichment Kit (StemCell Technologies, Vancouver, Canada) according to the protocol provided by the manufacturer.
Immature DCs (iDCs) were cultured with CB alone or in combination with TLR agonists in HB101 complete medium (Irvine Scientific, Santa Ana, CA) for 48 hr at the indicated CB:DC ratio. Naïve CD4+ T cells were co-cultured for 5–7 days with mature DCs at a DC:T-cell ratio of 1 : 10 in RPMI-1640 medium (Wisent Inc., St-Bruno, Canada) supplemented with 10% fetal calf serum (FCS) (Gibco–Invitrogen, Carlsbad, CA). For some experiments, T cells were cultured for 5 days with plate-bound anti-CD3 (10 mg/ml; OKT3; Janssen-Ortho, Tronto, Canada) in Yssel’s medium (Gemini Bio-product, West-Sacramento, CA) supplemented with 2% human AB serum (Wisent) in the presence of filtered culture supernatants of activated DCs. Primed T cells were expanded for 3–7 days in the presence of IL-2 (50 U/ml) (R&D Systems). In selected experiments, neutralizing monoclonal antibody (mAb) to IL-12 (clone C8.6.22 or clone 24910, specific to IL-12p70; R&D Systems) or isotype-matched control (10 μg/ml) was added to T-cell primary cultures.
To promote Th17 differentiation, naïve CD4+ T cells were stimulated on plate-bound anti-CD3 in Yssel’s medium supplemented with 2% human AB serum in the presence of anti-IFN-γ (1·5 μg/ml), IL-23 (10 ng/ml) and IL-1 (10 ng/ml) (R&D Systems) with or without IL-12 (60 pm) (F. Hoffman-La roche, Basel, Switzerland) for 5 days. Cells were expanded for 5 days in the presence of IL-2 (20 U/ml).
In vitrosuppression assay
To evaluate regulatory function, freshly isolated CD4+ T cells (2 × 105 cells/ml) were cultured alone (target cell × 1) or together with primed T cells at a 1 : 3 effector:target cell ratio and stimulated for 3–5 days with anti-CD3 (OKT3) (100 ng/ml) plus anti-CD28 (500 ng/ml) (BD Biosciences) in the presence of mitomycin C-treated monocytes (2 × 104 cells/ml). Proliferation of CD3-activated CD4+ T cells alone (target cell × 1) and proliferation of CD4+ T cells mixed at a cell ratio of 1 : 3 (target cell × 2) were used as reference values to evaluate the suppressive function of primed T cells. Cell proliferation was assessed using [3H]thymidine (GE Health Sciences) incorporation.
Antibodies and flow cytometry analysis
For intracellular cytokine staining, primed T cells were re-stimulated with 10 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich), 1 μg/ml of ionomycin (Calbiochem-Behring, San Diego, CA) and 3 μm of monensin (Calbiochem-Behring) for 6 hr. Cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) and permeabilized with 0·1% saponin. Cells were stained for intracellular IFN-γ, IL-4 (BD Biosciences) and T-bet (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
Statistical analysis was performed using the two-tailed Student’s t-test assuming equal variance between the compared groups, or a one-way analysis of variance (anova) for multiple groups. Statistical significance is indicated in the figures (*P < 0·05; **P < 0·01; ***P < 0·001).
Results
The unique combination of L. casei plus poly(I:C) selectively enhances IL-12p70 production
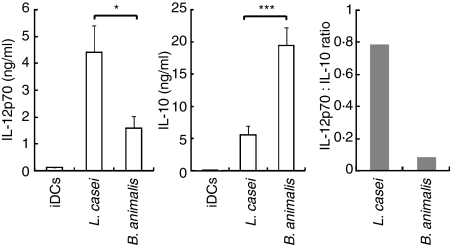
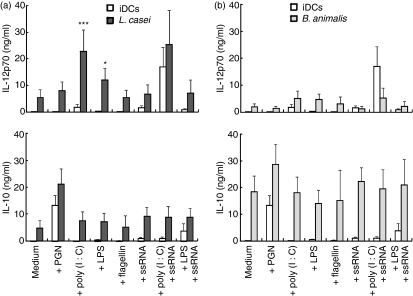
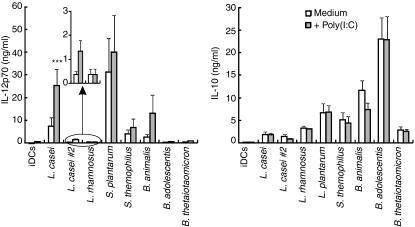
Human monocyte-derived DCs sustain IL-12p70 production in vitro solely in response to the activation of TRIF-coupled receptors (TLR3 or TLR4) combined with a delayed or concomitant endosomal receptor (TLR7, TLR8 or TLR9) signal.18,21 We here investigated whether direct exposure of DCs to a TLR signal combined with CB augmented IL-12p70 production. We first selected L. casei and B. animalis CB strains as they are representative of two major groups of CB strains that can be distinguished by their contrasting IL-12:IL-10 cytokine ratios (Fig. 1). Lactobacillus casei (Fig. 2a) but not B. animalis (Fig. 2b) added to DCs 4 hr prior to poly(I:C) (TLR3 ligand) resulted in a fivefold increase in IL-12p70 secretion as compared with bacteria or TLR alone. A small but significant enhancing effect was also observed when L. casei was combined with LPS (TLR4 ligand) (Fig. 2a). Addition of IFN-γ further augmented L. casei plus poly(I:C)-induced IL-12p70 production (data not shown). The L. casei/poly(I:C) combination was rather selective for IL-12p70 in as much as it did not facilitate IL-10, CXCL10 or IL-1β release and only slightly enhanced that of IL-6 (L. casei/TLR agonist versus L. casei or TLR agonist alone) (Fig. 2a and Fig. S1a). Flagellin (TLR5 ligand), PGN (TLR2 or NOD ligand) and ssRNA (TLR8) triggered cytokine release when used alone but did not augment L. casei-induced release of IL-12p70, IL-10, CXCL10, IL-1β or IL-6 (Fig. 2a and Fig. S1a). In contrast, none of the TLR agonists modulated the IL-10 secretion induced by B. animalis-treated DCs (Fig. 2b). As reported by Napolitani et al.,18 we confirmed that addition of poly(I:C) 4 hr prior to ssRNA strongly increased IL-12p70 production, which was not further augmented by L. casei. Six additional CB were examined and could not substitute for L. casei to enhance poly(I:C) or LPS-induced IL-12p70 production, nor did they facilitate IL-10, IL-6 or IL-1β production (Fig. 3 and Fig. S2). These included two potent IL-12 inducers (L. plantarum and S. thermophilus), an IL-10 inducer (B. adolescentis) and a weak IL-10 and IL-12 inducer (the Gram-negative B. thetaiotaomicron). However, a small increase in IL-12p70 secretion was noted with the L. casei no.2 (DN-114 086 strain)/poly(I:C) combination. As reported by others,22 our data revealed that L. rhamnosus and L. plantarum decreased LPS-induced IL-10 and IL-6 secretion.
Figure 1.
Cytokine profiles of dendritic cells (DCs) stimulated with Lactobacillus casei and Bifidobacterium animalis. Interleukin (IL)-12p70 and IL-10 secretion and the IL-12p70:IL-10 ratio in the 48-hr culture supernatants (CSNs) of DCs stimulated in the absence [immature DCs (iDCs)] or the presence of L. casei or B. animalis (bacteria : DC ratio at 200 : 1) were determined. The mean ± standard error of the mean (SEM) for 20 independent experiments is shown. Student’s t-test was performed: ***P < 0·001; *P < 0·05.
Figure 2.
Cytokine profiles of dendritic cells (DCs) stimulated with Lactobacillus casei or Bifidobacterium animalis in combination with various Toll-like receptor (TLR) agonists. Interleukin (IL)-12p70 and IL-10 production in 48-hr culture supernatants (CSNs) was determined. DCs were stimulated with L. casei (a) or B. animalis (b) 4 hr prior to the addition of medium or TLR agonists [peptidoglycan (PGN), poly(I:C), lipopolysaccharide (LPS), flagellin, and single-stranded RNA (ssRNA) alone and combined with poly(I:C) or LPS]. The mean ± standard error of the mean (SEM) for three to 18 independent experiments is shown. In the analysis of variance (ANOVA), the effect of commensal-related bacteria (CB) plus TLR agonists was compared with that of a single agent, i.e. CB or TLR agonists alone: ***P < 0·001; *P < 0·05.
Figure 3.
Cytokine profiles of dendritic cells (DCs) stimulated with poly(I:C) in combination with various strains of commensal-related bacteria (CB). Interleukin (IL)-12p70 and IL-10 release in 48-hr culture supernatants (CSNs) of DCs stimulated in the absence or presence of Lactobacillus casei, Lactobacillus casei no.2, Lactobacillus rhamnosus, Lactobacillus plantarum, Streptococcus thermophilus, Bifidobacterium animalis, Bifidobacterium adolescentis and Bacteroides thetaiotaomicron, a Gram-negative CB, 4 hr prior to exposure to medium or poly(I:C). The mean ± standard error of the mean (SEM) for five independent experiments is shown. ***P < 0·001 (analysis of variance). iDC, immature dendritic cell.
Lactobacillus casei synergizes with TLR3 signals to selectively enhance IL-12p70 production by DCs
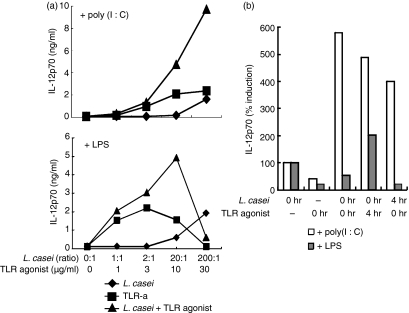
We next explored whether this unique signalling through TLR3/TLR4 and L. casei exerted a synergistic or an additive effect on IL-12p70 production (Fig. 4a). In support of a synergistic mode of action, a combination of suboptimal doses of poly(I:C) or LPS plus L. casei induced IL-12p70 production at concentrations that were higher than those induced by optimal doses of a single agonist. LPS at 30 μg/ml alone or combined with L. casei (200 : 1) decreased cytokine production and cellular viability. We next investigated the window of time in which L. casei and TLR displayed synergistic properties (Fig. 4b). Lactobacillus casei added simultaneously, 4 hr prior to or after poly(I:C), increased IL-12p70 production. In contrast, DCs absolutely required to be exposed to L. casei 4 hr prior to the addition of LPS to produce significant amounts of IL-12p70; the addition of LPS before L. casei rendered DCs refractory to LPS stimulation of IL-12p70 production and that of other cytokines.
Figure 4.
Dose–response and temporal requirements of Lactobacillus casei/Toll-like receptor (TLR) agonist combinatorial stimulation. (a) Interleukin (IL)-12p70 production by dendritic cells (DCs) stimulated with a range of concentrations (as indicated on the x-axis of the figure) of L. casei, poly(I:C), lipopolysaccharide (LPS) or combinations of L. casei plus TLR agonists. (b) Temporal requirement for L. casei/poly(I:C) or LPS synergy. TLR agonists were added to DC cultures simultaneously 4 hr after (0 hr, 4 hr) or 4 hr prior to (4 hr, 0 hr) addition of L. casei. IL-12p70 is shown as the percentage induction compared with L. casei alone. One representative experiment of three is shown.
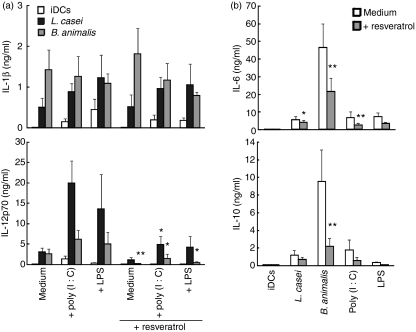
The signalling pathway used by CB to activate human DCs is still a matter of debate. Lactobacilli are unable to activate any of the TLR family, whereas culture supernatant of B. breve activates the TLR2 pathway.14,15 Here we examined whether resveratrol, an inhibitor of TRIF-coupled receptor and TLR2 signalling,23,24 interfered with IL-12 release induced by L. casei, L. casei plus TLR3 or L. casei plus TLR4 (Fig. 5a). Notably, resveratrol abolished poly(I:C)- or LPS-induced IL-12p70 production and significantly decreased the L. casei/poly(I:C) combinatorial-induced IL-12p70 production to concentrations equivalent to those induced by L. casei alone. IL-1β release was not regulated by resveratrol in any of these conditions. However, resveratrol reduced B. animalis- but not L. casei-induced IL-6 and IL-10 secretion in the absence of a TLR agonist (Fig. 5b), supporting the finding that resveratrol also inhibits TLR2 signalling.14,24
Figure 5.
Lactobacillus casei/poly(I:C) or lipopolysaccharide (LPS) synergy in the production of interleukin (IL)-12p70 is inhibited by resveratrol. (a) IL-1β and IL-12p70 secretion in culture supernatants (CSNs) of DCs stimulated in the absence or presence of L. casei 4 hr prior to the addition of Toll-like receptor (TLR) agonists [poly(I:C) or LPS] with or without resveratrol. (b) IL-6 and IL-10 secretion in CSNs of DCs stimulated with single agents with or without resveratrol. The mean ± standard error of the mean (SEM) for five independent experiments is shown. Student’s t-test (for the comparison of cytokine production with and without resveratrol) was performed: **P < 0·01; *P < 0·05.
We thus identified a unique and rather selective combination of one particular Lactobacillus strain (i.e. L. casei DN-114 001) and TLR3/TLR4 signals that acted in synergy to specifically enhance IL-12p70 production by DCs.
Lactobacillus casei/poly(I:C) synergy instructs DCs to boost Th1-polarized responses without inducing Th17
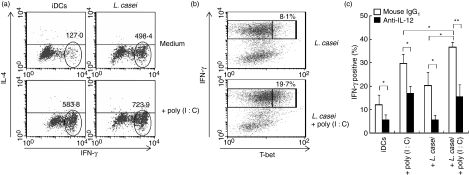
Direct PAMP recognition by DCs with the ensuing TLR signalling is an obligatory condition for the development of a strong Th1 response to pathogens in vivo.25In vitro, co-operation between selected TLR agonists triggers a Th1 programme in human DCs.18,21 We thus hypothesized that L. casei plus poly(I:C), an in vitro model that mimics the context of viral intestinal infection, instructs DCs to boost Th1 polarization. DCs were exposed to L. casei 4 hr prior to the addition of poly(I:C), following which they were incubated for 40 hr, and then used to prime naïve CD4+ T cells. After 5–7 days, T cells were expanded in the presence of IL-2 and re-stimulated with PMA plus ionomycin. DCs stimulated with L. casei plus poly(I:C) induced higher amounts of IFN-γ than DCs exposed to L. casei or poly(I:C) alone (Fig. 6a). We found that T cells primed with DCs exposed to L. casei plus poly(I:C) showed an increased proportion of T-bet-positive cells among cells positive for high IFN-γ, providing additional evidence for enhanced Th1 development (Fig. 6b). To investigate the role of IL-12 in the ability of DCs exposed to poly(I:C), L. casei alone or a combination of poly(I:C) and L. casei to increase IFN-γ production, we evaluated the effect of an antibody neutralizing IL-12. The presence of anti-IL-12 monoclonal antibody (mAb) during DC/T-cell co-cultures impaired Th1 differentiation, indicating that the IFN-γ production was critically dependent on the ability of DCs to secrete IL-12p70 (Fig. 6c). In a recent study, we found that DCs exposed to L. casei in the absence of a TLR agonist converted naïve T cells into suppressor T cells;16 in the present study, we found that T cells primed with L. casei plus poly(I:C)-treated DCs lost their capacity to exert an inhibitory function but T cells primed with L. casei-treated DCs did not (Fig. S3). These data provide evidence for two different and opposite effects of L. casei on DCs and demonstrate that L. casei-treated DCs require a TLR3 signal to become immunogenic.
Figure 6.
The combination of Lactobacillus casei and poly(I:C) instructs dendritic cells (DCs) to facilitate T helper type 1 (Th1) responses. DCs were stimulated with L. casei 4 hr prior to the addition of poly(I:C). Activated DCs were cultured for 5 days with allogeneic naïve CD4+ T cells and expanded with interleukin (IL)-2. After 3 days, primed T cells were re-stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin for 6 hr in the presence of monensin. (a) Interferon (IFN)-γ [mean fluorescence intensity (MFI)] and (b) the percentage of IFN-γ+ T-bet+ cells were assessed using intracytoplasmic staining. One representative experiment of three is shown. (c) Neutralizing anti-IL-12 or isotype-matched control monoclonal antibody (mAb) was added during primary cultures. The percentage of IFN-γ+ cells is shown as the mean ± standard error of the mean (SEM) for five independent experiments. Student’s t-test was performed: **P < 0·01; *P < 0·05. iDC, immature dendritic cell.
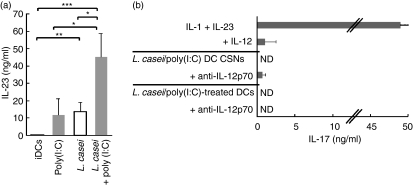
Perturbation of homeostatic conditions by pathogen invasion drives the development of an immune response that must result in protection of the host without inducing inflammatory disorders. Th17 may be associated with the development of chronic inflammatory disorders26,27 as well as with host defence against selected bacterial infections of the gut.28 Although DCs exposed to L. casei alone or together with TLR agonists released substantial amounts of Th17-polarizing cytokines that included IL-6, IL-1β (Fig. S1a) and IL-23 (Fig. 7a), they were unable to drive a Th17-polarizing programme. Indeed, neither culture supernatants of L. casei/poly(I:C)-activated DCs nor the DCs themselves promoted Th17 polarization (Fig. 7b). As L. casei and poly(I:C) acted in synergy to induce IL-12p70 release, which inhibited IL-1- and IL-23-induced Th17 differentiation, we examined the impact of one mAb that selectively neutralized IL-12p70 but not IL-23 on Th17 development. IL-12p70 neutralization strongly inhibited IFN-γ production in T-cell primary cultures (Fig. S4). We found that anti-CD3-activated naïve T cells stimulated in the presence of culture supernatants of L. casei/poly(I:C)-exposed DCs and anti-IL-12p70 mAb secreted small quantities of IL-17, which generally remained lower than those produced by IL-1- and IL-23-stimulated Th17 cells. Nevertheless, in spite of IL-12p70 neutralization at T-cell priming, L. casei/poly(I:C)-exposed allogeneic DCs consistently failed to induce Th17 differentiation.
Figure 7.
Lactobacilluscasei/Toll-like receptor 3 (TLR3) signal co-operation does not promote T helper type 17 (Th17) responses. (a) Interleukin (IL)-23 secretion in the culture supernatants (CSNs) of dendritic cells (DCs) stimulated with L. casei and/or poly(I:C). The mean ± standard error of the mean (SEM) for five experiments is shown. Student’s t-test was performed: ***P < 0·001; **P < 0·01; *P < 0·05. (b) Plate-bound anti-CD3 activated naïve T cells were cultured in the presence of anti-interferon (IFN)-γ, IL-23 and IL-1 with or without IL-12 [top: mean ± standard deviation (SD) of two experiments] or the CSNs of L. casei/poly(I:C)-activated DCs with or without anti-IL-12p70 monoclonal antibody (mAb) (middle: mean ± SD of four experiments). Purified naïve T cells were co-cultured with L. casei/poly(I:C)-activated allogeneic DCs with or without anti-IL-12p70 mAb (bottom: mean ± SD of four experiments). IL-17 production was measured by enzyme-linked immunosorbent assay (ELISA) after phorbol 12-myristate 13-acetate (PMA)/ionomycin re-stimulation. ND, not detectable.
We thus conclude that unique L. casei/TLR3 signalling on DCs induces large amounts of IL-12p70 and IL-23 which favour Th1 without driving a Th17 response.
Discussion
The mechanisms by which DCs translate information received from the microbial products they encounter are of significance in the maintenance of homeostasis and the establishment of protective immune responses.8,25 The intestinal mucosa is permanently exposed to trillions of potentially harmful bacteria, but the protective response of the host is selectively directed against pathogens.1,2 Following pathogen intrusion, the mechanisms by which DCs discriminate between virulent and non-virulent bacteria or viruses and then adapt their responses to elicit immunity versus tolerance remain to be elucidated.
Here we demonstrated that DCs exposed to at least one species of commensal-related bacterium, in this case L. casei, can trigger a robust IL-12-dependent Th1 response, a process that is dictated by DC encounter with TLR3 agonists. However, naïve T cells activated in the presence of cell-free supernatants of L. casei combined with a TLR3 agonist or DCs themselves were not polarized towards Th17 in spite of the release by DCs of substantial amounts of IL-6, IL-1β and IL-23, and neutralization of IL-12p70 in T-cell primary cultures. This may reflect inappropriate DC signalling and/or the inability of monocyte-derived DCs to direct Th cell fate towards Th17.12 In this regard, a recent study reported that the supernatant of yeast zymozan-activated monocyte-derived DCs induced IL-17 secretion by human CD45RO− T cells.29 It has been clearly demonstrated that commensal bacteria interact with the TLR expressed by gut epithelial cells to indirectly control DC function and dendrite extension.13,30 Nonetheless, a direct non-invasive bacterium/DC interaction can be visualized in vivo30 and the importance of DC-associated antigen in the induction of oral tolerance or a protective immune response is well documented.31 Our data indicate that exposure to poly(I:C) after, at the same time as, or prior to exposure to L. casei had a synergistic effect on DCs, selectively inducing IL-12p70 secretion. The latter was down-regulated by the addition of resveratrol, suggesting the involvement of TRIF-coupled receptor signalling pathways. We then postulated that this synergy was not restricted to L. casei and the TLR3 agonist. We found that L. casei moderately facilitated LPS-induced IL-12p70 production. In contrast to poly(I:C), when LPS was added together with or prior to L. casei, it inhibited IL-12p70 production. As the gut is constantly loaded with large amounts of LPS, DCs may be refractory to CB-induced IL-12p70 release, unable to induce a Th1 programme and thus poised to maintain gut tolerance. However, direct contact between DCs and CB and viruses may be occurring in vivo. Because DC exposure to L. casei and poly(I:C) boosted IL-12-driven Th1 responses, we propose acute viral intestinal disease as a relevant in vivo context for our in vitro observations. This proposal is based on the consensus that probiotics contribute significantly to the relief of acute diarrhoea caused by rotavirus [a double-stranded RNA (dsRNA) virus] but not to that caused by coronarovirus (an ssRNA virus).32Lactobacillus casei in fermented milk attenuates the incidence and duration of diarrhoea in infants.33 Similarly, elderly patients who regularly consumed a probiotic drink containing L. casei displayed a reduced incidence of diarrhoea.34 Finally, L. casei administered in food protects rats from rotavirus-associated diarrhoea.35 The manipulation of the dominant microbial community by probiotics is a prerequisite for the exertion of such potentially beneficial effects. In this regard, recent reports have demonstrated the existence of functional interactions between the common Gram-negative commensal-related bacterium B. thetaiotaomicron and a selective Bifidobacterium strain that result in changes in the bacterial and host genes.36,37
The importance of TLR3/TLR4 signalling in vivo is further illustrated by the control of viral infection in IL-1R-associated kinase-4 (IRAK)-4 deficient patients lacking TLR7/TLR8/TLR9-dependent pathways.38 Because two TLR signals (one TRIF-coupled TLR signal plus one endosomal receptor signal) must be delivered to human monocyte-derived DCs to sustain their IL-12 production and induce strong Th1-polarizing capacity,18,21 here we propose that L. casei may substitute for one signal to facilitate IL-12p70 and Th1 protective responses. Signalling pathways through MyD88 and interferon regulatory factor 5 (IRF)5 are required for IL-12p40 and IL-23p19 gene activation, respectively, whereas TRIF/IRF3 transactivates and amplifies IL-12p35.39,40 Here we found that L. casei/poly(I:C) co-operation enhanced IL-12p70 and IL-23 secretion. However, the molecular pathways involved in L. casei/TLR3 synergy remain to be elucidated. Taken together, our results show that DCs may integrate signals received from commensal flora and viral products, extending the concept that selected TLR agonist combinations synergistically trigger IL-12 production by DCs and the Th1-polarizing programme.18,21 Therefore, the search for additional critical CB/TLR combinatorial codes and elucidation of the molecular pathways involved may open avenues for the use of probiotics as potential adjuvants for the delivery of efficient mucosal vaccines.
Acknowledgments
We are grateful to Dr A. K. Palucka for critical comments on the manuscript. This work was supported by grants in aid from Danone Research and the Canadian Institute of Health Research (no. MOP-53152).
Author contributions
NB performed research, analysed data, and wrote the paper; MR performed research; RB-S and SS contributed vital new reagents; MS designed the research, analysed data and wrote the paper.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Cytokine profile of dendritic cells (DCs) stimulated with Lactobacillus casei or Bifidobacterium animalis in combination with various Toll-like receptor (TLR) agonists.
Figure S2. Cytokine profile of dendritic cells (DCs) stimulated with poly(I:C) or lipopolysaccharide (LPS) in combination with various strains of commensal-related bacteria (CB).
Figure S3. Lactobacillus casei/Toll-like receptor 3 (TLR3)- treated dendritic cells (DCs) lose their capacity to induce suppressive T cells.
Figure S4. Anti-interleukin (IL)-12p70 strongly inhibits interferon (IFN)-γ production in T-cell primary culture.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–62. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazmanian SK, Kasper DL. The love–hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849–58. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 3.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Mo JH, Shen C, Rucker AN, Raz E. Toll-like receptor signaling in intestinal epithelial cells contributes to colonic homoeostasis. Curr Opin Gastroenterol. 2007;23:27–31. doi: 10.1097/MOG.0b013e3280118272. [DOI] [PubMed] [Google Scholar]
- 5.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–29. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G, Sher A. Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 7.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182–93. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454 doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8 doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 13.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–26. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 14.Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a toll-like receptor 2 pathway. J Allergy Clin Immunol. 2006;117:696–702. doi: 10.1016/j.jaci.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol. 2008;84:468–76. doi: 10.1189/jlb.0108017. [DOI] [PubMed] [Google Scholar]
- 17.Smits HH, van Beelen AJ, Hessle C, et al. Commensal gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–80. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 18.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varol C, Landsman L, Fogg DK, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–80. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164:2193–9. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 21.Gautier G, Humbert M, Deauvieau F, et al. A type I interferon autocrine–paracrine loop is involved in toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880–5. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–46. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 24.Iyori M, Kataoka H, Shamsul HM, Kiura K, Yasuda M, Nakata T, Hasebe A, Shibata K. Resveratrol modulates phagocytosis of bacteria through an NF-kappaB-dependent gene program. Antimicrob Agents Chemother. 2008;52:121–7. doi: 10.1128/AAC.00210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 29.Gerosa F, Baldani-Guerra B, Lyakh LA, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–61. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szymanski H, Pejcz J, Jawien M, Chmielarczyk A, Strus M, Heczko PB. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains – a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23:247–53. doi: 10.1111/j.1365-2036.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 33.Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J Clin Pract. 2000;54:568–71. [PubMed] [Google Scholar]
- 34.Hickson M, D’Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerin-Danan C, Meslin JC, Chambard A, Charpilienne A, Relano P, Bouley C, Cohen J, Andrieux C. Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. J Nutr. 2001;131:111–7. doi: 10.1093/jn/131.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajzer M, Seeley RJ. Physiology: obesity and gut flora. Nature. 2006;444:1009–10. doi: 10.1038/4441009a. [DOI] [PubMed] [Google Scholar]
- 38.Yang K, Puel A, Zhang S, et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–78. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–6. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 40.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–5. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.