Abstract
Experimental autoimmune myasthenia gravis (EAMG), an animal model of myasthenia gravis (MG), is a rare organ-specific autoimmune disease targeting the autoantigen nicotinic acetylcholine receptor (AChR). We show here that the balance of T helper type 1 (Th1), Th2, Th17 and regulatory T (Treg) subsets of CD4+ helper T cells were redistributed during the development of EAMG and that the interleukin-17 (IL-17) cytokine is involved in this disease. The ratio of Th17 cells changed most notably with disease progression accompanied by an up-regulated level of IL-17. Moreover, the proliferative ability of AChR peptide-specific T cells and the anti-AChR antibody-secreting cells increased when stimulated by IL-17 in vitro. These findings suggested that the disequilibrium of the CD4+ helper T-cell subsets could promote the development of EAMG, and the pathogenic mechanism by which Th17 cells drives autoimmune responses by secreting cytokine IL-17 provides a new target for myasthenia gravis therapy.
Keywords: CD4+ helper T-cell subsets, disequilibrium, experimental autoimmune myasthenia gravis, interleukin-17, T helper type 17
Introduction
Autoreactive T-cell responses may be responsible for the pathogenesis of various autoimmune diseases.1 Previously, CD4+ helper T (Th) cells were subdivided into Th1, Th2 and regulatory (Treg) cell subsets, and the cellular responses of these subpopulations were initially implicated in the pathogenesis of many organ-specific autoimmune diseases.2–5 The Th1 cells secrete pro-inflammatory cytokines such as interferon-γ (IFN-γ) and interleukin-2 (IL-2), which can transfer cell-mediated immunity. The Th2 cells secrete anti-inflammatory cytokines like IL-4 and IL-10, which also stimulate the growth and differentiation of B cells and induce the production of antibodies. Additionally, Treg cells inhibit certain immune cell responses and are also considered to be anti-inflammatory. However, a new distinct subset of Th cells has been discovered and designated Th17 based on its secretion of the novel pro-inflammatory cytokine IL-17.6–11 The Th17 cells probably evolved to enhance host clearance of a range of pathogens distinct from those targeted by Th1 and Th2. Studies have revealed that Th17 cells are the major pathogenic T-cell subset in experimental autoimmune encephalitis, which was previously considered to be a Th1-mediated disease.12,13 It is also thought that Th17 cells play an important role in host defence against extracellular pathogens, which are not effectively cleared by Th1 or Th2 cells. Being highly pro-inflammatory, Th17 cells directed against self antigens cause severe autoimmune diseases such as collagen-induced arthritis.13,14 Meanwhile, studies in several experimental models have indicated that IL-17 is a major mediator of tissue inflammation.4,5 Augmented expression of this cytokine is observed in patients with various diseases, such as rheumatoid arthritis,15 systemic lupus erythematosus,16 allograft rejection,17 nephritic syndrome,18 asthma19 and multiple sclerosis.20 Additionally, overproduction of IL-17 has been noted in a variety of animal models.21–23 However, the immunoregulation of IL-17 and the relationship among Th1, Th2, Treg and Th17 cells during the initiation and development of autoimmune diseases have not yet been clearly investigated.
Myasthenia gravis (MG) is one of the rare organ-specific autoimmune diseases for which the target autoantigen, the nicotinic acetylcholine receptor (AChR) of the neuromuscular junctions, has been well characterized.24 Immunization in susceptible mouse and rat strains with AChR emulsified in complete Freund’s adjuvant (CFA) resulted in experimental autoimmune myasthenia gravis (EAMG), which is an animal model of MG.25 The AChR-specific Th cells are associated with autoreactive antibody production and hence the autoimmune response in both EAMG and MG. Wang et al.26 demonstrated that Th17 participate in the process of mouse EAMG in IL-12/IL-23 knockout mouse. However, the immunoregulatory roles of IL-17 and Th17 cells have not been elucidated in EAMG. In this study we demonstrate that the balance of the CD4+ helper T cells was redistributed, while IL-17 and Th17 cells played a critical role in co-ordinating cognate autoreactive T cells and B cells. These interactions appear to be critical for the genesis of autoantibodies and the subsequent development of EAMG.
Materials and methods
Animals
Female Lewis rats, 6–8 weeks old and 160–180 g in weight, were purchased from Peking Vital River Laboratory Animal Ltd. (Beijing, China). All rats were bred and maintained in accordance with the Care and Use of Laboratory Animals guidelines published by the China National Institute of Health.
Reagents
The peptide corresponding to the α97–116 region of the rat AChR α-subunit, R97–116 (DGDFAIVKFTKVLLDYTGHI), was synthesized by AC Scientific, Inc. (Xian, China). The rat myelin basic protein (MBP) 68–86 peptide (YGSLPQKSQRSQDENPV) was synthesized by Sangon Ltd. (Shanghai, China). The IL-17 cytokine was purchased from PeproTech EC Ltd. (London, UK). Mycobacterium tuberculosis (strain H37RA) was purchased from Difco (Detroit, MI) and CFA was purchased from Sigma-Aldrich (St Louis, MO). The following antibodies were purchased from commercially available sources: fluorescein isothiocyanate (FITC)-conjugated anti-rat CD4 and phycoerythrin (PE)-conjugated anti-rat Foxp3 (eBioscience, San Diego, CA); PE-conjugated anti-rat IFN-γ; PE-conjugated anti-rat IL-4 and mouse anti-rat CD3 (BD Biosciences, San Jose, CA); rabbit anti-rat IL-17 (Santa Cruz Biotechnology, Santa Cruz, CA); Cy3-conjugated goat anti-rabbit immunoglobulin G (IgG) and PE-Cy5-conjugated goat anti-mouse IgG (Caltag Laboratories, Burlingame, CA). The fixation and permeabilization kit used for flow cytometry was supplied by eBioscience.
Induction and clinical scoring of EAMG
The EAMG rats were anaesthetized and immunized subcutaneously in the tail with 200 μl inocula containing 50 μg R97-116 peptides in 100 μl CFA supplemented with 1 mg M. tuberculosis and 100 μl phosphate-buffered saline (PBS) on day 0, and then the rats were boosted on day 30 with the same peptide in incomplete Freund’s adjuvant. The control rats, designated as the CFA group, received the same emulsion except PBS was used instead of the peptide.
After the first immunization, each animal was weighed on alternate days until killed (6–8 weeks). The severity of clinical signs of EAMG was scored by measuring muscular weakness in a blinded fashion. Clinical scoring was based on the presence of tremor, hunched posture, muscle weakness, and fatiguability. Fatiguability was assessed after exercise (repetitive paw grips on the cage grid) for 30 seconds. Disease severity was expressed as follows26: Grade 0 = normal muscle strength; Grade 1 = mildly decreased activity, weak grip, fatiguable; Grade 2 = weakness, hunched posture at rest, decreased body weight, tremor; Grade 3 = severe generalized weakness, marked decrease in body weight, moribund; Grade 4 = dead. Rats with intermediate signs were assigned grades of 1·5, 2·5 or 3·5, as appropriate. Results are expressed as the mean score of each group at each time-point.
Preparation of mononuclear cells from lymph nodes
Mononuclear cells (MNC) were obtained from the popliteal, inguinal and para-aortic lymph nodes isolated from killed rats of both EAMG and CFA groups on day 14 and day 42 after the first immunization. Cells were washed three times in RPMI-1640, then cultured in EAMG lymphocyte culture medium, which was RPMI-1640 medium (Hyclone, Logan, UT) containing 10% fetal bovine serum (Gibco, Paisley, UK), 1%l-glutamine, 1% sodium pyruvate, 1% non-essential amino acids, 2 × 10−5 m 2-mercaptoethanol (Amresco, Solon, OH) and 1% penicillin–streptomycin (Gibco). Lymph node MNC were then adjusted to 2 × 106 cells/ml.
T-cell proliferation assay
Triplicate aliquots (200 μl) of MNC suspensions containing 4 × 105 cells were placed in 96-well, round-bottom microtitre plates, and stimulated with R97–116 peptides (10 μg/ml), R97–116 peptides (10 μg/ml) + IL-17 (0·05 μg/ml), or PBS. Concanavalin A was used at 5 μg/ml as a positive control. After 54 hr of incubation, the cells were pulsed for another 18 hr with 10 μl PBS containing 1 μCi [3H]methylthymidine (specific activity 60 Ci/mmol; China Institute of Atomic Energy, Beijing, China), and results were expressed as mean counts per minute of triplicate cultures.
Measurement of cytokines
To detect the cytokine levels in serum, EAMG and CFA rats were killed on day 14 and day 42 after the first immunization. The serum was collected for IFN-γ, IL-4, IL-17, transforming growth factor-β (TGF-β) and IL-6 detection by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Senxiong Biotech Industry Company, Shanghai, China). Each sample was tested in triplicate.
To detect the cytokine levels in lymphocyte culture supernatant, single-cell suspensions of lymph nodes cells from EAMG and CFA rats were cultured as described above, and then cultured in the presence of R97–116 peptides (10 μg/ml). Supernatants from different cell culture groups were collected after 72 hr of stimulation for IFN-γ, IL-4, IL-17, TGF-β and IL-6 detection by ELISA as above. Values were expressed as the mean cytokine concentration (pg/ml) ± SD.
Enumeration of AChR-specific IgG-secreting cells
A solid-phase enzyme-linked immunospot (ELISPOT) assay was employed with some modifications to detect AChR-specific IgG-secreting cells. Splenocyte suspensions were prepared and then adjusted to a cell concentration of 2 × 106 cells/ml as described above. The wells were coated with R97–116 peptides (10 μg/ml) or MBP 68–86 peptides (10 μg/ml) in 100 μl/well overnight at 4°. Aliquots of 100 μl containing 2 × 105 MNC were added to individual wells. Then, IL-17 was added to selected wells at a concentration of 0·025 μg/ml as a stimulating cytokine. After incubation for 24 hr, the wells were emptied, followed by the addition of rabbit anti-rat IgG, biotinylated swine anti-rabbit IgG, and avidin-biotin-peroxidase complex (ABC). After peroxidase staining, the red-brown immunospots which corresponded to the cells that had secreted anti-AChR or anti-MBP IgG antibodies were counted under a dissection microscope and standardized to numbers of spots per 105 MNC.
Determination of Th lymphocyte subsets by FACS
EAMG and CFA rats were killed on day 14 and day 42 after the first immunization. Lymph node MNC were harvested and prepared as described above. To evaluate distribution profiles of the CD4+ T-cell subsets, we performed a standard flow cytometry assay. Brefeldin A, a protein transport inhibitor that inhibits cytokine secretion, was added into the cell culture media and incubated for 5 hr. After washing twice with staining buffer, cells were stained extracellularly with mouse anti-rat CD3 followed by PE-Cy5-conjugated goat anti-mouse IgG or FITC-conjugated anti-CD4. After fixation and permeabilization, cells were stained intracellularly with PE-conjugated anti-rat-IFN-γ, anti-rat-IL-4, anti-rat-Foxp3, or rabbit anti-IL-17 followed by Cy3-conjugated goat anti-rabbit IgG. Isotype-matched antibodies conjugated with FITC or PE were used as negative controls. Samples were tested with a FACSCalibur flow cytometer and data were analysed with cell quest pro software (BD Biosciences, San Jose, CA).
Immunohistochemistry
Frozen spleen sections were stained with rabbit anti-rat IL-17 followed by a horseradish peroxidase-labelled anti-rabbit secondary antibody and a 3,3′-Diaminobenzidine (DAB) substrate to detect IL-17 expression. Section areas and positive cells were measured from digital images using image pro plus software (Media Cybernetics, Silver Springs, MD). The results were averaged and expressed as cells/mm2 tissue section.
Statistical analysis
Data are expressed as the means ± SD. Differences between pairs of groups were analysed by two-tailed Student’s t-test. Clinical scores were analysed using the non-parametric Mann–Whitney U-test. The level of significance was set as P< 0·05.
Results
EAMG induction
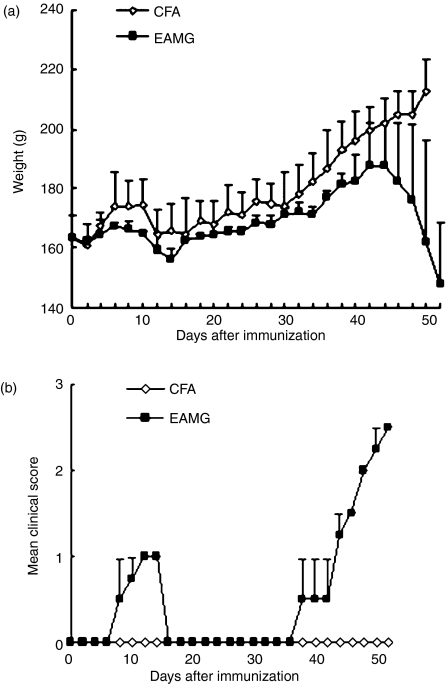
EAMG is routinely induced in Lewis rats by a single immunization of purified AChR from Torpedo electroplax in CFA. In this study we used the peptides R97–116 of the rat AChR α-subunit in CFA to establish the EAMG.27 The immunization protocol used was as follows: one immunization with the peptide (50 μg) in CFA subcutaneously in the tail base on day 0, followed by a booster injection of the same peptide (50 μg) in incomplete Freund’s adjuvant on day 30. The onset and progression of the disease are shown in Fig. 1. The graph shows two typical clinical phases. The EAMG rats exhibited muscular weakness around 8 days post-immunization. The weakness was moderately severe (0–1) and transient, disappearing after 14 days. Weakness recurred from 38 day after the first immunization (8 days after the booster) and was progressive (0–3); at the same time the average weight decreased. In contrast, CFA rats developed no obvious EAMG symptoms.
Figure 1.
Variations in body weight (a) and clinical scores of rats immunized with R97-116 peptides. (b) experimental autoimmune myasthenia gravis (EAMG) rats developed mild symptoms on day 8 after the first immunization and lasted about 8 days, then recovered. Clinical manifestations of EAMG such as loss of body weight, tremor, and muscle weakness were evident gradually from day 42 after the first immunization and rats developed severe EAMG. Rats given complete Freund’s adjuvant (CFA rats) showed no muscle weakness (n = 8 rats/group).
The altered distribution of subsets of CD4+ T cells during the development of EAMG
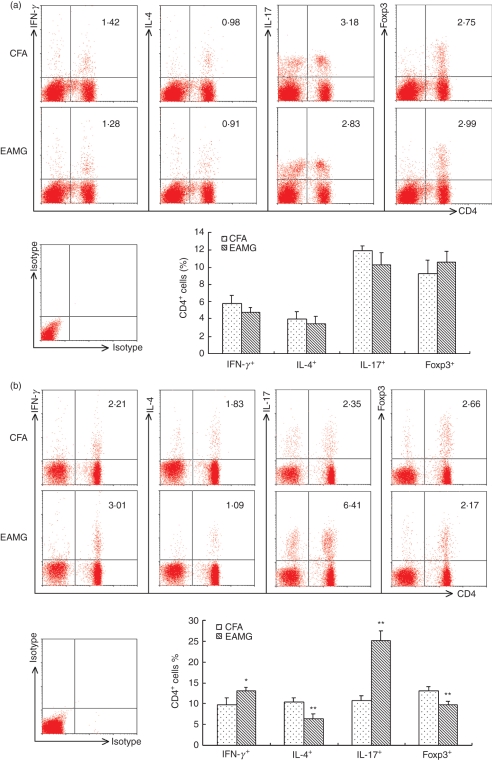
To examine the altered distribution of subsets of CD4+ T cells, we isolated lymph node MNC from CFA and EAMG rats on days 14 and 42 after the first immunization. The cells were cultured for 72 hr in the presence of R97–116 peptides, and stained with anti-rat-CD3 to set the gate, then costained with anti-rat-CD4 and anti-rat-IFN-γ, anti-rat-IL-4, anti-rat-IL-17, or anti-rat-Foxp3. CD4+ IFN-γ+ T cells represented Th1 cells; CD4+ IL-4+ T cells represented Th2 cells; CD4+ IL-17+ T cells represented Th17 cells; and CD4+ Foxp3+ T cells represented Treg cells. We first compared the percentage of these four subsets of CD4+ T cells in the early phase. As shown in Fig. 2(a), the percentage of Th1 and Th17 cells of EAMG rats was lower than that of CFA rats. However, the percentages of Treg and Th2 cells were higher in the EAMG rats, although there was no statistically significantly difference between the two groups.
Figure 2.
The altered distribution of subsets of CD4+ T cells during the development of experimental autoimmune myasthenia gravis (EAMG). Lymph node mononuclear cells (MNC) from EAMG rats and from rats given complete Freund’s adjuvant (CFA rats) were isolated on day 14 (a) and day 42 (b) after the first immunization. Expressions of interferon-γ (IFN-γ), interleukin-4 (IL-4), IL-17 and Foxp3 in CD4+ T cells were detected by fluorescence-activated cell sorting, all plots are gated on live CD3+ T cells. The figures are representative of three experiments repeated with similar results and the bar graphs summarize the data. (*P< 0·05, **P< 0·01 versus CFA group, n = 8 rats/group).
Interestingly, as shown in Fig. 2b, increased percentages of Th1 cells (P< 0·05) and Th17 cells (P< 0·01) together with reduced percentages of Th2 cells (P< 0·01) and Treg cells (P< 0·01) were observed in EAMG rats compared with the CFA rats in the late phase. These data suggest that disequilibrium of the four CD4+ helper T-cell subsets occurred in the late phase of EAMG.
Cytokine levels in rat serum and culture supernatants
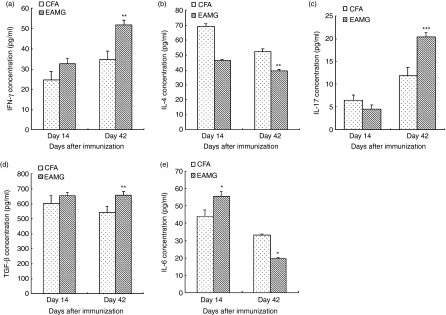
To detect whether the IFN-γ, IL-4, IL-17, TGF-β and IL-6 cytokines were involved in the disease processes, serum was collected from EAMG and CFA rats killed on day 14 and day 42 after the first immunization, and the cytokine levels in serum were detected by ELISA. As shown in Fig. 3, serum IL-4 and IL-17 levels were slightly lower while IFN-γ, TGF-β and IL-6 levels were higher in the EAMG group on day 14. However, on day 42, we found increased levels of IFN-γ (P< 0·01), IL-17 (P< 0·001) and TGF-β (P< 0·01) and reduced levels of IL-4 (P< 0·01) and IL-6 (P< 0·05) in the EAMG group compared with the CFA group.
Figure 3.
Cytokine concentrations in serum. Experimental autoimmune myasthenia gravis (EAMG) rats and rats given complete Freund’s adjuvant (CFA rats) were killed on day 14 and day 42 after the first immunization. Interferon-γ (IFN-γ) (a), interleukin-4 (IL-4) (b), IL-17 (c), transforming growth factor-β (TGF-β) (d) and IL-6 (e) concentrations in serum were detected by enzyme-linked immunosorbent assay. Data are the means ± SD of triplicates of three independent experiments. (*P< 0·05, **P< 0·01, ***P< 0·001 versus CFA group, n = 8 rats/group).
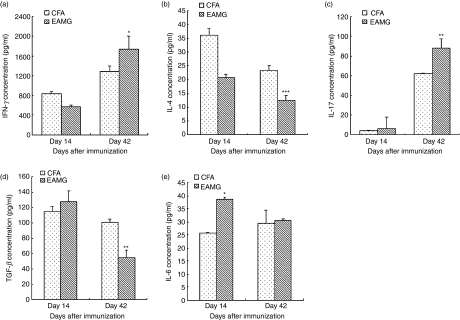
In the culture supernatants, as shown in Fig. 4, the IL-17, TGF-β and IL-6 concentrations were increased in the EAMG rats, while the IFN-γ and IL-4 levels were nearly the same between the CFA and EAMG rats on day 14. Subsequently, on day 42, IFN-γ (P< 0·05), IL-4 (P< 0·001) and IL-17 (P< 0·01) levels were significantly increased in the EAMG group compared with the CFA group, although the production of TGF-β (P< 0·01) was reduced. The average IL-6 concentration was still higher in the EAMG rats, but there was no statistically significant difference between the two groups.
Figure 4.
Cytokine concentrations in acetylcholine receptor (AChR)-specific mononuclear cell (MNC) culture supernatants. MNC were isolated from experimental autoimmune myasthenia gravis (EAMG) rats and rats given complete Freund’s adjuvant (CFA rats) killed on day 14 and day 42 after the first immunization and cultured in the presence of R97–116 peptides for 72 hr. The supernatants were collected and used to detect the interferon-γ (IFN-γ) (a), interleukin-4 (IL-4) (b), IL-17 (c), transforming growth factor-β (TGF-β) (d) and IL-6 (e) concentrations by enzyme-linked immunosorbent assay. Data are the means ± SD of triplicates of three independent experiments. (*P< 0·05, **P< 0·01 versus CFA group, n = 8 rats/group).
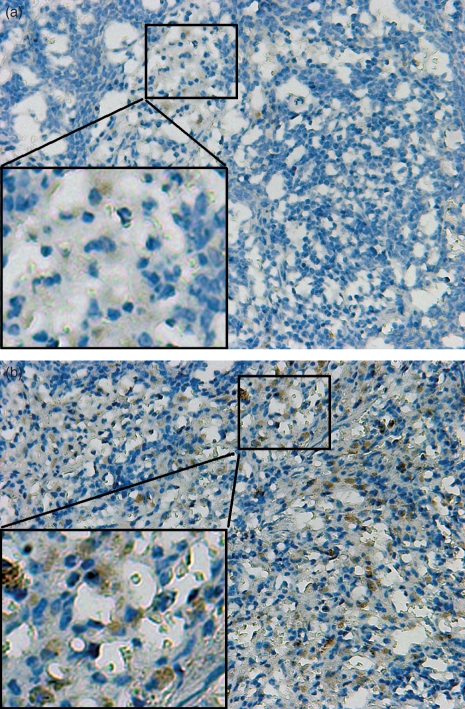
Increased expression of IL-17 in the spleen tissue of EAMG rats
The spleens from the rats were processed as indicated in the Materials and methods section for the detection of IL-17 by immunohistochemistry. The expression of IL-17 in the spleen tissue in the EAMG rats (15·82 ± 0·47 cells/mm2) versus the CFA group (6·01 ± 0·40 cells/mm2) was significantly increased (Fig. 5, P< 0·05).
Figure 5.
Expression of interleukin-17 (IL-17) in spleen tissue sections from experimental autoimmune myasthenia gravis (EAMG) rats and rats given complete Freund’s adjuvant (CFA rats) on day 42 post-immunization. Immunohistochemical staining of IL-17-positive cells in rat splenic tissues. (a) Expression of IL-17-positive cells in CFA rats. (b) Expression of IL-17-positive cells in EAMG rats. Magnification: × 200.
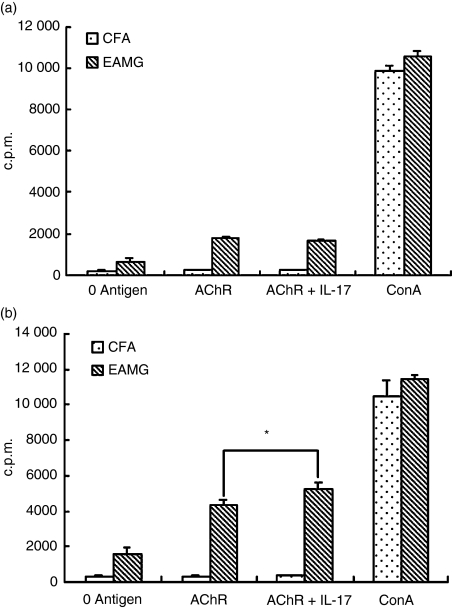
IL-17 enhancement of AChR-specific T-cell proliferation
To find whether the cytokine IL-17 can affect the AChR-specific T-cell proliferation, we immunized Lewis rats with R97–116 peptides. In the early phase on day 14 after the first immunization, or in the late phase on day 42 when EAMG was evident, lymph node MNC were prepared and analysed for proliferative responses as described in the method. In the early phase the cells from EAMG rats showed no obvious proliferative activity in response to IL-17 (Fig. 6a). However, the specific response against IL-17 became evident in the later phase of EAMG. As shown in Fig. 6(b), IL-17 significantly increased the T-cell proliferative responses of EAMG rats to rat AChR 97–116 peptides compared with those of the CFA rats (P< 0·05). There was no significant difference between EAMG and CFA rats when MNC were cultured in the absence of antigen or with concanavalin A.
Figure 6.
Proliferative responses of lymph node mononuclear cells (MNC) from experimental autoimmune myasthenia gravis (EAMG) rats and rats given complete Freund’s adjuvant (CFA rats) stimulated by interleukin-17 (IL-17). Lymph node MNC were isolated from CFA and EAMG rats on day 14 (a) and day 42 (b) after the first immunization. Triplicate MNC were challenged in vitro with R97–116 peptides with or without IL-17. Cells were incubated for 72 hr with the addition [3H]methylthymidine for the final 18 hr of culture. Values are expressed as the mean counts per minute (c.p.m.) ± SD of three independent experiments. (*P < 0·05 versus EAMG group in the absence of IL-17, n = 8 rats/group).
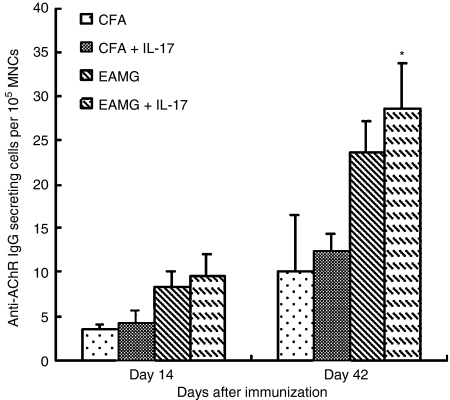
Effects of IL-17 on B-cell responses assessed by anti-AChR IgG antibody-secreting cells
The anti-AChR IgG antibody-secreting B cells were detected by ELISPOT. As the EAMG developed, the numbers of anti-AChR antibody-secreting B cells gradually increased (Fig. 7). Compared with the cells which had no stimulation, an increased number of anti-AChR antibody-secreting B cells were found under IL-17 stimulation (P< 0·05) in the EAMG group in the late phase. In the early phase more anti-AChR antibody-secreting cells were observed after adding IL-17, but there were no significant statistical differences (P> 0·05). The IL-17-stimulated levels were significantly lower in the CFA rats when examined in both early and late phases. No significant statistical differences were found in experiments when cells were cultured with MBP or left without antigen between the EAMG rats and the CFA rats (data not shown).
Figure 7.
Measurement of acetylcholine receptor (AChR)-specific antibody-secreting cells/105 splenocytes by an ELISPOT assay. Splenocytes from experimental autoimmune myasthenia gravis (EAMG) rats and rats given complete Freund’s adjuvant (CFA rats) were harvested and cultured in vitro in the presence of R97–116 peptides. Interleukin-17 (IL-17) was placed in every second well in parallel. Comparisons were made between the early and late phases, and results are expressed as mean values ± SD of three independent experiments. (*P< 0·05 versus EAMG group in the absence of IL-17, background spots 0–3, n = 8 rats/group).
Discussion
During the development of rat EAMG, there are two clinical phases. The early phase of EAMG begins about 7 days post-immunization and is self-limiting. Shi et al.28 demonstrated that the macrophages colocalized in the end-plate regions during the early phase of EAMG in Lewis rats. Apoptosis of the infiltrating macrophages is a major cause for their elimination during the early phase. The late phase of EAMG is mediated by anti-AChR self-reactive antibodies. Recently, it was demonstrated that the AChR-specific CD4+ helper T-cell subsets played a critical role during the MG/EAMG pathogenic process. They induced autoreactive B cells to produce pathogenic autoantibodies by producing pro-inflammatory cytokines and delivering costimulatory signals.29
Historically, Th1 cells (CD4+ IFN-γ+), Th2 cells (CD4+ IL-4+) and Treg cells (CD4+ Foxp3+) were characterized as classic CD4+ helper T-cell subsets and participated in the autoimmune disease by the cytokine they secreted. The Th1 cytokine IFN-γ is required during the initiation and development of an organ-specific autoimmune disease, the localized expression of the IFN-γ transgenic in the neuromuscular junction or administration of IFN-γ had more severe EAMG,30,31 and gene knockout of IFN-γ leads to a susceptibility to EAMG.32 However, the role of IL-4 in EAMG has been disputed. Balasa et al. indicated that IL-4 was not required for EAMG,33 while Milani et al. stated that IL-4 could prevent the development of EAMG and its progression to a self-maintaining, chronic autoimmune disease.34,35 Nevertheless, the disequilibrium of these two subsets has been considered to be the major contributor to pathogenesis in the development of EAMG.36 The Treg cells were identified as another CD4+ Th cell subset that was implicated in the tolerance of EAMG.37 Administration of ex-vivo-generated Treg cells to myasthenic rats inhibited the progression of experimental autoimmune MG and led to the down-regulation of humoral acetylcholine receptor-specific responses, the subpopulation of CD4+ CD25+ cells expressing Foxp3 cells in the spleen of treated rats was significantly elevated.38 Meanwhile, the cytokine TGF-β was suggested to play a role in strain-associated resistance to EAMG.30
Currently, the new CD4+ helper T-cell subset of Th17 cells and its effector cytokine IL-17 are considered to have demonstrated roles in promoting inflammation and autoimmunity.8,39 However, the effect of Th17 and the IL-17 cytokine in EAMG is not clear. In this study, the ratio of Th1 (CD4+ IFN-γ+) cells, Th2 (CD4+ IL-4+) cells, Treg (CD4+ Foxp3+) cells, and Th17 (CD4+ IL-17+) cells between EAMG and CFA groups did not change significantly on day 14. However, in the late phase of EAMG on day 42, the ratio of Th1 and Th17 cells was increased while the ratio of Th2 and Treg cells was decreased. Interestingly, the change of Th17 cells was the most notable. In other words, the balance among these four CD4+ helper T-cell subsets was redistributed. To obtain further evidence, we detected IFN-γ, IL-4, IL-17 and TGF-β secretions by AChR-specific MNC in vitro. We showed that the pathogenic factors IFN-γ and IL-17 were increased, and the potential protective factors, IL-4 and TGF-β, were decreased in the EAMG group. However, the serum TGF-β level was higher in the EAMG group; therefore, we investigated the IL-6 concentration in the next step. Interleukin-6 is involved in the generation of AChR-specific T- and B-cell response40 and simultaneously, IL-6 is referred to differentiation of Th17 cells.8 Interestingly, the IL-6 level in the culture supernatant was higher in the EAMG group than in the CFA group throughout the development of the disease. Naive CD4+ T cells induced by TGF-β express Foxp3 and develop into Treg cells, while the appearance of IL-6 diverts the development of Foxp3+ regulatory cells towards the Th17 lineage.38 In our study the increased IL-6 and TGF-β promoted the development of Th17 and aggravated EAMG, and in the late phases suppressed the Treg cells. The results of serum concentrations of cytokines are mixed and that could be because of the complex network of cytokines that could be secreted by many other cells beside T lymphocytes. Together, our results indicated that the disequilibrium of Th1, Th2, Th17 and Treg cells was the major factor during the development of EAMG. Yet, therapeutic approaches targeting AChR-specific T cells have not been successfully used, leading to non-specific therapy to EAMG. The pathogenesis of EAMG was related to the imbalance of Th1/Th2/Th17/Treg cell subsets, so promoting the differentiation of Th1 and Treg cells and inhibiting the role of Th1 and Th17 cells may become a new therapeutic goal for EAMG and MG. Meanwhile, our findings demonstrated that Th17 cells could play a crucially pathogenic role in the generation of high levels of IL-17 during the development of EAMG. These results are consistent with the previous description that Th17 is the major driver of autoimmune syndromes such as collagen-induced arthritis, experimental autoimmune encephalitis,41 and lupus.42,43
Specifically, IL-17 is a pleiotropic pro-inflammatory cytokine that enhances T-cell priming and stimulates epithelial, endothelial and fibroblastic cells to produce multiple pro-inflammatory mediators, including IL-1, IL-6, TNF-α and chemokines.42,44 Consequently, the effects of IL-17 are potentially hazardous because the adaptive immune system uses it to communicate with the innate immune system to promote inflammation. Previous studies have demonstrated that IL-17 was involved in many autoimmune diseases, including rheumatoid arthritis, asthma, systemic lupus erythematosus and allograft rejection.16,45–47 Therefore, we sought to characterize the immune regulatory function of IL-17 in terms of T-cell proliferation and the production of AChR-specific antibody secreting B cells. The T-cell proliferation induced by R97–116 peptide was observed to be increased under IL-17 stimulation in vitro; that is, IL-17 can up-regulate T-cell responses.
Hsu et al.48 indicated that IL-17 could lead to the initial accumulation of B cells in germinal centres by the expression of G protein signalling Rgs13 and Rgs16, and the absence of IL-17 resulted in a smaller germinal centre response and a reduced numbers of B cells. In this study, we showed that the AChR-specific B cells increased after stimulation by IL-17 in vitro. The induction of IL-17 suggested that it could aggravate the process of EAMG by regulating the antibody-secreting ability of B cells.
In summary, the findings in our study highlight the importance of Th17 cells and IL-17 in EAMG. All of these results show that in EAMG, an antibody-mediated disease, the changed ratio of Th17 cells was the most conspicuous among the disequilibrium of Th1, Th2, Treg and Th17 cells, while the level of IL-17 was up-regulated. The increased IL-17 can effectively promote the autoreactivity of T cells, as well as B cells. The activated T and B cells could then influence each other through cytokines and aggravate the disease. Although IL-17 seemed not to be involved in the early phases of EAMG, IL-17 and Th17 cells could aggravate the disease in the late phases. Therefore, rebuilding the balance of the CD4+ helper T-cell subsets could become a new therapeutic goal for EAMG and MG.
Acknowledgments
This research was supported by National Nature Science Foundation of China (30770665), Harbin Medical University Innovation Found Harbin Medical University cell biological engineering centre (1151gzx05), Harbin Medical University Youth Science Fund (060039), and Harbin Science & Technology Bureau Creative Talent Fund (2008RFQXS085).
Disclosures
The authors have no financial conflict of interest.
References
- 1.McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100:295–306. doi: 10.1111/j.1471-4159.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzaki J, Tsuji T, Imazeki I, Ikeda H, Nishimura T. Immunosteroid as a regulator for Th1/Th2 balance: its possible role in autoimmune diseases. Autoimmunity. 2005;38:369–75. doi: 10.1080/08916930500124122. [DOI] [PubMed] [Google Scholar]
- 3.Keino H, Takeuchi M, Usui Y, Hattori T, Yamakawa N, Kezuka T, Sakai JI, Usui M. Supplementation of CD4+CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:105–10. doi: 10.1136/bjo.2006.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen CH, Hegedüs L, Rieneck K, Moeller AC, Leslie RG, Bendtzen K. Production of interleukin (IL)-5 and IL-10 accompanies T helper cell type 1 (Th1) cytokine responses to a major thyroid self-antigen, thyroglobulin, in health and autoimmune thyroid disease. Clin Exp Immunol. 2007;147:287–95. doi: 10.1111/j.1365-2249.2006.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–91. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 11.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 12.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 13.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 17.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–84. [PubMed] [Google Scholar]
- 18.Matsumoto K, Kanmatsuse K. Increased urinary excretion of interleukin-17 in nephrotic patients. Nephron. 2002;91:243–9. doi: 10.1159/000058399. [DOI] [PubMed] [Google Scholar]
- 19.Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 21.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 22.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 23.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–90. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner T, Nizri E, Irony-Tur-Sinai M, Hamra-Amitay Y, Wirguin I. Acetylcholinesterase inhibitors and cholinergic modulation in myasthenia gravis and neuroinflammation. J Neuroimmunol. 2008;202:121–7. doi: 10.1016/j.jneuroim.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clin Immunol. 2000;94:75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Milani M, Ostlie N, Okita D, Agarwal RK, Caspi RR, Conti-Fine BM. C57BL/6 mice genetically deficient in IL-12/IL-23 and IFN-gamma are susceptible to experimental autoimmune myasthenia gravis, suggesting a pathogenic role of non-Th1 cells. J Immunol. 2007;178:7072–80. doi: 10.4049/jimmunol.178.11.7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baggi F, Annoni A, Ubiali F, et al. Breakdown of tolerance to a self-peptide of acetylcholine receptor alpha-subunit induces experimental myasthenia gravis in rats. J Immunol. 2004;172:2697–703. doi: 10.4049/jimmunol.172.4.2697. [DOI] [PubMed] [Google Scholar]
- 28.Shi FD, Bai XF, Li HL, Link H. Macrophage apoptosis in muscle tissue in experimental autoimmune myasthenia gravis. Muscle Nerve. 1998;21:1071–4. doi: 10.1002/(sici)1097-4598(199808)21:8<1071::aid-mus13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol. 2000;165:3136–44. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 30.Wang HB, Shi FD, Li H, van der Meide PH, Ljunggren HG, Link H. Role for interferon-gamma in rat strains with different susceptibility to experimental autoimmune myasthenia gravis. Clin Immunol. 2000;95:156–62. doi: 10.1006/clim.2000.4850. [DOI] [PubMed] [Google Scholar]
- 31.Gu D, Wogensen L, Calcutt NA, et al. Myasthenia gravis-like syndrome induced by expression of interferon gamma in the neuromuscular junction. J Exp Med. 1995;181:547–57. doi: 10.1084/jem.181.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balasa B, Deng C, Lee J, Bradley LM, Dalton DK, Christadoss P, Sarvetnick N. Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med. 1997;186:385–91. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasa B, Deng C, Lee J, Christadoss P, Sarvetnick N. The Th2 cytokine IL-4 is not required for the progression of antibody-dependent autoimmune myasthenia gravis. J Immunol. 1998;161:2856–62. [PubMed] [Google Scholar]
- 34.Milani M, Ostlie N, Wu H, Wang W, Conti-Fine BM. CD4+ T and B cells cooperate in the immunoregulation of experimental autoimmune myasthenia gravis. J Neuroimmunol. 2006;179:152–62. doi: 10.1016/j.jneuroim.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Milani M, Ostlie N, Wang W, Conti-Fine BM. T cells and cytokines in the pathogenesis of acquired myasthenia gravis. Ann N Y Acad Sci. 2003;998:284–7. doi: 10.1196/annals.1254.032. [DOI] [PubMed] [Google Scholar]
- 36.Saoudi A, Bernard I, Hoedemaekers A, Cautain B, Martinez K, Druet P, De Baets M, Guéry JC. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1- or Th2-type immune response in rats. J Immunol. 1999;162:7189–97. [PubMed] [Google Scholar]
- 37.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 38.Aricha R, Feferman T, Fuchs S, Souroujon MC. Ex vivo generated regulatory T cells modulate experimental autoimmune myasthenia gravis. J Immunol. 2008;180:2132–9. doi: 10.4049/jimmunol.180.4.2132. [DOI] [PubMed] [Google Scholar]
- 39.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Tüzün E, Li J, Wanasen N, Soong L, Christadoss P. Immunization of mice with T cell-dependent antigens promotes IL-6 and TNF-alpha production in muscle cells. Cytokine. 2006;35:100–6. doi: 10.1016/j.cyto.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 42.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849–58. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 43.Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–25. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 44.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Asquith DL, McInnes IB. Emerging cytokine targets in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:246–51. doi: 10.1097/BOR.0b013e3280eec78c. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida S, Haque A, Mizobuchi T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–35. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 48.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]