Abstract
It is well recognized that tissue microenvironments are involved in regulating the development and function of dendritic cells (DC). Oxygen supply, which varies in different tissues, has been accepted as an important microenvironmental factor in regulating the biological functions of several immune cells and as being involved in tumour progression and metastasis. However, little is known about the effect of hypoxia on the biological functions of DC and the effect of these hypoxia-conditioned DC on tumour metastasis. In this study, we analysed the transcriptional profiles of human monocyte-derived immature DC (imDC) and mature DC (mDC) cultured under normoxia and hypoxia by microarray, and found a body of potential targets regulating the functions of DC during hypoxia. In addition, the phagocytic ability of hypoxic imDC markedly decreased compared with that of normoxic imDC. Importantly, hypoxic DC poorly induced the proliferation of allogeneic T cells, but polarized allogeneic CD4+ naive T cells into a T helper type 2 (Th2) response. Moreover, hypoxic DC secreted large amounts of osteopontin, which were responsible for the enhanced migration of tumour cells. Therefore, our study provides new insights into the biological functions of DC under hypoxic conditions and one of mechanisms underlying tumour immune escape during hypoxia.
Keywords: cancer, dendritic cells, hypoxia, T helper type 2
Introduction
Tissue oxygen status is an important factor in regulating cell behaviour. Oxygen tension varies in different tissues, from 90–100 mmHg in arterial blood, to 20–70 mmHg in most normal tissues, and as low as 4 mmHg in lymphoid organs.1 Extremely low oxygen tension (< 1%) has been found in many compartments of normal, inflamed and tumour tissues. The procession of tumour progression is characterized by rapid cellular growth, leading to an imbalance between cellular O2 consumption and the O2 supply and thereby a hypoxic microenvironment.2,3 Hypoxia worsens as the diffusion distances increase with tumour expansion.4 Adaptation to hypoxia promotes the progression of a malignant tumour, including promoting angiogenesis and metastasis and rendering tumours more resistant to radiation and chemotherapy.5–7
Tumour progression results from cross-talk between different cell types in the tumour and the surrounding supporting tissue, the stroma, comprising fibroblast-like cells and specific extracellular matrix components, as well as inflammatory and immune cells. Immune cells, including T cells, macrophages, B cells and granulocytes, must adapt to differing oxygen concentrations as they develop and migrate in different tissues. Hypoxia impacts immune cell development as well as function. For example, B cells still produce immunoglobulins under hypoxia,8 whereas T-cell functions are downregulated by low oxygen levels in vitro. Although cytotoxic T cells that develop at low oxygen levels have a higher lytic capacity, fewer T cells become activated at low oxygen levels, and hypoxia could cause prolonged impairment of T-cell cytokine expression.9 When mice are exposed to hypoxic conditions, the proinflammatory response of neutrophils is inhibited, which protects lung tissue against excessive damage.10 Hypoxia also not only decreases the phagocytic activity of macrophages, but also can kill them.11,12 So, hypoxia may contribute to homeostasis and maintenance of tolerance by controlling and regulating inflammatory and immune cell functions.
Dendritic cells (DC) are important in the control of the developing immune response because they govern both initiation and polarization of adaptive immunity.13 Their functions and polarizing capacities are decisive for the outcome of T helper-mediated immunity.14–16 In their immature state, DC have a high capacity to capture antigens, but a low T-cell stimulatory capacity.17,18 Upon antigen uptake, DC undergo a progression of maturation, expressing high levels of major histocompatibility complex (MHC), CD40, CD80 and CD86 and having a strong T-cell stimulatory ability. Then DC migrate to secondary lymph nodes as their maturation progresses, where they prime T-cell responses and drive their differentiation toward T helper type 1 (Th1), Th2, Th17 or regulatory T cells.14–16 The activation stimulus in concert with tissue environmental factors encountered by the DC instructs them to polarize T cells toward a phenotype that initiates Th1, Th2, or regulatory T cells.19 During migration, DC will experience rapid changes of oxygen supply in different tissues, so, adaptation to hypoxia is an important mechanism for DC to fulfil their functions. It is still unclear about the phenotype and functional characteristics of DC under hypoxic conditions.
Most tumours can efficiently escape from the surveillance of the host immune system. Inhibition of DC function by the tumour environment could be involved in the escape of tumour cells from host immune surveillance. A recent report has asserted that injection of DC generated in vitro into tumour tissues cannot initiate a systemic response because the DC cannot migrate normally to regional lymph nodes.20 These data suggest that the migration and antigen-presenting function of DC may be inhibited in tumour environments. Therefore, it remains to be determined whether hypoxic conditions for tumours modified the functions of some DC. Moreover, there is limited knowledge on whether hypoxia-modified DC can promote the progression and metastasis of tumour cells. Herein, we report for the first time that hypoxia inhibits the maturation of DC and direct DC to polarize T cells to a Th2 response, and osteopontin (OPN) derived from hypoxia-conditioned DC promotes the migration of tumour cells.
Materials and methods
Reagents, monoclonal antibodies and cell culture
Recombinant human interleukin-4 (IL-4), recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF), OPN-neutralizing antibody and the isotype-matched control were purchased from R&D Systems. (Minneapolis, MN) Lipolysaccharide (LPS) from Escherichia coli was purchased from Sigma-Aldrich (St Louis, MO). Antibodies specific for CD14, CD80, CD86, human leucocyte antigen DR (HLA-DR), CD1a, CD40, CD209, CCR7 and their isotype-control antibodies were purchased from BD-Pharmingen. (San Diego, CA) The sources of other reagents is indicated in the text.
RPMI-1640 was supplemented with 10% heat-inactivated fetal calf serum (FCS), 1 mm non-essential amino acids, 45 μg/ml penicillin and streptomycin, and 2 mm l-glutamine (all from Gibco, Gaithersburg, MD complete RPMI medium). Dulbecco’s modified Eagle’s minimal essential medium (DMEM) was purchased from Gibco. The human breast tumour cell line MDA-MD-231 and mouse embryonic fibroblast cell line NIH/3T3 were routinely grown in DMEM supplemented with 100 U/ml penicillin and streptomycin and 10% fetal bovine serum at 37° in humidified air containing 5% CO2.
Generation of human monocyte-derived DC
The use of human peripheral blood monocytes from healthy donors was approved by the Institutional Review Board of Shandong University. Monocyte-derived DC were prepared as described previously.21 Briefly, CD14+ cells from peripheral blood mononuclear cells were enriched with a bead-labelled anti-CD14 monoclonal antibody (mAb; Miltenyi Biotec, Bergisch-Gladbach, Germany) using the magnetic antibody cell sorting (MACS) system (Miltenyi Biotec). The purity of CD14+ monocytes was routinely over 93%. CD14+ monocytes were cultured for 5 days in complete RPMI medium containing GM-CSF (1000 units/ml) and IL-4 (500 units/ml) under hypoxia or normoxia. According to the previous definition of tumour hypoxia,6 the cells in the hypoxic group were incubated at 1% O2 in a humidified incubator (HERA Cell 150; Heraeus, Osterode, Germany) with 5% CO2, and 94% N2. To induce maturation, LPS (1 μg/ml) was added on day 5, and the cells were cultured for another 2 days. Cell morphology and viability were determined by light microscopy (Olympus CKX31, Tokyo, Japan) and flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA).
Flow cytometry
Surface receptor expression on DC was detected on days 5 and 7. Cells were stained using mAbs labelled with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or PE-carbocyanin 5. Isotype controls were run in parallel. After incubation, the antigenic expression on DC was detected using a FACSCalibur flow cytometer (Becton Dickinson, CA) and mean fluorescence intensities were determined with cellquest software (Becton Dickinson).
RNA preparation and complementary RNA synthesis
Total RNA was prepared from three different donor-derived immature DC (imDC) or mature DC (mDC) using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA) and purified using RNeasy mini spin columns (Qiagen Inc.) according to the manufacturer’s protocol. Sample concentrations and quality were assessed by measuring the optical density (OD) at 260 nm, and 280 nm with an Aligent 2100 Bioanalyzer (Aligent Technologies, Palo Alto, CA). The 260/280 nm ratios of the samples were > 1·8. Sample purity was confirmed by electrophoresis on an agarose gel. All samples contained 18S and 28S ribosomal RNA peaks with no visible degradation products. A minimum of 20 μg of pooled RNA from each experimental condition was subsequently processed. RNA was reverse transcribed into double-stranded complementary DNA (cDNA) on a GeneAmp polymerase chain reaction system 2700 thermal cycler (Applied Biosystems, Foster City, CA) using the SuperScript Double-Stranded cDNA Synthesis kit (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. The cDNA was purified and used for in vitro transcription with the BioArray High Yield RNA Transcript Labeling kit (Enzo Life Sciences Framingdale, New York). Labelled complementary RNA (cRNA) was purified using the Qiagen RNeasy Mini kit (Qiagen Inc.).
Genechip hybridization and data analysis
Purified cRNA probes were used for hybridization to Affymetrix U133 plus2·0 GeneChips (Affymetrix Inc., Santa Clara, CA). Each RNA pool was hybridized to an individual chip. The hybridization was performed at 45° for 16 hr in the presence of herring sperm DNA. Chips were then stained with streptavidin-PE and scanned using an Agilent Genearray Scanner (Agilent technologies, Palo Alto, CA). The genechip® microarray suite v5·0 software program (Affymetrix Inc.) was used to analyse the data for subsequent statistical analysis. Differential expression was assessed by pairwise comparisons of normoxic samples to hypoxic samples. The primary index of gene expression was determined by the average difference in fluorescence between matched positive and negative control probes for each gene product on the array to determine the relative expression level. An average difference > 500 indicates detectable expression of a given gene product. Differential expression required a greater than twofold increase or decrease in messenger RNA expression levels.22 The significance of gene expression differences between the two experimental conditions was calculated using a one-way analysis of variance (anova).
Endocytic ability assay
FITC-dextran (molecular weight 40 000; Molecular Probes, Eugene, OR) was used to assess mannose receptor-mediated endocytosis as previously described.23 Briefly, approximately 2 × 105 cells per sample were incubated in medium containing 1 mg/ml FITC-dextran for 0, 60 or 120 min at 37° or 4°. After incubation, uptake was stopped by adding ice-cold phosphate-buffered saline containing 5 mm ethylenediamine tetraacetic acid and 2% FCS followed by extensive washes. Then the samples were analysed by flow cytometry as described above. At least 10 000 cells per sample were analysed. The level of antigen uptake by imDC was assessed on the FITC channel and expressed as the difference in mean fluorescence intensity between the test (37°) and control (4°) tubes for each sample.
Mixed lymphocyte reaction (MLR)
T cells were enriched by immunomagnetic positive selection from peripheral blood mononuclear cells using a bead-labelled anti-CD3 mAb (Miltenyi Biotec) and MACS separation columns (Miltenyi Biotec). The DC were incubated with CD3+ T cells at ratio of 1 : 20 under normoxia or hypoxia for 96 hr. Supernatants were obtained after 72 hr to determine cytokine secretion. To assess T-cell proliferation, cells were pulsed with 0·5 μCi of [3H]thymidine (Amersham Biosciences) during the last 16 hr of culture and [3H]thymidine incorporation was measured using a TopCount (Canberra Pacard, Dreieich, Germany).
Th-cell polarization assay
Naive CD4+ CD45RA+ T cells were enriched using an immunomagnetic naive CD4+ T-cell isolation kit (Miltenyi Biotec). The purity of naive CD4+ CD45RA+ T cells was > 95% as determined by flow cytometry. Naive T cells were cultured with DC at a ratio of 5 : 1 under normoxic or hypoxic conditions. On day 5, IL-2 (50 U/ml; R&D Systems) was added. Cytokine secretion was induced by phorbol 12-myristate 13-acetate (PMA, 100 nm) and ionomycin (1 μg/ml; both from Sigma-Aldrich) on day 9. The supernatant was obtained on day 10 and the secretions of IL-4 and interferon-γ (IFN-γ) were determined using a Bio-Plex Protein Array system (Bio-Rad Laboratories, Hercules, CA).
Bio-Plex analysis
Supernatants from DC, MLR and Th-cell polarization assay were harvested at the indicated time-points and were assayed in duplicate. Concentrations of IL-4, IL-10, IL-12 (p70), IFN-γ, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), and tumour necrosis factor-α (TNF-α) were simultaneously evaluated using commercially available multiplex bead-based sandwich immunoassay kits (Bio-Rad Laboratories). The assay was performed following the manufacturer’s instructions. Briefly, samples (50 μl/ml) or standards (50 μl/ml) were incubated with 50 μl of premixed beads into the wells of a 96-well microtitre plate. After incubation for 30 min at room temperature with agitation, the mixture was washed with wash buffer. Then 25 μl of detection antibody was added to each well and incubated for another 30 min at room temperature with agitation. After washing and incubation with streptavidin-PE, the mixtures were washed and resuspended in assay buffer. For each cytokine, eight standards with concentrations ranging from 0·2 to 3200 pg/ml were used. The plate was read using the Bio-Plex Protein Array system.
Enzyme-linked immunosorbent assay (ELISA)
The OPN protein concentration in the culture supernatants harvested from DC was measured using the Quantikine ELISA kit (R&D Systems) according to the manufacturer’s instructions. Briefly, a mouse mAb specific for OPN was coated onto a microplate. Standards and samples proteins were added to each well and incubated for 2 hr at room temperature. After washing to remove unbound proteins, an enzyme-linked polyclonal antibody specific for OPN was added to the wells and incubated for 2 hr at room temperature. Following a wash to remove unbound antibody–enzyme reagent, the substrate solution was added to the wells and incubated for 30 min at room temperature in the dark. The OD (450 nm) of each sample was determined using a microplate reader and the mean concentration of OPN was calculated.
Matrigel invasion assay
The ability of the tumour cells to migrate was quantified using Transwell inserts fitted with polycarbonate filters (8-μm pore size, Costar, Cambridge, MA) coated with 30 μg Matrigel diluted in phosphate-buffered saline as described previously.24 Briefly, tumour cells preincubated for 4 hr in complete DMEM or DC-conditioned medium collected from imDC or mDC cultured under hypoxia or normoxia (1 × 105 cells in 100 μl incubation media) were added to the upper compartment of the insert. The lower compartment was filled with 600 μl of conditioned medium collected from 3 × 106 NIH/3T3 fibroblasts incubated overnight in growth medium containing 10% FCS. After incubation at 37° for 12 hr, non-migrating cells that remained on the upper of surface of the filter were removed and the migrated cells on the lower surface of the filter were stained with Eosin Y (Sigma-Aldrich) and counted using a light microscope (Olympus CKX31).
Statistical analysis
Data are presented as mean ± SD. The Student’s t-test was used for statistical analysis where P-values of < 0·05 were considered statistically significant.
Results
Viability and phenotype of DC cultured under hypoxic conditions
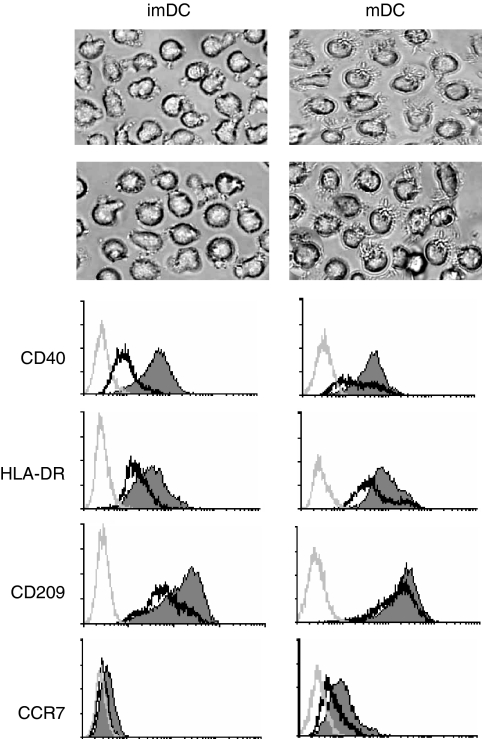
After 5 days of culturing with GM-CSF plus IL-4 or stimulation by LPS for another 2 days, the viability of both hypoxic and normoxic DC exceeded 90% (normoxic imDC: 95·2%, hypoxic imDC: 94·5%, normoxic mDC: 93·2%, hypoxic mDC: 92·35%). The cells had typical DC morphology: were non-adherent, clustered and had protruding veils (Fig. 1a). Flow cytometry revealed that CD14 was not expressed, while CD80, CD86 and CD1a were expressed on both immature and mature DC cultured under normoxia and hypoxia. There were no significant changes in the expression levels (data not shown). However, hypoxia significantly inhibited the expression of CD40 and HLA-DR on immature or LPS-stimulated mDC. The expression of CD209 on immature DC and the CCR7 expression on mature DC were also reduced under hypoxic conditions (Fig. 1b). These results suggest that hypoxia inhibited DC maturation, migration and the ability to capture antigen.
Figure 1.
Morphology and phenotypes of human monocyte-derived dendritic cells (DC) under normoxia or hypoxia. Human monocyte-derived DC were cultured under normoxic (N) or hypoxic (H) conditions for 5 days in the presence of granulocyte–macrophage colony-stimulating factor and interleukin-4 and induced for further maturation by the treatment with lipopolysaccharide for 48 hr, as described in the Materials and methods section. (a) Morphology of immature DC (imDC) and mature DC (mDC) under normoxic or hypoxic condition (200× magnification). (b) Surface marker expression on immature and mature DC under normoxia (thin and filled line) and hypoxia (bold and open line). The grey lines represent staining with a control isotype-matched antibody. Results are representative of four separate experiments.
Gene expression profile of DC cultured in a hypoxic microenvironment
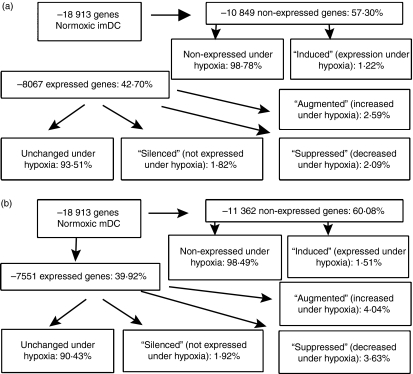
The transcriptional profile of hypoxic DC was investigated by microarray analysis. Equal amounts of RNA from the different samples were pooled and hybridized to human Affymetrix U133 plus2·0 GeneChips. The results showed that 8067 genes (42·7%) were expressed by imDC and 7551 genes (39·9%) were expressed in mDC cultured under normoxia. After restricting the profile to those sequences exhibiting at least twofold expression differences between hypoxic and normoxic samples, we identified that hypoxia modulated the expression of 658 genes (up to 3·48%) in imDC and 896 genes (up to 4·73%) in mDC relative to normoxic conditions. These modulated genes were grouped into four categories:
Silenced genes, which were detectable in normoxic DC but undetectable in hypoxic DC, included 147 genes (1·82%) in imDC and 145 genes (1·92%) in mDC
Induced genes, which were undetectable in normoxic DC but detectable in hypoxic DC, included 133 genes (1·22%) in imDC and 172 genes (1·51%) in mDC
Downregulated genes, which exhibited a greater than twofold decrease in expression in hypoxic DC relative to normoxic DC, included 169 genes (2·09%) in imDC and 274 genes (3·63%) in mDC
Upregulated genes, which exhibited a greater than twofold increase in expression in hypoxic DC relative to normoxic DC, included 209 genes (2·59%) in imDC and 305 genes (4·04%) in mDC (Fig. 2).
Figure 2.
Gene expression profiles in dendritic cells (DC) modulated by hypoxia environment. This schematic illustrates gene expression profiles in normoxic DC and hypoxic DC. Based on a greater than twofold difference in expression in hypoxic DC relative to normoxic DC, genes significantly altered were categorized as ‘silenced’, ‘downregulated’, ‘upregulated’ and ‘induced’. (a) The gene expression profiles in immature DC (imDC) cultured under normoxia or hypoxia. (b) The gene expression profiles in mature DC (mDC) cultured under normoxia or hypoxia.
Hypoxia-modulated genes were classified according to their known biological functions, such as apoptosis, cell growth and maintenance, signal transduction, immune response, biosynthesis, cell adhesion, cell–cell signalling and their response to stress and metabolism. Genes encoding products involved in protein metabolism were prominent among the hypoxia-modulated genes in both immature and mature DC. However, imDC had more alterations than mDC in hypoxia-modulated genes, implicated mainly in immune response, cell–cell signalling and the cellular response to stress, suggesting that imDC were more sensitive to hypoxia.
Classification of known DC function-associated genes modulated by hypoxia
To better understand the biological functions of products encoded by these hypoxia-modulated genes, we further classified these genes according to known DC-associated biological behaviours, including antigen uptake and presentation, cytokine and chemokine secretion and receptor production, secretion of extracellular matrix and adhesion molecules, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), regulation of both apoptosis and the cell cycle, and signal transduction (Tables 1 and 2). We found that genes encoding receptors for antigen-uptake, such as CD209 and CLECSF12 (DECTIN-1), and genes for non-classical antigen-presentation, such as CD1b and CD1E, were significantly downregulated in hypoxia-conditioned imDC, suggesting a reduced ability to capture and present bacterial antigens in imDC under hypoxic conditions. However, the expression of the HLA-E gene, which encodes the cellular ligand of CD94/NKG2A, was enhanced in imDC under hypoxic conditions. Killing of imDC is confined to the CD94/NKG2A(+)KIR(−) natural killer (NK) cell subset.25 So, the upregulation of HLA-E in hypoxia-conditioned imDC might enhance their resistance to autologous NK cell-mediated lysis. As for mDC, hypoxia inhibited the transcription of genes encoding TAP and LAMP implicated in assembling the MHC-I–peptide complex, and the gene encoding CD80, responsible for costimulating T cells, suggesting that hypoxia-modulated DC might be poor stimulators for T-cell activation. On the other hand, hypoxia upregulated the expression of the HLA-DQB1 and CD74 (invariant chain Ii) genes in mDC, both of which were involved in the presentation of MHC-II-restricted antigenic peptide. Such an increase in HLA-DQ expression can also be seen in interstitial cells and endothelium in the synovial membrane of patients with rheumatoid arthritis, a typical hypoxic disease.26 Similar to imDC, hypoxia-conditioned mDC expressed a higher level of HLA-E than normoxic DC. Of the genes related to survival and apoptosis, we found a marked reduction of the Bax/Bcl2 ratio in both immature and mature DC, suggesting that hypoxia-conditioned DC were more resistant to apoptosis.
Table 1.
Relative expression of selected genes in hypoxic dendritic cells (DC) compared to normoxic DC
| Immature DC | Mature DC | ||||||
|---|---|---|---|---|---|---|---|
| UniGene ID | Gene symbol | Gene description | Fold change | UniGene ID | Gene symbol | Gene Description | Fold change |
| Antigen uptake, procession, and presentation | |||||||
| Hs.381008 | HLA-E | MHC, class I, E | 2·5 | Hs.409934 | HLA-DQB1 | MHC, class II, DQ beta 1 | 4·5 |
| Hs.1310 | CD1B | CD1B antigen, b polypeptide | 0·32 | Hs.446471 | CD74 | CD74 antigen | 2·2 |
| Hs.278694 | CD209 | CD209 antigen | 0·57 | Hs.381008 | HLA-E | MHC, class I, E | 2·0 |
| Hs.143929 | CLECSF12 | C-type (calcium dependent, carbohydrate- recognition domain) lectin, superfamily member 12 | 0·50 | Hs.502 | TAP2 | Transporter 2, ATP-binding cassette, sub-family B (MDR/TAP) | 0·50 |
| Hs.249217 | CD1E | CD1E antigen, e polypeptide | 0·25 | Hs.838 | CD80 | CD80 antigen (CD28 antigen ligand 1, B7-1 antigen) | 0·50 |
| Hs.10887 | LAMP3 | Lysosomal-associated membrane protein 3 | 0·45 | ||||
| Apoptosis and cell cycle | |||||||
| Hs.79241 | BCL2 | B-cell CLL/lymphoma 2 | 2·12 | Hs.79241 | BCL2 | B-cell CLL/lymphoma 2 | 2·0 |
| Hs.159428 | BAX | BCL2-associated X protein | 0·31 | Hs.159428 | BAX | BCL2-associated X protein | 0·23 |
| Cytokine/chemokine and receptors | |||||||
| Hs.624 | IL8 | Interleukin 8 | 8·2 | Hs.511794 | CCR2 | Chemokine (C-C motif) receptor 2 | 9·3 |
| Hs.511794 | CCR2 | Chemokine (C-C motif) receptor 2 | 6·5 | Hs.506190 | CCR3 | Chemokine (C-C motif) receptor 3 | 5·6 |
| Hs.1722A | IL1 | Interleukin 1α | 6·0 | Hs.407995 | MIF | Macrophage migration inhibitory factor | 5·7 |
| Hs.506190 | CCR3 | Chemokine (C-C motif) receptor 3 | 3·5 | Hs.73793 | VEGF | Vascular endothelial growth factor | 4·5 |
| Hs.407995 | MIF | Macrophage migration inhibitory factor | 3·5 | Hs.54443 | CCR5 | Chemokine (C-C motif) receptor 5 | 4·3 |
| Hs.73793 | VEGF | Vascular endothelial growth factor | 3·5 | Hs.313 | OPN | Secreted phosphoprotein 1 | 3·5 |
| Hs.2161 | C5R1 | Complement component 5 receptor 1 (C5a ligand) | 2·2 | Hs.1652 | CCR7 | Chemokine (C-C motif) receptor 5 | 0·50 |
| Hs.54443 | CCR5 | Chemokine (C-C motif) receptor 5 | 2·1 | Hs.674 | IL12B | Interleukin 12B (natural killer cell stimulatory factor 2, cytotoxic lymphocyte maturation factor 2, p40) | 0·35 |
| Hs.313 | OPN | Secreted phosphoprotein 1 | 2·0 | Hs.414629 | CCL13 | Chemokine (C-C motif) ligand 13 | 0·28 |
| Hs.174142 | CSF1R | Colony stimulating factor 1 receptor | 1·8 | Hs.303649 | CCL2 | Chemokine (C-C motif) ligand 2 (MCP-1) | 0·28 |
| Hs.414629 | CCL13 | Chemokine (C-C motif) ligand 13 | 0·25 | Hs.169191 | CCL23 | Chemokine (C-C motif) ligand 23 | 0·1 |
| Hs.303649 | CCL2 | Chemokine (C-C motif) ligand 2 (MCP-1) | 0·28 | Hs.75703 | CCL4 | Chemokine (C-C motif) ligand 4 | 0·5 |
| Hs.75703 | CCL4 | Chemokine (C-C motif) ligand 4 | 0·5 | ||||
| Adhesive molecular | |||||||
| Hs.149609 | ITGA5 | Integrin α5 (fibronectin receptor, α-polypeptide) | 2·3 | Hs.149609 | ITGA5 | Integrin α5 (fibronectin receptor, α-polypeptide) | 4·6 |
| Hs.375957 | ITGB2 | Integrin β2 (antigen CD18 (p95) | 2·1 | Hs.172631 | ITGAM | Integrin αM (complement component receptor 3, α | 3·0 |
| Hs.423077 | SELPLG | Selectin P ligand | 2·0 | Hs.375957 | ITGB2 | Integrin β2 (antigen CD18 (p95), | 2·5 |
| Hs.145140 | ITGA4 | Integrin α4 (antigen CD49D, α4 subunit of VLA-4 receptor) | 0·50 | ||||
| MMPs/TIMPs | |||||||
| Hs.446641 | TIMP1 | Tissue inhibitor of metalloproteinase 1 | 3·0 | Hs.446641 | TIMP1 | Tissue inhibitor of metalloproteinase 1 | 3·8 |
| Hs.245188 | TIMP3 | Tissue inhibitor of metalloproteinase 3 | 0·33 | Hs.151738 | MMP9 | Matrix metalloproteinase 9 | 0·50 |
| Others | |||||||
| Hs.126384 | FCGR2B | Fc fragment of IgG, low affinity IIb, receptor for (CD32) | 10·9 | Hs.126384 | FCGR2B | Fc fragment of IgG, low affinity IIb, receptor for (CD32) | 94·1 |
| – | FCGR2C | Fc fragment of IgG, low affinity IIc, receptor for (CD32) | 8·8 | – | FCGR2C | Fc fragment of IgG, low affinity IIc, receptor for (CD32) | 44 |
| Hs.897 | FCER1A | Fc fragment of IgE, high affinity I, receptor for; alpha polypeptide | 3·0 | Hs.445466 | FPRL2 | Formyl peptide receptor-like 2 | 4·9 |
| Hs.433300 | FCER1G | Fc fragment of IgE, high affinity I | 2·6 | ||||
| Hs.897 | FCER1A | Fc fragment of IgE, high affinity I | 2·4 | ||||
| Hs.97199 | C1QR1 | Complement component 1, q subcomponent, receptor 1 | 2·4 | ||||
IgG, immunoglobulin; MHC, major histocompatibility complex.
Table 2.
Hypoxia signal pathway protein expression by dendritic cells (DC) under hypoxic conditions
| Immature DC |
Mature DC |
||||||
|---|---|---|---|---|---|---|---|
| UniGene ID | Gene symbol | Gene Description | Fold change | UniGene ID | Gene symbol | Gene Description | Fold change |
| Hs.509554 | HIF1A | Hypoxia-inducible factor 1, α subunit | 0·48 | Hs.509554 | HIF1A | Hypoxia-inducible factor 1, α subunit | 0·41 |
| Hs.73793 | VEGF | Vascular endothelial growth factor | 3·12 | Hs.73793 | VEGF | Vascular endothelial growth factor | 4·5 |
| Hs.473721 | SLC2A1 | Solute carrier family 2, member 1 | 3·1 | Hs.473721 | SLC2A1 | Solute carrier family 2, member 1 | 3·7 |
| Hs.441047 | ADM | Adrenomedullin | 2·4 | Hs.441047 | ADM | Adrenomedullin | 5·2 |
| Hs.45743 | ADORA2B | Adenosine A2b receptor | 2·5 | Hs.45743 | ADORA2B | Adenosine A2b receptor | 22·1 |
| Hs.445563 | ADRBK2 | Adrenergic β receptor kinase 2 | 2·1 | Hs.78465 | JUN | v-jun sarcoma virus 17 oncogene homologue (avian) | 4·9 |
| Hs.288555 | ELK3 | ELK3, ETS-domain protein (SRF accessory protein 2) | 3·7 | Hs.172674 | NFATC3 | Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 3 | 4·3 |
| Hs.34516 | CERK | Ceramide kinase | 2·5 | Hs.288555 | ELK3 | ELK3, ETS-domain protein (SRF accessory protein 2) | 2·5 |
| Hs.135457 | USP53 | Ubiquitin specific protease 53 | 2·3 | Hs.9754 | ATF5 | Activating transcription factor 5 | 2·1 |
| Hs.272210 | ATF7IP | Activating transcription factor 7 interacting protein | 2·2 | Hs.64056 | PAK1 | p21/Cdc42/Rac1-activated kinase 1 (STE20 homologue, yeast) | 2·1 |
| Hs.64056 | PAK1 | p21/Cdc42/Rac1-activated kinase 1 (STE20 homologue, yeast) | 2·0 | Hs.271980 | MAPK6 | Mitogen-activated protein kinase 6 | 2·1 |
| Hs.284275 | PAK2 | p21 (CDKN1A)-activated kinase 2 | 2·0 | Hs.246842 | ATF1 | Activating transcription factor 1 | 2·0 |
| Hs.297343 | CAMKK2 | Calcium/calmodulin-dependent protein kinase kinase 2 β | 2·1 | Hs.268675 | MEF2A | MADS box transcription enhancer factor 2, polypeptide A (myocyte enhancer factor 2A) | 2·0 |
| Hs.170610 | MAP3K1 | Mitogen-activated protein kinase kinase kinase 1 | 2·0 | Hs.515157 | JUNB | jun B proto-oncogene | 2·0 |
| Hs.46743 | MKKS | McKusick–Kaufman syndrome | 0·45 | Hs.50649 | TP53I3 | Tumour protein p53 inducible protein 3 | 0·48 |
| Hs.324178 | MAPKAP1 | Mitogen-activated protein kinase associated protein 1 | 0·5 | Hs.324178 | MAPKAP1 | Mitogen-activated protein kinase associated protein 1 | 0·5 |
| Hs.413901 | MAPKAPK5 | Mitogen-activated protein kinase-activated protein kinase 5 | 0·46 | Hs.134106 | MAP2K4 | Mitogen-activated protein kinase kinase 4 | 0·5 |
| Hs.9754 | ATF5 | Activating transcription factor 5 | 2·7 | Hs.114062 | PTPLA | Protein tyrosine phosphatase-like (proline instead of catalytic arginine), member a | 0·46 |
| Hs.515157 | JUNB | jun B proto-oncogene | 2·0 | Hs.397465 | HIPK2 | Homeodomain interacting protein kinase 2 | 0·48 |
| Hs.78465 | JUN | v-jun sarcoma virus 17 oncogene homologue (avian) | 2·1 | Hs.440315 | MAP3K14 | Mitogen-activated protein kinase kinase 14 | 0·4 |
| Hs.5613 | CRKL | v-crk sarcoma virus CT10 oncogene homologue (avian)-like | 2·0 | Hs.135457 | USP53 | Ubiquitin-specific protease 53 | 0·41 |
| Hs.81328 | NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α | 2·0 | Hs.11114 | TP53INP2 | Tumour protein p53 inducible nuclear protein 2 | 0·29 |
| Hs.433046 | ATF6 | Activating transcription factor 6 | 0·5 | Hs.81328 | NFKBIA | Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor α | 2·1 |
| Hs.460 | ATF3 | Activating transcription factor 3 | 0·45 | Hs.431926 | NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (p105) | 0·47 |
Among the cytokines and chemokines and their receptors related to inflammatory and immune responses, hypoxia significantly inhibited the expression of chemokine (C-C motif) ligand 2 (CCL2), CCL4 and CCL13 in imDC. In mDC, hypoxia upregulated the expression of C-C chemokine receptor 2 (CCR2), CCR3, and CCR5, which are normally downregulated in mDC, and, in contrast, markedly inhibited the expression of CCR7 and IL-12p40, which are normally upregulated in mDC. Hypoxia led to a severe reduction in the expression of chemokine genes, including CCL2, CCL13 and CCL23. Interestingly, vascular endothelial growth factor, a well-established hypoxia-modulated molecule able to impair DC maturation and function,27 also was induced significantly in both immature and mature DC under hypoxic conditions. These data suggest that hypoxia may interfere with DC maturation.
The majority of hypoxia-modulated adhesion molecules were upregulated, including integrin α5 (ITGA5) and integrin β2 (ITGB2) in both immature and mature DC, selectin P ligand (SELPLG) in imDC, and integrin αM (ITGAM) in mDC. However, ITGA4 was downregulated in mDC. Hypoxia enhanced TIMP-1 expression in both immature and mature DC, but inhibited MMP9 expression in mDC and increased macrophage migration inhibitory factor expression in imDC, suggesting that hypoxia might impair the migratory ability of DC, as we reported previously.28
Interestingly, hypoxia-inducible factor 1α (HIF-1α), which is induced in response to the stress of oxygen depletion, was downregulated. It is critically involved in the function of phagocytes, especially at sites of inflammation and HIF-1α gene-depleted phagocytes were compromised in their ability to migrate, to aggregate and to kill bacteria.29 The decreased expression level of HIF-1α indicated that the hypoxic environment has an impact on the function of DC. Nevertheless, adenosine A2b receptor (ADORA2B), one of the HIF-α-inducible elements, displayed a threefold and 21-fold increase in both immature and mature DC under hypoxia, respectively (Table 2).
Impaired maturation and production of cytokines in DC cultured under hypoxic conditions
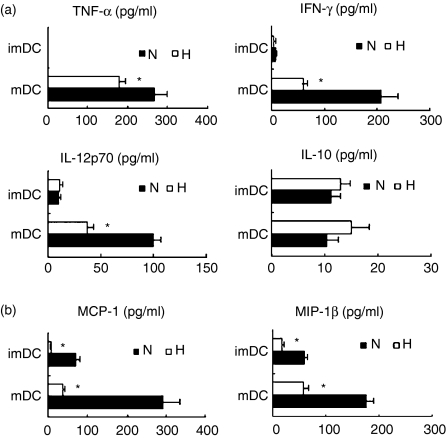
To determine whether hypoxia affects the maturation and activation of DC, we examined the phenotype and cytokine production of DC. As shown in Fig. 1(b), hypoxia significantly inhibited the expression of CD40 and HLA-DR on both immature and mature DC. Under hypoxic conditions, the secretion of Th1-type cytokines, including IFN-γ, TNF-α and IL-12p70, was inhibited significantly in mDC. In contrast, the secretion of IL-10, the antagonist of IL-12-induced Th1-cell polarization, was not significantly modulated by hypoxia (Fig. 3a). In addition, CCR7 expression was also downregulated in hypoxia-conditioned mDC (Fig. 1b). Thus, hypoxia may inhibit DC maturation and induce a Th2 response by DC.
Figure 3.
Cytokine secretion by dendritic cells (DC). The DC were induced under hypoxia (H) or normoxia (N). The supernatants were analysed by Bio-Plex Protein Array system for the secretion of tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-12 (IL-12) p70, IL-10 (a), and monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β) (b). The results are shown as the mean ± SD. *P< 0·05.
CCL2 (MCP-1) and CCL4 (MIP-1β) are involved in the recruitment of T cells, NK cells or monocytes and macrophages. The results from the microarray analysis show a significant downregulation of CCL2 and CCL4 genes in hypoxic DC. To confirm these results, we analysed the production of CCL2 and CCL4 using the Bioplex Protein Array system. We found that hypoxia significantly inhibits the production of MCP-1 and MIP by both immature and mature DC (Fig. 3b). These findings suggest that hypoxia affects the recruitment of other immune cells to the tissue environment.
Hypoxia affected the phagocytic ability of imDC and skewed mDC to mediate a Th2-polarizing response
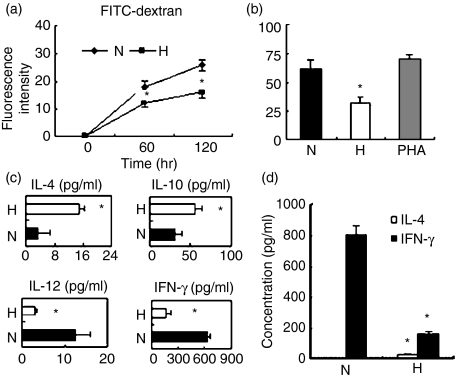
Antigen uptake is a functional characteristic of imDC. To study the endocytic ability of hypoxic imDC, we used FITC-dextran, which is taken up mainly through the mannose receptor. Dextran endocytosis of hypoxic DC was decreased markedly compared to normoxic DC (Fig. 4a). Confirming the microarray data, the expression level of CD209 in imDC was decreased in hypoxic DC as compared with normoxic DC (Fig. 1b).
Figure 4.
Hypoxia inhibited the phagocytic ability of immature dendritic cells (imDC) and skewed mature dendritic cells (mDC) to mediate a T helper type 2 (Th2)-polarizing response. (a) The imDC were induced under hypoxia (H) or normoxia (N). The cells endocytic ability was evaluated by the uptake of 1 mg/ml fluorescein isothiocyanate (FITC)-dextran measured using flow cytometry. Results were expressed as the mean ± SD of the fluorescence intensity. (b) The mixed lymphocyte reaction (MLR) test was performed with allogeneic T cells and hypoxic mDC (H) or normoxic mDC (N). [3H]Thymidine was added 16 hr before cells were collected and proliferation was assessed. (c) The supernatants were collected after 72 hr of culture and analysed for the production of interleukin-4 (IL-4), IL-10, IL-12p70 and interferon-γ (IFN-γ). (d) Naive CD4+ CD45RA+ T cells isolated by immunomagnetic negative depletion were polarized by culture with hypoxic or normoxic mDC. Production of the cytokines IL-4 and IFN-γ was induced by adding phorbol 12-myristate 13-acetate and ionomycin on day 9. The supernatant was collected after 24 hr and analysed using the Bio-Plex Protein Array system as described in the Materials and methods section. Data are expressed as the mean ± SD of four independent experiments. *P< 0·05.
To further investigate the effect of hypoxia on the DC-mediated T-cell response, we first examined the effect of hypoxia on the ability of mDC to stimulate the proliferation of allogeneic T cells using [3H]thymidine incorporation. Hypoxia severely inhibited the ability of DC to induce the proliferation of allogeneic T cells (Fig. 4b). We next analysed the cytokine expression in the MLR system, including IFN-γ, IL-4, IL-12p70 and IL-10. We found that IL-10 and IL-4 obviously increased, whereas IFN-γ and IL-12p70 significantly decreased in a DC–T-cell coculture system (Fig. 4c). The DC have the unique ability to induce a primary immune response through activation and polarization of naive T cells. Therefore, we evaluated the ability of hypoxia-conditioned DC to stimulate the primary allogeneic T-cell response. When cytokine secretion was induced by the addition of PMA and ionomycin, we found that naive T cells cocultured with hypoxic DC preferentially secreted the Th2 cytokine IL-4, but minimal amounts of the Th1 cytokine IFN-γ (Fig. 4d). The same experiment was performed with autologous T cells and culture supernatant; it obtained the same trend, although with a lower rate of polarization (data not shown). These results indicate that hypoxia skewed DC to polarize the T-cell response toward a Th2 phenotype.
Hypoxic DC promoted the migration of tumour cells through production of OPN
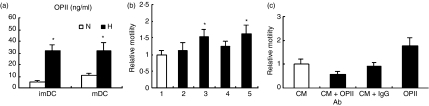
Genechip analysis showed that hypoxic DC expressed more OPN than normoxic DC and OPN plays an important role in enhancing tumour cell migration, therefore, we first determined the amount of OPN expressed by DC under hypoxic conditions. Compared with normoxia, hypoxia significantly increased the production of OPN in both imDC and mDC (Fig. 5a). We next used the Transwell system (Costar) to determine the migration ability of tumour cells in the presence or absence of DC-conditioned medium. Hypoxic DC-conditioned medium significantly enhanced the migratory ability of tumour cells (Fig. 5b). To confirm whether OPN was responsible for the enhanced migration of tumour cells, a neutralizing antibody against OPN was added to the DC-conditioned medium. As shown in Fig. 5(c), the OPN neutralizing antibody obviously prevented the increased migration of tumour cells. These results indicate that hypoxia-conditioned DC promote tumour cell migration in an OPN-dependent manner.
Figure 5.
Hypoxic dendritic cells (DC) promoted the motility of the breast tumour cells MDA-MD-231 by producing osteopontin (OPN). (a) The migratory activity of tumour cells was analysed in Transwell chambers in vitro as described in the Materials and methods section. Tumour cells were preincubated with or without DC-conditioned media collected from normoxic immature DC (imDC) (2), hypoxic imDC (3), normoxic mature DC (mDC) (4) or hypoxic mDC (5) for 4 hr. Complete RPMI medium served as a control (1). The number of cells migrating through the Matrigel-coated membranes was counted. (b) OPN production by imDC and mDC induced under normoxia (N) or hypoxia (H). Human CD14+ monocytes were incubated for 5 days and matured using lipopolysaccharide for another 2 days. OPN in the culture supernatant was quantified with using an enzyme-linked immunosorbent assay kit. (c) Tumour cells preincubated with imDC-conditioned medium with or without the OPN neutralizing antibody. (CM) hypoxic DC-conditioned medium; (IgG) normal goat immunoglobulin G served as isotype-matched control (10 μg/ml); (OPN Ab) OPN neutralizing antibody (10 μg/ml); (OPN) recombinant human OPN served as positive control (5 μg/ml). Data are the mean ± SD of five independent experiments. *P< 0·05.
Discussion
This study, for the first time, provides a wealth of information on hypoxia-modulated genes for further investigation of the molecular mechanisms underlying the adaptation of DC to hypoxia. We observed that monocytes still differentiated into DC under hypoxia, but these hypoxia-conditioned DC displayed poor T-cell-stimulatory activity and shifted towards a Th2-stimulating phenotype, possibly as the result of a marked reduction in expression of MHC class II and costimulatory molecules as well as of Th1 cytokines. Furthermore, we confirmed that hypoxic DC did promote tumour cell migration through increased secretion of OPN.
Immature DC sense chemotactic signals from inflammatory sites by constitutive expression of CCR2, CCR3, CCR5, C5R1 and FMLPR.30–32 These genes were upregulated in imDC by hypoxia, suggesting that imDC had a strong ability to sense inflammatory chemokines under hypoxic conditions. Mature DC upregulate the expression of CCR7, which allows DC to migrate to draining lymph nodes in response to lymph node-derived chemokines such as MIP-3β.33,34 In our study, we found a decrease in CCR7 expression in mDC under hypoxia, and also confirmed that hypoxia-modulated mDC displayed poor chemotactic ability in response to MIP-3β.28 However, hypoxia-conditioned imDC also displayed poor chemotaxis to RANTES, a primary chemoattractant for imDC (data not shown). This is not surprising, because the trafficking of DC is a complicated event, not only dependent on the expression of chemokine receptors but also on the extracellular matrix, adhesion molecules and reorganization of the cytoskeleton. Our previous studies, as well as those of others,35 showed that hypoxic DC exhibit markedly reduced expression of MMP9 and upregulated TIMP-1 transcripts, which was confirmed by the DNA array.
We identified a number of hypoxia-responsive genes involved in intracellular signalling cascades or transcription factor activity. One of the most intensively studied is HIF-1α, whose stability is regulated by oxygen. However, we found that HIF-1α was downregulated in hypoxia-conditioned immature and mature DC. This might result from long-term hypoxia exposure, because the monocytes were cultured under hypoxia from the onset of differentiation into DC. Nevertheless, the genes of some proteins involved in oxygen delivery and glucose metabolism were upregulated, such as vascular endothelial growth factor, adrenomedullin and GLUT-1, which are also the targets of HIF-1. The upregulation of these genes shows that, from monocytes to DC, common mechanisms are used to overcome oxygen deficiency. It has been shown that extracellular adenosines through binding the A2 class of adenosine receptors inhibit the Th1-cell response that is induced by antigen-presenting DC.36 Moreover, we observed that some members of the ERK, JNK and p38 pathways, as well as transcription factors, were inhibited or enhanced in DC under hypoxia, suggesting that hypoxia-conditioned DC had different responses to inflammatory cytokines, such as IL-1 and TNF-α, growth factors, mitogens, or other extracellular stresses compared to normoxia-conditioned DC.
Data from the microarray give a strong indication that DC are reprogrammed at transcriptional levels under hypoxia, and suggest that hypoxic DC have different bioactivities. Immature DC have a potent ability to capture antigens from bacteria, viruses and dead or dying cells by phagocytosis, pinocytosis and receptor-mediated endocytosis. Our results reveal that hypoxia-conditioned DC have a weaker phagocytic ability than normoxic DC. DC-SIGN (CD209) and dectin play key roles in the control of pathogenic infection by recognizing and binding to antigens derived from bacteria and fungi.37,38 Microarray data demonstrated that DC-SIGN and dectin were downregulated, whereas FcγRII FcεRI, and C1qR1 were upregulated in hypoxia-conditioned DC. The results from flow cytometry also confirmed that the expression level of CD209 was decreased in hypoxic DC as compared with normoxic DC. Thus, long-term hypoxia may decrease the ability of DC to capture foreign pathogenic antigens. On the other hand, some FcRs and C1qRs bind to and mediate the clearance of immune complex or C1q-coated apoptotic cells. Hypoxia upregulated the expression of FcγRII and C1qR1 in DC at the mRNA level, suggesting that hypoxic DC might assist in maintaining immunological homeostasis by eliminating immune complexes or apoptotic cells.
After antigen uptake, DC undergo maturation and activation with upregulation of MHC class II molecules, CD80, CD83, CD86 and CD40, which not only trigger the TCR-CD3 first signal but also provide costimulatory signals to T cells. Data from the microarray and flow cytometry analysis showed that MHC class II and the costimulatory molecule CD40 were downregulated, suggesting that hypoxia-modulated DC were poor stimulators of T cells. Indeed, we observed a marked reduction in the proliferation of allogeneic T cells stimulated by hypoxia-conditioned DC. Recently, Jantsch et al.39 reported that hypoxia combined with LPS led to marked induction of allogeneic lymphocyte proliferation compared with LPS alone. These differences in data may result from the different methods used. In our study, DC were induced under hypoxic conditions and Jantsch et al. first derived immature DC under normoxia, and then subjected them to hypoxia.
In addition, DC also govern the polarization of Th responses via the secretion of different cytokine profiles. In our study, we found that hypoxia caused a shift towards a Th2-driving phenotype of DC, decreasing the secretion of IFN-γ and increasing the secretion of IL-10 and IL-4 from DC-stimulated T cells, possibly as the result of a reduction of IL-12 and upregulation of IL-10 by hypoxia-conditioned DC. These results suggest that under physiological hypoxia, DC may be mainly involved in the maintenance of immune homeostasis and tolerance, and that under pathogenic hypoxia, such as in a tumour, the inhibition of DC function can be one of the reasons why the tumour escapes immune surveillance.
Hypoxia is reported to promote the metastasis of tumour cells. Accumulative evidence suggests that OPN overexpression is closely associated with the metastatic phenotype of cancer cells.40 We found that hypoxic DC produced significantly elevated amounts of OPN and hypoxic DC-conditioned medium could promote the motility of tumour cells. These findings suggest that stromal environments are not the bystanders but rather the active regulators of tumour metastasis.
In this study, we provide large amounts of information on the gene expression of human DC modulated by hypoxia and document the inhibitory regulatory effect of hypoxia on the function of DC, providing new insight into the mechanisms underlying the role of hypoxia in immune homeostasis or tumour progression. Better understanding of the biological characteristics of hypoxia-conditioned DC will offer new targets for DC-based anti-tumour therapy. Simultaneously targeting non-malignant cells within the tumour stroma should be considered whenever developing anticancer strategies.
Acknowledgments
This work was supported by grants from the National Key Basic Research Programme of China (No. 2006CB504100), and the National Natural Science Foundation of China (No. 30472261, No. 30671902 and No. 30121002).
Glossary
Abbreviations:
- imDC
immature dendritic cells
- mDC
mature dendritic cells
- OPN
osteopontin
References
- 1.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–9. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 3.Brahimi-Horn MC, Pouyssegur J. The hypoxia-inducible factor and tumor progression along the angiogenic pathway. Int Rev Cytol. 2005;242:157–213. doi: 10.1016/S0074-7696(04)42004-X. [DOI] [PubMed] [Google Scholar]
- 4.Vaupel P, Briest S, Hockel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wochenschr. 2002;152:334–42. doi: 10.1046/j.1563-258x.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- 5.Okunieff P, Fenton B, Chen Y. Past, present, and future of oxygen in cancer research. Adv Exp Med Biol. 2005;566:213–22. doi: 10.1007/0-387-26206-7_29. [DOI] [PubMed] [Google Scholar]
- 6.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 7.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl. 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 8.Biselli R, Le Moli S, Matricardi PM, Farrace S, Fattorossi A, Nisini R, D’Amelio R. The effects of hypobaric hypoxia on specific B cell responses following immunization in mice and humans. Aviat Space Environ Med. 1991;62:870–4. [PubMed] [Google Scholar]
- 9.Zuckerberg AL, Goldberg LI, Lederman HM. Effects of hypoxia on interleukin-2 mRNA expression by T lymphocytes. Crit Care Med. 1994;22:197–203. doi: 10.1097/00003246-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Thiel M, Chouker A, Ohta A, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun JK, McCormick TS, Villabona C, Judware RR, Espinosa MB, Lapetina EG. Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia. Proc Natl Acad Sci USA. 1997;94:13903–8. doi: 10.1073/pnas.94.25.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal AW, Geisow M, Garcia R, Harper A, Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981;290:406–9. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 14.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 15.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 16.Moser M. Regulation of Th1/Th2 development by antigen-presenting cells in vivo. Immunobiology. 2001;204:551–7. doi: 10.1078/0171-2985-00092. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–19. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 20.Triozzi PL, Khurram R, Aldrich WA, Walker MJ, Kim JA, Jaynes S. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer. 2000;89:2646–54. doi: 10.1002/1097-0142(20001215)89:12<2646::aid-cncr18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Renkl AC, Wussler J, Ahrens T, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–55. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Gordon JR, Zhang X, Xiang J. Analysis of the gene expression profiles of immature versus mature bone marrow-derived dendritic cells using DNA arrays. Biochem Biophys Res Commun. 2002;290:66–72. doi: 10.1006/bbrc.2001.6147. [DOI] [PubMed] [Google Scholar]
- 23.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Guo J, Yang M, et al. Cyclosporin A impairs dendritic cell migration by regulating chemokine receptor expression and inhibiting cyclooxygenase-2 expression. Blood. 2004;103:413–21. doi: 10.1182/blood-2003-07-2412. [DOI] [PubMed] [Google Scholar]
- 25.Campos-Martin Y, Colmenares M, Gozalbo-Lopez B, Lopez-Nunez M, Savage PB, Martinez-Naves E. Immature human dendritic cells infected with Leishmania infantum are resistant to NK-mediated cytolysis but are efficiently recognized by NKT cells. J Immunol. 2006;176:6172–9. doi: 10.4049/jimmunol.176.10.6172. [DOI] [PubMed] [Google Scholar]
- 26.Barkley D, Allard S, Feldmann M, Maini RN. Increased expression of HLA-DQ antigens by interstitial cells and endothelium in the synovial membrane of rheumatoid arthritis patients compared with reactive arthritis patients. Arthritis Rheum. 1989;32:955–63. doi: 10.1002/anr.1780320804. [DOI] [PubMed] [Google Scholar]
- 27.Boissel N, Rousselot P, Raffoux E, et al. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia. 2004;18:1656–61. doi: 10.1038/sj.leu.2403474. [DOI] [PubMed] [Google Scholar]
- 28.Qu X, Yang MX, Kong BH, et al. Hypoxia inhibits the migratory capacity of human monocyte-derived dendritic cells. Immunol Cell Biol. 2005;83:668–73. doi: 10.1111/j.1440-1711.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- 29.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63:1164–71. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 31.Guriec N, Daniel C, Le Ster K, Hardy E, Berthou C. Cytokine-regulated expression and inhibitory function of FcgammaRIIB1 and -B2 receptors in human dendritic cells. J Leukoc Biol. 2006;79:59–70. doi: 10.1189/jlb.0305155. [DOI] [PubMed] [Google Scholar]
- 32.O’Mahony L, O’Callaghan L, McCarthy J, et al. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am J Physiol Gastrointest Liver Physiol. 2006;290:G839–45. doi: 10.1152/ajpgi.00112.2005. [DOI] [PubMed] [Google Scholar]
- 33.Hume DA. Comment on “CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes”. J Immunol. 2006;177:2035–6. doi: 10.4049/jimmunol.177.4.2035. [DOI] [PubMed] [Google Scholar]
- 34.Hansson M, Lundgren A, Elgbratt K, Quiding-Jarbrink M, Svennerholm AM, Johansson EL. Dendritic cells express CCR7 and migrate in response to CCL19 (MIP-3beta) after exposure to Helicobacter pylori. Microbes Infect. 2006;8:841–50. doi: 10.1016/j.micinf.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Darmanin S, Fu Q, et al. Hypoxia suppresses the production of matrix metalloproteinases and the migration of human monocyte-derived dendritic cells. Eur J Immunol. 2005;35:3468–77. doi: 10.1002/eji.200526262. [DOI] [PubMed] [Google Scholar]
- 36.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–90. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 37.Serrano-Gomez D, Leal JA, Corbi AL. DC-SIGN mediates the binding of Aspergillus fumigatus and keratinophylic fungi by human dendritic cells. Immunobiology. 2005;210:175–83. doi: 10.1016/j.imbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Induction of CD8+ T cell responses through targeting of antigen to Dectin-2. Cell Immunol. 2006;239:87–91. doi: 10.1016/j.cellimm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Jantsch J, Chakravortty D, Turza N, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1087–97. doi: 10.1158/1055-9965.EPI-06-1008. [DOI] [PubMed] [Google Scholar]