Abstract
While our previous studies have demonstrated that complement activation induced by complement receptors type 2 (CR2/CD21) and 1 (CR1/CD35) results in C3-fragment deposition and membrane attack complex (MAC) formation in human B cells, the consequences of these events for B-cell functions remain unknown. In the present study, we show that CR2-induced complement activation results in membrane depolarization, as indicated by annexin V binding, with kinetics similar to those of C3-fragment deposition and different from those of MAC formation. On the other hand, like MAC formation, depolarization requires activation of complement via the alternative pathway, as indicated by total inhibition upon neutralization of factor D, and is abrogated by combined blockade of CR1 and CR2, but not of either receptor alone. The membrane depolarization is not associated with the apoptosis of B cells, as examined by co-staining with APO-2.7 or by the TdT-mediated biotin–dUTP nick-end labelling (TUNEL) assay. Confocal microscopy revealed that depolarization and C3 deposition, unlike MAC deposition, are limited to restricted areas on the B-cell surface. Double staining revealed a close association between the C3-fragment patches and membrane depolarization, as well as redistribution of lipid rafts to these areas. We propose that these events may play a role in the regulation of B-cell signalling and cross-talk with T cells.
Keywords: B cells, C3 deposition, CD21, lipid rafts, membrane depolarization
Introduction
Normal human B lymphocytes (B cells) activate the complement cascade,1 resulting in covalent attachment of C3b fragments to complement receptor 2 (CR2/CD21) itself and possibly also to other acceptor sites on the cell surface.2–4 This activation also results in the formation of membrane attack complexes (MAC) in the cell membrane.5 Activation occurs both via the classical pathway (CP) and the alternative pathway (AP), where the latter plays the predominant role.6 AP activation has been shown to be mediated primarily by CR2/CD211,5 as a result of the receptor’s ability to bind the hydrolysed form of C3 (C3i), which then generates a C3 convertase by binding factor B.4 Complement receptor 1 (CR1/CD35) assists in this process, first, by binding C3i, generated in the fluid phase, for presentation to CR2 and, second, by stabilizing the C3i–CR2 interaction through forming a ternary complex with both molecules.4,7 Many of the C3b fragments deposited on the B cells are subsequently degraded via iC3b to C3dg, in a process involving CR1 as a cofactor in the factor I-mediated cleavage of iC3b.8,9 Other C3b fragments, by attaching to the AP C3 convertases, convert these to C5 convertases and thereby initiate MAC formation.
Evidence that CR2-mediated complement activation occurs in vivo is provided by the observation that B cells freshly isolated from blood bear small, but significant, amounts of C3dg on their surface (approximately 10% of that observed after in vitro activation). The low level of complement deposition on circulating B cells can be accounted for by the inhibitory action of the CR1-bearing erythrocytes, which compete for the C3i spontaneously generated in the plasma.1,6
MAC formation causes the death, through lysis, of a wide variety of infectious micro-organisms, and has been implicated as a destructive factor in a range of neurodegenerative disorders,10–12 in renal disease13,14 and in atherosclerosis.15 Conversely, MAC, at sublytic doses, may exert protection against apoptotic stimuli16,17 and promote a wide variety of cellular activities,18–21 including cell proliferation.22 The consequences of spontaneous C3b deposition and MAC formation on normal human B cells remain unclear. To address this question, we first examined the cells for signs of destruction and then, in the absence of such evidence, we examined more closely the kinetics and distribution patterns of C3-fragment deposition, MAC formation and complement-induced membrane depolarization detected as enhanced annexin V binding. In addition, the relationship between these parameters and the disposition of lipid raft signal complexes was investigated. Our findings indicate that depolarization occurs concomitantly with C3-fragment deposition and re-arrangement into larger aggregates, and that these aggregates may act as focal points for lipid raft migration. The implications of these findings for B-cell function are discussed.
Materials and methods
Cells and serum
Mononuclear cells (MNC) were isolated by centrifugation, over Lymphoprep (Nycomed, Oslo, Norway), of blood drawn from healthy consenting donors into evacuated citrate–phosphate–dextrose (CPD)-containing tubes (Terumo, Leuvan, Belgium). Serum was harvested from the same donors, by collecting blood in anticoagulant-free tubes, which were held for 1 hr at 20° before centrifugation for 5 min at 400 g. Whole blood cells were freed of plasma by washing three times with veronal buffer (VB).
Complement activation by B cells and blockade of the complement pathways
MNC were washed three times in 10 ml of VB or RPMI-1640 supplemented with glutamine and streptavidin, and suspended in low absorbing polypropylene tubes (Life Technologies, Paisley, UK), at a density of 106 cells/ml, in RPMI-1640 containing 30% (v/v) autologous serum (as a complement source). The cells were then incubated at 37° for the indicated times. To achieve total blockade of complement activation, 20 mm EDTA was added to the serum prior to incubation with the MNC. Similarly, 4 mmol/l MgCl2/20 mmO,O′-bis(2-aminoethyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid (Mg/EGTA) or 5 μg/ml sheep anti-human factor D (The Binding Site, Birmingham, AL, UK) was used to block the CP or the AP, respectively. Following incubation the reaction was stopped by transfer of the samples to an ice bath and addition of EDTA to a final concentration of 20 mm. The cells were then washed three times in phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) at 4°.
Antibodies against complement receptors
The binding site blocking the anti-CR2 monoclonal antibody (mAb) FE8 (IgG1) was prepared as described previously23 and used for receptor blockade at a concentration of 1 μg/ml. The 3D9 mAb (IgG1), which blocks the C3b-binding site of CR1,24 was a gift from Dr J. O′Shea (Frederick Cancer Research and Development Centre, Frederick, USA), and was used either singly or in combination with FE8 at a final concentration of 1 μg/ml. HB135 (IgG2a, anti-CR2, non-blocking, 1 μg/ml) and HB8592 (IgG1, anti-CR1, non-blocking, 1 μg/ml) were purchased from the American Type Culture Collection (Manassas, VA) and served as controls. The antibodies were added to the cell samples prior to mixture with serum, and were present during the entire incubation period.
Flow cytometric detection of C3 fragment- and C9-deposition, annexin V binding and lipid rafts
After incubation with serum, the cells were incubated for 2 hr at 4° with: (i) fluorescein isothiocyanate (FITC)-conjugated polyclonal rabbit anti-human C3d (Dako, Copenhagen, Denmark) at a final concentration of 1 μg/ml; (ii) the anti-human C9 mAb E11, directed against a neo-epitope only exposed on MAC-associated C9 (kindly donated by Dr T. E. Mollnes, Nordland Central Hospital, Bodø, Norway), conjugated to FITC to an FITC : antibody ratio of 4·3 : 1 (specific activity = 1·79 soluble fluorescence equivalents/mAb) in this laboratory, at a final concentration of 2 μg/ml; (iii) FITC-conjugated annexin V (Clontech, Palo Alto, CA); or (iv) the FITC-conjugated β subunit of cholera toxin (FITC–CTB; List Biological Laboratories, Campbell, CA) at a final concentration of 1 μg/ml. B cells, in the MNC preparations, were labelled concurrently by inclusion of phycoerythrin (PE)-conjugated anti-CD19 (Dako) in the incubation mix.
After one further wash, the cells were suspended in FACSFlow (BD Biosciences, Glostrup, Denmark) and analyzed by flow cytometry, using a FACScalibur cytometer (BD Biosciences) and the cellquest software. B cells were identified by a combination of morphological (forward-light and side-light scatter) and fluorescence gating. Specific C3-fragment deposition, C9 incorporation into MAC and annexin V binding on B cells were measured as median fluorescence.
Confocal microscopy
MNC were incubated for 30 min at 4° with: (i) FITC-conjugated polyclonal rabbit anti-human C3d (Dako), (ii) FITC–CTB, (iii) Alexa Fluor 647–Annexin V (Invitrogen, Taastrup, Denmark), or (iv) tetramethyl rhodamine iso-thiocyanate (TRITC)–CTB (List Biological Laboratories), singly or in combination. After staining, the B cells were negatively selected using the Human B cell enrichment kit (StemCell Technologies, Grenoble, France) using the procedure recommended by the manufacturer. The B cells were then fixed for 30 min at 20° in 2% paraformaldehyde solution, centrifuged and washed twice, and then placed in Nunc® Lab-Tek® Chamber Slides (Sigma-Aldrich, Brøndby, Denmark) for analysis on a scanning confocal microscope system (model LSM 510; Carl Zeiss, Jena, Germany).
Measurement of apoptosis
TUNEL assay (APO-DIRECT™ kit)
MNC were incubated at 37° for 4 or 20 hr in RPMI-1640 containing 30% autologous serum and the relevant inhibitors. After incubation, 1 × 106 cells were suspended in 1% (v/v) paraformaldehyde in PBS and placed on ice. After 30 min, the cells were washed twice in PBS, resuspended in 70% (v/v) ice-cold ethanol and stored overnight at −20° in a freezer. Staining and analyses were performed using the protocol in the APO-DIRECT™ kit (Pharmingen, San Diego, CA).
APO-2.7 expression assay
MNC were incubated at 37° overnight in RPMI-1640 containing 30% (v/v) autologous serum and the relevant inhibitors. After incubation, the cells were washed twice in PBS. The cells (2·5 × 105) were incubated at 4° for 20 min with FITC-conjugated APO-2.7 mAb (Immunotech, Marseilles, France), PE–CD4 (BD Biosciences) and peridinin–chlorophyll–protein (PerCP)-conjugated CD19 (BD Biosciences) in PBS and then washed and fixed in PBS containing 1% formaldehyde.
Statistics
Unpaired t-tests were used for comparisons between groups, and the one-sample t-test was used to evaluate whether inhibitions differed from 0%, or if fluorescence values differed from those to which they were normalized (set as 100). Mean ± standard deviation values are shown unless stated otherwise. Two-sided P-values of < 0·05 were considered significant. All calculations were performed using graphpad prism for Windows, v. 4.00 (GraphPad, San Diego, CA).
Results
Spontaneous complement activation leads to exposure of phosphatidylserine on the surface of B cells
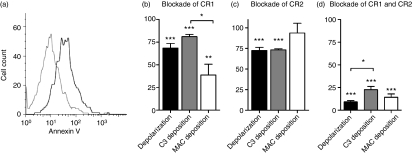
Following incubation of MNC with serum under circumstances known to elicit spontaneous C3 deposition and subsequent MAC deposition,5,7 B cells were examined for signs of apoptosis or necrosis on the basis of annexin V binding, DNA fragmentation and APO-2.7 expression. A moderate increase in annexin V binding to exposed phosphatidyl serine (PS) on the B cells was observed after incubation of MNC with autologous serum for 30 min (Fig. 1a), and the binding displayed a dependence on CR1/CD35 and CR2/CD21 activity that was similar to those for C3-fragment deposition and MAC formation, in that it was moderately inhibited by blockade of either receptor alone, whilst combined blockade resulted in its abrogation (Fig. 1b–d). By contrast, the low level of apoptosis observed under the incubation conditions (Fig. S1) was not altered through complement receptor blockade (Table 1 and Fig. 1b). Thus, it may be concluded that the PS exposure on the surface of B cells, following spontaneous complement activation, is not a consequence of apoptosis but, rather, of membrane depolarization arising directly from the complement-activation process.
Figure 1.
Spontaneous, complement receptor-dependent B-cell membrane depolarization and deposition of complement fragments. (a) A flow cytometric histogram showing membrane depolarization, as assessed by the binding of fluorescein isothiocyanate (FITC)-labelled annexin V to normal CD19+ B cells before (grey line) and after (black line) incubation with autologous serum (30%, v/v) for 30 min. (b) The effect of CR1 blockade on membrane depolarization, C3-fragment deposition and membrane attack complex (MAC) formation. Mononuclear cells (MNC) were pre-incubated with a CR1-binding site-blocking monoclonal antibody (mAb), 3D9, prior to incubation with autologous serum. The effect of receptor blockade on membrane depolarization (as assessed by the binding of annexin V), C3-fragent deposition (as assessed by the binding of anti-C3d) and MAC formation (as assessed by the binding of anti-C9) is shown. The data shown are normalized to the values obtained with a non-function blocking mAb, HB8592, used as the negative control. (c) The corresponding effect of neutralization of CR2 with a binding site-blocking mAb, FE8, is shown. The control here was the non-function blocking mAb, Hb135. (d) The effect of combined blockade of CR1 and CR2 with 3D9 and FE8 is shown. The control was incubation with the combination of H8592 and HB135. The data shown are mean ± standard error of the mean (SEM) of five experiments. *, **, ***: P < 0·05, 0·01 and 0·005, respectively, for significance of difference from 100% or between barred values.
Table 1.
Effect of complement receptor blockade on spontaneous B-cell apoptosis
| Serum (%) | Serum + FE8 and 3D9 (%) | |
|---|---|---|
| TUNEL assay | ||
| After 4 hr1 | 5·6 ± 2·3 | 6·5 ± 3·9 |
| After 20 hr1 | 14 ± 6 | 12 ± 7 |
| APO-2.7 | ||
| After 20 hr1 | 9·3 ± 4·4 | 8·8 ± 5·6 |
Shown is the percentage of apoptotic CD19+ B cells in a preparation of mononuclear cells incubated with 30% serum alone, or with 30% serum containing a combination of blocking antibodies to CR2 (FE8) and CR1 (3D9) for the given periods of time. The data are shown as mean ± standard deviation (SD) of three experiments.
TUNEL, TdT-mediated biotin–dUTP nick-end labeling.
Membrane depolarization shows kinetic similarities to that of C3-fragment deposition
The rise in annexin V binding upon complement activation, and its dependency on CR1/CD35 and CR2/CD21, suggests a connection between PS exposure and C3-fragment deposition or MAC formation. We therefore investigated the kinetics of the three processes to establish whether any correlation existed among them.
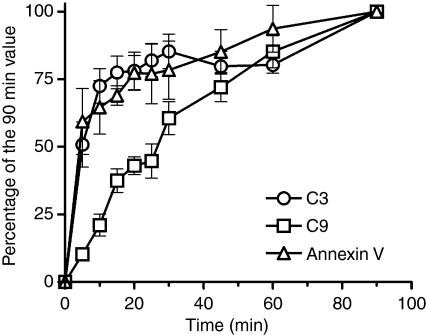
C3-fragment deposition and annexin V binding displayed the same biphasic kinetics, rising rapidly to an initial peak value after 20 min followed by a more gradual increase up to 90 min, indicating a relationship between C3-fragment deposition and membrane depolarization. The rate of C9 incorporation was somewhat slower, in keeping with the dependence of this process on C3 activation, and did not display the same biphasic pattern (Fig. 2).
Figure 2.
Kinetics of B-cell membrane depolarization and the deposition of complement on B cells. Mononuclear cells (MNC) were incubated with autologous serum (30%, v/v) for up to 90 min. B cells were identified by a combination of morphological (forwardlight and side-light scatter) and fluorescence gating. The resulting membrane depolarization, C3-fragment deposition and membrane attack complex (MAC) formation was detected by flow cytometry, by means of fluorescein isothiocyanate (FITC)-conjugated polyclonal rabbit antibodies to human C3d (circles), and C9 (squares) and FITC-conjugated annexin V (triangles), respectively. The fluorescence intensities were normalized to the signal measured after 90 min of incubation. C3-fragment deposition and annexin V binding display the same biphasic kinetics, rising rapidly over 20 min, followed by a more gradual increase. MAC formation, assessed as C9 incorporation, showed a somewhat slower monophasic increase. The data shown are mean values and 95% confidence intervals of six experiments. The curves differed significantly [P < 0·0001, as determined by a repeated-measures analysis of variance (anova)].
Membrane depolarization is primarily dependent on AP activation of complement
Our previous studies have shown that spontaneous complement activation on B cells occurs via both the AP and the CP, and that the activation pathway involved has a major influence both on the nature of the C3 fragments deposited (i.e. C3b/iC3b versus C3dg) and on the efficiency of MAC formation.7 Thus, activation via the AP results in only limited C3-fragment degradation and in more effective MAC initiation. We therefore chose to investigate whether the AP or the CP is primarily responsible for the increase in annexin V binding.
Blockade of the AP with anti-factor D resulted in total abrogation of both MAC formation and annexin V binding, while C3-fragment deposition was virtually unaffected (Table 2). By contrast, blockade of CP with Mg/EGTA resulted in more limited and variable diminution of all three parameters. In experiments where both CP and AP were blocked, C3-fragment deposition and MAC formation were totally abrogated, whereas annexin V binding was inhibited by 74% (Table 2).
Table 2.
Inhibition of B-cell membrane depolarization, C3-fragment deposition and membrane attack complex (MAC) formation mediated by blockade of the alternative and classical pathways of complement activation
| Percentage of inhibition mediated by |
|||
|---|---|---|---|
| aFD | Mg/EGTA | EDTA | |
| Membrane depolarization | 83 ± 7 (P< 0·0001) | 36 ± 7 (P< 0·0005) | 74 ± 5 (P< 0·0001) |
| C3-fragment deposition | 10 ± 3 (P< 0·0005) | 29 ± 7 (P< 0·0005) | 96 ± 4 (P< 0·0001) |
| MAC formation | 89 ± 4 (P< 0·0001) | 22 ± 5 (P< 0·002) | 99 ± 1 (P< 0·0001) |
Mononuclear cells were incubated with 30% autologous serum for 30 min at 37°. Shown is the inhibition of B-cell membrane depolarization (as measured by annexin V binding), C3 deposition and membrane attack complex (MAC) formation (as measured by the binding of anti-C3d and anti-C9, respectively) caused by disruption of the alternative pathway with anti-factor D (aFD), of the classical pathway with Mg/EGTA, and of both pathways with EDTA. The data represent the mean ± standard deviation (SD) of five experiments. P-values indicate the probability for the null hypothesis that no inhibition occurs.
Annexin V binds to restricted areas on the surface of complement-activated B cells
Our results indicate that annexin V binding to B cells, seen upon complement activation, is not a consequence of apoptosis but rather a structural change in the B-cell surface membrane, arising in conjunction with C3-fragment deposition. To examine more closely the nature and extent of this depolarization, we conducted simultaneous flow cytometric and confocal microscopy experiments on B cells both before and after incubation with autologous serum.
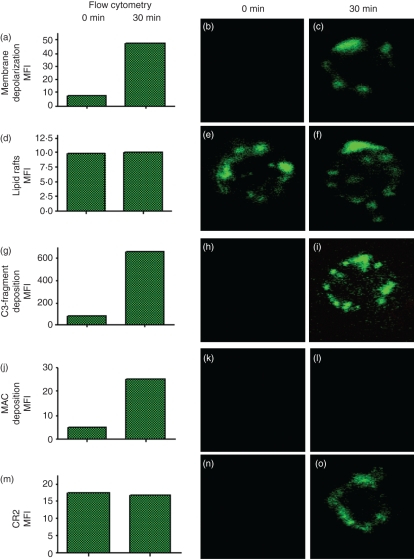
Flow cytometric analysis, following complement activation, showed an increase of approximately 10-fold over unstimulated B cells in the amount of annexin V bound to their surface membranes (Fig. 3a). Using confocal microscopy, annexin V binding to the B cells was barely visible in the absence of complement activation (Fig. 3b), indicating that the level of annexin V binding recorded by flow cytometry in this situation lay below the detection limit for the confocal microscope. After incubation with autologous serum for 30 min, however, annexin V was clearly located in restricted areas on the surface of the B cells (Fig. 3c).
Figure 3.
Visualization of membrane depolarization, lipid rafts, C3-fragment deposition, membrane attack complex (MAC) deposition and CR2 redistribution. Mononuclear cells (MNC) were incubated without (0 min) and with autologous serum for 30 min to allow spontaneous complement activation on B cells. B cells were then purified using magnetic beads. Using flow cytometry (left panels) and confocal microscopy (middle and right panels) the cells were analysed for (a–c) membrane depolarization, (d–f) lipid rafts, (g–i) C3 fragment deposition, (j–l) MAC deposition, and (m–o) the expression of CR2. Representative results of triplicate experiments are shown. MFI = mean fluorescent intensity units.
Dillon et al.25,26 have previously shown that annexin V binds to the signal-transducing lipid rafts on the B cells. To test whether the restricted regions to which annexin V binds were lipid rafts, we conducted simultaneous flow cytometric and confocal microscopic analyses using the specific lipid raft marker, CTB, and compared these to the staining with annexin V. Flow cytometric analysis revealed that the level of bound CTB was unaffected by 30 min of complement activation (Fig. 3d), as were the pattern and intensity of staining as visualized by confocal microscopy (Fig. 3e,f), leading us to conclude that areas of depolarization were not necessarily constituents of lipid rafts.
C3 deposition, but not MAC deposition, occurs in restricted areas
Prior to spontaneous complement activation, no C3 could be detected on the B-cell surface with either anti-C3d (Fig. 3h) or anti-C3c (data not shown), by confocal microscopy, even though the amount of C3d present (79 mean fluorescence intensity units (MFI) per B cell, as determined by flow cytometry) should have been sufficient for detection, if the fragments were deposited in clusters. Following complement activation, however, C3 was visible in patches similar to those seen with CTB (Fig. 3i). In contrast to C3, MAC deposition after complement activation could not be detected using confocal microscopy (Fig. 3k,l), despite the fact that the MAC fluorescence intensity (25 MFI per B cell, Fig. 3j) was similar in magnitude to that generated by FITC–annexin V or FITC–CTB (Fig. 3a,d). This could be accounted for by a uniform distribution of MAC fragments over the B-cell surface as a result of their rapid diffusion, at 37°, away from the sites of formation.
CR2/CD21 translocates into restricted areas upon spontaneous activation of complement
CR2/CD21 contributes to C3b-fragment deposition on B cells by acting as a privileged site for the assembly of the C3 convertase of the AP.2–4 Because it is well established that CR2/CD21 translocates with the B-cell antigen receptor (BCR) into lipid rafts upon cross-linking of the BCR by antigen,27 we next examined whether translocation of CR2/CD21 also occurred upon complement activation.
As might be expected, the expression of CR2 by B cells was unaffected by complement activation, generating a constant flow-cytometric signal of 17 MFI (Fig. 3m). Prior to complement activation, however, no fluorescence was detectable by confocal microscopy, indicating a uniform dispersal of CR2 molecules on the B-cell surface (Fig. 3n). However, after complement activation, CR2/CD21 was detectable in restricted regions similar to those revealed with anti-C3, annexin V and CTB (Fig. 3o).
The topographical relationship among membrane depolarization, C3-fragment deposition and lipid rafts
The observation that membrane depolarization (as detected by annexin V binding) is dependent upon spontaneous complement activation and C3-fragment deposition at the B-cell surface, while the staining intensity of lipid rafts with CTB is unaffected by these events, raises the question as to whether these two phenomena are in any way related. In order to examine more closely the topographical relationship among C3-fragment deposition, membrane depolarization and the distribution of lipid rafts, double labelling of the B cells, following complement activation, was undertaken using FITC-labelled anti-C3d and Alexa Fluor 647-conjugated annexin V or CTB.
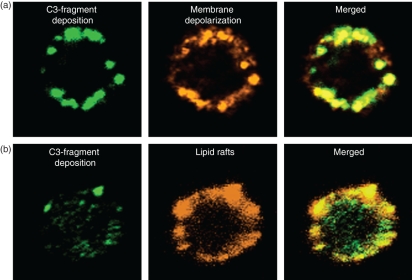
Whereas annexin V binding was primarily focussed in the centres of the C3-fragment aggregates (reflected by the intense yellow staining of their cores, Fig. 4a), the co-localization of cholera toxin with the aggregates was more diffuse and consistent with congregation of lipid rafts both throughout and around the major clusters of deposited C3 fragments (Fig. 4b).
Figure 4.
Topographical relationship among membrane depolarization, C3-fragment deposition and lipid rafts. Purified B cells were incubated with autologous serum for 30 min at 37°. (a) Localization of C3 fragments (left) and membrane depolarization (middle), as assessed by staining with fluorescein isothiocyanate (FITC)-coupled anti-C3d and Alexa fluor 647-coupled annexin V, respectively. Merged figures (right) show co-localization (yellow colour). (b) Staining for C3 fragments with FITC–anti-C3d (left) and for lipid rafts, as assessed using tetramethyl rhodamine iso-thiocyanate (TRITC)-coupled cholera toxin subunit B (middle). Merged figures (right) show co-localization (greenish yellow).
Discussion
The purpose of this study was to establish whether spontaneous complement activation at the B-cell surface, and the consequent deposition of C3 fragments and formation of MAC,1 give rise to biochemical or physiological changes in the B cells that might affect their function in an immune response. MAC formation on the surface of B cells could lead to loss of membrane integrity, possibly resulting in cell death. When we tested this, using the binding of annexin V as read-out, we observed that incubation of B cells with fresh serum resulted in spontaneous membrane depolarization as well as MAC deposition, and that both events were dependent on AP activation and functional CR1/CD35 and CR2/CD21. As the degradation of deposited C3b fragments to C3dg is limited when complement activation occurs via the AP, it may be inferred that depolarization and MAC formation are concomitant events dependent on the persistence of C3b (or iC3b) at the cell surface. On the other hand, the findings that membrane depolarization displayed much faster kinetics than MAC formation, and that there was no concordance in their distribution at the cell surface, appeared to rule out the possibility of depolarization being an apoptosis-related event. Confirmation of this conclusion was obtained from investigations with APO-2.7 mAb and TUNEL staining, which were negative.
In contrast to MAC formation, membrane depolarization showed near synchrony with the deposition of C3 fragments on the B-cell membrane, and a close association was observed between discrete areas of depolarization and the primary centres of C3-fragment deposition (Fig. 4a).
As it has been reported that lipid rafts on viable B cells bind annexin V,25,26 we chose to investigate, by confocal microscopy, whether the distribution of annexin V binding could be related to the presence of lipid rafts detected by CTB, which binds to ganglioside GM1,28 a raft component quite distinct from PS, which is involved in annexin V binding. While annexin V binding showed a pattern similar to that for CTB in single colour staining, it differed in one notable respect, namely that its binding required prior complement activation at the cell surface, whereas staining of the lipid rafts with CTB was complement-independent. Furthermore, a clear difference was observed in the distribution patterns of membrane depolarization and lipid rafts in that depolarization was intimately associated with the C3-fragment aggregates, whereas the distribution of lipid rafts in and around the C3 deposits was more diffuse (Fig. 4b). Given the identical kinetics for C3-fragment deposition and depolarization (as shown in Fig. 2), our interpretation of these findings is that the membrane depolarization is directly associated with aggregation of the C3 fragments, rather than a result of any internalization and processing event (which would be expected to involve a much larger time-frame), and is succeeded by migration of lipid rafts to these aggregates. It remains to be established whether the membrane depolarization, occurring within the aggregates, has any role in the assembly of lipid rafts.
The confocal studies also revealed a complement-dependent redistribution of the initiating CR2/CD21 from a diffuse pattern, invisible by microscopy, to visible patches similar to those of both the C3 fragments and annexin V.
Taken together, our observations are consistent with a model whereby C3 fragments deposited on, or in the vicinity of, the CR2 molecules responsible for complement activation aggregate to generate foci for membrane depolarization. This is succeeded by association of the aggregates with lipid raft signalling units by an, as yet, unknown mechanism. The finding of only terminally degraded C3 fragments (C3dg), in small amounts, on freshly isolated peripheral B cells, indicates that this process may be blocked in the circulation, where erythrocytes compete via their CR1 for any iC3 generated in the plasma.6 However, the process is likely to occur in inflamed tissue and in the draining lymph nodes, if complement is available in sufficient quantities. The formation of such complexes could be of relevance for B-cell activation and antigen presentation to T cells. To test this hypothesis, further studies are required to establish whether other components of the presentation process, such as BCR, CD19 and major histocompatibility complex II (MHC II), are found in association with the C3-fragment foci.
Elliott et al.29 have previously reported that PS functions as a non-apoptotic signalling mechanism in subpopulations of T cells, and it remains to be established whether the same is true for B cells. A possible option is that the assembly of CR2, C3 fragments and lipid rafts in depolarized regions on the B-cell surface prepares the B cells for subsequent uptake and presentation of antigen, and that the focusing of C3 fragments in these complexes may serve as a T-cell regulatory event. Relevant in this regard is the finding that naive T cells are driven to become regulatory T cells (Treg1) by C3b engagement of CD46 on their surfaces.30 Given that deposited C3 fragments on the surface on the antigen-presenting B cells are capable of engaging CD46 on naive T cells, this would help to explain an earlier observation that primary antigen presentation by B cells to T cells suppresses an immune response.31
Acknowledgments
We thank Jorge Bernardino de la Santa at MEMPHYS-Center for Membrane Physics, University of Southern Denmark, for help with the confocal images. The study was approved by the local ethics committee. This work received funding from the Danish Rheumatism Association, The Novo Nordisk Foundation, and The A. P. Møller and Spouse Chastine Mc-Kinney Møller Foundation.
Disclosures
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Viability of leucocytes before and after spontaneous complement activation.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Marquart HV, Svehag SE, Leslie RG. CR2 is the primary acceptor site for C3 during alternative pathway activation of complement on human peripheral B lymphocytes. J Immunol. 1994;153:307–15. [PubMed] [Google Scholar]
- 2.Mold C, Nemerow GR, Bradt BM, Cooper NR. CR2 is a complement activator and the covalent binding site for C3 during alternative pathway activation by Raji cells. J Immunol. 1988;140:1923–9. [PubMed] [Google Scholar]
- 3.Olesen EH, Johnson AA, Damgaard G, Leslie RG. The requirement of localized, CR2-mediated, alternative pathway activation of complement for covalent deposition of C3 fragments on normal B cells. Immunology. 1998;93:177–83. doi: 10.1046/j.1365-2567.1998.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwendinger MG, Spruth M, Schoch J, Dierich MP, Prodinger WM. A novel mechanism of alternative pathway complement activation accounts for the deposition of C3 fragments on CR2-expressing homologous cells. J Immunol. 1997;158:5455–63. [PubMed] [Google Scholar]
- 5.Nielsen CH, Marquart HV, Prodinger WM, Leslie RG. CR2-mediated activation of the complement alternative pathway results in formation of membrane attack complexes on human B lymphocytes. Immunology. 2001;104:418–22. doi: 10.1046/j.1365-2567.2001.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen CH, Pedersen ML, Marquart HV, Prodinger WM, Leslie RG. The role of complement receptors type 1 (CR1, CD35) and 2 (CR2, CD21) in promoting C3 fragment deposition and membrane attack complex formation on normal peripheral human B cells. Eur J Immunol. 2002;32:1359–67. doi: 10.1002/1521-4141(200205)32:5<1359::AID-IMMU1359>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Leslie RG, Prodinger WM, Nielsen CH. Complement receptors type 1 (CR1, CD35) and 2 (CR2, CD21) cooperate in the binding of hydrolyzed complement factor 3 (C3i) to human B lymphocytes. Eur J Immunol. 2003;33:3311–21. doi: 10.1002/eji.200324330. [DOI] [PubMed] [Google Scholar]
- 8.Leslie RG. The influence of complement receptor type 1 (CD35) and decay-accelerating factor (CD55) on complement receptor type 2- (CD21) mediated alternative pathway activation by B cells. Immunology. 1999;97:371–3. doi: 10.1046/j.1365-2567.1999.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross GD, Lambris JD. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J Exp Med. 1982;155:96–110. doi: 10.1084/jem.155.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGeer EG, McGeer PL. The future use of complement inhibitors for the treatment of neurological diseases. Drugs. 1998;55:739–46. doi: 10.2165/00003495-199855060-00001. [DOI] [PubMed] [Google Scholar]
- 11.McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26(Suppl. 1):94–7. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Morgan BP, Chamberlain-Banoub J, Neal JW, Song W, Mizuno M, Harris CL. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol. 2006;146:294–302. doi: 10.1111/j.1365-2249.2006.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nangaku M, Couser WG. Mechanisms of immune-deposit formation and the mediation of immune renal injury. Clin Exp Nephrol. 2005;9:183–91. doi: 10.1007/s10157-005-0357-8. [DOI] [PubMed] [Google Scholar]
- 14.Quigg RJ. Complement and autoimmune glomerular diseases. Curr Dir Autoimmun. 2004;7:165–80. doi: 10.1159/000075692. [DOI] [PubMed] [Google Scholar]
- 15.Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30:73–80. doi: 10.1385/IR:30:1:073. [DOI] [PubMed] [Google Scholar]
- 16.Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- 17.Zwaka TP, Torzewski J, Hoeflich A, Dejosez M, Kaiser S, Hombach V, Jehle PM. The terminal complement complex inhibits apoptosis in vascular smooth muscle cells by activating an autocrine IGF-1 loop. FASEB J. 2003;17:1346–8. doi: 10.1096/fj.02-0814fje. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Yang C, Jin N, Xie Z, Tang Y, Fei L, Jia Z, Wu Y. Terminal complement complex C5b-9-treated human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization. Eur J Immunol. 2007;37:167–76. doi: 10.1002/eji.200636285. [DOI] [PubMed] [Google Scholar]
- 19.Viedt C, Hansch GM, Brandes RP, Kubler W, Kreuzer J. The terminal complement complex C5b-9 stimulates interleukin-6 production in human smooth muscle cells through activation of transcription factors NF-kappa B and AP-1. FASEB J. 2000;14:2370–2. doi: 10.1096/fj.00-0468fje. [DOI] [PubMed] [Google Scholar]
- 20.Burger A, Wagner C, Hug F, Hansch GM. Up-regulation of intracellular calcium, cyclic adenosine monophosphate and fibronectin synthesis in tubuar epithelial cells by complement. Eur J Immunol. 1999;29:1188–93. doi: 10.1002/(SICI)1521-4141(199904)29:04<1188::AID-IMMU1188>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Lupia E, Del SL, Bergerone S, Emanuelli G, Camussi G, Montrucchio G. The membrane attack complex of complement contributes to plasmin-induced synthesis of platelet-activating factor by endothelial cells and neutrophils. Immunology. 2003;109:557–63. doi: 10.1046/j.1365-2567.2003.01692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fosbrink M, Niculescu F, Rus H. The role of c5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol Res. 2005;31:37–46. doi: 10.1385/IR:31:1:37. [DOI] [PubMed] [Google Scholar]
- 23.Prodinger WM, Schwendinger MG, Schoch J, Kochle M, Larcher C, Dierich MP. Characterization of C3dg binding to a recess formed between short consensus repeats 1 and 2 of complement receptor type 2 (CR2; CD21) J Immunol. 1998;161:4604–10. [PubMed] [Google Scholar]
- 24.O’Shea JJ, Brown EJ, Seligmann BE, Metcalf JA, Frank MM, Gallin JI. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985;134:2580–7. [PubMed] [Google Scholar]
- 25.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–32. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 26.Dillon SR, Constantinescu A, Schlissel MS. Annexin V binds to positively selected B cells. J Immunol. 2001;166:58–71. doi: 10.4049/jimmunol.166.1.58. [DOI] [PubMed] [Google Scholar]
- 27.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–60. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Heyningen S. Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974;183:656–7. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- 29.Elliott JI, Surprenant A, Marelli-Berg FM, et al. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol. 2005;7:808–16. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 30.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–9. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.