Abstract
Interleukin-27 (IL-27) is a new IL-12-related heterodimeric cytokine comprising a novel p28 molecule and the Epstein–Barr-virus-induced gene 3 (EBI3) molecules. It augments initiation of T helper type 1-mediated immunity by enhancing the proliferation and cytokine production of T cells. In this study, we examined whether a secreted form of IL-27 subunits would inhibit IL-27-mediated immunological responses. COS-7 cells transduced with the mouse (m) p28 gene secreted a monomeric mp28 protein; however, those transduced with the mEBI3 gene did not detect a mEBI3 protein in the culture supernatants. The secreted mp28 prevented the IL-27-mediated signal transduction and activator of transcription 1 phosphorylation and subsequently inhibited the IL-27-mediated intercellular adhesion molecule-1 induction and interferon-γ production in CD4+ T cells. We generated mp28-expressing murine carcinoma Colon 26 cells and inoculated a mixture of the mp28- and mIL-27-expressing Colon 26 cells into syngeneic BALB/c mice. Simultaneous production of mp28 and mIL-27 from Colon 26 cells suppressed IL-27-mediated anti-tumour effects in the mice. We examined the p28-mediated immune suppression by inoculating mp28-expressing myoblasts into allogeneic mice. Forced production of mp28 suppressed the allogeneic cytotoxic T-lymphocyte induction and subsequently retarded the graft rejection. Furthermore, production of both mp28 and mp40, which inhibits the functions of IL-12 and IL-23, prolonged the graft survival longer than the grafts expressing either mp28 or mp40. We propose that p28 can be a regulatory subunit for IL-27-mediated cellular immune responses and a possible therapeutic agent to suppress unfavourable immune responses.
Keywords: anergy, cytokine receptors, cytokines, T cells, tolerance
Introduction
Interleukin-27 (IL-27) is a novel heterodimeric cytokine belonging to the IL-12 family, which includes IL-12 and IL-23, and consists of p28, a newly discovered protein related to the p35 subunit of IL-12 (IL-12p35), and the Epstein–Barr-virus-induced gene 3 (EBI3) protein related to the p40 subunit of IL-12 and IL-23 (p40).1 Interleukin-27 is produced by activated antigen-presenting cells and preferentially activates naïve CD4+ T cells to enhance their proliferation and IL-12-mediated interferon-γ (IFN-γ) production.1,2 It also induces functional maturation of CD8+ T cells to effector cytotoxic T lymphocytes (CTL) and promotes immunoglobulin G2a (IgG2a) production in B cells.3–5 The IL-27 receptor is also a heterodimeric protein composed of gp130 and the orphan receptor WSX-1/T-cell cytokine receptor (TCCR), a homologue of the IL-12 receptor β2 subunit.6,7 Recent studies have shown that IL-27 activates signal transduction and activator of transcription (STAT) transcription factors (STAT1, STAT2, STAT3, STAT4 and STAT5) to induce the expression of T helper type 1 (Th1) -specific transcription factor T-bet, while it suppressed the expression of Th2-specific transcription factor GATA-binding protein 3 (GATA3) by down-regulating STAT6 expression.2,3,8,9 In WSX-1/TCCR or the EBI3 gene knockout mice IL-27 was critical for initiating efficient anti-parasite Th1 immunity in the early phase of a parasite infection.7,10,11 In contrast, the same WSX-1/TCCR-deficient mice demonstrated long-lasting severe inflammation because of a parasite burden;12 in this manner, IL-27 might play dual roles to initiate or to attenuate the immune responses.13
IL-12p35 and the p19 subunit of IL-23 (IL-23p19) are biologically inactive and hardly secreted when p40 is not expressed.14,15 In contrast, p40 is secreted when neither IL-12p35 nor IL-23p19 is expressed, and can inhibit the biological functions of IL-12 as a result of competitive binding of p40 to the IL-12 receptor.14,16 The forced expression of the p40 gene in donor cells subsequently retarded IL-12-mediated graft rejections in allogeneic host animals.17 Our report also showed that murine p40 (mp40) inhibited biological functions of mIL-23 as well as mIL-12 in vitro and in vivo.18 These findings suggest that a secreted subunit of the IL-12 family cytokines might play a role in the regulation of immune responses.
Subunits of IL-27 seemed to be secreted without making the heterodimeric protein1 and the molecular composition of the IL-27 receptor complex is similar to that of IL-12 and IL-23 receptors. We therefore presumed that a secreted subunit of IL-27 could impair the IL-27-mediated responses and examined possible inhibitory effects of p28 in vitro and in vivo in this study.
Materials and methods
Animals and cells
C57BL/6 and BALB/c mice (6- to 8-week-old females) were purchased from Japan SLC (Hamamatsu, Japan). Murine thymoma EL-4 cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS). The β-galactosidase-expressing murine myoblast C2C12 (C2Z) cells, the mp40-expressing C2Z (C2Zp40) cells,17 murine colon carcinoma Colon 26 cells, the bicistronic mp28-internal ribosomal entry site (IRES)-mEBI3 gene-expressing Colon 26 (Colon 26/IL-27) cells19 and simian kidney COS-7 cells were cultured in Dulbecco’s modified Eagle’s minimum essential medium (DMEM) supplemented with 10% heat-inactivated FCS.
Reagents
Fluorescein isothiocyanate-conjugated anti-mouse intercellular adhesion molecule-1 (mICAM-1) (YN1/1.7.4) and anti-H-2Ld (28-14-8) monoclonal antibodies (mAbs), purified anti-mCD28 (37.51) and anti-mouse interferon-γ (mIFN-γ) (XMG1.2) mAbs, and horseradish peroxidase (HRP)-conjugated anti-mIFN-γ (R4-6A2) mAb were purchased from e-Bioscience (San Diego, CA). Fluorescein isothiocyanate-conjugated anti-H-2Kd (SF1-1.1), anti-H-2Dd (34-2-12), anti-H-2Kk (AF3-12.1) and anti-H-2Dk (15-5-5) mAbs, and anti-mCD4-conjugated magnetic beads, and purified anti-mCD3 (17A2) mAb were obtained from BD Biosciences (San Jose, CA). Anti-myc (9E10) and anti-phosphotyrosine (pY)-STAT1 (A-2) mAbs and anti-HA (Y-11) and anti-STAT1p91 (M-23) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The HRP-conjugated anti-mIgG1 and anti-rabbit IgG antibodies were obtained from Southern Biotechnology (Birmingham, AL) and Invitrogen (Carlsbad, CA), respectively. Recombinant (r) mIL-27 and rmIFN-γ were obtained from R&D Systems (Minneapolis, MN).
Detection of secreted tagged-subunit proteins
For the construction of vectors expressing myc-tagged mp28 and mEBI3 (mp28-myc and mEBI3-myc, respectively), mp28 and mEBI3 genes generated with reverse transcription–polymerase chain reaction (RT-PCR)19 were cloned into pcDNA3.1-myc-His vector (Invitrogen). COS-7 cells were transfected with the vectors with lipofectin (Invitrogen) and then the supernatants and the cell lysates were collected after 48 hr. These samples were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reduced and non-reduced conditions and to standard Western blot analyses using appropriate antibodies and an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Piscataway, NJ). For detection of heterodimeric IL-27 secretion, the mEBI3 gene was fused with a haemagglutinin (HA) epitope at the 3′-end and was cloned into pcDNA3 vector (Invitrogen). The supernatants and cell lysates of the COS-7 cells transfected with both the mp28-myc and mEBI3-HA genes were subjected to SDS–PAGE under reducing conditions for Western blot analyses.
Assays for IL-27-stimulated T cells
Splenic CD4+ T cells of naïve C57BL/6 mice were purified using magnetic sorting. For the analysis of IFN-γ production, the CD4+ T cells were stimulated with rmIL-27 (10 ng/ml) and the supernatants [50% volume/volume (v/v)] of either the mp28-transfected or parental COS-7 cells. The viable cells were collected 24 hr later and re-stimulated with the supernatants (50% v/v) of mIL-12-transfected COS-7 cells for a further 24 hr.18 The amounts of IFN-γ in the supernatants were measured with an enzyme-linked immunosorbent assay. The IFN-γ production was statistically analysed using Student’s t-test. For an analysis of ICAM-1 expression, the CD4+ T cells were stimulated with plate-coated anti-CD3 (2 μg/ml) mAb and soluble anti-CD28 (0·5 μg/ml) mAb in the presence of rmIL-27 (10 ng/ml) and the supernatants (50%, v/v) of the mp28-transfected or parental COS-7 cells. The cells were harvested 16 hr later and the ICAM-1 expression profiles were cytometrically analysed with Cell Quest software (BD Biosciences). For STAT1 tyrosine phosphorylation, CD4+ T cells were stimulated for 15 min with rmIL-27 (100 ng/ml) and the supernatants (50%, v/v) of either the mp28-transfected or parental COS-7 cells. The cell lysates were subjected to Western blot analysis with anti-pY-STAT1 and anti-STAT1 antibodies.2,7 The intensity of respective proteins was determined with an image analysis using the public domain Image J program (available on the web site at http://rsb.info.nih.gov/ij). An arbitrary unit of phosphorylation was calculated according to the formula [intensity of pY-STAT1/intensity of STAT1] and the phosphorylation level was statistically analysed using Student’s t-test (n = 3).
Tumorigenesis of mp28-expressing tumour cells
Colon 26 cells were transduced with a LXSN retroviral vector bearing the mp28 DNA and G418 (600 μg/ml) resistant cells (Colon 26/p28) were selected. The gene expression was determined with Northern blot analyses using the mp28-specific radioactive probe.19 The expression levels of major histocompatibility complex (MHC) class I molecules on the Colon 26/IL-27, Colon 26/p28 and Colon 26 cells were determined with flow cytometry. Colon 26 cells (1 × 106) or the mixtures (1 × 106 in total) of Colon 26/IL-27 (2 × 105) and either Colon 26 or Colon 26/p28 (8 × 105) cells were inoculated subcutaneously or intraperitoneally into syngeneic BALB/c mice. The volume of subcutaneous tumours was calculated according to the formula [1/2 × length × width2]. Survival period of the tumour-inoculated mice were statistically analysed with a log rank test.
Generation of mp28-expressing myoblasts and myoblast transplantation
C2Z cells were transduced with the LXSN retroviral vector bearing the mp28 DNA and G418-resistant cells (800 μg/ml G418; C2Zp28) were selected. The mp28 and mp40 expression in myoblasts was determined with RT-PCR.18,19 Expression profiles of MHC class I molecules on each myoblast were analysed with flow cytometry. A β-galactosidase assay with a chromogenic substrate chlorophenol red-β-d-galactoside was carried out as previously described with small modifications.20 The cell lysates were prepared by sonication in 0·25 m Tris–HCl buffer (pH 7·5) and cleared supernatants were mixed with the substrate solution. Absorbance at 574 nm was measured with a microplate reader. Enzymatic activities were calculated with a purified-β-galactosidase protein (Calbiochem, San Diego, CA). Total protein concentrations were quantified with the Bradford method following the manufacturer’s guidelines (Bio-Rad, Hercules, CA). For the myoblast transplantation, myoblasts (1 × 106 cells) were inoculated into the quadriceps muscles of naïve C57BL/6 mice. On day 10 and 21 after the inoculation, the muscles were fixed with 10% formaldehyde/0·5% glutaraldehyde/phosphate-buffered saline for 1 day and then were stained with X-gal.17 We determined the β-galactosidase-expressing cells with an image analysis using the public domain Image J program. The percentages of β-galactosidase-expressing cells were calculated according to the formula (the area of β-galactosidase-expressing cells/the area of cross sections of the muscles × 100) and the β-galactosidase-positive areas of the C2Z-inoculated muscles were statistically analysed with Student’s t-test and Welch’s t-test.
Cytolytic assay
For evaluation of an allogeneic immune response, naïve C57BL/6 mice were intraperitoneally inoculated with C2Z, C2Zp28 or C2Zp40 cells (1 × 106 cells/head). Two weeks later, splenocytes of naïve or immunized mice were cultured with C2Z cells treated with mitomycin C (60 μg/ml) for 5 days. Cytolytic activities against C2Z and EL-4 cells were measured with the standard 6-hr 51Cr-release assays at various effector : target (E : T) ratios. Differences of the CTL activities were statistically analysed with Student’s t-test. For evaluation of a primary alloreactive CTL induction, splenocytes of naïve C57BL/6 mice were cultured with C2Z or C2Zp28 cells treated with mitomycin C (60 μg/ml) for 5 days. Cytolytic activities against C2Z and EL-4 cells were measured with the standard 6 hr 51Cr release assays at various E : T ratios. The mitomycin C treatment inhibited a cell division but not the mp28 production up to 7 days.
Results
Murine p28 but not mEBI3 was secreted as a monomer
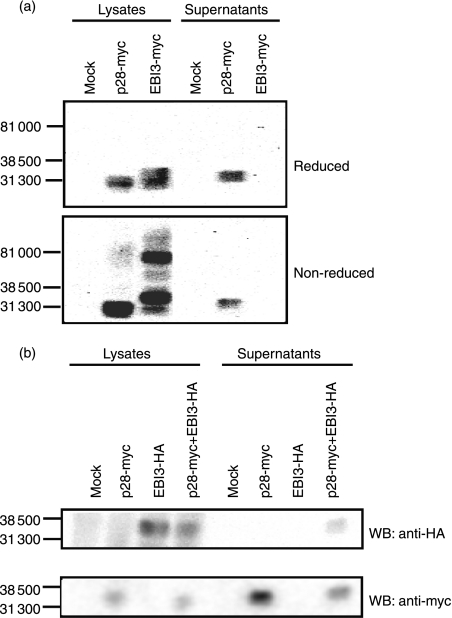
We investigated a possible multimer formation and secretion of mp28 and mEBI3 molecules. Cell lysates and supernatants of the COS-7 cells, which did not produce endogenous p28 and EBI3 proteins, transiently transfected with the mp28-myc or mEBI3-myc gene were subjected to Western blot analyses. The mp28-myc was detected in the supernatants as well as the cell lysates and its molecular weight under reduced and non-reduced conditions was approximately 30 000 (Fig. 1a). In contrast, mEBI3-myc was detected in the cell lysates but not in the supernatants of the transfected cells, and the molecular weights of the cytoplasmic mEBI3-myc under non-reduced and reduced conditions were approximately 70 000 and 33 000, respectively (Fig. 1a). Murine EBI3-myc was not detected in the supernatants so we examined the secretion of heterodimers comprising mp28-myc and mEBI3-HA in COS-7 cells. The mEBI3-HA was detected in the supernatants of the cells transfected with both the mp28-myc and mEBI3-HA genes but not of those transfected with the mEBI3-HA gene alone (Fig. 1b). These data suggest that mp28 can be secreted as monomers whereas mEBI3 is secreted only as heterodimers with mp28. Interestingly, we found that neither the homodimer nor the heterodimeric mEBI3-HA was detected in the non-reduced condition (data not shown) whereas the mEBI3-myc homodimer was formed (Fig. 1a). A conformational change of the mEBI3 tagged with HA could inhibit the anti-HA antibody binding.
Figure 1.
Murine p28 but not mEBI3 was secreted as a monomeric protein. (a) COS-7 cells were transfected with mp28-myc or mEBI3-myc DNA. The cell lysates and supernatants were separated with sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) under reduced or non-reduced conditions. The myc-tagged protein was examined with Western blot analysis. (b) COS-7 cells were transfected with mp28-myc, mEBI3-haemagglutinin (HA) or both DNAs. The culture supernatants were separated by SDS–PAGE under reduced conditions. The myc- or HA-tagged protein was examined with Western blot analyses.
Murine p28 inhibited IL-27 functions in vitro
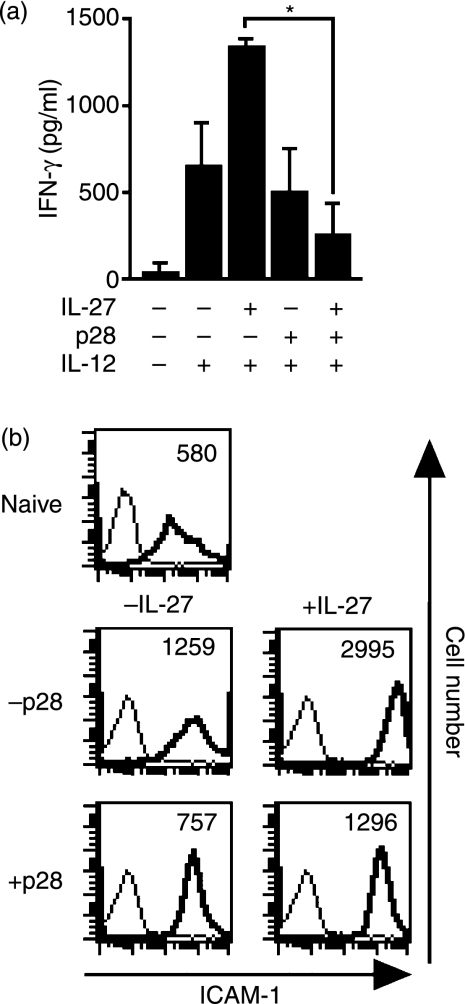
Interleukin-27 induced the expression of IL-12 receptor and subsequently enhanced the IL-12-induced IFN-γ production in CD4+ T cells,1,2 so we evaluated the IL-12-induced IFN-γ production in CD4+ T cells that were pre-incubated with mIL-27 and mp28 (Fig. 2a). Pre-incubation with mIL-27 enhanced the IL-12-induced IFN-γ production in CD4+ T cells, whereas pre-incubation with both mIL-27 and mp28 cancelled the IL-27-mediated enhancement of IL-12-induced IFN-γ production (P= 0·0024). The pre-incubation with mp28 alone did not affect the IL-12-induced IFN-γ production. Consistent with the previous report,1 naïve CD4+ T cells produced minimal amounts of IFN-γ when stimulated with either IL-27 alone or with both mIL-27 and mp28 (not shown data). Interleukin-27 also increased the expression level of ICAM-1 on CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs.3 We found that mp28 diminished the IL-27-induced ICAM-1 up-regulation (Fig. 2b). These results suggest that mp28 does not have biological functions but specifically prevents the IL-27 functions.
Figure 2.
Murine (m) p28 inhibited interleukin-27 (IL-27)-dependent biological responses. (a) Naïve CD4+ T cells were stimulated with recombinant mIL-27 in the presence of the supernatants from the mp28-transfected or parental COS-7 cells for 24 hr and then restimulated with IL-12 for 24 hr. Interferon-γ (IFN-γ) in the cell-free supernatants was measured and the data represent mean ± standard deviation (SD) of triplicate samples. Asterisk indicates the statistical significance (P< 0·01 with Student’s t-test). (b) Naïve CD4+ T cells were stimulated with plate-coated anti-CD3 and anti-CD28 monoclonal antibodies and were cultured with or without IL-27 in the presence of the supernatants from the mp28-transfected or parental Chinese hamster ovary cells for 16 hr. The cells were analysed for their surface expression of intercellular adhesion molecule 1 (ICAM-1) with flow cytometry. The number in each panel showed the mean fluorescence intensity of ICAM-1 expression.
Murine p28 blocked the IL-27-mediated STAT1 phosphorylation
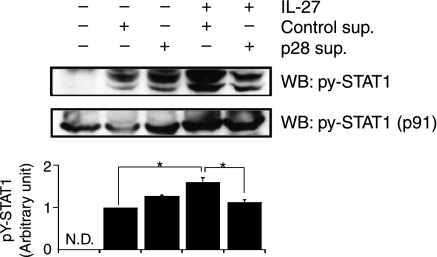
STAT1 is one of the key molecules in the IL-27 signalling pathways.2,8 We thereby investigated what effect mp28 would have on the level of STAT1 phosphorylation in IL-27-stimulated CD4+ T cells (Fig. 3). Recombinant mIL-27 induced the STAT1 phosphorylation in CD4+ T cells and the up-regulated IL-27-mediated phosphorylation was cancelled when the cells were stimulated in the presence of mp28. A certain level of STAT1 phosphorylation was found in the cells stimulated with the supernatants of parental COS-7 cells, which was probably because FCS was included in the supernatants. We observed a minimal increase of STAT1 phosphorylation with the supernatants of mp28-producing COS-7 cells compared with those of parental COS-7 cells; this remains unexplained.
Figure 3.
Murine (m) p28 inhibited interleukin-27 (IL-27)-mediated signal transducer and activator of transcription 1 (STAT1) activation. Naïve CD4+ T cells were stimulated with or without recombinant mIL-27 in the presence of the supernatants from the mp28-transfected (p28 sup.) or parental COS-7 cells (Control sup.) for 15 min. The cell lysates were subjected to Western blot analyses with anti-tyrosine-phosphorylated STAT1 (pY-STAT1) and anti-STAT1 (p91) antibodies. STAT1 phosphorylation levels were calculated as described in the Materials and methods and are summarized in the graph under the pictures using arbitrary units that were standardized with control supernatants as 1. Asterisks indicate statistical significance (P< 0·01). N.D.: not detected.
Murine p28 abrogated the IL-27-induced tumour rejection in vivo
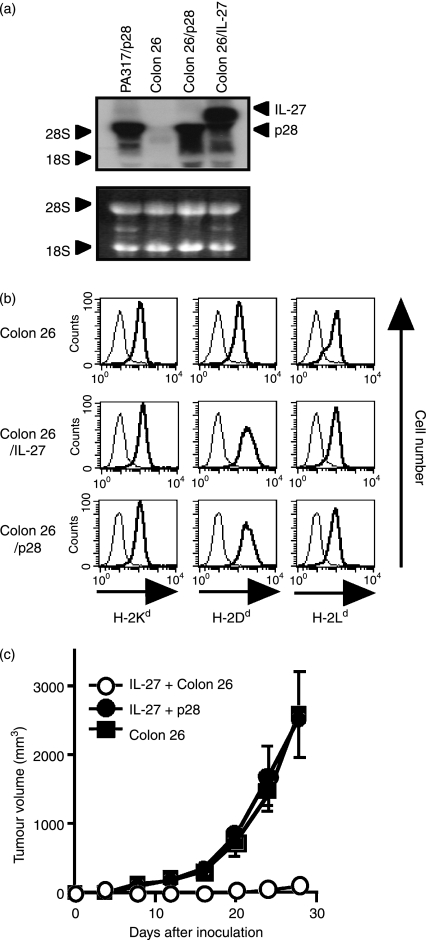
Inoculation of IL-27-producing tumour cells produced anti-tumour immune responses in host animals.19,21–23 We thereby generated mp28-expressing murine colon carcinoma Colon 26 (Colon 26/p28) cells and examined whether mp28 could impair the IL-27-mediated anti-tumour effects in vivo. Expressions of the mp28 and mIL-27 genes in Colon 26/p28 and Colon 26/IL-2719 cells were confirmed with Northern blot analyses using the p28-specific probe (Fig. 4a). The transcript in Colon 26/IL-27 cells was larger than that in Colon 26/p28 cells because the p28 gene was connected with the IRES sequence followed by the EBI3 gene. Expression levels of H-2Kd, H-2Dd and H-2Ld on Colon 26/p28 cells remained almost the same as those on Colon 26 and Colon 26/IL-27 cells (Fig. 4b). The in vitro proliferation rate and the in vivo tumour growth rate of Colon 26/p28 cells were not different from those of Colon 26 cells (data not shown). We subcutaneously inoculated the mixtures of Colon 26/IL-27 cells with either Colon 26/p28 or Colon 26 cells into mice, in which both mIL-27 and mp28 were produced at the same tumour site. The mixtures of Colon 26/IL-27 and Colon 26/p28 cells developed tumours and the growth was the same as that of Colon 26 cells in the inoculated mice, whereas the mixtures of Colon 26/IL-27 and Colon 26 cells completely regressed (Fig. 4c). We next investigated the survival period of the mice that were intraperitoneally inoculated with the mixtures of Colon 26/IL-27 with Colon 26/p28 cells (Table 1). All the mice inoculated with the mixtures of Colon 26/IL-27 with Colon 26 cells survived until the end of the observation, but none of the mice inoculated with the mixtures of Colon 26/IL-27 with Colon 26/p28 cells survived. These data indicate that mp28 suppresses the IL-27-mediated anti-tumour effects in vivo. We also found that the survival periods of the Colon 26/p28-inoculated mice were significantly shorter than those of parental Colon 26-inoculated mice (Table 1, P = 0·037 with log-rank test). This result implies that endogenous IL-27 was produced in the tumour-inoculated mice.
Figure 4.
Murine (m) p28-producing tumour cells prevented interleukin-27 (IL-27)-dependent anti-tumour effects in vivo. (a) Northern blot analysis of the mp28 and IL-27 gene expressions in Colon 26, Colon 26/p28 and Colon 26/IL-27 cells with the p28-specific probe (upper panel). Ribosomal RNAs were shown as a loading control (lower panel). PA317/p28 is a positive control for the p28 gene. (b) Flow cytometric analysis of major histocompatibility complex (MHC) class I molecules on Colon 26, Colon 26/IL-27 and Colon 26/p28 cells. The cells were stained with anti-H-2Kd, anti-H-2Dd and anti-H-2Ld (thick line), or control monoclonal antibodies (thin line). (c) Tumour growth of the IL-27- and p28-producing tumour cells. The mixtures of Colon 26/IL-27 cells with either Colon 26 (open circles) or Colon 26/p28 (closed circles) (total 1 × 106 cells), or the equal cell number of Colon 26 cells (closed squares) were inoculated subcutaneously into syngeneic mice. Data represent mean ± SD (n = 3).
Table 1.
Suppression of interleukin-27 (IL-27) -mediated anti-tumour activity with mp28
| Inoculated cells1 (number of mice) | Survived days | Average days ± SD |
|---|---|---|
| Colon 26 (n = 6) | 14, 17, 19, 23, 28, 28 | 21·5 ± 5·8 |
| Colon 26/p28 (n = 6) | 13, 13, 15, 15, 16, 22 | 15·7 ± 3·3 |
| Colon 26/IL-27 + Colon 262 (n = 6) | > 60, > 60, > 60, > 60, > 60, > 60 | > 60 |
| Colon 26/IL-27 + Colon 26/p282 (n = 7) | 22, 22, 23, 24, 42, 44, 55 | 33·1 ± 13·6 |
Tumour cells (1 × 106) were inoculated intraperitoneally.
Colon 26/IL-27 cells (2·5 × 105) were mixed with either Colon 26 (7·5 × 105) or Colon 26/p28 (7·5 × 105).
Murine p28 prolonged allograft survival
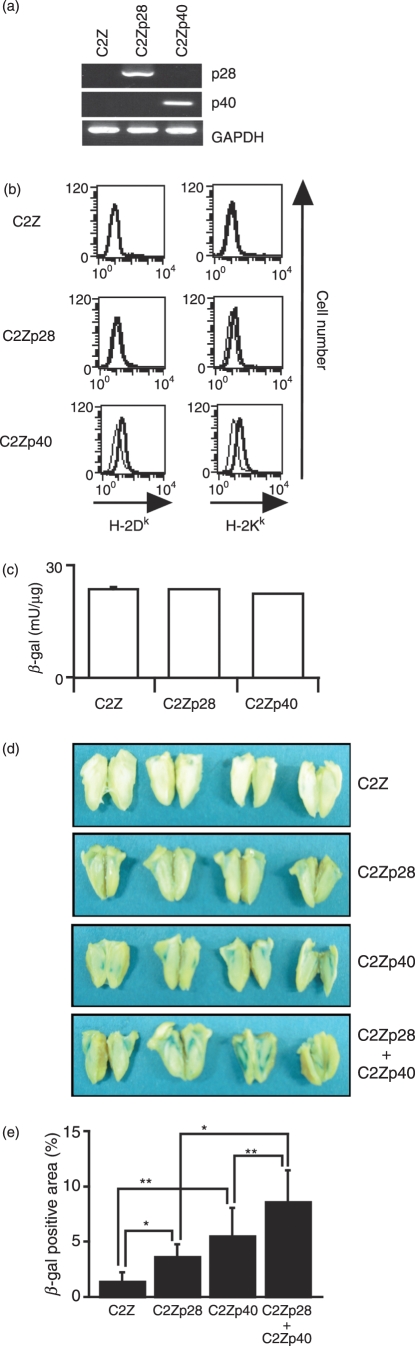
The allograft of p40-expressing C2C12 myoblast (C2Zp40) cells, which was able to trace the transplanted cells in host with the X-gal staining, survived in C57BL/6 mice longer than that of parental C2Z cells.17 We thereby generated the p28-expressing C2Z (C2Zp28) cells and examined whether mp28 would impair an allograft rejection. The expressions of the mp28 and mp40 genes in C2Zp28 and C2Zp40 cells, respectively, were confirmed with RT-PCR (Fig. 5a). The expression levels of H-2Kk and H-2Dk of C2Zp28 cells remained the same as those of C2Z cells, but C2Zp40 cells expressed MHC class I molecules slightly more than C2Z and C2Zp28 cells (Fig. 5b). β-Galactosidase activity in C2Zp28 cells was the same as in C2Z and C2Zp40 cells (Fig. 5c). The in vitro proliferation rate of C2Zp28 cells was identical to that of C2Z cells (data not shown). We inoculated C2Z, C2Zp28 or C2Zp40 cells into the quadriceps muscles of C57BL/6 mice and determined the allograft survival with residual β-galactosidase activities in the muscles 21 days after the inoculation. Muscles of naïve mice did not show any β-galactosidase activities (data not shown). The percentages of the residual C2Zp28 and C2Zp40 grafts were significantly higher than those of the C2Z grafts (Fig. 5d,e, P= 0·029 with Student’s t-test and P= 0·044 with Welch’s t-test, respectively). We then examined whether combined secretion of mp28 and mp40 could further suppress the graft rejection. C2Zp28 cells were mixed with an equal number of C2Zp40 cells and inoculated into allogeneic mice. The survival of mixed grafts was significantly prolonged when compared with that of C2Zp28 grafts (P= 0·003 with Student’s t-test) or C2Zp40 grafts (P= 0·044 with Welch’s t-test). These data suggest that local production of mp28 prolonged graft survival and that the simultaneous production of mp28 and mp40 additively suppressed host immune responses.
Figure 5.
Production of murine (m) p28 and mp40 prolonged allograft survival in allogeneic host animals. (a) Reverse transcription–polymerase chain reaction analysis of the gene expression of mp28, mp40 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, as a control) in C2Z, C2Zp28 or C2Zp40 cells. (b) Expression of H-2Kk and H-2Dk on C2Z, C2Zp28 and C2Zp40 cells. They were stained with anti-H-2Kk and anti-H-2Dk (thick line), or control antibodies (thin line). (c) The β-galactosidase activities in C2Z, C2Zp28 and C2Zp40 cells. Data indicate mean ± SD of triplicate experiments. (d) C2Z, C2Zp28 or C2Zp40 cells (total 1 × 106 cells), or mixture of C2Zp28 and C2Zp40 cells (5 × 105 C2Zp28 and 5 × 105 C2Zp40) were inoculated into the quadriceps of naïve C57BL/6 mice (n = 4) and killed 21 days after inoculation. The formaldehyde-fixed tissues were stained with X-gal. (e) The percentage of β-galactosidase-positive areas of each tissue was measured. Data indicated mean ± SD. Asterisks indicate statistical significances when compared with the C2Z-inoculated mice. (*P< 0·05 with Student’s t-test and **P< 0·05 with Welch’s t-test).
Murine p28 suppressed the alloreactive CTL induction
We next examined the mechanisms for prolonged allograft survival. The graft rejection was mainly caused by the alloreactive CTL induction, which is a typical Th1-dominant immune response; thereby, the C2Z-specific cytolytic activity of splenocytes of the mice inoculated with C2Z, C2Zp28 or C2Zp40 cells was investigated. Splenocytes of C2Zp28- or C2Zp40-inoculated mice showed less cytolytic activity against C2Z cells than did splenocytes of the mice inoculated with C2Z cells (Fig. 6a). The reduced cytotoxic activities were not observed against irrelevant EL-4 tumour cells (Fig. 6a).We also examined the primarily alloreactive CTL induction in vitro. Splenocytes of naïve C57BL/6 mice were cultured with mitomycin C-treated allogeneic C2Z or C2Zp28 cells for 5 days and were evaluated for cytolytic activity against C2Z cells. The cytolytic activity of splenocytes stimulated with C2Zp28 cells was lower than that of splenocytes stimulated with C2Z cells (Fig. 6b). We also observed lower cytotoxic activities against Colon 26 cells in C57BL/6 mice when stimulated with Colon 26/p28 cells (data not shown). These data indicate that mp28 impairs primary CTL induction in vitro.
Figure 6.
Murine p28 suppressed allogeneic cytotoxic T lymphocyte (CTL) induction. (a) Seven days after myoblast inoculation, the splenocytes of C57BL/6 mice that were inoculated with C2Z (open circles), C2Zp28 (closed circles) or C2Zp40 cells (open squares) were cultured for 5 days with mitomycin C-treated C2Z cells. Cytolytic activities against C2Z and EL-4 cells were measured at various effector : target (E : T)ratios. Data represent mean ± SD of triplicate samples. Asterisks indicate statistical significances when compared with the C2Z-inoculated mice (P< 0·05 with Student’s t-test). (b) Splenocytes of naïve C57BL/6 mice were cultured with mitomycin C-treated C2Z (open circles) or C2Zp28 (closed circles) cells. Cytolytic activities against C2Z and EL-4 cells were measured at various E : T ratios. Data represent mean ± SD of triplicate samples. Asterisks indicate statistical significances when compared with the C2Z-stimulated splenocytes (P< 0·05 with Student’s t-test).
Discussion
Modulation of the functions of IL-12 family cytokines could be effective in controlling adverse immune responses because these cytokines are key mediators in the initiation of various immune responses. Previous reports demonstrated that p40 inhibited biological functions of IL-12 and IL-23.14,16–18 We therefore examined the inhibitory effects of subunits of mIL-27. Murine p28 and mEBI3 could be secreted independently,1 so we first investigated a multimer formation of these subunit proteins. Our experiments showed that the monomeric, but not multimeric, mp28 was secreted. In contrast, mEBI3 formed a homodimer but none of the monomer was detected and the homodimer of mEBI3 was hard to detect in the cell supernatants of our experimental system. This result was consistent with the previous study, which showed that the secretion of mEBI3 was increased when it was coexpressed with mp28.1 We speculate that amounts of secreted mEBI3 could be lower than the detection limit of our experimental system. Human EBI3, although it can be secreted,1 was shown to be associated with calnexin, a resident molecular chaperone, and was retained in the endoplasmic reticulum.24 The poor secreting ability of mEBI3 might be attributable to the mechanism of calnexin association solely operating in the mouse system.
A previous study showed that p28 suppressed the IL-17 production in transforming growth factor-β- and IL-6-stimulating CD4+ T cells although not as efficiently as IL-27;25 however, the study did not describe the inhibitory action of p28 in the presence of IL-27. We here demonstrated that the secreted mp28 was inhibitory to the IL-27-mediated immune responses in vitro, such as the enhancement of both IFN-γ production and ICAM-1 expression. The analysis of IFN-γ production revealed that mp28 did not affect the IL-12-induced IFN-γ production, suggesting that p28 is a specific inhibitor for the IL-27-mediated responses. The analysis of STAT1 phosphorylation demonstrated that IL-27 signalling was repressed by mp28, albeit experiments with a purified soluble p28 protein would be required to examine whether p28 itself was able to stimulate STAT1 phosphorylation. We also demonstrated that the local production of mp28 suppressed IL-27-mediated immune responses in vivo, when we observed anti-tumour effects resulted from IL-27 production at the tumour site.19,21,22 Interleukin-27 has also been observed as an inducer of anti-angiogenic events.26 Although we did not investigate the angiogenesis and the production of anti-angiogenic cytokines, such as IFN-γ-inducible protein (IP-10) and monokine-induced by IFN-γ (MIG), at the tumour site that produces both mIL-27 and mp28, it could be possible that the local production of mp28 also diminished the angiogenesis by inhibition of the IL-27-mediated anti-angiogenic cytokine production. As p40 inhibited the IL-12 functions because of its competitive binding to the IL-12R complex,14,16 we presume that p28 binds to the IL-27R complex and competitively prevents the binding of IL-27 to the receptor. Furthermore, the mp28 monomer could be sufficient to inhibit IL-27 functions although the p40 monomer inhibits IL-12 functions more weakly than the p40 homodimer.14,16
A high expression of IL-27 was reported in the cases of inflammatory responses caused by viral and bacterial infections, and self antigens.27–32 These studies suggest that IL-27 is a critical molecule for the pathogenesis of relevant inflammatory diseases as well as IL-12 and IL-23, which are key mediators for these inflammatory diseases because of their Th1 and Th17 responses promoting activities.25,33 Experimental autoimmune encephalomyelitis and adjuvant-induced arthritis in rats were ameliorated by an immunization with mp28: the anti-mp28 antibodies neutralized endogenous rat IL-27 because of its cross-reactivity.34,35 We therefore propose that inhibition of IL-27 by the soluble form of p28 could be applicable for inflammatory diseases. Furthermore, the local and simultaneous production of mp28 and mp40 showed inhibitory effects greater than those of either mp28 or mp40 alone. p40, which is an inhibitor for IL-12 and IL-23, showed therapeutic effects in autoimmune disease models.36,37 We therefore presume that the combined administration of p28 and p40 could reduce unfavourable immune responses because IL-12 family cytokines orchestrate the regulation of Th1 immune responses by the sequential activation of naïve CD4+ T cells.38
In summary, we first demonstrated that soluble mp28 inhibited IL-27-dependent immune responses. The present study implies a potential therapeutic use of soluble p28 molecules as an immunosuppressant for autoimmune diseases and an anti-inflammatory agent for treatment of patients with severe systemic inflammatory diseases. p28 may circumvent the non-specific immune suppression caused by current immunosuppressive medicines, which often results in adverse reactions.
Acknowledgments
The authors thank Dr A. D. Miller (Fred Hutchinson Cancer Research Center, Seattle, WA) for the LXSN vector, Dr J. Hamuro (Ajinomoto, Tokyo, Japan) for Colon 26 cells and Dr H. Yagita (Juntendo University, Tokyo, Japan) for C2Z and C2Zp40 cells. This work was supported by a grant-in-aid for Centre of Excellence (COE) Program of the Japanese Ministry of Education, Culture, Sports, Science and Technology, grant-in-aid for scientific research from Japan Society for the Promotion of Science, the grant-in-aid for cancer research (18-10) from the Ministry of Health, Labour and Welfare, and a grant-in-aid from the Nichias Corporation and the Futaba Electronics Memorial Foundation.
Disclosures
None declared.
References
- 1.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Lucas S, Ghilardi N, Li J, Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–93. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 4.Matsui M, Moriya O, Belladonna M, Kamiya S, Lemonnier F, Yoshimoto T, Akatsuka T. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J Virol. 2004;78:9093–104. doi: 10.1128/JVI.78.17.9093-9104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimoto T, Okada K, Morishima N, et al. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–85. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 6.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–20. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 8.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–7. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 9.Hibbert L, Pflanz S, Malefyt RW, Kastelein R. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–22. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 10.Zahn S, Wirtz S, Birkenbach M, Blumberg R, Neurath M, Stebut E. Impaired Th1 responses in mice deficient in Epstein–Barr virus-induced gene 3 and challenged with physiological doses of Leishmania major. Eur J Immunol. 2005;35:1106–12. doi: 10.1002/eji.200425926. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Hamano S, Senaldi G, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–78. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 12.Villarino A, Hibbert L, Lieberman L, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 13.Villarino A, Huang E, Hunter C. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173:715–20. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 14.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 15.Oppmann B, Lesley R, Blorn B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 16.Gillessen S, Carvajal D, Ling P, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 17.Kato K, Shimozato O, Hoshi K, Wakimoto H, Hamada H, Yagita H, Okumura K. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc Natl Acad Sci USA. 1996;93:9085–9. doi: 10.1073/pnas.93.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimozato O, Ugai S, Chiyo M, et al. The secreted form of p40 subunit of interleukin (IL) -12 inhibits IL-23 functions and abrogates IL-23-mediated antitumour effects. Immunology. 2006;117:22–8. doi: 10.1111/j.1365-2567.2005.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–42. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 20.Oda Y, Funasaka K, Kitano M, Nakano A, Yoshikura T. Use of a high-throughput umu-microplate test system for rapid detection of genotoxicity produced by mutagenic carcinogens and airborne particle matter. Environ Mol Mutagen. 2004;43:10–9. doi: 10.1002/em.10209. [DOI] [PubMed] [Google Scholar]
- 21.Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res. 2004;24:3763–7. [PubMed] [Google Scholar]
- 22.Hisada M, Kamiya S, Fujita K, Belladonna M, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–6. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 23.Salcedo R, Stauffer J, Lincoln E, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–82. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 24.Deverge O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–53. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu M, Shimamura M, Owaki T, et al. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176:7317–24. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 27.Segal BM. Experimental autoimmune encephalomyelitis: cytokines, effector T cells, and antigen-presenting cells in a prototypical Th1-mediated autoimmune disease. Curr Allergy Asthma Rep. 2003;3:86–93. doi: 10.1007/s11882-003-0017-6. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Gran B, Zhang G, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Wirtz S, Becker C, Fantini M, et al. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-κB activation. J Immunol. 2005;174:2814–24. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 30.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–7. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 31.Larousserie F, Pflanz S, Coulomb-L’Hermine A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–71. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer S, Stallmach A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis. 2004;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal SA, Ghilardi N, Xie MH, Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–71. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–8. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima A, Seroogy CM, Sandora MR, et al. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest. 2001;107:1293–301. doi: 10.1172/JCI12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa GL, Sandora MR, Nakajima A, et al. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–87. doi: 10.4049/jimmunol.167.4.2379. [DOI] [PubMed] [Google Scholar]
- 38.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–12. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]