Abstract
Anti-myelin basic protein (-MBP) autoantibodies have generally been considered to be absent from sera from healthy individuals, but to be detectable in sera from some patients with multiple sclerosis (MS). However, their pathogenic role is uncertain. We demonstrate the presence of MBP-reactive autoantibodies in sera from 17 healthy individuals and 17 MS patients. The addition of MBP to the sera caused a dose-dependent deposition of MBP and co-deposition of immunoglobulin M (IgM) and fragments of complement component 3 (C3) on allogeneic monocytes. Calcium chelation abrogated the immunoglobulin deposition, indicating that formation of complement-activating immune complexes played a role in the binding process. Furthermore, MBP elicited tumour necrosis factor (TNF)-α and interleukin (IL)-10 production by normal mononuclear cells in the presence of serum from both patients and controls. Mononuclear cells from MS patients responded to MBP with the production of interferon (IFN)-γ, IL-4 and IL-5, in addition to TNF-α and IL-10. The production of IFN-γ and IL-5 was increased when MS serum was added rather than normal serum. Denaturation of MBP strongly inhibited MBP deposition and the MBP-induced IgM deposition and cytokine production, indicating that these events were facilitated by autoantibodies recognizing conformational epitopes on MBP. We infer that MBP-elicited TNF-α and IL-10 responses are promoted to equal extents by naturally occurring MBP autoantibodies and autoantibodies contained in MS sera. However, the latter seem to be more efficient in facilitating the production of IFN-γ and IL-5.
Keywords: autoantibodies, complement, cytokines, multiple sclerosis, myelin basic protein
Introduction
While it is well established that T cells with specificity for myelin proteins, such as myelin basic protein (MBP), play a pathogenic role in multiple sclerosis (MS),1,2 it is still a matter of debate whether myelin-reactive autoantibodies play a pathogenic role. Some authors claim that autoantibodies against MBP are present in cerebrospinal fluid (CSF)3–5 and serum4,6–8 from MS patients, although not all authors have been able to demonstrate their presence in serum.3 Their presence has been associated with earlier and more frequent relapses.7 Cells secreting high-affinity anti-myelin antibodies have been detected in CSF from patients with MS, but circulating anti-MBP antibodies appear to be of low affinity.6,9 Anti-myelin antibodies may be implicated in demyelination in MS by activating complement and thereby initiating complement-mediated tissue damage.10–14 Whereas there is evidence of activation of the early parts of the complement cascade in a wide range of MS patients, full activation with generation of the terminal complement complex appears to be restricted to patients with more advanced disease.15 There is no clear correlation between the presence of autoantibody-secreting cells and activation of the complement cascade.16 Indeed, autoantibodies may only be relevant for demyelination in a subgroup of patients with MS.17,18 Another mechanism by which autoantibodies and complement may participate in the pathogenesis of autoimmune diseases is the opsonization of self-antigens for presentation by antigen-presenting cells (APCs), as has been shown for the acetylcholine receptor19,20 and thyroglobulin.21
The presence of immunoglobulin M (IgM)- and IgA-containing circulating immune complexes (ICs) has been demonstrated in over 60% of MS patients, using an assay reliant upon complement component 3 (C3) fragments as a complex constituent.22,23 However, the extent to which these complexes are autoimmune in nature remains a matter of dispute. One study24 failed to identify MBP in any of the complexes examined, while the presence of viral antigens and/or antiviral antibodies was recorded in the majority of ICs from MS patients in another study.23 Nevertheless, two independent investigations demonstrated that ICs from one-third23 to a half25 of the MS patients examined contained MBP.
A few studies on MS patients have used healthy individuals as controls and assessed the content of anti-MBP antibodies or MBP-containing ICs in normal sera.6,23,24,26 The general conclusion from these studies was that sera from healthy individuals are devoid of antibodies to MBP.6,23,24,26 In one study, however, 5% of the healthy control population had anti-MBP antibodies of all four IgG subtypes, as well as IgM.27
Normal sera contain so-called natural autoantibodies (NAAs) belonging to a group of polyreactive antibodies, referred to as natural antibodies, which are encoded by germline variable region genes without extensive somatic mutations28,29 (reviewed by Dighiero)30. NAAs may have a homeostatic function by clearing senescent cells31 and/or metabolic waste products32 from the body. They may also play a role in regulating T-cell responses to self-antigens in the periphery by facilitating the uptake and presentation of self-antigens by APCs.21 A number of self-antigens, including tubulin, actin, thyroglobulin, myoglobin, fetuin, albumin, transferrin, collagen and cytochrome c, have been demonstrated to bind NAAs.33 To our knowledge, there is no evidence of NAA reactivity with myelin.
In the present study, we demonstrate the presence of IgM and IgG antibodies reactive with MBP in sera from healthy individuals as well as in sera from MS patients, and show that IgM and C3 fragments co-deposit with MBP onto monocytes. We show that these phenomena, as well as the MBP-induced release of cytokines from mononuclear cells (MNCs), require an intact tertiary structure of MBP (indicative of a role for antibody recognition in the process) and the presence of free calcium ions (indicative of a role for complement).
Materials and methods
Subjects
The study included sera and MNCs isolated from 17 MS patients [four male and 13 female; age 33 ± 7 years (mean ± standard error of the mean (SEM))] and 17 sex- and age-matched healthy controls (four male and 13 female; age 33 ± 7 years). Fifteen of the patients were diagnosed with relapsing-remitting MS and the remaining two patients were diagnosed with clinically isolated syndrome. None of the patients had received any medication within 4 weeks of study entry and none of them had ever been treated with immunosuppressive drugs such as azathioprine, methotrexate, cyclophosphamide or mitoxantrone. A further 10 healthy, blood group O donors donated MNCs for analyses involving stimulation of cells in cultures containing allogeneic sera. The study was approved by the local ethics committee and the informed consent of all participating subjects was obtained.
Cells and serum
Sera were prepared from venous blood drawn in BD Vacutainer™ non-coagulant-containing tubes (BD Bioscience, Brøndby, Denmark). After coagulation, the blood was spun at 814 g and 4°, and the serum was harvested after 10 min. The sera were stored at −80° before use.
MNCs were isolated from whole blood in heparinized BD Vacutainer tubes (BD Bioscience) or from buffy coat cells kindly provided by the Blood Bank at Copenhagen University Hospital. The cells were centrifuged (at 814 g for 30 min) on a Lymphoprep gradient (Axis-Shield PoC A/S, Oslo, Norway). The MNCs were used in experiments assessing immunoglobulin/complement deposition and MBP-elicited cytokine production, respectively.
Antigens
Human MBP (>95% pure) was purchased from Insight Biotechnology Ltd (Wembley, UK). The preparation was tested by mass spectrometry, kindly performed by Jan J. Enghild at the Center for Insoluble Protein Structures, University of Aarhus, Denmark and the contaminant was found to be haemoglobin. The MBP was diluted in 0·0025% NaN3 (Bie & Berntsen, Rødovre, Denmark) in phosphate-buffered saline (PBS) (Dulbecco’s; Gibco, Paisley, UK) to a final concentration of 1 mg/ml before use. In some experiments, MBP was denatured by boiling for 15 min.
Fluorescein isothiocyanate (FITC) conjugation of MBP was carried out as described by Terada et al.34 In brief, 100 μg of FITC was conjugated to 1 mg of MBP in buffer containing 50 mm borate, 200 mm NaCl, 20 mm CaCl2 (all purchased from Merck, VWR International ApS, Rødovre, Denmark) and 100 mm mannose (Sigma-Aldrich Denmark A/S, Brøndby, Denmark), pH 9·2, for 18 hr at 4°. Following conjugation, MBP was dialysed against PBS, pH 7·5. Human serum albumin (HSA; ZLB GmbH, Springe, Germany) and tetanus toxoid (TT; Statens Serum Institute, Copenhagen, Denmark) were used as control antigens.
Measurement of MBP-reactive IgM and IgG in serum
Microspheres (Luminex Corp., Austin, TX) were coupled with MBP, HSA or TT according to the Luminex protocol for a two-step carbodiimide coupling of proteins to carboxylated microspheres (http://www.luminexcorp.com/uploads/data/Protein%20Protocols%20FAQs/Protein%20Coupling%20Protocol%200407%2010207.pdf). In brief, the microspheres were incubated with 100 μg of antigen during the coupling procedure, unless otherwise stated.
Two thousand antigen-coated microspheres per bead set were incubated for 30 min with 0·5% casein (Sigma-Aldrich Denmark A/S) in PBS-Tween (0·1% Tween; Merck, VWR International ApS), washed three times in PBS-Tween and incubated overnight with 40× diluted serum at 4°. Following three washes in PBS-Tween, the beads were incubated overnight at 4° with either 4 μg/ml biotinylated anti-human IgM or 32 μg/ml biotinylated anti-human IgG antibodies (both purchased from Sigma-Aldrich). After three washes in PBS-Tween, the beads were incubated for 30 min with 4 μg/ml streptavidin-phycoerythrin (Phycolink® PJ31S, Prozyme; Europe Bioproducts Ltd, Cambridge, UK), washed three times with PBS-Tween and re-suspended in 100 μl of Luminex sheath buffer (Ramcon, Birkerød, Denmark) before Luminex measurement.
Measurement of immunoglobulin and complement deposition on monocytes
Sera from eight MS patients or eight healthy individuals were diluted in a Veronal buffer containing 141 mm NaCl, 0·5 mm MgCl2 (all purchased from Merck, VWR International ApS), 0·15 mm CaCl2, 0·1% gelatine and 12·5 mm HEPES (all purchased from Sigma-Aldrich), pH 7·4. For experiments involving assessment of complement activity, ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich) was added to a concentration of 10 mm. MBP or TT was added to the serum, diluted 1 : 2 in buffer, to a final concentration of 200 μg/ml and incubated for 45 min at 37°, and the mixtures were cooled to 4° for 10 min.
The antigen-containing sera were incubated with normal MNCs for 90 min at 4° at a final serum concentration of 30% [volume/volume (v/v)]. Following washing in PBS, monocytes were labelled by incubation with 5 μl of peridinin chlorophyll protein (PerCP)-conjugated anti-CD14 (BD Bioscience) and the samples were subdivided into tubes containing FITC-conjugated F(ab′)2 fragments of polyclonal rabbit anti-human IgG (5·1 μg/ml) or FITC-conjugated F(ab′)2 fragments of polyclonal rabbit anti-human IgM (5·5 μg/ml); both were purchased from Dako (Glostrup, Denmark). After incubation for 30 min at 4°, each sample was washed in fluorescence-activated cell sorter (FACS) sheath buffer (BD Bioscience) before measurement with a FACSCalibur flow cytometer (BD Bioscience). The measured fluorescence intensity per cell was converted to soluble fluorescein equivalents (SFEq) after calibration of the flow cytometer with the Quantum 24 quantification kit (Flow Cytometry Standards Corporation, San Juan, Puerto Rico).
In a different set of experiments, sera from four MS patients and four healthy donors were incubated with normal MNCs (from a male donor, aged 28 years) as described above, except that deposition of IgM and C3 fragments was assessed instead of IgG/IgM. FITC-conjugated polyclonal rabbit anti-human C3 antibodies (Dako) that recognize native C3, C3b, iC3b and C3dg were used.
Furthermore, deposition of MBP on monocytes was assessed by preincubating sera from four MS patients and four healthy donors with FITC-conjugated MBP, and thereafter incubating the mixture with normal MNCs from a healthy blood group O donor for 90 min at 4°, before washing and flow cytometric assessment of the binding of FITC-MBP to CD14+ monocytes. MNCs from two male donors (63 and 32 years of age) were used for titration and examination of the influence of EDTA/MBP denaturation, respectively.
Induction of cytokine responses with MBP
In the allogeneic design, MNCs from a healthy blood group O donor (female, aged 24 years) were seeded in a 96-well Nunclon Surface plate (Nunc, Roskilde, Denmark), each well containing 500 000 cells in 125 μl of RPMI, 10 μl of Dulbecco’s PBS and 65 μl of 30% sera (v/v) from patients or healthy controls. In the autologous design, MNCs from the included patients and controls were cultivated in the presence of media containing autologous sera or serum from a healthy blood group AB donor. The cells were stimulated with 30 μg/ml MBP, 30 μg/ml boiled MBP or 30 μg/ml TT and culture supernatants were collected at days 1 and 7.
Measurement of cytokines
Using a Luminex 100 IS (Luminex Corp.) and StarStation version 2.0 software (Applied Cytometry Systems, Sheffield, UK) we measured interleukin (IL)-2, IL-4, IL-5, IL-10, interferon (IFN)-γ and tumour necrosis factor (TNF)-α by means of the T helper type 1 (Th1)/Th2 five-plex and TNF-α single-plex bead array (Biosource, Invitrogen, Taastrup, Denmark). More than 50 events were acquired per bead set. In experiments involving cultivation of MNCs in autologous serum and standard blood group AB serum, the cytometric bead array Th1/Th2 kit and the corresponding software (BD Bioscience) for flow cytometry were used.
Statistics
prism4 (GraphPad, San Diego, CA) was used for statistical analysis. Unpaired and paired t-tests were employed. For comparison of differences in curves obtained with MS sera and control sera, respectively, a repeated measures analysis of variance (ANOVA) was used.
Results
The presence of MBP-reactive IgM and IgG in sera from MS patients and healthy controls
To determine whether MS patients and healthy controls expressed anti-MBP antibodies, sera from both groups were incubated with human MBP-coated microspheres, which were subsequently assessed for IgM and IgG uptake using biotinylated anti-human IgM and IgG class-specific antibodies and streptavidin-phycoerythrin as the probing reagents. In order to assess the specificity of the test, preliminary dose–response assays were performed using various concentrations of MBP to coat the beads (Fig. 1a) or different dilutions of patient and control sera to vary the amounts of IgM and IgG introduced into the assay (Fig. 1b,c). Under both circumstances, a direct relationship between IgG/IgM uptake and the concentrations of the reactants was observed, effectively excluding an interaction between the probing reagents and the MBP-coated beads as a significant contributor to the observed responses.
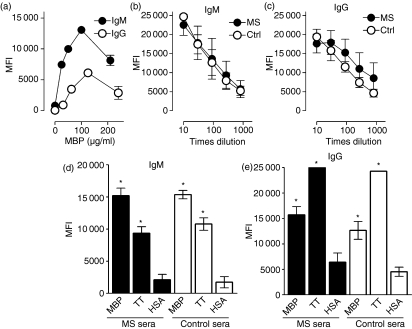
Figure 1.
Antibodies reactive with myelin basic protein (MBP) in sera from multiple sclerosis (MS) patients and healthy controls. (a) Carboxylated microspheres were conjugated with the given amounts of MBP and incubated with normal serum. The binding of immunoglobulin M (IgM) (black circles) and IgG (white circles) was assessed with biotinylated rabbit anti-human-IgM and -IgG antibodies, respectively and subsequent probing with phycoerythrin-coupled streptavidin. The mean fluorescence intensities (MFIs) of duplicate experiments are shown. (b, c) The effect of serum dilution on the captured amount of IgM and IgG is shown. The dose-dependent uptake of IgG differed significantly between four MS sera and four control sera [P< 0·02, analysis of variance (ANOVA)] whereas the corresponding curves for IgM did not (P = 0·80). (d) Microspheres coupled with 100 μg of MBP, 100 μg of tetanus toxoid (TT) or 100 μg of human serum albumin (HSA) were incubated with sera from 17 patients with MS (black bars) and 17 healthy controls (open bars) and assessed for the binding of IgM. (e) The corresponding binding of IgG is shown. The IgG binding to TT-coupled microspheres exceeded the upper detection limit of the analysis, at approximately 25·000 MFI. Bars and error bars represent mean ± standard error of the mean (SEM). *MFI values significantly different from those associated with HSA microspheres (P< 0·0001).
The assay was then applied on a larger scale to assess the relative levels of IgM and IgG antibodies in patients and controls (Fig. 1d,e). High concentrations of both coating MBP and sera were employed to minimize the influence of antibody affinity on the interaction. Microspheres coupled with TT, a recall antigen with which the background population had been vaccinated, served as a positive control, while HSA-coated microspheres were used as the most appropriate negative control, based on the assumption that the binding of any anti-HSA autoantibodies present would be effectively blocked by the relatively large amount of HSA (approx. 1 mg/ml final concentration) contained in the test sera.
Sera from MS patients and healthy individuals contained MBP-reactive IgM, in approximately equal amounts (Fig. 1d). The sera also contained significant amounts of IgM reactive with TT, although the content of TT-reactive IgM was lower than that of IgM reactive with MBP in both groups (P< 0·0001).
Both patient and control sera showed significantly more binding of IgG to MBP beads than to HSA beads (Fig. 1e), indicating the presence of anti-MBP-IgG antibodies in both groups. The MS sera contained slightly higher amounts of MBP-reactive IgG than did the control sera, but the difference was non-significant (P= 0·10). As expected, high levels of TT-specific IgG were found in both groups of sera.
Of potential interest is the additional finding that the serum dilution assay curves (Fig. 1b,c) displayed a significant difference in the dose-dependent uptake of IgG between MS and control sera, whereas the corresponding curves for IgM did not (P = 0·80). A corresponding difference was also observed in the slopes of the curves for IgG (slope = −2·90 × 10−4 versus −4·38 × 10−4 for MS and control, respectively), although the limited number of assays performed did not permit unequivocal determination of the significance of this finding (P = 0·27, n = 4).
Antigen-mediated deposition of IgG and IgM on monocytes
We next examined the influence of serum on the capture of MBP and immunoglobulins by APCs, represented by monocytes (Fig. 2). As shown in Fig. 2a, MBP bound to monocytes in a dose-dependent manner in the presence of both types of serum.
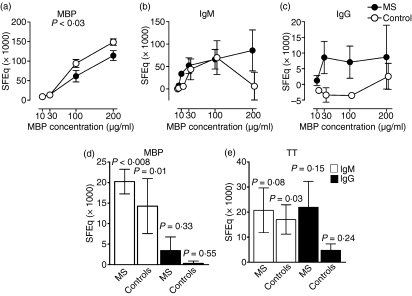
Figure 2.
Deposition of immunoglobulins on monocytes following incubation of sera with myelin basic protein (MBP). Sera from multiple sclerosis (MS) patients and controls were incubated with fluorescein isothiocyanate (FITC)-conjugated or unlabelled MBP for 45 min at 37° to allow formation of MBP/anti-MBP complexes. The sera were subsequently incubated with normal blood group O mononuclear cells for 90 min at 4°. (a) The consequent deposition of FITC-conjugated MBP on CD14+ monocytes, as measured by flow cytometry, is shown along with (b) the corresponding deposition of immunoglobulin M (IgM) and (c) IgG, induced by the addition of unlabelled MBP to the sera. The immunoglobulin deposition was measured using FITC-conjugated F(ab′)2 fragments of polyclonal rabbit antibodies against human IgM or IgG as a probe. The experiments were performed in triplicate. (d) Sera from 13 MS patients and 13 healthy individuals (controls) were preincubated with MBP (200 μg/ml) before addition to normal blood group O mononuclear cells. The resulting deposition of IgM (white bars) and IgG (black bars) on CD14+ monocytes is shown. (e) The corresponding deposition observed after preincubation of sera from six patients and six controls with tetanus toxoid (TT). All fluorescence intensities were converted to soluble fluorescein equivalents (SFEq) after subtraction of the background signal occurring without preincubation of the sera with antigen. The mean ± standard error of the mean (SEM) is shown. P-values indicate the probability for the null hypothesis that MS sera and control sera mediated MBP deposition to equal extents in (a) (as determined by a repeated measures analysis of variance) and that the mean value was 0 SFEq in (d) and (e) (as determined by a one-sample t-test).
Both IgM and IgG co-deposited with MBP on the monocytes (Fig. 2b,c). While a similar deposition of IgM occurred in the presence of MS sera and control sera, the patients’ sera mediated IgG deposition most efficiently (Fig. 2c). However, when tested with a larger number of sera, only the deposition of IgM was significantly higher than the background binding (Fig. 2d), irrespective of whether MS sera or control sera were employed. The deposition of IgM did not differ significantly between the two groups of sera (P= 0·50), nor did the deposition of IgG (P= 0·36), although the IgG deposition was ninefold higher in the presence of MS sera than in the presence of control sera (3·5 SFEq versus 0·4 SFEq, on average; Fig. 2e).
Considerable deposition of IgG and IgM was observed upon preincubation of both groups of sera with a control antigen, TT, but only the deposition of IgM in the control group reached significance (Fig. 2d).
Calcium dependence of MBP-mediated immunoglobulin deposition on monocytes
Addition of EDTA to the sera caused a marked inhibition of MBP deposition and MBP-mediated IgM deposition on monocytes, as well as a borderline-significant inhibition of the co-deposition of IgG in the presence of control sera. Thus, the MBP/immunoglobulin deposition was Ca2+-dependent (Table 1). This suggests a role for an interaction between complement components and complement receptors in the binding process.
Table 1.
Effect of calcium chelation and denaturation of myelin basic protein (MBP) on MBP-induced deposition of immunoglobulins, complement component 3 (C3) fragments and MBP on monocytes
| % inhibition by EDTA1 |
||
|---|---|---|
| Deposition on normal monocytes | MS sera (n = 8) | Control sera (n = 8) |
| IgM | 93 ± 8 (P < 0·0001) | 67 ± 17 (P < 0·007) |
| IgG | 27 ± 13 (P < 0·08) | 37 ± 16 (P = 0·05) |
| MBP2 | 46 ± 7 (P < 0·009)3 | 36 ± 8 (P < 0·02)4 |
| % inhibition by denaturation of MBP4 |
||
| MS sera (n = 4) | Control sera (n = 4) | |
| IgM | 92 ± 1 (P < 0·0001) | 80 ± 15 (P < 0·007) |
| C3 fragments | 98 ± 1 (P < 0·0001) | 67 ± 23 (P = 0·06) |
| MBP3 | 41 ± 11 (P < 0·03) | 53 ± 8 (P < 0·007) |
EDTA, ethylenediaminetetraacetic acid; MS, multiple sclerosis; Ig, immunoglobulin.
Sera from MS patients and healthy individuals were preincubated with MBP in the presence or absence of 10 mm EDTA before addition of the mixture to normal mononuclear cells. The subsequent deposition of IgM and IgG on CD14+ monocytes was measured by flow cytometry using fluorescein isothiocyanate (FITC)-labelled rabbit anti-human IgM and IgG, respectively, as probes.
For assessment of MBP deposition, FITC-conjugated MBP was used.
As the fluorescence was not normally distributed in the presence of EDTA-containing sera, median fluorescence intensity values were used.
Denaturation of MBP was achieved by boiling for 15 min.
All results are shown as mean ± standard error of the mean (SEM) after subtraction of the values obtained without incubation of the sera with MBP.
Co-deposition of C3-fragments and IgM on monocytes, and the requirement for an intact tertiary structure of MBP in the process
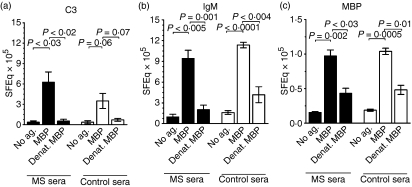
Probing with FITC-anti-C3d confirmed that C3 fragments were indeed co-deposited on monocytes following addition of MBP to both patient and control sera (Fig. 3a). Moreover, the deposition of C3 fragments as well as that of IgM and MBP on monocytes was abrogated by disruption of the tertiary structure of MBP by boiling (Fig. 3a–c, Table 1). This was to be expected, if complex formation depends upon the interaction of the anti-MBP antibodies with conformational epitopes on MBP.
Figure 3.
Deposition of complement and immunoglobulin M (IgM) on monocytes, elicited by intact or denatured myelin basic protein (MBP). Sera from multiple sclerosis (MS) patients (black bars) and healthy controls (white bars) were preincubated without antigen (No ag.), with intact MBP, or with MBP denatured by boiling (Denat. MBP), prior to addition of the mixtures to mononuclear cells from a healthy blood group O donor. Shown is the resulting deposition of (a) complement component 3 (C3) fragments and (b) IgM induced by unlabelled MBP and (c) fluorescein isothiocyanate (FITC)-labelled MBP on CD14+ monocytes. C3 fragments and IgM were probed using FITC-conjugated polyclonal rabbit anti-C3d antibodies recognizing C3b, iC3b and C3dg and F(ab′)2 fragments of FITC-conjugated polyclonal rabbit anti-human IgM antibodies, respectively. The mean fluorescence intensities for MBP and IgM and the median fluorescence intensities for C3 (which the cells bound heterogenously) were converted to soluble fluorescein equivalents (SFEq). The data are shown as mean ± standard error of the mean (SEM) for four experiments.
Cytokine responses of normal MNCs to MBP
Next, we assessed whether the sera from MS patients and healthy controls differed in their influence on the MBP-elicited cytokine responses of MNCs. To this end, we stimulated MNCs from a healthy donor with MBP in the presence of both types of sera, which contained similar amounts of IFN-γ (17 ± 2 pg/ml in the MS group versus 16 ± 4 pg/ml in the control group) and IL-10 (3·2 ± 0·5 and 3·8 ± 1·2 pg/ml, respectively) and of TNF-α, IL-4, IL-5 and IL-2 (all below 3 pg/ml; data not shown).
In the presence of all sera, the MNCs responded to MBP with a significant release of TNF-α and IL-10 (Fig. 4a,b), but not IFN-γ, IL-5, IL-4 or IL-2 (not shown). The levels of the first two cytokines were not significantly altered when MS sera were employed rather than sera from healthy controls (Fig. 4a,b).
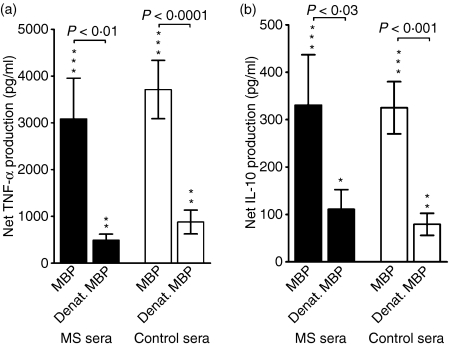
Figure 4.
The influence of serum on the myelin basic protein (MBP)-elicited cytokine responses of normal blood cells. Mononuclear cells from a healthy donor with representative cytokine responses to MBP were suspended in sera from 16 patients with multiple sclerosis (MS, black bars) or 17 healthy controls (white bars) and incubated for 1 day with untreated MBP or MBP denatured by boiling (denat. MBP). The resulting net production of cytokines, after subtraction of the background production occurring in the absence of stimulating antigen, is shown. Only tumour necrosis factor (TNF)-α (a) and interleukin (IL)-10 (b) were produced in detectable amounts. Data are shown as mean ± standard error of the mean (SEM). *P< 0·05, **P< 0·01, ***P< 0·0001 for the null hypothesis that the cytokine production in the presence of MBP equals the background production.
As with the deposition of MBP and the co-deposition of IgM and C3 on monocytes, the MBP-elicited release of IL-10 and TNF-α by MNCs depended on an intact tertiary structure of MBP. Thus, denaturation of MBP by boiling significantly reduced these responses, irrespective of whether MS sera or control sera were present in the cultures (Fig. 4, Table 2).
Table 2.
Effect of denaturation of myelin basic protein (MBP) on the cytokine response of mononuclear cells (MNCs)
| % inhibition by denaturation of MBP1,2 |
||
|---|---|---|
| Cytokine responses of normal MNCs in media containing allogenic sera3 | Sera from patients with multiple sclerosis (n = 16)4 | Sera from healthy controls (n = 17) |
| TNF-α | 68 ± 9 (P < 0·0001) | 55 ± 23 (P < 0·03) |
| IL-10 | 63 ± 8 (P < 0·0001) | 67 ± 9 (P < 0·0001) |
| Cytokine responses of MNCs in media containing autologous sera5 | Multiple sclerosis patients (n = 3) | Controls (n = 3) |
| TNF-α | 98 ± 1 (P < 0·0001) | 91 ± 4 (P < 0·002) |
| IL-10 | 87 ± 2 (P < 0·0004) | 55 ± 8 (P < 0·03) |
| IFN-γ6 | 83 | ND |
ND, not detectable; TNF, tumour necrosis factor; IFN, interferon; IL, interleukin.
Denaturation of MBP was achieved by boiling for 15 min.
The inhibition was calculated from the cytokine production induced by intact versus boiled MBP, after subtraction of the background production in the absence of MBP. Mean ± standard error of the mean (SEM) values are shown.
MNCs from a healthy blood group O individual were suspended in medium containing allogeneic serum (30% volume/volume) and stimulated for 1 day with MBP (30 μg/ml). The resulting cytokine production was measured using Luminex technology.
One patient serum was omitted for technical reasons.
Mononuclear cells, suspended in medium containing autologous serum (30% volume/volume), were stimulated with MBP (30 μg/ml) for 1 day before measurement.
Production of IFN-γ following stimulation with boiled MBP occurred in only one experiment.
IL-4- and IL-5 were non-detectable in all experiments.
The influence of serum composition on the cytokine responses to MBP in patient and control MNC cultures
As normal MNCs do not respond to challenge with MBP with the production of IFN-γ and IL-5, while MNCs from MS patients may do so,35 we decided to assess cytokine responses of MNCs from 17 MS patients and 17 sex- and age-matched controls cultured in media containing autologous or normal serum (derived from a healthy donor of blood group AB to avoid interaction of anti-A and anti-B antibodies).
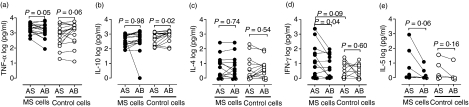
As shown in Fig. 5b,c, the type of serum did not influence the MBP-induced production of IL-10 or IL-4 in cultures of MS MNCs. By contrast, the IFN-γ response was significantly higher in media containing autologous serum as compared with normal AB serum, and similar trends were observed for the TNF-α and IL-5 responses (Fig. 5a,d,e).
Figure 5.
The influence of serum on the myelin basic protein (MBP)-elicited cytokine responses of blood cells from multiple sclerosis (MS) patients or healthy controls. Mononuclear cells from eight patients with MS (filled circles) and eight sex- and age-matched healthy controls (open circles) were cultivated for 7 days with 30 μg/ml of MBP in media containing autologous serum (AS) or standard serum from a healthy blood type AB donor (AB). The content of cytokines in the culture supernatants was assessed at days 1 and 7. Peak values were found on day 1 for tumour necrosis factor (TNF)-α (a), interleukin (IL)-10 (b) and IL-4 (c) and on day 7 for interferon (IFN)-γ (d) and IL-5 (e). The production of IL-2 was negligible. Shown are log10 transformed values, and P indicates the probability of equal production of cytokines in the presence of autologous serum and standard AB serum, as determined by a paired t-test.
The enhancing effect of the patients’ own sera on TNF-α- and IL-10 production could not be explained by a lack of stimulatory factors in AB serum, as TNF-α production by MNCs from healthy controls tended to be higher in AB serum than in autologous serum (Fig. 5a), and IL-10 production was significantly increased in the AB serum (Fig. 5b). Normal MNCs exhibited only poor IL-4, IFN-γ and IL-5 responses, making comparison of the influence of autologous serum and AB serum with respect to these responses infeasible.
Consistent with the findings for allogeneic cell cultures, the MBP-elicited production of TNF-α and IL-10 exhibited a strong dependence on an intact conformation of MBP (Table 2).
Discussion
Anti-MBP antibodies have generally been considered to be absent in sera from healthy individuals,6,8,23,24,26,27 and it has been a matter of controversy whether they exist in sera from MS patients.3,4,6,7 Here we demonstrate that sera from both MS patients and healthy controls contained MBP-reactive IgM and IgG antibodies, and that the amount of these antibodies did not differ significantly between the two groups. We used a novel semiquantitative assay based on the linking of intact MBP to microspheres for the detection of antibody binding, and the difference between our results and those obtained previously may arise from a loss of conformational epitopes upon binding of MBP to the solid phase in, for example, enzyme-linked immunosorbent assays, resulting in decreased sensitivity. Moreover, others have defined antibody positivity as readings above 2 standard deviations from the mean of healthy controls, and thereby by definition find the vast majority of healthy subjects antibody-negative.8 The antibodies in normal sera may be considered as NAAs, which are usually of the IgM isotype although IgG and IgA NAAs have also been described.36 The NAAs of healthy individuals are considered harmless, while IgM autoantibodies in MS patients may be of pathogenic relevance as synthesis of IgM in the central nervous system predicts the onset of relapse.37–39
Although the overall amount of anti-MBP antibodies did not differ significantly between MS patients and healthy individuals in the present study, the possibility remains that the antibodies from the two groups differ qualitatively. Indeed, our preliminary findings on antibody-dose responsiveness suggest that the anti-MBP IgG class antibodies from MS patients may display higher affinity for the autoantigen than the IgG antibodies from controls, while IgM antibodies from the two groups are indistinguishable in this respect (compare slopes in Fig. 1b,c). Anti-MBP antibodies from the two groups may also display distinct properties with respect to epitope specificity that may modulate presentation of self-peptides by APCs.40 Such modulation may operate on at least two different levels: (i) enhancement of the uptake of self-antigen by APCs,19–21,41 and/or (ii) an influence on antigen processing itself.41–43 Finally, disease-associated antibodies may have increased proteolytic activity.44
As regards the first option, we observed that control sera mediated the binding of MBP to APCs as efficiently as MS sera. Moreover, MBP induced co-deposition of IgM, IgG and C3 fragments to a similar extent in the presence of MS sera and control sera, suggesting that complement-activating MBP/anti-MBP complexes formed in both types of serum. Accordingly, EDTA-mediated inhibition of calcium-dependent serum factors, including complement,45 caused a marked inhibition of the deposition of IgM and IgG on monocytes. However, the apparent lack of distinction between MS and control sera, as inducers of MBP immune complex deposition, argues against antibody affinity as a potential pathogenic factor.
MBP induced the production of TNF-α and IL-10 by normal MNCs in the presence of all sera tested. As with the deposition of IgM and C3 fragments, this production did not depend on whether the serum present came from patients or controls. Moreover, the cytokine production depended on an intact conformation of MBP, indicating that antibodies were involved in the induction process. Monocytes are the major source of TNF-α and IL-10 production,46–48 so deposition of ICs on these cells presumably results in the release of TNF-α as well as IL-10 from the monocytes per se. The influence of T cells on the response requires further investigation. Also, it remains unresolved why the production of IL-10 by normal MNCs was lower in autologous serum than in the reference serum from a healthy blood group AB donor.
We also examined cytokine release induced by MBP in MNC cultures from MS patients and healthy individuals in the presence of autologous serum or the AB reference serum. As the production of TNF-α tended to be higher in autologous serum than in the normal serum, it is possible that the response was promoted by factors contained in the MS patients’ sera. The fact that the TNF-α responses of control MNCs showed the opposite tendency, i.e. being lower in the presence of autologous serum, supports this possibility. Again, a virtual abrogation of the response by denaturation of MBP suggests that the serum factors that promoted TNF-α- and IL-10 responses were indeed anti-MBP antibodies. The IFN-γ production by MS cells was promoted significantly by the patients’ own sera, and a similar tendency was observed for IL-5. Consistent with previous studies on the self-antigen thyroglobulin,21,41,49 there seems to be a promoting effect of disease-associated anti-MBP antibodies on antigen uptake and/or processing by APCs and a consequent enhanced stimulation of MBP-reactive T cells.
The present study shows a number of unexpected similarities between antibodies from MS patients and healthy individuals. Firstly, sera from the two groups contained comparable quantities of IgM and IgG reactive with MBP. Accordingly, addition of MBP to sera from both groups mediated a dose-dependent deposition of IgM, IgG and MBP itself on the surface of monocytes, indicating that formation of MBP/anti-MBP complexes had occurred. Supporting this notion, denaturation of MBP strongly reduced its own deposition and its ability to induce IgM deposition. A role for complement in the process was indicated by the deposition of C3 fragments along with IgM and MBP and by the observation that chelation of calcium strongly inhibited MBP/IgM/C3 fragment deposition. Secondly, disease-associated autoantibodies did not facilitate the production of TNF-α and IL-10 by normal MNCs to a greater extent than NAAs contained in normal sera. The responses did seem to be antibody-dependent, however, as they were abrogated by denaturation of MBP. While the propensity of MS-derived MNCs to produce IFN-γ and IL-5, in addition to TNF-α and IL-10, appears to be independent of the serum source driving antigen uptake and presentation, and, as such, to be programmed at the cellular level, the enhanced synthesis of these cytokines in the presence of the patient’s own serum does suggest that disease-associated antibodies can amplify the inflammatory cytokine response. More investigation is required to further characterize these antibodies and establish their proinflammatory role.
Acknowledgments
We thank Winnie Hansen for expert technical assistance. CJH received support from the Danish Biotechnology Program, The Lundbeck Foundation, The Warwara Larsens Foundation, The Danish Multiple Sclerosis Society, the Merchant L.F. Foghts Fund, The Augustinus Fund, a Former C.E.O. Leo Nielsen and Spouse Karen Margrethe Nielsen Grant for basic medical science, a Horse Dealer Ole Jakobsens Memorial Grant, and a Doctor Sofus Carl Emil Friis and Spouse Olga Doris Friis’ Grant.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- C3
complement component 3
- Ca
calcium
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- EDTA
ethylenediaminetetraacetic acid
- FACS
fluorescence-activated cell sorter
- FITC
fluorescein isothiocyanate
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- HSA
human serum albumin
- IC
immune complex
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- MBP
myelin basic protein
- MFI
mean fluorescence intensity
- MNC
mononuclear cell
- MS
multiple sclerosis
- NAA
natural autoantibody
- PBS
phosphate-buffered saline
- PerCP
peridinin chlorophyll protein
- RPMI
Roswell Park Memorial Institute
- SEM
standard error of the mean
- SFEq
soluble fluorescent equivalents
- TNF
tumour necrosis factor
- TT
tetanus toxoid
References
- 1.Pette M, Fujita K, Kitze B, Whitaker JN, Albert E, Kappos L, Wekerle H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770–6. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- 2.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 3.Olsson T, Baig S, Hojeberg B, Link H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann Neurol. 1990;27:132–6. doi: 10.1002/ana.410270207. [DOI] [PubMed] [Google Scholar]
- 4.Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe W, Berger T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 1999;122(Pt 11):2047–56. doi: 10.1093/brain/122.11.2047. [DOI] [PubMed] [Google Scholar]
- 5.Warren KG, Catz I, Johnson E, Mielke B. Anti-myelin basic protein and anti-proteolipid protein specific forms of multiple sclerosis. Ann Neurol. 1994;35:280–9. doi: 10.1002/ana.410350307. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor KC, Chitnis T, Griffin DE, Piyasirisilp S, Bar-Or A, Khoury S, Wucherpfennig KW, Hafler DA. Myelin basic protein-reactive autoantibodies in the serum and cerebrospinal fluid of multiple sclerosis patients are characterized by low-affinity interactions. J Neuroimmunol. 2003;136:140–8. doi: 10.1016/s0165-5728(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 7.Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–45. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 8.Angelucci F, Mirabella M, Frisullo G, Caggiula M, Tonali PA, Batocchi AP. Serum levels of anti-myelin antibodies in relapsing-remitting multiple sclerosis patients during different phases of disease activity and immunomodulatory therapy. Dis Markers. 2005;21:49–55. doi: 10.1155/2005/826817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellebjerg F, Madsen HO, Frederiksen JL, Ryder LP, Svejgaard A. Acute optic neuritis: myelin basic protein and proteolipid protein antibodies, affinity, and the HLA system. Ann Neurol. 1995;38:943–50. doi: 10.1002/ana.410380616. [DOI] [PubMed] [Google Scholar]
- 10.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 11.Rus H, Cudrici C, Niculescu F. C5b-9 complement complex in autoimmune demyelination and multiple sclerosis: dual role in neuroinflammation and neuroprotection. Ann Med. 2005;37:97–104. doi: 10.1080/07853890510007278. [DOI] [PubMed] [Google Scholar]
- 12.Tran GT, Hodgkinson SJ, Carter N, Killingsworth M, Spicer ST, Hall BM. Attenuation of experimental allergic encephalomyelitis in complement component 6-deficient rats is associated with reduced complement C9 deposition, P-selectin expression, and cellular infiltrate in spinal cords. J Immunol. 2002;168:4293–300. doi: 10.4049/jimmunol.168.9.4293. [DOI] [PubMed] [Google Scholar]
- 13.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–71. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 14.Gay FW. Early cellular events in multiple sclerosis. Intimations of an extrinsic myelinolytic antigen. Clin Neurol Neurosurg. 2006;108:234–40. doi: 10.1016/j.clineuro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Sellebjerg F, Jaliashvili I, Christiansen M, Garred P. Intrathecal activation of the complement system and disability in multiple sclerosis. J Neurol Sci. 1998;157:168–74. doi: 10.1016/s0022-510x(98)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.Sellebjerg F, Christiansen M, Garred P. MBP, anti-MBP and anti-PLP antibodies, and intrathecal complement activation in multiple sclerosis. Mult Scler. 1998;4:127–31. doi: 10.1177/135245859800400307. [DOI] [PubMed] [Google Scholar]
- 17.Sellebjerg F, Jensen CV, Christiansen M. Intrathecal IgG synthesis and autoantibody-secreting cells in multiple sclerosis. J Neuroimmunol. 2000;108:207–15. doi: 10.1016/s0165-5728(00)00292-7. [DOI] [PubMed] [Google Scholar]
- 18.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Melms A, Weissert R, Klinkert WE, Schalke BC, Tzartos S, Wekerle H. Specific immune complexes augment in vitro acetylcholine receptor-specific T-cell proliferation. Neurology. 1993;43:583–8. doi: 10.1212/wnl.43.3_part_1.583. [DOI] [PubMed] [Google Scholar]
- 20.Schalke BC, Klinkert WE, Wekerle H, Dwyer DS. Enhanced activation of a T cell line specific for acetylcholine receptor (AChR) by using anti-AChR monoclonal antibodies plus receptors. J Immunol. 1985;134:3643–8. [PubMed] [Google Scholar]
- 21.Nielsen CH, Leslie RG, Jepsen BS, Kazatchkine MD, Kaveri SV, Fischer E. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur J Immunol. 2001;31:2660–8. doi: 10.1002/1521-4141(200109)31:9<2660::aid-immu2660>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Coyle PK, Banks N, Schutzer SE. A microELISA Raji cell assay to detect immune complexes. J Immunol Methods. 1984;74:191–7. doi: 10.1016/0022-1759(84)90380-6. [DOI] [PubMed] [Google Scholar]
- 23.Coyle PK, Procyk-Dougherty Z. Multiple sclerosis immune complexes: an analysis of component antigens and antibodies. Ann Neurol. 1984;16:660–7. doi: 10.1002/ana.410160607. [DOI] [PubMed] [Google Scholar]
- 24.Geffard M, Boullerne A, Brochet B. Seric immune complexes in multiple sclerosis do not contain MBP epitopes. Brain Res Bull. 1993;30:365–8. doi: 10.1016/0361-9230(93)90266-e. [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta MK, Catz I, Warren KG, McPherson TA, Dossetor JB, Carnegie PR. Myelin basic protein: a component of circulating immune complexes in multiple sclerosis. Can J Neurol Sci. 1983;10:239–43. doi: 10.1017/s0317167100045078. [DOI] [PubMed] [Google Scholar]
- 26.Corsico B, Croce MV, Mukherjee R, Segal-Eiras A. Identification of myelin basic proteins in circulating immune complexes associated with lepromatous leprosy. Clin Immunol Immunopathol. 1994;71:38–43. doi: 10.1006/clin.1994.1049. [DOI] [PubMed] [Google Scholar]
- 27.Piyasirisilp S, Hemachudha T, Griffin DE. B-cell responses to myelin basic protein and its epitopes in autoimmune encephalomyelitis induced by Semple rabies vaccine. J Neuroimmunol. 1999;98:96–104. doi: 10.1016/s0165-5728(99)00065-x. [DOI] [PubMed] [Google Scholar]
- 28.Satoh J, Prabhakar BS, Haspel MV, Ginsberg-Fellner F, Notkins AL. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983;309:217–20. doi: 10.1056/NEJM198307283090405. [DOI] [PubMed] [Google Scholar]
- 29.Haspel MV, Onodera T, Prabhakar BS, McClintock PR, Essani K, Ray UR, Yagihashi S, Notkins AL. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983;304:73–6. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- 30.Dighiero G. Natural autoantibodies, tolerance, and autoimmunity. Ann N Y Acad Sci. 1997;815:182–92. doi: 10.1111/j.1749-6632.1997.tb52059.x. [DOI] [PubMed] [Google Scholar]
- 31.Lutz HU, Bussolino F, Flepp R, Fasler S, Stammler P, Kazatchkine MD, Arese P. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc Natl Acad Sci USA. 1987;84:7368–72. doi: 10.1073/pnas.84.21.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol. 1975;4:453–66. doi: 10.1016/0090-1229(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 33.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–87. [PubMed] [Google Scholar]
- 34.Terada M, Khoo KH, Inoue R, et al. Characterization of oligosaccharide ligands expressed on SW1116 cells recognized by mannan-binding protein. A highly fucosylated polylactosamine type N-glycan. J Biol Chem. 2005;280:10897–913. doi: 10.1074/jbc.M413092200. [DOI] [PubMed] [Google Scholar]
- 35.Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–9. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 37.Villar L, Garcia-Barragan N, Espino M, Roldan E, Sadaba M, Gomez-Rial J, Gonzalez-Porque P, Alvarez-Cermeno J. Influence of oligoclonal IgM specificity in multiple sclerosis disease course. Mult Scler. 2008;14:183–7. doi: 10.1177/1352458507082046. [DOI] [PubMed] [Google Scholar]
- 38.Villar LM, Masjuan J, Gonzalez-Porque P, Plaza J, Sadaba MC, Roldan E, Bootello A, Alvarez-Cermeno JC. Intrathecal IgM synthesis is a prognostic factor in multiple sclerosis. Ann Neurol. 2003;53:222–6. doi: 10.1002/ana.10441. [DOI] [PubMed] [Google Scholar]
- 39.Villar LM, Masjuan J, Gonzalez-Porque P, Plaza J, Sadaba MC, Roldan E, Bootello A, Alvarez-Cermeno JC. Intrathecal IgM synthesis predicts the onset of new relapses and a worse disease course in MS. Neurology. 2002;59:555–9. doi: 10.1212/wnl.59.4.555. [DOI] [PubMed] [Google Scholar]
- 40.Sellebjerg F, Frederiksen JL, Olsson T, Link H, Madsen HO, Ryder LP, Svejgaard A. Peptide specificity of anti-myelin basic protein antibodies in patients with acute optic neuritis and the HLA system. Scand J Immunol. 1994;39:575–80. doi: 10.1111/j.1365-3083.1994.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen CH, Hegedus L, Leslie RG. Autoantibodies in autoimmune thyroid disease promote immune complex formation with self antigens and increase B cell and CD4+ T cell proliferation in response to self antigens. Eur J Immunol. 2004;34:263–72. doi: 10.1002/eji.200324413. [DOI] [PubMed] [Google Scholar]
- 42.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–63. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quaratino S, Ruf J, Osman M, Guo J, McLachlan S, Rapoport B, Londei M. Human autoantibodies modulate the T cell epitope repertoire but fail to unmask a pathogenic cryptic epitope. J Immunol. 2005;174:557–63. doi: 10.4049/jimmunol.174.1.557. [DOI] [PubMed] [Google Scholar]
- 44.Ponomarenko NA, Durova OM, Vorobiev II, et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci USA. 2006;103:281–6. doi: 10.1073/pnas.0509849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziccardi RJ, Cooper NR. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976;116:496–503. [PubMed] [Google Scholar]
- 46.Burger D, Dayer JM. The role of human T-lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res. 2002;4(Suppl. 3):S169–76. doi: 10.1186/ar558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng X, Yau D, Holbrook C, Reder AT. Type I interferons inhibit interleukin-10 production in activated human monocytes and stimulate IL-10 in T cells: implications for Th1-mediated diseases. J Interferon Cytokine Res. 2002;22:311–9. doi: 10.1089/107999002753675730. [DOI] [PubMed] [Google Scholar]
- 48.Hamamcioglu K, Reder AT. Interferon-beta regulates cytokines and BDNF: greater effect in relapsing than in progressive multiple sclerosis. Mult Scler. 2007;13:459–70. doi: 10.1177/1352458506069672. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen CH, Hegedus L, Rieneck K, Moeller AC, Leslie RG, Bendtzen K. Production of interleukin (IL)-5 and IL-10 accompanies T helper cell type 1 (Th1) cytokine responses to a major thyroid self-antigen, thyroglobulin, in health and autoimmune thyroid disease. Clin Exp Immunol. 2007;147:287–95. doi: 10.1111/j.1365-2249.2006.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]