Abstract
The relationship between the p53 signal pathway and the response of human peripheral blood mononuclear cells (PBMC) to interferon (IFN)-β has hitherto not been examined. Using an oligonucleotide microarray, we found differential expression of at least 70 genes involved in the p53 signal pathway, including p53, which regulate cell proliferation and cell death following stimulation with IFN-β. We verified our observations on a limited set of p53-regulated genes at the transcriptional and translational levels. We also examined the consequences of the activation of the p53 signal pathway by IFN-β in PBMC. When cultured in the presence of T-cell mitogens, IFN-β restricted the entry of lymphocytes from the G0/G1 phase to the S phase and reduced the number of cells in the G2 phase. The addition of IFN-β alone did not increase apoptosis. However, in the presence of actinomycin D, a DNA-damaging agent, addition of IFN-β enhanced the susceptibility of PBMC to apoptosis. These observations suggest that, in spite of the activation of a number of mutually overlapping pathways mediating cell death, cell cycle arrest was the most evident consequence of IFN-β signalling in PBMC.
Keywords: apoptosis, interferon-β, cell cycle arrest, lymphocytes, p53
Introduction
Interferon (IFN)-β belongs to a family of naturally occurring molecules that have pleiotropic effects on immune and non-immune cells.1,2 The receptor for IFN-β is widely expressed in tissues, and the interaction of IFN-β with its receptor leads to oligomerization of the receptor and phosphorylation of the receptor-associated tyrosine kinases Janus kinase 1 (Jak1) and tyrosine kinase 2 (Tyk2). This then leads to the phosphorylation of signal transducers and activators of transcription 1 (STAT1) and STAT2, which subsequently dimerize, translocate to the nucleus and activate the transcription of a number of IFN-stimulated genes.3,4 Most of the type 1 interferon-stimulated genes have IFN-stimulated response element (ISRE) sequences in the promoter region.5,6 Activation of the IFN-stimulated genes requires the binding of the activated STAT proteins with p48 to form a trimeric complex that is responsible for regulating IFN actions.
IFN-β is currently used as a therapeutic agent in the treatment of hepatitis induced by the hepatitis C virus, multiple myeloma and multiple sclerosis.7–9 In the three major clinical applications of IFN-β, therapeutic benefits have in large part been derived from strategies focused on the proliferation and expansion of the target cells. Not surprisingly, a number of studies that examined the activation of genes by IFN-β have focused on the expression and regulation of proteins that mediate cell proliferation and apoptosis. These studies have shown increased expression of tumour necrosis factor (TNF), Fas ligand, and TNF-related apoptosis-inducing ligand (TRAIL) by IFN-β.10–14 IFN-β has also been shown to decrease the expression of Fas-associated death domain-like interleukin-1β-converting enzyme inhibitory protein (FLIP) and immunosuppressive acidic protein (IAP), two proteins that inhibit apoptosis.15,16 Although the induction of death receptors and their ligands has been surmised to be one of the principal mechanisms of action of IFN-β, direct evidence of the role of IFN-β in cell proliferation and apoptosis in human lymphocytes is lacking. More recently, IFN-β was shown to increase the induction of p53, a key protein involved in the activation of apoptosis in murine fibroblasts; however, the response of human peripheral blood mononuclear cells (PBMC) to the effects of IFN-β and in particular activation of the p53 pathway remains unexplored.14,17–20
The tumour suppressor protein p53 is a key transcription factor that is involved in the regulation of cell proliferation and cell death.21,22 By preventing the proliferation of cells bearing damaged DNA, which if left unattended can lead to neoplasia, p53 facilitates repair of DNA and, if the damage cannot be repaired, it directs the cells towards apoptosis. Thus, p53 acts as a tumour suppressor, and this has been confirmed by the presence of mutations of the p53 gene in cancer.23–25 It was believed that IFN-β-mediated activation of p53 following viral infection of tumour cells would lead to rapid apoptosis before viral expansion could occur, and thus restrict viral spread.26 This mechanism of action might explain the beneficial effects of IFN-β in the treatment of hepatitis caused by the hepatitis C virus.27 The mechanism of activation of p53 and its downstream signal pathway in PBMC and their role in regulating autoimmune diseases, including multiple sclerosis, remain unknown.
In our study, for the first time, we set out to examine, in a cohort of normal healthy individuals, the expression patterns and functions of the genes involved in the p53 signal pathway following culture with IFN-β and their effects on lymphocyte survival. Such studies, we believe, are important for the following reasons: (i) a detailed study of the activation of the p53 signal pathway in the PBMC of healthy donors by IFN-β is currently lacking; (ii) understanding of the outcome of p53 activation of PBMC in vitro will provide a basis for recognition of p53 activation pathways in the PBMC of patients on treatment with IFN-β for viral or autoimmune diseases, and (iii) evidence of defects in the p53 activation pathway may allow the identification of patients who show sub-therapeutic responses to IFN-β.
We show that, despite the activation of a number of proteins that have pro-apoptotic functions by IFN-β, the predominant effect on cell division was the induction of cell cycle arrest, and not apoptosis. These novel results have implications for the mechanism of action of IFN-β in the regulation of lymphocyte function in vivo.
Materials and methods
Subjects
The study group comprised 12 healthy volunteers who had no history of autoimmune disease and were not on any immunotherapy. The male to female ratio was 1 : 1; the ages of the subjects ranged from 30 to 60 years. Human subject studies were approved by the Committee for the Protection of Human Subjects of the Vanderbilt University Institutional Review Board.
Reagents
The RNA isolation kit and RNAse-free DNAse set were from Qiagen (Valencia, CA). cDNA was generated using Reverse Transcription Reagents (Applied Biosystems, Foster City, CA), and the iQ SYBR Green Supermix was from Bio-Rad Laboratories (Hercules, CA). The following primary antibodies were obtained and used in the indicated dilutions: mouse anti-human p53 antibody (DO-1) (1 : 2000), rabbit anti-human p21 antibody (1 : 2000), rabbit anti-human Bcl-2-associated X protein (Bax) antibody (1 : 2000), rabbit anti-human STAT1 antibody (1 : 5000), rabbit anti-human STAT2 antibody (1 : 5000), rabbit anti-human β-actin antibody (1 : 10 000), secondary horseradish peroxidase linked anti-mouse immunoglobulin (IgG) and anti-rabbit IgG (1 : 10 000); all these antibodies were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). The CD3-fluorescein isothiocyanate (FITC)-conjugated anti-human antibody and the Annexin V-FITC & 7-amino-actinomycin D (AAD) apoptosis detection kit were from BD Biosciences Pharmingen (San Jose, CA). DNAse-free RNAse and propidium iodide were from Roche Applied Science (Indianapolis, IN). IFN-β-1a was a gift from Serono Inc., (Rockland, MA). Actinomycin-D (Act D), phytohaemagglutinin (PHA) and Ficoll-Hypaque was purchased from Sigma-Aldrich (St Louis, MO).
Isolation and culture of PBMC
PBMC were isolated by density gradient centrifugation with Ficoll-Hypaque from freshly heparinized blood. The cells were washed in phosphate-buffered saline (PBS) and re-suspended at 1 × 106 cells/ml in complete RPMI-1640 medium containing 2 mmol glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum (Invitrogen, Carlsbad, CA). The induction of p53 was examined at the following doses of IFN-β: 100, 1000 and 5000 IU/ml. The PBMC were cultured in the presence of 10 μg/ml PHA and Act D was used at a single dosage of 50 ng/ml.
Total RNA extraction and reverse transcription
Total RNA was extracted from PBMC using the RNeasy mini kit (Qiagen, Valencia, CA) and treated with the RNAse-free DNAse set, following the manufacturer’s recommendations. A Bioanalyzer microfluidic assay (Agilent Technologies, Palo Alto, CA) was applied to test RNA integrity. Spectrophotometric and fluorometric methods were combined to quantify RNA. cDNA was generated from RNA using Reverse Transcription Reagents (Applied Biosystems). One microgram of total RNA was reverse-transcribed in a total volume of 25 μl using 100 units of reverse transcriptase, 2·5 μl of 10 × reverse transcription buffer, 2·5 μl of 10 × random primer and 1·5 μl of 20 U/μl RNase inhibitor. The mixture was incubated for 10 min at 25°, 120 min at 37° and 5 seconds at 85° and then rapidly cooled on ice. The cDNA samples were stored at −20°.
Microarray analysis
To determine the differentially expressed genes in PBMC following culture with IFN-β, we used the GeneChip® Human Gene 1·0 ST (Affymetrix Inc., Santa Clara, CA). This chip contains 764 885 probes representing 28 869 genes, each of which is represented on the array by approximately 26 probes spread across the full length of the gene. Peripheral blood mononuclear cells were obtained from five healthy individuals. The isolated PMBC were cultured with IFN-β (1000 IU/ml) for 0, 24 and 48 hr. RNA samples were submitted to the Vanderbilt Microarray Shared Resource (Vanderbilt University, Nashville, TN, USA) for microarray analysis using the GeneChip Whole Transcript (WT) Sense Target Labeling Assay protocol (Affymetrix Inc., Santa Clara, CA). Briefly, a total of 100 ng of total RNA was reverse-transcribed to cDNA which was then used as a template in an in vitro transcription reaction followed by fragmentation of the single-stranded cDNA and labelling through a terminal deoxy-transferase reaction. The biotinylated cDNA (5 μg) was fragmented and hybridized to the Human Gene 1·0 ST Array, which was then scanned using genechip scanner 3000 7G Plus 2 and command console Software (AGCC) version 1·0 (Affymetrix Inc.). Generated CEL files (raw Affymetrix data) were imported into expression console (Affymetrix Inc.) and normalized by robust multi-array average (RMA)-sketch for quality control purposes.28 Normalized data were uploaded into partek genomics suites (Partek Inc., St Louis, MI) for statistical analysis. To identify significant differences in gene expression level among the groups, log2gene expression measurements for each gene on each chip were modelled using a multifactor mixed model in the partek genomics suites software. In order to increase sensitivity and allow identification of potentially important biological changes, we employed a lower level of stringency and set an adjusted P-value [false discovery rate (FDR)] cut-off of 0·2. The lists of differentially expressed genes were then classified according to their biological pathway and biological processes. This was achieved using the protein analysis through evolutionary relationships (PANTHER) Classification System to compare them with reference lists to look for enriched functional categories.29
Real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR)
Real-time quantitative PCR was carried out in an iCycler detection system (Bio-Rad laboratories, Hercules, CA) in a volume of 25 μl. The reaction mixture consisted of 12·5 μl of iQ SYBR Green Supermix, 200 nm of each primer, and 1 μl of cDNA template. Reactions were performed for 45 cycles (95° for 15 seconds, 60° for 30 seconds and 72° for 30 seconds) after an initial 3-min incubation at 95°. Primers for the different genes amplified are shown in Table 1. The primers for p53 comprised regions that overlapped the full length and the beta/gamma isoform of p53. All reactions were performed in duplicate. Values for each gene were normalized to the values of the internal control β-actin using the threshold cycle (Ct) method, and the fold change compared with the culture control was calculated.
Table 1.
Primers for quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR)
| Forward primer | Reverse primer | |
|---|---|---|
| p53 | CGTCAGAAGCACCCAGGACT | CATCCTCCTCCCCACAACAA |
| p21 | TCCTCTAGCTGTGGGGGTGA | GAAGGTCGCTGGACGATTTG |
| BAX | CAGCAAACTGGTGCTCAAGG | CGGAGGAAGTCCAATGTCCA |
| MDM235 | CAAGTTACTGTGTATCAGGCAGGG | TCTGTTGCAATGTGATGGAAGG |
| NOXA | ACCGCTGGCCTACTGTGAAG | TGTGCTGAGTTGGCACTGAAA |
| PUMA | GACCTCAACGCACAGTACGAG | AGGAGTCCCATGATGAGATTGT |
| STAT1 | TGCAAATGCTGTATTCTTCTTTGG | TATGCAGTGCCACGGAAAGC |
| STAT2 | CCTGCTGTGCTGGGAGGTAT | GAAAGAAGCCACTGCCCTGA |
| β-actin | GCCGAGGACTTTGATTGCAC | TGGACTTGGGAGAGGACTGG |
BAX, Bcl-2 associated X protein; MDM2, murine double minute 2; STAT, signal transducers and activators of transcription.
Western blot analysis
Cell lysates for western blotting were prepared by treating PBMC with 50 mm Tris (pH 8·0), 200 mm NaCl, 1% NP40 supplemented with 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mm NaF, 20 mmβ-glycerophosphate, 1 mm sodium vanadate, 1 mm dithiothreitol and 1 mm phenylmethysulphonyl fluoride. The cells were incubated on ice for 30 min and sonicated, before being centrifuged at 18 000 g for 15 min. The total protein concentration was measured according to the Bradford assay method (Bio-Rad Laboratories). Equal amounts of protein were loaded onto a 12% sodium dodecyl sulphate (SDS)–polyacrylamide gel in electrophoresis buffer (25 mm Tris-HCl, 250 mm glycine and 0·1% SDS) and separated at 100 V. Proteins were then transferred onto polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA) by electroblotting for 1 hr at 100 V. After blocking with blotto (1 × Tris buffer solution (TBS), 0·05% Tween-20 and 5% non-fat milk powder) for 2 hr, the membranes were probed with primary antibodies. After three washes, secondary horseradish peroxidase linked anti-mouse IgG or anti-rabbit IgG was added for 1 hr. Specific bands were visualized using enhanced chemiluminescence reagent and exposed to X-ray film. The intensity of the bands was quantified using wcif image j software (Wright Cell Imaging Facility, Toronto, Canada.). The ratio of the intensity of the band of the tested protein and that of β-actin was measured on the same membrane.
Detection of apoptosis
Apoptosis was analysed by labelling with the Annexin V-FITC & 7-AAD apoptosis detection kit. PBMC were cultured with IFN-β for 48 hr in either the presence or absence of DNA-damaging agent Act D for 24 hr before harvesting. At the end of the culture period, the cells were washed and stained with Annexin V-FITC and 7-AAD, and then were submitted to the BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Data were analysed using bd facsdiva software (BD Biosciences) and cell apoptosis was determined by Annexin V+ and 7-AAD−.
Cell cycle analysis
PBMC (2 × 106) were stained with CD3-FITC for 30 min, washed twice and fixed in 75% ethanol at 4° for 2 hr, and then washed in PBS and subjected to digestion with DNAse-free RNAse for 0·5 hr at 37°. Cells were re-suspended in 500 μl of PBS with propidium iodide, and then submitted to the BD LSRII flow cytometer. Flow cytometry data were analysed using flowjc software (FlowJo, Ashland, OR).
Statistic analysis
Results are expressed as mean ± standard deviation. Statistically significant differences among groups were identified using analysis of variance (anova). Specifically, we employed repeated measures anova for the data obtained in the western blotting, real-time RT-PCR and cell cycle experiments. The PROC MIXED procedure in sas (version 9·1; SAS Institute, Cary, NC) and the SIMULATE adjustment were used to compute adjusted P-values of all pairwise differences of three time point's measurements for each parameter such as p53, p21 and Bax. The data from apoptosis experiments were analysed using one-way anova in spss 11.0 software (SPSS, Chicago, IL) and P-values < 0·05 were considered significant.
Results
Activation of the p53 signal pathway by IFN-β
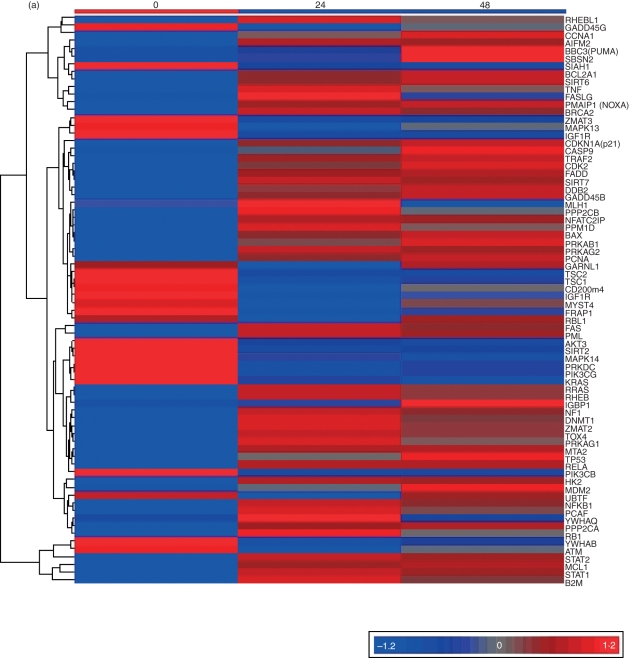
We examined the genes involved in the p53 signal pathway that were targeted by IFN-β using GeneChip® Human Gene 1·0 ST. A list of 8060 genes that showed a statistically significant change from baseline (FDR < 0·2) was generated. Among the 74 genes that were recognized as being involved in the p53 signal pathway, apoptosis and the cell cycle were two of the most highly represented biological processes (Tables 2 and 3, Fig. 1a). Of these genes, 16 were involved in the cell cycle process, 15 in apoptosis and 10 in both (Table 3). As shown in the heat map (Fig. 1a), there was an increase in p53 expression in cells cultured with IFN-β. The previously recognized downstream targets of p53, such as p21, PUMA, NOXA, Bax and growth arrest and DNA damage inducible gene 45 (Gadd45), were all shown to be induced by IFN-β. Genes that regulate the expression of death receptor-associated genes, such as those belonging to the TNF superfamily [Fas-associated protein with death domain (FADD), TNF receptor-associated factor 2 (TRAF2), TNF, apoptosis stimulating fragment (FAS), Fas ligand (FASLG)] and those involved in the common apoptotic pathway (apoptosis inducing factor 2 and Caspase 9), were also up-regulated. We also noted increased expression of murine double minute 2 (MDM2), a protein known to down-regulate apoptosis by inhibiting the actions of p53.
Table 2.
Genes in the p53 signalling pathway are involved in the response of PBMC to interferon (IFN)-β
| Gene | GenBank accession number | P-value | Definition |
|---|---|---|---|
| PML | NM_033240 | 7·78E-11 | Promyelocytic leukaemia |
| MCL1 | NM_021960 | 8·60E-09 | Myeloid cell leukaemia sequence 1 (BCL2-related) |
| BRCA2 | NM_000059 | 1·44E-08 | Breast cancer 2, early onset |
| STAT2 | NM_005419 | 1·84E-07 | Signal transducer and activator of transcription 2, 113 kDa |
| FAS | NM_000043 | 6·30E-07 | Fas (TNF receptor superfamily, member 6) |
| CDKN1A(p21) | NM_078467 | 7·47E-07 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) |
| PMAIP1(NOXA) | NM_021127 | 2·21E-06 | Phorbol-12-myriSTATe-13-acetate-induced protein 1 |
| IGF1R | NM_000875 | 3·39E-06 | Insulin-like growth factor 1 receptor |
| TSC2 | NM_000548 | 1·12E-05 | Tuberous sclerosis 2 |
| CCNA1 | NM_003914 | 1·13E-05 | Cyclin A1 |
| FASLG | NM_000639 | 1·40E-05 | Fas ligand (TNF superfamily, member 6) |
| AIFM2 | NM_032797 | 2·30E-05 | Apoptosis-inducing factor, mitochondrion-associated, 2 |
| PRKDC | NM_006904 | 2·68E-05 | Protein kinase, DNA-activated, catalytic polypeptide |
| SIRT7 | NM_016538 | 2·83E-05 | Homo sapiens sirtuin (silent mating type information regulation 2 homologue) 7 |
| AKT3 | NM_181690 | 4·22E-05 | v-akt murine thymoma viral oncogene homologue 3 (protein kinase B, gamma) |
| RELA | NM_021975 | 4·99E-05 | v-rel reticuloendotheliosis viral oncogene homologue A (avian) |
| TSC1 | NM_000368 | 6·25E-05 | Tuberous sclerosis 1 |
| C20orf74 | NM_020343 | 8·63E-05 | Chromosome 20 open reading frame 74 |
| B2M | NM_004048 | 0·000127075 | Beta-2-microglobulin |
| MDM2 | NM_002392 | 0·000162073 | MDM2 p53 binding protein homologue (mouse) |
| MYST4 | NM_012330 | 0·000243101 | MYST histone acetyltransferase (monocytic leukaemia) 4 |
| GADD45B | NM_015675 | 0·000253463 | Growth arrest and DNA-damage-inducible, beta |
| PRKAG2 | NM_016203 | 0·000278452 | Protein kinase, AMP-activated, gamma 2 non-catalytic subunit |
| RB1 | NM_000321 | 0·000334025 | Retinoblastoma 1 (including osteosarcoma) |
| PIK3CB | NM_006219 | 0·000547966 | Phosphoinositide-3-kinase, catalytic, beta polypeptide |
| MAPK13 | NM_002754 | 0·000598988 | Mitogen-activated protein kinase 13 |
| IGF1R | NM_000875 | 0·000800872 | Insulin-like growth factor 1 receptor |
| HK2 | NM_000189 | 0·000908611 | Hexokinase 2 |
| TRAF2 | NM_021138 | 0·00102911 | TNF receptor-associated factor 2 |
| PCNA | NM_002592 | 0·0011794 | Proliferating cell nuclear antigen |
| BBC3(PUMA) | AF354654 | 0·00126057 | BCL2 binding component 3 |
| GARNL1 | NM_014990 | 0·00131323 | GTPase activating Rap/RanGAP domain-like 1 |
| NFATC2IP | NM_032815 | 0·00131522 | Nuclear factor of activated T-cells, cytoplasmic, calcin |
| PCAF | NM_003884 | 0·0014721 | p300/CBP-associated factor |
| PRKAG1 | NM_212461 | 0·00155707 | protein kinase, AMP-activated, gamma 1 non-catalytic subunit |
| CASP9 | NM_001229 | 0·00194233 | Caspase 9, apoptosis-related cysteine peptidase |
| CDK2 | NM_001798 | 0·00232801 | Cyclin-dependent kinase 2 |
| DDB2 | NM_000107 | 0·00252077 | Damage-specific DNA-binding protein 2, 48 kDa |
| SIRT6 | NM_016539 | 0·00268096 | Sirtuin (silent mating type information regulation 2 homologue) 6 (S. cerevisiae) |
| BAX | NM_004324 | 0·00335452 | BCL2-associated X protein |
| STAT1 | NM_007315 | 0·00344978 | Signal transducer and activator of transcription 1, 91 kDa |
| SESN2 | NM_031459 | 0·00395825 | Sestrin 2 |
| PPP2CA | NM_002715 | 0·00404361 | Protein phosphatase 2 (formerly 2A), catalytic subunit, alpha isoform |
| UBTF | NM_014233 | 0·00431895 | Upstream binding transcription factor, RNA polymerase I |
| SIAH1 | NM_001006610 | 0·00479628 | Seven in absentia homologue 1 (Drosophila) |
| ATM | NM_000051 | 0·00500669 | Ataxia telangiectasia mutated |
| IGBP1 | NM_001551 | 0·00532907 | Immunoglobulin (CD79A) binding protein 1 |
| RHEBL1 | NM_144593 | 0·00552916 | Ras homologue enriched in brain like 1 |
| FADD | NM_003824 | 0·00561322 | Fas (TNFRSF6)-associated via death domain |
| FRAP1 | NM_004958 | 0·00782508 | FK506 binding protein 12-rapamycin associated protein 1 |
| TP53 | NM_000546 | 0·00814257 | Tumour protein p53 (Li-Fraumeni syndrome) |
| PIK3CG | NM_002649 | 0·00840817 | Phosphoinositide-3-kinase, catalytic, gamma polypeptide |
| PPM1D | BC042418 | 0·00952229 | Protein phosphatase 1D magnesium-dependent, delta isoform |
| RHEB | NM_005614 | 0·0104375 | Ras homologue enriched in brain |
| DNMT1 | NM_001379 | 0·0106164 | DNA (cytosine-5-)-methyltransferase 1 |
| RRAS | NM_006270 | 0·0108314 | Related RAS viral (r-ras) oncogene homologue |
| SIRT2 | NM_012237 | 0·0115687 | Sirtuin (silent mating type information regulation 2 homologue) 2 |
| GADD45G | NM_006705 | 0·013215 | Growth arrest and DNA-damage-inducible, gamma |
| PPP2CB | NM_001009552 | 0·0150046 | Protein phosphatase 2 (formerly 2A), catalytic subunit, beta isoform |
| ZMAT3 | NM_022470 | 0·015343 | Zinc finger, matrin type 3 |
| YWHAB | NM_003404 | 0·0166223 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, beta polypeptide |
| YWHAQ | NM_006826 | 0·0168976 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta polypeptide |
| TNF | NM_000594 | 0·0177639 | Tumour necrosis factor (TNF superfamily, member 2) |
| RPKAB1 | NM_006253 | 0·020311 | Protein kinase, AMP-activated, beta 1 non-catalytic subunit |
| NF1 | NM_001042492 | 0·0224542 | Neurofibromin 1 |
| MLH1 | NM_000249 | 0·0237558 | mutL homologue 1, colon cancer, nonpolyposis type 2 (E. coli) |
| TOX4 | NM_014828 | 0·0242946 | TOX high-mobility group box family member 4 |
| MAPK14 | NM_001315 | 0·0277024 | Mitogen-activated protein kinase 14 |
| ZMAT2 | NM_144723 | 0·0279075 | Zinc finger, matrin type 2 |
| KRAS | NM_033360 | 0·0351255 | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homologue |
| MTA2 | NM_004739 | 0·0357651 | Metastasis associated 1 family, member 2 |
| BCL2A1 | NM_004049 | 0·0384042 | BCL2-related protein A1 |
| RBL1 | NM_002895 | 0·0424297 | Retinoblastoma-like 1 (p107) |
| NFKB1 | NM_003998 | 0·0430098 | Nuclear factor of kappa light polypeptide gene enhancer |
Table 3.
Identification of p53 response pathway genes that play a role in cell cycle arrest and apoptosis
| Gene | GenBank accession number | Regulation by interferon-β | P-value | Definition |
|---|---|---|---|---|
| Apoptosis | ||||
| PIK3CG | NM_002649 | − | 0·008408 | Phosphoinositide-3-kinase, catalytic, gamma polypeptide |
| PIK3CB | NM_006219 | − | 0·000548 | Phosphoinositide-3-kinase, catalytic, beta polypeptide |
| NFKB1 | NM_003998 | + | 0·04301 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 |
| FADD | NM_003824 | + | 0·005613 | Fas (TNFRSF6)-associated via death domain |
| TRAF2 | NM_021138 | + | 0·001029 | TNF receptor-associated factor 2 |
| TNF | NM_000594 | + | 0·017764 | Tumour necrosis factor (TNF superfamily, member 2) |
| BCL2A1 | NM_004049 | + | 0·038404 | BCL2-related protein A1 |
| CASP9 | NM_001229 | + | 0·001942 | Caspase 9, apoptosis-related cysteine peptidase |
| AIFM2 | NM_032797 | + | 2·3E-05 | Apoptosis-inducing factor, mitochondrion-associated, 2 |
| MCL1 | NM_021960 | + | 8·6E-09 | Myeloid cell leukaemia sequence 1 (BCL2-related) |
| FASLG | NM_000639 | + | 1·4E-05 | Fas ligand (TNF superfamily, member 6) |
| MDM2 | NM_002392 | + | 0·000162 | MDM2 p53 binding protein homologue (mouse) |
| FAS | NM_000043 | + | 6·3E-07 | Fas (TNF receptor superfamily, member 6) |
| BBC3(PUMA) | AF354654 | + | 0·001261 | BCL2 binding component 3 |
| PMAIP1 (NOXA) | NM_021127 | + | 2·21E-06 | Phorbol-12-myriSTATe-13-acetate-induced protein 1 |
| Cell cycle arrest | ||||
| PRKDC | NM_006904 | − | 2·68E-05 | Protein kinase, DNA-activated, catalytic polypeptide |
| C20orf74 | NM_020343 | − | 8·63E-05 | Chromosome 20 open reading frame 74 |
| TSC2 | NM_000548 | − | 1·12E-05 | Tuberous sclerosis 2 |
| TSC1 | NM_000368 | − | 6·25E-05 | Tuberous sclerosis 1 |
| MYST4 | NM_012330 | − | 0·000243 | MYST histone acetyltransferase (monocytic leukaemia) 4 |
| GARNL1 | NM_014990 | − | 0·001313 | GTPase activating Rap/RanGAP domain-like 1 |
| FRAP1 | NM_004958 | − | 0·007825 | FK506 binding protein 12-rapamycin associated protein 1 |
| RBL1 | NM_002895 | − | 0·04243 | Retinoblastoma-like 1 (p107) |
| YWHAB | NM_003404 | − | 0·016622 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, beta polypeptide |
| YWHAQ | NM_006826 | + | 0·016898 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta polypeptide |
| RB1 | NM_000321 | + | 0·000334 | Retinoblastoma 1 (including osteosarcoma) |
| SESN2 | NM_031459 | + | 0·003958 | Sestrin 2 |
| CDK2 | NM_001798 | + | 0·002328 | Cyclin-dependent kinase 2 |
| PCNA | NM_002592 | + | 0·001179 | Proliferating cell nuclear antigen |
| CCNA1 | NM_003914 | + | 1·13E-05 | Cyclin A1 |
| CDKN1A | NM_078467 | + | 7·47E-07 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) |
| Overlapping | ||||
| IGF1R | NM_000875 | − | 3·39E-06 | Insulin-like growth factor 1 receptor |
| AKT3 | NM_181690 | − | 4·22E-05 | v-akt murine thymoma viral oncogene homologue 3 (protein kinase B, gamma) |
| IGF1R | NM_000875 | − | 0·000801 | Insulin-like growth factor 1 receptor |
| ATM | NM_000051 | − | 0·005007 | Ataxia telangiectasia mutated |
| GADD45G | NM_006705 | − | 0·013215 | Growth arrest and DNA-damage-inducible, gamma |
| TP53 | NM_000546 | + | 0·008143 | Tumour protein p53 (Li-Fraumeni syndrome) |
| NFATC2IP | NM_032815 | + | 0·001315 | Nuclear factor of activated T-cells, cytoplasmic, calcin |
| RELA | NM_021975 | + | 4·99E-05 | v-rel reticuloendotheliosis viral oncogene homologue A |
| BAX | NM_004324 | + | 0·003355 | BCL2-associated X protein |
| GADD45B | NM_015675 | + | 0·000253 | Growth arrest and DNA-damage-inducible, beta |
+, up-regulation; −, down-regulation.
Figure 1.
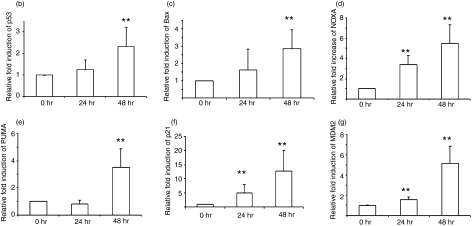
Activation of the p53 signal pathway by interferon (IFN)-β in peripheral blood mononuclear cells (PBMC) at the transcription level. (a) Hierarchical cluster of 74 differentially regulated genes in the p53 signal pathway after culture of PBMC with IFN-β. Each row corresponds to a single gene and each column corresponds to the average relative expression level at each time-point, with 0, 24 and 48 hr from left to right. The values were transformed to a log2 scale and converted into colour intensity. Red indicates increased expression and blue indicates reduced expression. (b–g) Real-time reverse transcription–polymerase chain reaction (RT-PCR) values for p53 and its target genes following culture with IFN-β: (b) p53, (c) Bcl-2-associated X protein (Bax), (d) NOXA, (e) PUMA, (f) p21 and (g) MDM2; pooled data from seven individuals. The y-axis represents the fold increase in real-time values after normalization to β-actin. **P < 0·001; *P < 0·05 when compared with unstimulated cells at 0 hr.
To determine levels of p53 mRNA in cells cultured with IFN-β, we performed real-time RT-PCR on RNA isolated from the PBMC of seven individuals, which were cultured with 1000 IU/ml of IFN-β, using primers specific for p53, p21, PUMA, NOXA and MDM2. After 48 hr of culture with IFN-β, there were significant increases (P < 0·05) in the expression of p53 (2·3-fold), p21 (12-fold), PUMA (3·5-fold), NOXA (5·5-fold), MDM2 (5-fold) and Bax (2·8-fold), compared with PBMC cultured in medium alone. At 24 hr, only NOXA, p21 and MDM2 showed a statistically significant difference from 0 hr (Fig. 1b–g).
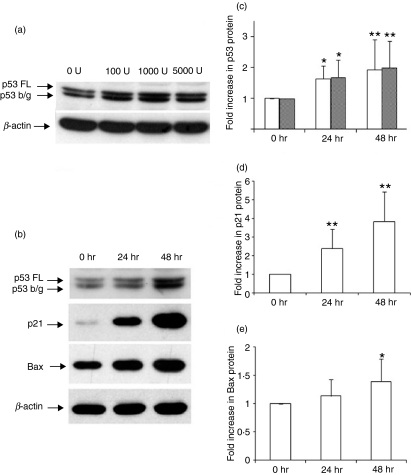
Induction of p53 and p53 targeted proteins was also examined using western blot assays. We examined the kinetics of induction of p53 following in vitro culture of PBMC with IFN-β from 12 healthy volunteers. The addition of 1000 U/ml IFN-β was sufficient for significant induction of p53 at 48 hr (Fig. 2a,b) and there was a time-dependant increase in the full-length and beta/gamma isoforms of p53. The increase in protein level was already significant at 24 hr and increased further at 48 hr (P < 0·05 compared with baseline). Densitometric studies for 12 individuals showed a 1·92-fold increase in the amount of full-length p53 and a 1·98-fold increase in the amount of the beta/gamma isoform, which was significant at 48 hr (Fig. 2b). Induction of p21 and Bax proteins using western blot assays was performed for 12 individuals. Densitometric analysis of western blots showed a 3·8-fold increase in p21 (P < 0·001) and a 1·39-fold increase in Bax (P < 0·05) following 48 hr of culture with IFN-β (Fig. 2d,e). These studies support the hypothesis that IFN-β may activate the p53 signal pathway in PBMC which is critical for cell proliferation and apoptosis.
Figure 2.
(a) Western blots showing the dose–response of p53 expression in response to interferon (IFN)-β at 48 hr, (b) western blots showing the induction of p53, p21 and Bcl-2-associated X protein (Bax) in peripheral blood mononuclear cells (PBMC) cultured with 1000 IU/ml IFN-β, (c–e) densitometric values of western blots of (c) p53, (d) p21 and (e) Bax, for 12 individuals, normalized to β-actin. **P < 0·001; *P < 0·05 when compared with unstimulated cells at 0 hr.
Differences in the induction of p53 and p53 isoforms following gamma irradiation (IR) and upon culture with IFN-β
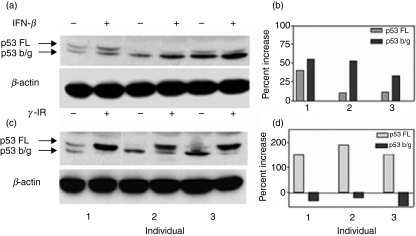
As gamma IR is a potent inducer of p53, we compared the induction of p53 and its beta/gamma isoform following either treatment with gamma IR or the addition of 1000 IU/ml IFN-β for 48 hr. As shown in Fig. 3(a,c), gamma IR (10 Gy) of PBMC induced the expression principally of full-length p53. Treatment with IFN-β, in contrast, induced both the full-length and beta/gamma isoforms of p53. In three volunteers, the beta/gamma isoform was dominant over the full-length isoform (Fig. 3b,d). These studies suggest that the p53 activation patterns of IFN-βare different from those of genotoxic stress, the most well-known inducer of p53.
Figure 3.
Induction of p53 in peripheral blood mononuclear cells (PBMC) following gamma irradiation (IR) or after culture with interferon (IFN)-β. (a) Induction of p53 in PBMC from three individuals following culture with IFN-β (1000 IU/ml for 48 hr) and probing with anti-p53 antibody. (b) Densitometric analysis of p53 after normalization to β-actin. (c) Induction of p53 in PBMC from the same three individuals following gamma irradiation of PBMCs (10 Gy). (d) Densitometric analysis of p53 and its isomers, after normalization to β-actin. The y-axis in (b) and (d) represents the per cent increase in the signal of the full-length (FL) and beta/gamma (b/g) isoforms in cells subjected to either gamma irradiation or culture with IFN-β when compared with cells cultured in medium alone.
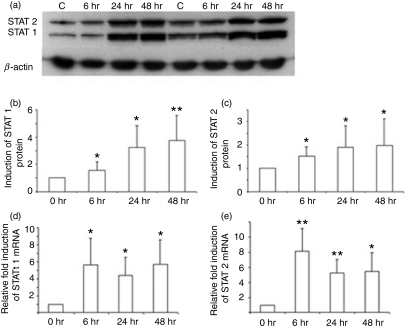
Induction of STAT1 and STAT2 by IFN-β
The IFN-β receptor uses the Jak-STAT pathway to transduce signals necessary for the transcription of IFN-responsive genes. Also, STAT1 and STAT2 form part of the heterotrimeric complex that binds to the promoter regions of p53. We examined whether STAT1 and STAT2 are targets for IFN-β, and thus act to amplify the IFN-β signalling pathway. We examined the expression of STAT1 and STAT2 following culture of PBMC with IFN-β from 12 healthy volunteers. As shown in Fig. 4(a–c), there was a significant increase in protein levels of both STAT1 and STAT2 as early as 24 hr after culture using western blotting techniques. Densitometric studies showed a twofold increase over baseline for the induction of STAT2, while STAT1 showed a 3·7-fold increase (P < 0·05 compared with untreated cells for both STAT1 and STAT2).
Figure 4.
Induction of signal transducers and activators of transcription 1 (STAT1) and STAT2 by interferon (IFN)-β. (a) Western blots of STAT1 and STAT2 proteins following culture of peripheral blood mononuclear cells (PBMC) with IFN-β from two individuals. (b) Densitometric values of protein levels for STAT1 and (c) densitometric values for STAT 2; pooled analysis for 12 individuals. (d, e) Results of real-time reverse transcription–polymerase chain reaction (RT-PCR) for (d) STAT1 and (e) STAT2 gene expression following culture of PBMC with IFN-β; pooled analysis for seven individuals. Results are expressed as fold increase in mRNA levels over that seen following culture of PBMC in medium alone. *P < 0·05; **P < 0·001 when compared with cells at 0 hr.
To determine whether the increased expression of STAT1 and STAT2 was attributable to an increase in the mRNA of the respective STAT1 and STAT2 genes, real-time RT-PCR using primers specific for STAT1 and STAT2 was performed on PBMC. In mRNA obtained from the PBMC of seven individuals cultured with IFN-β, real-time RT-PCR values showed a 5·6-fold increase in mRNA levels over baseline for STAT1 and a 5·4-fold increase for STAT2 at 48 hr (Fig. 4d,e). Kinetic studies showed that the increase in mRNA for both STAT1 and STAT2 was seen early, at 6 hr (P < 0·05 compared with untreated cells). These studies show that IFN-β induces rapid transcription of STAT1 and STAT2, thereby increasing the constitutive levels of the key signalling proteins necessary for the activation of the IFN-β receptor signalling pathway.
Induction of apoptosis in PBMC cultured with IFN-β
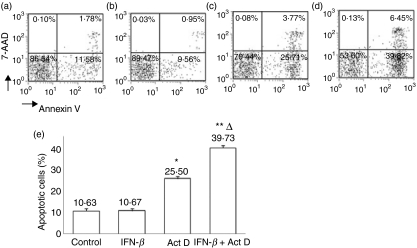
To examine the functional consequences of activation of p53, we investigated the apoptosis of PBMC following culture with IFN-β using flow cytometry. The addition of IFN-β to PBMC and culture for 48 hr did not increase apoptosis when compared with cells cultured in medium alone. As Act D is a known inducer of apoptosis in a number of cell lines,30 we examined the effects of addition of Act D to PBMC cultured with IFN-β. The addition of IFN-β did not increase the number of Annexin V-stained cells. The percentage of Annexin V+ 7-AAD− cells increased from 10·63 to 25·50% in the presence of Act D. In the presence of both ActD and IFN-β, the percentage of Annexin V+ 7-AAD− cells was 39·73% (P < 0·05; Fig. 5). These experiments showed that, although there was an increase in the expression of pro-apoptotic genes following culture with IFN-β (Fig. 1), a direct effect of IFN-β on apoptosis was not evident unless a DNA-damaging agent was added.
Figure 5.
Flow cytometric analysis of induction of apoptosis by interferon (IFN)-β: (a) cells treated with medium alone, (b) cells treated with 1000 IU/ml IFN-β for 48 hr, (c) cells treated with actinomycin D (50 ng/ml) for 24 hr, and (d) cells treated with IFN-β for 48 hr with actinomycin D added for the last 24 hr of culture. (e) Bar graph representing the apoptosis of peripheral blood mononuclear cells (PBMC) following culture with IFN-β in the presence or absence of actinomycin D. Data are representative of seven independent experiments. *P < 0·05; **P < 0·001 when compared with cells that were cultured with medium alone; ΔP < 0·05 compared with cultured with actinomycin D alone.
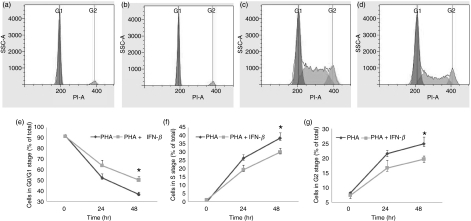
IFN-β prevents exit from the G0/G1 stage of the cell cycle
Our microarray analysis, along with the real-time RT-PCR and western blot experiments, showed that the expression of p21 was significantly elevated following culture with IFN-β. As p21 plays a critical role in inducing cell cycle arrest, we examined the effect of IFN-β on cell cycle progression. As the majority of fresh PBMC are non-proliferating (arrested in the G0/G1 stage), the cells were cultured with PHA to promote cell division in T cells, and the effect of the addition of IFN-β on cell cycle progression was examined using flow cytometry (Fig. 6). As expected, after the addition of PHA (10 μg/ml), the percentage of CD3+ lymphocytes in the G0/G1 stage in control cultures dropped from 92·2% at 0 hr to 52·2% at 24 hr, and was 36·7% at 48 hr (Fig. 6e). In CD3+ lymphocytes that were cultured with IFN-β (1000 IU/ml) and PHA (10 μg/ml), the percentage of cells in G0/G1 decreased from 91·7% at 0 hr to 63·9% at 24 hr and reduced further to 50·3% at 48 hr (Fig. 6e; P < 0·05 compared with cells that did not receive IFN-β, but were cultured with PHA). Also, the percentage of cells in G2 decreased from 24·9% when cultured with PHA alone to 19·65% when IFN-β was added with PHA (Fig. 6g,c,d; P < 0·05). The percentages of cells entering the S phase in cells that were treated with PHA and IFN-β were also lower compared with cells treated with PHA alone (Fig. 6c,d,f). These results show that, in the presence of PHA, IFN-β induces cell cycle arrest at G0/G1 and decreases the transition to the S phase, and thereby decreases the number of cells in the G2 phase.
Figure 6.
Flow cytometric analysis of cell cycle dynamics of CD3+ T lymphocytes stimulated with phytohaemagglutinin (PHA) in the presence or absence of interferon (IFN)-β: (a) control, cells cultured in medium alone; (b) cells cultured with IFN-β for 48 hr; (c) cells cultured with PHA for 48 hr and (d) cells cultured with IFN-β and PHA for 48 hr. The figure shows the profile for one representative from six individuals. Regulation of cell cycle progression by IFN-β: (e) G0/G1 phase, (f) S phase and (g) G2 phase. Error bars represent the mean and standard deviation of values for six individuals. *P < 0·05 for the comparison between cells cultured with PHA alone and cells cultured with PHA plus IFN-β.
Discussion
Using microarray techniques, complemented by real-time RT-PCR and western blot analyses, we show that IFN-β is capable of the activation of a number of genes involved in the p53 signalling pathway in human PBMC. The proteins that were activated downstream of p53 by IFN-β in our study are to some degree similar to those previously described as being activated by genotoxic stress.21,31,32 These include genes that control apoptosis, such as PUMA, NOXA and Bax, and those that induce cell cycle arrest, such as p21 and Sestrin 2. However, unlike the induction of apoptosis that follows activation of p53 after genotoxic stress, IFN-β induces cell cycle arrest in activated lymphocytes. The addition of IFN-β increased the sensitivity of lymphocytes to apoptosis in the presence of Act D. These observations suggest that DNA damage or other additional signals of cellular stress or damage may be necessary for IFN-β to mediate apoptosis in human lymphocytes.
The prevailing view regarding cell lines is that an increase in the constitutive levels of p53 allows time for DNA repair by inducing cell cycle arrest, or instructs the initiation of the cell death if the damage appears irreparable. In our study, the addition of IFN-β to PBMC cultured with PHA restricted the transition of cells from the G0/G1 phase to the S phase (induction of cell cycle arrest) and reduced the number of cells in the G2 phase. Inhibition of the transition from the G1 phase to the S phase involves the activation of a number of genes, of which p21 has been most extensively studied and is a key molecule involved in inhibiting cyclin-dependent kinase 1/2 (CDK1/2)33 The 12-fold increase in the expression of p21 suggests that this protein, along with other genes that regulate cell cycle arrest, as shown in Table 3, is critical for impeding the transition from G0/G1 to S in cells cultured with IFN-β. However, although a number of genes involved in the apoptotic process, such as BAX, PUMA and NOXA, were up-regulated, the cell death programme was not initiated.
The answer to the fundamental question of how activation of p53 leads to either cell cycle arrest or apoptosis is unclear, especially in light of the finding that induction and activation of the p53 signal pathway are not the result of double-stranded DNA breaks such as are seen with IFN-β. One possibility might relate to the activation of different isoforms of p53. Studies on tumour cell lines showed that transcription of p53 was regulated by a single promoter, producing the full-length transcript and two isoforms.34 More recently, an internal promoter of p53 was described, and at least six additional isoforms, some of which act to interfere with the transcription of the full-length protein, have been described.35 We have shown that IFN-β induces the expression of the full-length and beta/gamma isoforms of p53 in PBMC. We also observed that the pattern of induction of the full-length and beta/gamma isoforms seen following stimulation with IFN-β is distinct and different from that seen following genotoxic stress, which predominantly induces full-length p53 only. Thus, the expression of different isoforms of p53 induced by genotoxic injury or IFN-β may also alter the potency of the expression of target genes, which would skew the response towards either cell cycle arrest or apoptosis.
Another possibility might relate to the ability of p53 to bind additional transcription factors and recruit them to the promoter regions of p53 target genes.36–39 A study examining the binding of p53 to different DNA-binding sites in yeast and mammalian systems showed a difference in the ability of p53 to bind sites derived from genes involved in cell cycle arrest and/or DNA repair when compared with genes regulating mitochondrial apoptotic pathways.40 These results suggest that, whereas only the binding site sequences are required for p53-dependent activation of the cell cycle arrest genes, additional transcription factors are needed for the induction and expression of many of the pro-apoptotic genes. Hence, recruitment of additional transcription factors to p53 targeted genes may differ between cells cultured with IFN-β and cells subjected to genotoxic stress. Activation of haematopoietic zinc finger protein (Hzf), a p53 target protein, results in the transactivation of pro-arrest genes over that of pro-apoptotic genes,41 and may be favoured in cells stimulated with IFN-β.
Cellular levels of p53 are regulated tightly at the levels of transcription, post-translational modification and degradation. The ISRE that is present in the promoter region of the p53 gene binds to the heterotrimeric complex consisting of STAT1 and STAT2, thereby regulating the transcription of p53.19,42 Our studies showed a rapid induction of STAT1 and STAT2 mRNA and proteins following culture with IFN-β. Considering that the induction of both p53 mRNA and protein was modest at 24 hr and significant at 48 hr, this suggests that the initial amplification of STAT1 and STAT2 is necessary for optimal transcription of the p53 gene. Post-translational modifications of p53 are a critical step in the regulation of cellular levels of p53. In normal resting cells, p53 is rapidly degraded following binding to MDM2, thus ensuring cell integrity.43 IFN-β appears to regulate the expression of p53 both at transcription, by increasing mRNA levels, and also at degradation, by increasing the levels of MDM2. Although the increase in the amount of p53 protein in IFN-β-activated cells may be modest, the cellular consequences of activation of downstream targets, especially p21, and the induction of cell cycle arrest were significant.
Our study indicates additional mechanisms by which IFN-β may provide therapeutic benefits in human disease. Although the potency and kinetics of p53 induction varied among donors, all donor cells showed an increase in p53 expression 48 hr after culture with IFN-β. In autoimmune diseases such as multiple sclerosis, by activating p53 targeted genes, IFN-β can induce cell cycle arrest and thereby restrict the expansion of putative autoreactive lymphocytes. Whether the p53 response governs the optimal clinical response is at present not known. In patients being treated for hepatitis caused by hepatitis C virus, activation of the p53 pathway may dictate the antiviral response in hepatocytes. Acting as an adjuvant, IFN-β, by inducing p53-related pro-apoptotic genes, can enhance the actions of chemotherapeutic drugs in inducing cell death and improve outcomes in the treatment of human neoplastic diseases.
Acknowledgments
This study was supported by a postdoctoral fellowship award from the National MS Society (FG 1737A1/1) to FZ and by an investigator originated grant from Serono Inc, and the William Weaver Fund.
Disclosures
Neither author has any conflict of interest to disclose.
References
- 1.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282:20053–7. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- 2.Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282:20047–51. doi: 10.1074/jbc.R700004200. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8:907–22. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 4.Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 5.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 6.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 7.Moriyama M, Arakawa Y. Treatment of interferon-alpha for chronic Hepatitis C. Expert Opin Pharmacother. 2006;7:1163–79. doi: 10.1517/14656566.7.9.1163. [DOI] [PubMed] [Google Scholar]
- 8.Mihelic R, Kaufman JL, Lonial S. Maintenance therapy in multiple myeloma. Leukemia. 2007;21:1150–7. doi: 10.1038/sj.leu.2404633. [DOI] [PubMed] [Google Scholar]
- 9.Ann Marrie R, Rudick RA. Drug insight: interferon treatment in multiple sclerosis. Nat Clin Pract Neurol. 2006;2:34–44. doi: 10.1038/ncpneuro0088. [DOI] [PubMed] [Google Scholar]
- 10.Juang SH, Wei SJ, Hung YM, Hsu CY, Yang DM, Liu KJ, Chen WS, Yang WK. IFN-beta induces caspase-mediated apoptosis by disrupting mitochondria in human advanced stage colon cancer cell lines. J Interferon Cytokine Res. 2004;24:231–43. doi: 10.1089/107999004323034105. [DOI] [PubMed] [Google Scholar]
- 11.Oehadian A, Koide N, Mu MM, Hassan F, Islam S, Yoshida T, Yokochi T. Interferon (IFN)-beta induces apoptotic cell death in DHL-4 diffuse large B cell lymphoma cells through tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Cancer Lett. 2005;225:85–92. doi: 10.1016/j.canlet.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Wandinger KP, Lunemann JD, Wengert O, et al. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet. 2003;361:2036–43. doi: 10.1016/S0140-6736(03)13641-0. [DOI] [PubMed] [Google Scholar]
- 13.Wandinger KP, Sturzebecher CS, Bielekova B, Detore G, Rosenwald A, Staudt LM, McFarland HF, Martin R. Complex immunomodulatory effects of interferon-beta in multiple sclerosis include the upregulation of T helper 1-associated marker genes. Ann Neurol. 2001;50:349–57. doi: 10.1002/ana.1096. [DOI] [PubMed] [Google Scholar]
- 14.Zipp F. Apoptosis in multiple sclerosis. Cell Tissue Res. 2000;301:163–71. doi: 10.1007/s004410000179. [DOI] [PubMed] [Google Scholar]
- 15.Sharief MK, Semra YK, Seidi OA, Zoukos Y. Interferon-beta therapy downregulates the anti-apoptosis protein FLIP in T cells from patients with multiple sclerosis. J Neuroimmunol. 2001;120:199–207. doi: 10.1016/s0165-5728(01)00422-2. [DOI] [PubMed] [Google Scholar]
- 16.Sharief MK, Semra YK. Upregulation of the inhibitor of apoptosis proteins in activated T lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 2001;119:350–7. doi: 10.1016/s0165-5728(01)00365-4. [DOI] [PubMed] [Google Scholar]
- 17.Weinstock-Guttman B, Bhasi K, Badgett D, et al. Genomic effects of once-weekly, intramuscular interferon-beta 1a treatment after the first dose and on chronic dosing: relationships to 5-year clinical outcomes in multiple sclerosis patients. J Neuroimmunol. 2008;205:113–25. doi: 10.1016/j.jneuroim.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Hilpert J, Beekman JM, Schwenke S, et al. Biological response genes after single dose administration of interferon beta-1b to healthy male volunteers. J Neuroimmunol. 2008;199:115–25. doi: 10.1016/j.jneuroim.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–23. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 20.O’Doherty C, Villoslada P, Vandenbroeck K. Pharmacogenomics of Type I interferon therapy: a survey of response-modifying genes. Cytokine Growth Factor Rev. 2007;18:211–22. doi: 10.1016/j.cytogfr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 22.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 23.Kastan MB. Wild-type p53: tumors can’t stand it. Cell. 2007;128:837–40. doi: 10.1016/j.cell.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–34. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Fontela C, Macip S, Martinez-Sobrido L, Brown L, Ashour J, Garcia-Sastre A, Lee SW, Aaronson SA. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 2008;205:1929–38. doi: 10.1084/jem.20080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberti A, Boccato S, Vario A, Benvegnu L. Therapy of acute hepatitis C. Hepatology. 2002;5(Suppl. 1):S195–200. doi: 10.1053/jhep.2002.36808. [DOI] [PubMed] [Google Scholar]
- 28.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 29.Thomas PD, Campbell MJ, Kejariwal A, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleeff J, Kornmann M, Sawhney H, Korc M. Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells. Int J Cancer. 2000;86:399–407. doi: 10.1002/(sici)1097-0215(20000501)86:3<399::aid-ijc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 32.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 33.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 34.Baumbusch LO, Myhre S, Langerod A, et al. Expression of full-length p53 and its isoform Deltap53 in breast carcinomas in relation to mutation status and clinical parameters. Mol Cancer. 2006;5:47. doi: 10.1186/1476-4598-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–37. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vousden KH. Outcomes of p53 activation – spoilt for choice. J Cell Sci. 2006;119:5015–20. doi: 10.1242/jcs.03293. [DOI] [PubMed] [Google Scholar]
- 37.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg RL, Veprintsev DB, Bycroft M, Fersht AR. Comparative binding of p53 to its promoter and DNA recognition elements. J Mol Biol. 2005;348:589–96. doi: 10.1016/j.jmb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Koutsodontis G, Vasilaki E, Chou WC, Papakosta P, Kardassis D. Physical and functional interactions between members of the tumour suppressor p53 and the Sp families of transcription factors: importance for the regulation of genes involved in cell-cycle arrest and apoptosis. Biochem J. 2005;389:443–55. doi: 10.1042/BJ20041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian H, Wang T, Naumovski L, Lopez CD, Brachmann RK. Groups of p53 target genes involved in specific p53 downstream effects cluster into different classes of DNA binding sites. Oncogene. 2002;21:7901–11. doi: 10.1038/sj.onc.1205974. [DOI] [PubMed] [Google Scholar]
- 41.Das S, Raj L, Zhao B, Kimura Y, Bernstein A, Aaronson SA, Lee SW. Hzf Determines cell survival upon genotoxic stress by modulating p53 transactivation. Cell. 2007;130:624–37. doi: 10.1016/j.cell.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilcek J. Boosting p53 with interferon and viruses. Nat Immunol. 2003;4:825–6. doi: 10.1038/ni0903-825. [DOI] [PubMed] [Google Scholar]
- 43.Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–50. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]