Abstract
Adenosine is a well-described anti-inflammatory modulator of immune responses within peripheral tissues. Extracellular adenosine accumulates in inflamed and damaged tissues and inhibits the effector functions of various immune cell populations, including CD8 T cells. However, it remains unclear whether extracellular adenosine also regulates the initial activation of naïve CD8 T cells by professional and semi-professional antigen-presenting cells, which determines their differentiation into effector or tolerant CD8 T cells, respectively. We show that adenosine inhibited the initial activation of murine naïve CD8 T cells after αCD3/CD28-mediated stimulation. Adenosine caused inhibition of activation, cytokine production, metabolic activity, proliferation and ultimately effector differentiation of naïve CD8 T cells. Remarkably, adenosine interfered efficiently with CD8 T-cell priming by professional antigen-presenting cells (dendritic cells) and semi-professional antigen-presenting cells (liver sinusoidal endothelial cells). Further analysis of the underlying mechanisms demonstrated that adenosine prevented rapid tyrosine phosphorylation of the key kinase ZAP-70 as well as Akt and ERK1/2 in naïve αCD3/CD28-stimulated CD8 cells. Consequently, αCD3/CD28-induced calcium-influx into CD8 cells was reduced by exposure to adenosine. Our results support the notion that extracellular adenosine controls membrane-proximal T-cell receptor signalling and thereby also differentiation of naïve CD8 T cells. These data raise the possibility that extracellular adenosine has a physiological role in the regulation of CD8 T-cell priming and differentiation in peripheral organs.
Keywords: cell activation, signal transduction, T-cell receptor, T cells
Introduction
T cells exert their effector function in peripheral tissues where they contribute to the control of viral or bacterial pathogens.1 A balanced control of immune responses within peripheral tissues, however, is necessary to avoid over-reactive immunity that may lead to tissue damage and loss of organ function. Likewise, sufficient strength of immunity has to be maintained to contain and eliminate infectious micro-organisms. To control inflammatory reactions in peripheral tissues numerous endogenous anti-inflammatory factors have been described, such as lipocortin-1,2 lipoxins3 and protectins.4 Extracellular adenosine is a further well-described inflammatory modulator of immune responses. Inflamed and damaged tissues are characterized by high extracellular adenosine levels induced by disruption of the local microcirculation and subsequent onset of hypoxia.5 Hypoxia induces intracellular adenosine triphosphate (ATP) degradation, leading to an increase in adenosine-5′-monophosphate (AMP) and adenosine concentrations in cells.6 The accumulation of intracellular adenosine is further increased by hypoxia-induced inhibition of adenosine kinase.7 Accumulated adenosine is then released into the extracellular space.8 Additionally, a hypoxia-induced increase in the expression of the membrane-bound enzymes ecto-nucleoside triphosphate diphosphohydrolase (NTPDase1, apyrase, CD39) and ecto-5′-nucleotidase (CD73) leads to dephosphorylation of adenine nucleotides to adenosine,9,10 which further increases the extracellular adenosine concentration.
Adenosine itself protects tissues against damage through different mechanisms initiated by inflammation.11,12 It stimulates anti-inflammatory signalling pathways, such as activation of cyclo-oxygenase-2 (COX-2).13,14 A direct adenosine-mediated interference with immune reactions in the tissue occurs by activation of adenosine receptors on various immune cells. Among the family of adenosine receptors, the importance of the adenosine A2A receptor for the regulation of T cells has been recognized as most important. Various T-cell receptor (TCR) controlled effector functions are regulated by adenosine.15–17 Based on these findings Sitkovsky and Ohta postulated a so-called ‘2-Danger-signal’ model.18 They suggested that a first signal indicates danger caused by the presence of pathogens and leads to the activation of immune cells and thereby evokes defensive effector functions. A second signal, i.e. adenosine, indicates danger from overactive immune responses and triggers down-regulation of pro-inflammatory activities of the immune system to prevent excessive collateral damage and destruction of normal tissues. These reports clearly illustrated the potent activity of adenosine to restrain the effector function of activated T cells.
It was recently reported that naïve T cells migrate through non-lymphoid organs19 and that priming of CD8 and CD4 T-cell responses can occur outside the secondary lymphatic tissue by professional and non-professional antigen-presenting cells (APC).20,21 Although extensive investigations on the regulation of effector or memory T-cell functions by adenosine have been published,16,17,22 so far no clear characterization of the direct effect of adenosine on naïve murine CD8 T cells has been reported. Therefore, we characterized the influence of adenosine on naïve CD8 T-cell priming and further elucidated the molecular mechanisms underlying adenosine-induced inhibition of TCR-triggered T-cell activation.
Materials and methods
Mice and reagents
All animal experiments were performed in accordance with German law regarding the protection of animals and complied with institution guidelines and the principles of laboratory animal care (NIH publication 86-23, revised 1985). C57BL/6 mice were purchased from Elevage Janvier (Strasbourg, France). H2-KbSIINFEKL-restricted TCR-transgenic animals (OT-I), and H2-Kb-restricted DesTCR mice (recognizing three endogenous peptides derived from C57BL/6 background) were bred under specific pathogen-free conditions to the FELASA guidelines in the central animal facility of the University Hospital Bonn. OT-I mice were injected with 300 μg αNK1.1 antibody (clone PK136) 2 days before killing.
Antibodies for flow cytometry and enzyme-linked immunosorbent assay (ELISA) were purchased from BD Bioscience (Heidelberg, Germany) or eBioscience (San Diego, CA). αKi67 antibody was kindly provided by Dr Elmar Endl (University Hospital, Bonn, Germany). Phospho-specific antibodies for Western blot analysis were obtained from Cell Signaling (Danvers, MA) and anti-β-actin antibody from Sigma (Deisenhofen, Germany). Adenosine, dibutyryl-cAMP and Hoechst-33258 were purchased from Sigma. 6-NBDG, Fluo-4, carboxyfluorescein succinimidyl ester (CFSE), αCD3/CD28 beads and ionomycin were obtained from Invitrogen (Karlsruhe, Germany). Streptavidin was bought at Dianova (Hamburg, Germany) and antibody-coated beads for magnetic cell separation were obtained from Miltenyi Biotec (Bergisch Gladbach, Germany). CGS21680 was provided by C. Müller (Bonn). The cAMP ELISA was purchased from R&D Systems, Minneapolis, MN and used according to the manufacturer’s protocol.
Cell isolation
Highly pure liver sinusoidal endothelial cell (LSEC) isolation has been described by our group previously.20 For separation of splenic DC, spleens were digested with 0·05% collagenase solution and CD11c+ cells were isolated by immunomagnetic separation with αCD11c-labelled magnetic antibody cell sorting (MACS) microbeads. Splenic CD8 T cells were isolated by positive (αCD8 MACS beads) or negative selection.
Flow cytometry
Between 1 × 103 and 1 × 106 cells were stained with specific antibodies and 10 μg/ml Fc-Block (clone 2.4G2) for 15 min on ice. For staining of intracellular antigens cells were fixed with 4% [weight/volume (w/v)] paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. Afterwards cells were permeabilized with 0·5% (w/v) saponin in PBS for 15 min on ice, followed by 30 min incubation with antibody on ice and then analysed. Dead cells were excluded by Hoechst-33258 staining and total cell numbers were calculated using fluorescent labelled microbeads (BD Bioscience) as follows: (beadstotal × cellssample)/beadssample. Acquisition and analysis was conducted with fluorescence-activated cell sorting CantoII (BD Bioscience) and flowjo software (Tree Star Inc, Ashland, OR). For analysis of T-cell proliferation, naïve freshly isolated CD8 T cells were labelled for 20 min with CFSE (1 μm) (Invitrogen) and washed twice with PBS. After 3 days, proliferation was assessed by flow cytometric analysis of CFSE dilution in Hoechst-33258negative CD8 cells. Cell division index, which indicates the average number of cell divisions undergone by the responding T cells, was calculated from Hoechstnegative CFSE+ CD8 cells using flowjo software according to the manufacturer’s protocol.
Analysis of glucose metabolism activity
After staining of 106 T cells/ml with Hoechst-33258 a baseline was recorded for 10 seconds with a flow cytometer, 30 μm 6-NBDG was added and fluorescence was measured for a further 60 seconds. αCD3/CD28-stimulated CD8 T cells were activated for 24 hr, living cells were regained with gradient centrifugation (LSM 1077 Lymphocyte; PAA, Pasching, Germany) and 1 × 105 cells were seeded without stimulation in 96-well plate in RPMI-1640 medium containing 0·5% (w/v) bovine serum albumin over night. Subsequently, the supernatant was analysed for lactate levels with a ‘Lac’ reaction kit (Randox Laboratories, Antrim, UK) according to the manufacturer’s guideline.
Western blot analysis
Negatively selected OT-I/RAG−/− CD8 T cells were incubated with biotinylated αCD3/CD28 antibodies and cells were activated with 40 μg/ml streptavidin, either alone or after 5 min, before incubation with 1 mm adenosine. The reaction was stopped by adding protein sample buffer [0·58 m sucrose, 4% (w/v) sodium dodecyl sulphate, 0·04% (v/v) bromphenol blue, 62·5 mm Tris–HCl pH 6·8]. Equal amounts of protein were used for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SE 600 Ruby; Amersham Biosciences, Piscataway, NJ). Proteins were blotted on a polyvinlyidene difluoride membrane (Hybond P; Amersham Biosciences) with a semi-dry transfer unit (TE77 ECL; Amersham Biosciences) and protein phosphorylation was detected with phospho-specific antibodies and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Inc, Santa Cruz, CA). Blots were developed using a chemiluminescent detection kit (AppliChem, Darmstadt, Germany).
Determination of calcium influx
Negatively selected CD8 T cells were incubated for 45 min at 37° with phenol-red free RPMI-1640 medium containing 10% (v/v) fetal bovine serum and 3·5 μm Fluo-4 dye. Cells were washed twice and again incubated for 45 min at 37° with phenol-red-free RPMI-1640 medium/10% (v/v) fetal bovine serum. T cells were incubated with biotinylated αCD3/CD28 antibodies for 15 min on ice, washed and baseline fluorescence was recorded. Activation was initiated by adding 40 μg/ml streptavidin immediately or after pretreatment with 1 mm adenosine. Afterwards, fluorescence intensity was recorded for a further 390 seconds. Control stimulation was performed with 1·5 μm ionomycin for each sample.
Statistics
Data are shown as mean ± standard error of the mean (SEM). For statistical analysis a Student’s t-test was used. P-values < 0·05 were considered significant.
Results
Adenosine inhibits αCD3/CD28-induced activation of naïve CD8 T cells
As adenosine is known to modulate the function of various immune cell populations, we used αCD3/CD28-coated microbeads as ‘artificial’ APC to unequivocally investigate the effects of extracellular adenosine on activation of naïve CD8 T cells. Increased expression of activation markers, such as CD25 and CD44, within 24 hr after αCD3/CD28 stimulation was prevented by incubation of naïve CD8 T cells with adenosine at high (1 mm) but not at low (0·1 mm) concentrations (Fig. 1a). As cytokine production is crucial for CD8 T-cell activation, expansion and differentiation we next examined whether adenosine influenced interleukin-2 (IL-2) and interferon-γ (IFN-γ) expression. T-cell activation in the presence of 1 mm adenosine was followed by the absence of detectable IL-2-release (Fig. 1b) and by a dramatic reduction in IFN-γ-producing T cells (Fig. 1c). Typically, T-cell activation is accompanied by increased metabolic function.23 We observed that naïve CD8 T cells activated in the presence of adenosine showed a reduced increase in cell size compared with untreated T cells (Fig. 1d) or naïve T cells (data not shown). Furthermore, adenosine-exposed T cells also showed reduced glucose uptake and undetectable levels of lactate production over the following 24 hr (Fig. 1e,f), indicating that adenosine markedly influenced metabolic activity. It is important to mention that adenosine was removed before glucose uptake or lactate production by T cells was determined.
Figure 1.
Adenosine inhibits early activation processes in naïve CD8 T cells after αCD3/CD28 activation. Naïve CD8 T cells were activated in the absence or presence of adenosine with αCD3/CD28 beads for 24 hr. (a) Phenotypic analysis of activation markers on CD8 T cells by flow cytometry (shaded: no adenosine; thick black line: 1 mm adenosine; black line: 0·1 mm adenosine; dashed: unstimulated). (b) Interleukin-2 (IL-2) concentration was determined in cell culture supernatants after 24 hr by enzyme-linked immunosorbent assay and calculated to the numbers of viable T cells at the end of the experiment. (c) Intracellular staining for interferon-γ (IFN-γ) in CD8 T cells after 24 hr of stimulation. (d) Flow cytometric analysis of CD8 T-cell size at 24 hr post-activation. (e) Uptake of the fluorescently labelled glucose analogue 6-NBDG (30 μm) was determined by flow cytometry in living CD8 T cells. (f) Lactate release into the cell culture supernatant over a period of 16 hr was determined from 105 CD8 T cells isolated at 24 hr post-activation with αCD3/CD28 beads in the absence or presence of adenosine. *P < 0·05, ***P < 0·001.
We analysed the influence of adenosine on consecutive events such as proliferation and expansion of T cells. A complete inhibition of T-cell proliferation after 48 hr in the presence of 1 mm adenosine during the initial αCD3/CD28-stimulation was observed (Fig. 2a). As expected the loss of proliferation correlated with a decreased percentage of divided cells as well as a reduced number of cell divisions and total cell numbers (Fig. 2b). To elucidate whether adenosine-induced inhibition of T-cell proliferation was caused by inhibition of entry into the cell cycle or by blockade within the cell cycle, we determined the intracellular levels of Ki67 in αCD3/CD28-stimulated CD8 T cells. We observed a remarkable reduction in the percentage of Ki67-positive T cells after activation in the presence of adenosine (Fig. 2c). Therefore we concluded that T cells did not enter the cell cycle. Inhibition of T-cell proliferation depended on the continuous presence of high concentrations of adenosine, because T cells regained the capacity to proliferate at later time-points if adenosine was not further supplemented (data not shown). The striking reduction in the absolute number of living cells in the presence of adenosine after 48 hr compared with the number initially added into the culture is probably related to increased apoptotic cell death. The induction of apoptosis is a known feature of various nucleosides including adenosine24 and was also observed in our experiments (data not shown).
Figure 2.
Adenosine inhibits expansion and effector differentiation of naïve CD8 T cells after αCD3/CD28 activation. Naïve carboxyfluorescein succinimidyl ester (CFSE)-loaded CD8 T cells were activated with αCD3/CD28 beads in the absence or presence of adenosine. (a) After 48 hr proliferation was analysed by flow cytometry. (b) Percentage of divided cells, division index and total number of living CD8 cells. (c) Intracellular staining of CD8 T cells for Ki-67 protein after stimulation with αCD3/CD28 beads in the absence or presence of adenosine for 48 hr (shaded: no adenosine; black line: 1 mm adenosine). (d) Restimulation of living CD8 T cells 72 hr post-activation with αCD3 in adenosine-free culture media. After 16 hr the cell culture supernatant was assessed for interferon-γ (IFN-γ) and interleukin-2 (IL-2) concentrations. *P < 0·05, **P < 0·01, ***P < 0·001.
To analyse the impact of adenosine on T-cell differentiation we restimulated adenosine-exposed T cells after 72 hr of αCD3/CD28 stimulation. Notably, equal numbers of living T cells were subjected to αCD3 restimulation for 24 hr in the absence of adenosine and effector cytokine levels were determined in the cell culture supernatant by ELISA. In line with our previous observations, CD8 T cells activated in the presence of adenosine (1 mm) showed reduced capacity to produce IFN-γ and IL-2 (Fig. 2d). This indicates a failure of T-cell differentiation into effector T cells. However, CD8 T cells initially exposed to low adenosine concentrations showed a slight increase in IFN-γ production, which may be related to a less exhaustive state of the cells. Collectively, these experiments reveal a potent inhibitory function of high concentrations of adenosine on the priming and differentiation of naïve CD8 T cells.
Adenosine blocks the priming of naïve CD8 T cells by APC
Initial stimulation of naïve CD8 T cells by professional APC, such as DC, or by organ-resident APC, such as LSEC, determines differentiation into effector or tolerant CD8 T cells, respectively.20,25 This process of tolerogenic or immunogenic stimulation of naïve CD8 T cells is driven by active signalling through either costimulatory or coinhibitory molecules. We investigated whether adenosine modulated such immunogenic as well as tolerogenic T-cell priming. We cocultured H2-Kb-restricted DesTCR transgenic CD8 T cells with antigen-presenting splenic H2-Kb+ DC or H2-Kb+ LSEC in the presence or absence of adenosine. Expression of activation markers on CD8 T cells was analysed after 24 hr and proliferation after 48 hr. Similar to the results of αCD3/CD28 stimulation we observed reduced T-cell expression of CD25 and CD44 in the presence of a high concentration of adenosine after priming by antigen-presenting DC and LSEC (Fig. 3a). After 48 hr a clear reduction in APC-induced proliferation of T cells was observed in the presence of adenosine (Fig. 3b). Detailed analysis revealed that the percentage of divided cells, division index and total cell numbers of living CD8 T cells were significantly reduced after 48 hr, regardless of the stimulating APC (Fig. 3c). Compared with αCD3/CD28 stimulation, the inhibitory effects of adenosine were less pronounced, which may have been caused by more rapid degradation of adenosine by coculture of APC and T cells. We next investigated whether adenosine also affected the differentiation into effector CD8 T cells. Indeed, T cells primed by DC in the presence of high concentrations of adenosine failed to express IFN-γ after restimulation through the TCR (Fig. 3d). However, similar experiments to directly reveal adenosine-mediated inhibition of tolerance induction also demonstrated lack of IFN-γ expression (data not shown) but were not conclusive because tolerant T cells are already non-responsive to TCR stimulation.20,26 Nevertheless, as induction of tolerance by LSEC is an active process26 our observation that adenosine inhibited the initial tolerogenic priming of T cells indicates control of adenosine on tolerance induction. Taken together, these results support the notion that adenosine not only controlled priming by APC but also prevented effector cell differentiation in T cells.
Figure 3.
Adenosine blocks tolerogenic and immunogenic priming of naïve CD8 T cells. Antigen-presenting liver sinusoidal endothelial cells (LSEC; 106) or splenic dendritic cells (DC; 5 × 105) were cultured with 106 CD8 DesTCR transgenic T cells in the absence or presence of adenosine. (a) Flow cytometric analysis of activation markers after 24 hr (shaded: no adenosine; black line: 1 mm adenosine; dashed line: 0·1 mm adenosine) and (b) proliferation of DesTCR T cells after 48 hr of stimulation. (c) Percentage of divided cells, division index and total number of living DesTCR cells after 48 hr. (d) Ovalbumin (OVA) -loaded splenic DCs (5 × 105) were cultured with 106 CD8 OT-I T-cell receptor transgenic T cells in the absence or presence of adenosine for 48 hr. After 24 hr adenosine in indicated concentrations was added again. After 48 hr living CD8 T cells were restimulated with αCD3 in adenosine-free culture media for 4 hr and stained intracellularly for interferon-γ (IFN-γ). **P < 0·01, ***P < 0·001.
Inhibition of membrane-proximal signalling events in TCR/CD28-induced naïve CD8 T-cell activation
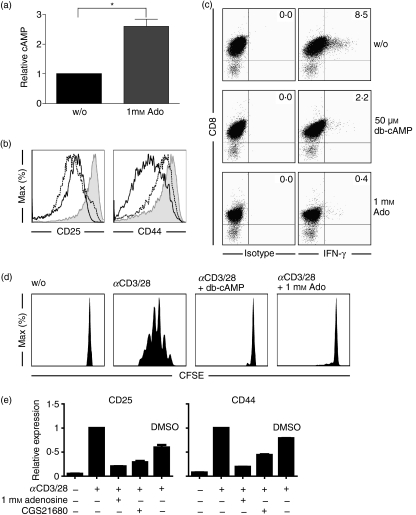
It was reported that adenosine increased intracellular cAMP in T cells.15,17 We also detected an increase in cAMP production in naïve CD8 T cells within 5 min after exposure to adenosine (Fig. 4a). To further investigate whether increased levels of cAMP influenced T-cell stimulation, we incubated αCD3/CD28-stimulated naïve CD8 T cells with a membrane-permeable cAMP analogue. The addition of dibutyryl-cAMP (db-cAMP) was associated with a decrease in CD25 and CD44 expression (Fig. 4b) and a reduction in IFN-γ expression in αCD3/CD28-stimulated naïve CD8 T cells (Fig. 4c). Moreover, in response to the addition of db-cAMP the proliferation of αCD3/CD28-stimulated naïve CD8 T cells was dramatically reduced (Fig. 4d). Consistent with a dominant effect of the A2A-receptor on T-cell function and its described capacity to induce cAMP in T cells we found that the A2A-receptor-specific agonist CGS21680 impeded the αCD3/CD28-induced activation of naïve CD8 T cells (Fig. 4e). These results suggested that cAMP generation induced by adenosine interfered with signalling events in T-cell activation. We therefore aimed to elucidate these events in more detail.
Figure 4.
Increase in intracellular cAMP levels interferes with T-cell activation. (a) 4 × 106 naïve CD8 T cells were treated with adenosine (1 mm) for 5 min and intracellular cAMP levels were analysed. (b) T cells were activated with αCD3/CD28 beads in the absence or presence of adenosine or db-cAMP. After 24 hr, expression levels of CD25 and CD44 on living CD8 T cells were analysed by flow cytometry (shaded: no adenosine; black line: 1 mm adenosine; dashed line: 50 μm db-cAMP) (c) T cells from (b) were stained intracellularly for interferon-γ (IFN-γ). (d) Naïve carboxyfluorescein succinimidyl ester (CFSE) -loaded 106 CD8 T cells were activated with αCD3/CD28 beads in the presence of db-cAMP (50 μm). After 48 hr CFSE profiles of living CD8 cells were analysed by flow cytometry. (e) Naïve CD8 T cells were treated with adenosine (1 mm) or CGS21680 (100 μm) and activated with αCD3/CD28 beads for 24 hr. Activation markers (CD25, CD44 and PD1) on CD8 T cells were analysed by flow cytometry and are shown as relative expression levels compared with untreated cells. *P < 0·05.
Naïve CD8 T-cell stimulation requires simultaneous activation of TCR- and CD28-signalling pathways to achieve full activation. We assessed classical signalling events related to TCR/CD28 signalling. As cAMP has been reported to inhibit TCR signalling already at the level of the TCR–CD3 complex,27 we investigated the effects of adenosine on tyrosine phosphorylation of membrane-proximal signalling molecules. Adenosine treatment of naïve CD8 T cells clearly reduced tyrosine phosphorylation of ZAP-70 within 5 min after αCD3/CD28 stimulation (Fig. 5a). This is of particular relevance as ZAP-70 is a key player in TCR signalling. Reduced levels of tyrosine phosphorylation were also observed for Akt and ERK1/2 (Fig. 5a). We further confirmed the interception of adenosine with proximal TCR signalling by measuring calcium influx. Adenosine pre-treatment for 5 min significantly reduced calcium influx into αCD3/CD28-stimulated naïve CD8 T cells (Fig. 5b). Taken together, these results suggest that adenosine similar to cAMP interferes with membrane-proximal TCR signalling events.
Figure 5.
Adenosine-induced inhibition of membrane-proximal signalling events in T-cell receptor (TCR)/CD28-induced naïve CD8 T-cell activation. (a) Naïve OT-I/RAG−/− CD8 T cells were stimulated with cross-linked αCD3/CD28 antibodies for the times indicated immediately or after 5 min pre-incubation with 1 mm adenosine. Afterwards, T cells were lysed and specific protein-phosphorylation was determined. (b) Flow cytometric analysis of calcium influx in fluo-4-loaded naïve CD8 T cells after stimulation with streptavidin cross-linked αCD3/CD28 antibodies (red line: αCD3/CD28 / no adenosine; blue line: αCD3/CD28/adenosine (1 mm); black line: isotype control). Equal loading with Fluo-4 was controlled by control stimulation with 1·5 μm ionomycin.
Discussion
Several reports have shown, that adenosine most efficiently controls the effector functions of various immune cells including fully differentiated effector CD8 T cells.15–17,22,28 This led to the well-established ‘2-Danger-signal’ model of Sitkovsky and Ohta, in which adenosine acts as a modulator of immune responses in peripheral tissues.12,18 Here, we investigate the early and membrane-proximal mechanisms mediating the effects of extracellular adenosine on the priming of naïve CD8 T cells.
We provide evidence that adenosine suppressed the activation of naïve CD8 T cells by inhibiting TCR signalling events. We observed reduced calcium influx as well as decreased tyrosine phosphorylation of signalling molecules like ERK1/2 and Akt after TCR activation. Moreover, tyrosine phosphorylation of the TCR key kinase ZAP-70 was strongly reduced in the presence of adenosine. This argues for a membrane-proximal inhibition of TCR signalling by adenosine. We assume that reduced ZAP-70 tyrosine phosphorylation is a consequence of a lack of Lck activity, which is required for recruitment of ZAP-70 to the CD3/TCR signal transduction complex.29 Tyrosine kinase activity of Lck is under tight control of the kinase Csk.30,31 Mustelin and Tasken have described a model for the regulation of Lck function by Csk. In this model, phosphorylation of Lck by Csk is a downstream event initiated by cAMP-dependent activation of the protein kinase A (PKA) type I.32 Indeed, we also observed induction of increased intracellular cAMP levels in CD8 T cells after adenosine exposure. Moreover, db-cAMP treatment of naïve CD8 T cells before αCD3/CD28 stimulation also inhibited ZAP-70 phosphorylation. Therefore, our experimental data support the notion that this mechanism contributes to early adenosine-induced inhibition of membrane-proximal TCR signalling. Nevertheless, it remains to be determined if other PKA-dependent mechanisms also play a role, like inhibition of Raf, phospholipase C-γ or activation of cAMP-induced transcriptional repressor ICER.33–38 It is likely that membrane-proximal inhibition of TCR signalling by adenosine is not restricted to naïve CD8 T cells but is also operative in fully differentiated effector T cells.
We and others observed cAMP induction in CD8 T cells after adenosine exposure. Coupling to an intracellular increase in cAMP formation is a known feature of two members of the adenosine receptor family, namely adenosine A2A and A2B receptors.15–17,39 Most likely, the observed cAMP induction is related to activation of the adenosine A2A receptor, which has been reported to mediate the efficient regulation of T-cell functions.15–18,40 Support for an adenosine A2A receptor-dependent inhibition of T-cell activity in our experiments came from observations that the A2A receptor CGS impeded activation of naïve CD8 T cells and that naïve CD8 T cells derived from adenosine A2B receptor knockout mice showed similar adenosine-induced inhibition of T-cell activity after αCD3/CD28 stimulation (data not shown). Additionally, in line with previous reports, we measured reduced T-cell activation after αCD3/CD28 stimulation in the presence of 5′-(N-ethylcarboxamido) adenosine (NECA) (data not shown), which is a known potent agonist of adenosine A1, A2A and A3 receptors, and a weaker agonist at the A2B receptor subtype, but inactive at P2 receptor subtypes, so excluding a contribution by P2 receptors.41,42 Inhibitory effects were observed for concentrations of 1 mm and higher (data not shown) but not of 0·1 mm adenosine. Measuring exact concentrations of adenosine in tissue is difficult so it is not known what concentrations of adenosine are actually encountered by T cells in the tissue, and hence we cannot compare these concentrations with tissue levels. However, we hypothesize that the adenosine concentrations used in our studies are likely to be present in damaged tissue in vivo.
Taken together, these results strongly suggest that inhibition of membrane-proximal signalling in naïve CD8 T cells was mediated through the adenosine A2A receptor. The prominent role of adenosine A2A receptors in controlling immune responses within peripheral tissues has been emphasized unequivocally in several in vivo studies using adenosine A2A receptor knockout animals. In particular, adenosine A2A receptors were shown to restrict anti-tumour effector functions of tumour-specific CD8 T cells giving rise to the paradox of the parallel existence of tumour and tumour-specific CD8 T cells.39 Along the same line, A2A receptor knockout animals develop uncontrolled immunity even after weak stimulation,43,44 Furthermore, A2A receptors also control IL-2 secretion and IL-2-driven expansion of effector CD8 T cells in vivo45 and the colitogenic function of adoptively transferred CD45RBhi T cells.46
Here, we report experiments suggesting that adenosine has yet another function in controlling immune responses, i.e. the prevention of priming of naïve CD8 T cells. Under defined in vitro conditions we observed that CD8 T-cell priming by professional antigen-presenting DC was inhibited by adenosine. While we doubt that the high concentrations of adenosine required for the inhibition of T-cell priming can be reached in secondary lymphatic tissue, we would assume that adenosine levels in peripheral tissues are sufficient to achieve regulation. Migration through non-lymphoid organs and priming of naïve CD8 T cells has been reported to occur outside the lymphatic system within the bone marrow by DC or within the liver by semi-professional LSEC or hepatocytes, giving rise to fully activated effector or tolerant CD8 T cells, respectively.19–21,47 As tolerance induction in peripheral tissues is often associated with clonal deletion of potentially autoreactive CD8 T cells,48 the presence of high concentrations of adenosine in peripheral tissue in response to damage or infection may prevent inadvertent deletion of rare precursor CD8 T cells with specificity for infectious micro-organisms. Moreover, adenosine-mediated inhibition of tolerogenic T-cell-priming by antigen-presenting LSEC, which involves initial stimulation and expansion of naïve CD8 T cells,26 may allow for the prevention of accidental tolerance induction towards circulating antigens during situations where increased adenosine concentrations are caused by local infection in the liver. Similar results were obtained recently for CD4 T cells, where adenosine was shown to promote long-term anergy and generation of regulatory T cells.49 Furthermore, activation of A2A receptors has been shown to attenuate T-cell-mediated allograft rejection in vivo,50 which supports our findings that adenosine is operational in the restriction of differentiation of naïve CD8 T cells.
Taken together, our results provide evidence for the inhibition of proximal TCR signalling events by adenosine, which prevents priming and differentiation of naïve CD8 T cells and presumably is also operative in restricting effector functions of already activated CD8 T cells. Our data raise the question of the physiological relevance for inhibition of naïve CD8 T-cell activation by adenosine. Although our in vitro findings need to be examined in appropriate in vivo models, our data suggest a potential new function for adenosine in the prevention of peripheral CD8 T-cell priming in situations of local tissue damage or infection.
Acknowledgments
The authors are grateful for the technical support of the House for Experimental Therapy (HET) of the Medical Faculty of Bonn University and the Flow Cytometry Core Facility at the Institute for Molecular Medicine and Experimental Immunology. This work was supported by the DFG to P.A.K. (GRK 804, SFB 704) and the Studienstiftung des deutschen Volkes to C.L. and F.A.S.
Disclosures
None.
References
- 1.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 2.Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol. 1993;150:992–9. [PubMed] [Google Scholar]
- 3.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–72. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 4.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–83. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Winn HR, Rubio R, Berne RM. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981;241:H235–42. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- 6.Zetterstrom T, Vernet L, Ungerstedt U, Tossman U, Jonzon B, Fredholm BB. Purine levels in the intact rat brain studies with an implanted perfused hollow fibre. Neurosci Lett. 1982;29:111–5. doi: 10.1016/0304-3940(82)90338-x. [DOI] [PubMed] [Google Scholar]
- 7.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–64. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 8.Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, Molina M. Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol Membr Biol. 2001;18:81–5. doi: 10.1080/096876800110033783. [DOI] [PubMed] [Google Scholar]
- 9.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 13.Pouliot M, Fiset ME, Masse M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–86. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- 14.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–10. [PubMed] [Google Scholar]
- 16.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–80. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 17.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–9. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 18.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–33. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 20.Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–54. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 21.Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–7. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 22.Dong RP, Kameoka J, Hegen M, Tanaka T, Xu Y, Schlossman SF, Morimoto C. Characterization of adenosine deaminase binding to human CD26 on T cells and its biologic role in immune response. J Immunol. 1996;156:1349–55. [PubMed] [Google Scholar]
- 23.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 24.Meisel H, Gunther S, Martin D, Schlimme E. Apoptosis induced by modified ribonucleosides in human cell culture systems. FEBS Lett. 1998;433:265–8. doi: 10.1016/s0014-5793(98)00927-2. [DOI] [PubMed] [Google Scholar]
- 25.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 26.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology (Baltimore, Md) 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 27.Skalhegg BS, Tasken K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR–CD3 complex. Science. 1994;263:84–7. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- 28.Hoskin DW, Butler JJ, Drapeau D, Haeryfar SM, Blay J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int J Cancer. 2002;99:386–95. doi: 10.1002/ijc.10325. [DOI] [PubMed] [Google Scholar]
- 29.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–93. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 30.Bergman M, Mustelin T, Oetken C, et al. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992;11:2919–24. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- 32.Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J. 2003;1:15–27. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065–9. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 34.Hafner S, Adler HS, Mischak H, et al. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994;14:6696–703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park DJ, Min HK, Rhee SG. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992;267:1496–501. [PubMed] [Google Scholar]
- 36.Liu M, Simon MI. Regulation by cAMP-dependent protein kinase of a G-protein-mediated phospholipase C. Nature. 1996;382:83–7. doi: 10.1038/382083a0. [DOI] [PubMed] [Google Scholar]
- 37.Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–86. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 38.Bodor J, Spetz AL, Strominger JL, Habener JF. cAMP inducibility of transcriptional repressor ICER in developing mature human T lymphocytes. Proc Natl Acad Sci USA. 1996;93:3536–41. doi: 10.1073/pnas.93.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grader-Beck T, van Puijenbroek AA, Nadler LM, Boussiotis VA. cAMP inhibits both Ras and Rap1 activation in primary human T lymphocytes, but only Ras inhibition correlates with blockade of cell cycle progression. Blood. 2003;101:998–1006. doi: 10.1182/blood-2002-06-1665. [DOI] [PubMed] [Google Scholar]
- 41.Fredholm BB, Jzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 42.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–83. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–20. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 44.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–82. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 45.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–14. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–9. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 47.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–12. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–6. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 49.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, Lobo PI, Okusa MD. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–9. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]