Abstract
Strongyloides stercoralis is an intestinal nematode capable of chronic, persistent infection and hyperinfection of the host; this can lead to dissemination, mainly in immunosuppressive states, in which the infection can become severe and result in the death of the host. In this study, we investigated the immune response against Strongyloides venezuelensis infection in major histocompatibility complex (MHC) class I or class II deficient mice. We found that MHC II−/− animals were more susceptible to S. venezuelensis infection as a result of the presence of an elevated number of eggs in the faeces and a delay in the elimination of adult worms compared with wild-type (WT) and MHC I−/− mice. Histopathological analysis revealed that MHC II−/− mice had a mild inflammatory infiltration in the small intestine with a reduction in tissue eosinophilia. These mice also presented a significantly lower frequency of eosinophils and mononuclear cells in the blood, together with reduced T helper type 2 (Th2) cytokines in small intestine homogenates and sera compared with WT and MHC I−/− animals. Additionally, levels of parasite-specific immunoglobulin M (IgM), IgA, IgE, total IgG and IgG1 were also significantly reduced in the sera of MHC II−/− infected mice, while a non-significant increase in the level of IgG2a was found in comparison to WT or MHC I−/− infected mice. Together, these data demonstrate that expression of MHC class II but not class I molecules is required to induce a predominantly Th2 response and to achieve efficient control of S. venezuelensis infection in mice.
Keywords: immune response, immunosuppression, MHC class I, MHC class II, Strongyloides venezuelensis, strongyloidiasis
Introduction
Strongyloides stercoralis is an intestinal nematode that inhabits the human small intestine. It is capable of chronic, persistent infection or hyperinfection of the host, involving the pulmonary and gastrointestinal tracts and leading, in some cases, to dissemination to other organs. Disseminated Strongyloides stercoralis infection occurs mainly in certain immunosuppressive states, such as haematological malignancies, human immunodeficiency virus (HIV) infection/acquired immune deficiency syndrome (AIDS), T-cell leukaemia virus type-1 (HTLV-1) infection and long-term corticosteroid use. In these cases, the infection can become severe and result in the death of the immunocompromised host.1–3
Little is known about the protective immune response against this nematode, but infection is generally characterized by the development of a T helper type 2 (Th2) immune response. In human and murine models, Strongyloides sp. induces the production of cytokines such as interleukin (IL)-3, IL-4 and IL-5, with subsequent secretion of specific immunoglobulin M (IgM), IgG, IgA and IgE, which is essential for the elimination of the parasite. The inflammatory response in the intestine is usually accompanied by intestinal eosinophilia, mastocytosis and increased numbers of goblet cells4–8 which together induce changes in gut physiology which act in concert to create an environment that is hostile to the worm.6,9
During thymic selection for development of the T-cell repertoire, major histocompatibility complex (MHC) class II molecules are required for CD4+ commitment, while self antigen recognition on the surface of the MHC class I molecule leads to CD8+ T-cell selection.10,11 Subsequently, in the peripheral tissues, the immune response against foreign antigens, for example in helminth infections, involves the interaction of peptide–MHC class II complexes with CD4+ T cells,12 which differentiate into Th2 lymphocytes and provide the basis for the protection against the nematode infection.13–16 Conversely, the interaction between peptide–MHC class I complexes and CD8+ T cells seems to be involved in the suppression of immune responses in chronic helminthiasis.17,18 In MHC class I deficient (MHC I−/−) mice, which are unable to express β2 microglobulin, infection with Nippostrongylus brasiliensis induces an intact Th2 response,19 while MHC II−/− mice are completely susceptible to this worm.14,15 Moreover, strongyloidiasis is usually asymptomatic and restricted to the gastrointestinal tract in the majority of patients; however, the failure of an effective host immune response in cases of reduced or absent CD4+ or CD8+ T cells in immunocompromised hosts may culminate in the hyperinfection syndrome, dissemination and death. Nevertheless, the roles of class I and II MHC molecules in the induction of a specialized CD8+ or CD4+ T-cell response to Strongyloides are still poorly understood. Accordingly, in this study, we investigated the role of MHC molecules in the development of the immune response in immunocompromised mice infected with Strongyloides venezuelensis.
Materials and methods
Animals
C57BL/6 wild-type (WT) mice weighing 20–25 g were obtained from the animal facilities of the Centro Multidisciplinar para Investigação Biológica (CEMIB), Universidade Estadual de Campinas, SP, Brazil. C57BL/6 MHC I/ mice, lacking β2 microglobulin (B6·129P2-BMtmlunc) and MHC II/ mice lacking the C2TA transactivation molecule (B6·129 S2-CD4tm1mark) were purchased from Jackson Laboratories (Bar Habor, ME) and maintained in the Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Brazil. All animals used for the experiments were male and aged 8–10 weeks. Experimental procedures were approved by the Animal Ethics Committee of the Universidade Federal de Uberlândia.
Parasites
An S. venezuelensis strain was isolated from the wild rodent Bolomys lasiurus in April 1986 and since then it has been maintained in the Departamento de Parasitologia, Instituto de Biologia, Universidade Estadual de Campinas, Brazil, by serial passages in Wistar rats (Rattus norvegicus).
Infection of mice with S. venezuelensis
Strongyloides venezuelensis third-stage infective larvae (L3) were obtained from charcoal cultures of infected rat faeces. The cultures were incubated at 28° for 72 hr, and the infective larvae were collected and concentrated using a Baermann apparatus. The recovered larvae were then counted and C57BL/6 WT, MHC I−/− or MHC II−/− mice were individually inoculated by subcutaneous (s.c.) injection with 3000 S. venezuelensis L3 larvae. Uninfected mice were used as controls (day 0).
Egg and adult worm counts
Eggs per gram of faeces were counted according to Machado et al.20 on days 5, 8, 13 and 21 post-infection (p.i.). The parasitological examination was performed three times, and the average of the three results was recorded. At the same time-points, groups of six mice were placed individually on clean, moist absorbent paper and allowed to defecate to recover faeces. For recovery adult worms, the upper half of the small intestine from each infected mouse was removed after killing of the mouse, washed, cut open longitudinally, placed in Petri dishes containing saline, and incubated for 2 hr at 37°. The worms were counted under light stereomicroscopy and worm fecundity was estimated by dividing the number of eggs eliminated in faeces by the number of worms recovered from the intestine of each mouse on days 5, 8, 13 and 21 p.i.21
Experimental procedure and histological analysis
Tissue samples of the small intestine were collected, fixed in 10% buffered formalin and processed routinely for paraffin embedding and sectioning. The small intestine was cut into four pieces and rolled to make a ‘Swiss roll’. Tissue sections (4 μm) were mounted on slides and stained with haematoxylin and eosin. The entire length of the small intestine was examined histologically. Lesions were scored in a blind manner and graded based on the severity of inflammation according to inflammatory cell infiltration in the lamina propria (LP), epithelium and submucosa, as previously described.22 The inflammatory score was represented by arbitrary units: 0–2, mild; 2–4, moderate; 4–6, severe; above 6, very severe. Eosinophil numbers were determined by counting the cells in 40 microscopic fields (1 × 400) for two intestinal histological sections from each of six mice per group.
Blood and serum collection
On days 5, 8, 13 and 21 p.i., mice were anaesthetized and blood samples were collected by retrorbital puncture. Total blood cell counts were immediately assayed in a Neubauer chamber. Differential counts were obtained using Panotico staining (Renylab, Barbacena, MG, Brazil). Blood was then centrifuged, and the serum was stored at −20° until use.
Cytokine measurement in intestinal homogenates and sera
Cytokines were quantified in homogenates prepared from the small intestine and sera. Gut samples (100 mg of duodena) obtained from WT, MHC I−/− or MHC II−/− mice on days 5, 8, 13 and 21 p.i. were homogenized in 500 ml of phosphate-buffered saline (PBS) plus 0·05% Tween 20 containing protease inhibitors [1·6 mm phenylmethylsulphonyl fluoride, 100 μg/ml leupeptin hemisulphate, 10 μg/ml aprotinin, 1 mm benzamidine hydrochloride hydrate and 10 mm ethylenediaminetetraacetic acid (EDTA)]. Each sample was then centrifuged for 10 min at 3000 g, and the supernatant was used for the assays. Concentrations of IL-4, IL-5, IL-12 and interferon (IFN)-γ were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Duoset; R&D Systems, Minneapolis, MN), according to manufacturer’s instructions. The detection sensitivity ranged from 15 to 31 pg/ml for each assay.
Alkaline parasite extracts
Strongyloides venezuelensis alkaline extracts were prepared according to the protocol previously described.23 In brief, 1 ml of 0·15 m NaOH was added to ∼300·000 S. venezuelensis L3 larvae, which were maintained under gentle shaking for 6 hr at 4°. Subsequently 0·3 m HCl was added until pH 7·0 was reached. This preparation was then centrifuged at 12 400 g for 30 min at 4°. The protein content of the supernatant was determined by the Lowry method.24 The antigenic extract was used for ELISA.
Measurement of antibodies in sera
Parasite-specific IgM, IgA, IgE, total IgG, IgG1 and IgG2a were quantified by ELISA in serum samples of S. venezuelensis-infected and non-infected mice. Briefly, high-binding microtitre plates (Corning-Costar; Laboratory Sciences Company, New York, NY) were coated with 10 μg/ml S. venezuelensis alkaline extract in 0·06 m carbonate buffer (pH 9·6) for 18 hr at 4°. After three washes in PBS containing 0·05% Tween 20 (PBS-T), non-specific binding sites were blocked with PBS-T plus 1% bovine serum albumin (BSA; Sigma Chemical Co., St Louis, MO) (PBS-T-BSA) for 1 hr at 37°. Serum samples (50 μl each) were added (50 μl/well) at dilutions of 1/10 (total IgG, IgG1, IgG2a and IgE), 1/80 (IgA), and 1/100 (IgM) in block buffer, except for IgA which was diluted in PBS-T. After incubation for 45 min (IgM, IgA and total IgG) or 1 hr (IgG1, IgG2a and IgE) at 37°, plates were washed and peroxidase goat anti-mouse IgM (1/1500; Sigma), IgA (1/100; Sigma), total IgG (1/1000; Sigma), IgG1 (1/4000; Santa Cruz Biotechnology, Paso Robles, CA) or IgG2a (1/2000; Santa Cruz Biotechnology) conjugates were added to the plates which were incubated for 45 min (IgM, IgA and total IgG) or 2 hr (IgG1 and IgG2a) at 37°. For IgE assays, biotinylated goat anti-mouse IgE (1/250; Caltag Lab, San Francisco, CA) was added and plates were incubated for 2 hr at 37°, and then streptavidin-peroxidase conjugate (Sigma) (1/1000) was added and plates were incubated for 30 min at 37°. The reaction was developed with 0·03% H2O2 in 0·1 m citrate-phosphate buffer (pH 4·5) containing 3,3′5,5′ tetramethylbenzidine (Sigma). For development of IgM, IgA, total IgG, IgG1 and IgG2a reactions, the enzyme substrate, consisting of 0·05% H2O2 in 0·1 m citrate-phosphate buffer (pH 5·0) containing 0·4 mg/ml o-phenylenediamine (Sigma-Aldrich Co., Deisenhofen, Germany), was added to the plates. Absorbance was read at 450 (IgE) or 492 nm (IgM, IgA, IgG, IgG1 and IgG2a) in a plate reader (Titertek Multiskan; Flow Laboratories, McLean, VA). The results were expressed as an ELISA index (EI), as follows: EI = absorbance of test sample/cut-off, where the cut-off was the mean absorbance of three negative sera plus three standard deviations. Values of EI > 1·0 were considered positive.
Statistical analysis
Data are reported as mean ± standard error of the mean (SEM) and analysed using one-way analysis of variance (anova), followed by Tukey’s test. Student’s t-test was used in the analysis of parasite and egg numbers. Values of P < 0·05 were considered statistically significant.
Results
MHC II−/− mice have increased numbers of eggs in the faeces and delayed elimination of S. venezuelensis
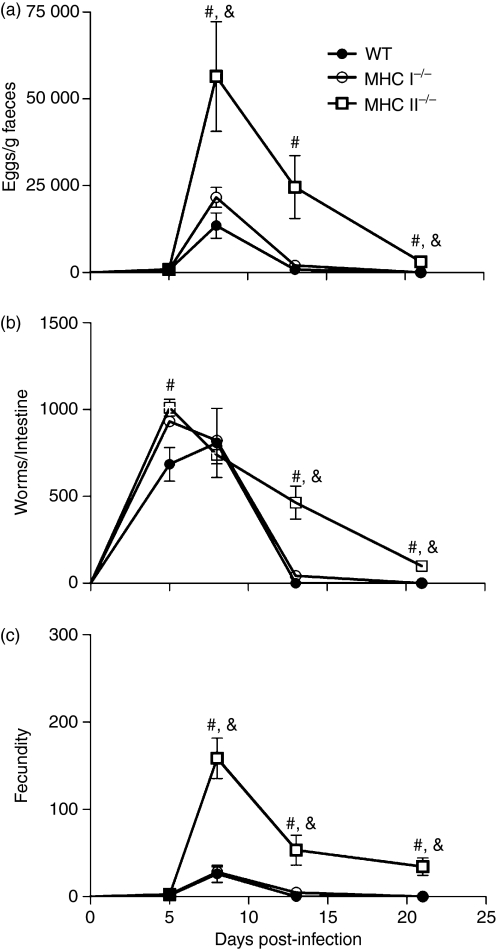
To determine whether immunocompromised mice were more susceptible to S. venezuelensis infection, the numbers of eggs in faeces and adult worms recovered from the small intestine and the fecundity rate were evaluated in WT, MHC I−/− and MHC II−/− infected mice (Fig. 1). There were no differences in the number of eggs excreted in the faeces at 5 days p.i. among the groups studied. However, at 8 days p.i. the number of eggs recovered in the faeces of MHC II−/− infected mice was significantly higher than that in WT or MHC I−/− mice (Fig. 1a). At 13 days p.i. a significant reduction in the number of eggs excreted in the faeces was observed in WT and MHC I−/− mice and the infection was completely eliminated at 21 days p.i. in these animals. In contrast, at 21 days p.i. the number of eggs recovered in the feces of MHC II−/− mice was significantly higher than that in WT or MHC I−/− animals (Fig. 1a). A significant difference was found in the number of adult worms that were established in the small intestine at 5 days p.i. in MHC II−/− mice compared with WT mice (Fig. 1b). At 8 days p.i., the number of worms recovered from the small intestine was similar in all groups studied. However, at 13 days p.i., S. venezuelensis infection in WT and MHC I−/− mice was significantly reduced compared with that in MHC II−/− animals. The infection was completely eliminated at 21 days p.i. in WT mice and we observed a significant reduction in the number of worms recovered from the small intestines of MHC I−/− mice compared with MHC II−/− mice (Fig. 1b). The fecundity of worms from MHC II−/− mice was significantly higher than that of worms from WT and MHC I−/− animals at 8, 13 and 21 days p.i. (Fig. 1c).
Figure 1.
Parasite burdens in wild-type (WT), major histocompatibility complex (MHC) I−/− and MHC II−/− mice after subcutaneous (s.c.) infection with 3000 Strongyloides venezuelensis L3 larvae. The number of eggs per gram of faeces eliminated in faeces (a), adult worms recovered from the small intestine (b), and the fecundity rate (c) were evaluated at 0, 5, 8, 13 and 21 days post-infection. Data are expressed as mean ± standard error of the mean (SEM) (n = 6). Results are representative of two or three independent experiments. *WT versus MHC I−/−; #WT versus MHC II−/−; &MHC I−/− versus MHC II−/−; P < 0·05.
Inflammatory damage was reduced in the intestines of S. venezuelensis-infected MHC II−/− mice
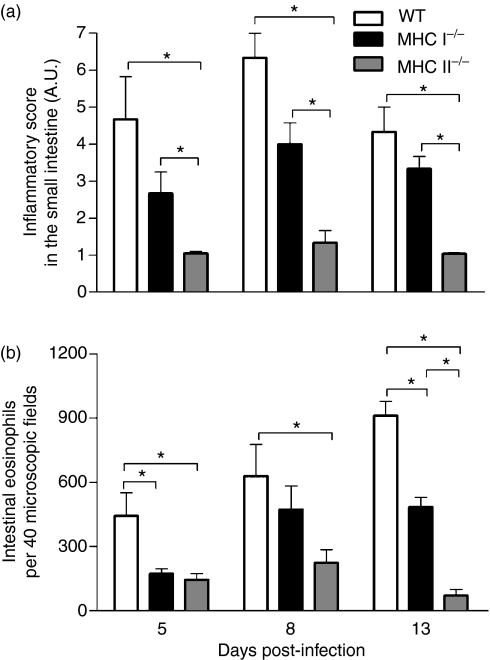
The infection of WT mice induced higher inflammatory infiltration in the LP and submucosa of the small intestine compared with MHC II−/− animals on days 5, 8 and 13 p.i., this infiltration consisting of polymorphonuclear eosinophils and mononuclear cells. Inflammatory lesions, localized mainly in the duodena, were observed on day 5 p.i., and were more severe on day 8 p.i. These lesions were associated with elevated numbers of worms in the mucosa in addition to the inflammatory reaction and the presence of oedema at the ends of villi in the LP. On day 13 p.i. the inflammatory reaction was reduced in the small intestine of WT mice, coinciding with the clearance of the parasite from the organ (Fig. 2a).
Figure 2.
Histopathological analysis of the small intestines of wild-type (WT), major histocompatibility complex (MHC) I−/− and MHC II−/− mice infected with 3000 Strongyloides venezuelensis L3 larvae. (a) Inflammatory scores on days 5, 8 and 13 post-infection, represented in arbitrary units (A.U.): 0–2, mild; 2–4, moderate; 4–6, severe; above 6, very severe. (b) Eosinophil counts in the intestinal tissues in the same time periods. The number of eosinophils was determined by counting the cells in 40 microscopic fields (1 × 400) for two intestinal histological sections from each mouse. Data are expressed as mean ± standard error of the mean (SEM) (n = 6). Results are representative of two or three independent experiments. *P < 0·05.
The presence of inflammatory infiltration was also observed in the duodena of MHC I−/− mice. The lesions were observed as early as 5 days p.i., peaked at day 8 and decreased at day 13 p.i. (Fig. 2a), and eosinophils were also found in the inflammatory lesions (Fig. 2b). In contrast, the MHC II−/− mice presented mild inflammatory infiltration in all portions of the small intestine and oedema in the LP at the ends of villi, which was associated with the presence of worms (Fig. 2a). The MHC II−/− mice presented statistically lower numbers of polymorphonuclear eosinophils on days 5, 8 and 13 p.i. compared with WT mice and lower numbers on day 13 p.i. compared with MHC I−/− mice (Fig. 2b).
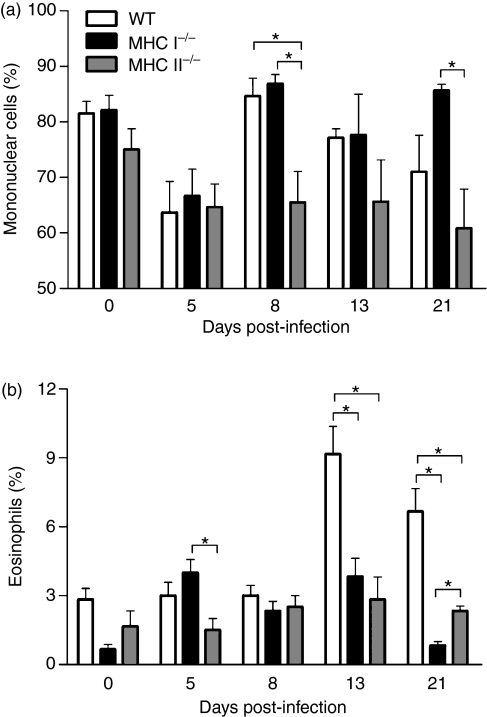
Circulating mononuclear cells and eosinophils are decreased in the blood of S. venezuelensis-infected MHC II−/− mice
As S. venezuelensis infection affected the inflammatory infiltrate in the intestine, we next investigated whether other parameters such as circulating leucocytes were also affected in mice deficient in MHC I or MHC II molecules. Then, after counting total leucocyte numbers in the blood (data not shown), differential cell counts were performed to quantify the numbers of mononuclear cells and eosinophils. There were noticeable differences in the frequency of mononuclear cells at 8 days p.i., when WT and MHC I−/− mice showed higher cell counts than MHC II−/− mice. At 21 days p.i., a significant difference in mononuclear cell counts was found between MHC I−/− and MHC II−/− mice (Fig. 3a). On day 5 p.i., eosinophils were diminished in MHC II−/− mice compared with MHC I−/− animals. In addition, a significant increase in the eosinophil percentage was seen in WT mice compared with both deficient animals at 13 and 21 days p.i. (Fig. 3b). Interestingly, we also observed an elevated frequency of eosinophils in MHC II−/− animals in comparison to MHC I−/− mice on day 21 p.i. (Fig. 3b).
Figure 3.
Leucocyte counts in the blood of wild-type (WT), major histocompatibility complex (MHC) I−/− and MHC II−/− mice, after subcutaneous (s.c.) infection with 3000 Strongyloides venezuelensis L3 larvae. Mononuclear cells (a) and eosinophils (b) were evaluated at 0, 5, 8, 13 and 21 days post-infection. Data are expressed as mean ± standard error of the mean (SEM) (n = 6). Results are representative of two or three independent experiments. *P < 0·05.
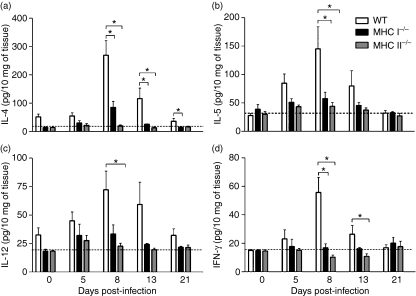
Gut inflammation and systemic response are modulated by differential cytokine production in S. venezuelensis-infected mice
To determine whether the histopathological features found in WT and MHC-deficient mice were related to differential modulation of intestinal immunity, we quantified the cytokines produced at the site of worm establishment. The results showed that levels of IL-4, IL-5, IL-12 and IFN-γ remained unchanged throughout the infection in MHC II−/− mice, similar to the situation in non-infected controls (Fig. 4a–d). WT infected mice presented the greatest increase in IL-4, IL-5 and IFN-γ on day 8 p.i. when compared with the other groups (Fig. 4a,b and d). Moreover, at the same time-point, IL-12 levels were significantly elevated in WT mice compared with MHC II−/− mice (Fig. 4c). On day 13 p.i., the concentration of IL-4 in the WT gut was higher than that observed in the intestines of MHC I−/− or MHC II−/− mice (Fig. 4a). IL-12 detection in WT infected animals was also elevated in comparison to the other groups on day 13 p.i., although these differences were not statistically significant (Fig. 4c). The IFN-γ concentration was also significantly increased in WT infected mice compared with MHC II−/− infected mice on day 13 p.i. (Fig. 4d).
Figure 4.
Levels of interleukin (IL)-4 (a), IL-5 (b), IL-12 (c) and interferon (IFN)-γ (d) determined by enzyme-linked immunosorbent assay (ELISA) in small intestine homogenates from wild-type (WT), major histocompatibility complex (MHC) I−/− and MHC II−/− mice, after subcutaneous (s.c.) infection with 3000 Strongyloides venezuelensis L3 larvae. Analyses were performed at 0, 5, 8, 13 and 21 days post-infection. Dashed lines indicate the detection limit for the cytokines. Data are expressed as mean ± standard error of the mean (SEM) (n = 6). Results are representative of two or three independent experiments. *P < 0·05.
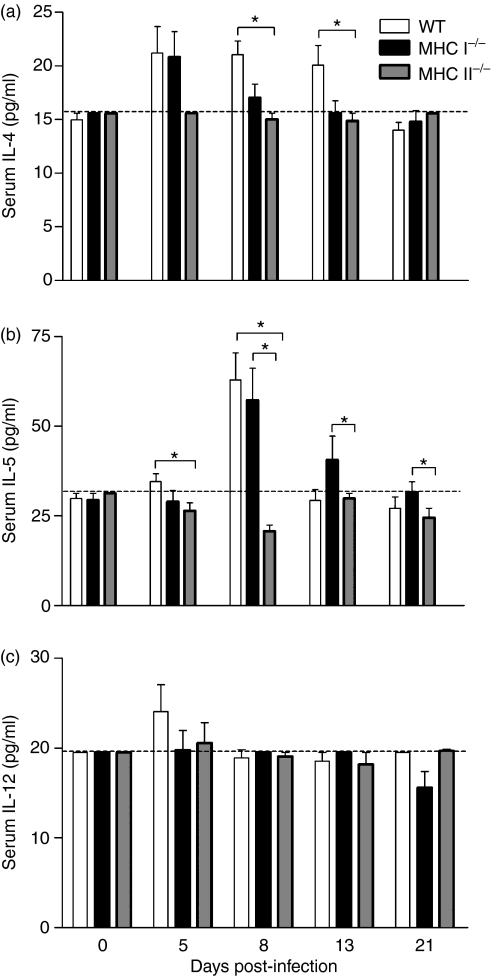
In order to evaluate the systemic consequences of S. venezuelensis infection in immunocompetent and compromised animals, cytokine concentrations were also measured in mice sera. Similarly to IL-4 detected in the intestine homogenates, the concentration of IL-4 in WT sera exhibited a significant increase on days 8 and 13 p.i., compared with MHC II−/− mice (Fig. 5a). Moreover, at 5 days p.i., the concentration of IL-5 was significantly higher in WT than in MHC II−/− infected sera. The concentration of IL-5 exhibited a significant increase in WT and MHC I−/− mice compared with MHC II−/− mice at 8 days p.i. On days 13 and 21 p.i., MHC I−/− animals exhibited higher levels of IL-5 compared with MHC II−/− mice (Fig. 5b). We did not find any significant difference in IL-12 levels among the groups studied, which presented similar production of this cytokine at all the time-periods evaluated (Fig. 5c). Additionally, the concentration of IFN-γ was below the limit of detection in the sera of all infected animals analysed (data not shown).
Figure 5.
Levels of interleukin (IL)-4 (a), IL-5 (b) and IL-12 (c) determined by enzyme-linked immunosorbent assay (ELISA) in the sera of wild-type (WT), major histocompatibility complex (MHC) I−/− and MHC II−/− mice, after subcutaneous (s.c.) infection with 3000 Strongyloides venezuelensis L3 larvae. Analyses were performed at 0, 5, 8, 13 and 21 days post-infection. Dashed lines indicate the detection limit for the cytokines. Data are expressed as mean ± standard error of the mean (SEM) (n = 6). Results are representative of two or three independent experiments. *P < 0·05.
Production of antibodies is reduced in the sera of S. venezuelensis-infected MHC II−/− mice
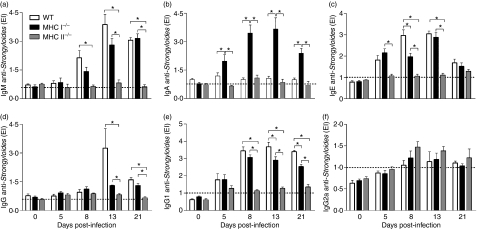
To evaluate the humoral response against S. venezuelensis, parasite-specific IgM, IgA, IgE, total IgG, IgG1 and IgG2a antibodies were measured in the sera of infected mice on days 5, 8, 13 and 21 p.i., and compared with values obtained in controls (day 0). Parasite-specific IgM was significantly higher in WT sera than in MHC II−/− sera on days 8, 13 and 21 p.i., and was also significantly higher in MHC I−/− sera than in MHC II−/− sera at 13 and 21 days p.i. (Fig. 6a). Interestingly, there was a greater increase in the levels of parasite-specific IgA in the sera of MHC I−/− mice compared with WT or MHC II−/− animals on days 5, 8, 13 and 21 p.i. (Fig. 6b).
Figure 6.
Levels of specific immunoglobulin M (IgM) (a), IgA (b), IgE (c), total IgG (d), IgG1 (e) and IgG2a (f) in serum samples of wild-type (WT), major histocompatibility complex (MHC) I−/− and MHC II−/− mice, after subcutaneous (s.c.) infection with 3000 Strongyloides venezuelensis L3 larvae. The results are expressed as enzyme-linked immunosorbent assay (ELISA) index (EI) values (values of EI > 1·0 are considered positive; horizontal dashed line) and are given as mean ± standard error of the mean (SEM) (n = 6). Results are representative of two or three independent experiments. *P < 0·05.
Levels of parasite-specific IgE in the sera of WT and MHC I−/− infected mice increased significantly on days 5, 8 and 13 p.i., and on days 8 and 13 p.i. there was a significant difference between WT and MHC II−/− animals (Fig. 6c). Moreover, we observed elevated levels of IgE in MHC I−/− sera compared with MHC II−/− sera on days 5, 8 and 13 p.i. Regarding total IgG, WT and MHC I−/− infected mice presented significantly higher levels than MHC II−/− mice on days 13 and 21 p.i. (Fig. 6d). Parasite-specific IgG1 from MHC II−/− infected mice was also significantly lower than the values found in the other animals on days 8, 13 and 21 p.i. (Fig. 6e) and a significant increase was also observed in WT sera compared with MHC I−/− sera at 13 and 21 days p.i. A non-significant increase in IgG2a levels was detected in the sera of MHC II−/− infected mice, on days 8, 13 and 21 p.i., although differences relative to the other groups were not statistically significant (Fig. 6f).
Discussion
Although it is well established that Th2 cytokine responses are related to host protection against gastrointestinal nematodes,25–30 in Strongyloides experimental infection the role of MHC class I and II molecules in mediating worm elimination and host protection has not been clarified. Studies with other experimental nematode infections, for example Brugia malayai,31Nippostrongylus brasiliensis32 and Angiostrongylus costaricensis,33 demonstrated the role of MHC class II molecules in the induction of the Th2 response during infection.
Here, we demonstrated that intestinal establishment of S. venezuelensis was affected by the absence of MHC class II molecules during parasite infection. Moreover, MHC II−/− infected mice exhibited higher susceptibility to S. venezuelensis, with impaired responses to the worm, suggesting that the failure of MHC class II expression affects the antigen presentation to CD4+ T cells that is necessary for the initiation of an effective Th2 response against this parasite. In contrast, MHC I−/− mice presented an immune response against the parasite similar to that of WT mice. These findings are in agreement with those of previous studies, which showed that proteins secreted by N. brasiliensis drive specific IL-4 production, essentially confined to the CD4+ T-cell population,15 and that without CD4 ligation to MHC II molecules the response to N. brasiliensis infection is absent.14 In addition, MHC I−/− animals infected with N. brasiliensis presented an intact Th2 response.19
In human hosts and experimental models, infection with Strongyloides sp. is characterized by tissue eosinophilia5,6 and intestinal mastocytosis,4,7–9,34 with production of Th2-type antibodies 35,36 and cytokines.34,37 In the present study, for the first time, the roles of MHC I and MHC II molecules were investigated in S. venezuelensis infection in mice. We found that S. venezuelensis induced mild inflammatory changes in the small intestines of MHC II−/− infected mice, accompanied by reduced eosinophil counts. This strongly suggested that the lack of MHC class II expression may have accounted for the delayed intestinal elimination of the worms by reducing eosinophil accumulation and the local inflammatory response, as it is known that eosinophils play a role in the killing of this nematode.5,37,38 Moreover, eosinophils can also express MHC class II molecules,31 and the exact function of these molecules on eosinophils as well as their role in the regulation of CD4+ T-cell responses to helminths are still not known.
Infection with S. venezuelensis induced an intestinal Th2 response in WT mice. Although Th1 cytokines were predominantly observed at 8 days p.i., the parasite also induced a notable increase in IL-4 and IL-5 at the same time, and this was accompanied by eosinophil accumulation in the gut together with the peak of parasite recovery. Interestingly, MHC I−/− mice presented a Th2 response similar to that seen in WT mice, but with lower tissue levels of cytokine production. In contrast, MHC II−/− infected mice had overall reduced levels of cytokines, even in the absence of worm infection, demonstrating that these animals presented a global defect in cytokine production, with consequent failure in mounting effective immune responses. In fact, this defective response resulted in increased parasitism and a delayed elimination of adult worms. In agreement with these results, MHC II molecule activation has been demonstrated to drive specific Th2 cytokine production in helminth infection,14,31 inducing the differentiation and activation of basophils and mast cells,39,40 as well as stimulating B cells to produce specific IgG1 and IgE,27,41 which are required to expel gastrointestinal nematodes.20,42
The reduction in leucocyte recruitment to the blood (data not shown) accompanied by a low frequency of eosinophils and mononuclear cells in MHC II−/−S. venezuelensis-infected mice coincided with the peak in egg numbers in faeces and increased worm fecundity. Consistent with these results, a previous study showed that platelet-activating factor receptor-deficient (PAFR−/−) mice infected with S. venezuelensis presented reduced numbers of circulating leucocytes and eosinophils, coinciding with a delay in the elimination of adult worms and decrease in worm fecundity compared with infected WT controls.21
In this study, cytokine production was also evaluated in the sera of infected animals, and the results suggested that there was an increase in IL-5 just before the onset of an increase in the numbers of eosinophils in the blood of WT infected mice. This is in accordance with the facts that IL-5 induces eosinophil recruitment from the bone marrow to the blood43 and that these cells are involved in protective immunity against migrating larvae in tissues.6 As described above, MHC II−/− mice also exhibited an incapacity to mount effective systemic cytokine responses, while MHC I molecules did not appear to influence cytokine production, in particular that of IL-5, in response to S. venezuelensis, although reduced frequencies of circulating eosinophils were also found in these animals in the late response to the infection. It is possible that other immune mechanisms dependent on MHC I molecules, in addition to IL-5 production, are necessary to recruit eosinophils in these mice. Moreover, previous studies demonstrated that CD8-deficient hosts, in addition to having high serum IL-5 levels, show accumulation of eosinophils in the lymph nodes,44 suggesting that these cells may accumulate in other sites.
Immunity against S. stercoralis larvae has been shown to be dependent not only on eosinophil recruitment45,46 but also on immunoglobulin production and complement activation.47,48 As described here, together with increased production of Th2 cytokines, the production of parasite-specific antibodies seemed to be essential to induce a protective immune response mediating worm expulsion. These results support the hypothesis that class switching from IgM to IgG (and also to IgA or IgE) isotypes requires cognate interactions between CD4+ T cells and antigen-specific B cells,49 while the absence of MHC class II molecules accounted for the failure of the immune response mediated by these antibodies in the deficient mice. Interestingly, there was a significant increase in parasite-specific IgA in the sera of MHC I−/− infected mice, although the reasons for such an increase are still not known. Moreover, in the absence of Th2 cytokines, there is no induction of B-cell activation and differentiation into IgA-secreting plasma cells,50 as observed in the present experiments in MHC II−/− mice. In addition, our results indicated an elevated production of parasite-specific IgE and IgG1 in WT and MHC I−/− infected mice, in contrast to MHC II−/− animals, which presented lower levels of IgE and IgG1 and a non-significant IgG2a elevation. These results demonstrated that Strongyloides infection induces a predominantly Th2-type antibody response that contributes to the process of adult worm elimination, while in the absence of MHC class II molecules a tendency towards Th1 antibody production is observed. This is in accordance with the results of previous studies showing roles for IgG1 and IgE in S. venezuelensis elimination.20,51
Our results indicate that expression of MHC class II molecules is essential to the induction of protective Th2 immunity in S. venezuelensis elimination. The finding that MHC I−/− infected mice responded similarly to WT animals suggested that, as expected, immunosuppression through the depletion of CD8+ T cells has little effect on nematode control.17 In contrast, we have provided strong evidence to support a role for class II molecules in the development and function of CD4+ T cells, which are necessary for the generation of an effective immune response against Strongyloides infection.
Finally, we conclude that MHC class II but not MHC I molecules are required for the efficient control of S. venezuelensis infection in mice.
Acknowledgments
We are grateful to the technicians Antônio Tomaz Júnior from the Laboratório de Imunologia, Universidade Federal de Uberlândia (for helping in mouse infection), Maria do Rosário de Fátima Gonçalves Pires from the Laboratório de Parasitologia, Universidade Federal de Uberlândia (for helping with the preparation of S. venezuelensis alkaline extracts), João Batista A. Oliveira from the Departamento de Parasitologia, Universidade Estadual de Campinas (for helping with the maintenance and infection of the mice) and Richard Átila de Sousa from Laboratório de Histologia da Universidade Federal de Uberlândia (for helping with the formulation of histological preparations). This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- 1.Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004;26:487–97. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 2.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–7. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 4.Abe T, Nawa Y. Kinetic study of mast-cell growth factor production by lymphocytes during the course of Strongyloides ratti infection in mice. Parasitol Res. 1988;74:484–8. doi: 10.1007/BF00535150. [DOI] [PubMed] [Google Scholar]
- 5.El-Malky M, Maruyama H, Hirabayashi Y, et al. Intraepithelial infiltration of eosinophils and their contribution to the elimination of adult intestinal nematode, Strongyloides venezuelensis in mice. Parasitol Int. 2003;52:71–9. doi: 10.1016/s1383-5769(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 6.Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991;72:502–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Nawa Y, Ishikawa N, Tsuchiya K, et al. Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol. 1994;16:333–8. doi: 10.1111/j.1365-3024.1994.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 8.Onah DN, Nawa Y. Mucosal immunity against parasitic gastrointestinal nematodes. Korean J Parasitol. 2000;38:209–36. doi: 10.3347/kjp.2000.38.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol. 1993;23:551–5. doi: 10.1016/0020-7519(93)90159-v. [DOI] [PubMed] [Google Scholar]
- 10.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–43. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce EL, Shedlock DJ, Shen H. Functional characterization of MHC class II-restricted CD8+CD4− and CD8−CD4− T cell responses to infection in CD4−/− mice. J Immunol. 2004;173:2494–9. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 12.Martinic MM, van den Broek MF, Rulicke T, et al. Functional CD8+ but not CD4+ T cell responses develop independent of thymic epithelial MHC. Proc Natl Acad Sci USA. 2006;103:14435–40. doi: 10.1073/pnas.0606707103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angyalosi G, Neveu R, Wolowczuk I, Delanoye A, Herno J, Auriault C, Pancre V. HLA class II polymorphism influences onset and severity of pathology in Schistosoma mansoni-infected transgenic mice. Infect Immun. 2001;69:5874–82. doi: 10.1128/IAI.69.9.5874-5882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowell DJ, Magram J, Turck CW, Killeen N, Locksley RM. Impaired Th2 subset development in the absence of CD4. Immunity. 1997;6:559–69. doi: 10.1016/s1074-7613(00)80344-1. [DOI] [PubMed] [Google Scholar]
- 15.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–87. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Urban JF, Jr, Noben-Trauth N, Schopf L, Madden KB, Finkelman FD. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol. 2001;167:6078–81. doi: 10.4049/jimmunol.167.11.6078. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda S, Uchikawa R, Yamada M, Arizono N. Differentially enhanced cytokine gene expression in CD4+ and CD8+ T cells in mesenteric lymph nodes in rats infected with Nippostrongylus brasiliensis. Parasite Immunol. 1999;21:527–34. doi: 10.1046/j.1365-3024.1999.00252.x. [DOI] [PubMed] [Google Scholar]
- 18.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 19.Brown DR, Fowell DJ, Corry DB, Wynn TA, Moskowitz NH, Cheever AW, Locksley RM, Reiner SL. Beta 2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado ER, Ueta MT, Lourenco EV, et al. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol. 2005;175:3892–9. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- 21.Negrão-Corrêa D, Souza DG, Pinho V, Barsante MM, Souza AL, Teixeira MM. Platelet-activating factor receptor deficiency delays elimination of adult worms but reduces fecundity in Strongyloides venezuelensis-infected mice. Infect Immun. 2004;72:1135–42. doi: 10.1128/IAI.72.2.1135-1142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welter A, Mineo JR, Silva DA, Lourenco EV, Ferro EA, Roque-Barreira MC, da Silva NM. An opposite role is exerted by the acarian Myocoptes musculinus in the outcome of Toxoplasma gondii infection according to the route of the protozoa inoculation. Microbes Infect. 2006;8:2618–28. doi: 10.1016/j.micinf.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Machado ER, Ueta MT, de Fatima Goncalves-Pires Mdo R, Alves de Oliveira JB, Faccioli LH, Costa-Cruz JM. Strongyloides venezuelensis alkaline extract for the diagnosis of human strongyloidiasis by enzyme-linked immunosorbent assay. Mem Inst Oswaldo Cruz. 2003;98:849–51. doi: 10.1590/s0074-02762003000600024. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 25.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–61. [PubMed] [Google Scholar]
- 26.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–51. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 28.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–77. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence CE, Paterson JC, Higgins LM, MacDonald TT, Kennedy MW, Garside P. IL-4-regulated enteropathy in an intestinal nematode infection. Eur J Immunol. 1998;28:2672–84. doi: 10.1002/(SICI)1521-4141(199809)28:09<2672::AID-IMMU2672>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie GJ, Emson CL, Bell SE, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 31.Mawhorter SD, Pearlman E, Kazura JW, Boom WH. Class II major histocompatibility complex molecule expression on murine eosinophils activated in vivo by Brugia malayi. Infect Immun. 1993;61:5410–2. doi: 10.1128/iai.61.12.5410-5412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland MJ, Harcus YM, Balic A, Maizels RM. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC class I-related receptors. Immunol Lett. 2005;96:93–101. doi: 10.1016/j.imlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Geiger SM, Hoffmann WH, Soboslay PT, Pfaff AW, Graeff-Teixeira C, Schulz-Key H. Angiostrongylus costaricensis infection in C57BL/6 mice: MHC-II deficiency results in increased larval elimination but unaltered mortality. Parasitol Res. 2003;90:415–20. doi: 10.1007/s00436-003-0853-2. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Tsuchiya K, Hara T, Nakahata T, Kurokawa M, Ishiwata K, Uchiyama F, Nawa Y. Intestinal mast cell response and mucosal defence against Strongyloides venezuelensis in interleukin-3-hyporesponsive mice. Parasite Immunol. 1998;20:279–84. doi: 10.1046/j.1365-3024.1998.00142.x. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues RM, Sopelete MC, Silva DA, Cunha-Junior JP, Taketomi EA, Costa-Cruz JM. Strongyloides ratti antigenic components recognized by IgE antibodies in immunoblotting as an additional tool for improving the immunodiagnosis in human strongyloidiasis. Mem Inst Oswaldo Cruz. 2004;99:89–93. doi: 10.1590/s0074-02762004000100016. [DOI] [PubMed] [Google Scholar]
- 36.Rossi CL, Takahashi EE, Partel CD, Teodoro LG, da Silva LJ. Total serum IgE and parasite-specific IgG and IgA antibodies in human strongyloidiasis. Rev Inst Med Trop Sao Paulo. 1993;35:361–5. doi: 10.1590/s0036-46651993000400010. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama H, Yabu Y, Yoshida A, Nawa Y, Ohta N. A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J Immunol. 2000;164:3749–54. doi: 10.4049/jimmunol.164.7.3749. [DOI] [PubMed] [Google Scholar]
- 38.Ovington KS, McKie K, Matthaei KI, Young IG, Behm CA. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology. 1998;95:488–93. doi: 10.1046/j.1365-2567.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–45. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 40.Thienemann F, Henz BM, Babina M. Regulation of mast cell characteristics by cytokines: divergent effects of interleukin-4 on immature mast cell lines versus mature human skin mast cells. Arch Dermatol Res. 2004;296:134–8. doi: 10.1007/s00403-004-0486-z. [DOI] [PubMed] [Google Scholar]
- 41.Faquim-Mauro EL, Coffman RL, Abrahamsohn IA, Macedo MS. Cutting edge: mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. 1999;163:3572–6. [PubMed] [Google Scholar]
- 42.Negrão-Corrêa D, Pinho V, Souza DG, Pereira AT, Fernandes A, Scheuermann K, Souza AL, Teixeira MM. Expression of IL-4 receptor on non-bone marrow-derived cells is necessary for the timely elimination of Strongyloides venezuelensis in mice, but not for intestinal IL-4 production. Int J Parasitol. 2006;36:1185–95. doi: 10.1016/j.ijpara.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–7. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 44.de Lavareille A, Prigogine C, Paulart F, et al. Regulatory role of host CD8+ T lymphocytes in experimental graft-versus-host disease across a single major histocompatibility complex class II incompatibility. Transplantation. 2005;80:1293–9. doi: 10.1097/01.tp.0000178380.85521.75. [DOI] [PubMed] [Google Scholar]
- 45.Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2000;165:4544–51. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 46.Rotman HL, Yutanawiboonchai W, Brigandi RA, Leon O, Gleich GJ, Nolan TJ, Schad GA, Abraham D. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol. 1996;82:267–78. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 47.Kerepesi LA, Hess JA, Leon O, Nolan TJ, Schad GA, Abraham D. Toll-like receptor 4 (TLR4) is required for protective immunity to larval Strongyloides stercoralis in mice. Microbes Infect. 2007;9:28–34. doi: 10.1016/j.micinf.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Ligas JA, Kerepesi LA, Galioto AM, Lustigman S, Nolan TJ, Schad GA, Abraham D. Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2003;71:6835–43. doi: 10.1128/IAI.71.12.6835-6843.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macaulay AE, DeKruyff RH, Goodnow CC, Umetsu DT. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997;158:4171–9. [PubMed] [Google Scholar]
- 50.van Ginkel FW, Wahl SM, Kearney JF, Kweon MN, Fujihashi K, Burrows PD, Kiyono H, McGhee JR. Partial IgA-deficiency with increased Th2-type cytokines in TGF-beta 1 knockout mice. J Immunol. 1999;163:1951–7. [PubMed] [Google Scholar]
- 51.Fernandes A, Pereira AT, Eschenazi PD, Schilter HC, Sousa AL, Teixeira MM, Negrão-Corrêa D. Evaluation of the immune response against Strongyloides venezuelensis in antigen-immunized or previously infected mice. Parasite Immunol. 2008;30:139–49. doi: 10.1111/j.1365-3024.2007.01009.x. [DOI] [PubMed] [Google Scholar]