Abstract
Human haematopoietic progenitor/stem cells (HPCs) differentiate into functional T cells in the thymus through a series of checkpoints. A convenient in vitro system will greatly facilitate the understanding of T-cell development and future engineering of therapeutic T cells. In this report, we established a lentiviral vector-engineered stromal cell line (LSC) expressing the key lymphopoiesis regulator Notch ligand, Delta-like 1 (DL1), as feeder cells (LSC-mDL1) supplemented with Flt3 ligand (fms-like tyrosine kinase 3, Flt3L or FL) and interleukin-7 for the development of T cells from CD34+ HPCs. We demonstrated T-cell development from human HPCs with various origins including fetal thymus (FT), fetal liver (FL), cord blood (CB) and adult bone marrow (BM). The CD34+ HPCs from FT, FL and adult BM expanded more than 100-fold before reaching the β-selection and CD4/CD8 double-positive T-cell stage. The CB HPCs, on the other hand, expanded more than 1000-fold before β-selection. Furthermore, the time required to reach β-selection differed for the various HPCs, 7 days for FT, 14 days for FL and CB, and 35 days for adult BM. Nevertheless, all of the T cells developed in vitro were stalled at the double-positive or immature single-positive stage with the exception that some CB-derived T cells arrived at a positive selection stage. Consequently, the LSC-mDL1 culture system illustrated diverse T-cell development potentials of pre- and post-natal and adult human BM HPCs. However, further modification of this in vitro T-cell development system is necessary to attain fully functional T cells.
Keywords: bone marrow, cord blood, Delta-like 1, fetal tissue, lentiviral vector, lymphoid differentiation, T cells
Introduction
Haematopoietic precursor cells from bone marrow migrate to the thymus, where they undergo a series of lineage commitment events and developmental checkpoints before adopting a T-cell fate. A common in vivo model for the study of human T-cell development is based on humanized severe combined immunodeficiency mice.1,2 However, these mice are not convenient for the evaluation of molecular signalling pathways or of specific cell lineages over time. An alternative in vitro system to study T-cell differentiation is based on fetal thymus organ culture.3–7 In this model, the thymic lobes are depleted of endogenous thymocytes by deoxyguanosine treatment and reconstituted with progenitor cells of interest to assess thymocyte development. Fetal thymus organ culture can support the differentiation of haematopoietic stem/progenitor cells (HPCs) to CD8+ and CD4+ T lymphocytes.5 However, it faces numerous challenges including being cumbersome to set up and having a limited cellular yield.
The strong evidence that Notch signalling directs the fate of T cells rather than B cells has led to the establishment of a mouse OP9 stromal cell line expressing the Notch ligand Delta-like 1 (DL1) for the study of T-cell development.8–11 The OP9 cells lack macrophage colony-stimulating factor and can support B-cell differentiation from bone marrow (BM)-derived HPCs. The OP9 cells transduced with retroviral DL1 (OP9DL1) support T-cell differentiation from HPCs of murine origin,9,11 and similar results have been reported with human cord blood (CB) and adolescent BM, although with limited T-cell maturation potential.9,12–14 There have been reported differences in lymphopoiesis of murine fetal and adult origin,15 yet, comparable studies for human T-cell development are still lacking.
Here we used a lentiviral DL1-modified OP9 cell line (LSC-mDL1) for the study of thymopoiesis of human fetal thymus (FT), fetal liver (FL), CB and adult BM. The HPCs first differentiate into CD8− CD4− double-negative (DN) T cells, and undergo T-cell receptor (TCR) β gene rearrangement, the β-selection checkpoint, then transition to CD8+ CD4+ double-positive (DP) stage, and finally they mature after positive and negative selections (reviewed in ref. 16). We report that HPCs derived from human FL, FT, CB and adult BM go through the established T-cell differentiation pathway under LSC-mDL1 in vitro culture conditions. However, striking differences exist in timing and lineage commitment potential of T-cell development for human HPCs with different origins. These include proliferation, survival and maturation kinetics such as the ability to reach the TCR αβ+ CD3+ CD8+ CD4+ stage of T cells. The in vitro system is useful to delineate the role of various T-cell development regulators and is a convenient tool for the engineering of therapeutic T cells.
Materials and methods
Cells
OP9 cells were purchased from American Type Culture Collection (Manassas, VA). To establish LSC-mDL1 and LSC-GFP cell lines, OP9 cells were transduced with lentiviral vectors encoding mouse DL1 (mDL1) and green fluorescent protein (GFP), respectively. OP9 cells and its derived cell lines were maintained in α-minimal essential medium (Gibco, Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum and 1% penicillin–streptomycin. The adult BM and CB CD34+ cells were purchased from AllCells, LLC (Emeryville, CA), and Cambrex Corp. (Baltimore, MD) Aborted fetal liver and thymus tissues were purchased from Advanced Bioscience Resources Inc., (Alameda, CA) and processed with a tissue grinder and pressed through cell dissociation mesh sieves (Sigma, St Louis, MO). Single cell suspensions were labelled with anti-CD34 microbeads and purified using magnetic antibody cell sorting columns (Miltenyi Biotech Inc., Auburn, CA). The purity was determined using phycoerythrin-conjugated anti-CD34 (Clone 8G12; BD Biosciences, San Diego, CA) and analysed with FACSaria.
In vitro T-cell development
The purified CD34+ progenitors were seeded at 2 × 104 cells per well into 24-well plates containing a confluent monolayer of LSC-mDL1 or LSC-GFP cells. The cocultures were maintained in α-minimal essential medium with 20% fetal bovine serum, 1% penicillin–streptomycin, 5 ng/ml interleukin-7 (PeproTech Inc., Rocky Hill, NJ) and 5 ng/ml Flt3L (PeproTech, Inc.) and were fed with complete medium every 2–3 days. The coculture was transferred to a new well once the monolayer became overdifferentiated. Cells were harvested at the indicated time-points for analysis.
Lentiviral vector construction and transduction
Lentiviral vectors were generated with the NHP/TYF lentiviral vector system as previously described.17,18 Mouse DL1 complementary DNA (cDNA) was cloned into pTYF transducing vector behind a strong human EF1α promoter. Mouse DL1 cDNA was amplified using primers flanking the DL1 open reading frame containing an optimized initiation codon sequence (-CCACCAUG-), with the forward primer sequence AAG GAT CCA CCA TGG GCC GTC GGA GCG CGC and the reverse primer sequence AAA CTA GTT ACA CCT CAG TCG CTA TAA CAC ACT. OP9 cells were plated into 24-well dishes and transduced with pTYF-mDL1 or pTYF-GFP lentivirus at a multiplicity of infection of 10–100. The transduced cells were continuously propagated and confirmed for transgene expression. The expression of lentiviral transgene in OP9 cells is stable for more than 50 passages.
RNA extraction and quantitative reverse transcription–polymerase chain reaction
RNA was extracted with the Tri-reagent (MRC Inc., Cincinnati, OH) and oligo-dT (15-mer)-primed cDNA was made with Moloney murine leukaemia virus reverse transcriptase (Promega Corp., Madison, WI). Expression of mDL1 was determined by both semi-quantitative and real-time polymerase chain reaction (PCR). For the semi-quantitative PCR, all PCR amplifications used the same serially diluted cDNA normalized to mouse glyceraldehyde 3-phosphate dehydrogenase (mGAPDH). The PCR amplification conditions were as follows: denaturing temperature, 95°; annealing temperature, 55°; extension temperature, 72°; the amplification cycles were 25 cycles for mGAPDH, and 35 cycles for mDL1. Products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. For the real-time PCR, the reactions were performed using the QuantiTech SYBR green PCR kit (Qiagen Inc., Valencia, CA) and analysed with the Mx3000P QPCR system (Stratagene, San Diego, CA). For data analysis, standard curves were plotted for both mGAPDH and mDL1 primer sets with a 10-fold serial dilution of a positive sample. The Ct values were then converted to the relative cDNA amount based on the standard curve. To correct for the different inputs among samples, results were then normalized to equivalent levels of mGAPDH. Primer sequences were as follows (5′–3′): mGAPDH forward primer, TCA CCA CCA TGG AGA AGG C, and reverse primer, GCT AAG CAG TTG GTG GTG CA; mDL1 forward primer, GCT CTT CCC CTT GTT CTA ACG, and reverse primer, CAC ATT GTC CTC GCA GTACC.
Flow cytometry
Antibodies for CD4 [clone RPA-T4, conjugated with phycoerythrin (PE) and fluorescein isothiocyanate (FITC)], CD8 (clone RPA-T8, PE), CD7 (clone M-T701, FITC, PE), CD1a [clone HI149, with allophycocyanin (APC)], CD3 (clone SK7, PE-Cy7), TCR-αβ (clone T10B9.1A-31, FITC) and TCR-γδ (clone B1, FITC) were obtained from BD Biosciences. The antibody for CD28 (clone CD28.2, APC) was from eBioscience (San Diego, CA). Cells were first washed with phosphate-buffered saline with 2% fetal bovine serum and blocked with mouse and human serum at 4° for 30 min. For each antibody staining, cells were incubated with antibodies as described by the manufacturer’s instructions. Data were acquired using FACSCalibur and cellquest software (Becton Dickinson Immunocytometry Systems, San Diego, CA) and flowjo software (Tree Star Inc., Ashland, OR).
Results
Supraphysiological expression of DL1 in lentiviral vector-modified stromal cells (LSC-mDL1)
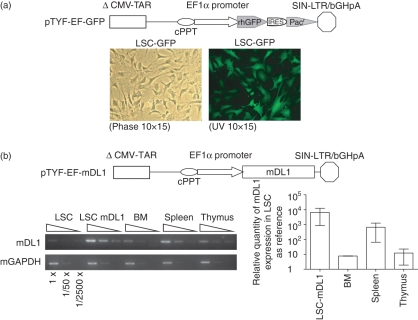
Murine OP9 cells transduced with an oncoretroviral vector expressing DL1 have been shown to support T-cell development.9 We have previously reported that lentiviral vectors mediate high levels of transgene expression.19 To generate cell lines expressing high levels of DL1, we transduced OP9 with a control GFP gene (LSC-GFP) or the mouse DL1 gene (LSC-mDL1). The OP9 cells expressed high levels of GFP after lentiviral transduction (Fig. 1a). The expression of mDL1 in LSC-mDL1 was compared to the native mDL1 expression in different mouse lymphoid organs by reverse transcription PCR (Fig. 1b). The results showed that LSC-mDL1 expressed markedly increased levels of mDL1 compared with mouse BM, spleen and thymus. The expression of mDL1 was approximately 10 000-fold higher in LSC-mDL1 than in control OP9 cells (Fig. 1b).
Figure 1.
Stromal cells transduced with lentiviral vectors expressing green fluorescent protein (GFP) and mouse Delta-like 1 (mDL1). (a) Lentiviral vector construct expressing recombinant humanized GFP reporter gene (rhGFP) and transduced OP9 stromal cells. The diagram illustrates a self-inactivating bicistronic lentiviral vector TYF-EF-rhGFP-Pac expressing GFP and a puromycin-resistant gene under the human EF1α promoter control. LSC-GFP cell line expressed GFP at near 100% efficiency. (b) Quantitative analysis of mDL1 expression in LSC-mDL1. The lentiviral vector construct expressing mDL1 is illustrated. The expression of mDL1 in LSC-mDL1 cells was compared with control LSC cells (LSC-GFP), mouse bone marrow, spleen and thymus. Semi-quantitative reverse transcription–polymerase chain reaction (RT-PCR) gel analysis is shown to the left and real-time RT-PCR to the right with control lentiviral vector-engineered stromal cell line (LSC) set as 1.
Differential proliferation and survival potentials of CD34+ HPCs of FT, FL, CB and adult BM on LSC-mDL1
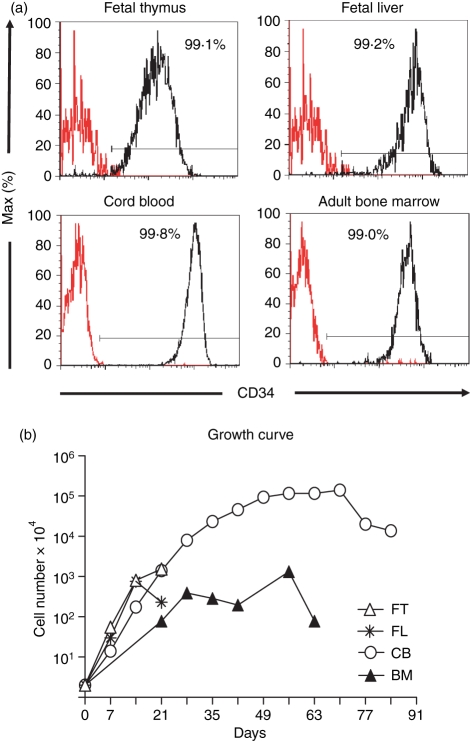
To see if LSC-mDL1 could support T-cell development, CD34+ cells were purified from human FT, FL, CB and adult BM. The four sources of CD34+ HPCs showed a purity of > 99%, as determined by post-sort flow cytometry analysis (Fig. 2a). Purified CD34+ cells were cocultured with LSC-GFP or LSC-mDL1 stromal cells in the presence of recombinant interleukin-7 and Flt3L. The HPCs cocultured with LSC-GFP showed very limited proliferation and a short survival period (data not shown). In contrast, HPCs cocultured with LSC-mDL1 exhibited exponential proliferation and prolonged survival (Fig. 2b). This suggests that Notch signalling not only promotes T-lineage commitment, but also supports progenitor cell survival. CD34+ cells derived from FT and FL displayed similar proliferation and survival kinetics on LSC-mDL1, with an approximately 1000-fold increase in cell number in 2 weeks, followed by a decrease in proliferation and cell death after 3 weeks. The CB-derived CD34+ cells expanded about 100 000-fold and survived for about 90 days on LSC-mDL1 (Fig. 2b), 100 times more than that reported on the oncoretroviral vector-transduced OP9mDL1.14 The adult BM-derived HPCs showed < 1000-fold increase in cell number, which was slightly lower than FT-derived and FL-derived HPCs, and significantly lower than CB-derived HPCs. The BM-derived HPCs survived for longer than those from FT and FL and for a shorter time than those from CB on LSC-mDL1. Therefore, the CB-derived HPCs had the most expansion and survival potential when compared with FT, FL and adult BM in LSC-mDL1 coculture.
Figure 2.
Proliferation and survival potential of developing T cells from human fetal thymus (FT), fetal liver (FL), cord blood (CB) and adult bone marrow (BM) CD34+ haematopoietic progenitor/stem cells (HPCs) on LSC-mDL1. (a) Analysis of CD34 expression. The starting HPCs were purified with anti-CD34 antibody magnetic affinity columns and confirmed by flow cytometry to contain ≥ 99% CD34+ cells. (b) Growth kinetics of developing T cells on LSC-mDL1. The CD34+ HPCs derived from FT, FL, CB and BM were cultured on LSC-mDL1 supplemented with interleukin-7 and Flt3L and representative growth kinetics of three independent experiments are shown.
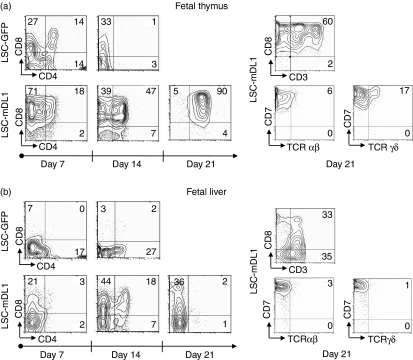
Rapid differentiation of FT-derived and FL-derived HPCs to CD8/CD4 DP cells on LSC-mDL1
The FL is a primary site of haematopoietic development until birth.20 T-cell differentiation has been illustrated using HPCs isolated from mouse FL.9 However, the T-cell development potential of human FT or FL in a stromal cell-based culture system has not been demonstrated. Here we report for the first time that HPCs of human FT and FL could develop into T cells on LSC-mDL1 in vitro (Fig. 3). The FT-derived CD34+ cells were able to rapidly differentiate into CD8/CD4 DP cells after just 1 week of coculture with LSC-mDL1 (Fig. 3a). The number of DP cells increased over time and peaked at 3 weeks. About 90% of the cells were arrested in the DP stage on day 21 and did not differentiate further (Fig. 3a). These DP cells did not survive beyond 3 weeks and the population collapsed after day 21 of the coculture (Fig. 2b). Around 60% of the CD8+ cells expressed CD3; however, the expression of fully assembled TCR-αβ heterodimers was only a marginal 6%. TCR-γδ expression was slightly higher, about 17% (Fig. 3, right panel). As the TCR-αβ antibody was specific for a monomorphic determinant of TCR-αβ heterodimer, only the fully assembled TCR-αβ surface molecules were detected (see Fig. 7 illustration). All of the cells expressed high levels of CD7, a receptor expressed in early T cells (data not shown). Our results indicate that the FT-derived CD34+ HPCs rapidly differentiated into and then arrested at DP stage when cultured on LSC-mDL1. In addition, these cells could differentiate into both γδ and αβ T cells, with an inclination towards the γδ lineage.
Figure 3.
Kinetic and phenotype analyses of differentiating T cells of human fetal thyroid thymus (FT) and fetal liver (FL) haematopoietic progenitor/stem cells (HPCs) on LSC-mDL1. The HPCs were cultured on LSC-mDL1 and control LSC-GFP cells under the same conditions. (a) T-cell surface marker analysis of human FT-derived HPCs on LSC-mDL1. (b) T-cell surface marker analysis of human FL-derived HPCs on LSC-mDL1.
Figure 7.
Illustration of quantitative and qualitative differences of T-cell development of four sources of human haematopoietic progenitor/stem cells (HPCs) on LSC-mDL1.
The differentiation kinetics of human FL HPCs on LSC-mDL1 was similar to that of their murine counterpart on the stromal cell line OP9DL1.9 However, the T-cell developmental kinetics of FL-derived HPCs differed from those of FT-derived HPCs. The FL HPCs developed to DP stage after 1 week but the majority of cells remained in CD8+ immature single-positive stage (ISP), and only about 18% of the cells progressed to DP stage by day 14 (Fig. 3b). The DP population vanished on day 21 in the culture, leaving a population of cells arrested in the CD8+ ISP stage (Fig. 3b). All of the differentiating FL precursors were CD7+ on day 21 (not shown). A low percentage of CD8+ cells coexpressed CD3, and only 3% expressed TCR-αβ, while 1% expressed TCR-γδ (Fig. 3b, right panel). Overall, FT and FL HPCs could rapidly differentiate to DP cells, with a very small percentage expressing TCR-αβ. In addition, FT-derived and FL-derived HPCs differed in their potential to adopt TCR-γδ lineage.
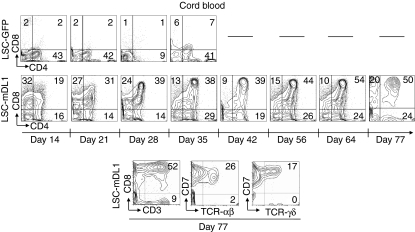
Robust proliferation, prolonged survival and rapid maturation of CB HPCs to DP T cells on LSC-mDL1
Human CB-derived HPCs can differentiate to TCR-αβ-bearing DP T cells on OP9DL1 stromal cells.13 On LSC-mDL1, we found that CB HPCs rapidly differentiated to DP stage with a marked increase in cell number (Figs 2b and 4). Cord blood CD34+ HPCs developed into DP stage in 2 weeks and remained in DP stage for approximately 70 days (Fig. 4), whereas the CD8+ ISP population gradually declined. The CB resembled FT in its loss of the CD8+ ISP population and accumulation of DP stage over time (Figs 3a and 4). The percentage of CD4+ ISP cells showed a slight increase, from 15 to 25%. The expression of CD3 and TCR-αβ peaked to 52% and 26%, respectively on day 56, suggesting a more mature phenotype (Fig. 4, bottom panel). Additionally, expression of TCR-γδ spiked to 17% on day 77, suggesting that CB progenitors can choose the αβ or γδ T-cell lineage pathway when cultured on LSC-mDL1. In summary, cord blood CD34+ HPCs rapidly developed into DP stage and advanced further, as shown by the expression of CD3, TCR-αβ, and TCR-γδ.
Figure 4.
Kinetic and phenotype analyses of differentiating T cells of human cord blood (CB) haematopoietic progenitor/stem cells (HPCs) on LSC-mDL1. The HPCs were cultured on LSC-mDL1 and control LSC-GFP cells under the same conditions.
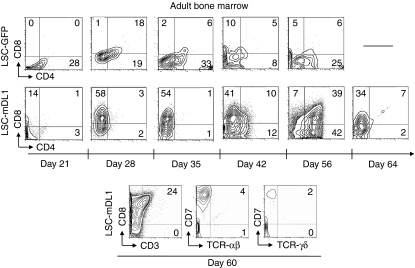
Prolonged survival but limited maturation potential of adult BM-derived CD34 HPCs on LSC-mDL1
In adult life, BM serves as a primary site of haematopoiesis. In vitro development of T cells from adult BM-derived HPCs in the stromal culture system has not been reported. Here, we examined the T-cell differentiation potential of adult BM CD34+ HPCs on LSC-mDL1. The BM HPC-derived T cells were able to survive for about 60 days and showed a delayed differentiation to DP stage compared to HPCs derived from FT, FL and CB (Fig. 5). However, the DP cells peaked around day 56 and were short-lived because they disappeared in just 1 week (Fig. 5a). The maximal expression of TCR-αβ was about 4% around day 60, suggesting that the majority of cells were arrested in the CD8+ ISP stage (Fig. 5, bottom panel). Similar to FL-derived HPCs, adult BM-derived HPCs did not differentiate toward the TCR-γδ lineage (Fig. 3, right panel). Hence, when cultured on LSC-mDL1, adult BM-derived HPCs developed to the DP stage with very limited potential of entering TCR-αβ and TCR-γδ lineages.
Figure 5.
Kinetic and phenotype analyses of differentiating T cells of human adult bone marrow (BM) haematopoietic progenitor/stem cells (HPCs) on LSC-mDL1. The HPCs were cultured on LSC-mDL1 and control LSC-GFP cells under the same condition.
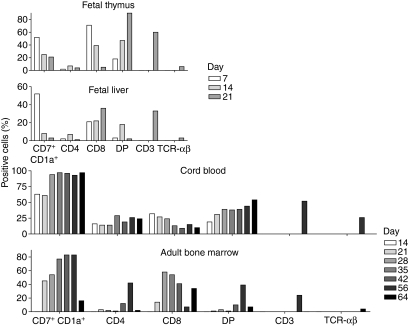
Diverse in vitro T-cell development potentials of HPCs derived from human FT, FL, CB and adult BM
We compared the T-cell development potential of four different sources of human CD34+ HPCs in the LSC-mDL1 coculture (Fig. 6). Both FT and CB resembled each other compared with FL and adult BM. The HPCs derived from FT and CB differentiated to the DP T-cell stage quickly, and the majority of cells remained arrested at the DP stage (Fig. 6a,c). Moreover, both FT and CB progenitors had the ability to differentiate into TCR-γδ T cells and expressed CD7 and CD1a at high levels. The CB-derived HPCs generated a significantly higher percentage of TCR-αβ-bearing T cells than did FT-derived HPCs. Furthermore, FT-derived HPCs exhibited much shorter differentiation kinetics and survival term (about 21 days) in the LSC-mDL1 coculture as opposed to CB cells that survived for about 70 days (Fig. 2b).
Figure 6.
Comparison of T-cell developmental kinetics of human fetal thymus (FT), fetal liver (FL), cord blood (CB) and adult bone marrow (BM) CD34+ haematopoietic progenitor/stem cells (HPCs) on LSC-mDL1. The different markers for T-cell development including CD7/CD1a double-positive, CD4, CD8, CD4/CD8 DP, CD3 and T-cell receptor (TCR)-αβ are depicted on the x-axis. The y-axis represents the percentage of positive cells at different time points of T-cell development indicated by the graded bars.
Adult BM-derived HPCs resembled FL-derived HPCs in their differentiation patterns; however, they differed in their survival ability in the LSC-mDL1 coculture (Figs 6 and 2b). Both precursors differentiated to short-lived DP cells, of which only 5–6% matured to TCR-αβ-bearing T cells (Fig. 6). The transition to DP stage from adult BM-derived HPCs occurred at a much later time-point than from FL-derived HPCs. Both precursors showed a decrease in CD7+ CD1a+ DP T cells before cell death, and both had an ending population of CD8+ ISP T cells, as opposed to FT-derived and CB-derived HPCs being arrested in the DP stage (Fig. 6a–d). These four sources of human haematopoietic progenitors differed in T-cell differentiation potential, with some similarities between CB and FT, and between FL and adult BM.
Discussion
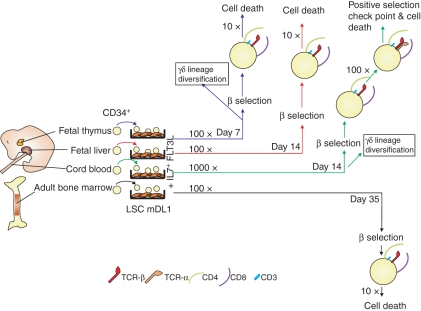
In vitro culture is a great tool for the study of the cellular mechanisms that mediate T-lymphocyte development. Here we establish a lentiviral-modified murine OP9 stromal cell culture system, LSC-mDL1, to study T-cell development, similar to the oncoretroviral OP9DL1 culture system.9 We found quantitative and qualitative differences in the T-cell development potential of four different sources of human CD34 HPCs on LSC-mDL1 (Fig. 7). Our finding is consistent with previous observations that the haematopoietic system has different developmental potentials in fetal and postnatal life.21,22
There are some differences in lymphoid development kinetics among the FT-, FL-, CB- and adult BM-derived HPCs. A striking difference was noted in the proliferation of the various HPCs before they reach the β-selection checkpoint. The CD8− CD4− DN T cells (DN3 stage) that have successfully rearranged their TCR-β chains are the only cells that can progress to the CD8+ CD4+ DP stage, a process called β-selection.23 The HPCs derived from FT, FL and adult BM were able to expand about 100-fold and the CB-derived HPCs expand about 1000-fold before they face the β-selection checkpoint (Fig. 7). Apparently, the intrinsic differences of these different sources of CD34+ progenitors contribute to their different expansion and developmental potentials. The FT-derived HPCs undergo β-selection within the first week of the coculture, followed by CB-derived and finally BM-derived HPCs. The robust expansion of CB HPCs is probably the result of the ability of such progenitors to generate large clones of progeny.24 There was a two-log greater increase in T-cell production from CB HPCs on LSC-mDL1 (100 000 ×) compared with similar studies using the oncoretroviral modified OP9DL1 system (1000 ×).13,14 A possible explanation is the extremely high level of lentiviral DL1 expression in LSC-mDL1 (Fig. 1). La Motte-Mohs et al.13 showed that the CB HPC-derived CD3/TCR-αβ-positive T cells could be activated with anti-CD3/CD28 monoclonal antibodies to express activation markers (CD27, CD28, CD69 and cytotoxic T-lymphocyte antigen-4). We have observed a similar response with adult BM HPC-derived T cells on LSC-mDL1, but noted that the ‘partially matured’ T cells failed to proliferate or to express functional effector cytokines (E. Patel and L.J. Chang, unpublished data).
There was a time difference in thymocyte development for FT, FL and CB compared with adult BM. The FT progenitors reached CD8+ CD4+ DP stage in the first week of the LSC-mDL1 coculture. Both FL-derived and CB-derived HPCs took about 2 weeks to reach the DP stage. The adult BM-derived HPCs were able to reach the DP stage in about 6 weeks. This could be because adult BM-derived HPCs may undergo sequential proliferation and migration before they reach the thymus and differentiate into T cells. On the other hand, HPCs of embryonic origin can differentiate into T cells without proliferation (self-renewal).25 Adult BM-derived HPCs take longer to reach the DP stage than embryonic and fetal HPCs. The delay in differentiation of adult BM-derived HPCs is longer than that observed in a previous report using paediatric BM-derived HPCs.14 This difference could be linked to the difference in the age of the donor, as Smedt et al. used BM from a 12- to 14-year-old donor, in our case the BM donors were all over 20 years of age. It is evident that donor age contributes significantly to the kinetics of T-cell development.21,26,27
An additional difference is the number of cells that reach DP stage and their ability to proliferate and survive. The HPCs derived from CB and FT produced as high as 54% and 90% DP cells, and those derived from FL and BM produced 18% and 39% DP cells, respectively (Fig. 6). As the DP lineage decision is closely linked to the strength and duration of cytokine, Notch and TCR signalling, the expression levels of various regulatory factors in the different progenitors may contribute to their different kinetics of development. After reaching the DP stage, the proliferation rate of FT-, FL- and adult BM-derived T cells declined, with about 10-fold expansion in cell number, whereas CB-derived HPC T cells expanded another 100-fold after reaching the DP stage (Figs 2 and 7). The final difference was in the elevated potential of the CB HPCs to differentiate into both TCR-αβ and TCR-γδ cells (Fig. 6). The role of Notch1 signalling and its influence on commitment to TCR-αβ and TCR-γδ cells have been controversial. Some have reported that sustained Notch1 signalling promotes αβ over γδ T cells,13,28 while others found it favouring γδ over αβ T cells.29–31 Apparently, Notch1 signalling supports the development of HPCs derived from murine FL and BM into both γδ and αβ T cells.9,32 Again, it is conceivable that the various expression levels of Notch receptors and their ligands may influence the cell fate decisions.33,34
Our report provides a paralleled overview of early T-cell development from different sources of human HPCs. It is clear that the in vitro stromal cell culture system is still limited in supporting the progression of T cells from DN to DP stage and maturing into functional single-positive CD4 and CD8 T cells. The latter requires optimal negative and positive selections engaging TCR signalling and major histocompatibility complex interactions between the developing thymocytes, thymic epithelial cells and various antigen-presenting cells entering the thymus. Although the current in vitro model may not fully recapitulate the in vivo thymic niche, future modifications may bring us closer to a more efficient system for the understanding and exploitation of developing T cells.
Acknowledgments
The authors thank Q. Yang, W. Chou and G. Eubanks for technical assistance. This work was supported by funds from Yongling Foundation and NIH-NHLBI grant HL59412.
Author contribution
All authors are accountable for the conception and integrity of the research, and analysis of the data; Patel and Chang are responsible for the execution and for data collection; Patel is responsible for the initial drafting of the manuscript and all authors are responsible for revisions of the manuscript.
References
- 1.McCune JM, Ramikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The scid-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–9. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 2.Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11742–7. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamarck ME, Gottlieb PD. Expression of thymocyte surface alloantigens in the fetal mouse thymus in vivo and in organ culture. J Immunol. 1977;119:407–15. [PubMed] [Google Scholar]
- 4.Jenkinson EJ, Franchi LL, Kingston R, Owen JJ. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982;12:583–7. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 5.Yeoman H, Gress RE, Bare CV, et al. Human bone marrow and umbilical cord blood cells generate CD4+ and CD8+ single-positive T cells in murine fetal thymus organ culture. Proc Natl Acad Sci USA. 1993;90:10778–82. doi: 10.1073/pnas.90.22.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plum J, De Smedt M, Defresne MP, Leclercq G, Vandekerckhove B. Human CD34+ fetal liver stem cells differentiate to T cells in a mouse thymic microenvironment. Blood. 1994;84:1587–93. [PubMed] [Google Scholar]
- 7.Poznansky MC, Evans RH, Foxall RB, et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat Biotechnol. 2000;18:729–34. doi: 10.1038/77288. [DOI] [PubMed] [Google Scholar]
- 8.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–56. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 10.Pear WS, Radtke F. Notch signaling in lymphopoiesis. Semin Immunol. 2003;15:69–79. doi: 10.1016/s1044-5323(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 11.Zuniga-Pflucker JC. T-cell development made simple. Nat Rev Immunol. 2004;4:67–72. doi: 10.1038/nri1257. [DOI] [PubMed] [Google Scholar]
- 12.Jaleco AC, Neves H, Hooijberg E, Gameiro P, Clode N, Haury M, Henrique D, Parreira L. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–9. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 14.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33:227–32. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Kincade PW, Owen JJ, Igarashi H, Kouro T, Yokota T, Rossi MI. Nature or nurture? Steady-state lymphocyte formation in adults does not recapitulate ontogeny. Immunol Rev. 2002;187:116–25. doi: 10.1034/j.1600-065x.2002.18710.x. [DOI] [PubMed] [Google Scholar]
- 16.Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–40. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- 17.Chang L-J, Liu X, He J. Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther. 2005;12:1133–44. doi: 10.1038/sj.gt.3302509. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, He J, Liu C, Chang L-J. An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens. Vaccine. 2006;24:3477–89. doi: 10.1016/j.vaccine.2006.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaiss A-K, Son S, Chang L-J. RNA 3′-readthrough of oncoretrovirus and lentivirus: implications in vector safety and efficacy. J Virol. 2002;76:7209–19. doi: 10.1128/JVI.76.14.7209-7219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumano A, Godin I. Pluripotent hematopoietic stem cell development during embryogenesis. Curr Opin Immunol. 2001;13:166–71. doi: 10.1016/s0952-7915(00)00200-4. [DOI] [PubMed] [Google Scholar]
- 21.Offner F, Kerre T, De Smedt M, Plum J. Bone marrow CD34 cells generate fewer T cells in vitro with increasing age and following chemotherapy. Br J Haematol. 1999;104:801–8. doi: 10.1046/j.1365-2141.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Guidos C. Thymus and T-lymphocyte development: what is new in the 21st century? Immunol Rev. 2006;209:5–9. doi: 10.1111/j.0105-2896.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3−CD4−CD8− thymocyte differentiation. J Immunol. 1994;152:4783–92. [PubMed] [Google Scholar]
- 24.Theunissen K, Verfaillie CM. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML-IC assay. Exp Hematol. 2005;33:165–72. doi: 10.1016/j.exphem.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Ling KW, Dzierzak E. Ontogeny and genetics of the hemato/lymphopoietic system. Curr Opin Immunol. 2002;14:186–91. doi: 10.1016/s0952-7915(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 26.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–6. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 27.Chidgey A, Dudakov J, Seach N, Boyd R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin Immunol. 2007;19:331–40. doi: 10.1016/j.smim.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Washburn T, Schweighoffer E, Gridley T, Chang D, Fowlkes BJ, Cado D, Robey E. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88:833–43. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 29.De Smedt M, Reynvoet K, Kerre T, Taghon T, Verhasselt B, Vandekerckhove B, Leclercq G, Plum J. Active form of Notch imposes T cell fate in human progenitor cells. J Immunol. 2002;169:3021–9. doi: 10.4049/jimmunol.169.6.3021. [DOI] [PubMed] [Google Scholar]
- 30.Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–57. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Peydro M, de Yebenes VG, Toribio ML. Sustained Notch1 signaling instructs the earliest human intrathymic precursors to adopt a gammadelta T-cell fate in fetal thymus organ culture. Blood. 2003;102:2444–51. doi: 10.1182/blood-2002-10-3261. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–7. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 33.Walker L, Carlson A, Tan-Pertel HT, Weinmaster G, Gasson J. The notch receptor and its ligands are selectively expressed during hematopoietic development in the mouse. Stem Cells. 2001;19:543–52. doi: 10.1634/stemcells.19-6-543. [DOI] [PubMed] [Google Scholar]
- 34.Karanu FN, Murdoch B, Miyabayashi T, et al. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97:1960–7. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]