Abstract
Staphylococcus aureus, a major source of nosocomial and community-acquired infections, has a nasal carriage rate exceeding 25% in the human population. To elucidate host–pathogen interactions pertaining to nasal carriage, we examined the role of interleukin-1 (IL-1) in the colonization of human nasal epithelial cells (NEC) by a nasal carrier strain and a non-carrier strain of S. aureus. Using an organotypic model of the nasal epithelium, we observed that inoculation with a non-carrier strain of S. aureus induced production of IL-1 from NEC, but the expression of this cytokine was significantly reduced when NEC were inoculated with a carrier strain. Moreover, both IL-1α and IL-1β significantly decreased the growth of the nasal carrier strain of S. aureus (P< 0·001, n= 17 to n= 25); however the growth of the non-carrier strain was unaffected. Interestingly, it was found that several nasal carrier strains of S. aureus form quorum-dependent biofilms, which can be partially inhibited when preincubated with IL-1α. Taken together these data suggest that, although nasal carrier strains of S. aureus are sensitive to IL-1, they display a significant colonization advantage by both preventing the host from expressing IL-1 and elaborating a protective biofilm.
Keywords: bacteria/bacterial immunity, cytokines, innate immunity
Introduction
Staphylococcus aureus is carried in the nasal passages of approximately one-quarter of the human population, predominantly colonizing the moist squamous epithelium on the septum adjacent to the nasal ostium.1,2 Since its discovery in the 1880s by Scottish surgeon Alexander Ogsten, S. aureus has proved to be a major cause of nosocomial and community-acquired infections and has become increasingly resistant to conventional antibiotics.3–6
The success of nasal carrier strains of S. aureus in colonizing the nasal epithelium depends upon their ability to evade host innate immunity. In the nasal passages, innate immunity consists of a diverse array of defensive strategies including mechanical clearance by the mucociliary escalator, detection of pathogens facilitated by pattern recognition receptors and the elaboration of antimicrobial peptides and proteins.7 Defensive secretions are elaborated in the form of cationic peptides such as lysozyme, secretory leucocyte protease inhibitor (SLPI) and lactoferrin (the three of which comprise the majority of the natural antibiotic activity of nasal secretions) as well as calcitermin, α-defensins (human neutrophil peptides 1–3: HNP 1–3) and human β-defensins (HBDs), of which HBD-3 is the most effective defensin against S. aureus.8–10
The nasal carrier strain of S. aureus has evolved a complex strategy to evade host innate immunity involving bacterial determinants and permissive host elements.2,11–13 Knowledge of host determinants includes our findings that the nasal fluid obtained from nasal carriers of S. aureus is defective in killing carrier strains but not laboratory strains of S. aureus.2,14 We also ascertained that host nasal fluid contains elevated levels of HBD-2 as well as HNP 1–3, indicating that although subclinical infections are prevalent in carriers of S. aureus, the innate immune response to this infection is ineffective.2
Evidence of bacterial determinants of staphylococcal nasal carriage includes our findings that the nasal carrier strain of S. aureus circumvents some host innate mechanical clearance mechanisms by colonizing a region of the nasal passage devoid of cilia and subepithelial glands.2Staphylococcus aureus also displays significantly greater attachment to nasal epithelial cells (NEC) than a non-carrier strain and has been observed to delay its detection by bacterial pathogen recognition receptors, namely Toll-like receptor 2, on naïve NEC in comparison to a non-carrier strain.13 We also noted that the nasal carrier strain compromised antibacterial effectors through a significant reduction in the levels of HBD-2 and HBD-3 in comparison to a non-carrier strain in the initial stages of colonization.13 However, we observed that the ability of the nasal carrier strain of S. aureus to attach and proliferate may be inhibited by interleukin-1 (IL-1) or by an IL-1-derived pathway.
Previous studies by our group and others revealed that certain chemokines can also serve an additional function apart from chemotaxis: to directly inhibit bacterial growth.15–17 In the current study, we expanded these experiments to investigate whether IL-1 affects the colonization of NEC by a highly characterized nasal carrier strain of S. aureus. We observed that naïve NEC, inoculated with a nasal carrier strain of S. aureus, expressed significantly less IL-1β than an infection with its non-carrier counterpart. The carrier strain was inhibited in subsequent planktonic culture experiments by the addition of both IL-1α and IL-1β but not of other members of the IL-1 superfamily or other acute-phase cytokines. Most importantly, we observed that nasal carrier strains of S. aureus elaborated quorum-dependent biofilms, which was not apparent in the non-carrier strain. The formation of a biofilm by carrier strains of S. aureus was directly inhibited by the addition of IL-1α. Taken together these studies suggest that although IL-1 can play a part in the inhibition of the growth of carrier strains of S. aureus, the ability of the nasal epithelium to express these cytokines may be compromised by colonization with nasal carrier strains.
Materials and methods
Reagents
Functionally active, 99% pure cytokines IL-1α, IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-10, tumour necrosis factor-α (TNF-α), all from R&D Systems Inc., Minneapolis, MN); IL-18 (MBL Co., Ltd, Woburn, MA) and IL-33 (Peprotech, Rocky Hill, NJ) were received in a lyophilized form and reconstituted in sterile phosphate-buffered saline. Bovine serum albumin (BSA), which was used as a stabilizer for cytokine preparations, was used as control in all experiments at the same concentrations found in the lyophilized preparations.
Bacterial strains and culture conditions
Staphylococcus aureus (SA) D30 which has been extensively characterized, was originally isolated from the anterior nares of a healthy donor, and served as the carrier strain in the experiments herein.2,13,14Staphylococcus aureus 930918-3, (from Ian Holder, Shriners Burn Hospital, Cincinnati, OH) served as the non-carrier strain of S. aureus in these experiments and S. epidermidis was kindly provided by Dr Robert I. Lehrer, University of California (Los Angeles).13,18 Other strains of S. aureus used in the biofilm experiments included S. aureus 502A (an intermittent carrier used in intervention studies in the 1960s), S. aureus D20, S. aureus D98 and S. aureus D85 (designated carrier strains).2,14,19 Bacteria were cultivated on trypticase soy agar (TSA, Bacto™; Becton Dickinson and Company, Sparks, MD) and subcultured in tryptic soy broth (TSB; Bacto™) from which stocks were prepared. For all experiments, snap-frozen (−80°) stocks were thawed rapidly and cultured at 37°. Levels of bacterial inocula were estimated by measuring the absorbance at 625 nm of a washed bacterial suspension in phosphate-buffered saline (Mediatech Inc., Herndon, VA). An optical density at 625 nm of 0·1 approximated 2·0 × 107 colony-forming units (CFU)/ml. Inocula were quantified by spreading 10-μl aliquots of the liquid culture on TSA and enumerating CFU, following 18 hr of incubation at 37°.
Broth culture experiments were performed typically using 1 ml of growth medium (TSB or cell culture growth medium; RPMI-1640; Mediatech Inc., with or without 10% fetal bovine serum). Minimal cell culture medium (RPMI-1640) was incubated to a final concentration of 10 ng/ml of cytokine together with very carefully defined levels of bacteria (1000 CFU), using BSA as a control.20–22 The reactions were incubated at 37° for 18 hr using a rotary bacterial shaker at 250 rpm. The resulting levels of bacterial growth were then assessed by diluting the incubation to the appropriate levels in the respective medium, spreading 10-μl aliquots on TSA and incubating overnight at 37°. Resulting bacterial colonies were counted manually and recorded as CFU.
Preparation of biofilms
Snap-frozen cultures of S. aureus were incubated in TSB until they had reached stationary phase (6–8 hr), the optical density at 625 nm was measured and aliquots of the bacteria were added to BD Falcon Microtest™ 96-well enzyme-linked immunosorbent assay (ELISA) plates (BD Biosciences, Bedford, MA) and incubated at 25° for 3 days. Biofilms were washed with 0·85% NaCl and then stained with crystal violet (1% for 20 min). The stain was rinsed thrice with 0·85% NaCl, air dried and the plate was imaged. To quantify biofilm production, 100 μl of 100% ethanol was added to solubilize the dye, and the absorbance was measured at 620 nm.22
Organotypic NEC culture and stimulation
Human NEC (RPMI 2650, American Type Culture Collection, Manassas, VA) were grown on porous collagen-coated cell culture inserts, (Transwell® COL, 24-mm diameter, polyester, 0·4-μm pore size; Corning Inc., Corning, NY). Cell culture medium consisted of Dulbecco’s modified Eagle’s minimal essential medium (DMEM; Mediatech Inc.) with high glucose (4·5 g/l) and sodium pyruvate (110 mg/l), supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (584 mg/l; Irvine Scientific, Santa Ana, CA) and 10% (v/v) fetal bovine serum (Gemini Bio-Products, West Sacramento, CA). Cells were seeded onto Transwell inserts with antibiotic-supplemented media but subsequent changes of media used antibiotic-free DMEM. Cells were cultivated for 4 days in 5% CO2 at 37° until confluence was achieved, the media being replaced every day. On the fourth day the organotypic cell layer was exposed to the air–liquid interphase and cultivated for an additional 4 days. For experimental procedures, organotypic cell layers were inoculated with 3 × 103 CFU and incubated for 0–30 hr at 37° in 5% CO2. Using the Trypan blue exclusion method, viability of NEC after incubation with bacteria was determined to be > 95%.
Quantification of IL-1β/IL-1Ra by ELISA
The apical surface of NEC and the basolateral supernatants were analysed by commercial ELISA for the presence of IL-1β using the BD Opt EIA™ kit (Pharmingen, San Diego, CA) and IL-1Ra detection kit (R&D Systems Inc.) according to the manufacturers’ protocols. The concentrations of cytokines in the media were quantified by comparison with a standard curve generated using the appropriate recombinant human cytokine.
Statistical analysis
Statistical analyses were performed using graphpad prism 4 software (Hearne Scientific Software Pty. Ltd, Melbourne, Vic., Australia). The CFU analyses were performed on three to 25 independent experiments with a minimum of three replicates for each experiment. Numerical values for CFU were transformed to log10 values for graphical representation purposes because these values were skewed. A two-tailed Student’s t-test was used to analyse the raw data (i.e. compare group means), assuming unequal variances. For the analysis of IL-1β/IL-1Ra peptides by ELISA, control baseline values were subtracted from experimental values.
Results
NEC express reduced levels of IL-1β and IL-1Ra when infected with the nasal carrier strain of S. aureus
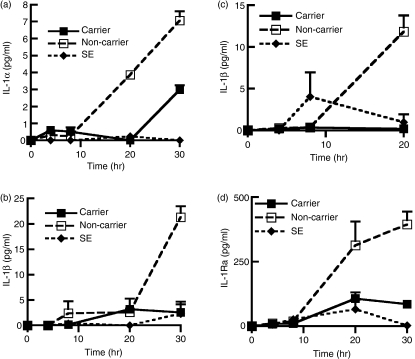
To ascertain the levels of IL-1β expressed upon staphylococcal infection, we inoculated NEC layers with nasal carrier and non-carrier strains of S. aureus and measured the expression of IL-1α and IL-1β by ELISA at various time-points between 0 and 30 hr. The results indicated that the levels of IL-1α and IL-1β expressed in the apical medium from naïve NEC were significantly higher in the non-carrier strain of S. aureus compared with the carrier strain after 30 hr incubation, (IL-1α, P= 0·007, n= 4, Fig. 1a; IL-1βP= 0·003, n= 3, Fig. 1b). Equally it was observed that the level of IL-1β at the basolateral surface was also reduced during infections by the carrier strain when compared with expression levels during infection by the non-carrier strain (P= 0·009, n= 4, Fig. 1c). The typical volume of the NEC layers was 45·2 mm2 (surface area of cells) × 0·015 (average depth of cells) = 0·678 mm3 or 0·678 μl. Similar volume estimates can be used for apical surface fluid, the in vivo depth of which has been estimated to be approximately 0·015 mm. Considering that apical and basolateral samples were diluted in approximately 1 ml of buffer, then the values indicated in Fig. 1 would be multiplied by 1475. The levels of IL-1 noted in our experiments were comparable with those found in vivo.23 The carrier strain of S. aureus elicited low expression of proinflammatory IL-1α and IL-1β from NEC in comparison with the non-carrier strain, so we postulated that there may be a concomitant decrease in the level of the anti-inflammatory IL-1Ra. Surprisingly, it was observed that the non-carrier strain of S. aureus induced expression of significantly higher levels of IL-1Ra than the nasal carrier strain after 20 hr incubation (P= 0·006, n= 4, Fig. 1d).
Figure 1.
Nasal epithelial cells (NEC) express reduced levels of interleukin-1α (IL-1α), IL-1β and IL-1 receptor antagonist (IL-1Ra) when infected with the nasal carrier strain of Staphylococcus aureus. The NEC layers were cultured at the air–liquid interphase in an antibiotic-free medium and inoculated with 3 × 103 ± 342 (mean ± SEM) colony forming units (CFU) per layer of NEC for a period of up to 30 hr. The organisms used were S. aureus, the carrier strain (D30), the non-carrier strain (930918-3); and S. epidermidis (SE). Levels of (a) IL-1α (b) IL-1β were assessed by enzyme-linked immunosorbent assay from the apical surface of the NEC. Levels of (c) IL-1β and (d) IL-1Ra were assessed from the basolateral cell culture supernatant. The results represent mean ± SEM of three or four separate experiments.
Levels of the acute-phase cytokine TNF-α were also measured after incubation of the carrier and non-carrier strains of S. aureus on NEC layers using the same protocol but there were no significant differences between the two different strains of S. aureus (data not shown). Levels of IL-1α, IL-1β and IL-1Ra were significantly reduced during infection by the nasal carrier strain of S. aureus so we investigated whether these cytokines had any direct effect on the growth of the nasal carrier or the non-carrier strain of S. aureus.
The growth of the nasal carrier strain of S. aureus is significantly inhibited by IL-1α and IL-1β but not by other members of the IL-1 family or other acute-phase cytokines
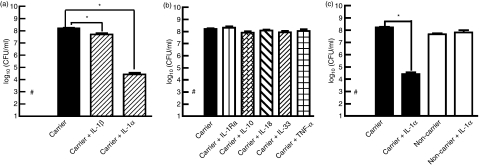
Previous studies from our laboratory and others have shown that certain chemokines can inhibit bacterial growth.15–17 To draw direct comparisons with these published experiments, we replicated their protocols exactly.20,22,24 Our results indicated that the nasal carrier strain of S. aureus used in these experiments was inhibited by 10 ng/ml IL-1α, (P< 0·001, n= 25) and to a lesser extent by IL-1β, (P< 0·001, n= 17, Fig. 2a).
Figure 2.
The growth of the nasal carrier strain of Staphylococcus aureus is significantly inhibited by interleukin-1α (IL-1α) and IL-1β. The S. aureus carrier strain was incubated in serum-free cell culture medium with (a) IL-1α and IL-1β and (b) other related cytokines including IL-1 receptor antagonist (IL-1Ra), IL-18, IL-33, IL-10 and tumour necrosis factor-α (TNF-α) and (c) compared with the non-carrier strain and IL-1α for a period of 20 hr. Bacteria were subsequently enumerated on trypticase soy agar. The results represent the mean ± SEM where n represents the number of independent experiments n = 17 to n = 25 for (a), n = 3 to n = 22 for (b) and n = 4 to n = 25 for (c). P values were calculated using a two-tailed unpaired t-test with a 95% confidence interval. #the inoculum and *P< 0·001.
Further tests using the same conditions revealed that other members of the IL-1 superfamily, IL-1Ra, IL-18 and IL-33, had no significant effects on the growth of the carrier strain of S. aureus, nor did other acute-phase cytokines, TNF-α and IL-10, (n= 3–25, Fig. 2b). When we compared the growth of the carrier and non-carrier strains of S. aureus in the presence of IL-1α (10 ng/ml), we found that this cytokine did not affect the growth of the non-carrier strain of S. aureus (P= 0·62 n= 3, Fig. 2c). Other investigators have reported that IL-1β can enhance the growth of selected strains of Gram-positive and Gram-negative bacteria so the antimicrobial effect might be strain specific.20,22,24,25
We have determined that growth of the carrier strain is inhibited by IL-1α and IL-1β in liquid media at moderate inocula but not by other members of the IL-1 family nor by the acute-phase cytokine TNF-α. Since this phenomenon was demonstrated by the planktonic growth form of the carrier strain of S. aureus, we questioned whether it would be exhibited by surface-associated sessile growth forms such as those found in biofilms.
Biofilm formation by the carrier strain of S. aureus is significantly greater than that of the non-carrier strain
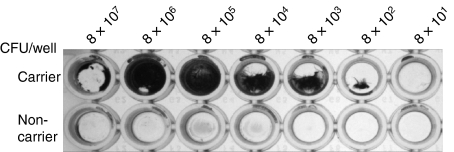
We have previously demonstrated that when the nasal carrier strain of S. aureus was incubated with nasal fluid from a carrier of S. aureus, the bacteria expressed an electron-dense layer after 2 hr which covered the peptidoglycan, as seen with transmission electron microscopy.2 It was notable that the non-carrier strain under the same conditions did not form an electron-dense layer. We postulated that this layer might represent the initial stages of biofilm formation by the carrier strain of S. aureus. To test this theory, we incubated both the carrier and non-carrier strains of S. aureus in 100 μl TSB for 3 days at 25° in a range of inocula in 96-well ELISA plates as indicated in the Materials and methods section. After 3 days of incubation, we observed that the carrier strain of S. aureus had formed a robust biofilm, which was not apparent in the non-carrier strain (Fig. 3). The carrier strain biofilm appeared to be a concentration-dependent phenomenon because wells with 8 × 106 CFU/well and higher showed a rapid decline in biofilm formation and inocula lower than this showed gradual diminishment of biofilm production. Other authors have used ethanol as a means of stimulating biofilm production; however, in these experiments we observed that maximal biofilm production could be achieved by a simple reduction in the incubation temperature to 25°.22 Importantly, this reflects the temperatures encountered in the anterior nasal passages, which range down to 25°.26
Figure 3.
Carrier but not non-carrier strains of Staphylococcus aureus elaborate a biofilm. Both the nasal carrier strain and the non-carrier strains S. aureus were inoculated into trypticase soy broth in 10-fold serial dilutions of stock starting at 8 × 107 colony-forming units (CFU) in a total volume of 100 μl. Biofilms were visualized after 3 days by the addition of crystal violet.
Biofilm formation by the carrier strain of S. aureus is significantly inhibited in the presence of IL-1α
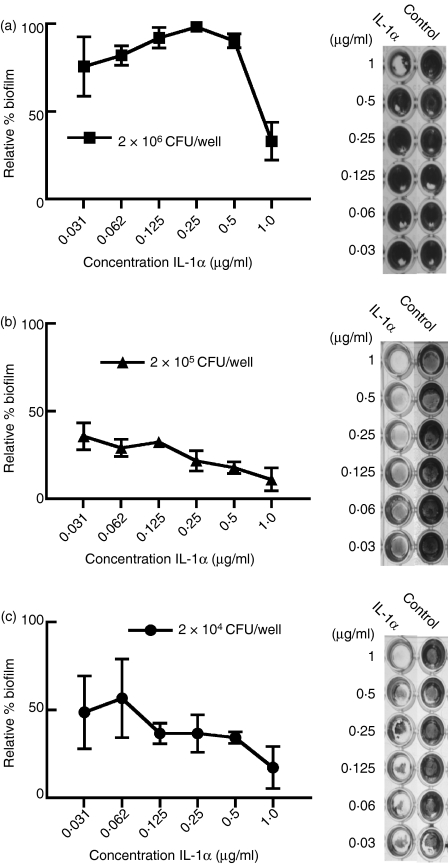
To assess biofilm formation under the effects of IL-1, we added a series of concentrations of IL-1α, the most potent inhibitor of the planktonic form of the nasal carrier strain of S. aureus, to each inoculum of the carrier strain of S. aureus. The inhibition of biofilms was quantified as described in the Materials and methods section. The results revealed that IL-1α inhibited the formation of biofilm by the carrier strain of S. aureus at an initial inoculum of 2 × 106 CFU compared to the BSA control (Fig. 4a). This inhibition by IL-1α was even more remarkable when the initial inoculum of the carrier strain was reduced to 2 × 105 CFU/well (Fig. 4b) and 2 × 104 CFU/well (Fig. 4c). Although we used a higher concentration of IL-1α in experiments involving the inhibition of biofilm in the carrier strain of S. aureus, we also used a correspondingly higher inoculum of bacteria. Collectively, these results suggest that although IL-1α can prevent biofilm formation by nasal carrier S. aureus, this strain retains its ability to colonize nasal epithelia, probably through its aforementioned ability to suppress IL-1 expression.
Figure 4.
Biofilm formation by the nasal carrier strain of Staphylococcus aureus is inhibited by interleukin-1α (IL-1α). A reduction in the biofilm formation of the nasal carrier strain was observed by the preincubation with various concentrations of IL-1α compared with a control. The figures represent a triplicate of experiments where an initial biofilm inoculum of a total number of bacteria at (a) 2 × 106 colony-forming units (CFU), (b) 2 × 105 CFU and (c) 2 × 104 CFU. The panels to the right of each graph are representative treated and control wells. Biofilm concentration was quantified by staining with crystal violet and measuring the absorbance at 620 nm.
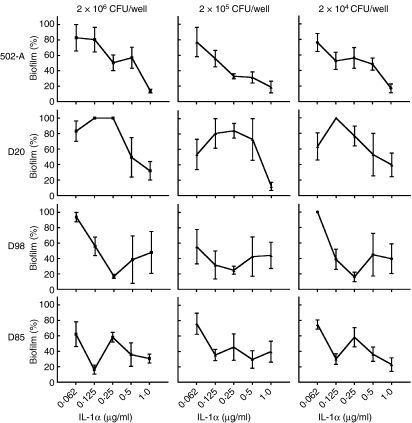
To draw broader conclusions from the results above, we conducted the experiment above on several other strains of S. aureus, namely D20, D98, D85 and 502-A (Fig. 5). The first three strains have been reported as carrier strains while 502-A has been termed an intermittent carrier strain.2,14,19 Under similar conditions, the non-carrier strain did not produce biofilm. The results further confirmed that the addition of IL-1α to carrier strains of S. aureus was responsible for the inhibition of biofilm formation (Fig. 5). Moreover this observation seemed to hold true irrespective of the level of inoculum. This reflects the results obtained from the nasal carriage strain D30 and strengthens our claim that IL-1α is likely to be responsible for the inhibition of nasal carriage strains of S. aureus in vitro.
Figure 5.
Biofilm formation by other strains of Staphylococcus aureus are inhibited by interleukin-1α (IL-1α). The S. aureus strains D20, D85, D98 and 502-A were incubated at inocula of 2 × 106, 2 × 105 and 2 × 104 colony-forming units (CFU)/well in the presence of IL-1α (0·62–1·0 μg/ml) over a period of 3 days. The panels indicate the relative biofilm formation in the presence of IL-1α in comparison to that obtained from using a similar concentration of bovine serum albumin. Biofilm formation was quantified by staining with crystal violet, measuring the absorbance at 620 nm and calculating percentage biofilm compared to the untreated control. The results represent mean ± SEM of three to six separate experiments.
Discussion
We examined the biological relevance of the direct innate IL-1-mediated host response to a nasal carrier strain of S. aureus. We demonstrated that the growth of a carrier strain of S. aureus was inhibited by the addition of IL-1α and IL-1β. Moreover, we also observed that NEC inoculated with a carrier strain of S. aureus expressed significantly lower levels of both IL-1α and IL-1β compared to inoculation with a non-carrier strain. Indeed, the ability of S. aureus D30 to prevent IL-1 upregulation in NEC suggests that this IL-1-sensitive strain has evolved a mechanism by which it can colonize the nasal epithelial surface.
The observation that IL-1α, IL-1β and IL-1Ra are downregulated in naïve NEC, which have been inoculated with the carrier strain of S. aureus, during the early stages of infection, is paralleled in previous experimental work in this laboratory that demonstrated a similar downregulation of the antimicrobial peptides HBD-2 and HBD-3 by the carrier strain of S. aureus.13 From an infection perspective, it logically follows that by blocking the IL-1 receptor, the carrier strain of S. aureus can effectively compromise the IL-1-based proinflammatory processes such as production of antimicrobial peptides, proinflammatory cytokines and chemokines, recruitment of neutrophils and mobilization of other effector cells to the site of infection. The manipulation of the initial innate immune response may be part of a wider strategy of the carrier strain of S. aureus to sustain its infectious capacity.
Before the current report, several other studies have suggested that IL-1β can increase bacterial growth of certain selected strains of bacteria in the culture medium.20,21,25,27 Using the same methodologies as reported for these observations of IL-1β,20,22,24 we have demonstrated that a nasal carrier strain of S. aureus can instead be inhibited by IL-1α and IL-1β. The group of Kanangat noted that IL-1β bound to the surface of S. aureus and used this observation as part of their hypothesis to explain an increase in the growth of the strains used in their experiments.21 The group of McLaughlin also noted a strain-specific phenomenon between two species of S. aureus in relation to their growth kinetics upon the addition of IL-1.22 The reasons for inhibition of the nasal carrier strain growth by IL-1 may be simply the result of strain variability, as proposed by McLaughlin, or it could be a multifactorial process involving inoculum size, amount, availability of nutrients in media or binding of IL-1 to the bacterial surface, possibly changing the kinetics of growth.22 For our studies, changes in bacterial growth kinetics in the presence of IL-1 were probably strain specific because we did not observe a difference in growth between the carrier and non-carrier strains in the absence of IL-1.
In the course of these experiments we also noted that the carrier strain of S. aureus and several other nasal carrier strains of S. aureus form biofilms. This is of prime importance in the process of nasal colonization because it has been reported that some biofilm-producing strains of S. aureus show survival rates against antibiotics > 500 times the level that is effective against planktonic populations, and they might, therefore, elaborate biofilms to subvert the natural antimicrobial polypeptides present on the host’s nasal mucosa.5 The influence of IL-1α on the inhibition of biofilms formed by several carrier strains may suggest an unexplored dynamic which requires further investigation.
In conclusion, we note that although IL-1 plays an important role in the inhibition of a nasal carriage strain of S. aureus, this host innate defence mechanism is effectively compromised by immunoevasion strategies of the nasal carrier strain.
Acknowledgments
These studies were supported by grant AI060753 from the National Institutes of Health, Bethesda, MD. We thank Dr AmyLiese Cole, Nitya Venkataraman, Karthikeyan Sivaraman and Julie Martellini for their expert technical assistance.
References
- 1.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 2.Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001;8:1064–9. doi: 10.1128/CDLI.8.6.1064-1069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogsten A. Micro-organisms in surgical diseases. BMJ. 1881;1:369–75. doi: 10.1136/bmj.1.1054.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogston A. Micrococcus poisoning. J Anat Physiol. 1882;16:526–67. [PMC free article] [PubMed] [Google Scholar]
- 5.Kostenko V, Ceri H, Martinuzzi RJ. Increased tolerance of Staphylococcus aureus to vancomycin in viscous media. FEMS Immunol Med Microbiol. 2007;51:277–88. doi: 10.1111/j.1574-695X.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 6.Pfeltz RF, Wilkinson BJ. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr Drug Targets Infect Disord. 2004;4:273–94. doi: 10.2174/1568005043340470. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol. 2006;18:355–62. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 8.Cole AM, Liao HI, Stuchlik O, Tilan J, Pohl J, Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–91. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 9.Midorikawa K, Ouhara K, Komatsuzawa H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole AM, Kim YH, Tahk S, Hong T, Weis P, Waring AJ, Ganz T. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001;504:5–10. doi: 10.1016/s0014-5793(01)02731-4. [DOI] [PubMed] [Google Scholar]
- 11.Shuter J, Hatcher VB, Lowy FD. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–18. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouwen J, Boelens H, van Belkum A, Verbrugh H. Human factor in Staphylococcus aureus nasal carriage. Infect Immun. 2004;72:6685–8. doi: 10.1128/IAI.72.11.6685-6688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn GA, Cole AM. Suppression of innate immunity by a nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology. 2007;122:80–9. doi: 10.1111/j.1365-2567.2007.02615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–75. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR–CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–7. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–55. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 17.Yount NY, Waring AJ, Gank KD, Welch WH, Kupferwasser D, Yeaman MR. Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochim Biophys Acta. 2007;1768:598–608. doi: 10.1016/j.bbamem.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Lee IH, Cho Y, Lehrer RI. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect Immun. 1997;65:2898–903. doi: 10.1128/iai.65.7.2898-2903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Light IJ, Walton RL, Sutherland JM, Shinefield HR, Brackvogel V. Use of bacterial interference to control a staphylococcal nursery outbreak. Deliberate colonization of all infants with the 502A strain of Staphylococcus aureus. Am J Dis Child. 1967;113:291–300. doi: 10.1001/archpedi.1967.02090180051001. [DOI] [PubMed] [Google Scholar]
- 20.Meduri GU, Kanangat S, Stefan J, Tolley E, Schaberg D. Cytokines IL-1beta, IL-6, and TNF-alpha enhance in vitro growth of bacteria. Am J Respir Crit Care Med. 1999;160:961–7. doi: 10.1164/ajrccm.160.3.9807080. [DOI] [PubMed] [Google Scholar]
- 21.Kanangat S, Bronze MS, Meduri GU, Postlethwaite A, Stentz F, Tolley E, Schaberg D. Enhanced extracellular growth of Staphylococcus aureus in the presence of selected linear peptide fragments of human interleukin (IL)-1beta and IL-1 receptor antagonist. J Infect Dis. 2001;183:65–9. doi: 10.1086/317645. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin RA, Hoogewerf AJ. Interleukin-1beta-induced growth enhancement of Staphylococcus aureus occurs in biofilm but not planktonic cultures. Microb Pathog. 2006;41:67–79. doi: 10.1016/j.micpath.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Leid JG, Shirtliff ME, Costerton JW, Stoodley AP. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–45. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanangat S, Postlethwaite A, Cholera S, Williams L, Schaberg D. Modulation of virulence gene expression in Staphylococcus aureus by interleukin-1beta: novel implications in bacterial pathogenesis. Microbes Infect. 2007;9:408–15. doi: 10.1016/j.micinf.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Porat R, Clark BD, Wolff SM, Dinarello CA. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254:430–2. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 26.Liener K, Leiacker R, Lindemann J, Rettinger G, Keck T. Nasal mucosal temperature after exposure to cold, dry air and hot, humid air. Acta Otolaryngol. 2003;123:851–6. doi: 10.1080/00016480310000601a. [DOI] [PubMed] [Google Scholar]
- 27.Kim KS, Le J. IL-1 beta and Escherichia coli. Science. 1992;258:1562–3. doi: 10.1126/science.1455239. [DOI] [PubMed] [Google Scholar]