Abstract
A large number of intercellular signaling molecules have been identified that orchestrate female reproductive physiology. However, with the exception of steroid hormone receptors, little information exists about the transcriptional regulators that mediate cellular responses to these signals. The transcription factor C/EBPβ (CCAAT/enhancer-binding protein β) is expressed in ovaries and testes, as well as many other tissues of adult mice. Here we show that mice carrying a targeted deletion of the C/EBPβ gene exhibit reproductive defects. Although these animals develop normally and males are fertile, adult females are sterile. Transplantation of normal ovaries into mutant females restored fertility, thus localizing the primary reproductive defect to the ovary proper. In normal ovaries, C/EBPβ mRNA is specifically induced by luteinizing hormone (LH/hCG) in the granulosa layer of preovulatory antral follicles. C/EBPβ-deficient ovaries lack corpora lutea and fail to down-regulate expression of the prostaglandin endoperoxidase synthase 2 and P450 aromatase genes in response to gonadotropins. These findings demonstrate that C/EBPβ is essential for periovulatory granulosa cell differentiation in response to LH. C/EBPβ is thus established as a critical downstream target of G-protein-coupled LH receptor signaling and one of the first transcription factors, other than steroid hormone receptors, known to be required for ovarian follicle development in vivo.
Keywords: C/EBPβ knockout, granulosa cells, ovulation, corpus luteum, ovary, female reproduction
The CCAAT/enhancer-binding protein (C/EBP) family of transcriptional regulators is composed of four functionally related basic leucine zipper (bZIP) DNA-binding proteins (C/EBPα, C/EBPβ, C/EBPδ, and CRP1/C/EBPε) that recognize the same palindromic DNA sequence and exhibit similar leucine zipper dimerization specificities (Johnson and Williams 1994). The expression patterns of the members of this family often overlap, making it difficult to discern the specific regulatory functions of each protein. To address the roles of the individual C/EBP family members in a mammalian system, we and others have begun to introduce targeted mutations of the C/EBP genes into the mouse germ line.
Mice lacking C/EBPα die shortly after birth due to hypoglycemia and an absence of stored glycogen in the liver (Wang et al. 1995). The mutant animals also fail to accumulate lipid in their fat tissue, in accordance with prior studies showing that C/EBPα is required for terminal differentiation of preadipocytes (Samuelsson et al. 1991; Lin and Lane 1992; Freytag et al. 1994). In addition, analysis of hematopoiesis in fetal liver of C/EBPα null animals showed that this protein is essential for development of the granulocytic compartment (Zhang et al. 1997). This defect most likely results from the loss of expression of the granulocyte colony-stimulating factor (G-CSF) receptor gene, whose promoter contains a binding site for C/EBPα (Smith et al. 1996).
Mice deficient for C/EBPβ (a.k.a. NF-IL6, IL-6DBP, LAP, CRP2 and NF-M; for review, see Johnson and Williams 1994) are viable but display immune defects, including lymphoproliferative disorders, imbalanced T-helper responses, impaired tumor cytotoxicity and bactericidal activity of macrophages, and increased susceptibility to infections (Screpanti et al. 1995; Tanaka et al. 1995). Defects in the myeloid lineage of the hematopoietic system had been anticipated because of previous reports of C/EBPβ up-regulation during monocyte/macrophage and neutrophilic differentiation (Natsuka et al. 1992; Scott et al. 1992; Katz et al. 1993). Although C/EBPβ has been implicated in the regulation of cytokine genes and in the induction of acute phase genes in hepatocytes, hepatic acute phase responses are not significantly impaired in homozygous mutant mice (Screpanti et al. 1995). However, liver-specific expression of a developmentally regulated cytochrome P450 gene, CYP2D11, is significantly reduced in mice that lack C/EBPβ (Lee et al. 1997).
In the present study, we show that C/EBPβ is also essential for female reproduction because of a critical role in ovarian follicle development. Folliculogenesis begins when the primordial germ cell recruits ovarian interstitial cells, forming a primordial follicle composed of pregranulosa cells surrounding the oocyte. Granulosa cells later begin to proliferate under the stimulation of follicle-stimulating hormone (FSH). The follicle eventually forms a fluid-filled cavity, the antrum, which enlarges as the follicle matures into the preovulatory stage. At this point the granulosa cells become responsive to luteinizing hormone (LH), whose levels increase transiently during the estrous cycle. LH triggers follicle rupture (ovulation) and also signals the granulosa cells to differentiate into luteal cells. The follicle subsequently transforms into the corpus luteum, which functions as a transient endocrine organ required for maternal development during pregnancy and is an essential source of progesterone (for review, see Freeman 1994). Our analysis of C/EBPβ-deficient mice shows that mutant granulosa cells are unable to transgress to the luteal stage, rendering females sterile. Thus, the use of gene targeting has revealed a hitherto unanticipated function of the C/EBPβ gene in murine development and physiology.
Results
Generation of C/EBPβ-deficient mice
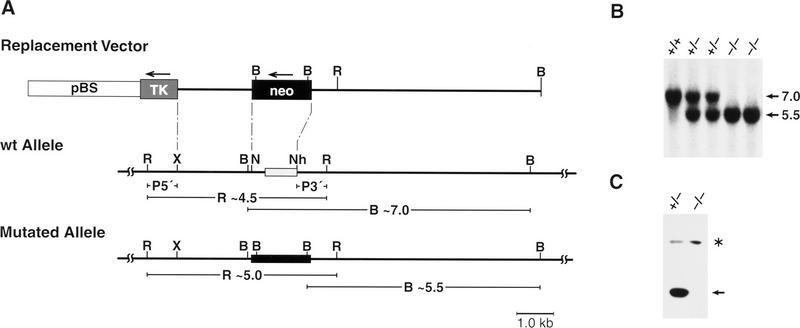
Embryonic stem (ES) cells were transfected with a replacement type targeting vector constructed from mouse genomic DNA (Fig. 1A). Two recombinant clones containing the predicted rearranged bands were used to generate chimeras that transmitted the mutated allele to their progeny (Fig. 1B). Animals from both independently derived lines were used for subsequent studies. As reported previously (Screpanti et al. 1995; Tanaka et al. 1995), C/EBPβ-deficient animals were viable but were born at approximately half the expected frequency. Western blot analysis of liver tissue confirmed that the C/EBPβ protein is not expressed in homozygous mutant mice (Fig. 1C).
Figure 1.
Targeted mutation of the C/EBPβ gene in mice. (A) Diagram of the targeting vector, wild-type allele, and mutated allele. The entire coding region and parts of the promoter were replaced by a neomycin resistance gene. Homologous recombination at the 5′ side of the gene was screened by a probe (P5′) that detects the conversion of a 4.5-kb EcoRI fragment into 5.0 kb and, at the 3′ side, a probe (P3′) that detects the alteration of a 7.0-kb BamHI fragment into a 5.5-kb fragment. (B) BamHI; (X) XbaI; (N) NotI; (Nh) NheI; (R) EcoRI. (B) Southern blot analysis of BamHI digested genomic mouse DNA probed with P3′ (see A). The 7.0-kb fragment diagnostic of the wild-type allele (+/+) and the 5.5-kb fragment diagnostic of the mutated allele (−/−) are indicated. (C) Western blot analysis of liver nuclear extracts from heterozygous or homozygous mutant animals probed with a C/EBPβ-specific antiserum (arrow). The asterisk identifies a nonspecific cross-reactivity of the antiserum.
C/EBPβ-deficient females are sterile
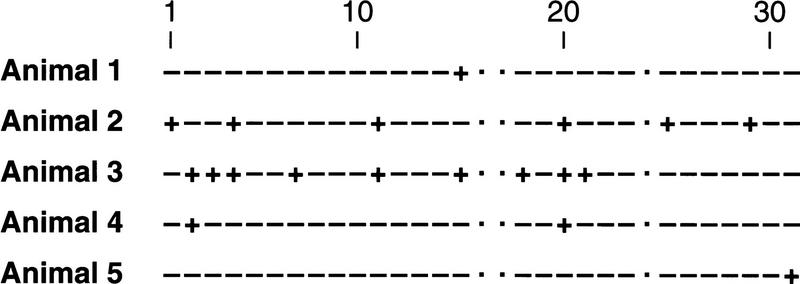
C/EBPβ−/− males and C/EBPβ+/− females were fertile and were mated routinely to maintain the mouse colony. However, C/EBPβ−/− females were completely infertile. Initially, eight mutant females were housed from age 6–8 weeks for 4 months with wild-type males, but no litters were recorded. To determine whether lack of mating was the primary cause of sterility, five more females were mated and monitored for the presence of seminal vaginal plugs. As shown in Figure 2, all of the animals mated at least once within this period (animals 1 and 5 received new males on day 14 of testing), demonstrating that C/EBPβ deficiency does not lead to asexual behavior of females. However, mating frequency was mostly acyclic, with periods of 2 days to >13 days between matings. In contrast, normal animals will enter estrous and mate every 4–5 days (Allen 1922). At the end of testing the females were analyzed for uterine content, but no embryos were detected.
Figure 2.
Seminal vaginal plug frequency in 5 C/EBPβ-deficient females. Mutant females were mated to males of proven fertility and analyzed for the presence of seminal vaginal plugs every morning for 31 days. (−) No plug; (+) plugged; (·) no record.
Two C/EBPβ−/− females were super-ovulated (see below) and mated to C57BL/6 males. Mating was confirmed by the presence of vaginal plugs on the following day, but the animals did not appear to become pregnant and no pups were delivered thereafter. We also subjected three mutant and three wild-type females to super-ovulation, mated them to wild-type males (confirmed by the presence of seminal vaginal plugs), and analyzed uterine contents on day 14 postcoitum. All wild-type animals carried several embryos as well as reabsorptions, whereas neither was detected in the C/EBPβ-deficient females (data not shown). In summary, these data show that C/EBPβ−/− female mice are unable to initiate or maintain pregnancy.
C/EBPβ-deficient ovaries lack corpora lutea and ovulate inefficiently
Comparative histology of uterine tissue from wild-type and C/EBPβ−/− mice did not reveal any abnormalities in mutant animals (data not shown). Furthermore, the wet weights of the uteri from super-ovulated mutant and wild-type animals were indistinguishable (Table 1). These data suggest that the uteri of virgin animals develop normally in the absence of C/EBPβ. However, histological examination of the ovaries of adult mice revealed a complete absence of mature corpora lutea in mutant animals (Fig. 3). In contrast, normal ovaries may contain three or more generations of corpora lutea, as their morphological life span is three to four times longer than each estrous cycle (Freeman 1994). A total of nine virgin C/EBPβ−/− females ages 5 weeks to 3 months were analyzed for ovarian histology. Mature corpora lutea, characterized as being larger than antral follicles with hypertrophic cells (depicted in Fig. 3A for a normal ovary), were never observed, whereas all stages of primary and antral follicles were present and histologically normal (Fig. 3B; data not shown). No other defects in ovarian development were apparent.
Table 1.
Uterus weights of super-ovulated females
| Number
|
Genotype
|
mg (± s.e.m.)
|
|---|---|---|
| 4 | +/+ | 99.5 ± 9.2 |
| 5 | +/− | 102.4 ± 4.4 |
| 4 | −/− | 106.5 ± 16.9 |
Four-month-old females were super-ovulated (see Materials and Methods), and uterine wet weights were determined 4–8 hr after injection of hCG.
Figure 3.
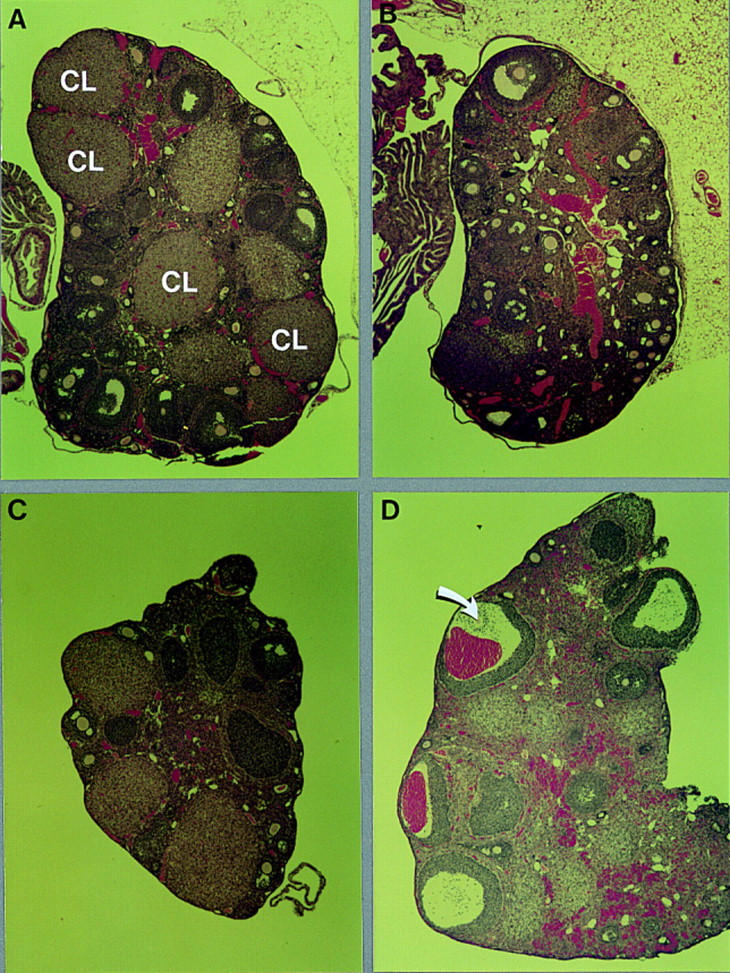
Morphology of normal and C/EBPβ-deficient ovaries from untreated animals or after treatment with gonadotropins in vivo. Photomicrographs (40×) of sections of hematoxylin/eosin-stained ovaries from heterozygous (A) and homozygous (B) mutant ovaries of 2- to 3-month old mice, and of heterozygous (C) and mutant (D) ovaries from mice treated for 2 days with PMSG and subsequently for 14 hr with hCG (see Table 2). The arrow indicates an oocyte that has failed to be expelled. (CL) Corpus luteum.
To investigate whether the ovarian defect in C/EBPβ-deficient mice is the result of impaired pre- or postovulatory mechanisms, we subjected animals to a super-ovulation protocol. Super-ovulation involves the sequential administration of pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG), substituting for FSH and LH (Greenwald and Roy 1994). This treatment results in follicular synchronization and ovulation of large numbers of oocytes. Injections of ovulatory doses of gonadotropins resulted in an average of 30 ovulated oocytes in heterozygous females. However, mutant animals produced only three to six oocytes that could be recovered from the oviducts after hormone treatment (Table 2). DAPI staining of nuclear DNA showed that the oocytes were arrested in metaphase II as expected for normal, ovulated, and unfertilized oocytes (data not shown).
Table 2.
C/EBPβ-deficient females exhibit reduced ovulation in response to gonadotropins
| Genotype
|
No. of ovulated oocytes (mean ± s.e.)
|
Number
|
|---|---|---|
| +/− | 30.3 ± 1.7 | 3 |
| −/− | 3.7 ± 0.7 | 4 |
Three-month-old mice were sacrificed 14 hr after injection with hCG, which was preceded 50 hr earlier by an injection of PMSG. Oviducts were collected, and the oocytes harvested and counted. The ovaries were fixed for histological analysis (see Fig. 3).
Histological analysis showed that after 14 hr of hCG treatment, large antral follicles had disappeared in control animals that had ovulated efficiently (Fig. 3C). The remaining cells were either undergoing luteinization or atresia (degeneration). In contrast, large, often hemorrhagic antral follicles were still present in mutant ovaries (Fig. 3D). Follicles beginning luteinization but containing oocytes that had failed to be expelled were also apparent (data not shown). Large antral follicles were rarely seen in normal super-ovulated ovaries and were never hemorrhagic. Taken together, the morphology of mutant super-ovulated ovaries and the low yield of ovulated oocytes after hormone treatment indicate that the mechanisms required to expel the oocyte and to support efficient luteinization are impaired in C/EBPβ−/− mice.
Sterility of C/EBPβ-deficient females is attributable to ovary-intrinsic defects
Reproductive performance of females depends on the proper function of and communication between several organs, as well as the metabolic condition and immunological fitness of the animal (Adashi and Leung 1993; Knobil and Neill 1994). To address whether the ovarian failure of C/EBPβ−/− mice was caused by intrinsic defects or systemic causes, we performed ovary transfer experiments. First, we exchanged ovaries between homozygous mutant females and normal (i.e., fertile) heterozygous littermates. When normal ovaries were implanted into C/EBPβ-deficient littermates, six out of seven animals became pregnant at least once and carried embryos to term (Table 3A). These results demonstrate that the uterus, hypothalamus, and pituitary of C/EBPβ−/− mice can support reproductive functions and suggest that defects in the ovary itself are the primary cause of sterility in mutant female mice.
Table 3.
Ovary transplants between mutant and wild-type mice
|
|
Number
|
Host
|
Ovaries
|
Litters/animal
|
Pups/litter
|
|
|---|---|---|---|---|---|---|
| A. | 2 | −/− | +/− | +/− | 1 | 1–2 |
| 1 | −/− | +/− | +/− | 2 | 2–3 | |
| 3 | −/− | −/− | +/− | 1 | 2 | |
| (+/−) (+/+) | ||||||
| B. | 2 | +/+ | −/− | +/+ | 3 | 7 37 |
| 3 | +/+ | −/− | +/+ | 1 | 4 10 | |
(A) One or two ovaries from 4- to 6-week-old C/EBPβ-deficient mice were replaced by ovaries from heterozygous littermates. The mice were mated 14 days postsurgery, and the first litters were born 20–30 days later. The animals were sacrificed at 3–4 months of age for histological analysis (see Fig. 4). (B) One ovary each from 4- to 6-week-old wild-type 129/SV × C57BL/6 females was replaced with one ovary from age-matched C/EBPβ-deficient mice. The mice were mated 14 days postsurgery to wild-type males, and the first litters were born 20–30 days later. The genotype of the pups was determined by tail DNA analysis. The mothers were sacrificed after one or three litters for histological analysis (see Fig. 4).
To investigate whether the C/EBPβ-deficient ovaries could give rise to viable oocytes and/or whether hormonal communication between the ovaries and neuroendocrine organs was impaired, we performed unilateral transplants of mutant ovaries into heterozygous or wild-type littermates. However, these transplants were complicated by frequent transplant rejections because the genetic background of donor and host animals was mixed 129/SV and C57BL/6. The observation that transplants into mutant animals were more successful than transplants into normal animals may be attributable to the impaired T-helper cell function in C/EBPβ−/− animals (Screpanti et al. 1995). To circumvent problems of histocompatibility, we used wild-type F1 females generated by crossing pure 129/SV and C57Bl/6 animals as hosts for the mutant ovary transplants. Five wild-type females containing unilateral mutant ovary transplants were mated to wild-type males, and nine litters from these matings were analyzed for their genotypes. Each of the females gave birth to heterozygous progeny (Table 3B). Thus, the mutant ovary can produce functional oocytes in the background of a wild-type host; in addition, the mutant ovary does not interfere with the reproductive functions of its wild-type host. Assuming 10% ovulation efficiency of mutant ovaries (Table 2), one would have predicted a maximum of 5% heterozygous pups after transplantation of one mutant ovary into a wild-type host. However, ∼20% of the progeny carried the mutant allele. These data suggest that the ovulation efficiency of mutant ovaries is improved within a wild-type host, at least in comparison to super-ovulated mutant animals.
Next, we analyzed whether transplantation of ovaries also affected the formation of corpora lutea. When heterozygous ovaries were implanted into mutant females, corpora lutea could be detected even 3 months after organ transfer, thus demonstrating that mutant host animals can support the formation and maintenance of corpora lutea in normal ovary implants (Fig. 4A). However, when mutant ovaries were implanted into normal (heterozygous and wild-type) hosts, corpora lutea were never observed (Fig. 4B), even when the host animal was pregnant (data not shown). These results confirm that the formation of the corpus luteum is impaired in C/EBPβ-deficient mice and that this phenotype is caused by intrinsic ovarian defects. Furthermore, these data suggest that the development of embryos derived from C/EBPβ-deficient ovary implants (Table 3B) was supported by corpora lutea formed in the contralateral wild-type ovary of the host.
Figure 4.
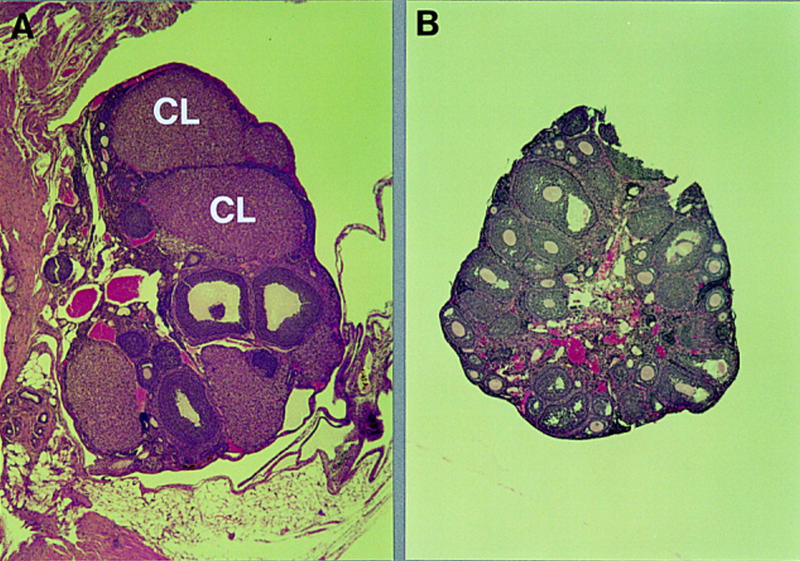
Morphology of transplanted ovaries. Photomicrographs (50×) of sections of hematoxylin/eosin-stained ovaries from a heterozygous ovary (A) 3 months after transplantation into a homozygous mutant host (see Table 3A) and a mutant ovary (B) 2 months after transplantation into a wild-type host animal (see Table 3B). (CL) Corpus luteum.
C/EBPβ is essential for granulosa cell function
In rats, C/EBPβ mRNA expression is rapidly induced in granulosa cells within 30 min after stimulation with LH/hCG in vivo (Sirois and Richards 1993). This finding implicates C/EBPβ as functioning downstream of the LH receptor in the granulosa cell compartment. To address whether ovarian cell types other than granulosa cells express C/EBPβ, we performed in situ hybridization analysis. Figure 5 shows that C/EBPβ is highly expressed in granulosa cells of antral follicles in animals that were treated for 7 hr with hCG. A high magnification image (Fig. 5F) demonstrates that C/EBPβ mRNA is not induced in theca cells. In addition, C/EBPβ expression in normal animals was undetectable in corpora lutea, the absence of which is the most evident histological defect in C/EBPβ-deficient ovaries. This observation suggests that C/EBPβ functions in granulosa cells prior to their maturation to the luteal phase. Although C/EBPβ may be expressed at low levels in other cell types of the ovary, it is clearly most abundant in granulosa cells of antral follicles. These findings support the proposed role of C/EBPβ in regulating differentiation and/or functional maturation of granulosa cells into luteal cells.
Figure 5.
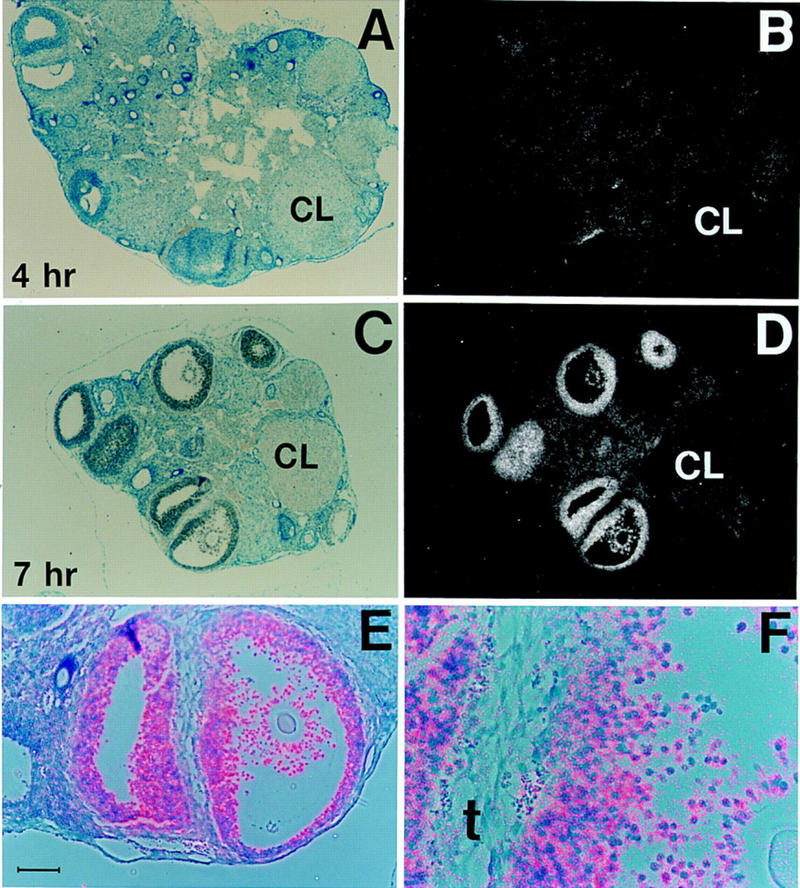
C/EBPβ expression is induced in granulosa cells 4–7 hr after hCG treatment. Bright-field (A,C), dark-field (B,D), and bright- plus dark-field (E,F) photomicrographs of sections from normal adult ovaries after in situ hybridization to a C/EBPβ-specific antisense cRNA probe. The animals were treated for 2 days with PMSG and then with hCG for 4 hr (A,B) and 7 hr (C,D), respectively. E and F show high magnification of the two follicles shown at the bottom of C and D; silver grains (predominantly over granulosa cells) are in pink. The scale bar in E represents 10 μm. (A,B) C/EBPβ+/− ovaries; (C–F) C/EBPβ+/+ ovaries. (CL) Corpus luteum; (t) theca layer. Magnifications, 62.5× (A–D); 100× (E); 320× (F).
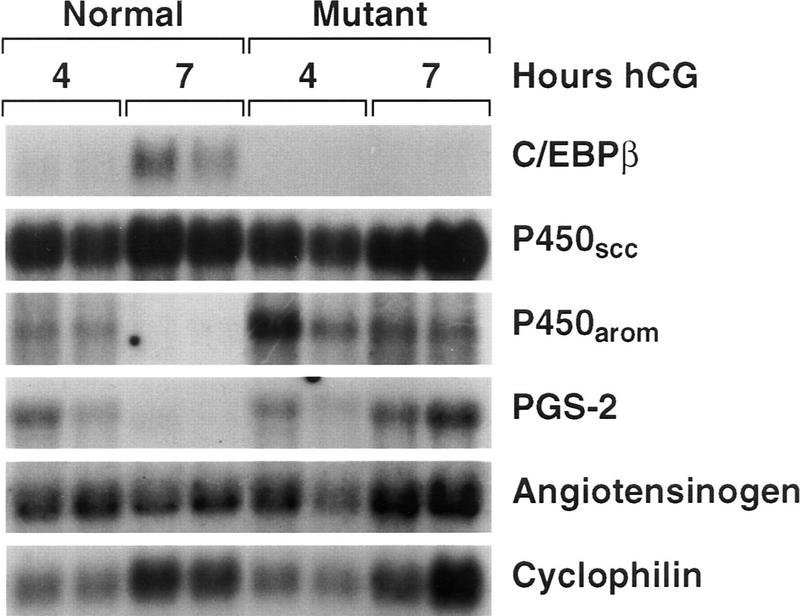
To begin to determine at the molecular level the consequences of C/EBPβ deficiency, we analyzed the expression of several potential target genes. C/EBP proteins can bind to the promoter of the prostaglandin endoperoxide synthase-2 gene (PGS-2, COX-2, PES-2), which encodes the rate-limiting enzyme in the conversion of arachidonic acid to prostaglandins, thromboxane, and prostacyclin. The C/EBPβ binding element is thus implicated in the regulation of PGS-2 expression in granulosa cells, as well as in osteoblastic and endothelial cell lines (Sirois and Richards 1993; Inoue et al. 1995; Yamamoto et al. 1995). In vivo, PGS-2 expression is induced by LH/hCG in granulosa cells, attains peak levels 4 hr after hormone treatment, and declines rapidly thereafter (Sirois et al. 1992). Targeted deletion of the PGS-2/COX-2 gene in mice leads to female infertility with ovarian histology similar to that described here (Dinchuk et al. 1995). We therefore examined whether induction of PGS-2 expression by LH/hCG was impaired in C/EBPβ−/− mice, thus directly causing female sterility. Figure 6 shows that similar levels of PGS-2 expression were observed in RNA of whole ovaries from mutant and control animals 4 hr after hCG treatment. In agreement with previous reports (Sirois et al. 1992), PGS-2 expression was essentially undetectable in normal ovaries at 7 hr of hCG treatment. However, at the same time point, mutant ovaries retained high levels of PGS-2 mRNA. Thus, induction of PGS-2 expression is normal in mutant animals but subsequent down-regulation of this gene is severely impaired.
Figure 6.
Gene expression analysis of normal and mutant ovaries after gonadotropin treatment. The animals were treated for 2 days with PMSG and with hCG for 4 hr (normal, C/EBPβ+/−) and 7 hr (normal, C/EBPβ+/+), as indicated. One ovary from each animal was fixed for histological analysis (Fig. 5); total RNA was isolated from the second ovary and 10 μg was analyzed by Northern blotting with sequential hybridization to radioactively labeled cDNA probes for the indicated genes.
We further analyzed the expression of P450 aromatase (P450arom) and angiotensinogen, both of which are specifically expressed in granulosa cells and are candidates for regulation by C/EBPβ according to previous promoter analyses (Thomas and Sernia 1990; Brasier and Li 1996; Toda et al. 1996). Expression of P450arom, which catalyzes the last step in the synthesis of estrogens, is induced by FSH/PMSG and declines upon hCG/LH receptor signaling (Richards 1994). As shown in Figure 6, P450arom mRNA was undetectable in normal ovaries after 7 hr of hCG treatment but was still present at this time in mutant ovaries. These data again indicate a failure of LH/hCG to shut off the expression of a gene in granulosa cells following its induction.
Regulation of the angiotensinogen gene by gonadotropins has not been observed, although inhibition of the ovarian renin–angiotensinogen system can interfere with ovulation (Kuji et al. 1996). Figure 6 shows that the levels of angiotensinogen mRNA are comparable in normal and mutant ovaries at both time points; therefore, expression of this gene does not appear to be altered significantly by the lack of C/EBPβ. P450scc, the enzyme catalyzing the first step of steroidogenesis from cholesterol, is expressed in most cell types of the ovary and is up-regulated by LH (Richards 1994). Like angiotensinogen, ovarian expression of P450scc was unaffected by deletion of the C/EBPβ gene (Fig. 6).
In summary, mRNA analyses revealed that total transcript levels of P450scc, which is widely expressed in the ovary, and angiotensinogen, which is expressed in granulosa cells, were not significantly altered in C/EBPβ-deficient ovaries at the time points analyzed. Furthermore, target genes of FSH and LH (P450arom, P450scc, and PGS-2) were expressed in the ovaries of mutant mice following hormone treatment. However, the PGS-2 and P450arom genes failed to undergo subsequent down-regulation in response to LH/hCG. These data demostrate that C/EBPβ deficiency impairs granulosa cell function subsequent to LH/hCG–receptor activation.
Discussion
The generation and analysis of C/EBPβ–null mice has revealed novel functions for this transcription factor in vivo. Although mutant males and heterozygous females are fertile, nullizygous females are sterile. Our analysis identifies the primary cause of infertility as defective ovarian granulosa cell function at postovulatory stages of follicular development, resulting in lack of corpora lutea. Ovulation was also impaired in mutant mice, occurring at ∼10% the efficiency of wild-type animals in super-ovulation experiments. However, single mutant ovaries that were implanted into wild-type hosts gave rise to 20% of the total progeny produced by these females. These results suggest that defective ovulation is not the primary cause of sterility. The data further indicate that super-ovulation experiments alone may not yield conclusive data on the effect of a mutation on ovulation efficiency. Although functional oocytes were produced by the transplanted mutant ovaries, no corpora lutea were formed. Therefore, the absence of corpora lutea is an intrinsic defect of C/EBPβ-deficient ovaries and explains the sterility of mutant female mice. This ovary-autonomous defect is consistent with the high levels of C/EBPβ observed in normal granulosa cells after LH stimulation.
Ovary transplants
When normal ovaries were implanted into mutant females, pregnancies could occur, demonstrating that the uterus, hypothalamus, and pituitary can support reproductive functions. In most cases, only one litter was produced by mutant females with wild-type ovary implants. This could be attributable to the high level of stress caused by the first pregnancy, which exacerbated their poor health, or to the stimulation of an immune response against the C/EBPβ-positive embryos or ovary implant after the first parturition. In support of the latter, no ovary implant was found at sacrifice in one animal (out of four animals analyzed) 2 months after it delivered a pup that by its genotype was derived from the implant. In addition, two animals appeared to have encapsulated the implant (data not shown).
The newborn pups delivered by mutant females with wild-type ovaries appeared normal. However, they did not have milk in their stomachs and were eaten by the mothers soon after birth. The appearance of the pregnant mutant animals suggested that they were carrying more than one to two embryos (Table 3A). It is therefore likely that most of the offspring were cannibalized before they could be rescued from the mothers. Although initial observations indicated that nurturing and suckling behavior were normal, the finding that mutant females do not or cannot feed their pups requires further investigation. In general, however, our data suggest that neural, hormonal, and uterine functions during pregnancy and parturition are normal in C/EBPβ-deficient females.
Communication between granulosa cells and the oocyte is required for normal oocyte development (Simon et al. 1997). Histological analysis of mutant ovaries suggested that follicular development is unaffected until the late antral stage. Furthermore, mutant ovaries gave rise to progeny when transplanted into a wild-type host animal. Thus, C/EBPβ-deficient granulosa cells function normally until the peri- or postovulatory stage. Cross talk between the ovaries and other reproductive organs is also essential for female reproductive functions. We show that the presence of a mutant ovary does not impair the function of the contralateral host ovary or the neuroendocrine control of reproduction. Furthermore, mutant ovaries were unable to form corpora lutea even in the presence of a normal ovary. These observations indicate that the mutant granulosa cells do not secrete hormones or other factors that inhibit reproduction and that the deleterious consequences of the C/EBPβ mutation are intrinsic to the affected follicles.
Mating frequency
In normal female mice, mating behavior is governed by cyclic hormonal changes and would normally occur at estrous, which is entered on average every 5 days (Allen 1922). However, when fertilization is unsuccessful, the coital stimuli alone can trigger pseudopregnancy, in which case mating would resume after ∼12 days (Ormandy et al. 1997). The normal estrous cycle is regulated by the hormonal cross talk between the hypothalamus, pituitary and the ovary. The failure of C/EBPβ-deficient ovaries to generate functional corpora lutea predicts impaired hormonal production from the ovary and thus irregularities in the estrous cycle. The irregular mating behavior of C/EBPβ–null females could thus be accounted for by the lack of the luteal phase. That cyclic changes do occur is suggested by several 4- to 5-day periods between matings (Fig. 2) as well as by the observation that vaginal smears obtained from mutant females at day of sacrifice varied in appearance (data not shown) in accordance with different stages of the cycle (Allen 1922).
In only a single case did we record a period of >10 days between matings. This observation suggests that mating does not efficiently trigger pseudopregnancy in C/EBPβ-deficient females, again supporting the notion of luteal dysfunction. The mating frequency data are similar to those reported for mice deficient in prolactin receptor expression, which also display luteal dysfunction (Ormandy et al. 1997). However, analysis of ovarian mRNA showed that the prolactin receptor gene is expressed in C/EBPβ−/− mice (data not shown), indicating that the absence of this receptor does not cause the mutant phenotype.
C/EBPβ expression in granulosa cells
Induction of C/EBPβ expression by LH/hCG specifically in granulosa cells of antral follicles is consistent with a role for C/EBPβ in late follicular development. In hypophysectomized rats, C/EBPβ mRNA expression is induced rapidly in granulosa cells 30 min after stimulation with LH/hCG in vivo (Sirois and Richards 1993). This finding indicates that the C/EBPβ gene is an immediate target of LH–receptor signaling. Our observation that C/EBPβ mRNA levels were induced between 4 and 7 hr of hCG treatment of intact mice may indicate that C/EBPβ gene expression is modulated by additional factors derived from or under the control of the hypophysis.
The finding that C/EBPβ is a nuclear target of LH signaling suggests that C/EBP−/− mice could have phenotypes similar to animals carrying mutations that affect LH or its receptor. Targeted mutations of the LH β-subunit or the LH–receptor in mice have not been reported. However, NGF1A-deficient mice, which do not express LH efficiently, display ovarian histology similar to that observed in C/EBPβ-deficient mice. In contrast to C/EBPβ-deficient mice, however, the formation of the corpus luteum could be initiated in these animals by hormone treatment (Lee et al. 1996). Analysis of ovarian mRNA confirmed that the LH-receptor is expressed in C/EBPβ-deficient mice (data not shown). These data support the notion that C/EBPβ exerts its critical function downstream of the LH–receptor.
C/EBPβ mRNA is strongly up-regulated in granulosa cells in response to LH/hCG (Fig. 5; Sirois and Richards 1993). LH–receptor signaling is known to elicit increased intracellular cAMP levels (Richards 1993), which activate the cAMP response element-binding (CREB) protein. Recently two CRE motifs were identified within the C/EBPβ promoter that bind CREB and mediate transcriptional induction by activated PKA (Niehof et al. 1997). It is likely that these elements in the C/EBPβ promoter also regulate induction by LH in granulosa cells. Other cell-specific transcriptional regulators may also contribute to the specific induction of C/EBPβ in granulosa cells.
C/EBPβ expression differs significantly from that of C/EBPα, which is abundant in most ovarian cell types (Piontkewitz et al. 1993). Sirois and Richards (1993) reported that C/EBPα expression is down-regulated by LH/hCG in rat granulosa cells and is thus reciprocal to C/EBPβ. Recently, it was demonstrated that inhibition of C/EBPα expression using antisense oligonucleotides interferes with ovulation (Piontkewitz et al. 1996). It is possible that C/EBPα expression is required for the subsequent induction of C/EBPβ by LH/hCG, thus accounting for the ovulation deficit observed when C/EBPα expression is ablated. Because the neonatal lethality of C/EBPα–null mice (Wang et al. 1995) precludes a straightforward test of this hypothesis, the development of conditional targeting strategies to inactivate the C/EBPα gene in granulosa cells may be necessary to define its role in follicular development.
Requirement for C/EBPβ during luteal differentiation
Super-ovulation experiments demonstrated that mutant ovaries are unable to respond appropriately to hormone treatment and that the follicles do not transgress to the luteal phase of development and instead tend to become hemorrhagic. The occurrence of hemorrhagic follicles is also observed in LH–transgenic mice (Risma et al. 1995) and ER-deficient mice, which have elevated levels of LH (Couse et al. 1995). Thus, hemorrhagic follicles may be indicative of overstimulated LH–receptor activity. As hemorrhagic follicles were not observed without hormone treatment, endogenous levels of LH may not be sufficient to trigger the hemorrhagic response in C/EBPβ-deficient mice. It is possible that LH overstimulation results from the inability of mutant granulosa cells to undergo maturation to luteal cells, thereby causing them to remain sensitive to LH stimulation. This notion is further supported by the fact that down-regulation of the P450arom and PGS-2 genes subsequent to induction by LH/hCG is defective in C/EBPβ−/− mice.
C/EBPβ potentially encodes a truncated polypeptide, LIP, that functions as a transcriptional repressor (Descombes and Schibler 1991). Therefore, an inhibitory product of the C/EBPβ gene could be responsible for down-regulation of PGS-2 and P450arom in normal animals. Recent studies also suggest that the C/EBPβ binding element inhibits PGS-2 promoter activity in transfected granulosa cells (Morris and Richards 1996). Alternatively, the fact that the granulosa cells do not mature into the luteal stage may explain the lack of transcriptional attenuation for these genes. Regardless of the mechanism, our findings demonstrate that although C/EBPβ is dispensable for induction of the PGS-2 and P450arom genes, it is required for their subsequent shut-off. It remains to be established whether unattenuated expression of either gene is the direct cause of sterility in C/EBPβ−/− females.
To our knowledge, the finding that C/EBPβ is a critical component of murine granulosa cell differentiation represents the first in vivo demonstration of an essential transcriptional regulator targeted by gonadotropin receptor signaling. The receptors for FSH and LH, which belong to the class of G protein-coupled, seven-transmembrane receptors, are related in sequence and structure and share similar signal transduction pathways (Adashi and Leung 1993). Based on these similarities, it is unclear how FSH and LH elicit such different responses in the same cells and why both are required for normal reproductive functions. C/EBPβ appears to act specifically as an LH–receptor responsive gene and may thus provide a tool to elucidate functional differences between the FSH and LH receptor systems. Furthermore, because follicles in mutant ovaries mature normally but are specifically blocked at a periovulatory stage, C/EBPβ-deficient mice should provide a unique model system to identify downstream genes that are essential for LH-elicited follicular maturation.
Materials and methods
Generation of C/EBPβ−/− mice
The replacement-type targeting vector consisted of 129/SV (Stratagene) mouse genomic DNA comprising a 2.0-kb XbaI–NotI fragment of 5′ homology and a 5.5-kb NheI–BamHI fragment of 3′ homology with the C/EBPβ locus. A 1.2-kb region comprising the entire coding sequence and promoter sequences was replaced with the pGKneobpA cassette, which was used as a positive selectable marker. The pGK–thymidine kinase cassette was included as negative selectable marker (Soriano et al. 1991).
Electroporation and selection were performed using the CJ7 ES cell line as described (Swiatek and Gridley 1993). DNAs derived from G418/FIAU-resistant ES clones were screened with a diagnostic BamHI restriction enzyme digestion using the 5′ probe external to the targeting vector sequence and the 3′ probe internal to the targeting vector sequence as indicated in Figure 1. Recombinant clones containing the predicted rearranged bands were obtained at a frequency of 1/24. Two independent ES cell C/EBPβ recombinant clones were injected into C57BL/6 blastocysts to generate chimeras, which transmitted the mutated allele to the progeny after mating to C57BL/6 females.
Immunoblotting
Nuclear extracts were prepared from liver as described (Gorski et al. 1986), and 20 μg of protein was electrophoresed on 10% SDS–polyacrylamide gels, transferred to nitrocellulose membranes, and probed with the C-19 anti-C/EBPβ antiserum (Santa Cruz, CA) and HRP-conjugated second antibody (Promega). The blot was developed using the ECL chemiluminescence system (Amersham Corp., Arlington Heights, IL).
Analysis of ovary tissue
Ovaries were fixed either in 10% neutral buffered formalin or with 4% paraformaldehyde. Five-micron sections were prepared from paraffin-embedded tissues and stained with hematoxylin/eosin according to standard procedures. In situ hybridization analysis was performed as described (Tessarollo and Parada 1995), using as a probe antisense cRNA transcribed from a 480-bp NheI–DraI fragment derived from the C/EBPβ 3′ untranslated region (UTR) (Williams et al. 1991).
Care and treatment of mice
The mice were housed and bred in a specific pathogen-free facility with a 12-hr light cycle starting at 7 a.m. and chow and water ad libitum. Procedures were conducted in compliance with the guidelines of the Animals Studies Committee of the National Cancer Institute. Where indicated, mice were given intraperitoneal injections of PMSG (5 IU, Sigma) or hCG (5 IU, Sigma). For the data in Table 2 and Figure 3, the animals received PMSG at 5 p.m. of day 1 and hCG at 7 p.m. of day 3 and were sacrificed at 9 a.m. of day 4. For all other super-ovulation experiments the mice were given PMSG at 4 p.m. of day 1 and hCG at 1 p.m. of day 3.
Ovary transplants
Ovary transplants were performed essentially as described (Cunliffe-Beamer 1983). Donor and recipient mice were anesthetized with Avertin. The ovarian fat pads were moved outside onto surgical drape through a small longitudinal incision through the skin. Where applicable, one skin incision was used for both ovaries, moving it over the position of each ovary for an incision through the peritoneum. The bursa was opened at one end, keeping most of the membrane intact, and the ovary was teased out and placed into PBS at 37°C. The bursa was held open with Dumont tweezers and the replacement ovary was pushed into the bursa. Blood was left around the bursa to help maintain the ovary in place by clotting. The ovary, oviduct, and uterus were then placed back into the body cavity while ensuring that the ovary was fully inserted inside the bursa. The skin incisions were closed with 9-mm wound clips, and the mice were given appropriate postoperative care.
RNA analysis
Total RNA was prepared from whole ovaries homogenized in 1 ml of RNA STAT-60 reagent (TEL-TEST “B”, Inc., Friendswood, TX) according to the manufacturer’s protocol. The RNA was analyzed by Northern blotting as described (Sterneck et al. 1996). Radiolabeled DNA probes were prepared from isolated cDNA clones for P450arom, P450scc, PGS-2 (Sirois et al. 1992), cyclophilin (Danielson et al. 1988), angiotensinogen [ATCC; (Lynch et al. 1986)], and a 480-bp NheI–DraI fragment representing the 3′ UTR of the rat C/EBPβ gene (Williams et al. 1991).
Acknowledgments
We thank J. Blair-Flynn, M.E. Palko, S. Reid, L. Sewell, B. Shankle, and D. Swing for expert technical assistance, Dr. L. Lock for help with the super-ovulation experiments, Dr. D. Wickramasinghe for help in analyzing chromosome condensation in oocytes, Dr. N.A. Jenkins for her suggestions concerning ovary-transplant experiments, Drs. M.A. Bedell, R. Cutler, Jr., P.J. Donovan, L.L., and D.W. for their interest and stimulating discussions, Drs. J.S. Richards (Baylor College of Medicine), Kelly E. Mayo (Northwestern University), and D. Segaloff (University of Iowa) for providing cDNA probes, and R.C., D.W., J.S.R., and Dr. E. Adashi (University of Utah) for helpful comments on earlier versions of the manuscript. Research sponsored by the National Cancer Institute, Department of Health and Human Services, under contract with ABL.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL johnsopf@ncifcrf.gov; FAX (301) 846-5991.
References
- Adashi EY, Leung PCK. The ovary. In: Martini L, editor. Comprehensive endocrinology. New York, NY: Raven Press; 1993. [Google Scholar]
- Allen E. The oestrous cycle in the mouse. Am J Anat. 1922;30:297–371. [Google Scholar]
- Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Eddy EM, Schomberg DW, Korach KS. Disruption of the mouse oestrogen receptor gene: Resulting phenotypes and experimental findings. Biochem Soc Trans. 1995;23:929–935. doi: 10.1042/bst0230929. [DOI] [PubMed] [Google Scholar]
- Cunliffe-Beamer TL. Biomethodology and surgical techniques. In: Foster HL, Small JD, Fox JG, editors. The mouse in biomedical research. New York, NY: Academic Press; 1983. pp. 401–437. [Google Scholar]
- Danielson PE, Forss-Petter S, Brow MA, Calvatta L, Milner RJ, Sutcliff JG. p1B15: A cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;1:261–269. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York, NY: Raven Press; 1994. pp. 613–658. [Google Scholar]
- Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes & Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- Gorski K, Carneiro M, Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Greenwald GE, Roy SK. Follicular development and its control. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York, NY: Raven Press; 1994. pp. 629–724. [Google Scholar]
- Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- Johnson P, Williams SC. CCAAT/enhancer binding (C/EBP) proteins. In: Yaniv M, Tronche F, editors. Liver gene expression. Austin, TX: R.G. Landes Company; 1994. pp. 231–258. [Google Scholar]
- Katz S, Kowenz-Leutz E, Muller C, Meese K, Ness SA, Leutz A. The NF-M transcription factor is related to C/EBP-β and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J. 1993;12:1321–1332. doi: 10.1002/j.1460-2075.1993.tb05777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobil E, Neill JD. The physiology of reproduction. New York, NY: Raven Press; 1994. [Google Scholar]
- Kuji N, Sueoka K, Miyazaki T, Tanaka M, Oda T, Kobayashi T, Yoshimura Y. Involvement of angiotensin II in the process of gonadotropin-induced ovulation in rabbits. Biol Reprod. 1996;55:984–991. doi: 10.1095/biolreprod55.5.984. [DOI] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- Lee YH, Williams SC, Baer M, Sterneck E, Gonzalez FJ, Johnson PF. The ability of C/EBP beta but not C/EBP alpha to synergize with an Sp1 protein is specified by the leucine zipper and activation domain. Mol Cell Biol. 1997;17:2038–2047. doi: 10.1128/mcb.17.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Lane MD. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes & Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Lynch KR, Simnad VI, Ben-Ari ET, Garrison JC. Localization of preangiotensinogen messenger RNA in the rat brain. Hypertension. 1986;8:540–543. doi: 10.1161/01.hyp.8.6.540. [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS. An E-box region within the prostaglandin endoperoxide synthase-2 (PGS-2) promoter is required for transcription in rat ovarian granulosa cells. J Biol Chem. 1996;271:16633–16643. doi: 10.1074/jbc.271.28.16633. [DOI] [PubMed] [Google Scholar]
- Natsuka S, Akira S, Nishio Y, Hashimoto S, Sugita T, Isshiki H, Kishimoto T. Macrophage differentiation-specific expression of NF-IL6, a transcription factor for interleukin-6. Blood. 1992;79:460–466. [PubMed] [Google Scholar]
- Niehof M, Manns MP, Trautwein C. CREB controls LAP/C/EBPβ transcription. Mol Cell Biol. 1997;17:3600–3613. doi: 10.1128/mcb.17.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Enerback S, Hedin L. Expression and hormonal regulation of the CCAAT enhancer binding protein-α during differentiation of rat ovarian follicles. Endocrinology. 1993;133:2327–2333. doi: 10.1210/endo.133.5.8404685. [DOI] [PubMed] [Google Scholar]
- ————— Expression of CCAAT enhancer binding protein-α (C/EBPα) in the rat ovary: Implications for follicular development and ovulation. Dev Biol. 1996;179:288–296. doi: 10.1006/dbio.1996.0258. [DOI] [PubMed] [Google Scholar]
- Richards JS. Gonadotropin-regulated gene expression in the ovary. In: Adashi EY, Leung PCK, editors. The ovary. New York, NY: Raven Press; 1993. pp. 93–110. [Google Scholar]
- ————— Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson L, Stromberg K, Vikman K, Bjursell G, Enerback S. The CCAAT/enhancer binding protein and its role in adipocyte differentiation: Evidence for direct involvement in terminal adipocyte development. EMBO J. 1991;10:3787–3793. doi: 10.1002/j.1460-2075.1991.tb04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LM, Civin CI, Rorth P, Friedman AD. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–1735. [PubMed] [Google Scholar]
- Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, Bistoni F, Frati L, Cortese R, Gulino A, Ciliberto G, Costantini F, Poli V. Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Sirois J, Richards JS. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBP beta promoter element. J Biol Chem. 1993;268:21931–21938. [PubMed] [Google Scholar]
- Sirois J, Simmons DL, Richards JS. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem. 1992;267:11586–11592. [PubMed] [Google Scholar]
- Smith LT, Hohaus S, Gonzalez DA, Dziennis SE, Tenen DG. PU.1 (Spi-1) and C/EBPα regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood. 1996;88:1234–1247. [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Kaplan DR, Johnson PF. Interleukin-6 induces expression of peripherin and cooperates with Trk receptor signaling to promote neuronal differentiation in PC12 cells. J Neurochem. 1996;67:1365–1374. doi: 10.1046/j.1471-4159.1996.67041365.x. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes & Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted distruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Parada LF. Oncogene techniques: In situ hybridization. Methods Enzymol. 1995;254:419–430. doi: 10.1016/0076-6879(95)54028-8. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Sernia C. The immunocytochemical localization of angiotensinogen in the rat ovary. Cell Tissue Res. 1990;261:367–373. doi: 10.1007/BF00318679. [DOI] [PubMed] [Google Scholar]
- Toda K, Nomoto S, Shizuta Y. Identification and characterization of transcriptional regulatory elements of the human aromatase cytochrome P450 gene (CYP19) J Steroid Biochem Mol Biol. 1996;56:151–159. doi: 10.1016/0960-0760(95)00232-4. [DOI] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBPα knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- Williams SC, Cantwell CA, Johnson PF. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes & Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]