Abstract
What are the molecular mechanisms of bacterial infections triggering or modulating lupus nephritis? In nephritic MRLlpr/lpr mice, transient exposure to bacterial cell wall components such as lipopeptide or lipopolysaccharide (LPS) increased splenomegaly, the production of DNA autoantibodies, and serum interleukin (IL)-6, IL-12 and tumour necrosis factor (TNF) levels, and aggravated lupus nephritis. Remarkably, bacterial lipopeptide induced massive albuminuria in nephritic but not in non-nephritic mice. This was associated with down-regulation of renal nephrin mRNA and redistribution from its normal localization at foot processes to the perinuclear podocyte area in nephritic MRLlpr/lpr mice. Bacterial lipopeptide activates Toll-like receptor 2 (TLR2), which we found to be expressed on cultured podocytes and glomerular endothelial cells. TNF and interferon (IFN)-γ induced TLR2 mRNA and receptor expression in both cell types. Albumin permeability was significantly increased in cultured podocytes and glomerular endothelial cells upon stimulation by bacterial lipopeptide. LPS also induced moderate albuminuria. In summary, bacterial lipopeptide and LPS can aggravate glomerulonephritis but only lipopeptide potently induces severe albuminuria in MRLlpr/lpr mice.
Keywords: albuminuria, autoimmunity, endothelial cells, podocytes, Toll-like receptors
Introduction
The immune system seeks to control pathogens with the minimum of immunity-mediated tissue damage; nevertheless, even local infections often trigger immune responses that cause remote tissue damage, for example in immune complex disease.1 As a second mechanism, circulating microbial molecules ligate innate pathogen recognition receptors which trigger systemic antimicrobial immunity by activating antigen-presenting cells enhancing, the costimulation of antigen presentation to T cells, modulating T-cell polarization, and promoting antibody production by activating B-cell proliferation.2 Furthermore, the interaction of circulating microbial products with innate pathogen recognition receptors in remote solid organs can boost local defence mechanisms which add to the immunity-mediated damage rather than to pathogen control.1
Toll-like receptors (TLRs) are one of several innate immunity receptor families that trigger antimicrobial immunity and contribute to immunity-related tissue pathology.2–4 For example, TLR7 and TLR9 recognize viral RNA and microbial CpG-DNA on B cells and plasmacytoid dendritic cells which can trigger immune complex disease and can cause severe glomerular pathology in pre-existing glomerulonephritis.5–10 Furthermore, non-immune cells express a restricted pattern of TLR family members, i.e. TLR1–6.11,12 For example, viral double-stranded RNA (dsRNA) is taken up into intracellular endosomes of glomerular mesangial cells, a compartment where mice and human mesangial cells express TLR3.13,14 Exposure to viral dsRNA activates mesangial cells in vitro and in vivo to produce large amounts of proinflammatory cytokines and to undergo apoptosis, both resulting in severe glomerular pathology.14 Viral RNA signalling in mesangial cells is enhanced by proinflammatory cytokines, through the induction of TLR3.14 Consistent with this finding, systemic exposure to viral dsRNA can aggravate a pre-existing glomerulonephritis but does not trigger the onset of de novo glomerulonephritis.7,14
A subgroup of TLRs specifically recognizes bacterial cell wall components. TLR2/-1 and TLR2/-6 heterodimers recognize bacterial lipopeptide and TLR4 is a crucial component of the lipopolysaccharide (LPS) receptor complex.15–18TLR2 or TLR4 agonists injected together with the antiserum can exacerbate the development of serum nephritis.19,20 Furthermore, TLR2 chimeric mice are largely protected from serum nephritis.21 However, these findings do not address the question of which mechanisms trigger lupus flares induced by transient exposure of bacterial cell wall components. We hypothesized that bacterial lipopeptide and LPS would modulate established lupus-like immune complex glomerulonephritis, and may give detailed insights into the mechanisms of bacterial infection-induced lupus flares.
Materials and methods
Animals, chemicals and experimental protocol
Female MRLlpr/lpr mice were obtained from Jackson Laboratories (Bar Harbor, MA). At 16 weeks of age mice were randomly distributed into three groups that received a total of seven intraperitoneal injections every alternate day for 18 weeks as follows. (1) 100 μl of sterile isotonic saline only. (2) 15 μg of N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-[R]-Cys-[S]-Serl-[S]-Lys trihydro-chloride (P3C or pam3cys; Invivogen, Toulouse, France), a synthetic tripalmitoylated lipopeptide that mimics the acylated amino terminus of bacterial lipopolysaccharide. To confirm the absence of contamination by LPS in P3C, polymyxin B (Invivogen) was incubated with P3C during in vitro stimulation in podocytes and endothelial cells. (3) 10 μg of ultrapure LPS (Invivogen). P3C and LPS were dissolved in sterile normal saline and injected in a volume of 100 μl. Blood was collected under ether anaesthesia 3 hr after the last injection and just before mice were killed at 18 weeks of age.
TLR3 ligand poly(inosinic:cytidylic acid)-RNA [poly(I:C)-RNA] (Invivogen), TLR9 ligand CpG-DNA-1668 (TIB Molbiol, Berlin, Germany) and TLR7 ligand imiquimod (Sequoia Research Products Ltd, Oxford, UK) were used for in vitro stimulation. TLR2-deficient C57/BL6 mice were a generous gift from Dr Shizuo Akira (Osaka University, Osaka, Japan). C57/BL6 mice were purchased from Charles River (Sulzfeld, Germany). All experimental procedures had been approved by the local government authorities.
Assessment of lupus disease activity
Lupus disease activity parameters were determined using enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-6, IL-12p40 (BD OptEiA, San Diego, CA), TNF (Biolegend, San Diego, CA), interferon (IFN)-α (PBL Biomedical Labs, Piscataway, NJ), immunoglobulin G1 (IgG1), IgG2a, IgG2b, IgG3 (Bethyl Lab, Montgomery, TX), and urinary albumin (Bethyl Lab). Urinary creatinine concentrations were determined using an automatic autoanalyser (Integra 800; Roche Diagnostics, Mannheim, Germany). DNA autoantibodies were determined by ELISA as previously described.10 For histopathological analysis, kidneys were fixed in 10% buffered formalin, processed, and embedded in paraffin. Sections of 3–4 μm for periodic acid-Schiff stains were prepared following routine protocols. The severity of the renal lesions was graded using the indices for activity and chronicity as described for human lupus nephritis.19 In brief, the activity index was calculated by assessing glomerular cell proliferation and leucocyte infiltration, fibrinoid necrosis, cellular crescents, hyaline thrombi, and tubulointerstitial leucocyte infiltrate with a score on a 0–3 scale. Fibrinoid necrosis and cellular crescents were weighted by a factor of 2. The chronicity index was calculated by assessing glomerular sclerosis, fibrous crescents, interstitial fibrosis and tubular atrophy with a score on a 0–3 scale.7 Immunostaining was performed on either paraffin-embedded or frozen sections as described previously10 using the following primary antibodies: anti-mouse Mac-2 (1 : 50; Cedarlane, Hornby, ON, Canada), anti-mouse CD3 (1 : 100; Serotec, Oxford, UK), anti-mouse Ki-67 (1 : 100, cell proliferation; Dianova, Hamburg, Germany); anti-mouse IgG (1 : 100, M32015; Caltag Laboratories, Burlingame, CA), anti-mouse C3c [1 : 20, goat anti-mouse (GAM)/C3c/fluorescein isothiocyanate (FITC); Nordic Immunological Laboratories, Tilburg, the Netherlands], anti-mouse nephrin [1 : 50, guinea pig polyclonal (GP-N2); Progen Biotechnik, Heidelberg, Germany]. For quantitative analysis glomerular cells were counted in 15 cortical glomeruli per section. Semiquantitative scoring of complement C3c or IgG deposits from 0 to 3 was performed on 15 cortical glomerular sections as described previously.10 For transmission electron microscopy (Zeiss EM 900; Zeiss, Oberkochen, Germany) a small piece of cortical tissue from a kidney pole was fixed in glutaraldehyde and embedded in araldite, cut with an ultramicrotome, and stained with osmiumtetroxide and lead citrate.
In vitro stimulation of primary splenocytes from TLR2−/− and wild-type (WT) BL6 mice
Primary splenocytes were isolated from the spleens of TLR2-deficient and WT C57/BL6 mice (8–9 weeks of age) after they had been killed under aseptic conditions in an aseptic hood. Spleens were cut in 2–3 ml of Hanks’ balanced salt solution (HBSS; pH 7·4) on ice using fine scissors and broken into fine pieces of tissue, and cell suspensions were then passed through 70-μm plastic filters (BD Biosciences, Franklin Lakes, NJ). Red blood cells (RBCs) were lysed with sterile 0·3 m NH4Cl followed by three washings with HBSS at 400 g at 4° for 6 min. The pellet was re-suspended in medium containing RPMI + 5% fetal calf serum (FCS) + 1% penicillin and streptomycin (PS) (2–4 ml) and 500 000 cells were added to each well of a 24-well plate, containing 0·5 ml of medium. The cells were stimulated with ligands or medium and incubated for 24 hr at 37°, and the supernatants were subsequently harvested for the analysis of IL-6 release. P3C (3 μg/ml), LPS (3 μg/ml), CpG (1 μg/ml), imiquimod (3 μg/ml), and poly(I:C)-RNA (30 μg/ml) were used for stimulation of splenocytes.
In vitro studies of glomerular visceral epithelial cells (GVECs) and glomerular endothelial cells (GENCs)
GENCs were prepared from ts A58 immorto mice as previously described, and grown in medium containing RPMI-1640 + Glutamax (Invitrogen, Paisley, UK), 10% heat-inactivated FCS, 100 units/ml penicillin and 100 μg/ml streptomycin (Biochrom KG, Berlin, Germany).22 GVECs, i.e. podocytes, were derived from conditionally immortalized mouse podocyte clones which proliferate only when cultured under permissive conditions at 33° as previously described.23,24 All cells were stimulated after 24 hr of FCS starvation in the presence or absence of TNF (500 units/ml) (Immunotools, Friesoythe, Germany) plus IFN-γ (200 units/ml) (Peprotech, London, UK) and together with TLR agonists as follows: TLR2 and P3C (1 and 10 μg/ml); TLR4 and ultrapure LPS (1 and 10μg/ml). IL-6 and monocyte chemotactic protein (MCP)-1 were measured in cell culture supernatants by ELISA using commercial kits (BD OptEiA). Proliferation of GENCs and GVECs was determined with a colorimetric assay (Promega, Mannheim, Germany) as described previously7 following the protocol provided by the manufacturer. Cell RNA was isolated 6 hr after stimulation using the Qiagen RNeasy kit (Qiagen, Helden, Germany) for mRNA expression analysis.
In vitro fluorescein-albumin permeability assay
GVECs or GENCs were grown to confluent monolayers in hanging cell culture inserts of 1 μm pore size (Millipore, Billerica, MA) placed inside 24-well plates with 0·5 ml of medium in inserts and wells, i.e. on both sides of the membrane as described previously.25 Fluorescein-labelled bovine serum albumin (Invitrogen, Karlsruhe, Germany) was added to the inserts and the cells were stimulated with 10 μg/ml P3C, 10 μg/ml ultrapure LPS or medium. The filtrate (100 μl) below each insert in the well was sampled at different time-points and the same volume of medium was added to the well as replacement. The fluorescence optical density (OD) was measured for excitation at 485 nm and emission at 535 nm in NUNC black 96-well plates (NUNC, Kamstrupvej, Denmark).
Flow cytometry
For flow cytometry, cells were stained with rat anti-mTLR2 (1 : 100; Biolegend), mouse anti-mTLR4 (1 : 100; Biolegend) and anti-mouse B7-1/CD80, and then phycoerythrin (PE) anti-mouse IgG (1 : 200), PE anti-rat IgG (1 : 200) or FITC goat anti-hamster IgG (1 : 200; all Biolegend) was used for detection. Isotype antibodies used were mouse IgG1 (1 : 100; Biolegend) and rat IgG2a (1 : 100; BD Pharmingen, Heidelberg, Germany).
Real-time quantitative (TaqMan) reverse transcription–polymerase chain reaction (RT-PCR)
To measure the mRNA expression pattern in cultured cells or renal tissue, real-time PCR was performed as previously described using TaqMan (Applied Biosystems, Foster City, CA).10 Controls consisting of double-distilled water (ddH2O) were negative for target and housekeeper genes. Oligonucleotide primer (300 nm) and probes (100 nm) used were from PE Biosystems (Weiterstadt, Germany) and are listed in Tables 1 and 2.
Table 1.
Probes used for real-time polymerase chain reaction (PCR)
| Gene | Accession number | Sequence |
|---|---|---|
| TLR1 | AF316985 | Forward primer: 5′-GTCAAAGCTTGGAAAGAATCTGAAG-3′ Reverse primer: 5′-AATGAAGGAATTCCACGTTGTTTC-3′ 6 FAM : 5′-ATCTTACCCTGAACAATG-3′ |
| TLR2 | AF124741 | Forward primer: 5′-CACCGGTCAGAAAACAACTTACC-3′ Reverse primer: 5′-CAAGATCCAGAAGAGCCAAAGAG-3′ 6 FAM : 5′-AGACAAAGCGTCAAATC-3′ |
| TLR3 | AF355152 | Forward primer: 5′-CGAAAGTTGGACTTGTCATCAAATC-3′ Reverse primer: 5′-ACTTGCCAATTGTCTGGAAACAC-3′ 6 FAM : 5′-CACTTAAAGAGTTCTCCC-3′ |
| TLR4 | AF110133 | Forward primer: 5′-TTCAGAACTTCAGTGGCTGGATT-3′ Reverse primer: 5′-CCATGCCTTGTCTTCAATTGTTT-3′ 6 FAM : 5′-ATCCAGGTGTGAAATT-3′ |
| TLR5 | AF186107 | Forward primer: 5′-CCCAGCTTGGATGAAATATCTGTAA-3′ Reverse primer: 5′-CCCAGTCTTTTCTTCTTGAACACTTA-3′ 6 FAM : 5′-CGGGCACCAGTACT-3′ |
| TLR6 | AB020808 | Forward primer: 5′-TGAATGATGAAAACTGTCAAAGGTTAA-3′ Reverse primer: 5′-GGGTCACATTCAATAAGGTTGGA-3′ 6 FAM : 5′-TGGTGAGTTCTGATAAAA-3′ |
| CCR1 | NM_009912 | Forward primer: 5′-TTAGCTTCCATGCCTGCCTTATA-3′ Reverse primer: 5′-TCCACTGCTTCAGGCTCTTGT-3′ 6 FAM : 5′-ACTCACCGTACCTGTAGCCCTCATTTCCC-3′ |
| CCR2 | NM_009917 | Forward primer: 5′-CAAGACAATCCTGATCGTGCAA-3′ Reverse primer: 5′-TCCTACTCCCAAGCTGCATAGAA-3′ 6 FAM : 5′-TCTATACCCGATCCACAGGAGAACATGAAGTTT-3′ |
| CCR5 | NM_009917 | Forward primer: 5′-CAAGACAATCCTGATCGTGCAA-3′ Reverse primer: 5′-TCCTACTCCCAAGCTGCATAGAA- 3′ 6 FAM : 5′-TCTATACCCGATCCACAGGAGAACATGAAGTTT-3′ |
| Nephrin EX7 (NPHS-1) | AF168466 | Forward primer: 5′-ACCCTCCAGTTAACTTGTCTTTGG-3′ Reverse primer: 5′- ATGCAGCGGAGCCTTTGA-3′ 6 FAM : 5′-TCCAGCCTCTCTCC-3′ |
| Podocin EX3 (NPHS-2) | AY050309 | Forward primer: 5′-CCACAGAGGATGGTGAAATCTA-3′ Reverse primer: 5′-AGGGCCAGTCAAAGGAACTTCT-3′ 6 FAM : 5′-ACGCTCAGGAGGAAT-3′ |
| VEGF | M95200 | Forward primer: 5′-GCTGTGCAGGCTGCTGTAAC-3′ Reverse primer: 5′-TGATGTTGCTCTCTGACGTGG-3′ 6 FAM : 5′ - ATTGCCGTCGCTGCGACCATG -3′ |
| iNOS | NM_010927 | Forward primer: 5′-GTGACGGCAAACATGACTTCAG -3′ Reverse primer: 5′- GCCATCGGGCATCTGGTA - 3′ 6 FAM : 5′-CTGGAATTCACAGCTCATCCGGTACGC-3′ |
| ICAM | NM_010493 | Forward primer: 5′-GCCCTGGTCACCGTTGTG-3′ Reverse primer: 5′-GGATGGATGGATACCTGAGCAT-3′ 6 FAM : 5′-TCCCTGGGCCTGGTG-3′ |
| VCAM | NM_011693 | Forward primer: 5′-AACCCAAACAGAGGCAGAGTGTAC-3′ Reverse primer: 5′-GACCCAGATGGTGGTTTCCTT-3′ 6 FAM : 5′-TGTCAACGTTGCCCC-3′ |
| ILK | NM_010562 | Forward primer: 5′-CCTTGCACTGGGCCTGC-3′ Reverse primer: 5′-CTCCACGCATGATCAGCATT-3′ 6 FAM : 5′-AGGCCGCTCTGCGGTGGTTG-3′ |
| FAT | XM_134149 | Forward primer: 5′-CCCCGAGAGGAGAAGTATAGCT-3′ Reverse primer: 5′-ACGAAGCTGTTTCCCGTGAA-3′ 6 FAM : 5′-ATGCCCAGGGAGCTCA-3′ |
| Claudin-1 | AF072127 | Forward primer: 5′-GATGTGGATGGCTGTCATTGG-3′ Reverse primer: 5′-CCATGCTGTGGCCACTAATGT-3′ 6 FAM : 5′-CGCCAGACCTGAAAT-MGBNFQ-3′ |
CCR, chemokine (C-C motif) receptor; FAT, FAT tumour suppressor homolog 1; ICAM, intercellular adhesion molecule; ILK, integrin-linked kinase; iNOS, inducible nitric oxide synthase; NPHS, nephrosis 1, congenital, Finnish type; TLR, Toll-like receptor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
Table 2.
Predeveloped TaqMan assay reagents from Applied Biosystems
| Gene | Accession number | Assay ID |
|---|---|---|
| CCL5 | NM_013653 | Mm01302428_m1 |
| CCL2 | NM_011333 | Mm00441242_m1 |
| IL-6 | NM_031168 | Mm00446190_m1 |
| IFN-γ | NM_008337 | Mm00801778_m1 |
| TNF | NM_013693 | Mm00443258_m1 |
| ZO-1 | NM_009366/D14340 | Mm00493699_m1 |
| B7-1/CD80 | M_009855 | Mm00711660_m1 |
| BMP-7 | NM_007557 | Mm00432102_m1 |
| IFN-β | NM_010510 | Mm00439546_s1 |
BMP, bone morphogenetic protein; CCL, chemokine (C-C motif) ligand; IFN, interferon; IL, interleukin; TNF, tumour necrosis factor; ZO, zonula occludens.
Statistical analysis
Statistics were obtained using graphpad prism version 4.03 (GraphPad, San Diego, CA). Data were analysed using the unpaired two-tailed t-test for comparison between two groups. One-way analysis of variance (ANOVA) followed by post-hoc Bonferroni’s test was used for multiple comparisons.
Results
Bacterial lipopeptide P3C selectively activates TLR2
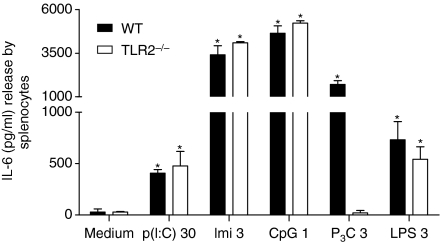
We first confirmed the selectivity of P3C by stimulating the splenocytes isolated from spleens of WT C57/BL6 and TLR2-deficient BL6 mice. We found that P3C selectively activated TLR2 and induced the release of IL-6 in WT cells. This response was completely abrogated in TLR2-deficient BL6 cells and was similar to that for medium control (Fig. 1). The IL-6 release upon stimulation with positive controls such as LPS, CpG, imiquimod and poly(I:C) was similar in WT and TLR2-deficient cells and was significantly higher than that for medium control (Fig. 1).
Figure 1.
In vitro stimulation of primary splenocytes. Primary splenocytes from spleens of Toll-like receptor 2 (TLR2)-deficient and wild-type (WT) C57/BL6 mice were cultured in a 24-well plate. Cells were stimulated with pam3cys (P3C; 3 μg/ml), lipopolysaccharide (LPS; 3 μg/ml), CpG (1 μg/ml), imiquimod (Imi; 3 μg/ml), poly(inosinic:cytidylic acid)-RNA [p(I:C); 30 μg/ml] or medium and incubated for 24 hr at 37°, and supernatants were harvested for analysis of interleukin (IL)-6 release. Note the abrogation of IL-6 release upon P3C stimulation in TLR2-deficient cells and note the increase in IL-6 release upon stimulation with LPS (3 μg/ml), CpG (1 μg/ml), imiquimod (3 μg/ml) or poly(I:C)-RNA (30 μg/ml). Data represent means ± standard deviation of three experiments, each performed in duplicate. *P < 0·05 versus medium for the respective group (TLR2−/− or WT).
P3C and LPS increase serum cytokines and lymphoproliferation in nephritic MRLlpr/lpr mice
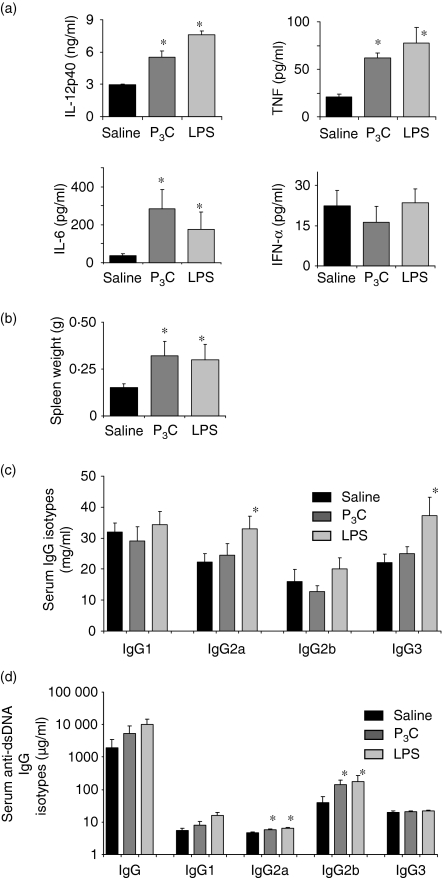
We investigated whether transient exposure to different bacterial cell wall components could aggravate immune complex disease, and if so, via which mechanisms. We used 16-week-old female MRLlpr/lpr mice as a model of active lupus nephritis and injected 15 μg of P3C or 10 μg of LPS intraperitoneally (i.p.) on every alternate day from week 16 to week 18 of age.14 We observed that serum levels of TNF, IL-6 and IL-12p40 were all significantly increased in 18-week-old MRLlpr/lpr mice injected with LPS or P3C as compared with mice in the saline group (Fig. 2a). In contrast, LPS and P3C did not affect serum levels of IFN-α in MRLlpr/lpr mice (Fig. 2a). LPS- and P3C-treated mice had significantly increased spleen weights (Fig. 2b). LPS increased serum total IgG2a and IgG3 isotype levels (Fig. 2c) and LPS and P3C both increased dsDNA autoantibodies of the IgG2a and IgG2b isotypes (Fig. 2d).
Figure 2.
Treatment with pam3cys (P3C) and lipopolysaccharide (LPS) and autoimmunity in 18-week-old MRLlpr/lpr mice. (a) Serum levels of interleukin (IL)-6, IL-12p40, tumour necrosis factor (TNF) and interferon (IFN)-α were measured by enzyme-linked immunosorbent assay (ELISA). (b) Spleen weights (g) of MRLlpr/lpr mice in all groups. Total serum immunoglobulin G (IgG) isotype levels (c) and serum anti-double-stranded DNA (dsDNA) IgG isotype levels (d) were measured by ELISA. Data in (a), (b), (c) and (d) represent mean ± standard error of the mean (n = 10 per group); *P < 0·05 versus saline.
P3C and LPS aggravate established lupus nephritis independently of the major effect on glomerular immune complex deposition and complement activation
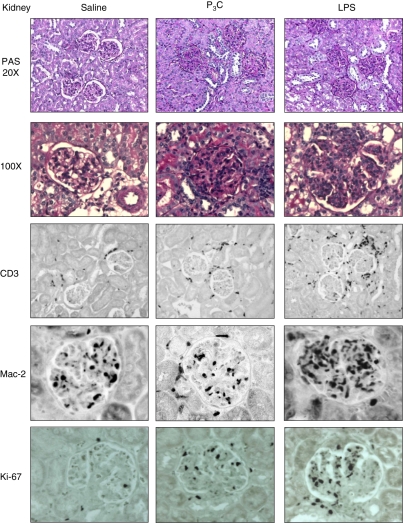
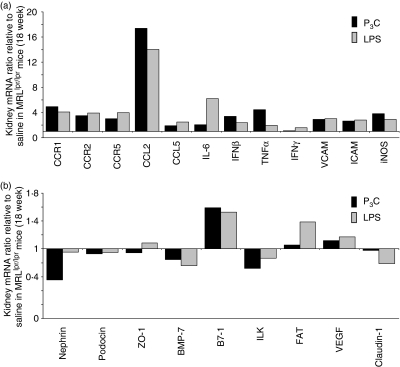
How do these effects of LPS and P3C on systemic autoimmunity translate to lupus nephritis in MRLlpr/lpr mice? LPS and P3C did not significantly increase glomerular IgG deposits or local complement factor C3c activation in kidneys compared with saline-treated MRLlpr/lpr mice (Table 3). Nevertheless, exposure to LPS and P3C caused significant aggravation of glomerular pathology, as indicated by the histopathological composite scores for activity and chronicity of lupus nephritis (Table 3 and Fig. 3). In LPS-treated MRLlpr/lpr mice the infiltration of macrophages and T cells into the glomerular and interstitial compartments was particularly increased (Table 3 and Fig. 3). This was associated with a significant increase in the number of Ki-67+ proliferating cells in both compartments (Table 3 and Fig. 3). Glomerular or diffuse interstitial B-cell infiltrates were not detected in kidneys of all groups (not shown). Renal mRNA levels of adhesion molecules [vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM)], chemokines [chemokine (C-C motif) ligand 2 (CCL2) and CCL5], their respective chemokine receptors (CCR1, CCR2 and CCR5), and cytokines such as TNF, IL-6, IFN-β, IFN-γ and injury marker inducible nitric oxide synthase (iNOS) were also increased in LPS- and P3C-treated kidneys of MRLlpr/lpr mice as compared with saline-treated controls (Fig. 4a, b). Thus, LPS and P3C aggravate lupus nephritis independent of a major effect on glomerular immune complex deposition and complement activation, which is consistent with their marginal effects on DNA autoantibody production.
Table 3.
Histological findings in 18-week-old MRLlpr/lpr mice
| Saline | P3C | LPS | |
|---|---|---|---|
| Histological scores | |||
| Kidney activity index | 5·1 ± 1·2 | 12·3 ± 4·51 | 14·0 ± 2·71 |
| Kidney chronicity index | 0·2 ± 0·3 | 2·9 ± 1·51 | 2·3 ± 1·11 |
| Lung | 0·9 ± 0·2 | 1·3 ± 0·41 | 1·4 ± 0·41 |
| Glom. IgG deposit score | |||
| IgG | 1·3 ± 0·1 | 1·2 ± 0·1 | 1·2 ± 0·1 |
| C3c | 1·4 ± 0·2 | 1·3 ± 0·2 | 1·4 ± 0·1 |
| Cellular response (cells/glom. or hpf) | |||
| Glom. Mac-2 + (cells/glom.) | 6·7 ± 0·8 | 6·2 ± 0·4 | 13·8 ± 0·61,2 |
| CD3 + (cells/glom.) | 0·4 ± 0·1 | 0·4 ± 0·1 | 3·0 ± 0·41,2 |
| Ki-67 + (cells/glom.) | 1·1 ± 0·1 | 3·3 ± 0·41 | 6·6 ± 0·71,2 |
| Interst. Mac-2 + (cells/hpf) | 3·3 ± 0·4 | 4·8 ± 0·6 | 6·6 ± 0·51 |
| CD3 + (cells/hpf) | 6·3 ± 0·5 | 7·5 ± 0·9 | 14·3 ± 1·61,2 |
| Ki-67 + (cells/hpf) | 2·8 ± 0·3 | 3·0 ± 0·2 | 7·0 ± 1·21 |
| Tubular Ki-67 + (cells/hpf) | 2·3 ± 0·2 | 3·2 ± 0·4 | 6·3 ± 1·21,2 |
Values are mean ± standard error of the mean; 1P < 0·05 versus saline; 2P < 0·05 versus P3C.
hpf, high-power field; IgG, immunoglobulin G; interst., interstitial; glom, glomeruli; LPS, lipopolysaccharide; P3C, pam3cys.
Figure 3.
Kidney histopathology analysis of MRLlpr/lpr mice. Kidney sections of 18-week-old MRLlpr/lpr mice of all groups were stained with periodic acid-Schiff (magnifications ×20 and ×100, respectively). For immunostaining of kidney sections, antibodies for Mac-2 (macrophages), CD3 (lymphocytes), or Ki-67 (proliferating cells) were used as indicated (magnification ×400). Images are representative of 10 mice in each group.
Figure 4.
RNA expression in kidneys of MRLlpr/lpr mice. RNA expression levels were determined by real-time reverse transcription–polymerase chain reaction (RT-PCR) as described in the Materials and methods using the primers and probes listed in Tables 1 and 2. (a, b) Total kidney RNA was pooled from 18-week-old MRLlpr/lpr mice (n = 10 per group), and analysed in duplicate. PCR results from (a) and (b) were first calculated for the respective 18S rRNA levels and then expressed as a ratio of either pam3cys (P3C)- versus saline-treated or lipopolysaccharide (LPS)- versus saline-treated mice. Hence, bars above the line representing a ratio of 1 indicate P3C- or LPS-induced induction of specific mRNA expression while bars below the line representing a ratio of 1 indicate P3C- or LPS-induced down-regulation of specific mRNA expression. Data in (a) and (b) represent the ratio relative to saline-treated mice. BMP-7, bone morphogenetic protein-7; CCL, chemokine (C-C motif) ligand; CCR, chemokine (C-C motif) receptor; FAT, FAT tumour suppressor homolog 1; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; ILK, integrin-linked kinase; iNOS, inducible nitric oxide synthase; TNF, tumour necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1.
P3C induces severe albuminuria in MRLlpr/lpr mice
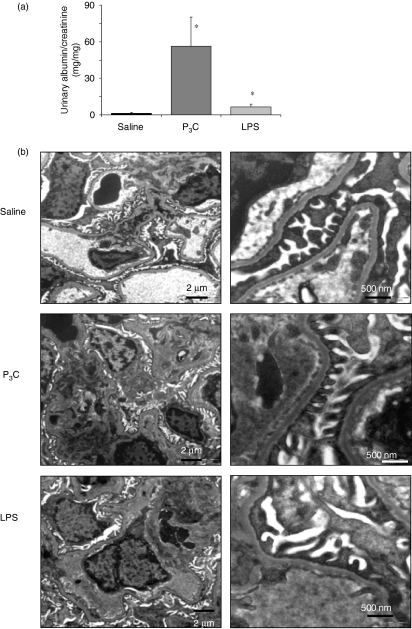
Under physiological conditions, the urinary loss of protein is prevented by an intact glomerular filtration barrier; thus, albuminuria is an important functional parameter of glomerular damage. LPS and P3C both significantly increased albuminuria in 18-week-old MRLlpr/lpr mice, and in the case of P3C the albuminuria induced was massive (Fig. 5a). In lupus nephritis massive albuminuria occurs as a manifestation of diffuse proliferative glomerulonephritis membranous glomerulonephritis or both.26,27 We could exclude P3C-induced membranous lupus nephritis because of the absence of epimembranous immune deposits as determined by IgG immunostaining (not shown) and electron microscopy (Fig. 5b). Together, LPS and P3C both aggravate lupus nephritis but only P3C induces massive albumiuria in nephritic MRLlpr/lpr mice. These findings are suggestive of specific effects of P3C on cells of the glomerular filtration barrier.
Figure 5.
Albuminuria and glomerular ultrastructural analysis of MRLlpr/lpr mice. (a) Albuminuria was assessed using the ratio of urinary albumin/creatinine (mg/mg) excretion in 10 mice in each group. Data represent the mean ± standard error of the mean (SEM) (n = 10 per group); *P < 0·05 versus saline. (b) Electron microscopy of glomerular capillary cross-sections of 18-week-old MRLlpr/lpr mice. Images show the fenestrated glomerular endothelial cells (GENCs) attached to the inner side of the glomerular basement membrane (GBM) of the capillary and the glomerular visceral epithelial cell (GVEC) foot processes attached to the outer part of the GBM of the capillary. The slit-diaphragm is the hardly visible membrane connecting the interdigitating foot processes next to the GBM. Treatment with pam3cys (P3C) and lipopolysaccharide (LPS) produced hypercellularity in the mesangial compartment but no marked ultrastructural differences in the structures of the glomerular filtration barrier. Black and white lines inside figures indicate scale or size in μm or nm.
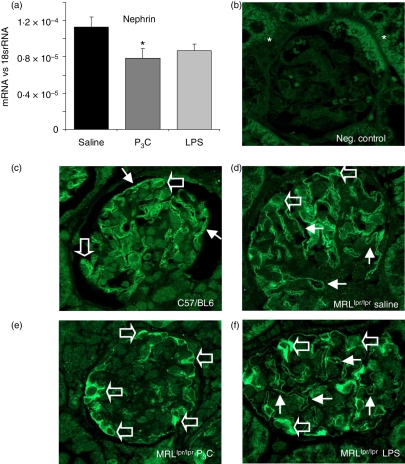
P3C modulates nephrin expression in GVECs of MRLlpr/lpr mice
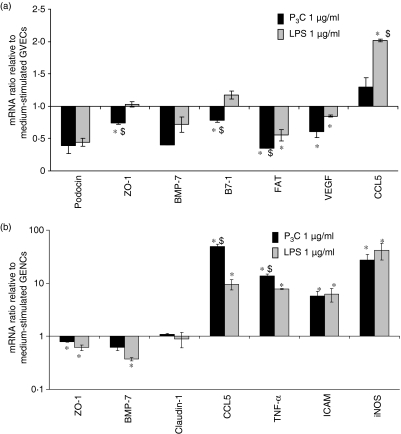
Massive albumiuria is often related to GVEC dedifferentiation indicated by foot process effacement, which could not be detected in P3C-treated MRLlpr/lpr mice with massive albuminuria (Fig. 5b).28 Hence, we hypothesized that P3C affects the functional properties of the GVECs without triggering foot process effacement.29 We therefore analysed the mRNA level of the slit-diaphragm-related protein nephrin in kidneys from all groups of MRLlpr/lpr mice. Interestingly, P3C significantly reduced the renal mRNA levels of nephrin (Fig. 6a), an important structural protein of the slit-diaphragm. Because the spatial distribution of nephrin is crucial for its function at the slit-diaphragm, we performed immunostaining to localize nephrin in the glomeruli of healthy C57/BL6 or nephritic MRLlpr/lpr mice.28 In the kidneys of healthy C57/BL6 mice, nephrin staining localized to GVEC foot processes along the outer glomerular basement membrane (Fig. 6b, c). The staining pattern was similar in nephritic MRLlpr/lpr mice (Fig. 6d) but exposure to P3C was associated with a redistribution of nephrin from foot processes to the perinuclear area of GVECs (Fig. 6e). In contrast, upon exposure to LPS, nephrin positivity was still robust in GVEC foot processes despite coincident positivity in the perinuclear area (Fig. 6f). We propose that one of the reasons for P3C-induced massive albuminuria is reduced expression of nephrin mRNA in the kidney and redistribution of nephrin protein in the GVECs of nephritic MRLlpr/lpr mice. The cell junction-associated proteins zonula occludens-1 (ZO-1) and FAT (FAT tumour suppressor homolog 1) were also significantly down-regulated in cultured GVECs (Fig. 7a). P3C also led to significant down-regulation of renal bone morphogenetic protein-7 (BMP-7) (Fig. 7a), a GVEC survival factor in cultured GVECs.29 These data suggest that bacterial cell wall components induce the activation of cultured GVECs as well as GVECs in nephritic MRLlpr/lpr mice. This modulates the expression of cell junction proteins which contribute to the functional integrity of the glomerular filtration barrier.
Figure 6.
Nephrin expression in kidneys of nephritic MRLlpr/lpr mice. (a) Nephrin mRNA levels were determined by real-time polymerase chain reaction (PCR) in duplicate using RNA isolated from kidneys of 18-week-old MRLlpr/lpr mice as described in the Materials and methods (n = 7 per group). Data are expressed as the mean ± standard error of the mean (SEM) versus the respective 18S rRNA. *P < 0·05 versus saline. (b, c) Kidneys of healthy C57/BL6 mice were stained either without (b) or with (c) a nephrin-specific antibody as negative and positive controls. Autofluorescence of tubular cross-sections is indicated by asterisks in (b). Note the pseudolinear nephrin positivity along the outer glomerular basement membrane illustrating its physiological expression pattern in glomerular visceral epithelial cell (GVEC) foot processes (arrows in c) while nephrin positivity is minimal in the perinuclear area of GVEC (open arrows in c). In kidneys of 18-week-old nephritic saline-treated MRLlpr/lpr mice (d) the distribution pattern of nephrin in GVECs is similar to that of healthy mice in (c). In P3C-treated MRLlpr/lpr mice the pseudolinear nephrin positivity along the outer glomerular basement membrane is much decreased (arrows in e) and the perinuclear GVEC positivity becomes very marked (open arrows in e). In lipopolysaccharide (LPS)-treated MRLlpr/lpr mice the pseudolinear nephrin positivity along the outer glomerular basement membrane is maintained (arrows in f) while the perinuclear GVEC positivity is also marked (open arrows in f) (n = 6 per group; magnification ×1000).
Figure 7.
RNA expression in cultured glomerular visceral epithelial cells (GVECs) and glomerular endothelial cells (GENCs). RNA expression levels were determined by real-time reverse transcription–polymerase chain reaction (RT-PCR) as described in the Materials and methods using the primers and probes listed in Tables 1 and 2. (a, b) Cultured GVECs (a) and GENCs (b) were incubated with 1 μg/ml of either pam3cys (P3C) or lipopolysaccharide (LPS) for 6 hr, when mRNA was obtained for RT-PCR duplicate analysis. The PCR results for (a) and (b) were first calculated for the respective 18S rRNA levels and then expressed as the ratio of either P3C- versus saline/medium-treated or LPS- versus saline/medium-treated mice. Hence, bars above the line representing a value of 1 indicate P3C- or LPS-induced induction of specific mRNA expression while bars below the line representing a value of 1 indicate P3C- or LPS-induced down-regulation of specific mRNA expression. Data in (a) and (b) represent the mean ± standard error of the mean for at least two independent experiments. *P < 0·05 versus medium; $P < 0·05 LPS versus P3C. BMP-7, bone morphogenetic protein-7; CCL, chemokine (C-C motif) ligand; FAT, FAT tumour suppressor homolog 1; ICAM, intercellular adhesion molecule; iNOS, inducible nitric oxide synthase; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1.
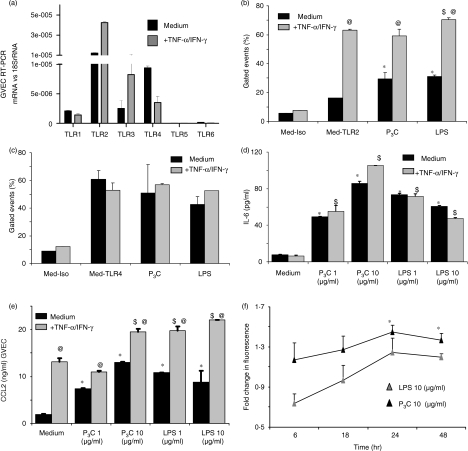
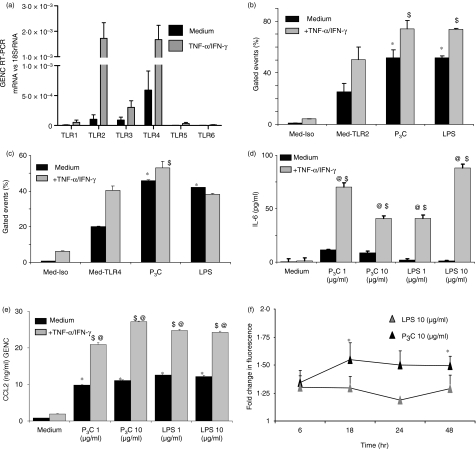
P3C and TNF/IFN-γ induce TLR2 expression and signalling in GVECs
TLR2 and TLR4 mRNA was strongly expressed by cultured GVECs (Fig. 8a). We used in vitro stimulation with the inflammatory mediators TNF and IFN-γ to mimic GVEC activation in glomerular inflammation. Interestingly, prestimulation with TNF/IFN-γ induced the expression of TLR2 mRNA (Fig. 8a), and was also observed to induce the surface expression of TLR2 protein by flow cytometry (Fig. 8b), suggesting that proinflammatory microenvironments significantly enhance TLR2 expression in GVECs. TLR2 surface expression was also induced by 1 μg/ml of P3C or LPS in GVECs (Fig. 8b), but neither of these factors induced TLR4 mRNA or protein expression (Fig. 8a, c). These findings were consistent with a robust effect of P3C stimulation on IL-6 and CCL2 production in GVECs (Fig. 8d, e). Does GVEC activation enhance albumin permeability? We addressed this question by testing the permeability of GVEC monolayers to fluorescein-labelled albumin. Albumin permeability was increased by P3C and LPS stimulation at 24 and 48 hr but only the increase induced by P3C was statistically significant (Fig. 8f). Furthermore, P3C (and LPS) significantly decreased mRNA expression of VEGF in GVECs (Fig. 7a), but LPS and P3C did not significantly affect the overall low proliferation rate of GVECs (data not shown). In summary, GVEC preactivation by proinflammatory cytokines such as TNF and IFN-γ induced TLR2 expression; in addition, P3C and LPS alone had significant effects on the expression of TLR2 and IL-6 or CCL2 release, and down-regulated VEGF.
Figure 8.
Expression and regulation of Toll-like receptors (TLRs) in glomerular visceral epithelial cells (GVECs). GVECs were expanded under permissive culture conditions (33°) and terminally differentiated at 37° as described in the Materials and methods. (a) RNA was isolated from GVECs kept in medium (black bars) or medium plus 500 units/ml tumour necrosis factor (TNF) and 200 units/ml interferon (IFN)-γ (grey bars) for 3 hr and TLR mRNA expression levels were determined by real-time polymerase chain reaction (PCR) and expressed as the ratio relative to the respective 18S rRNA levels. Data represent the mean ± standard error of the mean (SEM) of three independent experiments. (b, c) GVECs were stimulated as above for 24 hr and flow cytometry was performed for TLR2 (b) and TLR4 (c). Lipopolysaccharide (LPS) (1 μg/ml) and pam3cys (P3C) (1 μg/ml) were used for stimulation. Data are expressed as the percentage of gated events and represent the mean ± SEM of three independent experiments. Med-Iso, medium-treated cells stained with the isotype antibody; Med-TLR2, medium-treated cells stained with the TLR2 antibody; Med-TLR4, medium-treated cells stained with the TLR4 antibody; +TNF-α/IFN-γ, TNF-α/IFN-γ-treated cells; medium, medium-treated cells. (d, e) GVECs were stimulated as before and with increasing doses of P3C or LPS as indicated, and interleukin (IL)-6 and chemokine (C-C motif) ligand 2 (CCL2) were measured in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA). Data represent the mean ± SEM of three independent experiments. (f) GVEC monolayers were grown on hanging membrane inserts and the permeability of fluorescein-labelled bovine serum albumin loaded into inserts was determined in the medium from each well, as described in the Materials and methods. LPS (10 μg/ml) and P3C (10 μg/ml) were used for stimulation. Data illustrate the fluorescence optical density (OD) of the filterate of P3C- or LPS-treated groups versus the medium-treated group at different time-points as indicated. Data represent the mean ± SEM of four experiments, each performed in duplicate. *P < 0·05 versus medium; $P < 0·05 versus medium + TNF-α/IFN-γ; @P < 0·05 versus the respective ligand group with medium.
P3C and LPS both enhance TNF/IFN-γ-induced TLR2 and TLR4 signalling in GENCs
GENC activation may also cause proteinuria.29 Cultured GENCs expressed TLR2 and TLR4 mRNA and TNF/IFN-γ stimulation strongly induced both these TLR mRNAs (Fig. 9a). The same effect was noted at the protein level by flow cytometry (Fig. 9b, c). P3C stimulation had an additive effect on the surface expression of TLR2 and TLR4 whereas that of LPS was restricted to TLR2 (Fig. 9b, c). However, P3C and LPS both had strong additive effects with TNF/IFN-γ on GENC IL-6 and CCL2 production (Fig. 9d, e). P3C and LPS also significantly increased the mRNA expression of ICAM, CCL5, iNOS and TNF in cultured GENCs, indicating a severe endothelial cell injury response (Fig. 7b). P3C and LPS also induced up-regulation of CCL2 and iNOS levels in kidneys (Fig. 4). How does the potency of P3C and LPS translate to albumin permeability of GENCs? Albumin permeability was significantly increased in GENC monolayers upon stimulation with P3C at 18 and 48 hr (Fig. 9f). The effect of LPS was variable and did not reach statistical significance compared with medium at any time-point (Fig. 9f). LPS, P3C or TNF/IFN-γ did not significantly affect the proliferation of GENCs (data not shown). Together these data indicate that the proinflammatory environment induces TLR2 and TLR4, which has additive effects with P3C- and LPS to induce IL-6 and CCL2 release by GENCs. P3C increases albumin permeability in GENC monolayers and up-regulates iNOS, CCL5, TNF and ICAM levels in GENCs.
Figure 9.
Expression and regulation of Toll-like receptors (TLRs) in glomerular endothelial cells (GENCs). GENCs were cultured as described in the Materials and methods. (a) RNA was isolated from GENCs kept in medium (black bars) or medium plus 500 units/ml tumour necrosis factor (TNF) and 200 units/ml interferon (IFN)-γ (grey bars) for 3 hr, and TLR mRNA expression levels were determined by real-time polymerase chain reaction (PCR) and expressed as a ratio relative to the respective 18S rRNA levels. Data represent the mean ± standard error of the mean (SEM) of three independent experiments. (b, c) GENCs were stimulated as above for 24 hr and flow cytometry was performed for TLR2 (b) and TLR4 (c). Lipopolysaccharide (LPS) (1 μg/ml) and pam3cys (P3C) (1 μg/ml) were used for stimulation. Data are expressed as the percentage of gated events and represent the mean ± SEM of three independent experiments. Med-Iso, medium-treated cells stained with the isotype antibody; Med-TLR2, medium-treated cells stained with the TLR2 antibody; Med-TLR4, medium-treated cells stained with the TLR4 antibody; +TNF-α/IFN-γ, TNF-α/IFN-γ-treated cells; medium, medium-treated cells. (d, e) GENCs were stimulated as before and with increasing doses of P3C or LPS as indicated, and interleukin (IL)-6 and chemokine (C-C motif) ligand 2 (CCL2) were measured in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA). Data represent the mean ± SEM of three independent experiments. (f) Albumin permeability through GENC monolayers was determined as described in Fig. 6e. LPS (10 μg/ml) and P3C (10 μg/ml) were used for stimulation. Data illustrate the fold change in fluorescence optical density (OD) of the filterate of P3C- or LPS-treated groups versus the medium-treated group at different time-points as indicated. Data represent the mean ± SEM of four experiments, each performed in duplicate. *P < 0·05 versus medium; $P < 0·05 versus medium + TNF-α/IFN-γ; @P < 0·05 versus the respective ligand group with medium.
P3C or LPS does not trigger de novo glomerulonephritis
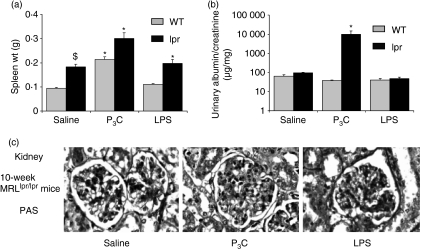
On the basis of in vitro data, one would predict that the local effects of P3C and LPS on GENCs and GVECs might in part depend on the presence of a proinflammatory environment, i.e. pre-existing glomerulonephritis. To test this hypothesis we injected 8-week-old non-nephritic female MRL WT or MRLlpr/lpr mice with LPS and P3C on alternate days for 14 days, as before. At 10 weeks of age, untreated MRLlpr/lpr mice had higher spleen weights than WT mice (Fig. 10a). Injection of P3C increased spleen weight in MRL and MRLlpr/lpr mice, an effect that was also observed in WT mice (Fig. 10a). However, LPS and P3C did not affect the production of dsDNA autoantibodies, glomerular IgG or complement deposits in 10-week-old MRL and MRLlpr/lpr mice (Table 4). LPS induced moderate glomerular hypercellularity with increased numbers of neutrophils in glomerular capillaries (not shown) which slightly increased the composite activity score (Table 4). However, LPS treatment did not affect the number of glomerular macrophages (not shown) or albuminuria (Fig. 10b). In contrast, P3C induced significant moderate albuminuria in MRLlpr/lpr mice but P3C-treated MRLlpr/lpr mice did not reveal any of the aforementioned histopathological glomerular abnormalities (Table 4 and Fig. 10c). In summary, P3C induced moderate albuminuria in 10-week-old MRLlpr/lpr mice but neither P3C nor LPS triggered de novo lupus nephritis.
Figure 10.
Treatment with pam3cys (P3C) and lipopolysaccharide (LPS) in 10-week-old MRLlpr/lpr mice. (a) Spleen weight (g) at 10 weeks of age in MRLlpr/lpr mice. Data are the mean ± standard error of the mean (SEM). *P < 0·05 versus the saline group (n = 5–7 per group). (b) Urinary albumin/creatinine ratios were measured as described in the Materials and methods and are expressed as a ratio in μg/mg. Data are the mean ± SEM (n = 5–7 per group). *P < 0·05 versus the saline group. (c) Kidney sections of 10-week-old MRLlpr/lpr mice of all groups were stained with periodic acid-Schiff (PAS) (magnifications ×400 and ×100, respectively); images are representative for 5–7 mice per group.
Table 4.
Histological findings in 10-week-old MRLlpr/lpr mice
| MRL WT mice |
MRLlpr/lpr mice |
|||||
|---|---|---|---|---|---|---|
| Saline | P3C | LPS | Saline | P3C | LPS | |
| Histological scores | ||||||
| Activity index | ND | ND | ND | 0·6 ± 0·5 | 0·7 ± 0·4 | 3·2 ± 0·62 |
| Chronicity index | ND | ND | ND | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 |
| Glomeruli IgG deposit score | ||||||
| IgG | ND | ND | ND | 1·3 ± 0·11 | 1·2 ± 0·11 | 1·2 ± 0·11 |
| C3c | ND | ND | ND | 0·4 ± 0·31 | 0·4 ± 0·31 | 0·4 ± 0·21 |
Values are mean ± standard error of the mean; 1P < 0·05 versus wild type (WT); 2P < 0·05 versus saline; IgG, immunoglobulin G; ND, not detectable; LPS, lipopolysaccharide; P3C, pam3cys.
Discussion
Bacterial cell wall components are potent triggers of innate and adaptive immune responses.2,3,30 In systemic autoimmunity such as systemic lupus erythematosus (SLE), one would expect that bacterial lipopeptides or LPS would aggravate immune complex disease, for example by activating B-cell proliferation, autoantibody production and the production of proinflammatory cytokines, as has been observed after transient exposure of autoimmune mice to microbial RNA and DNA.4,8,10 In this study we confirmed that LPS and P3C both aggravated lupus nephritis in MRLlpr/lpr mice by enhancing immune complex disease, but the finding that only P3C induced massive albuminuria suggests that P3C specifically modulates the glomerular filtration barrier.
The glomerular filtration barrier consists of three major components: the fenestrated GENC layer, the glomerular basement membrane and the interdigitating and slit-diaphragm forming GVECs. Massive proteinuria is commonly associated with GVEC dedifferentiation, often indicated by GVEC foot process effacement in humans but not always in proteinuric mice.28,29 Our data support the concept that bacterial cell wall components specifically activate GVECs to modulate their functional state. It is known that innate pathogen recognition and danger signalling are not restricted to immune cells; for example, most cell types respond to LPS, indicating that they express the TLR4 signalling complex.18 Here we show that the cell types of the glomerular filtration barrier express that subset of TLRs which allows recognition of additional bacterial cell wall components, i.e. TLR1, TLR2, TLR4 and TLR6.3,31 Our data demonstrate that LPS and P3C both activate GVECs and GENCs via TLR4 and TLR2, respectively. Exposure to very high doses of LPS (200 μg) triggers albuminuria in mice, a phenomenon that involves the induction of B7-1/CD80 (CD80 antigen) on GVECs.32 Our study shows that exposure of mice to lower doses of LPS (10 μg) remains associated with increased renal B7-1/CD80 expression and triggers significant albumiuria in nephritic MRLlpr/lpr mice. In contrast, low doses of P3C (15 μg) induced massive albuminuria in nephritic MRLlpr/lpr mice despite comparable effects of P3C and LPS on systemic autoimmunity and glomerular immune complex deposition. These data argue for different functional effects of TLR2 and TLR4 signalling on GVECs, GENCs or both.
GVECs express the structural and signalling proteins of the slit-diaphragm, a specialized contact structure between adjacent GVECs.28,32 Genetic deletions or acquired dysfunctions of slit-diaphragm-associated proteins are associated with proteinuria and, vice versa, proteinuric states are commonly associated with dysregulation of slit-diaphragm-associated proteins.28,34 For example, lack of functional nephrin, a major structural component of the slit-diaphragm, can cause congenital nephrotic syndrome of the Finnish type, a mostly fatal disease of the newborn.28 Our data show that bacterial lipopeptide modulated the physiological spatial expression of nephrin in GVEC foot processes and down-regulated nephrin mRNA expression in nephritic kidneys of MRLlpr/lpr mice. We also found that P3C treatment down-regulated the intracellular scaffolding proteins ZO-1 and FAT, which link the Neph family proteins of the slit-diaphragm to the cortical actin skeleton.28,33 This may have contributed to our finding that P3C significantly increased the albumin permability of GVEC monolayers and also increased the release of cytokine and chemokine by GVECs. However, the interpretation of these data is limited by the fact that GVEC monolayers do not entirely reproduce the morphology of GVECs in vivo. P3C also had significant potential to enhance albumin permeability in GENCs. Very strong activation of GENCs is associated with proteinuria, for example through down-regulation of tight junction proteins.31,35,36 P3C and LPS both down-regulated ZO-1 mRNA in cultured GENCs and induced the mRNA expression of endothelial cell (EC) activation or injury markers such as iNOS, ICAM, CCL5 and TNF in cultured GENCs. This mechanism parallels the increased EC permeability in Gram-positive or Gram-negative bacterial sepsis.37–40
Bacterial infections do not always trigger massive proteinuria or glomerulonephritis in humans. Consistently, low doses of bacterial lipopeptide or LPS did not trigger massive albuminuria or de novo glomerulonephritis in non-nephritic mice. It is known that bacterial infections often aggravate pre-existing glomerular disease, for example a flare of lupus nephritis. In this regard, our in vivo and in vitro data are consistent with the hypothesis that a proinflammatory microenvironment, such as that present in pre-existing glomerulonephritis, can enhance the recognition of bacterial cell wall components by inducing TLR2 and TLR4 expression in GENCs and TLR2 expression in GVECs. These data parallel our in vivo results which indicate bacterial lipopeptide-induced severe albuminuria only in mice with pre-established glomerular inflammation. This novel pathomechanism may explain why bacterial infections occasionally trigger massive proteinuria in patients with various clinical entities of glomerulopathies, including immune complex glomerulonephritis and renal vasculitis.
In summary, our data describe a novel molecular mechanism of bacterial infection-induced massive albuminuria in pre-established glomerular disease. We suggest that the potential of bacterial lipopeptide to induce massive albuminuria may be related to the effects of lipopeptide exposure and the presence of proinflammatory cytokines for enhancing TLR2 expression and TLR2-mediated effects which specifically modulate the functions of endothelial and epithelial cells of the glomerular filtration barrier.
Acknowledgments
The expert technical assistance of Dan Draganovic, Jana Mandelbaum, Ilka Edenhofer, Stephanie Pfeiffer, and Silvia Kaden is gratefully acknowledged. The authors are grateful to Dr Shizuo Akira, Osaka University, Japan for the generous gift of TLR2-deficient mice.
Glossary
Abbreviations:
- ANOVA
analysis of variance
- BMP-7
bone morphogenetic protein-7
- C3c
complement component C3c
- ddH2O
double-distilled water
- dsRNA
double-stranded RNA
- FCS
fetal calf serum
- GENC
glomerular endothelial cell
- GVEC
glomerular visceral epithelial cell
- ICAM
intercellular adhesion molecule
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- P3C
pam3cys
- poly(I:C)-RNA
poly(inosinic:cytidylic acid)-RNA
- TLR
Toll-like receptor
- VEGF
vascular endothelial growth factor
- ZO-1
zonula occludens-1
Grants
Deutsche Forschungsgemeinschaft (AN372/8-1, GRK 1202), EU Integrated Project INNOCHEM (FP6-518167), Else Kröner-Fresenius Stiftung and the Deutsche Forschungsgemeinschaft (SE888/4-1).
Disclosures
No conflict of interest.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signalling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Ehlers E, Ravetch JV. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007;28:74–9. doi: 10.1016/j.it.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 7.Pawar RD, Patole PS, Ellwart A, Lech M, Segerer S, Schlondorff D, Anders HJ. Ligands to nucleic acid-specific toll-like receptors and the onset of lupus nephritis. J Am Soc Nephrol. 2006;12:3365–73. doi: 10.1681/ASN.2006030263. [DOI] [PubMed] [Google Scholar]
- 8.Anders HJ, Vielhauer V, Eis V, et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL(Fas)lpr mice. FASEB J. 2004;18:534–6. doi: 10.1096/fj.03-0646fje. [DOI] [PubMed] [Google Scholar]
- 9.Anders HJ, Banas B, Linde Y, et al. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: role of TLR9-mediated expression of chemokines and chemokine receptors. J Am Soc Nephrol. 2003;14:317–26. doi: 10.1097/01.asn.0000042169.23931.73. [DOI] [PubMed] [Google Scholar]
- 10.Pawar RD, Patole PS, Zecher D, Segerer S, Kretzler M, Schlondorff D, Anders HJ. Toll-like receptor-7 modulates immune complex glomerulonephritis. J Am Soc Nephrol. 2006;17:141–9. doi: 10.1681/ASN.2005070714. [DOI] [PubMed] [Google Scholar]
- 11.Patole PS, Pawar RD, Lech M, et al. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol Dial Transplant. 2006;21:3062–73. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- 12.Tsuboi N, Yoshikai Y, Matsuo S, et al. Roles of Toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002;169:2026–33. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 13.Wörnle M, Schmid H, Banas B, et al. Novel role of Toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol. 2006;168:370–85. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patole PS, Grone HJ, Segerer S, et al. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol. 2005;16:1326–38. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- 15.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triantafilou M, Gamper FGJ, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem. 2006;281:31002–11. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 17.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipopeptides through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 18.Poltorak A, He X, Smirnova I, et al. Defective LPS signalling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 19.Brown HJ, Lock HR, Sacks SH, Robson MG. TLR2 stimulation of intrinsic renal cells in the induction of immune-mediated glomerulonephritis. J Immunol. 2006;177:1925–31. doi: 10.4049/jimmunol.177.3.1925. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Xie C, Chen J, Zhu J, Zhou H, Thomas J, Zhou XJ, Mohan C. Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-alpha production. J Immunol. 2006;176:632–9. doi: 10.4049/jimmunol.176.1.632. [DOI] [PubMed] [Google Scholar]
- 21.Brown HJ, Sacks SH, Robson MG. Toll-like receptor 2 agonists exacerbate accelerated nephrotoxic nephritis. J Am Soc Nephrol. 2006;7:1931–9. doi: 10.1681/ASN.2005111167. [DOI] [PubMed] [Google Scholar]
- 22.Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int. 2004;65:2223–7. doi: 10.1111/j.1523-1755.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 23.Mundel P, Reiser J, Borja AZM, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–58. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 24.Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- 25.Satchell SC, Buchatska O, Khan SB, et al. Interferon-beta reduces proteinuria in experimental glomerulonephritis. J Am Soc Nephrol. 2007;18:2875–84. doi: 10.1681/ASN.2006101104. [DOI] [PubMed] [Google Scholar]
- 26.Austin HA, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–95. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 27.Weening JJ, D’agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 28.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 29.Kalluri R. Proteinuria with and without renal glomerular podocyte effacement. J Am Soc Nephrol. 2006;17:2383–9. doi: 10.1681/ASN.2006060628. [DOI] [PubMed] [Google Scholar]
- 30.Mitu GM, Wang S, Hirschberg RR. BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am J Physiol Renal Physiol. 2007;293:F1641–8. doi: 10.1152/ajprenal.00179.2007. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki A, Medzitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;10:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 32.Camici M. Renal glomerular permselectivity and vascular endothelium. Biomed Pharmacother. 2005;59:30–7. doi: 10.1016/j.biopha.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–7. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiser J, Kritz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherence junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 35.Yanagida-Asanuma E, Asanuma K, Kim K, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signalling complexes in kidney podocytes. Am J Pathol. 2007;171:415–27. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280:H434–40. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- 37.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68:231–8. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 38.van Eijk L, Nooteboom A, Hendriks T, Sprong T, Netea M, Smits P, van der Hoeven J, Pickkers P. Plasma obtained during human endotoxemia increases endothelial albumin permeability in vitro. Shock. 2006;25:358–62. doi: 10.1097/01.shk.0000209527.35743.b0. [DOI] [PubMed] [Google Scholar]
- 39.Angelini DJ, Hyun SW, Grigoryev DN, et al. TNF- increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Pathol. 2005;167:1161–72. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- 40.Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738–43. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]