Abstract
The production of interferon-γ (IFN-γ) by infiltrating natural killer (NK) cells in liver is involved in the control of mouse hepatitis virus (MHV) infection. The objectives of this study were to identify the mechanisms used by MHV type 3 to modulate the production of IFN-γ by NK cells during the acute hepatitis in susceptible C57BL/6 mice. Ex vivo and in vitro experiments revealed that NK cells, expressing carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1a (the MHV receptor), can produce a higher level of IFN-γ in the presence of both L2-MHV3 and interleukin-12 (IL-12)/IL-18. The synergistic production of IFN-γ by NK cells depends on viral replication rather than viral fixation only, because it is inhibited or not induced in cells infected with ultraviolet-inactivated viruses and in cells from Ceacam1a−/− mice infected with virulent viruses. The synergistic IFN-γ production involves the p38 mitogen-activated protein kinase (MAPK) rather than the extracellular signal-regulated kinase-1/2 MAPK signalling pathway. However, the signal triggered through the engagement of CEACAM1a decreases the production of IFN-γ, when these molecules are cross-linked using specific monoclonal antibodies. These results suggest that control of acute hepatitis by IFN-γ-producing NK cells may depend on both production of IL-12 and IL-18 in the liver environment and viral infection of NK cells.
Keywords: CEACAM1a, coronavirus, hepatitis, interferon-γ, interleukin-12/interleukin-18, mitogen-activated protein kinase, natural killer cells
Introduction
Natural killer (NK) cells are an important arm of innate immunity against virus-infected cells, bacteria and tumour cells, and exert direct cytotoxicity functions as well as indirect antiviral functions through the secretion of interferon-γ (IFN-γ).1
The role of intrahepatic NK cells in the outcome of human viral hepatitis is not fully understood. NK cells are known to act as a first line to control many viral infections such as herpes simplex virus type 1, Epstein–Barr virus, human herpesvirus 6 and murine cytomegalovirus.2,3 However, human hepatitis C virus has developed strategies to evade the detection and elimination by NK cells,4 as is also suggested by the increased numbers of NK cells in the livers of patients during the immunotolerance phase of chronic hepatitis B virus infection.5 The liver is enriched in NK and NKT cells, which play major roles in the control of viral hepatitis infections by limiting viral replication via an IFN-γ-dependent mechanism. Recently, several studies have demonstrated the importance of the cytotoxic activities of NK cells in preventing the establishment of chronic human hepatitis C virus infections.6,7
Natural killer cells are rapidly recruited from the bone marrow and spleen during viral infections.8 Interleukin-12 (IL-12), IL-15, IL-18 and IFN-γ enhance the cytotoxic activity of NK cells and further increase the production of IFN-γ by these cells.1,2 Moreover, the production of IFN-γ by NK cells stimulated with IL-12 and IL-18 is dependent on the activation of the immunoreceptor tyrosine-based activation motifs (ITAMs) and is regulated through the p38 and extracellular signal-regulated kinase-1/2 mitogen-activated protein kinase (ERK-1/2 MAPK) pathways.9,10 However, little is known about the mechanism involved in the efficient production of antiviral IFN-γ by intrahepatic NK cells during the acute phase of viral hepatitis.
Mouse hepatitis virus (MHV) is an excellent model for studying the immunological disorders associated with viral hepatitis. The hepatotropic MHV3 serotype induces acute or chronic hepatitis according to the strain, age and immune status of the mouse. Moreover, MHV3 can replicate in hepatocytes, liver sinusoidal endothelial cells (LSEC) and Kupffer cells (KC), leading to virus-induced cell death, resulting in a fulminate hepatitis in susceptible C57BL/6 mice, and their death within 3–5 days postinfection (p.i.).11 We have previously reported that the development of hepatitis in pathogenic L2-MHV3-infected C57BL/6 mice is related to a decrease in splenic and myeloid NK cells because of the formation of syncytia and their subsequent apoptosis.12 Moreover, in vivo depletion of NK cells during MHV infections enhances virus replication, which in turn leads to a more pronounced hepatitis.13 In contrast, no or low decreases in NK cells were detected in mice infected with attenuated virus variants,12,14 suggesting a protective role of NK cells in hepatitis.
Interferon-γ is known to play a protective role against the hepatitis induced by various MHV serotypes.15,16 Very few studies have targeted the efficiency of NK cell functions during MHV infection. Recently, it was reported that enhanced protection in MHV-CXCL10-infected mice correlated with increased IFN-γ production by infiltrating NK cells within brain and liver.17 Furthermore, the addition of IL-12 and IL-18 in mice susceptible to MHV3 infection led to an increase in IFN-γ production in the liver and better control of the infection, although the IFN-γ-producing cells involved were not identified.18
Infection of susceptible cells by MHV depends on the fixation of viral surface proteins to a receptor, identified as the carcinoembryonic antigen-related cell adhesion molecule 1a (CEACAM1a). It was shown that CEACAM1 homotypic interactions between NK cells and various target cells inhibit NK cell cytotoxicity.19 In addition, the engagement of CEACAM1a leads to the inhibition of T-cell proliferation and IFN-γ production by NKT cells.20 In contrast, toll-like receptor-dependent activation of nuclear factor-κB upregulates the expression of CEACAM1a, whereas IFN-γ downregulates it, so decreasing the permissivity of susceptible cells to MHV infection.21,22 CEACAM1a is expressed at the surface of hepatic cells including LSEC, KC, hepatocytes and B and NK cells, but is scarce or not expressed by naive CD4+ or CD8+ T cells.23–26
It was reported that the p38 and ERK-1/2 MAPK pathways were activated in peritoneal macrophages infected with MHV3 within the first 30 min of infection, suggesting that this effect occurred in response to the fixation of the virus to its receptor.27 It was also suggested that the replication of the MHV-A59 virus was dependent on the activation of p38 but not the ERK-1/2 MAPK pathway, without further identifying the replication step involved.28 In this respect, the effect of the CEACAM1a engagement by MHV surface proteins and the further activation of the p38 or ERK-1/2 MAPK pathways on the antiviral function of NK cells remain unknown. The fact that Ceacam1a−/− mice are resistant to a MHV-A59 infection suggests the absolute requirement of CEACAM1a for viral infectivity.29
In this work, we demonstrate a synergistic production of IFN-γ by NK cells in the presence of both MHV3 and IL-12/IL-18, involving viral replication and the p38 MAPK signalling pathway, but decreased by the engagement of CEACAM1a.
Materials and methods
Mice
Wild-type C57BL/6 mice were purchased from Charles River Laboratories (St Constant, QC, Canada). Ceacam1a knockout (Ceacam1a−/−) mice were generated by Dr N. Beauchemin as previously described.30 The animals, certified as MHV3-free by the manufacturer, were housed under HEPA-filtered air (Forma Scientific, Marietta, OH). Female mice between 8 and 12 weeks of age were used in all the experiments. The study was conducted in compliance with the regulations of the Animal Committee of the Université du Québec à Montreal (UQAM).
Viruses
The pathogenic L2-MHV3 virus is a cloned substrain isolated from the liver of infected DBA2 mice and propagated in L2 cells as previously described.31 The pathogenic properties of the L2-MHV3 virus were assessed regularly.
In vivoviral infection
Groups of three or six C57BL/6 mice, according to the experiments, were infected by the intraperitoneal route with 1000 50% tissue culture infective dose (TCID50) of L2-MHV3. Mock-infected mice received a similar volume of RPMI-1640 (Gibco Laboratories, Grand Island, NY). After 72 hr of infection, the mice were anaesthetized by intraperitoneal injection using ketamine hydrochloride (200 mg/kg; Vetrepharm Canada Inc., Belleville, ON, Canada) and xylazine (10 mg/kg; Bayer Inc., Toronto, ON, Canada). Mice were bled by section of the portal vein and aortic artery. The livers were harvested following exsanguination and intrahepatic mononuclear cells (MNCs) were isolated as described by Watanabe et al.32
Cells
The continuous mouse fibroblast L2 cell line was grown in RPMI-1640 supplemented with l-glutamine (2 mm), antibiotics (penicillin 100 U/ml and streptomycin 100 mg/ml) (Gibco Laboratories) and 5% fetal calf serum (FCS). L2 cells were used for viral production.
Intrahepatic MNCs were isolated from the liver of mice from each experimental group as previously described.33 Briefly, the liver was pressed through a 70 μm cell strainer (Falcon Scientific Co., Montreal, QC, Canada) which was then washed with 10 ml RPMI-1640 supplemented with 20% FCS. The cell suspension was then deposited on 7 ml FCS to allow debris sedimentation; the top layer was then recovered and centrifuged for 10 min at 1000 g. The supernatants were collected for quantification of cytokines by enzyme-linked immunosorbent assay (ELISA) after been passed through a 0·45 μm filter (Sarstedt Inc., Montreal, QC, Canada). The cell suspension was deposited on the top of a discontinuous Percoll gradient [45% and 67% Percoll in phosphate-buffered saline (PBS)] (Amersham Pharmacia, Uppsala, Sweden; Gibco Laboratories) and centrifuged for 30 min at 1000 g. The MNCs were collected at the interface of the 45% and 67% Percoll layers and washed with RPMI-1640 supplemented with 20% FCS and finally adjusted to 0·5 × 106 or 1·0 × 106 cells/ml.
Myeloid MNCs were isolated from femurs of uninfected mice. Briefly, the femurs were soaked in 70% ethanol for 2–5 min and washed subsequently with RPMI-1640 supplemented with 20% FCS. The femurs were then excised from the surrounding muscle tissues. The marrow was flushed using a syringe filled with RPMI-1640 supplemented with 20% FCS and fitted with a needle calibre 26G3/8 (Becton Dickinson & Co., Franklin Lakes, NJ). The cell suspension was thereafter purified on a lymphoprep gradient (Cedarlane, Hornby, ON, Canada), washed and adjusted to 106 cells/ml.
Intrahepatic or myeloid NK cells were eliminated by positive selection using the CD49b (DX5 or Pan NK) antibody coupled to microbeads (StemCell Technologies, Vancouver, BC, Canada). Total myeloid MNCs, purified DX5+ cells or ‘DX5-depleted’ MNCs were resuspended in RPMI-1640 supplemented with 20% FCS and adjusted to 106 cells/ml.
In all experiments, the cell viability, ranging from 90% to 100%, was assayed by a trypan blue exclusion test.
Ex vivoviral infection
Intrahepatic MNCs were isolated from six mock- or L2-MHV3-infected C57BL/6 mice after 72 hr of infection and were seeded in 24-well plates at concentrations of 0·5 × 106 cells/ml in RPMI-1640 supplemented with 20% FCS. Recombinant murine IL-12 (rIL-12) or rIL-18 (BioSource, Montreal, QC, Canada) were added to a final concentration of 0·1 and 25 ng/ml, respectively. The cells were then incubated at 37°, under 5% CO2 for 24 hr. The supernatants were collected for IFN-γ quantification by ELISA.
In vitroviral infection
The MNCs were isolated from the livers or bone marrows from uninfected wild-type or Ceacam1a−/− C57BL/6 mice and cells were thereafter seeded in 24-well plates at a concentration of 106 cells/ml in RPMI-1640 supplemented with 20% FCS. Recombinant IL-12 or rIL-18 was added to a final concentration of 0·1 or 25 ng/ml, respectively. In some experiments, SB203580 and U0126, specific p38 and ERK-1/2 MAPK inhibitors (Calbiochem, San Diego, CA), were added to a final concentration of 10 μg/ml. Different concentrations of sodium stibogluconate (SS) were also added during 15 min to inhibit the SHP-1 phosphatase. A monoclonal murine anti-CEACAM1a antibody (AgB10, produced in rat and affinity-purified on a HiTrap protein G column) was also added at a concentration of 2 μg/106 cells. The cells were infected with a 0·1–1·0 multiplicity of infection of infectious L2-MHV3 or L2-MHV3 treated for 1 hr with ultraviolet (UV) light and then incubated at 37°, under 5% CO2 for 24 hr. The supernatants were collected for IFN-γ quantification by ELISA.
Flow cytometric analysis
Percent of intrahepatic NK1.1+ TCR-β– cells from in vivo mock- or L2-MHV3-infected C57BL/6 mice were determined by double immunolabelling. The intrahepatic MNCs were isolated and 106 cells were resuspended in 1 ml PBS and further incubated on ice in the presence of a CD16/CD32 blocker (Pharmingen, Toronto, ON, Canada) for 15 min. Thereafter, the cells were incubated for 30 min with 1 μg fluorescein isothiocyanate-conjugated NK1.1 [clone PK136; mouse immunoglobulin G2aκ (IgG2aκ); Pharmingen] and 1 μg phycoerythrin-conjugated T-cell receptor-β (TCR-β; clone H57-597; Armenian Hamster IgG2 λ1; Pharmingen). The cells were then washed in PBS and fixed overnight at 4° in PBS, pH 7·2 containing 1% formaldehyde (Fisher Scientific Co., Montréal, Qué, Canada). Flow cytometric analyses were performed on a fluorescence-activated cell flow cytometer (FACScan) with cell quest software (Becton-Dickinson, Moutain View, CA). Ten thousand cells were analysed per sample and the percentages of NK1.1+ TCR-β– cells were determined by a multiparametric analysis.
Cytokines determination by ELISA
Interferon-γ, IL-12 and IL-18 levels produced in supernatants from ex vivo or in vitro infections or in liver extracts were determined using mouse IL-12 (p70), mouse IL-18 and mouse IFN-γ BD OptEIA ELISA sets (BD Biosciences, Mississauga, ON, Canada).
Statistical analysis
For in vivo and ex vivo studies, statistical analyses were performed using an analysis of variance (anova) test. For in vitro studies, statistical analyses were performed using a Student’s t-test. All statistical comparisons were calculated with graphpad prism 4.03 software (GraphPad Software Inc., La Jolla, CA). Error bars represent standard errors and a value of P < 0·05 was considered significant.
Results
MHV3 infection synergizes the production of IFN-γ by intrahepatic MNCs in the presence of IL-12 and IL-18
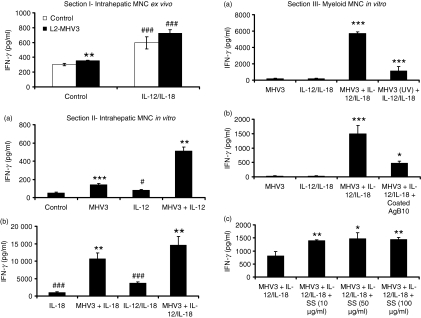
To verify the ability of intrahepatic MNCs from L2-MHV3-infected mice to produce IFN-γ during the acute phase of hepatitis, intrahepatic MNCs from six mock- or L2-MHV3-infected C57BL/6 mice were isolated after 72 hr of infection and activated ex vivo with rIL-12/rIL-18 for 24 hr. Levels of IFN-γ were then evaluated in cell supernatants. As shown in Fig. 1 (section I), unstimulated intrahepatic MNCs isolated from L2-MHV3-infected mice produced slightly more IFN-γ than cells from mock-infected mice (P < 0·01). Addition of rIL-12 and rIL-18, however, increased the production of IFN-γ by MNCs from both L2-MHV3- and mock-infected mice (P < 0·001).
Figure 1.
Section I – Production of interferon-γ (IFN-γ) from intrahepatic mononuclear cells (MNCs) purified from mock- and L2-murine hepatitis virus (MHV)-3-infected C57BL/6 mice after 72 hr and treated ex vivo for 24 hr with recombinant interleukin-12 (rIL-12) and rIL-18. Six mice were used in each experimental group. The IFN-γ levels were quantified in supernatants by enzyme-linked immunosorbent assay (**P < 0·01 when compared with untreated mock-infected cells; ###P < 0·001 when compared with respective untreated cells). Section II – Production of IFN-γ in in vitro mock-infected and L2-MHV3-infected intrahepatic MNCs from C57BL/6 mice at 24 hr postinfection in the absence or presence of rIL-12 (a), or with rIL-18 and rIL-12/rIL-18 (b) (**P < 0·01 and ***P < 0·001 when compared with respective uninfected cells, #P < 0·05 and ###P < 0·001 when compared with untreated uninfected cells). Section III – Production of IFN-γ at 24 hr postinfection in in vitro myeloid MNCs from C57BL/6 mice infected with infectious L2-MHV3 and ultraviolet-treated L2-MHV3 (a), pretreated or not with 2 μg coated AgB10 (b) or pretreated or not with different concentrations of SS (c) in the presence of rIL-12/rIL-18 at 24 hr postinfection (*P < 0·05, **P < 0·01 and ***P < 0·001).
To verify if the viral infection is involved in the production of IFN-γ by intrahepatic MNCs, these cells were isolated from uninfected C57BL/6 mice and infected in vitro with the L2-MHV3 for 24 hr in the presence of rIL-12, rIL-18 or both. The IFN-γ released in the cell supernatants was then quantified. As shown in the Fig. 1 (section II, a), L2-MHV3 infection of intrahepatic MNCs induced low but significant IFN-γ production (P < 0·001). Addition of rIL-12 (P < 0·05), rIL-18 (P < 0·001) or rIL-12/rIL-18 (P < 0·001) to uninfected intrahepatic MNCs further increased the IFN-γ production (Fig. 1, section II a and II b). Production of IFN-γ by intrahepatic MNCs, however, synergistically increased when the cells were simultaneously treated in vitro with both the L2-MHV3 and cytokines (P < 0·01 for all treatments when compared with the uninfected treated cells) (Fig. 1, section II a and II b). These results indicate that IL-12 and IL-18 act in synergy with the L2-MHV3 on one or more susceptible cell types present in the intrahepatic MNC culture from uninfected mice to produce IFN-γ.
NK cells are responsible for the production of IFN-γ in response to a combined treatment with the L2-MHV3 and IL-12/IL-18
It was previously demonstrated that the production of IFN-γ in the liver in response to IL-12 administration depends on NK cells.34 To verify if the IFN-γ response, induced in the presence of rIL-12 and rIL-18 during an in vitro L2-MHV3 infection, mainly depends on NK cells, intrahepatic MNCs were isolated from uninfected mice and NK cells were removed with an anti-DX5 antibody coupled to microbeads (< 2% DX5+ cells remaining in the DX5-depleted cell preparation). Total MNCs and DX5-depleted cell preparations were thereafter infected in vitro with L2-MHV3 in the presence of rIL-12/rIL-18. The IFN-γ level strongly decreased in DX5-depleted cells (1201 ± 146 pg/ml) compared with total intrahepatic MNCs (8236 ± 198 pg/ml) (P < 0·001), when infected with the L2-MHV3 and further treated with rIL-12/rIL-18. Indeed, the levels of IFN-γ were comparable to those measured with uninfected rIL-12/rIL-18-treated cells from total intrahepatic MNCs (1403 ± 316 pg/ml) or DX5-depleted cells (1047 ± 61 pg/ml). The number of positively selected intrahepatic NK cells from uninfected mice remains too low to allow detection of high levels of IFN-γ by ELISA, because the absolute numbers of intrahepatic NK1.1+ TCR– cells in normal C57BL/6 mice reached approximately 105 cells/liver.12
However, intrahepatic NK cells were recruited in the liver from the bone marrow or spleen in L2-MHV3-infected mice.12,14 The number of NK cells expressing the CEACAM1a receptor was significantly higher in the bone marrow when compared with the spleen or the liver,35 and so bone marrow MNCs were then used as a source of NK cells. MNCs from bone marrow were isolated and NK cells were thereafter purified with an anti-DX5 antibody coupled to microbeads. Total myeloid MNCs, purified DX5+ cell preparations (96% purity) and DX5-depleted cell preparations (< 2% DX5+ cells remaining) were then infected in vitro with L2-MHV3 in the presence of rIL-12 and rIL-18. As shown in Table 1, low IFN-γ was produced by total myeloid MNCs infected with L2-MHV3 or treated only with rIL-12/rIL-18. However, addition of these cytokines to L2-MHV3-infected myeloid MNCs induced a synergistic production of IFN-γ when compared with untreated L2-MHV3-infected cells (P < 0·001) or with uninfected rIL-12/rIL-18-treated cells (P < 0·001). No significant IFN-γ was produced by uninfected or L2-MHV3-infected DX5-depleted myeloid MNCs, treated or not with rIL-12 and rIL-18, suggesting that NK cells were the only cells involved in the production of IFN-γ. Purified DX5+ cells produced IFN-γ when stimulated with rIL-12/rIL-18 (P < 0·001 compared with untreated cells) and responded in a synergistic manner when infected with L2-MHV3 (P < 0·001 compared with rIL-12/rIL-18-treated cells). These results indicate that NK cells are involved in the synergistic production of IFN-γ induced both by the L2-MHV3 and the cytokines.
Table 1.
Production of interferon-γ at 24 hr postinfection in L2-MHV3-infected myeloid mononuclear cells from wild-type and Ceacam1a−/− C57BL/6 mice in the absence or presence of rIL-12/rIL-18 and p38 MAPK (SB203580) or ERK-1/2 MAPK (U0126) inhibitor
| IFN-γ (pg/ml) | ||||
|---|---|---|---|---|
| Myeloid cells1 | Control | L2-MHV3 | rIL12/rIL-18 | L2-MHV3+ rIL12/rIL-18 |
| C57BL/6 mice | ||||
| Total cells | < 31·32 | < 31·3 | < 31·3 | 799 ± 243*** |
| +SB2035803 | – | – | – | 79 ± 42*** |
| +U01263 | – | – | – | 577 ± 254 |
| DX5-depleted cells4 | < 31·3 | < 31·3 | 43 ± 33 | 127 ± 64 |
| Purified DX5+ cells4 | < 31·3 | < 31·3 | 646 ± 99*** | 1664 ± 277*** |
| +SB2035803 | – | – | – | 504 ± 99*** |
| +U01263 | – | – | – | 1361 ± 84 |
| Ceacam1a−/− mice | ||||
| Total cells | < 31·3 | < 31·3 | < 31·3 | < 31·3 |
ERK-1/2, extracellular signal-regulated kinase-1/2; IFN-γ, interferon-γ; MAPK, mitogen-activated protein kinase; MHV3, murine hepatitis virus 3; MNC, mononuclear cells; NK, natural killer; rIL-12, recombinant interleukin-12.
Myeloid MNCs were obtained from wild-type and Ceacam1a−/− C57BL/6 mice and infected with the L2-MHV3 virus in the presence of rIL-12/rIL-18 for 24 hr.
The IFN-γ levels were quantified in supernatants by enzyme-linked immunosorbent assay (***P < 0·001). The limit of sensitivity of the IFN-γ test was 31·3 pg/ml.
Total myeloid MNCs and purified NK cells were obtained from wild-type C57BL/6 mice, treated for 2 hr with a p38 MAPK (SB203580) or ERK-1/2 MAPK (U0126) inhibitor and thereafter infected with L2-MHV3 in the presence of rIL-12/rIL-18.
DX5-depleted cells and purified DX5+ cells were obtained by treatment of total myeloid MNCs with the CD49b (DX5 or Pan NK) monoclonal antibody coupled to microbeads (< 2% DX5+ cells remaining in the DX5-depleted cells preparation; 96% purity in the purified fraction).
Ceacam1a−/− NK cells do not promote the synergistic IFN-γ production in the presence of IL-12 and IL-18
To verify if the production of IFN-γ by L2-MHV3-infected NK cells, treated with IL-12 and IL-18 is initiated by the viral replication, cells from Ceacam1a−/− mice (the specific MHV receptor) were compared with those from wild-type C57BL/6 mice. Myeloid MNCs from wild-type and Ceacam1a−/− C57BL/6 mice were infected in vitro with L2-MHV3 for 24 hr in the presence of rIL-12/rIL-18. The IFN-γ levels in supernatants were further quantified. Results revealed that no IFN-γ was induced by myeloid MNCs from Ceacam1a−/− mice when treated with rIL-12/rIL-18 infected or not with L2-MHV3 (Table 1). In addition, no viral proteins were detected in myeloid NK cells from Ceacam1a−/− mice following a double immunolabelling using anti-DX5 and anti-MHV3 antibodies, indicating that no viral replication occurred in these cells.
Viral replication is essential to promote the synergistic IFN-γ production in NK cells
The failure of Ceacam1a−/− cells to produce IFN-γ suggests that viral fixation or viral replication is essential for this response. The role of viral replication was then verified using UV-inactivated viruses. Myeloid MNCs from wild-type C57BL/6 mice were infected with infectious or UV-inactivated L2-MHV3 in the presence of rIL-12/rIL-18. Levels of IFN-γ were thereafter determined in supernatants at 24 hr p.i. As shown in Fig. 1 (section III a), production of IFN-γ strongly decreased when UV-inactivated L2-MHV3 was added to cells in the presence of rIL-12/rIL-18 (P < 0·001). The absence of viral translation in myeloid NK cells infected with the UV-inactivated viruses was also confirmed by a double immunolabelling using anti-DX5 and anti-MHV3 antibodies, because no intracellular viral proteins were detected.
However, when bone marrow MNCs were treated with rIL-12/rIL-18 for 2 hr before infection with the virus, the synergistic IFN-γ production failed to occur (data not shown), indicating that the cellular signalling pathway(s) involved in this response may be simultaneously activated by cytokines and the first steps of viral replication.
The p38 MAPK pathway, rather than ERK-1/2 MAPK, is involved in synergistic IFN-γ production in NK cells
The p38 and ERK-1/2 MAPK pathways are mainly involved in the production of IFN-γ by NK cells when stimulated by IL-12 and IL-18.9,10 Moreover, unidentified early steps of MHV replication also depend on activation of the p38 and ERK-1/2 MAPK pathways,27,28 whereas CEACAM1a engagement predominantly induces the ERK-1/2 MAPK pathway,36 so verifying if the IFN-γ response depended on the p38 and/or ERK-1/2 MAPK pathways. Total bone marrow MNCs and purified DX5+ cells (96% purity) from wild-type C57BL/6 mice were treated for 2 hr with specific inhibitors of p38 (SB203580) and ERK-1/2 (U0126) MAPK and thereafter infected with L2-MHV3 in the presence of rIL-12 and rIL-18. The IFN-γ levels were then quantified 24 hr p.i. As shown in Table 1, production of IFN-γ by L2-MHV3-infected total bone marrow MNCs treated with rIL-12 and rIL-18 significantly decreased in the presence of the p38 MAPK inhibitor (P < 0·001), when compared with L2-MHV3-infected cells treated only with the cytokines. Such an effect was conserved in the purified myeloid DX5+ cell fraction (P < 0·001 for the p38 MAPK inhibitor). The ERK-1/2 MAPK inhibitor did not induce any effect.
Inhibitory role of CEACAM1a in the IFN-γ production in the presence of MHV3
It is known that cross-linking or homotypic interactions between CEACAM1a are essential to induce an inhibitory response in NK or NKT cells.19,20 We therefore studied if the cross-linking of the anti-CEACAM1a monoclonal antibody (AgB10) could provide this effect. We had verified first that the specific antibody AgB10 did not induce or inhibit by itself the synergistic IFN-γ production in the presence of rIL-12/rIL-18 only (results not shown). Then, AgB10 was coated on 24-well plates for 2 hr after which myeloid MNCs from wild-type C57BL/6 were seeded and infected with L2-MHV3 in the presence of rIL-12/rIL-18. The IFN-γ levels were then quantified after 24 hr p.i. As shown in Fig. 1 (section III b), the synergistic IFN-γ production was inhibited by coated AgB10, suggesting that cross-linking of CEACAM1a can downregulate the synergistic response induced by L2-MHV3 in the presence of IL-12/IL-18 or compete with the viral fixation to the CEACAM1a receptor (P < 0·05 when compared with L2-MHV3 treatment plus rIL-12/rIL-18).
To further support the hypothesis of the engagement of CEACAM1a in the downregulation of the IFN-γ response, bone marrow MNCs from wild-type C57BL/6 mice were treated with different concentrations of a SHP-1 phosphatase inhibitor (SS), which is involved in the inhibitory signalling pathway of CEACAM1a37 but not in viral replication, before infection with L2-MHV3 in the presence of rIL-12/rIL-18. The IFN-γ levels were thereafter quantified after 24 hr p.i. As shown in Fig. 1 (section III c), production of IFN-γ increased when myeloid cells were treated with the SHP-1 phosphatase inhibitor at concentrations ranging from 10 to 100 μg/ml (P < 0·01 at 10 and 100 μg/ml, P < 0·05 at 50 μg/ml). This result supports the inhibitory role of CEACAM1a rather than neutralization of viral fixation.
Impairment of IL-12, IL-18 and IFN-γ production and increase of apoptotic NK1.1+ TCR-β– cells in liver from L2-MHV3-infected C57BL/6 mice
We have recently demonstrated that the level of hepatitis in mice infected with L2-MHV3 depends on viral permissivity of KC and LSEC,16 involved in the production of intrahepatic IL-12 and IL-18 and the subsequent IFN-γ production by NK cells. Intrahepatic IFN-γ, IL-12 and IL-18 levels were then evaluated by ELISA tests in liver extracts from six C57BL/6 mice infected with 1000 TCID50 of the L2-MHV3 at 72 hr p.i. The intrahepatic IFN-γ was not induced in livers from L2-MHV3-infected mice at 72 hr p.i. compared with mock-infected mice (< 31·3 pg/ml in both mice). This absence of IFN-γ production may be related to the inability of hepatic cells to produce adequate levels of IL-12 and IL-18. Effectively, the intrahepatic IL-12 was not induced in L2-MHV3-infected mice after 3 days of infection compared with mock-infected mice (< 62·5 pg/ml in both mice). Furthermore, intrahepatic IL-18 levels decreased in L2-MHV3-infected mice during the acute hepatitis (mock-infected mice: 888 ± 175 pg/ml; L2-MHV3-infected mice: 608 ± 50 pg/ml) (P < 0·01). These cytokine decreases were also observed as early as 24 hr p.i. (results not shown).
In addition, we have previously reported that NK cells from C57BL/6 mice support viral replication and subsequent apoptosis. To verify if the decrease of IFN-γ production is related to apoptosis of intrahepatic NK cells during the acute phase of the viral hepatitis, three C57BL/6 mice were infected with 1000 TCID50 of L2-MHV3 and intrahepatic MNCs were thereafter isolated at different times p.i. Intrahepatic MNCs were then labelled with fluorescein isothiocyanate-conjugated anti-NK1.1 and phycoerythrin-conjugated anti-TCR-β antibodies and analysed by fluorometry. Two distinct cell populations were observed according to the forward/side scatter (FSC/SSC) parameters. Normal lymphoid cells were characterized by normal FSC and SSC parameters (TUNEL+ cells < 10·0 ± 2·4%), whereas apoptotic lymphoid cells, characterized by lower FSC and higher SSC parameters, were confirmed by a TUNEL test (97·0 ± 3·0% positive cells). Intrahepatic NK1.1+ TCR-β– cells from L2-MHV3-infected mice underwent apoptosis from 36 hr p.i. (mock-infected mice: 1·8 ± 0·1%; L2-MHV3-infected mice: 10·4 ± 1·6%; P < 0·01) to 72 hr p.i. (mock-infected mice: 4·8 ± 1·1%; L2-MHV3-infected mice: 20·3 ± 2·5%; P < 0·01) and peaking at 48 hr p.i. (mock-infected mice: 6·0 ± 0·2%; L2-MHV3-infected mice: 25·5 ± 1·6%; P < 0·001).
Discussion
In this study, we report that L2-MHV3, in the presence of IL-12 and IL-18, induces a synergistic IFN-γ production by NK cells from Ceacam1a+/+ mice. This effect was shown to be dependent on viral replication, and was under the control of the p38 MAPK but not the ERK-1/2 MAPK signalling pathway. However, the signal triggered through the engagement of CEACAM1a inhibits the synergistic production of IFN-γ.
In this work, we have demonstrated that MHV3 can induce a synergistic IFN-γ response, both in intrahepatic and myeloid MNCs, in the presence or absence of IL-12/IL-18. This property was mainly the result of NK (DX5+) cells because it was lost when NK cells were depleted, whereas purified NK cells exhibit this synergistic IFN-γ response. It was important to demonstrate the role of NK cells in the production of IFN-γ, since CD8+ T cells, or NKT cells, which are also presents in the liver and/or the bone marrow, can be other sources for IFN-γ in response to IL-12/IL-18 treatment.38,39 However, neither CD4+ nor CD8+ T cells expressed CEACAM1a, or have a very low level of expression,24 and can be infected by L2-MHV3.40 On the other hand, B cells, which may be infected by MHV3,41 are not known to be IFN-γ-producing cells.
We have previously reported that NK cells are a new cell target for MHV.12 These cells can express the viral CEACAM1 receptor.42 In this context, the synergistic production of IFN-γ by NK cells in the presence of a coronavirus requires CEACAM1a. Effectively, NK cells from Ceacam1a−/− mice do not produce IFN-γ in a synergistic manner in response to IL-12/IL-18 and L2-MHV3. The absence of a synergistic response of cells from Ceacam1a−/− mice did not result from functional anomalies of NK cells because no significant differences in the intrahepatic lymphoid cell subpopulation, or in NK cell functions, have been observed in cells from Ceacam1a−/− mice when compared with wild-type C57BL/6 mice. Moreover, no maturation defects of NK cells have been detected in the bone marrow of Ceacam1a−/− mice compared with wild-type C57BL/6 mice (unpublished observations from Dr Beauchemin’s team). However, our results suggest that L2-MHV3 may induce this synergistic IFN-γ production independently of CEACAM1a, but requires this molecule to enter into NK cells. This hypothesis is also supported by the involvement of viral replication rather than viral fixation, as demonstrated by the use of UV-inactivated MHV3 and the p38 MAPK inhibitor.
In addition, CEACAM1a receptor does not directly trigger the production of IFN-γ by NK cells, as the addition of a specific anti-CEACAM1a antibody (AgB10) did not induce production of this cytokine. AgB10 binds an area between the first and second immunoglobulin domains of the CEACAM1a.29,43 However, experiments using plates bound with anti-CEACAM1a AgB10 antibodies, or the SHP-1 inhibitor, revealed that engagement of CEACAM1a receptors activates the SHP-1 signalling pathway, which in turn leads to the inhibition of IFN-γ production by NK cells in the presence of L2-MHV3. The CEACAM1a receptor exists under two isoforms constituted either of a long inhibiting cytoplasmic domain, which contains immunoreceptor tyrosine-based inhibitory motifs (ITIMs), or a short activating cytoplasmic domain, which does not possess ITIMs or ITAMs.44 Ortaldo et al.45 have recently demonstrated that IL-12 and IL-18 override the inhibitory mechanisms of the Ly-49 inhibitory receptors containing ITIMs, so enabling IFN-γ production. These authors have nevertheless noticed that cross-linking of activating Ly49 receptors which possess ITAMs is essential for a response to IL-12 and IL-18. CEACAM1a receptor has already been associated with the inhibition of the IFN-γ production by human NKT cells.20 In our study, CEACAM1a may play an inhibitory role, by cross-linking or homotypic interactions, in IFN-γ production by NK cells. On the other hand, inhibition of SHP-1, which is associated with ITIMs,35 increased the production of this cytokine. CEACAM1a may therefore be involved in the modulation of an IL-12/IL-18-dependent IFN-γ pathway in NK cells. We can hypothesize that the high number of viral S proteins produced by MHV3-infected hepatic cells may bind to CEACAM1a molecules expressed at the NK cell surface attenuating the secretion of IFN-γ.
Our results indicate that the synergistic response of IFN-γ in L2-MHV3-infected NK cells, in the presence of rIL-12 and rIL-18, is completely dependent on the p38, but not the ERK-1/2, MAPK pathway. The p38 MAPK pathway therefore links the viral infection in the NK cells with activation mediated by rIL-12 and rIL-18. The production of IFN-γ by NK cells stimulated with IL-12 and/or IL-18 is dependent on the activation of ITAMs and is regulated through the p38 and ERK-1/2 MAPK pathways.9,10 Pretreatment of NK cells with IL-12 for 15 min is sufficient for IFN-γ induction.10 Simultaneously, the p38 MAPK pathway is essential for the first steps of MHV replication,28 whereas the ERK-1/2 MAPK pathway is predominantly activated by CEACAM1a.36 We have observed that UV-inactivated L2-MHV3 fails to induce IFN-γ production compared with the infectious L2-MHV3, indicating that a RNA-dependent phase of the viral replication, rather than viral fixation to CEACAM1a receptor, is involved in the synergistic IFN-γ production. The UV-inactivated virus may bind to the CEACAM1a receptor and be internalized, but cannot induce the translation of viral messenger RNA. Effectively, Banerjee et al.28 have demonstrated that UV-inactivated MHV failed to activate MAPKs, whereas activation of p38 MAPK and c-Jun terminal kinase are essential for phosphorylation of the translation initiation factor 4E involved in the enhancing of translation rates of cap-containing messenger RNA.
An absence of synergistic IFN-γ production was observed between L2-MHV3 and rIL-12/rIL-18 added at an interval of 2 hr p.i. This result reinforces the implication of the synergistic production of IFN-γ by NK cells occurring at an early stage in viral replication. Recently, Barr et al.46 have reported a rapid and transient activation of NK cells and IFN-γ production following infection with herpes simplex virus type 1, resulting from a release of IL-18 by dendritic cells. We cannot exclude the possibility that the loss of synergistic production of IFN-γ when NK cells were pretreated with rIL-12/rIL-18 for 2 hr before infection with L2-MHV3 may be consequent to impaired expression of CEACAM1a. Indeed, IFN-γ reduces the expression of CEACAM1a, as reported by Vassao et al.22 Consequently, NK cells may become resistant to a subsequent fixation of MHV3, which in turn would affect IFN-γ production.
During the acute phase of viral infection, recruitment of NK cells from the bone marrow or spleen to the affected organ is generally observed.8 Several studies have demonstrated that myeloid or splenic NK cells are essential during acute hepatitis, as these cells determined antiviral protection.3,12,14,17 For the induction of an efficient antiviral response by recruited NK cells, the intrahepatic microenvironment must be adequate to activate them. Hepatic NK cells normally colocalize at sites expressing viral antigen and IFN-γ during infection.47 This environment may allow NK cells to receive cell-to-cell or cell-to-matrix contact signals that favour an IL-12-dependent IFN-γ secretion, as NK cells are differently activated by IL-18 depending on their localization.48 However, recruited NK cells in liver require activation by adequate levels of IL-12 and IL-18 to become high producers of IFN-γ. It is known that liver KC produced IL-12 and IL-18 following various stimuli and activated intrahepatic NK and NKT cells to produce IFN-γ.49 The results that we have provided are in agreement with a previous in vivo study, reporting that treatment with rIL-12 and rIL-18 of susceptible mice creates an anti-MHV3 state by significantly increasing IFN-γ production.18 However, IL-12 and IL-18 were not induced in livers from L2-MHV3-infected C57BL/6 mice, preventing the synergistic IFN-γ production by recruited NK cells. In addition, the recruited intrahepatic NK cells from spleen and bone marrow may be infected by L2-MHV3 produced by infected LSEC or KC, leading to a virus-induced apoptosis phenomenon. The susceptibility of C57BL/6 mice to acute hepatitis reflects an inefficient innate antiviral response by low IFN-γ production because of deficient IL-12/IL-18 production and apoptosis of NK cells. The apoptosis of intrahepatic recruited NK cells, such as previously reported for spleen and bone marrow,12–14 is an additional evading mechanism of the innate antiviral defence. Our results suggest that intrahepatic NK cells from resistant mice, as A/J or C3H mice, would produce an efficient IFN-γ response in the presence of adequate intrahepatic IL-12 and IL-18 levels.
On other hand, hepatic NK cells also exert their functions in a tolerant environment and indeed, immunosuppressive cytokines such as transforming growth factor-β and IL-10 determine the levels of IFN-γ inhibition.50 However, we have recently observed that these immunosuppressive cytokines decrease in the liver of L2-MHV3-infected mice but not in mice infected with attenuated KC+ LSEC– and KC– LSEC–virus variants.14 The inability of intrahepatic NK cells to produce IFN-γ may be related to their apoptosis, which is dependent on viral permissivity of KC and LSEC to MHV3 replication. In fact, viral infection of KC and LSEC may favour the viral infection of NK cells when these cells are in contact. These observations suggest that IL-12/IL-18 produced in vivo by KC may be essential to preserve the antiviral functions of NK cells, and the integrity of LSEC favours the survival of NK1.1 cells recruited to the liver. However, the synergistic production of IFN-γ occurred during the first steps of viral replication when the most of KC and LSEC were not yet infected.
Further work is in progress to identify the step of viral replication involved in the synergistic IFN-γ production by NK cells and the role of L2-MHV3-infected hepatocytes, KC and LSEC in the intrahepatic functions of NK and NKT cells.
Acknowledgments
This work was supported by a grant from NSERC-Canada (LL) and the Canadian Institutes of Health Research (NB). A. Jacques was supported by a NSERC fellowship from the Canadian government. The authors thank Dr Tatiana Scorza (Université du Québec à Montréal) for revising this manuscript and her critical comments.
Acknowledgments
All the authors have no potential conflicts of interest, including financial interests in any company or institution that might benefit from the publication of this work.
References
- 1.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–64. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 2.Gosselin J, Tomoiu A, Gallo RC, Flamand L. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood. 1999;94:4210–19. [PubMed] [Google Scholar]
- 3.Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–28. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies. Liver Transpl. 2006;12:363–72. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 5.Sprengers D, Van der Molen RG, Kusters JG, Hansen B, Niester HG, Schalm SW, Janssen HL. Different composition of intrahepatic lymphocytes in the immune-tolerance and immune-clearance phase of chronic hepatitis. Br J Med Virol. 2006;78:561–8. doi: 10.1002/jmv.20576. [DOI] [PubMed] [Google Scholar]
- 6.Herzer K, Falk CS, Encke J, Eichhorst ST, Ulsenheimer A, Seliger B, Krammer PH. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang T, Ho C, Gravy JS, Douglas SD, Ho WZ. Natural killer cells inhibit C virus expression. J Leukoc Biol. 2004;76:1171–9. doi: 10.1189/jlb.0604372. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Xu JW, Zhang WC, Wei HM, Tian ZG. TLR3 Ligand-induced accumulation of activated splenic natural killer cells into liver. Cell Mol Immunol. 2005;2:449–53. [PubMed] [Google Scholar]
- 9.Mavropoulos A, Sully G, Cope AP, Clark AR. Stabilization of IFN-gamma mRNA by MAPK p38 in IL-12 and IL-18-stimulated human NK cells. Blood. 2005;105:282–8. doi: 10.1182/blood-2004-07-2782. [DOI] [PubMed] [Google Scholar]
- 10.Ortaldo JR, Winkler-Pickett R, Wigginton J, et al. Regulation of ITAM positive receptors: role of IL-12 and IL-18. Blood. 2006;107:1468–75. doi: 10.1182/blood-2005-04-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JP, Chen W, Koehren F, Pereira CA. The virulence of mouse hepatitis virus 3, as evidenced by permissivity of cultured hepatic cells toward escaped mutants. Res Virol. 1994;145:297–302. doi: 10.1016/S0923-2516(07)80034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehoux M, Jacques A, Lusignan S, Lamontagne L. Murine viral hepatitis involves NK cell depletion associated with virus-induced apoptosis. Clin Exp Immunol. 2004;137:41–51. doi: 10.1111/j.1365-2249.2004.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–8. [PubMed] [Google Scholar]
- 14.Jacques A, Bleau C, Martin JP, Lamontagne L. Intrahepatic endothelial and Kupffer cells involved in immunosuppressive and NK/NK-T cell disorders in the viral acute hepatitis. Clin Exp Immunol. 2008;152:298–310. doi: 10.1111/j.1365-2249.2008.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mello IG, Vassao RC, Pereira CA. Virus specificity of the antiviral state induced by IFN gamma correlates with resistance to MHV 3 infection. Arch Virol. 1993;132:281–9. doi: 10.1007/BF01309539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucchiari MA, Modolell M, Eichmann K, Pereira CA. In vivo depletion of interferon-gamma leads to susceptibility of A/J mice to mouse hepatitis virus 3 infection. Immunobiology. 1992;185:475–82. doi: 10.1016/S0171-2985(11)80089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh AB, Lodoen MB, Edwards RA, Lanier LL, Lane TE. Evidence for differential roles for NKG2D receptor signalling in innate host defense against coronavirus-induced neurological and liver disease. J Virol. 2008;82:3021–30. doi: 10.1128/JVI.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira C, Tsuhako MH, de Franco MT, Modolell M, Pereira CA. Arginine metabolism during macrophage autocrine activation and infection with mouse hepatitis virus 3. Immunobiology. 2004;209:585–98. doi: 10.1016/j.imbio.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markel G, Lieberman N, Katz G, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–10. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 20.Markel G, Wolf D, Hanna J, Gazit R, Goldman-Wohl D, Lavy Y, Yagel S, Mandelboim O. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest. 2002;110:943–53. doi: 10.1172/JCI15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muenzner P, Naumann M, Meyer TF, Gray-Owen SD. Pathogenic Neisseria trigger expression of their carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1: previously CD66a) receptor on primary endothelial cells by activating the immediate early response transcription factor, nuclear factor-κB. J Biol Chem. 2001;276:24331–40. doi: 10.1074/jbc.M006883200. [DOI] [PubMed] [Google Scholar]
- 22.Vassao RC, de Franco MT, Hartz D, Modolell M, Sippel AE, Pereira CA. Down-regulation of Bpg1 viral receptor by interferon-γ is related to the antiviral state and resistance to mouse hepatitis virus 3 infection. Virology. 2000;274:278–83. doi: 10.1006/viro.2000.0463. [DOI] [PubMed] [Google Scholar]
- 23.Coutelier JP, Godfraind C, Dveksler GS, Wysocka M, Cardellichio CB, Noel H, Holmes KV. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994;24:1383–90. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godfraind C, Coutelier JP. Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression. Histol Histopathol. 1998;13:181–99. doi: 10.14670/HH-13.181. [DOI] [PubMed] [Google Scholar]
- 25.Kammerer R, Hahn S, Singer BB, Luo JS, Von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–74. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Moller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65:740–5. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.McGilvray ID, Lu Z, Wei AC, Dackiw AP, Marshall JC, Kapus A, Levy G, Rotstein OD. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J Biol Chem. 1998;273:32222–9. doi: 10.1074/jbc.273.48.32222. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S, Narayanan K, Mizutani T, Makino S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J Virol. 2002;76:5937–48. doi: 10.1128/JVI.76.12.5937-5948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmila E, Turbide C, Olson M, Jothy S, Kolmes KV, Beauchemin N. Ceacam1a−/− mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J Virol. 2004;78:10156–65. doi: 10.1128/JVI.78.18.10156-10165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 2006;25:5527–36. doi: 10.1038/sj.onc.1209541. [DOI] [PubMed] [Google Scholar]
- 31.Dupuy JM, Rodrigue D. Heterogeneity in evolutive patterns of inbred mice infected with a cloned substrain of mouse hepatitis virus type 3. Intervirology. 1981;16:114–17. doi: 10.1159/000149255. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Ohtsuka K, Kimura M, et al. Details of an isolation method for hepatic lymphocytes in mice. J Immunol. 1992;146:145–54. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- 33.Lamontagne L, Massicotte E, Page C. Mouse hepatitis viral infection induces an extrathymic differentiation of the specific intrahepatic alpha beta-TCRintermediate LFA-1high T-cell population. Immunology. 1997;90:402–10. doi: 10.1111/j.1365-2567.1997.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pien GC, Biron CA. Compartmental differences in NK cell responsiveness to IL-12 during lymphocytic choriomeningitis virus infection. J Immunol. 2000;164:994–1001. doi: 10.4049/jimmunol.164.2.994. [DOI] [PubMed] [Google Scholar]
- 35.Thirion G, Feliu AA, Coutelier JP. CD66a (CEACAM1) expression by mouse natural killer cells. Immunology. 2008;125:535–40. doi: 10.1111/j.1365-2567.2008.02867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Q, Chow EM, Wong H, Gu J, Mandelboim O, Gray-Owen SD, Ostrowski MA. CEACAM1a (CD66a) promotes human monocyte survival via a phosphatidylinositol 3-kinase- and AKT-dependent pathway. J Biol Chem. 2006;281:39179–93. doi: 10.1074/jbc.M608864200. [DOI] [PubMed] [Google Scholar]
- 37.Chen T, Zimmermann W, Parker J, Chen I, Maeda A, Bolland S. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukoc Biol. 2001;70:335–40. [PubMed] [Google Scholar]
- 38.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, Wei P, Targan SR. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002–7. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 39.Uchida T, Kinoshita M, Fukasawa M, Habu Y, Shinomiya N, Seki S. IL-18 time-dependently modulates Th1/Th2 cytokine production by ligand-activated NKT cells. Eur J Immunol. 2007;37:966–77. doi: 10.1002/eji.200636465. [DOI] [PubMed] [Google Scholar]
- 40.Lamontagne L, Jolicoeur P. Low-virulent mouse hepatitis viruses exhibiting various tropisms in macrophages, T and B cell subpopulations, and thymic stromal cells. Lab Anim Sci. 1994;44:17–24. [PubMed] [Google Scholar]
- 41.Jolicoeur P, Lamontagne L. Mouse hepatitis virus 3 pathogenicity expressed by a lytic viral infection in bone marrow 14.8+ mu+ B lymphocyte subpopulations. J Immunol. 1989;143:3722–30. [PubMed] [Google Scholar]
- 42.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, Mandelboim O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 43.Daniels E, Letourneau S, Turbide C, et al. Biliary glycoprotein 1 expression during embryogenesis: correlation with events of epithelial differentiation, mesenchymal–epithelial interactions, absorption, and myogenesis. Dev Dyn. 1996;206:272–90. doi: 10.1002/(SICI)1097-0177(199607)206:3<272::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 44.Beauchemin N, Draber P, Dveksler G, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–9. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 45.Ortaldo JR, Young HA. Expression of IFN-γ upon triggering of activating Ly49D NK receptors in vitro and in vivo: costimulation with IL-12 or IL-18 overrides inhibitory receptors. J Immunol. 2003;170:1763–9. doi: 10.4049/jimmunol.170.4.1763. [DOI] [PubMed] [Google Scholar]
- 46.Barr DP, Belz GT, Reading PC, Wojtasiak M, Whitney PG, Heath WR, Carbone FR, Brooks AG. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur J Immunol. 2007;37:1334–42. doi: 10.1002/eji.200636362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, Spengler U. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology. 2002;36:1001–8. doi: 10.1053/jhep.2002.35532. [DOI] [PubMed] [Google Scholar]
- 48.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental for IFN-γ responses during viral infection. J Immunol. 2000;165:4787–91. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 49.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–6. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 50.Schröder M, Meisel C, Buhl K, Profanter N, Sievert N, Volk HD, Grutz G. Different modes of IL-10 and TGF-β to inhibit cytokine-dependant IFN-γ production: consequences for reversal of lipopolysaccharide desensitization. J Immunol. 2003;170:5260–7. doi: 10.4049/jimmunol.170.10.5260. [DOI] [PubMed] [Google Scholar]