Abstract
Accumulating evidence suggests that T cells and autoantibodies reactive with myelin oligodendrocyte glycoprotein (MOG) play a critical role in the pathogenesis of multiple sclerosis (MS). In the present study, we have tried to elucidate the pathomechanisms of development and progression of the disease by analysing T cells and autoantibodies in MOG-induced rat experimental autoimmune encephalomyelitis (EAE), which exhibits various clinical subtypes mimicking MS. Analysis using overlapping peptides revealed that encephalitogenic epitopes resided in peptide 7 (P7, residue 91–108) and P8 (residue 103–125) of MOG. Immunization with MOGP7 and MOGP8 induced relapsing–remitting or secondary progressive EAE. T cells taken from MOG-immunized and MOGP7-immunized rats responded to MOG and MOGP7 and sera from MOG-immunized rats reacted to MOG and MOGP1. Significant epitope spreading was not observed at either T-cell or antibody levels. Interestingly, sera from MOGP7-immunized rats with clinical signs did not react to MOG and MOG peptides throughout the observation period, suggesting that disease development and relapse in MOGP7-induced EAE occur without autoantibodies. However, MOGP7 immunization with adoptive transfer of anti-MOG antibodies aggravated the clinical course of EAE only slightly. Analysis of antibodies against conformational epitope (cme) suggests that anti-MOGcme may play a role in the pathogenicity of anti-MOG antibodies. Collectively, these findings demonstrated that relapse of a certain type of MOG-induced EAE occurs without autoantibodies but that autoantibodies may play a role in disease progression. Relapses and the progression of MS-mimicking EAE are differently immunoregulated so immunotherapy should be designed appropriately on the basis of precise information.
Keywords: experimental autoimmune encephalomyelitis, LEW.1AV1 rat, myelin oligodendrocyte glycoprotein
Introduction
Multiple sclerosis (MS) is believed to be an autoimmune demyelinating disease of the central nervous system (CNS) characterized by the presence of a variety of clinical subtypes such as relapsing–remitting (RR), primary-progressive (PP), secondary-progressive (SP) and relapsing–progressive courses.1 In most cases, the disease begins at about 30 years of age with episodes of acute worsening of neurological function, followed by a variable degree of recovery between relapses during the RR phase of the disease. In approximately half of patients the clinical course changes from RRMS to SPMS after 10 years and this has occurred in almost 90% by 25 years.2 The shift from RRMS to SPMS is a serious problem because SPMS responds poorly to medications that are effective in RRMS.3 Elucidation of the pathomechanisms is critical for the development of effective immunotherapy for MS.
Relapse and remission are characteristic features of MS but the precise pathomechanisms remain poorly understood. Epitope spreading, which was first described in detail by Lehmann et al.,4 may explain the presence of multiple relapses during the disease course. T cells reactive with the primary antigen induce CNS tissue destruction, which augments the release of the second antigen. This second antigen then activates T cells that respond to the second antigen during the relapse.5–7 However, it is difficult to explain all the relapses by epitope spreading because there are relapses without epitope spreading (discussed in detail later).8 Administration of interleukin-12,9 staphylococcal enterotoxin B or tumour necrosis factor-α10 was reported to induce the relapse of experimental autoimmune encephalomyelitis (EAE).
Although it is suggested that neuroantigens such as myelin basic protein (MBP), proteolipid protein and myelin oligodendrocyte glycoprotein (MOG) are involved in the pathogenesis of MS, recent analysis of MS and its animal models suggests that MOG is critically involved in disease progression.11–14 Therefore, it is important to analyse disease relapse and progression using the MOG-induced animal model. In a previous study, we succeeded in producing EAE, which mimics RRMS and SPMS, in LEW.1AV1 rats.15 This animal model has an advantage over MOG-EAE induced in B6 mice to analyse the pathomechanisms of relapse because the latter shows only chronic persistent EAE without relapse.16
In the present study, we characterized the nature of pathogenic T cells and autoantibodies from MOG-immunized LEW.1AV1 rats at various time-points. Special attention was paid to the presence or absence of epitope spreading at both T-cell and B-cell levels and the role of anti-MOG antibodies. Consequently, we found that T-cell and B-cell epitope spreading within the MOG molecule and to MBP was minimal or absent during the course of EAE even when rats showed evidence of relapse. Furthermore, MOG peptide-immunized rats developed RR- or SP-EAE without anti-MOG antibody elevation. These findings strongly suggest that although anti-MOG antibodies play an important role in disease progression, the relapse of EAE, and probably MS, could occur without autoantibody involvement and T- and B-cell epitope spreading is not essential for this event.
Materials and methods
Unless otherwise indicated, all reagents and apparatus were obtained in Tokyo, Japan.
Animals and reagents
Male and female LEW.1AV1 rats aged 8–12 weeks were used for the experiments. All animal experiments were approved by the institute committee and performed in accordance with institutional guidelines.
Recombinant rat MOG was prepared as follows. The gene encoding the extracellular domain (amino acids 1–125) of MOG was amplified using primers specific for the corresponding MOG sequence. Polymerase chain reaction products were then digested with SphI and HindIII and subcloned into pQE30 (Qiagen) for large-scale preparation. The sequence of the construct was confirmed by sequencing. Recombinant MOG produced in transformed Escherichia coli were isolated under denaturing conditions and purified using Ni-NTA agarose (Qiagen). Then, purified MOG was diluted and refolded in phosphate-buffered saline containing 1 m l-arginine, 2 mm glutathione (reduced form), and 0·2 mm glutathione (oxidized form). As a final step, recombinant protein was incubated with Detoxi-Gel (Pierce, Funakoshi) overnight to remove endotoxins. The obtained protein contained endotoxins at < 10 EU/1 mg protein as determined with a Toxinometer ET-2000 (Wako).
Overlapping 18–23-mer peptides were prepared using a peptide synthesizer, PSSM-8 (Shimadzu Biotech, Kyoto, Japan). The purity of each peptide was determined, and the peptide was purified if necessary, by high-performance liquid chromatography (Waters 486, Waters 600 and Bondasphere C18 column; Waters) and all peptides were > 90% pure.
EAE induction and clinical evaluation
The LEW.1AV1 rats were immunized in the tail base with MOG or MOG peptides emulsified with complete Freund’s adjuvant (CFA). In some experiments, pertussis toxin (2 μg) was injected intraperitoneally at the time of immunization. Clinical signs were evaluated as the total score comprising the sum of the degrees of paresis of each limb and the tail (partial paresis, 0·5; complete paresis, 1·0). Therefore, the clinical score for complete paralysis of the four limbs plus tail or the moribund condition was 5. The majority of rats reaching a score of 5 died or were killed under ether anaesthesia for histological examination.
Histological and immunohistochemical examination
The optic nerve and the cervical, thoracic and lumbar spinal cord were routinely examined. The cerebrum, brainstem and cerebellum were also examined in some cases. The tissues were fixed in 4% paraformaldehyde and processed for paraffin embedding. Six-micrometre sections were cut and stained with haematoxylin & eosin (H&E) and using Kruever and Barrera’s method. Inflammatory lesions were graded using sections stained with H&E and W3/13 for T cells into four categories (Grade 1, leptomeningeal and adjacent subpial cell infiltration; Grade 2, mild perivascular cuffing; Grade 3, extensive perivascular cuffing; Grade 4, extensive perivascular cuffing and severe parenchymal cell infiltration). Demyelinating lesions were graded using sections stained using the Kruever and Barrera method and ED1 for macrophages into five categories (Grade 1, trace of perivascular or subpial demyelination; Grade 2, focal demyelination; Grade 3, demyelination involving a quarter of tissues examined, i.e. the spinal tract, brainstem, cerebellar white matter or optic tract; Grade 4, massive confluent demyelination involving half of the tissue; Grade 5, extensive demyelination involving the entire tissues) according to Storch et al.17 with a few modifications.
Single immunoperoxidase staining was performed as described previously.18,19 Briefly, paraffin-embedded sections were deparaffinized and rehydrated. After blocking the endogenous peroxidase activity with methanol containing 0·3% hydrogen peroxide, sections were incubated with monoclonal antibody (mAb) W3/13 (Dainippon Pharm, Osaka, Japan) for T-cell staining or with ED1 (purified from the hybridoma supernatant) for macrophages. After washing, sections were incubated with biotinylated anti-mouse immunoglobulin G (IgG) (Vector, Burlingame, CA) followed by use of a horseradish peroxidase (HRP) -labelled VECTSTAIN Elite ABC Kit (Vector). The HRP-binding sites were detected in 0·005% diaminobenzidine and 0·01% hydrogen peroxide. To confirm the specificity of the staining, the primary antibodies were omitted or replaced with normal mouse IgG. The controls did not show any specific staining.
Proliferative responses of T cells against MOG, MBP and MOG peptides
Proliferative responses of lymph node cells were assayed in microtitre wells by uptake of [3H]thymidine. After being washed with phosphate-buffered saline, lymph node cells (2 × 105 cells/well) were cultured with the indicated concentrations of MOG, MBP and MOG peptides for 3 days, with the last 18 hr in the presence of 0·5 μCi [3H]thymidine (Amersham Pharmacia Biotech). In some experiments, the proliferative responses of MOG-specific or MOG peptide-specific T line cells (3 × 104 cells/well) were assayed in the presence of the antigens and antigen-presenting cells (5 × 105 cells/well). The cells were harvested on glass-fibre filters, and the label uptake was determined using standard liquid scintillation techniques.
Enzyme-linked immunosorbent assay
The level of anti-MOG protein, anti-MOG peptide and anti-MBP antibodies was measured using standard enzyme-linked immunosorbent assay (ELISA). Recombinant rat MOG protein, MOG peptides or purified rat MBP (10 μg/ml) was coated onto microtitre plates and diluted sera (1 : 100) from normal and immunized animals were applied. After washing, appropriately diluted HRP-conjugated anti-rat IgG, IgG1 or IgG2a was applied. The reaction products were then visualized after incubation with the substrate. Absorbance was read at 450 nm.
Generation of anti-MOG mAbs
Anti-MOG rat mAbs were generated following Kishiro et al.20 Briefly, LEW.1AV1 rats were immunized with 250 μg recombinant rat MOG1–125 emulsified with CFA and killed 2 weeks after immunization. Iliac lymph node cells were taken from the rats and hybridized with myeloma cells using polyethylene glycol/dimethyl sulphoxide (PEG/DMSO solution Hybri-Max, Sigma). SP2/0-Ag14, kindly provided by Dr Sado (Shigei Medical Research Institute, Okayama, Japan), was used as hybridizing cells. After seeding the microplates, hybridoma cells were selected in the HAT medium. Ten to 14 days later, antibody-producing cells were screened by ELISA and cloned by limiting dilution. Established clones were expanded in culture and a large volume of supernatant containing anti-MOG mAbs was obtained. The mAbs were purified on a HiTrap protein G column (GE Healthcare Bio-Sciences).
In vivo treatment with anti-T-cell and anti-B-cell mAbs
In some experiments, MOG-immunized or MOG peptide-immunized rats were treated by intraperitoneal injection of anti-T-cell (R73) or anti-B-cell (9D3 or OX33) mAbs. The mAbs at a dose of 100 μg were given 6 days a week for the indicated period.
Generation of polyclonal antibodies against MOG and MOG peptides and transfer experiments
Polyclonal antibodies against MOG and MOG peptides were raised by immunizing rats with the antigens/CFA four times on a weekly basis. Sera were obtained 1 week after the last immunization and ammonium sulphate-precipitated preparations were used for transfer experiments. The presence of antibodies against the indicated antigens was confirmed by ELISA.
For antibody transfer, MOGP7 was immunized and then sera or mAb at the indicated dose was injected intravenously twice a week. Rats were examined histologically 5 weeks after immunization.
Production of native MOG-expressing cells and flow cytometric analysis of antibodies against conformational epitopes of the MOG molecule
Native-MOG-expressing cells21,22 were produced with the Flp-In T-REx system (Invitrogen). Full-length MOG complementary DNA including the leader sequence was amplified by reverse transcription–polymerase chain reaction using messenger RNA derived from rat brain and inserted into pCR4-Blunt TOPO vector (Invitrogen). After cloning and sequencing, the appropriate construct was inserted into pcDNA5/FRT/TO vector. The plasmid vectors were grown in E. coli and then purified using an Endofree Plasmid Maxi Kit (Qiagen). The plasmid vector and pOG44 vector which contains the Flp recombinase sequence were cotransfected into Flp-In T-REx 293 cells with Lipofectamine 2000 (Invitrogen). Native MOG-expressing cells were obtained after selection with Hygromycin B.
Native MOG was induced on the surface of Flp-In T-REx 293 cells by the addition of tetracycline 48 hr before use. Staining the cells with anti-MOG mAb (8-18C5, provided by Dr Gold, Wuetzburg, Germany), which recognizes the conformational epitope, revealed that more than 90% of cells were positive for 8-18C5, indicating that almost all of the cells expressed native MOG on their surface. For titration of antibodies against conformational epitopes, native MOG-expressing cells were incubated with the sera to be examined (serially diluted from × 4 to × 4096), followed by incubation of fluorescein isothiocyanate-labelled anti-rat IgG antibodies (KPL, Gaithersburg, MD). The percentages of positive cells were determined by FACScan (BD Bioscience).
Statistical analysis
Data were analysed using Student’s t-test or Mann–Whitney’s U-test. P-values < 0·05 were considered statistically significant.
Results
Encephalogenicity of MOG peptides for LEW.1AV1 rats
For detailed analysis of the pathomechanisms of MOG-induced EAE, we synthesized peptides that encompass the extracellular domain of the rat MOG molecule (residues 1–125) (Table 1) and tested their encephalitogenicity for LEW.1AV1 rats. Table 2 summarizes the results. Screening with the peptide mixtures revealed that Mixtures 1 and 2 containing P1, P2, P3, P4, P5 and P6 (Groups B and C) did not induce clinical EAE. Only Mixture 3 containing P7 and P8 induced clinical EAE (Group D). Separate immunization revealed that both P7 and P8 induced EAE with mean clinical scores of 3·3 ± 1·5 for P7 (Group E) and 1·7 ± 1·0 for P8 (Group F). Compared with MOG-induced EAE, the onset of the disease induced by peptide immunization was much earlier. This was probably because MOG peptides are more hydrophilic than MOG1–125 used in the present study. The soluble form of MOG generally induces acute EAE with early onset.15
Table 1.
Sequences of myelin oligodendrocyte glycoprotein peptides used in the present study1
| Peptide | Residue | Sequence |
|---|---|---|
| P1 | 1–23 | GQFRVIGPGHPIRALVGDEAELP |
| P2 | 18–40 | DEAELPCRISPGKNATGMEVGWY |
| P3 | 35–55 | MEVGWYRSPFSRVVHLYRNGK |
| P4 | 50–70 | LYRNGKDQDAEQAPEYRGRTE |
| P5 | 65–84 | YRGRTELLKESIGEGKVALR |
| P6 | 79–96 | GKVALRIQNVRFSDEGGY |
| P7 | 91–108 | SDEGGYTCFFRDHSYQEE |
| P8 | 103–125 | HSYQEEAAVELKVEDPFYWINPG |
Amino acid 1–125 residues in the extracellular domain of rat myelin oligodendrocyte glycoprotein.
Table 2.
Severity of experimental autoimmune encephalomyelitis (EAE) induced by myelin oligodendrocyte glycoprotein (MOG) peptides in LEW.1AV1 rats1
| Group | Immunogen | Incidence | Onset | Max. clinical score | Mild monophasic | Acute lethal | Relapsing– remitting | Secondary progressive | Chronic |
|---|---|---|---|---|---|---|---|---|---|
| A | MOG | 6/6 | 24·7 ± 6·2 | 4·2 ± 0·52 | 0 | 0 | 5 | 1 | 0 |
| B | Mix 1 (P1, P2, P3) | 0/3 | (–) | 0 | 0 | 0 | 0 | 0 | 0 |
| C | Mix 2 (P4, P5, P6) | 0/3 | (–) | 0 | 0 | 0 | 0 | 0 | 0 |
| D | Mix 3 (P7, P8) | 3/3 | 11·3 ± 1·5 | 2·8 ± 1·9 | 2 | 1 | 0 | 0 | 0 |
| E | P7 | 5/5 | 11 | 3·3 ± 1·52 | 1 | 2 | 2 | 0 | 0 |
| F | P8 | 9/9 | 17·3 ± 6·5 | 1·7 ± 1·02 | 4 | 0 | 2 | 1 | 2 |
Recombinant rat MOG or MOG peptides (100 μg) emulsified with complete Freund’s adjuvant were immunized into the tail base. To maximize the encephalogenicity of the peptides, pertussis toxin (2 μg) was injected intraperitoneally at the time of peptide immunization. Mixes 1, 2 and 3 were mixtures of 100 μg of each peptide. Clinical subtypes were classified into five categories.
Statistical analysis was performed with regard to the maximal clinical score. When all values were compared, statistical significance was noted between MOG (a) and P8 (f) immunization (P = 0·0005). Comparison of chronic EAE, including relapsing–remitting and secondary progressive, revealed that clinical signs of the P7-immunized group (e) were significantly milder than those of the MOG-immunized group (a) (P = 0·005).
Clinicopathological features of MOG- and MOG peptide-induced EAE in LEW.1AV1 rats
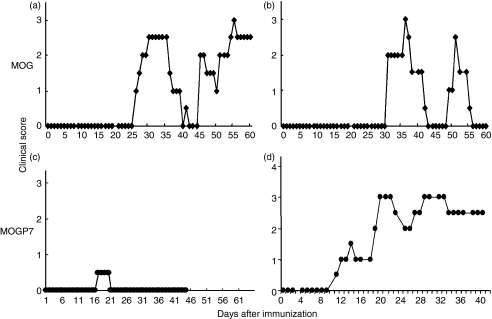
As reported in a previous paper,15 immunization of LEW.1AV1 rats with recombinant rat MOG induced various types of EAE mimicking MS. As shown in Fig. 1(a,b), rats that had been immunized with MOG developed SP-EAE (Fig. 1a) or RR-EAE (Fig. 1b). Interestingly, MOGP7 immunization induced similar SP-EAE (Fig. 1d) or RR-EAE (not shown) in some rats (Table 2, Group A versus E). The clinical subtypes of EAE induced by immunization with MOG, MOGP7 or MOGP8 are shown in Table 2. In this series of experiments, five of six MOG-immunized rats developed RR-EAE and only one developed SP-EAE. Immunization with MOGP7 induced EAE including two cases of the acute lethal form and two cases of the RR subtype, whereas MOGP8 immunization induced relatively mild EAE including two cases of RR, one of SP and three chronic subtypes. It should be noted that 41·1% of rats that had been immunized with MOGP7 or MOGP8 developed mild monophasic EAE (representative clinical course is shown in Fig. 1c). When all maximal clinical scores from MOG-, MOGP7- and MOGP8-immunized rats were compared, statistical significance was noted only between MOG (Group A) and MOGP8 (Group F) immunization (P = 0·0005). Comparison of maximal clinical scores in chronic EAE including RR and SP forms revealed that the clinical signs of the P7-immunized group (Group E) were significantly milder than those of the MOG-immunized group (Group A) (P = 0·005). These findings indicate that although disease relapse and progression occur in MOG peptide-immunized rats, the disease is generally milder than that in MOG-immunized rats.
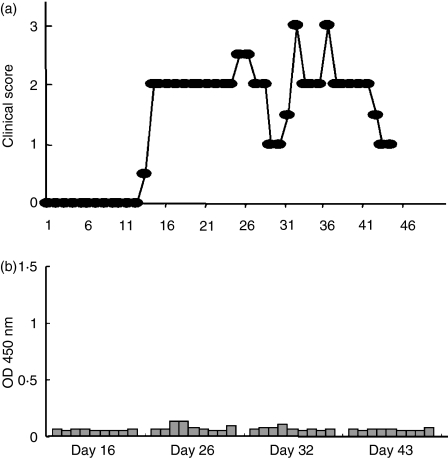
Figure 1.
The clinical course of experimental autoimmune encephalomyelitis (EAE) induced by immunization with myelin oligodendrocyte glycoprotein (MOG) protein (a, b) and MOG peptide (c, d). Representative clinical courses of the disease are shown. While MOG protein-immunized rats usually develop secondary progressive (a) or relapsing–remitting (b) EAE, the majority of MOG peptide-immunized rats show acute monophasic EAE (c). However, a small number of rats develop chronic EAE (d). Observation of the clinical signs of MOG peptide-induced EAE (c, d) was terminated on day 45 for histological examinations.
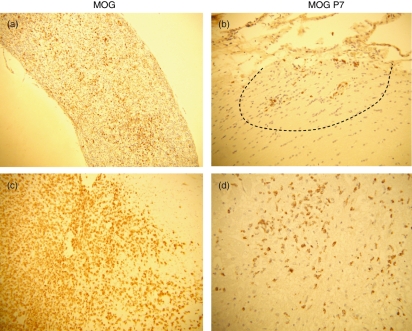
Consistent with the clinical features, histological examinations revealed relatively mild pathology in MOG peptide-immunized rats. ED1 staining for macrophages demonstrated mild and localized macrophage infiltration in the optic nerve (Fig. 2b) and spinal cord (Fig. 2d) of MOG peptide-immunized rats, while there was extensive macrophage infiltration in the same regions of MOG-immunized rats (Fig. 2a,c). Demyelination in MOG peptide-immunized rats was minimal (data not shown).
Figure 2.
Pathology of optic nerve (a, b) and spinal cord (c, d) lesions in rats that had been immunized with myelin oligodendrocyte glycoprotein (MOG) (a, c) and MOG peptides (P7 and P8) (b, d). Histological examinations revealed relatively mild pathology in MOG peptide-immunized rats. ED1 staining for macrophages demonstrated extensive macrophage infiltration in the optic nerve (a) and spinal cord (c) of MOG-immunized rats and mild and localized (dotted line) macrophage infiltration in MOG peptide-immunized rats (b, d). (a, b) MOG immunization, day 14; (c, d) MOG peptides (P7 + P8) immunization, day 12. (a) × 60; (b–d) × 120.
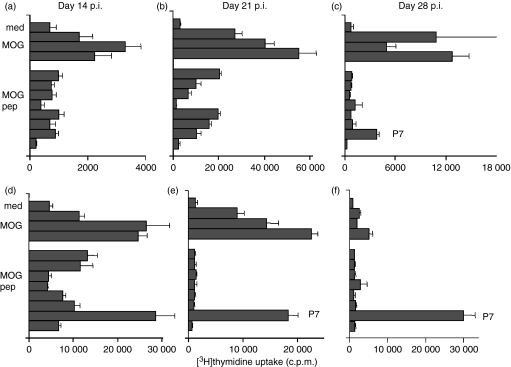
Characterization of T cells activated by MOG and MOG peptide immunization
The proliferative responses of lymph node T cells from rats that had been immunized with MOG (Fig. 3a–c) and MOGP7 (Fig. 3d–f) to MOG, MBP and MOG peptides were assayed by [3H]thymidine uptake on days 14, 21 and 28 postimmunization (p.i.). At each time-point two rats were examined and essentially the same results were obtained. On day 14 of MOG immunization, T cells showed modest but significant responses to MOG, whereas the responses to MOG peptides were marginal (stimulation index < 2; Fig. 3a). On day 21 p.i., T cells responded vigorously to MOG and significantly to MOGP1, P2, P5, P6 and P7 (stimulation index > 3; Fig. 3b). On day 28 p.i., T cells responded only to MOG and MOGP7 (Fig. 3c). Immunization with MOGP7 induced vigorous T-cell responses to MOG and MOGP7 on days 14 (Fig. 3d) and 21 (Fig. 3e) p.i. but responses to MOG were not present on day 28 p.i. (Fig. 3f). These findings suggest that the T-cell epitope in MOGP7 is rather weak but immunodominant in the MOG molecule. When the mixture of P7 and P8 was used for immunization, only the MOGP7 response was detected in the regional lymph node T cells (data not shown). Collectively, it was demonstrated that intramolecular epitope spreading was mild and transient in MOG-immunized rats and was completely absent in MOGP7-immunized rats. To determine the presence or absence of intermolecular epitope spreading, we also examined T-cell responses to MBP in MOG- and MOGP7-immunized rats. At all time-points examined, there was no MBP response (data not shown).
Figure 3.
Proliferative responses of lymph node cells of rats immunized with myelin oligodendrocyte glycoprotein (MOG) (a–c) and MOG-P7 (d–f). Lymph node cells (2 × 105 cells/well) were cultured in triplicate with MOG (10, 33 and 100 μg/ml), myelin basic protein (MBP; 10, 33 and 100 μg/ml) or MOG-P1–8 (100 μg/ml) for 3 days, with the last 18 hr in the presence of 0·5 μCi [3H]thymidine. On day 14 postimmunization (p.i.) of MOG immunization, T cells showed modest but significant responses to MOG protein, whereas the responses to MBP and MOG peptides were marginal (stimulation index < 2) (a). On day 21 p.i., T cells responded vigorously to MOG protein and significantly to MOG P1, P2, P5 P6 and P7 (stimulation index > 3) (b). On day 28 p.i., T cells responded only to MOG protein and P7 (c). Immunization with MOG P7 induced vigorous T-cell responses to MOG protein and MOG P7 on days 14 (d) and 21 p.i. (e) but such responses were not present on day 28 p.i. (f).
Characterization of antibodies against MOG and MOG peptides in MOG- and MOG peptide-immunized rats
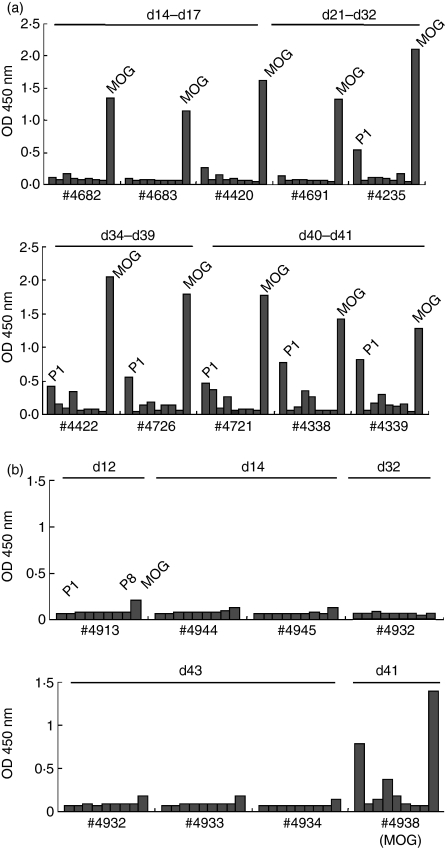
We next determined the levels of antibodies against MOG and MOG peptides in sera of MOG-immunized rats from day 14 to day 43 p.i. (Fig. 4a). During the early stage (day 14–21), anti-MOG antibodies were elevated, while anti-MOG peptide antibodies were not detected. From day 32 and thereafter, antibodies against MOGP1 were also detected. Similar findings were obtained on days 58 and 77 (data not shown). Importantly, there was no B-cell epitope spreading against other MOG peptides (Fig. 4a).
Figure 4.
Kinetics of anti-myelin oligodendrocyte glycoprotein (anti-MOG) (a) and anti-MOG peptide (b) antibodies in MOG-immunized rats. The levels of anti-MOG and anti-MOG peptide antibodies were measured in triplicate by enzyme-linked immunosorbent assay. Recombinant MOG or MOG peptides (P1–P8) (10 μg/ml) were coated onto microtitre plates and diluted sera (1 : 100) from MOG-immunized rats were applied. After washing, horseradish peroxidase-conjugated anti-rat immunoglobulin G was allowed to react. The reaction products were then visualized after incubation with the substrate and the absorbance was read at 450 nm. During the early stage (day 14–21), anti-MOG antibodies were elevated, while anti-MOG peptide antibodies were not detected. From day 32 antibodies against P1 were also detected. All SDs were within 10% of the mean values.
We also performed a similar analysis using sera from MOG peptide-immunized rats (Fig. 4b). Rats were immunized with MOGP7 and examined on days 12, 14, 16, 32 and 43 p.i. Representative results from days 12, 14, 32 and 43 are shown in Fig. 4(b). To our surprise, immunization with MOGP7 did not elicit a significant elevation of antibodies against MOG and MOG peptides including MOGP7. To confirm that the results were reliable, we repeated the assay with positive control serum from a MOG-immunized rat (#4338 in Fig. 4a,b) and obtained almost identical values in two assays. Therefore, in MOGP7-induced EAE, antibodies against MOG and MOG peptides were not generated and therefore were not involved in its pathogenesis. We also analysed the relationship between the clinical course and levels of anti-MOG and anti-MOG peptide antibodies in the longitudinal examination of individual rats. A representative result is shown in Fig. 5. Rats were immunized with MOGP7 and observed daily for clinical signs. In parallel, sera were obtained via the tail vein on days 16, 26, 32 and 43. As clearly shown, MOGP7-immunized rats developed RR-EAE without the elevation of anti-MOG and anti-MOG peptide antibodies. It was demonstrated that under certain conditions, relapses of EAE occur without autoantibody elevation.
Figure 5.
Kinetics of anti-myelin oligodendrocyte glycoprotein (anti-MOG) protein and anti-MOG peptide antibodies in MOG peptide-immunized rats. Rats were immunized with MOG P7, P8 or P7 + P8 and examined on days 12, 14, 16, 32 and 43 postimmunization. Immunization with P7 or P8 alone or in combination did not elicit the significant elevation of antibodies against P7, P8 and MOG. The assay with positive control serum from a MOG protein-immunized rat (#4338) showed high antibody titres against MOG and MOG-P1 that were identical to those shown in Fig. 4. The #4932 rat was examined multiple times by drawing blood from the tail vein. All SDs were within 10% of the mean values.
Anti-T-cell and B-cell immunotherapy of MOG- and MOG peptide-induced EAE
Characterization of T cells and antibodies in MOG- and MOGP7-induced EAE suggested that MOGP7-induced EAE developed in a T-cell-dependent manner, whereas the role of T cells in MOG-induced EAE remained unclear. To clarify the role of T cells in the development of MOG- and MOGP7-induced EAE in more detail, we performed treatment experiments with anti-T-cell receptor αβ mAb (R73). In a previous study, we reported that R73 treatment successfully suppressed the development of acute EAE.23 As clearly shown in Table 3, daily administration of R73 for 21 days from day 0 completely suppressed the development of EAE in both MOG-immunized (Group A) and MOGP7-immunized (Group E) rats. The shorter duration of treatment resulted in incomplete suppression (Group B) or no suppression (Group C) of disease development. These findings indicated that not only in MOGP7-induced EAE, but also in MOG-induced EAE, T cells play a pivotal role in disease development.
Table 3.
Modulation of myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) by anti-T-cell receptor αβ monoclonal antibody (mAb) (R73)1
| Group | Immunogen | mAb | Tx (day) | Incidence | Onset | Max. clinical score |
|---|---|---|---|---|---|---|
| A | MOG | R73 | 0–21 | 0/3 | (−) | (−) |
| B | MOG | R73 | 0–14 | 2/4 | 28, 35 | 2·5, 5 |
| C | MOG | R73 | 7–21 | 4/4 | 26·0 ± 4·53 | 3·7 ± 1·04 |
| D | MOG | Control2 | 7–21 | 4/4 | 35·8 ± 5·83 | 2·3 ± 0·44 |
| E | MOGP7 | R73 | 0–21 | 0/3 | (−) | (−) |
| F | MOGP7 | Control2 | 0–21 | 3/3 | 14·0 | 1·8 ± 0·2 |
R73 at a dose of 100 μg was injected intraperitoneally 6 days a week for the indicated period and observed daily for clinical signs by day 50 postimmunization.
As a control, saline was administered during the indicated period.
There was no significant difference between the two groups.
We wanted to identify the role of B cells in disease development and relapse by depleting this cell type with anti-B-cell antibodies. Unfortunately, however, two anti-B-cell mAbs tested did not deplete B cells at all (data not shown) and we failed to obtain meaningful data with this approach.
Role of anti-MOG antibodies and the presence or absence of antibodies against conformational epitope of MOG (anti-MOGcme antibodies)
The MOGP7-induced EAE would provide useful information with regard to the role of anti-MOG antibodies because this type of EAE develops without anti-MOG antibody involvement. If antibodies play an important role in the pathogenesis of EAE, then MOGP7 immunization with cotransfer of the antibodies would exacerbate the clinical and pathological features of EAE. For this purpose, we produced polyclonal anti-MOG and anti-MOG peptide antisera and injected them into MOGP7-immunized rats. As shown in Table 4, MOGP7 immunization with the transfer of anti-MOG sera slightly exacerbated clinical EAE. However, the difference was not statistically significant. Cotransfer of anti-MOG mAbs had no effect (data not shown).
Table 4.
Myelin oligodendrocyte glycoprotein peptide 7 (MOGP7) immunization with adoptive transfer of anti-MOG or anti-MOG-peptide antisera
| Group | Immunogen | Sera | Incidence | Onset | Max. clinical score |
|---|---|---|---|---|---|
| A | MOGP7 | Anti-MOG | 3/3 | 14·0 ± 3·8 | 1·8 ± 0·9 |
| B | MOGP7 | Anti-MOGP1 | 3/3 | 14·3 ± 3·8 | 1·1 ± 0·9 |
| C | MOGP7 | (−) | 3/3 | 15·0 ± 5·0 | 1·3 ± 1·2 |
MOGP7 was immunized and then 1 ml of × 5 diluted anti-MOG or anti-MOGP1 sera was injected intravenously twice a week. To evaluate the effects of antibodies, pertussis toxin was not used in this series of experiments.
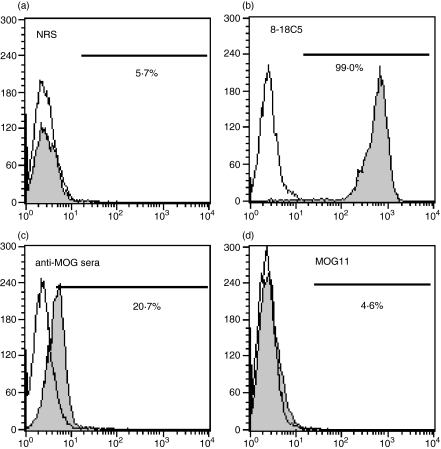
To explore these results in more detail, we established an assay system to evaluate the titres of antibodies against conformational epitopes (cme) of the MOG molecule in sera and mAbs used for the transfer experiment. As shown in Fig. 6, the 8-18C5 mAb, which recognizes MOGcme24 and is pathogenic upon adoptive transfer,25 showed a very high titre (Fig. 6b), whereas normal rat serum (Fig. 6a) and MOG11 mAb (Fig. 6d) did not react with native MOG. Polyclonal anti-MOG antisera used for the transfer experiment showed low but definite native-MOG binding activities (Fig. 6b). Taken together, there is a tendency for the titres of anti-MOGcme to correlate with the pathogenicity of anti-MOG antibodies. Unfortunately however, we were unable to perform transfer experiments with 8-18C5 because we did not possess a sufficient amount for the experiments.
Figure 6.
Detection of antibodies against conformational epitopes of the myelin oligodendrocyte glycoprotein (MOG) molecule. Native MOG was induced on the surface of Flp-In T-REx 293 cells by the addition of tetracycline 48 hr before use. Sera or antibodies to be examined (serially diluted from × 4 to × 4096 for sera) followed by incubation of fluorescein isothiocyanate-labelled anti-rat immunoglobulin G antibodies. The per cent of positive cells was determined by FACScan. Normal rat serum (a) stained only 5·7% of native MOG-expressing cells, whereas anti-MOG monoclonal antibody (mAb), which recognizes the conformational epitope, stained 99% of the cells, indicating that almost of all the cells express native MOG on their surface. MOG-11 mAb did not react with native MOG (d). Anti-MOG antisera showed an intermediate value. (a) Normal rat serum × 64; (b) 8–18C5 × 10 000; (c) sera used for transfer, × 64; (d) MOG11 mAb, × 64.
Discussion
In the present study, we characterized the nature of T and B cells involved in MOG- and MOG peptide-induced EAE in LEW.1AV1 rats. As described previously,15 MOG-induced EAE in rats has an advantage over mouse EAE that is induced in the B6 strain with MOG35-55 peptide, which is popularly used for analysis. While mice usually develop chronic persistent EAE, MOG-immunized rats develop the RR or SP form of EAE mimicking the clinical course of MS. Therefore, EAE in rats would be more suitable for analysis of the pathomechanism of relapse and secondary progression. Using this model, we obtained the following findings with regard to the role of T and B cells in MOG-induced rat EAE. First, the major encephalitogenic epitope resides within residues 91–108 (MOGP7) of the MOG molecule. This finding was consistent with that reported previously by Weissert et al.26 Second, T cells taken from MOG-immunized and MOGP7-immunized rats responded to MOG and MOGP7. While anti-MOG and anti-MOGP1 antibodies were generated by MOG immunization, MOGP7 immunization did not elicit antibodies against MOG and any MOG peptides. At both T-cell and B-cell levels, intra- and intermolecular epitope spreading was minimal and was not associated with the relapse and progression of EAE. Finally, MOGP7 immunization plus anti-MOG antisera transfer slightly aggravated EAE compared with MOGP7 immunization alone but the difference was not significant. Flow cytometric analysis also revealed that the antibodies used in the present study contained lower titres of antibodies against conformational epitopes. Collectively, these findings suggest that relapses of EAE could be triggered by pathogenic T cells alone and that autoantibodies, especially those against the conformational epitopes, may be involved in disease progression.
It was of interest to examine how much epitope spreading at the T-cell and B-cell levels was involved in relapses in our EAE model. Epitope spreading was first described in detail by Lehmann et al.4 as a key process in the development of chronic autoimmune diseases. Initially, this immunological process was intensively investigated and was thought to be highly involved in the relapse and chronicity of EAE.5,7,27 However, later reports demonstrated epitope spreading without disease progression28 or relapse without epitope spreading8 so it would seem that the relationship between relapses of EAE and epitope spreading is classified into three different categories, i.e. (1) ‘Functional epitope spreading’: T cells reacting with the second epitope play an essential role in relapses and blocking of the function of these T cells results in suppression of relapses,5,7 (2) ‘Non-functional epitope spreading’: although epitope spreading occurs, blocking this process does not suppress the development of relapses,29,30 (3) ‘Relapse without epitope spreading’.8,31 In the present study, it was clearly demonstrated that T-cell and B-cell epitope spreading was minimally involved in relapses of MOG-induced EAE in LEW.1AV1 rats. In particular, in MOGP7-induced EAE, T cells reacted only to MOG and MOGP7 and there was no generation of anti-MOG and anti-MOG peptide antibodies throughout the disease course. Even under such conditions, two of five rats showed RR-EAE (Table 2), clearly demonstrating that T-cell and B-cell epitope spreading is not involved in relapse, at least in this type of EAE. Although epitope spreading is generally observed to occur during the course of MS,28,32–34 some studies showed no correlation between disease severity and the number of epitopes recognized.28,34
With regard to the role of B cells in MOG-induced EAE, several reports have demonstrated different outcomes. Initially, it was reported that B-cell-deficient mice develop EAE with demyelination after MOG sensitization.35 Later, it was demonstrated that B cells are essential for the development of MOG-induced, but not MOG peptide-induced, EAE.36 Furthermore, B-cell-deficient mice that had been immunized with MOG (EAE never develops under this condition) developed clinical EAE after adoptive transfer of anti-MOG antisera,36 demonstrating the importance of anti-MOG antibodies in EAE development. In the present study, analysis of antibodies against conformational epitopes revealed that the titres of anti-MOGcme correlate well with the pathogenicity of anti-MOG antibodies. Collectively, it is suggested that generation of autoantibodies is essential for exacerbation and persistence of disease.
Whether anti-MOG antibodies are involved in the pathogenesis of MS is controversial. It was first reported that anti-myelin autoantibodies, including anti-MOG antibodies, are increased after the first demyelinating event.13 However, later reports rejected a relationship between anti-MOG antibody elevation and the disease status of MS.37,38 Although these results were obtained by standard ELISA detecting antibodies directed to linear epitopes, several groups have demonstrated the importance of autoantibodies against conformational epitopes of the MOG molecule in MS and EAE.11,21,22,39 Detailed analysis of this type of autoantibody should be performed to elucidate the pathomechanisms of MS.
Beside epitope spreading, which cannot explain all relapses as described above, the molecular basis of disease relapse remains poorly elucidated. There are at least two mechanisms underlying this event. The first is related to enhanced survival of pathogenic T cells. Hur et al.40 clearly demonstrated that osteopontin induced relapse and progression of EAE through enhanced survival of encephalitogenic T cells. Tbet, a transcription factor that controls T helper type 1 and type 17 cells,41 plays an essential role in EAE progression.42–44 Furthermore, osteopontin expression in activated T cells is regulated by Tbet.45 The second factor is related to macrophage migration into the CNS. In chronic, but not acute, EAE lesions, dense macrophage infiltration was frequently observed.15 In such lesions, MCP-1/CCL2 was significantly upregulated46 and blocking MCP-1 with antibodies,47 or decoy chemokine gene48 and chemokine receptor49 inhibited relapse and progression of EAE.
In summary, we induced chronic (RR and SP) EAE by immunizing LEW.1AV1 rats with MOG and MOGP7 and examined the role of T and B cells in its pathogenesis. Since rats with EAE induced by the major encephalitogenic MOG peptide, MOGP7, developed disease relapse without MOG antibody elevation, this process could occur without autoantibody involvement. However, autoantibodies against the conformational epitopes of MOG were detected in MOG-induced, but not in MOG peptide-induced, EAE, suggesting the importance of such antibodies in disease progression. Therefore, multiplexed analysis of the disease status is important not only for understanding the pathogenesis, but also for the application of appropriate immunotherapies against autoimmune diseases of the CNS.
Acknowledgments
This study was supported in part by Health and Labour Sciences Research Grants for Research on Psychiatric and Neurological Diseases and Mental Health from the Ministry of Health and Labour and by Grants-in-Aid from the Ministry of Education and Science, Japan. Y. M. was also supported by ‘Welfare and Health Fund’ of Tokyo Metropolitan Government.
Glossary
Abbreviations:
- EAE
experimental autoimmune encephalomyelitis
- CME
conformational epitope
- CNS
central nervous system
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- MOGP7
MOG peptide 7
- p.i.
postimmunization
- RR
relapsing and remitting
- SC
spinal cord
- SP
secondary progressive
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 5.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller SD, Vanderlugt CL, Begolka WS, Pao W, Neville KL, Yauch RL, Kim BS. Epitope spreading leads to myelin-specific autoimmune responses in SJL mice chronically infected with Theiler’s virus. J Neurovirol. 1997;3(Suppl. 1):S62–5. [PubMed] [Google Scholar]
- 7.Vanderlugt CL, Neville KL, Nikcevich KM, Eagar TN, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:670–8. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 8.Jones RE, Bourdette D, Moes N, Vandenbark A, Zamora A, Offner H. Epitope spreading is not required for relapses in experimental autoimmune encephalomyelitis. J Immunol. 2003;170:1690–8. doi: 10.4049/jimmunol.170.4.1690. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed Z, Gveric D, Pryce G, Baker D, Leonard JP, Cuzner ML. Myelin/axonal pathology in interleukin-induced serial relapse of experimental allergic encephalomyelitis in the Lewis rats. Am J Pathol. 2001;158:2127–38. doi: 10.1016/s0002-9440(10)64684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisi GM, Santambrogio L, Houchwald G, Smith S, Carlino JA, Jeanette-Thorbecke G. Staphylococcal enterotoxin B and tumor-necrosis factor-α-induced relapses of experimental allergic encephalomyelitis: protection by transforming growth factor-β and interleukin-10. Eur J Immunol. 1995;25:3035–40. doi: 10.1002/eji.1830251108. [DOI] [PubMed] [Google Scholar]
- 11.von Budingen HC, Hauser SL, Fuhrmann A, Nabavi CB, Lee JI, Genain CP. Molecular characterization of antibody specificities against myelin/oligodendrocyte glycoprotein in autoimmune demyelination. Proc Natl Acad Sci U S A. 2002;99:8207–12. doi: 10.1073/pnas.122092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koehler NK, Genain CP, Giesser B, Hauser SL. The human T cell response to myelin oligodendrocyte glycoprotein: a multiple sclerosis family-based study. J Immunol. 2002;168:5920–7. doi: 10.4049/jimmunol.168.11.5920. [DOI] [PubMed] [Google Scholar]
- 13.Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–45. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor KC, Appel H, Bregoli L, et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J Immunol. 2005;175:1974–82. doi: 10.4049/jimmunol.175.3.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakuma H, Kohyama K, Park IK, Miyakoshi A, Tanuma N, Matsumoto Y. Clinicopathological study of a myelin oligodendrocyte glycoprotein-induced demyelinating disease in LEW.1AV1 rats. Brain. 2004;127:2201–13. doi: 10.1093/brain/awh260. [DOI] [PubMed] [Google Scholar]
- 16.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–9. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 17.Storch MK, Stefferl A, Brehm U, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–94. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Fujiwara M. The immunopathology of adoptively transferred experimental allergic encephalomyelitis (EAE) in Lewis rats. Part 1. Immunohistochemical examination of developing lesion of EAE. J Neurol Sci. 1987;77:35–47. doi: 10.1016/0022-510x(87)90204-8. [DOI] [PubMed] [Google Scholar]
- 19.Ohmori K, Hong Y, Fujiwara M, Matsumoto Y. In situ demonstration of proliferating cells in the rat central nervous system during experimental autoimmune encephalomyelitis. Evidence suggesting that most infiltrating T cells do not proliferate in the target organ. Lab Invest. 1992;66:54–62. [PubMed] [Google Scholar]
- 20.Kishiro Y, Kagawa M, Naito I, Sado Y. A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct Funct. 1995;20:151–6. doi: 10.1247/csf.20.151. [DOI] [PubMed] [Google Scholar]
- 21.Lalive PH, Menge T, Delarasse C, Della Gaspera B, Pham-Dinh D, Villoslada P, von Budingen HC, Genain CP. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:2280–5. doi: 10.1073/pnas.0510672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou D, Srivastava R, Nessler S, et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:19057–62. doi: 10.1073/pnas.0607242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Tsuchida M, Hanawa H, Abo T. Successful prevention and treatment of autoimmune encephalomyelitis with short-term administration of anti-T cell receptor αβ antibody. Immunology. 1994;41:1–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, Jacob U. Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci USA. 2003;100:9446–51. doi: 10.1073/pnas.1133443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–54. [PMC free article] [PubMed] [Google Scholar]
- 26.Weissert R, de Graaf KL, Storch MK, Barth S, Linington C, Lassmann H, Olsson T. MHC class II-regulated central nervous system autoaggression and T cell responses in peripheral lymphoid tissues are dissociated in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7588–99. doi: 10.4049/jimmunol.166.12.7588. [DOI] [PubMed] [Google Scholar]
- 27.Miller SD, Vanderlugt CL, Begolka WS, et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–6. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 28.Ristori G, Giubilei F, Giunti D, Perna A, Gasperini C, Buttinelli C, Salvetti M, Uccelli A. Myelin basic protein intramolecular spreading without disease progression in a patient with multiple sclerosis. J Neuroimmunol. 2000;110:240–3. doi: 10.1016/s0165-5728(00)00342-8. [DOI] [PubMed] [Google Scholar]
- 29.Takacs K, Chandler P, Altmann DM. Relapsing and remitting experimental allergic encephalomyelitis: a focused response to the encephalitogenic peptide rather than epitope spread. Eur J Immunol. 1997;27:2927–34. doi: 10.1002/eji.1830271127. [DOI] [PubMed] [Google Scholar]
- 30.Smith PA, Morris-Downes M, Heijmans N, et al. Epitope spread is not critical for the relapse and progression of MOG 8-21 induced EAE in Biozzi ABH mice. J Neuroimmunol. 2005;164:76–84. doi: 10.1016/j.jneuroim.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Jones RE, Mass M, Bourdette DN. Myelin basic protein-specific T lymphocytes induce chronic relapsing experimental autoimmune encephalomyelitis in lymphocyte-deficient (SCID) mice. J Neuroimmunol. 1999;93:92–101. doi: 10.1016/s0165-5728(98)00205-7. [DOI] [PubMed] [Google Scholar]
- 32.Tejada-Simon MV, Zang YCQ, Ynag D, et al. Aberrant T cell response to myelin antigen during clinical exacerbation in patients with multiple sclerosis. Int Immunol. 2000;12:1641–50. doi: 10.1093/intimm/12.12.1641. [DOI] [PubMed] [Google Scholar]
- 33.Goebels N, Hofstetter H, Schmidt S, Brunner C, Wekerle H, Hohlfeld R. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain. 2000;123:508–18. doi: 10.1093/brain/123.3.508. [DOI] [PubMed] [Google Scholar]
- 34.Davis S, Nicholson T, Laura M, Giovannoni G, Altmann DM. Spread of T lymphocyte immune response to myelin epitopes with duration of multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:371–7. doi: 10.1093/jnen/64.5.371. [DOI] [PubMed] [Google Scholar]
- 35.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–3. [PubMed] [Google Scholar]
- 36.Lyons JA, Ramsbottom MJ, Cross AH. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur J Immunol. 2002;32:1905–13. doi: 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Lampasona V, Franciotta D, Furlan R, Zanaboni S, Fazio R, Bonifacio E, Comi G, Martino G. Similar low frequency of anti-MOG IgG and IgM in MS patients and healthy subjects. Neurology. 2004;62:2092–4. doi: 10.1212/01.wnl.0000127615.15768.ae. [DOI] [PubMed] [Google Scholar]
- 38.Kuhle J, Pohl C, Mehling M, et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med. 2007;356:371–8. doi: 10.1056/NEJMoa063602. [DOI] [PubMed] [Google Scholar]
- 39.von Budingen HC, Hauser SL, Ouallet JC, Tanuma N, Menge T, Genain CP. Epitope recognition on the myelin/oligodendrocyte glycoprotein differentially influences disease phenotype and antibody effector functions in autoimmune demyelination. Eur J Immunol. 2004;34:2072–83. doi: 10.1002/eji.200425050. [DOI] [PubMed] [Google Scholar]
- 40.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 41.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–8. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–31. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Nath N, Prasad R, Giri S, Singh AK, Singh I. T-bet is essential for the progression of experimental autoimmune encephalomyelitis. Immunology. 2006;118:384–91. doi: 10.1111/j.1365-2567.2006.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci USA. 2005;102:17101–6. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jee Y, Yoon WK, Okura Y, Tanuma N, Matsumoto Y. Upregulation of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in the central nervous system is closely associated with relapse of autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol. 2002;128:49–57. doi: 10.1016/s0165-5728(02)00147-9. [DOI] [PubMed] [Google Scholar]
- 47.Karpus WJ, Kennedy KJ. MIP-1α and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol. 1997;62:681–7. [PubMed] [Google Scholar]
- 48.Park IK, Hiraki K, Kohyama K, Matsumoto Y. Differential effects of decoy chemokine (7ND) gene therapy on acute, biphasic and chronic autoimmune encephalomyelitis: implication for pathomechanisms of lesion formation. J Neuroimmunol. 2008;194:34–43. doi: 10.1016/j.jneuroim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto Y, Sakuma H, Miyakoshi A, Tsukada Y, Kohyama K, Park I, Tanuma T. Characterization of relapsing autoimmune encephalomyelitis and its treatment with decoy chemokine receptor gene. J Neuroimmunol. 2005;170:49–61. doi: 10.1016/j.jneuroim.2005.08.022. [DOI] [PubMed] [Google Scholar]