Abstract
Systemic lupus erythematosus (SLE) is characterized in its early stages by the expansion of autoreactive T cells that trigger B-cell activation with subsequent multi-organ injury. Dendritic cells (DCs) in lupus were found to display an aberrant phenotype with higher expression of the maturation markers major histocompatibility complex (MHC) class II, CD80 and CD86, as well as higher production of proinflammatory cytokines including interleukin-12 (IL-12), resulting in an increased ability to activate T cells. A peptide (hCDR1) based on the complementarity determining region-1 of an anti-DNA antibody ameliorated SLE in both induced and spontaneous lupus models by downregulating T-cell functions. Our objectives were to determine whether DCs play a role in promoting the beneficial effects of hCDR1. We showed here that treatment with hCDR1 lowered the expression levels of MHC class II, CD80 and CD86 on DCs. The latter effect was associated with downregulation of messenger RNA expression and secretion of IL-12, a cytokine that upregulated T-cell proliferation and interferon-γ (IFN-γ) secretion. Moreover, DCs derived from hCDR1-treated mice downregulated proliferation and IFN-γ secretion by T cells from untreated mice. Upregulation of transforming growth factor-β (TGF-β) secretion by T cells, following treatment with hCDR1, resulted in downregulation of IFN-γ production and contributed to the phenotypic changes and magnitude of IL-12 secretion by DCs. The ameliorating effects of hCDR1 are therefore mediated at least partially by the upregulated secretion of TGF-β by T cells that contribute to the induction of DCs with immature phenotype and suppressed functions. The resulting DCs further downregulate autoreactive T-cell functions.
Keywords: cytokines, dendritic cells, murine lupus, T-cell functions, tolerogenic peptide
Introduction
Antigen-presenting cells (APCs), mainly dendritic cells (DCs), govern the balance between tolerance and immunity of T cells.1,2 Two well-established maturation states for DCs include the immature and mature states.3 Immature DCs function as antigen-capturing cells and express relatively low levels of surface major histocompatibility complex (MHC) molecules and costimulatory molecules such as CD80 and CD86.2,4,5 Mature DCs are immunogenic in that they express high levels of these cell surface molecules, which are important for T-cell activation.2,6 These phenotypic changes, that occur following maturation and activation of DCs in response to infection or inflammation, are accompanied by the production of cytokines, of which interleukin-12 (IL-12) is necessary for T helper type 1 (Th1) activation and interferon-γ (IFN-γ) production by T cells.3,7–9 In addition, maturation and activation of DCs following infection or inflammation changes the status of these cells from tolerogenicity to immunogenicity.6,7
Systemic lupus erythematosus (SLE) is a disease characterized, at early stages, by the expansion of autoreactive T cells that trigger polyclonal B-cell activation with subsequent hypergammaglobulinaemia and organ injury mainly as a result of immune complex deposits and cell infiltration.10–12 It was recently reported that DCs from patients with SLE display an aberrant phenotype with higher expression of the maturation markers MHC class II, CD80 and CD86 compared with DCs of controls.13–15 Moreover, the T-cell hyperactivity in murine lupus was reported to be a consequence of hyperstimulatory DCs that were significantly more mature/activated and more proinflammatory with elevated production of various cytokines including IL-12, and with the ability to increase T-cell proliferation and activation compared with the DCs of controls.13–15 These properties enable them to breach tolerance.15
Experimental SLE can be induced in naïve, non-SLE-prone mice, by immunization with monoclonal anti-DNA antibodies that express the major idiotype (Id), designated 16/6Id.16,17 The 16/6Id-induced disease in mice is manifested by high levels of autoantibodies (anti-double-stranded DNA and anti-nuclear protein antibodies), and by SLE-associated clinical symptoms.16,17 Furthermore, a peptide (hCDR1) based on the sequence of the complementarity determining region 1 (CDR1) of the human 16/6Id was shown to downregulate in vitro and in vivo autoreactive T-cell responses, and to ameliorate the clinical manifestations of spontaneous (NZB × NZW)F1 and induced models of SLE in mice.18–22 The latter was associated with downregulation of the cytokines that play a key role in the pathogenesis of lupus (e.g. IFN-γ, IL-10 and IL-1β) and with upregulation of the immunosuppressive cytokine transforming growth factor-β (TGF-β).19,20,22 Moreover, treatment with hCDR1 inhibited T-cell adhesion and chemotaxis by downregulating extracellular signal-regulated kinase phosphorylation23 which was found to be involved in 16/6Id stimulated T-cell proliferation. Interferon-γ was also found to play an important role in the 16/6Id-stimulated proliferation24 and to be associated with downregulation of T-cell receptor signalling, T-bet expression and nuclear factor-κB activation.25
The DCs of lupus-afflicted mice and patients were found to be more mature/activated, with higher production capacity of proinflammatory cytokines including IL-12, and with increased ability to activate T cells,13,15 the downregulation of the properties of the latter DCs might be of importance in decreasing their immunogenicity. Hence, the objective of the present study was to determine whether hCDR1, which has been shown to be beneficial in lupus, affects the phenotype and function of DCs. We show here that treatment with hCDR1 reduced the expression levels of MHC class II, CD80 and CD86 on DCs (CD11c-positive) in association with downregulated secretion of IL-12 and downregulated expression of IL-12 messenger RNA (mRNA). Moreover, the hCDR1-affected DCs were shown to downregulate T-cell functions as they inhibited T-cell proliferation and Th1 activation. The upregulated secretion of TGF-β by T cells of hCDR1-treated mice contributed to the observed changes in DCs.
Materials and methods
Mice
Female BALB/c mice (Harlan, Indianapolis, IN) were used at the age of 8–10 weeks. (NZB × NZW)F1 female mice were purchased from Jackson Laboratory (Bar Harbor, ME). The study was approved by the Animal Care and Use Committee of the Weizmann Institute of Science.
Synthetic peptides
A peptide (GYYWSWIRQPPGKGEEWIG) based on the sequence of the CDR1 of an anti-DNA monoclonal antibody (mAb) that bears the major idiotype, designated 16/6Id, was synthesized by Polypeptide Laboratories (Los Angeles, CA).
Antibodies and reagents
The human anti-DNA mAb that bears the 16/6Id (IgG1/κ) was previously described.26 The antibody is secreted by hybridoma cells that are grown in culture and purified by using a protein G–Sepharose column (Pharmacia, Uppsala, Sweden). Phycoerythrin-conjugated anti-CD11c, fluorescein isothiocyanate-conjugated anti-CD80, anti-CD86 and anti-I-Ad were purchased from Pharmingen (BD Bioscience, Mountain View, CA). Cells were stained with the latter reagents according to the manufacturer’s instructions. A recombinant human TGF-β and an anti-TGF-β1, -β2 and -β3 neutralizing mAb (clone 1D11) and its isotype control were purchased from R & D Systems (Minneapolis, MN).
Immunization and treatment of mice
BALB/c mice were immunized with 1 μg of the human mAb 16/6Id in complete Freund’s adjuvant (CFA) with or without a concomitant subcutaneous injection of hCDR1 (50 μg/mouse) in phosphate-buffered saline (PBS). (NZB × NZW)F1 mice with a full-blown disease (8 months old) were treated for 10 weeks, once a week, with either the vehicle Captisol [sulphobutylether beta cyclodextrin that has been designed by Cydex (Lenexa, KS) to enhance the solubility and stability of drugs] or hCDR1 (50 μg/mouse).
Purification of DCs
Lymph node (LN)-derived DCs were isolated as follows: LN cells were released by tearing and treatment with collagenase D (Roche Diagnostics Corp, Indianapolis, IN). CD11c+ cells were isolated using anti-CD11c-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions [purity of DCs determined by fluorescence-activated cell sorting (FACS) was 75–85%].
Purification of T cells
Preparation of an enriched population of T cells either from LN (BALB/c mice) or spleen (NZB × NZW)F1 was performed as previously described.25 The purified cells, which were mainly T cells (> 92% as assessed by FACS analysis), were then collected and washed in RPMI-1640.
Cell stimulation with 16/6Id in vitro
The LN cells (5 × 106) were incubated in the presence of 16/6Id (25 μg/ml). Purified T cells were stimulated using either irradiated (3000 rads) syngeneic splenocytes from normal mice (as a source of APCs) or irradiated purified DCs, in the presence of 16/6Id (25 μg/ml).
Proliferation assays
Ten days after immunization, LN-derived T cells were incubated with either irradiated (3000 rads) syngeneic splenocytes from normal mice or with irradiated purified DCs in the presence of the 16/6Id (5 μg/well). Cultures were set up (in triplicate) in 200 μl of RPMI-1640-enriched medium containing 1% syngeneic normal mouse serum in 96-well microtitre plates (Nunc, Roskilde, Denmark). After 96 hr, [3H]thymidine was added to the wells. Eighteen hours later, the plates were harvested onto a filter plate. Incorporation of [3H]thymidine was measured using a beta-counter.
Detection of IL-12 and IFN-γ secretion
Cells (5 × 106/ml) of 16/6Id-immunized mice were incubated in enriched medium and stimulated with 16/6Id (25 μg/ml). Supernatants were removed after 48 hr and analysed for cytokine content. The levels of IL-12 (p70) or IFN-γ were determined by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (BD Bioscience).
Real-time reverse transcription-polymerase chain reaction
Quantitative real-time reverse transcription-polymerase chain reaction using a LightCycler (Roche, Mannheim, Germany) was performed as previously described.25 The following primer sequences were used (forward and reverse respectively): IL-12 (5′-CTTCAGTGCCTAGAAGATG-3′ and 5′-AGTCCACCACAGTTGC-3′), IFN-γ (5′-GAACGCTACACACTGC-3′ and 5′-CTGGACCTGTGGGTTG-3′), and β-actin (5′-GTGACGTTGACATCCG-3′ and 5′-GAGCGTTTGTTGTACCT-3′). The levels of β-actin were used for normalization while calculating the expression levels of other genes. The results are expressed as the relative expression levels for each gene.
Statistical analysis
The non-parametric Mann–Whitney U-test was used for statistical analyses of the data. A value of P ≤ 0·05 was considered statistically significant.
Results
Treatment with hCDR1 downregulated MHC class II, CD80 and CD86 on dendritic cells
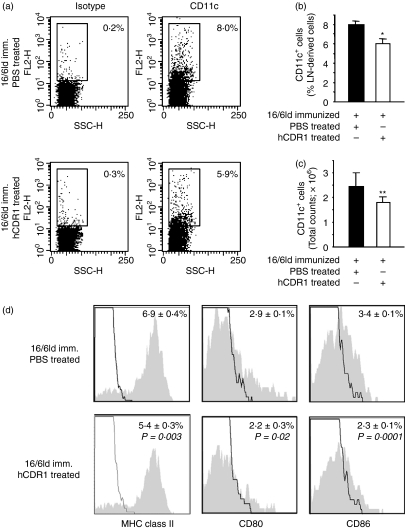
Treatment with hCDR1 has been shown to downregulate SLE-associated T-cell functions. Because DCs are important players in T-cell activation, we studied the effect of treatment with hCDR1 on DCs. To this end, 10 days after immunization, LN cells, derived from mice that were immunized with 16/6Id and treated concomitantly with either the vehicle (PBS) or hCDR1, were double stained with antibodies to CD11c and to either MHC class II, CD80, or CD86. The results were analysed by FACS. Dot plots of CD11c expression in LN cells of representative mice can be seen in Fig. 1(a). The mean (± SD) percentage expression (Fig. 1b) and the absolute counts (Fig. 1c) of CD11c-expressing cells indicated that the percentage and the absolute counts of DCs were significantly reduced in the 16/6Id-immunized mice that were treated with hCDR1 in comparison to the mice that received PBS. The effects of treatment with hCDR1 on the phenotype of DCs were also studied on gated CD11c cells, and the results are shown in Fig. 1(d). Treatment with hCDR1 resulted in significantly lower percentages of CD11c DCs that express MHC class II and the costimulatory molecules CD80 and CD86. Although the relative expression of MHC class II molecules on DCs was slightly reduced following treatment with hCDR1 (i.e. 77% compared with 80% for hCDR1-treated and PBS-treated mice, respectively), the mean fluorescence intensity (MFI) of this molecule was significantly reduced (i.e. 237 ± 6 versus 270 ± 25 in hCDR1-treated and PBS-treated mice, respectively). Furthermore, treatment with hCDR1 was associated with a diminished expression of both CD80 (P = 0·05) and CD86 (P = 0·01) molecules in the DCs (i.e. ∼ 22% versus ∼ 35% of DCs from hCDR1-treated and PBS-treated, 16/6Id immunized mice, respectively). The results are representative of four independent experiments. It is noteworthy that treatment with hCDR1 downregulated the expression levels of these molecules (Fig. 1) to levels comparable to those of the negative control, namely CFA-injected mice (5·0%, 2·6%, 2·3% for MHC class II, CD80 and CD86, respectively). Consequently, treatment with hCDR1 downregulated the expression of maturation markers on DCs.
Figure 1.
Treatment with hCDR1 downregulated major histocompatibility complex (MHC) class II, CD80 and CD86 on dendritic cells (DCs). Ten days after immunization, lymph node (LN) cells derived from mice that were immunized with 16/6Id with or without concomitant treatment with hCDR1, were double-stained with antibodies to CD11c and to either MHC class II, CD80 or CD86. The results were analysed by fluorescence-activated cell sorting. The results of one representative experiment of four are depicted. (a) Dot plots depicting staining with CD11c and isotype control of LN cells from individual mice per treatment group (n= 6 or n = 7/group). The mean (± SD) percentages (b) and the mean (± SD) total cell counts (c) of CD11c+ cells within the LN-derived cells from 16/6Id-immunized mice of each treatment group. (d) The expression of MHC class II, CD80 and CD86 on gated CD11c+ cells. Representative grey histograms and black lines depict staining with the relevant monoclonal antibodies and isotype control, respectively. *P < 0·0001, **P < 0·02.
Treatment with hCDR1 downregulated IL-12 secretion
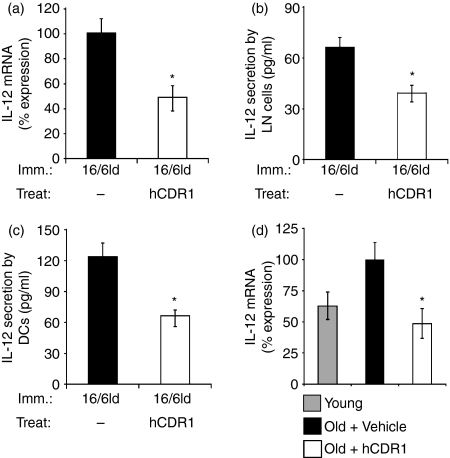
To determine whether the phenotypic changes in DCs are accompanied by modulation of their function, we studied the effect of treatment with hCDR1 on the secretion of IL-12, a cytokine that is important for T-cell activation during antigen presentation.8 We first studied the effect of hCDR1 on mRNA expression of IL-12 in LN cells derived from mice that were immunized with 16/6Id and either concomitantly treated or not with hCDR1. As shown in Fig. 2(a), treatment with hCDR1 significantly downregulated IL-12 mRNA expression (100% versus 48·4% in 16/6Id immunized mice that were either not treated or treated with hCDR1, respectively). This effect was accompanied by a significant downregulation of IL-12 secretion (from 66 pg/ml to 39 pg/ml, Fig. 2b). IL-12 secretion by LN cells derived from the negative control (CFA-injected) mice was determined to be 36 ± 2·8 pg/ml.
Figure 2.
Treatment with hCDR1 downregulated interleukin-12 (IL-12) secretion and messenger RNA (mRNA) expression. Lymph node (LN) cells (a, b) and purified dendritic cells (DCs) (c) were derived from 16/6Id-immunized BALB/c mice (a, b, c) that were either treated or not with hCDR1. Expression of IL-12 mRNA was determined by real-time reverse transcription–polymerase chain reaction (mean % expression ± SD). Secretion of IL-12 (b, c) was measured in supernatants of the cell cultures (see Materials and methods) by enzyme-linked immunosorbent assay (mean pg/ml ± SD). Expression of IL-12 mRNA was also determined in splenocytes derived from young (2 months old), disease-free mice and old (8 months old) SLE-afflicted (NZB × NZW)F1 mice (d) that were either treated with vehicle (Captisol) or hCDR1. The results of one representative experiment of the three performed are depicted. *P < 0·05.
Because DCs are a main source for IL-12, we studied the effect of hCDR1 on its secretion by DCs derived from mice that were immunized with 16/6Id and were either concomitantly treated or not treated with hCDR1. Figure 2(c) demonstrates that treatment with hCDR1 significantly inhibited IL-12 secretion by DCs (123 pg/ml versus 64 pg/ml following immunization with 16/6Id without or with concomitant treatment with hCDR1, respectively). Therefore, the downregulation of maturation markers on DCs is associated with the inhibition of IL-12 expression and secretion.
Next, to confirm the role of IL-12 in SLE-afflicted mice, we also determined the effect of treatment with hCDR1 in (NZB × NZW)F1 mice that develop lupus spontaneously. To this end, splenocytes of young (2 months old) and old (8 months old) SLE-afflicted (NZB × NZW)F1 mice that were either treated with hCDR1 or with vehicle were compared for mRNA expression of IL-12. Figure 2(d) shows a significant upregulation of IL-12 expression in splenocytes derived from old SLE-afflicted (NZB × NZW)F1 mice that were treated with the vehicle compared with young, disease-free, mice. Moreover, in vivo treatment of old, SLE-afflicted (NZB × NZW)F1 mice, with hCDR1 significantly downregulated IL-12 mRNA expression to levels detected in young mice.
In vitro addition of IL-12 upregulated IFN-γ secretion and proliferation by T cells
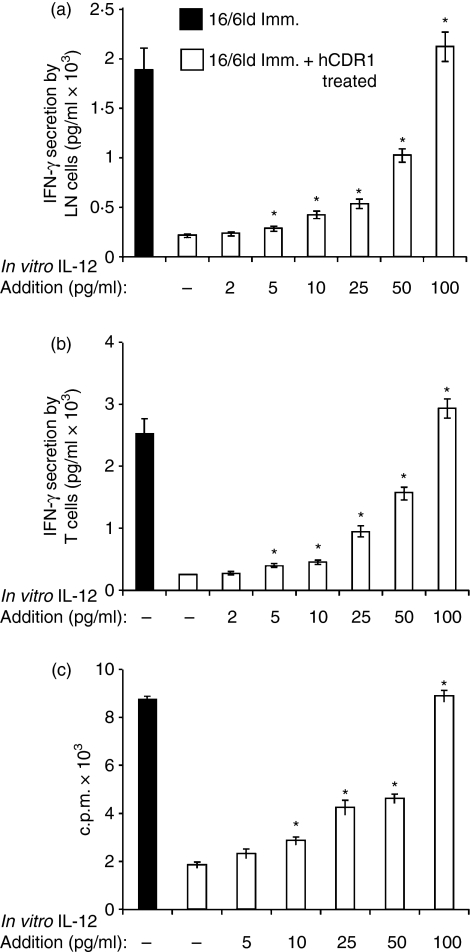
To determine whether the observed inhibition of IL-12 secretion is also functionally significant, we studied the effect of IL-12 on T-cell functions. To this end, LN cells derived from mice that were treated with hCDR1 concomitant with their immunization with 16/6Id were incubated with various concentrations of IL-12 and in the presence of 16/6Id. Forty-eight hours later IFN-γ secretion was measured in the supernatants by enzyme-linked immunosorbent assay. As shown in Fig. 3(a), in vitro IL-12 addition upregulated IFN-γ secretion in a dose-dependent manner up to a level (with 100 pg/ml of IL-12) comparable to that determined in supernatants of 16/6Id-immunized mice. Next, we studied the effect of IL-12 on IFN-γ secretion from T cells incubated with irradiated normal splenocytes in the presence of 16/6Id. As shown in Fig. 3(b), a similar dose-dependent increase in IFN-γ secretion was observed following the addition of IL-12.
Figure 3.
Interleukin-12 (IL-12) upregulated 16/6Id triggered interferon-γ (IFN-γ) secretion and proliferation by T cells. Lymph node (LN) cells (a), derived from mice that were treated with hCDR1 concomitant with their immunization with 16/6Id were incubated with various concentrations of IL-12 in the presence of 16/6Id (25 μg/ml). Forty-eight hours later, IFN-γ secretion was measured in the supernatants by enzyme-linked immunosorbent assay. Purified T cells (b, c) were incubated with irradiated syngeneic splenocytes from normal mice with various concentrations of IL-12 in the presence of 16/6Id. Secretion of IFN-γ (b) was measured in the supernatants by enzyme-linked immunosorbent assay. Cell proliferation (c) was measured by incorporation of [3H]thymidine (see Materials and methods). Results of proliferation are expressed as mean counts per min (c.p.m.) ± SD. The results of one representative experiment out of the three performed are shown. *P < 0·05.
To assess the effect of IL-12 on T-cell proliferation, we incubated the cells with irradiated normal splenocytes in the presence of 16/6Id and various concentrations of IL-12. As shown in Fig. 3(c), T-cell proliferation was upregulated following the addition of IL-12. A significant increase in T-cell proliferation was detected already after the addition of 10 pg/ml of IL-12. Consequently, the addition of low concentrations of IL-12 significantly affected the proliferation of and IFN-γ secretion by 16/6Id-stimulated T cells.
The effects of DCs from different sources on T-cell functions
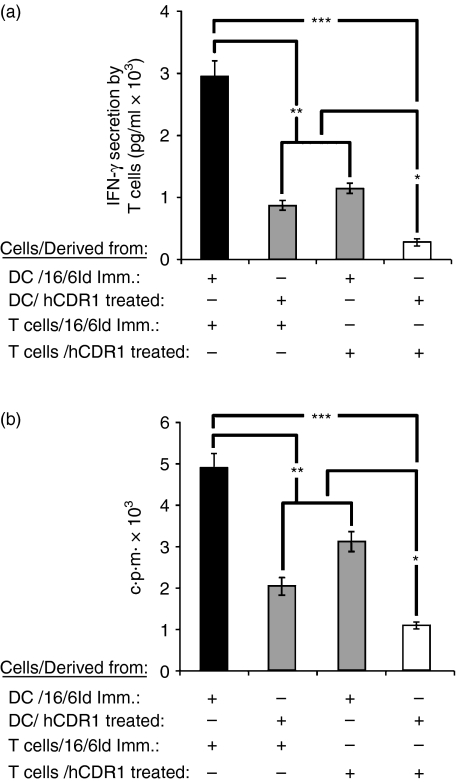
Next, we wanted to evaluate the effect of DCs derived from hCDR1-treated mice on T cells. To this end, DCs derived from mice that were immunized with 16/6Id and concomitantly treated with hCDR1 or PBS were incubated with T cells derived from either group in the presence of 16/6Id. Incubation of DCs and T cells from hCDR1-treated mice (Fig. 4a) significantly downregulated IFN-γ secretion, as compared with DCs and T cells derived from 16/6Id-immunized mice. Incubation of DCs from hCDR1-treated mice with T cells from 16/6Id-immunized mice, or of DCs from 16/6Id-immunized mice with T cells from hCDR1-treated mice resulted in significantly lower secretion levels of IFN-γ than those secreted by mixtures of DCs and T cells derived from 16/6Id-immunized mice. However, the former levels were higher than the levels observed when the two types of cells were derived from hCDR1-treated mice.
Figure 4.
The effects of dendritic cells (DCs) from different sources on T-cell function. Irradiated DCs purified from mice that were immunized with 16/6Id and treated with phosphate-buffered saline or hCDR1 were incubated with T cells derived from either of the latter groups in the presence of 16/6Id (25 μg/ml). Interferon-γ (IFN-γ) secretion (a) was measured in the supernatants by enzyme-linked immunosorbent assay, and cell proliferation (b) was measured by incorporation of [3H]thymidine (see Materials and methods). Black bars indicate DCs and T cells from 16/6Id-immunized mice. White bars indicate DCs and T cells from hCDR1-treated mice. Grey bars indicate mixtures of DCs and T cells from either group. The results of one representative experiment out of the three performed are shown. *P < 0·05, **P < 0·01, ***P < 0·005.
To evaluate the direct effect of DCs on T-cell proliferation, we studied the effect of the same four combinations of cells on the 16/6Id-stimulated proliferation. A significant downregulation in the 16/6Id-triggered T-cell proliferation was observed when both the T cells and DCs were derived from mice that were treated with hCDR1 (Fig. 4b), as compared with the proliferation of cell mixtures from 16/6Id-immunized mice (Fig. 4b). Coincubation of DCs from hCDR1-treated mice with T cells from 16/6Id-immunized mice or vice versa (Fig. 4b) significantly downregulated T-cell proliferation compared with that of cells derived from 16/6Id-immunized mice;it also downregulated, although to a lesser degree, the proliferation in comparison to the mixure of DCs and T cells derived from hCDR1-treated mice (white bars).
The role of TGF-β in the effect of hCDR1 on the phenotype and function of DCs
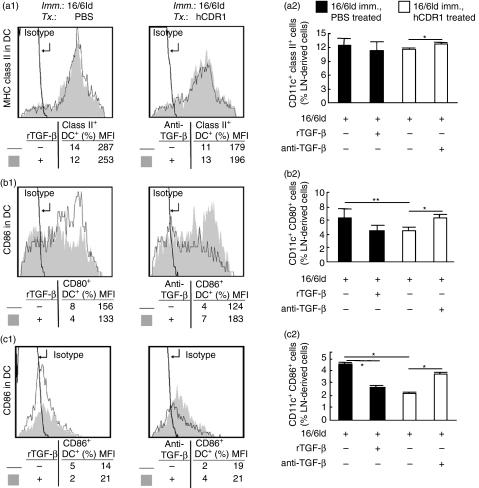
Because TGF-β plays an important role in the inhibitory effect of hCDR1 on T-cell functions,23–25,27 we studied the effect of this cytokine on maturation markers of DCs. To this end, LN cells derived from either PBS-treated or hCDR1-treated, 16/6Id-immunized mice were incubated with 16/6Id for 24 hr in the presence of TGF-β (250 pg/ml) or anti-TGF-β mAb (10 μg/ml). The cultured cells were then double-stained with antibodies to CD11c and to MHC class II, CD80 or CD86. The expression of the maturation markers was determined on CD11c-gated cells, and the results were analysed by FACS. Representative histograms from individual mice are presented in Fig. 5(a1–c1) and the mean (± SD) expression of DC maturation markers from all mice within a group (n= 4/group) is summarized in Fig. 5(a2–c2). These results indicated that treatment of 16/6Id-immunized mice with hCDR1 resulted in lower expression of MHC class II and a significant downregulation in the expression of CD80 (P < 0·05) and CD86 (P < 0·0001) on LN-derived DCs as compared to DCs from PBS-treated mice. Furthermore, the addition of recombinant TGF-β to the cultures of LN cells from PBS-treated, 16/6Id-immunized mice, caused a reduced expression of MHC class II molecules on the DCs in association with a decreased expression of the costimulatory molecules, CD80 and CD86. Moreover, the decrement in the expression of these maturation markers on DCs from hCDR1-treated, 16/6Id-immunized mice was abrogated when anti-TGF-β neutralizing mAb was added to the cultures (Fig. 5). The results were reproducible in four independent experiments.
Figure 5.
The role of transforming growth factor-β (TGF-β) in the effects of hCDR1 on dendritic cells (DCs). Lymph node (LN) cells derived from 16/6Id-immunized mice that were treated with (Tx) phosphate-buffered saline or hCDR1 were incubated for 24 hr with recombinant TGF-β (250 pg/ml) or an anti-TGF-β neutralizing monoclonal antibody (mAb; 10 μg/ml). The cultured cells were then double stained for CD11c and either major histocompatibility complex (MHC) class II, CD80 or CD86. The expression of the latter three molecules on gated CD11c+ cells is depicted in representative histograms (a1–c1) where grey-filled and black line histograms depict the state following incubation, respectively, with or without the presence of rTGF-β and the anti-TGF-β mAb in the cultures. Statistical data are presented at the bottom of histograms. (a2–c2) Mean (± SD) percentages of MHC-class II-, CD80- and CD86-expressing CD11c+ cells within the LN-derived cells from all mice per treatment group (n= 4/group). Results of one representative experiment out of four are shown. *P < 0·05, **P < 0·005.
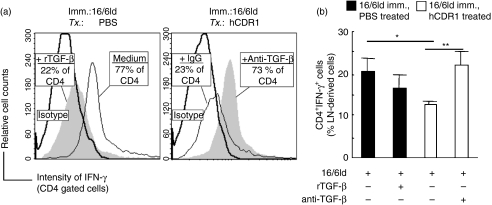
Because secretion of IFN-γ is downregulated by DCs from hCDR1-treated mice (Fig. 4), we wanted to evaluate the effect of TGF-β on IFN-γ production. To this end, LN cells derived from PBS-treated or hCDR1-treated, 16/6Id-immunized mice were incubated in the presence of 16/6Id for 24 hr with TGF-β (250 pg/ml) or anti-TGF-β mAb (10 μg/ml), respectively. Then, the cultured cells were double-stained with antibodies to CD4 and intracellular IFN-γ. The results were analysed by FACS and are shown in Fig. 6. Representative histograms from individual mice are presented in Fig. 6(a), and the mean (± SD) percentage of CD4+ IFN-γ+ cells in the LN from all mice within a group (n= 4/group) is summarized in Fig. 6(b). It can be seen that the percentage of CD4+ cells (77%) that express IFN-γ in the LN cells of PBS-treated, 16/6Id-immunized mice was significantly higher (P = 0·005) in comparison to that (23%) of CD4+ cells in the LN from hCDR1-treated, 16/6Id-immunized mice. The addition of recombinant TGF-β to the culture of LN cells from PBS-treated, 16/6Id-immunized mice resulted in a diminished expression of IFN-γ in the CD4+ cells whereas the addition of anti-TGF-β neutralizing mAb to the culture of LN cells from hCDR1-treated, 16/6Id-immunized mice abrogated the suppressive effect on IFN-γ. The results were reproducible in four independent experiments.
Figure 6.
The role of transforming growth factor-β (TGF-β) in the effects of hCDR1 on interferon-γ (IFN-γ) -expressing CD4 cells. Lymph node (LN) cells derived from 16/6Id-immunized mice that were treated (Tx) with phosphate-buffered saline or hCDR1 were incubated for 24 hr with rTGF-β (250 pg/ml) or anti-TGF-β neutralizing monoclonal antibody (mAb) (10 μg/ml), respectively. The cultured cells were then double-stained for CD4 and intracellular IFN-γ. The expression of IFN-γ on gated CD4+ cells is depicted in representative histograms (a) where grey-filled and black line histograms depict the state following incubation with or without the presence of either rTGF-β or the anti-TGF-β mAb in the cultures, respectively. (b) Mean (± SD) percentages of IFN-γ-expressing CD4+ cells within the LN-derived cells from all mice per treatment group (n= 4/group). The results of one representative experiment out of the four performed are depicted. *P < 0·005, **P < 0·0005.
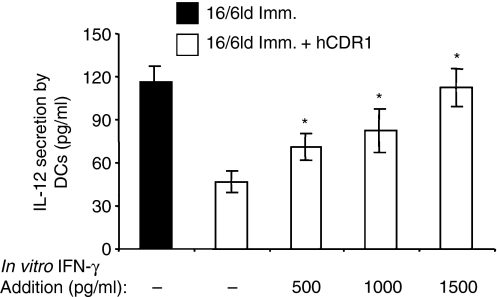
To assess the effect of IFN-γ on IL-12 secretion, DCs derived from mice that were immunized with 16/6Id and concomitantly treated with hCDR1 were incubated with various concentrations of IFN-γ and the secretion of IL-12 was measured in the supernatants of the cell culture. As shown in Fig. 7, in vitro incubation with IFN-γ upregulated IL-12 secretion from DCs in a dose-dependent manner. Notably, an in vitro addition of 500 pg/ml of IFN-γ significantly upregulated IL-12 secretion whereas 1500 pg/ml of IFN-γ elevated IL-12 secretion to a level equivalent to that secreted by DCs derived from mice that were immunized with 16/6Id without treatment with hCDR1 (Fig. 2c).
Figure 7.
The role of interferon-γ (IFN-γ) in the effect of hCDR1 on interleukin-12 (IL-12) secretion. Dendritic cells (DCs) derived from mice that were immunized with 16/6Id and concomitantly treated with hCDR1 were incubated with various concentrations of IFN-γ and the secretion of IL-12 was measured. Results of one representative experiment out of three are shown. *P < 0·05.
Discussion
The main findings of this study are that hCDR1 inhibited T-cell proliferation and IFN-γ secretion by downregulating the expression of maturation markers of DCs, namely, MHC class II, CD80 and CD86, as well as the secretion and mRNA expression of IL-12. The hCDR1 induced upregulation of TGF-β secretion by T cells contributed to these effects.
Our previous studies demonstrated the significant effect of hCDR1 on autoreactive T cells. In the present study, evaluation of the relative contribution to the inhibition of IFN-γ secretion and proliferation indicated that DCs play a key role in addition to that of T cells following treatment with hCDR1. The downregulation of the phenotype and function of DCs following treatment with hCDR1 is an additional mechanism in its ameliorating clinical effects. The beneficial effects were shown previously to be associated with upregulation of the immunosuppressive cytokine TGF-β and with inhibition of T-cell proliferation and secretion of pathogenic cytokines (e.g. IFN-γ, IL-10, IL-1β, tumour necrosis factor-α).20–25 Furthermore, induction of functional CD4 and CD8 regulatory T cells following treatment with hCDR1 and the interaction between the two populations of regulatory T cells played a critical role in the amelioration of lupus manifestations by hCDR1.27–29 Moreover, downregulation of the elevated T-cell apoptosis in lupus through several pathways including the p21Ras, Fas/Fas ligand, and the molecules caspases and Bcl-xL, is an additional mechanism by which hCDR1 suppressed SLE-associated autoimmune responses.30–32 The hCDR1 was demonstrated to be a T-cell epitope,23–25,33 and based on its specific suppressive effects on the one hand and its ability to upregulate regulatory cells and immunosuppressive molecules on the other hand,23–25,27,29,32 this tolerogenic peptide has been characterized as a partial agonist.
In the present study we showed that treatment with hCDR1 downregulated the expression of MHC class II, CD80 and CD86 on DCs (Fig. 1). In agreement, the latter were shown to be upregulated in lupus.13,15 Because T-cell stimulation and activation by antigen is dependent on two signals34 mediated by T-cell interaction with these molecules on DCs, it is suggested that hCDR1 exerts its beneficial effects by inhibiting the ability of DCs to present antigen through MHC class II and to costimulate through CD80 and CD86. Furthermore, the effect of hCDR1 was not restricted just to the phenotype of DCs but rather, it was accompanied by a significant downregulation of IL-12 secretion and its mRNA expression by LN (Fig. 2a,b) from 16/6Id-immunized mice. The main source for IL-12 secretion and its inhibition was DCs (Fig. 2c). The production of this cytokine was reported to be elevated in patients with lupus and its essential role in murine lupus-like disease was demonstrated.35,36 Indeed, treatment with hCDR1 also significantly inhibited IL-12 mRNA expression by splenocytes from mice with established lupus because its expression was downregulated in old (8 months old) SLE-afflicted (NZB × NZW)F1 mice treated with hCDR1 (Fig. 2d). The latter downregulation in IL-12 mRNA expression in SLE-afflicted (NZB × NZW)F1 mice was accompanied by improvement in the serological, clinical and immunohistological manifestations of the disease (data not shown). Taken together, these effects may suggest that hCDR1 promotes the immature state of DCs. Interleukin-12 is necessary for T-cell proliferation, Th1 activation and IFN-γ production by T cells.8,9,37,38 Indeed, the addition of IL-12 to T cells derived from mice that were treated with hCDR1, concomitant with their immunization with 16/6Id, upregulated T-cell proliferation and IFN-γ secretion (Fig. 3). The addition of low concentrations of IL-12 (as low as 5 pg/ml) to LN cells or T cells derived from mice that were treated with hCDR1, concomitant with their immunization with 16/6Id, was enough to significantly upregulate IFN-γ secretion. The observed reduction of IL-12 secretion following treatment with hCDR1 appears to be significant and relevant for the inhibition of T-cell functions.
TGF-β is an immunosuppressive cytokine that is associated with autoimmune diseases.39,40 Its levels were shown to be low in patients with active SLE.41 Treatment of SLE-prone mice, which led to the upregulation of TGF-β, ameliorated the serological and clinical manifestations.42,43 The hCDR1 was shown to upregulate TGF-β secretion by T cells and this cytokine by itself inhibited T-cell proliferation.24 The TGF-β was found to inhibit DC activation, IL-12 secretion, and the expression of various maturation markers including MHC class II, CD80 and CD86.44–46 Indeed, in vitro addition of TGF-β downregulated the expression of these markers of DCs (Fig. 5), and inhibited the production of IFN-γ by T cells (Fig. 6), and the addition of anti-TGF-β neutralizing antibody to hCDR1-treated, 16/6Id-immunized cells reversed the inhibitory effects on both DCs and T cells. The pathogenic role of IFN-γ was further demonstrated in the present study because its addition to DCs derived from mice that were treated with hCDR1 concomitant with 16/6Id immunization abrogated the inhibitory effect of hCDR1 on IL-12 secretion (Fig. 5).
In conclusion, the apparent downregulation of murine autoreactive T-cell functions, including proliferation and IFN-γ secretion, is at least partially a consequence of the ability of hCDR1 to downregulate DC phenotype and function, which are important in the stimulation and activation of T cells during the interaction between these two types of cells. The downregulation in the expression of markers characteristic of DCs by hCDR1 is mediated by a direct effect of TGF-β, whereas downregulation of IL-12 secretion by DCs is mediated indirectly via the inhibition of IFN-γ secretion by TGF-β. These phenotypic and functional effects of hCDR1 on DCs contribute to the inhibition of T-cell functions. The downregulation of IL-12 secretion was observed both early in disease induction (10 days after immunization with 16/6Id), and in SLE-afflicted (NZB × NZW)F1 mice. Therefore, the above described mechanism appears to play a role in the amelioration of SLE manifestations following treatment with hCDR1.
Glossary
Abbreviations:
- APCs
antigen-presenting cells
- CDR
complementarity-determining region
- CFA
complete Freund’s adjuvant
- DCs
dendritic cells
- Id
idiotype
- IFN
interferon
- IL
interleukin
- LN
lymph node
- mAb
monoclonal antibody
- SLE
systemic lupus erythematosus
- TGF
transforming growth factor
- Th
T helper
References
- 1.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Tan JK, O’Neill HC. Maturation requirements for dendritic cells in T cell stimulation leading to tolerance versus immunity. J Leukoc Biol. 2005;78:319–24. doi: 10.1189/jlb.1104664. [DOI] [PubMed] [Google Scholar]
- 4.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–7. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 6.De Heusch M, Oldenhove G, Urbain J, Thielemans K, Maliszewski C, Leo O, Moser M. Depending on their maturation state, splenic dendritic cells induce the differentiation of CD4(+) T lymphocytes into memory and/or effector cells in vivo. Eur J Immunol. 2004;34:1861–9. doi: 10.1002/eji.200424878. [DOI] [PubMed] [Google Scholar]
- 7.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 8.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 10.Lang TJ, Nguyen P, Papadimitriou JC, Via CS. Increased severity of murine lupus in female mice is due to enhanced expansion of pathogenic T cells. J Immunol. 2003;171:5795–801. doi: 10.4049/jimmunol.171.11.5795. [DOI] [PubMed] [Google Scholar]
- 11.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501–37. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Fujio K, Sadakata A, Okamoto A, Yu R, Yamamoto K. Identification of systemically expanded activated T cell clones in MRL/lpr and NZB/W F1 lupus model mice. Clin Exp Immunol. 2004;136:448–55. doi: 10.1111/j.1365-2249.2004.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol. 2006;177:5878–89. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 14.Decker P, Kötter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology. 2006;45:1087–95. doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, Wakeland EK, Mohan C. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Inves. 2005;115:1869–78. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendlovic S, Brocke S, Shoenfeld Y, Ben-Bassat M, Meshorer A, Bakimer R, Mozes E. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci USA. 1988;85:2260–4. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waisman A, Mendlovic S, Ruiz PJ, Zinger H, Meshorer A, Mozes E. The role of the 16/6 idiotype network in the induction and manifestations of systemic lupus erythematosus. Int Immunol. 1993;5:1293–300. doi: 10.1093/intimm/5.10.1293. [DOI] [PubMed] [Google Scholar]
- 18.Waisman A, Ruiz PJ, Israeli E, Eilat E, Konen-Waisman S, Zinger H, Dayan M, Mozes E. Modulation of murine systemic lupus erythematosus with peptides based on complementarity determining regions of a pathogenic anti-DNA monoclonal antibody. Proc Natl Acad Sci USA. 1997;94:4620–5. doi: 10.1073/pnas.94.9.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinger H, Eilat E, Meshorer A, Mozes E. Peptides based on the complementarity-determining regions of a pathogenic autoantibody mitigate lupus manifestations of (NZB × NZW)F1 mice via active suppression. Int Immunol. 2003;15:205–14. doi: 10.1093/intimm/dxg026. [DOI] [PubMed] [Google Scholar]
- 20.Eilat E, Dayan M, Zinger H, Mozes E. The mechanism by which a peptide based on complementarity-determining region-1 of a pathogenic anti-DNA auto-Ab ameliorates experimental systemic lupus erythematosus. Proc Natl Acad Sci USA. 2001;98:1148–53. doi: 10.1073/pnas.98.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eilat E, Zinger H, Nyska A, Mozes E. Prevention of systemic lupus erythematosus-like disease in (NZB × NZW)F1 mice by treating with CDR1- and CDR3-based peptides of a pathogenic autoantibody. J Clin Immunol. 2000;20:268–78. doi: 10.1023/a:1006663519132. [DOI] [PubMed] [Google Scholar]
- 22.Luger D, Dayan M, Zinger H, Liu JP, Mozes E. A peptide based on the complementarity determining region 1 of a human monoclonal autoantibody ameliorates spontaneous and induced lupus manifestations in correlation with cytokine immunomodulation. J Clin Immunol. 2004;24:579–90. doi: 10.1007/s10875-004-6245-2. [DOI] [PubMed] [Google Scholar]
- 23.Sela U, Hershkoviz R, Cahalon L, Lider O, Mozes E. Down-regulation of stromal cell-derived factor-1alpha-induced T cell chemotaxis by a peptide based on the complementarity-determining region 1 of an anti-DNA autoantibody via up-regulation of TGF-beta secretion. J Immunol. 2005;174:302–9. doi: 10.4049/jimmunol.174.1.302. [DOI] [PubMed] [Google Scholar]
- 24.Sela U, Mauermann N, Hershkoviz R, et al. The inhibition of autoreactive T cell functions by a peptide based on the CDR1 of an anti-DNA autoantibody is via TGF-beta-mediated suppression of LFA-1 and CD44 expression and function. J Immunol. 2005;175:7255–63. doi: 10.4049/jimmunol.175.11.7255. [DOI] [PubMed] [Google Scholar]
- 25.Sela U, Dayan M, Hershkoviz R, Cahalon L, Lider O, Mozes E. The negative regulators Foxj1 and Foxo3a are up-regulated by a peptide that inhibits systemic lupus erythematosus-associated T cell responses. Eur J Immunol. 2006;36:2971–80. doi: 10.1002/eji.200636137. [DOI] [PubMed] [Google Scholar]
- 26.Waisman A, Shoenfeld Y, Blank M, Ruiz PJ, Mozes E. The pathogenic human monoclonal anti-DNA that induces experimental systemic lupus erythematosus in mice is encoded by a VH4 gene segment. Int Immunol. 1995;7:689–96. doi: 10.1093/intimm/7.4.689. [DOI] [PubMed] [Google Scholar]
- 27.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF- β. Proc Natl Acad Sci USA. 2006;103:8810–15. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharabi A, Mozes E. The suppression of murine lupus by a tolerogenic peptide involves foxp3-expressing CD8 cells that are required for the optimal induction and function of foxp3-expressing CD4 cells. J Immunol. 2008;181:3243–51. doi: 10.4049/jimmunol.181.5.3243. [DOI] [PubMed] [Google Scholar]
- 29.Sharabi A, Azulai H, Shtoeger ZM, Mozes E. Clinical amelioration of murine lupus by a peptide based on the complementarity determining region-1 of an autoantibody and by cyclophosphamide: similarities and differences in the mechanisms of action. Immunology. 2007;121:248–57. doi: 10.1111/j.1365-2567.2007.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapoport MJ, Sharabi A, Aharoni D, Bloch O, Zinger H, Dayan M, Mozes E. Amelioration of SLE-like manifestations in (NZB × NZW)F1 mice following treatment with a peptide based on the complementarity determining region 1 of an autoantibody is associated with a down-regulation of apoptosis and of the pro-apoptotic factor JNK kinase. Clin Immunol. 2005;117:262–70. doi: 10.1016/j.clim.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Sharabi A, Haviv A, Zinger H, Dayan M, Mozes E. Amelioration of murine lupus by a peptide, based on the complementarity determining region-1 of an autoantibody as compared to dexamethasone: different effects on cytokines and apoptosis. Clin Immunol. 2006;119:146–55. doi: 10.1016/j.clim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Sharabi A, Luger D, Ben-David H, Dayan M, Zinger H, Mozes E. The role of apoptosis in the ameliorating effects of a CDR1-based peptide on lupus manifestations in a mouse model. J Immunol. 2007;179:4979–87. doi: 10.4049/jimmunol.179.8.4979. [DOI] [PubMed] [Google Scholar]
- 33.Sthoeger ZM, Dayan M, Tcherniack A, Green L, Toledo S, Segal R, Elkayam O, Mozes E. Modulation of autoreactive responses of peripheral blood lymphocytes of patients with systemic lupus erythematosus by peptides based on human and murine anti-DNA autoantibodies. Clin Exp Immunol. 2003;131:385–92. doi: 10.1046/j.1365-2249.2003.02058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller DL. T cells: a proliferation of costimulatory molecules. Curr Biol. 2000;10:R227–30. doi: 10.1016/s0960-9822(00)00400-0. [DOI] [PubMed] [Google Scholar]
- 35.Capper ER, Maskill JK, Gordon C, Blakemore AI. Interleukin (IL)-10, IL-1ra and IL-12 profiles in active and quiescent systemic lupus erythematosus: could longitudinal studies reveal patient subgroups of differing pathology? Clin Exp Immunol. 2004;138:348–56. doi: 10.1111/j.1365-2249.2004.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai J, Liu B, Cua DJ, Li Z. Essential roles of IL-12 and dendritic cells but not IL-23 and macrophages in lupus-like diseases initiated by cell surface HSP gp96. Eur J Immunol. 2007;37:706–15. doi: 10.1002/eji.200636643. [DOI] [PubMed] [Google Scholar]
- 37.Maruo S, Toyo-oka K, Oh-hora M, et al. IL-12 produced by antigen-presenting cells induces IL-2-independent proliferation of T helper cell clones. J Immunol. 1996;156:1748–55. [PubMed] [Google Scholar]
- 38.Ahn HJ, Tomura M, Yu WG, Iwasaki M, Park WR, Hamaoka T, Fujiwara H. Requirement for distinct Janus kinases and STAT proteins in T cell proliferation versus IFN-gamma production following IL-12 stimulation. J Immunol. 1998;161:5893–900. [PubMed] [Google Scholar]
- 39.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 40.Dean GS, Tyrrell-Price J, Crawley E, Isenberg DA. Cytokines and systemic lupus erythematosus. Ann Rheum Dis. 2000;59:243–51. doi: 10.1136/ard.59.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtsuka K, Gray JD, Stimmler MM, Toro B, Horwitz DA. Decreased production of TGF-beta by lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1998;160:2539–45. [PubMed] [Google Scholar]
- 42.Raz E, Dudler J, Lotz M, Baird SM, Berry CC, Eisenberg RA, Carson DA. Modulation of disease activity in murine systemic lupus erythematosus by cytokine gene delivery. Lupus. 1995;4:286–92. doi: 10.1177/096120339500400409. [DOI] [PubMed] [Google Scholar]
- 43.Sato MN, Minoprio P, Avrameas S, Ternynck T. Changes in the cytokine profile of lupus-prone mice (NZB/NZW)F1 induced by Plasmodium chabaudi and their implications in the reversal of clinical symptoms. Clin Exp Immunol. 2000;119:333–9. doi: 10.1046/j.1365-2249.2000.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–70. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 45.Geissmann F, Revy P, Regnault A, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic langerhans cells. J Immunol. 1999;163:1737–41. [PubMed] [Google Scholar]
- 46.Strobl H, Knapp W. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–90. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]