Abstract
The chemokine (C-C motif) receptor CCR5 and its ligand CCL5 play key roles in the intra-articular recruitment of peripheral blood mononuclear cells (PBMC) in rheumatoid arthritis (RA). Therefore, using quantitative cytofluorometry, we followed T4 cell surface CCR5 density in 27 subjects with RA before and after treatment with the anti-CD20 monoclonal antibody rituximab. We observed low T4 cell surface CCR5 densities before treatment, which correlated positively with disease activity, as determined using a disease activity score evaluated on 28 joints (DAS 28), and negatively with CCL5 mRNA concentrations in PBMC, contrasting with a high proportion of intracellular CCR5 molecules, a pattern compatible with ligand-induced CCR5 internalization. At 3 months post-treatment, CCL5 mRNA expression in PBMC declined, whereas T4 cell surface CCR5 densities increased proportionally to the decrease in DAS 28. Thus, peripheral blood T4 cell surface CCR5 density is a good surrogate marker of RA activity and of the efficiency of anti-CD20 therapy.
Keywords: cell surface expression, chemokine, chemokine receptor, T lymphocytes
Introduction
The chemokine (C-C motif) receptor CCR5 and its three major ligands, the chemokines CCL3, CCL4 and particularly CCL5, seem to play key roles in rheumatoid arthritis (RA). CCL3, CCL4 and CCL5 are produced by synovial macrophages and fibroblasts as well as by chondrocytes, particularly under stimulation by tumour necrosis factor (TNF)-α,1–5 a key cytokine in RA. Consequently, the synovial fluid concentrations of CCL3, CCL4 and CCL5 are increased in RA.1,6–10 The overproduction of these C-C chemokines in the pathological joint is thought to be involved in the attraction of CCR5-positive monocytes and T helper type 1 (Th1) as well as Th17 lymphocytes, an event that could increase inflammation. In support of this hypothesis, anti-CCL5 antibodies and CCR5 antagonists inhibit the development of arthritis in animal models of RA.11–13 Moreover, a mutation occurring within the promoter region of the CCL5 gene has been linked to RA.14 Last but not least, subjects heterozygous for the Δ32 deletion in the CCR5 gene, who express low cell surface densities of CCR5, seem to be partially protected from RA,15–18 and particularly from severe forms of the disease.19
The more CCR5 molecules a cell expresses at its surface, the more intensively it is attracted by CCR5 ligands.20 In particular, surface CCR5 density determines the level of chemotaxis of a T cell towards RA synovial cells producing CCL5.21 Therefore, the level of expression of CCR5 at the surface of circulating T4 cells should be an important factor deciding whether or not they are recruited to the inflamed joints.
B cells have been shown to be key players in RA immunopathology. In addition to their properties of antigen presentation, B cells are major co-activators of T cells in synovitis. Moreover, B cells are source of cytokines, including TNF-α and lymphotoxin, contributing to local inflammation.22 Rituximab, a monoclonal antibody (mAb) targeting B cells, has been shown to induce long-term clinical responses in active RA refractory to TNF-α blockade.23 Rituximab therapy results in early and dramatic B-cell depletion, but also modifies T-cell activation, the Th1:Th2 ratio, the regulatory T-cell subpopulation and the apoptotic pathway in T cells.24–26 Thus, rituximab could exert its effect on Th1-mediated autoimmune diseases via cellular as well as humoral immunity.
For these reasons, we measured peripheral blood T4 cell surface CCR5 expression in subjects with active RA, and followed its evolution under therapy targeting CD20.
Materials and methods
Patients
A total of 27 patients were evaluated at the University Hospital of Montpellier, and included in this study, all satisfying the American College of Rheumatology (ACR) criteria revised in 1987 for RA diagnosis. The criteria for patient eligibility were: RA, resistance to at least one disease-modifying antirheumatic drug (DMARD) and at least one TNF-α antagonist, age over 18 years and written consent. Exclusion criteria were: a history of severe or recurrent infectious disease, absence of contraception, pregnancy, a diagnosis of cancer, and prior rituximab treatment. Among these 27 patients, 14 treated with rituximab as recommended by the manufacturer and the French Drug Agency AFSSAPS (1000 mg; two perfusions within 15 days) were followed up.
Flow cytometry
B cells from whole blood were quantified after direct labelling with a phycoerythrin (PE)-conjugated anti-CD19 mAb (Beckman Coulter, Roissy CDG Cedex, France). CCR5 density at the surface of peripheral blood T4 cells was quantified as previously described.27 Briefly, blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes and processed immediately. Peripheral blood mononuclear cells (PBMC) were directly labelled with a PE-conjugated anti-CD4 mAb (Beckman) and indirectly labelled with the anti-CCR5 mAb 2D7 (Pharmingen, San Diego, CA) and a fluorescein isothiocyanate (FITC)-conjugated anti-immunoglobulin probe (Beckman). After gating on CD4+ cells, the intensity of CCR5 expression on CCR5-expressing cells was analysed by converting FITC fluorescence into the mean number of cell surface-bound mAb molecules per cell, using populations of standard microbeads pre-coated with different well-defined quantities of mAb (QIFIKIT; Dako, Glostrup, Denmark) and concurrently labelled with the same FITC-conjugated probe. For CCR5 intracellular detection, cells were first permeabilized using phosphate-buffered saline containing 0·2% Saponin (Sigma-Aldrich, St Quentin Fallavier, Cedex, France). CCR5 monoclonal antibody or isotypic control was then added and incubated for 30 min on ice. After two washes, cells were incubated for 30 min on ice with the FITC-conjugated anti-immunoglobulin probe. Finally, cells were labelled with the PE-conjugated anti-CD4 mAb.
mRNA isolation and quantitative reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated from freshly collected PBMC using the QIAamp RNA blood mini kit (Qiagen SA, Courtaboeuf Cedex, France) as recommended by the supplier. Extracted RNA (500 ng) was then reverse transcribed using the High Capacity cDNA Archive kit (Applied Biosystems, Courtaboeuf, France) according to the manufacturer’s instructions. Quantitative RT-PCR was then carried out with the ABI PRISM 7900HT sequence detection system (Applied Biosystems). Data were collected with instrument spectral compensations using the Applied Biosystems sds 2.1 software, and analysed using the threshold cycle (Ct) relative quantification method. The content of cDNA samples was normalized by subtracting the number of copies of the endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference gene from that of the target gene (ΔCt = Ct of target gene −Ct of GAPDH) and expressed as 2−ΔCt.
Statistical analysis
CCR5 expression on T4 cells, CCR5 and CCL5 mRNA concentrations in PBMC from healthy subjects or those with RA, and data on subjects with RA at inclusion and after 3 months of treatment were compared using the Mann–Whitney test. Variations in B-cell count, and in CCR5 and CCL5 mRNA concentrations in PBMC after anti-CD20 therapy were analysed with a paired version of the Wilcoxon signed-rank test. Pearson rank correlations were used to evaluate the link between CCR5 density and a disease activity score evaluated on 28 joints (DAS 28).
Results
Peripheral blood T4 cell surface CCR5 density is down-regulated in RA
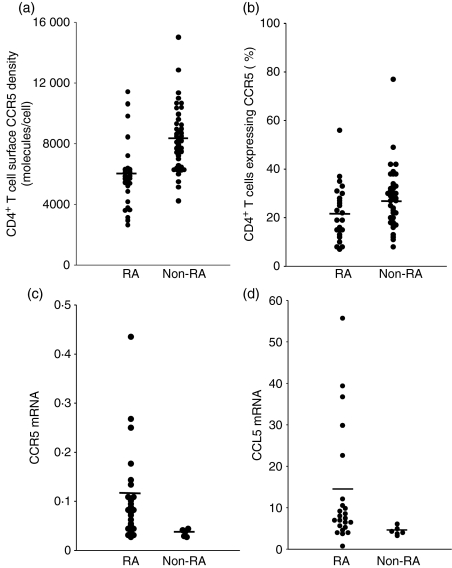
We measured by quantitative flow cytometry the mean number of CCR5 molecules at the surface of peripheral blood T4 cells in 27 subjects with active RA and compared these CCR5 densities with those for age- and sex-matched healthy volunteers. The bioclinical characteristics of the patients are indicated in Table 1. Figure 1(a) shows that in RA CCR5 is drastically under-expressed at the surface of circulating T4 cells [arithmetic means of 5671 (95% confidence interval (CI) 4860–6482) and 8257 (95% CI 7675–8840) CCR5 molecules per cell in RA and non-RA individuals, respectively; P< 0·001]. Similarly, the percentage of T4 cells expressing CCR5 was lower in individuals with RA than in healthy volunteers [arithmetic means of 22% (95% CI 17–27) and 27% (95% CI 24–30) in RA and non-RA individuals, respectively; P= 0·044; Fig. 1b].
Table 1.
Demographic, clinical and biological data for the 27 subjects with rheumatoid arthritis (RA) included in the study
| Parameter | Mean (SD)1 | Minimum | Maximum |
|---|---|---|---|
| Age (years) | 60·33 (11·55) | 37 | 82 |
| Sex ratio (%, female:male) | 89:11 | – | – |
| RA duration (years) | 13·3 (8·3) | 1 | 37 |
| Erosive RA (%) | 73 | ||
| Rheumatoid factor > 30 UI/l (%) | 85 | – | – |
| Prior DMARD therapy (years) | 3·7 (1·9) | 0 | 9 |
| Prior biotherapy (years) | 1·2 (1·4) | 0 | 4 |
| Associated DMARD therapy (%) | 70 | – | – |
| Prednisone (mg/day) | 5·5 (4·9) | 0 | 15 |
| Patient’s assessment of pain (0–100 mm visual analogue scale) | 53 (23) | 12 | 85 |
| DAS 28 | 5·8 (1·7) | 1·9 | 8·5 |
| C-reactive protein plasma level (mg/l) | 32·1 (30·7) | 0 | 106 |
Unless otherwise indicated.
DAS 28, disease activity score evaluated on 28 joints; DMARD, disease-modifying antirheumatic drug; SD, standard deviation.
Figure 1.
(a) Chemokine (C-C motif) receptor 5 (CCR5) density on peripheral blood T4 cells, measured on CCR5-positive T4 cells by quantitative flow cytometry, and (b) the percentage of peripheral blood T4 cells expressing CCR5 in subjects with rheumatoid arthritis (RA) and in healthy controls. (c) CCR5 mRNA and (d) chemokine (C-C motif) ligand 5 (CCL5) mRNA expression in PBMC from subjects with RA and from healthy controls.
CCL5 mRNA is over-expressed in PBMC from RA subjects
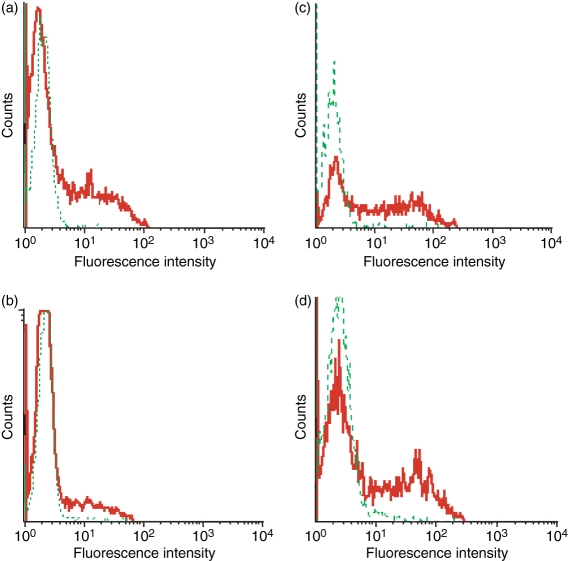
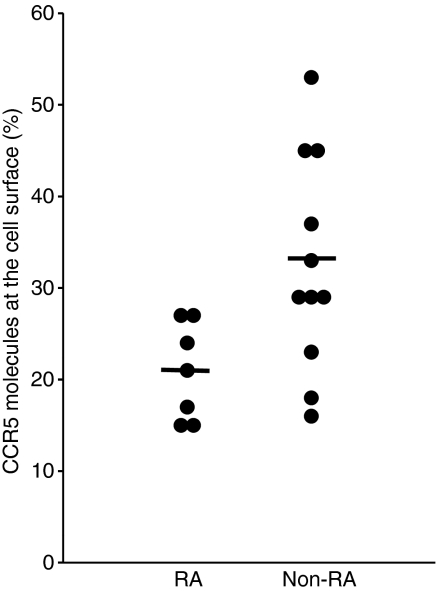
Next, we tried to determine which factors might be responsible for the low T4 cell surface CCR5 density we observed in subjects with RA. First, we measured by quantitative PCR the amount of CCR5 mRNA present in their PBMC. We noted an increase in CCR5 mRNA concentration in the PBMC of patients as compared with healthy volunteers [arithmetic means of 0·11 (95% CI 0·07–0·15) and 0·04 (95% CI 0·03–0·04) in RA and non-RA individuals, respectively; P= 0·005; Fig. 1c]. Thus, the decrease in CCR5 expression at the T4 cell surface does not seem to be a consequence of a decrease in CCR5 gene expression. As CCR5 ligands produced by PBMC permanently induce CCR5 internalization into T4 cells,28,29 we hypothesized that the low level of CCR5 expression at the surface of T4 cells might be a consequence of a CCR5 over-internalization as a result of overproduction of CCR5 ligands. To test this possibility, we measured the mRNA amount of the most abundant CCR5-binding chemokine, CCL5, in the PBMC of these patients. As compared with the controls, we observed that RA PBMC over-expressed CCL5 mRNA [arithmetic means of 14·07 (95% CI 8·41–19·74) and 4·33 (95% CI 3·24–5·42) in RA and non-RA individuals, respectively; P= 0·011; Fig. 1d]. In order to further test the hypothesis that T4 cell surface CCR5 under-expression in RA is the consequence of CCR5 internalization, we compared the amount of CCR5 molecules present inside RA T4 cells with the amount of CCR5 molecules present inside non-RA T4 cells. For this purpose, we permeabilized the cells before labelling them with the anti-CCR5 mAb. Figure 2 illustrates such an experiment. The number of CCR5 molecules exposed at the T4 cell surface of the subject with RA, measured on CCR5-positive non-permeabilized cells, was 4747 (Fig. 2b), whereas the total number of CCR5 molecules present on the inside and outside of the cell, measured on CCR5-positive permeabilized cells, was 17 858 (Fig. 2d). By contrast, for the healthy donor, T4 cell surface CCR5 density was 5611 (Fig. 2a), whereas the total number of CCR5 molecules was 15 078 (Fig. 2c). Thus, the percentage of non-internalized CCR5 was only 27% for the subject with RA, whereas it was 37% for the healthy control. We performed the same experiment with seven subjects with RA and 11 healthy controls. Figure 3 shows that the proportion of CCR5 molecules expressed at the cell surface is actually reduced in the course of the disease [arithmetic means of 21% (95% CI 16–26%) and 33% (95% CI 25–40%) in RA and non-RA individuals, respectively; P= 0·015]. On the whole, these data are consistent with a model where, in active RA, the over-expression of CCR5-binding chemokines by PBMC results in the internalization of CCR5 inside the T4 cells.
Figure 2.
Surface (a, b) and total (c, d) chemokine (C-C motif) receptor 5 (CCR5) expression in peripheral blood T4 cells from a healthy donor (a, c) and a subject with rheumatoid arthritis (RA) (b, d). Cells permeabilized (c, d) or not (a, b) were indirectly labelled with an anti-CCR5 mouse monoclonal antibody (solid line) or with a negative control antibody (dotted line) and a fluorescein isothiocyanate (FITC)-labelled goat anti-mouse immunoglobulin probe, and directly labelled with a phycoerythrin (PE)-conjugated anti-CD4 antibody. PE-positive cells were analysed for green fluorescence. A representative experiment of three is shown.
Figure 3.
Proportion of chemokine (C-C motif) receptor 5 (CCR5) molecules expressed by T4 cells that are present at the cell surface in patients with rheumatoid arthritis (RA) as compared with healthy donors. This proportion was calculated from the ratio of the mean number of CCR5 molecules detected on non-permeabilized T4 cells to the mean number of CCR5 molecules detected in and on permeabilized T4 cells by quantitative flow cytometry.
Peripheral blood T4 cell surface CCR5 density is inversely correlated with RA activity
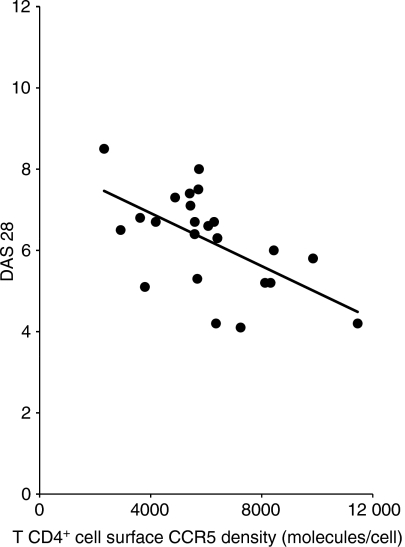
As CCR5 down-regulation appears to result from a high level of inflammatory chemokine production, we tested the hypothesis that this parameter could be linked to disease activity. For this purpose, we compared peripheral blood T4 cell surface CCR5 density with the DAS 28 values of the patients. We found a strong negative correlation (r = −0·532 and P= 0·007) between these two factors (Fig. 4).
Figure 4.
Correlation between chemokine (C-C motif) receptor 5 (CCR5) density on peripheral blood T4 cells and disease activity score evaluated on twenty eight points (DAS) 28 in subjects with active rheumatoid arthritis (RA).
Increase in peripheral blood T4 cell surface CCR5 density correlates with response to therapy targeting CD20
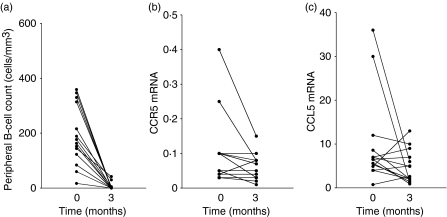
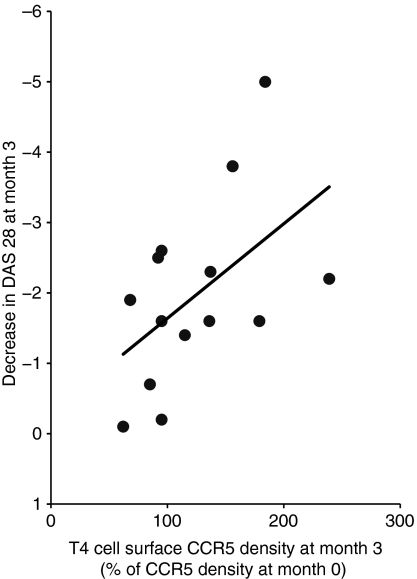
Fourteen of the patients elected for treatment with anti-CD20 were followed up. Eleven of these 14 patients responded to this therapy (decrease in DAS 28 ≥ 1·2). The changes in pain assessment, DAS 28, and C-reactive protein plasma level are indicated in Table 2. The peripheral blood B-cell counts of these patients dropped drastically under monoclonal antibody administration [arithmetic means of 192 (95% CI 123–261) and 6 (95% CI 0–14) B cells/μl before and after treatment, respectively; P< 0·001; Fig. 5a]. Concurrently, we monitored CCR5 and CCL5 mRNA expression in their PBMC. The biotherapy reduced to almost physiological levels the level of CCR5 mRNA [arithmetic means of 0·10 (95% CI 0·04–0·16) and 0·06 (95% CI 0·04–0·08) before and 3 months after treatment, respectively; P= 0·039; Fig. 5b] and that of CCL5 mRNA [arithmetic means of 9·94 (95% CI 4·07–15·82) and 4·79 (95% CI 2·60–8·98) before and 3 months after treatment, respectively; P= 0·057; Fig. 5c]. As a result, T4 cell surface CCR5 density increased under therapy, and this increase was correlated with the decrease in DAS 28 (r = −0·519 and P= 0·057; Fig. 6).
Table 2.
Bioclinical changes in 14 subjects with rheumatoid arthritis (RA) treated with rituximab
| Parameter | Day 0 Mean (SD) | Day 90 Mean (SD) | P-value |
|---|---|---|---|
| Patient’s assessment of pain (0–100 mm visual analogue scale) | 59 (23) | 31 (28) | 0·018 |
| DAS 28 | 6·4 (1·1) | 4·2 (1·7) | 0·002 |
| C-reactive protein plasma level (mg/l) | 31 (33) | 8 (7) | 0·008 |
DAS 28, disease activity score evaluated on 28 joints; SD, standard deviation.
Figure 5.
(a) Peripheral B-cell count, and (b) chemokine (C-C motif) receptor 5 (CCR5) mRNA and (c) chemokine (C-C motif) ligand 5 (CCL5) mRNA expression in peripheral blood mononuclear cells (PBMC) of subjects with rheumatoid arthritis (RA) before and after anti-CD20 therapy.
Figure 6.
Positive correlation between the increase in T4 cell surface chemokine (C-C motif) receptor 5 (CCR5) density and the decrease in a disease activity score evaluated on 28 joints (DAS 28) in subjects with rheumatoid arthritis (RA) treated with anti-CD20 monoclonal antibody.
Discussion
In the present study, we found that T4 cell surface CCR5 density was diminished in subjects with RA proportionally to their disease activity, and was restored proportionally to the response to an anti-CD20 therapy.
Physiologically, CCL3, CCL4 and particularly CCL5 are continuously produced by PBMC, bind to CCR5 and provoke its internalization.28,29In vitro, studying CCR5-positive cell clones producing CCL3, CCL4 and CCL5, Maeda et al.30 showed that the addition of anti-chemokine antibodies to the culture resulted in an increase in cell surface CCR5 expression. In vivo, the administration to healthy volunteers of a CCR5 antagonist able to block the CCR5–chemokine interaction rapidly provoked the increase in peripheral blood T4 cell surface CCR5 density.31 Here, we showed that RA PBMC over-expressed CCL5 mRNA. This observation is consistent with the data of Boiardi et al.,32 who reported high serum levels of CCL5 in active RA. CCL5 overproduction in RA might be attributable to the presence of binding sites for the inflammatory DNA-binding protein nuclear factor (NF)-κB in the CCL5 promoter.33 Thus, TNF-α has been shown to induce CCL5 gene expression.34–36 Hornung et al.34 previously reported that this resulted in CCR5 down-regulation in peripheral lymphocytes, and Lane et al.35 made the same observation in peripheral monocytes and alveolar macrophages. In the present study on subjects with RA, the inverse correlation between CCR5 expression on T4 cells and the number of CCL5 mRNA copies in PBMC, as well as the redistribution of CCR5 molecules from the surface to the inside of T4 cells, lends credence to the hypothesis that CCR5 down-modulation is a consequence of over-internalization of the chemokine receptor mediated by its ligands, which are over-produced.
An additional reason for the low CCR5 expression we observed on PBMC from subjects with RA could be the preferential intra-articular recruitment of T4 cells with high surface CCR5 densities. As the level of expression of CCR5 on T4 cells determines the intensity of attraction exerted by RA synovial cells on T4 cells,21 peripheral blood could be depleted in T4 cells expressing high levels of CCR5. Our observation that the percentage of circulating T4 cells expressing CCR5 is reduced in subjects with RA, an observation previously reported by some37,38 but not all39 groups, argues in favour of this hypothesis. This percentage of peripheral blood CCR5-positive T4 cells was reported by Nissinen et al.40 to be higher in patients who did not respond to another biotherapy, the anti-TNFα antibody infliximab, as compared with those who did. Here, we did not note such a predictive value (data not shown).
In addition to CCL5 mRNA over-expression in PBMC, we also noted CCR5 mRNA over-expression in active RA. This over-expression is probably a result of the fact that the Th1 cytokines interleukin (IL)-2 and interferon (IFN)-γ are able to activate CCR5 gene transcription.41,42 Moreover, the inflammatory cytokine TNF-α, a key actor in RA, is also known to induce CCR5 gene expression.43,44
We noted a reduction in the amount of CCL5 mRNA in PBMC after 3 months of anti-CD20 therapy. This finding is reminiscent of the decrease in the serum levels of CCL5 under methotrexate reported by another group.32 Interestingly, in this latter work a sustained level of this chemokine was predictive of radiological erosions after 1 year. The decrease in CCL5 mRNA expression might be a consequence of the anti-inflammatory effect of the B-cell depletion, either directly, for instance through the reduction of B cell-derived cytokine expression, or indirectly through an effect on T cells.
Of note, CCR5 down-regulation from the surface of circulating T4 cells induced by the over-production of CCR5 ligands by PBMC during active RA should result in a reduction of the intra-articular recruitment of these T4 cells, thus creating negative feedback. From a therapeutic point of view, the increase in peripheral production of CCR5-binding chemokines could represent a desirable goal. Finally, in practice, T4 cell surface CCR5 density could be a convenient surrogate marker of RA activity and of response to anti-CD20 therapy routinely measurable by flow cytometry.
Disclosures
No conflict of interest.
References
- 1.Hosaka S, Akahoshi T, Wada C, Kondo H. Expression of the chemokine superfamily in rheumatoid arthritis. Clin Exp Immunol. 1994;97:451–7. doi: 10.1111/j.1365-2249.1994.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin Immunol. 2001;98:39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- 3.Pulsatelli L, Dolzani P, Piacentini A, Silvestri T, Ruggieri R, Gualtieri G, Meliconi R, Facchini A. Chemokine production by human chondrocytes. J Rheumatol. 1999;26:1992–2001. [PubMed] [Google Scholar]
- 4.Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–9. [PubMed] [Google Scholar]
- 5.Whyte A, Binns RM. RANTES and chemokines in rheumatoid arthritis. Lancet. 1994;343:1291–2. doi: 10.1016/s0140-6736(94)92181-4. [DOI] [PubMed] [Google Scholar]
- 6.Robinson E, Keystone EC, Schall TJ, Gillett N, Fish EN. Chemokine expression in rheumatoid arthritis (RA): evidence of RANTES and macrophage inflammatory protein (MIP)-1 beta production by synovial T cells. Clin Exp Immunol. 1995;101:398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volin MV, Shah MR, Tokuhira M, Haines GK, Woods JM, Koch AE. Rantes expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89:44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 8.Borzi RM, Mazzetti I, Macor S, Silvestri T, Bassi A, Cattini L, Facchini A. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238–42. doi: 10.1016/s0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 9.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Strieter RM. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int Immunol. 1999;11:553–9. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 11.Barnes DA, Tse J, Kaufhold M, Owen M, Hesselgesser J, Strieter R, Horuk R, Perez HD. Polyclonal antibody directed against human RANTES ameliorates disease in the Lewis rat adjuvant-induced arthritis model. J Clin Invest. 1998;101:2910–9. doi: 10.1172/JCI2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–20. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 13.Youssef S, Maor G, Wildbaum G, Grabie N, Gour-Lavie A, Karin N. C-C chemokine-encoding DNA vaccines enhance breakdown of tolerance to their gene products and treat ongoing adjuvant arthritis. J Clin Invest. 2000;106:361–71. doi: 10.1172/JCI9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makki RF, al Sharif F, Gonzalez-Gay MA, Garcia-Porrua C, Ollier WE, Hajeer AH. RANTES gene polymorphism in polymyalgia rheumatica, giant cell arteritis and rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:391–3. [PubMed] [Google Scholar]
- 15.Cooke SP, Forrest G, Venables PJW, Hajeer A. The D32 deletion of CCR5 receptor in rheumatoid arthritis. Arthritis Rheum. 1998;41:1135–6. doi: 10.1002/1529-0131(199806)41:6<1135::AID-ART24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Reino JJ, Pablos JL, Carreira PE, et al. Association of rheumatoid arthritis with a functional chemokine receptor, CCR5. Arthritis Rheum. 1999;42:989–92. doi: 10.1002/1529-0131(199905)42:5<989::AID-ANR18>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Zapico I, Coto E, Rodriguez A, Alvarez C, Torre JC, Alvarez V. CCR5 (chemokine receptor-5) DNA-polymorphism influences the severity of rheumatoid arthritis. Genes Immun. 2000;1:288–9. doi: 10.1038/sj.gene.6363673. [DOI] [PubMed] [Google Scholar]
- 18.Prahalad S. Negative association between the chemokine receptor CCR5-Delta32 polymorphism and rheumatoid arthritis: a meta-analysis. Genes Immun. 2006;7:264–8. doi: 10.1038/sj.gene.6364298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garred P, Madsen HO, Petersen J, et al. CC chemokine receptor 5 polymorphism in rheumatoid arthritis. J Rheumatol. 1998;25:1462–5. [PubMed] [Google Scholar]
- 20.Desmetz C, Lin Y-L, Mettling C, Portalès P, Rabesandratana H, Clot J, Corbeau P. The strength of the chemotactic response to a CCR5 binding chemokine is determined by the level of cell surface CCR5 density. Immunology. 2006;119:551–61. doi: 10.1111/j.1365-2567.2006.02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desmetz C, Lin Y-L, Mettling C, Portales P, Noël D, Clot J, Jorgensen C, Corbeau P. Cell surface CCR5 density determines the intensity of T cell migration towards rheumatoid arthritis synoviocytes. Clin Immunol. 2007;123:148–54. doi: 10.1016/j.clim.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Dorner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol. 2003;15:246–52. doi: 10.1097/00002281-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Frampton JE, Scott LJ. Rituximab: in rheumatoid arthritis. BioDrugs. 2007;21:333–41. doi: 10.2165/00063030-200721050-00005. [DOI] [PubMed] [Google Scholar]
- 24.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell-depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–30. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 25.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid arthritis synovium is B-cell dependent. J Immunol. 2001;167:4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 26.Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, Malmström V, Trollmo C. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Reynes J, Portales P, Segondy M, et al. CD4+ cell surface CCR5 density as a determining factor of viral load in HIV-1-infected individuals. J Infect Dis. 2000;181:927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 28.Sabbe R, Picchio GR, Pastore C, Chaloin O, Hartley O, Offord R, Mosier DE. Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression. J Virol. 2001;75:661–71. doi: 10.1128/JVI.75.2.661-671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–94. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda N, Koyanagi Y, Misawa N, Miyano-Kurosaki N, Kira JI, Yamamoto N. Acquisition of HIV type 1 resistance by beta-chemokine-producing CD4+ T cells. AIDS Res Hum Retroviruses. 1999;15:1453–60. doi: 10.1089/088922299309964. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y-L, Mettling C, Portalès P, Rouzier R, Clot J, Reynes J, Corbeau P. The chemokine CCL5 regulates the in vivo cell surface expression of its receptor, CCR5. AIDS. 2008;22:430–3. doi: 10.1097/QAD.0b013e3282f46a6f. [DOI] [PubMed] [Google Scholar]
- 32.Boiardi L, Macchioni P, Meliconi R, Pulsatelli L, Facchini A, Salvarani C. Relationship between serum RANTES levels and radiological progression in rhumatoid arthritis patients treated with methotrexate. Clin Exp Rheumatol. 1999;17:419–25. [PubMed] [Google Scholar]
- 33.Moriuchi H, Moriuchi M, Fauci AS. Nuclear factor-kB potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–91. [PubMed] [Google Scholar]
- 34.Hornung F, Scala G, Leonardo MJ. TNF-a-induced secretion of C-C chemokines modulates C-C chemokine receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000;164:6180–7. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- 35.Lane BR, Markovitz DM, Woodford NL, Rochford R, Strieter RM, Coffey MJ. TNF-a inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163:3653–61. [PubMed] [Google Scholar]
- 36.Nelson PJ, Kim HT, Manning WC, Goralski TJ, Krensky AM. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–12. [PubMed] [Google Scholar]
- 37.Shadidi KR, Aarvak T, Henriksen JE, Natvig JB, Thompson KM. The chemokines CCL5, CCL2 and CXCL12 play significant roles in the migration of Th1 cells into rheumatoid arthritis. Scand J Immunol. 2003;57:192–8. doi: 10.1046/j.1365-3083.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang CR, Liu MF. Regulation of CCR5 expression and MIP-1alpha production in CD4+ T cells from patients with rheumatoid arthritis. Clin Exp Immunol. 2003;132:371–8. doi: 10.1046/j.1365-2249.2003.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruth JH, Rottman JB, Katschke KJ, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44:2750–60. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 40.Nissinen R, Leirasalo-Repo M, Peltomaa R, Palosuo T, Vaarala O. Cytokine and chemokine receptor profile of peripheral blood mononuclear cells during treatment with infliximab in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:681–7. doi: 10.1136/ard.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mo H, Monard S, Pollack H, et al. Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4+ T cells and monocytes from cord and adult blood. AIDS Res Hum Retroviruses. 1998;14:607–17. doi: 10.1089/aid.1998.14.607. [DOI] [PubMed] [Google Scholar]
- 42.Weissman D, Dybul M, Daucher MB, Davey RT, Walker RE, Kovacs JA. Interleukin-2 up-regulates expression of the Human Immunodeficiency Virus fusion coreceptor CCR5 by lymphocytes in vivo. J Infect Dis. 2000;181:933–8. doi: 10.1086/315303. [DOI] [PubMed] [Google Scholar]
- 43.Juffermans NP, Paxton WA, Dekkers PEP, Verbon A, de Jonge E, Speelman P, van Deventer SJH, van der Poll T. Up-regulation of HIV coreceptors CXCR4 and CCR5 on CD4+ T cells during human endotoxemia and after stimulation with (myco)bacterial antigens: the role of cytokines. Blood. 2000;96:2649–54. [PubMed] [Google Scholar]
- 44.Patterson BK, Czerniewski M, Andersson J, Sullivan Y, Su F, Jiyamapa D, Burki Z, Landay A. Regulation of CCR5 and CXCR4 expression by type 1 and 2 cytokines: CCR5 expression is downregulated by IL-10 in CD4-positive lymphocytes. Clin Immunol. 1999;91:254–62. doi: 10.1006/clim.1999.4713. [DOI] [PubMed] [Google Scholar]