Abstract
This study investigates the role of the homeobox gene Distal-less (Dll) in the development of the legs, antennae, and wings of Drosophila. Lack of Dll function causes a change in the identity of ventral appendage cells (legs and antennae) that often results in the loss of the appendage. Ectopic Dll expression in the proximal region of ventral appendages induces nonautonomous duplication of legs and antennae by the activation of wingless and decapentaplegic. Ectopic Dll expression in dorsal appendages produces transformation into corresponding ventral appendages; wings and halteres develop ectopic legs and the head–eye region develops ectopic antennae. In the wing, the exogenous Dll product induces this transformation by activating the endogenous Dll gene and repressing the wing determinant gene vestigial. It is proposed that Dll induces the development of ventral appendages and also participates in a genetic address that specifies the identity of ventral appendages and discriminates the dorsal versus the ventral appendages in the adult. However, unlike other homeotic genes, Dll expression and function is not defined by a cell lineage border. Dll also performs a secondary and late function required for the normal patterning of the wing.
Keywords: Drosophila distal appendages, dorsal-to-ventral limb transformation, Distal-less, vestigial
The adult structures of Drosophila are constituted by a main body or “trunk”, and a number of outgrowths or appendages such as wings, legs, antennae, etc. All these structures are differentiated by imaginal cells that are grouped in specific imaginal discs in the head and thorax (for review, see Cohen 1993). In the thorax, each adult segment is formed by the derivatives of two types of discs—one contributing to the dorsal and the other to the ventral part of the segment. The humeral, wing, and haltere discs form the dorsal prothoracic, mesothoracic, and metathoracic regions, respectively. Ventrally, there is a pair of leg discs per thoracic segment. In the head, most of the cephalic structures are differentiated by the eye–antennal disc, with the exception of the clypeous and the proboscis. These structures originate from other discs (Gehring and Seippel 1967). The eye–antennal disc is more complex than the thoracic discs because it is formed by precursors from more than one embryonic segment (Cohen and Jürgens 1991; González-Crespo and Morata 1995). Moreover, unlike the thoracic discs, it contains dorsal and ventral derivatives. The antennal part can be transformed into a complete leg in homeotic Antennapedia (Antp) mutations (Gehring 1966), whereas the eye part can be transformed into a wing by ophtalmoptera mutations. This suggests that the antenna is a ventral derivative and the eye a dorsal derivative (see Morata and Lawrence 1979).
Several developmental characteristics are common to dorsal and ventral appendages. For example, the role of engrailed (en), hedgehog (hh), and decapentaplegic (dpp) in the signalling mechanism responsible for morphogenesis (Basler and Struhl 1994). However, other genes such as wingless (wg), apterous (ap), vestigial (vg), and Distal-less (Dll) are expressed very differently in dorsal and ventral discs (Cohen 1993). Of these genes, Dll appears to have a critical role in the development of ventral appendages, legs, and antennae (Sunkel and Whittle 1987; Cohen and Jürgens 1987a,b). It is expressed in the central part of the leg and antennal discs, a region that contains the precursor cells of the more distal regions of both appendages (Cohen 1993). Activation of Dll expression in the leg and antennal discs is triggered by localized expression of hh (Díaz-Benjumea et al. 1994; Campbell and Tomlinson 1995) in the posterior compartment, which directs the expression of wingless (wg) in ventral–anterior cells and dpp in dorsal–anterior cells close to the anterior–posterior (A/P) compartment boundary (Basler and Struhl 1994; Díaz-Benjumea et al. 1994). The juxtaposition of wg- and dpp-expressing cells in the central region of the disc activates Dll (Díaz-Benjumea et al. 1994; Campbell and Tomlinson 1995). It has been proposed that the proximo-distal (P/D) axis of the limb is established by cell–cell interactions that maintain Dll expression (Díaz-Benjumea et al. 1994; Held et al. 1994, 1995; Campbell and Tomlinson 1995). These Wg and Dpp signals confer dorsalizing and ventralizing properties to the cells close to their respective expression domains (Peifer et al. 1991; Couso et al. 1993; Struhl and Basler 1993; Díaz-Bemjumea and Cohen 1994; Held and Heup 1996). Mutual repression by Wg and Dpp signalling systems generates a stable regulatory circuit by which each gene maintains its own expression in a spatially restricted domain (Brook and Cohen 1996; Jiang and Struhl 1996; Johnston and Schubiger 1996; Penton and Hoffman 1996; Theisen et al. 1996; Heslip et al. 1997). Ectopic expression of wg or dpp in the leg imaginal disc can induce ectopic expression of Dll and therefore duplication of the P/D axis (Díaz-Benjumea et al. 1994). However, it is not known whether Dll activity is able to induce the formation of the appendage.
Genetic and mosaic analyses have shown that Dll is required specifically in the areas defined by its expression pattern. The removal of Dll activity gives rise to a phenotype interpreted as the loss of most of the leg, from the trochanter to the tarsus (Cohen and Jürgens 1989a,b). A similar effect is found in the antennal cells that fail to develop in the absence of Dll function (Cohen and Jürgens 1989a,b). It has been argued (Cohen and Jürgens 1989b; Cohen 1993; González-Crespo and Morata 1996) that the region of the leg corresponding to Dll expression is the true appendage and that the proximal leg structures, coxa and pleurae, are formed by an expansion of the trunk. According to this theory, Dll expression would define the true appendage.
Although it is clear that Dll has an important role in appendage development, its specific function in the determination of leg and antennal patterns is uncertain. Dll− cells fail to develop in these appendages and consequently, it is not known whether its function is connected with a developmental switch as in other homeobox genes such as en, Ultrabithorax (Ubx), or ap (Morata and Lawrence 1975; Morata and García-Bellido 1976; Blair 1993; Díaz-Benjumea and Cohen 1993; Guillén et al. 1995; Tabata et al. 1995; for review, see Lawrence and Morata 1994). Moreover, Dll is also expressed in the wing imaginal disc (Díaz-Benjumea and Cohen 1995), although the functional significance of this expression is unknown.
To further investigate the developmental role of Dll, we have re-examined the phenotype of Dll− cells in the ventral and dorsal appendages and also expressed the Dll product ectopically in distinct locations of different imaginal discs. Dll was shown to have two separate functions: a primary function to induce the formation of ventral appendages and their identity and a secondary function involved in the differentiation of the wing margin pattern.
Results
Expression and requirements for Dll in the dorsal and ventral appendages
The eye–antennal, leg, and wing discs are of primary concern in this study, although Dll is also expressed in the genitalia (N. Gorfinkiel, G. Morata, and I. Guerrero, unpubl.). The Dll product accumulates in the central part of the leg and antennal discs. This region corresponds to the distal elements of the appendages (Fig. 1A,B) (Díaz-Benjumea et al. 1994). A more proximal ring of expression exists in the leg disc and is separated from the main body by an area of little or no expression (see also Cohen 1993). The wing disc has a very different expression pattern. The product is first detected in the early third instar in a few cells of the distal region of the wing pouch at both sides of the D/V border (Fig. 1C), long after full expression is established in the leg (Díaz-Benjumea et al. 1994). By the second half of the third instar the Dll product accumulates along the D/V border as described previously (Díaz-Benjumea and Cohen 1995), extending to the wing pouch (Fig. 1D). Therefore the activity of Dll in the wing not only differs from that of the antenna and leg in its topography of expression but also appears later. Dll expression in the third-instar haltere disc was also examined and was found to differ from that in the wing disc at the same stage; the Dll product accumulates in two regions in the anterior and posterior compartments, respectively, but there is no detectable expression along the D/V border (Fig. 1E). Because Ubx mutations transform the haltere into a wing disc, it is suggested that Ubx acts as a negative regulator of Dll in adult cells as reported for the embryo (Cohen et al. 1989).
Figure 1.
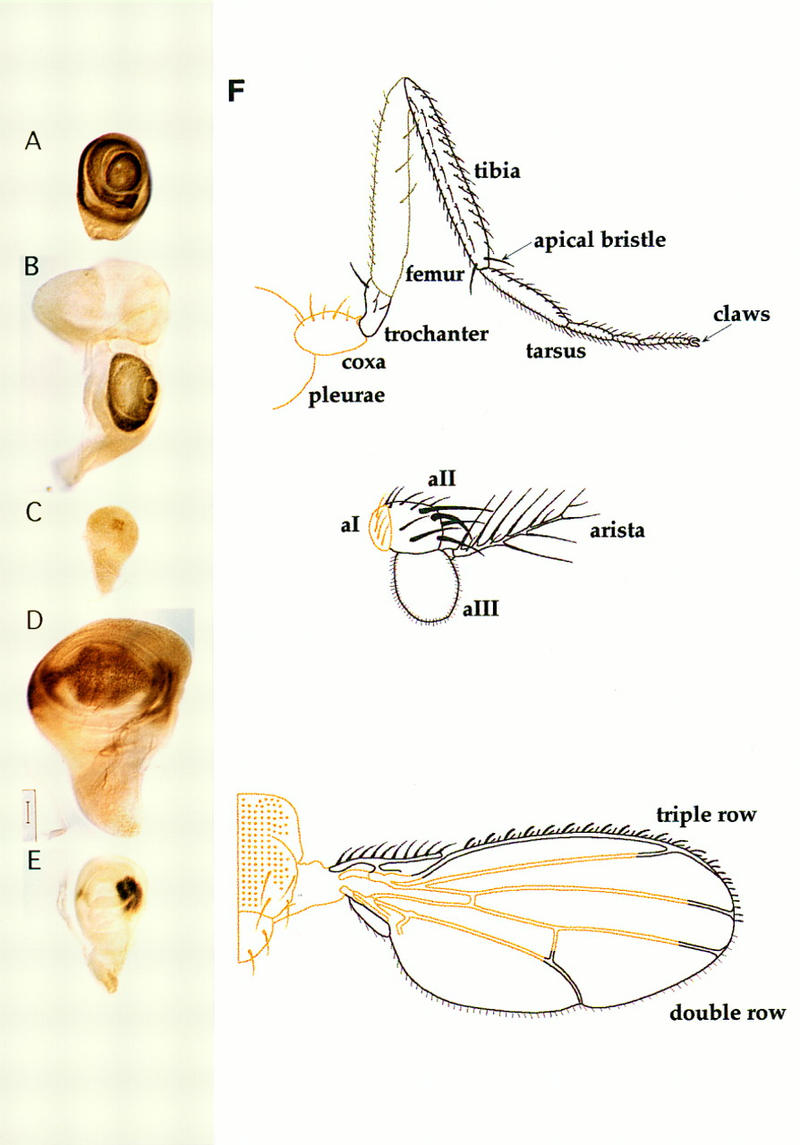
Dll expression domains. (A–E) Wild-type Dll expression patterns visualized with Dll antibody in late third instar imaginal discs (except for C). (A) Leg disc; (B) Eye–antennal disc; (C) early third instar wing disc; (D) wing disc; (E) haltere disc; (F) schematic representation of Dll expression domains in leg, antenna, and wing as visualized in Dll–GAL4/UAS–y+ flies. The brown color stands for the y+ rescue. Note the paler brown color indicating weak y+ rescue in the femur. See main text for a detailed description.
These expression patterns can be visualized directly in the adult structures using the GAL4/UAS–yellow+ (y+) method (Calleja et al. 1996). Several GAL4 insertions were found in the Dll locus allowing distinction of the adult regions where Dll is expressed according to the y+ rescue observed. These results are schematized in Figure 1F. In the adult leg, the coxa and pleurae do not show signs of y+ rescue, although there is clear rescue in part of the trochanter where some bristles are y+. There is weak rescue in the femur that appears to be restricted to the bristles that show intermediate pigmentation between y− and y+ and finally there is strong rescue in the region from the tibia to the tarsus. In the antenna, the Dll product is present in the aII and aIII antennal segments and the arista. The wings of Dll-GAL4/UAS–y+ flies show y+ rescue in nearly all the bristles and hairs along the anterior and posterior compartments of the wing margin. The y+ rescue also extends into some cells of the inner region of the wing blade, but the precise limit is difficult to estimate. The description of the adult Dll expression pattern is in accordance with that observed in imaginal discs.
According to the expression studies described above, Dll subdivides the appendages into two clearly defined regions; one containing and the other not containing the Dll product. Because homeotic genes expression is often defined by cell lineage (compartment) borders (for review, see Lawrence 1992), cell lineage analysis was performed to ascertain whether the border of Dll expression corresponds to a cell lineage restriction. Previous work (Steiner 1976) has already shown that there is no restriction. Using the FRT/FLP method (Golic 1991), y− clones were induced at different periods during larval development (see Materials and Methods). Special attention was paid to the leg clones in the proximity of the trochanter and to the antennae in the border between aI and aII. It was observed that even clones initiated at early third instar [72–96 hr after egg laying (AEL)] may extend to Dll expressing and nonexpressing cells. The same result is obtained by analysis of the behavior of armadillo (arm)–lacZ clones in the leg imaginal disc. Clones (marked by the lack of β-gal staining) induced after 72 hr of development can extend to both Dll-expressing and not expressing domains (Fig. 2A). Consequently, Dll expression is not maintained by cell lineage.
Figure 2.
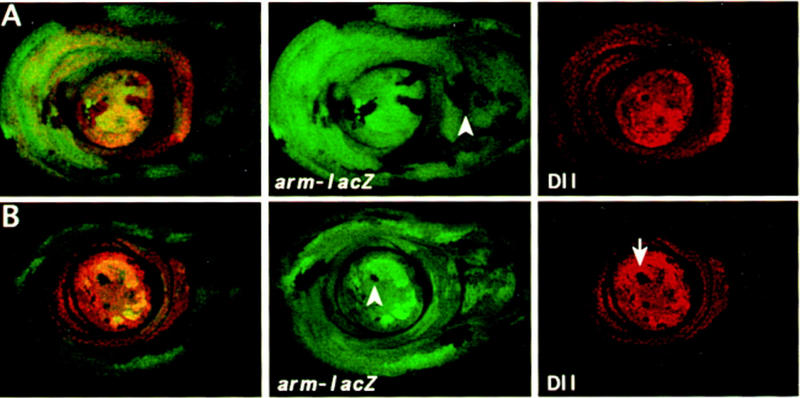
Mitotic recombinant clones in leg discs induced by the FRT–FLP system at 72–96 hr AEL (early third instar). Leg discs were stained with anti-β-gal (green) and anti-Dll (red). (A) Wild-type clones marked by the absence of β-gal expression. The arrowhead indicates a clone that extends to the boundary between Dll expressing and Dll nonexpressing cells. (B) DllSA1 clones marked by the absence of β-gal and Dll expression and their twin spots by the elevated level of β-gal. Note that the DllSA1 clones do not proliferate further. Here and in all remaining images of leg discs, dorsal is to the right.
Expression patterns suggest that Dll is required for the development of both ventral and dorsal appendages until late in development, although the distinct expression patterns in the antennal and leg discs with respect to the wing discs suggest different functions. Early requirements for Dll in the antennal and leg discs have been reported already (Cohen and Jürgens 1989b) and can be summarized as follows: Dll− cells cannot proliferate in these appendages with the exception of the more proximal structures, the pleurae and coxa of the leg and the first segment of the antenna. It is noteworthy that the coxa and aI antennal segment are considered homologous structures (Posthlewait and Schneidermann 1971). Therefore the leg and antennal discs exhibit homologous expression and requirement for Dll.
Using the FLP/FRT method, Dll− clones were induced during different developmental periods of the leg, eye–antennal, and wing discs (Fig. 3). The results of lack of Dll function in the legs are illustrated in Figure 3A–C. Early clones, induced during the first and second instar (24–72 hr AEL), behave as reported by Cohen and Jürgens (1989b)—they only appear in the pleural and coxa regions and produce no morphological alteration. Very few small and abnormal clones were found in the femur to tarsus region. Although clones were undoubtedly produced in these regions, they appear to be eliminated from the region (see also Cohen and Jürgens 1989b). In the antennae, first-instar Dll− clones (Fig. 3D–F) are detected because they are able to differentiate aI antennal and a small part of the aII segment but fail to form the rest of aII and aIII segments and the arista. This is in agreement with previous observations (Cohen and Jürgens 1989b).
Figure 3.
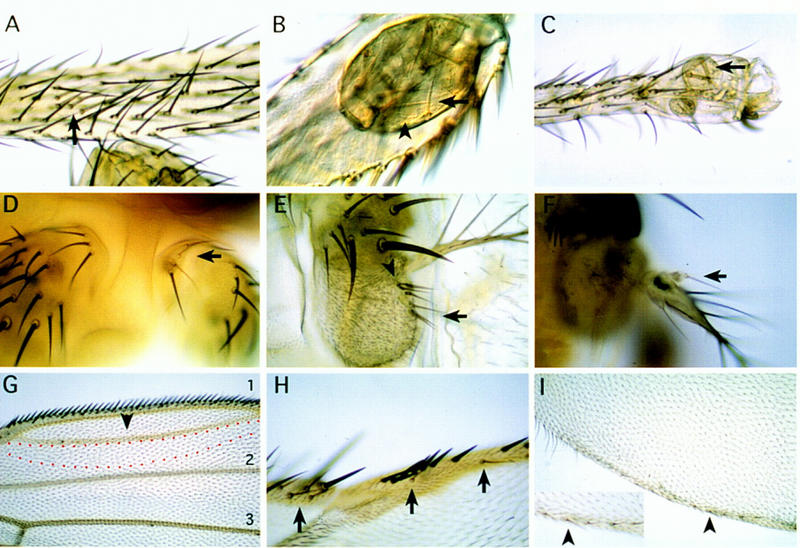
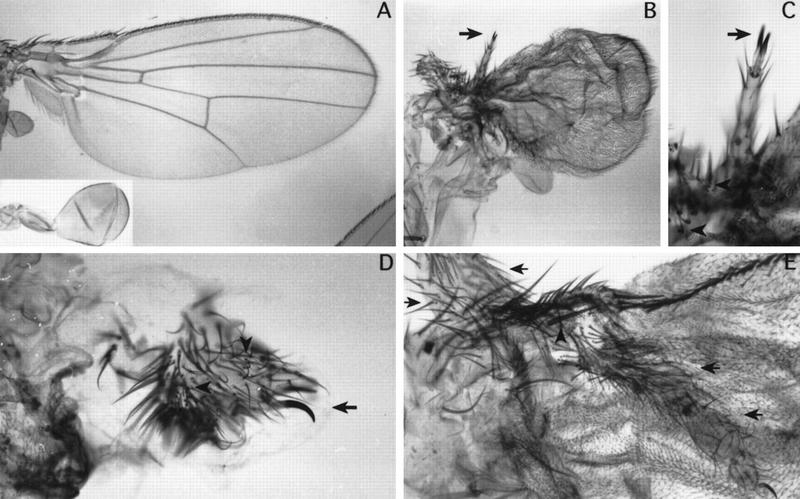
Phenotypic effects of DllSA1 clones in the leg (A–C), antenna (D–F), and wing (G–I). The clones were induced during 24–120 hr AEL and marked with y except for G where clones were marked with forked36(f36) (see Materials and Methods). (A–C) Clones in the leg. Early induced clones (24–48 hr AEL) only appear in the coxa as it has been described previously. Clones induced later (72–120 hr AEL), however, are able to proliferate and differentiate nonbracted bristles in the proximal tibia (A) and vesicles of y− tissue that segregate from the surrounding wild-type tissue in the distal tibia (B) and tarsus (C). Arrows indicate y bristles; arrowheads indicate trichomes that are not present in the distal leg. (D–F) Clones in the antenna. An early (24–48 hr AEL) clone in aI (D) does not produce a mutant phenotype as aI does not require Dll activity. Late clones (72–120 hr AEL) in the aIII antennal segment (E) and arista (F) develop bristles sometimes with an associated bract. Arrows indicate y− bristles; arrowheads indicate bracted bristles. (G–I) Clones in the wing. The clones near the D/V margin give rise to extra-vein tissue. The red dashed line indicates a dorsal clone marked with f close to vein 1. Normal veins 1, 2, and 3 are indicated. Arrowhead indicates extra-vein (G). Clones that abut the D/V boundary also eliminate bristles of the triple row in the A compartment (H) and long hairs of the double row in the P compartment (I). (Inset in I) Magnification showing the y− bristles with socket in the P compartment (arrowhead). Arrows indicate y bristles.
In contrast with early clones, those induced during the third-larval (72–120 hr AEL) periods are recovered frequently in the distal regions of both legs and antennae. In the trochanter and the tibia–tarsus region of the leg, the majority of Dll− clones form vesicles that invaginate inside the appendage. These often differentiate y− bristles and trichomes that do not resemble those in the vicinity of the clone, indicating that lack of Dll function produces a change in the cell type (Fig. 3B,C). Interestingly, the clones in the intervening region, the femur and proximal tibia, behave differently. These clones differentiate bristles of the corresponding type, but are often unable to induce a neighbor cell to differentiate a bract, an accompanying structure of many of the leg bristles (Fig. 3A). It is possible that Dll is required only in the bristle mother cells of this region and explains why this requirement has not been visualized by antibody or lacZ staining of the disc. Late clones in the antennae are able to differentiate, but in the aII and aIII segments they tend to segregate, forming vesicles that separate from the surrounding wild-type tissue. It is difficult to establish the identity of the patterns formed by these clones but these often differentiate bracted bristles in the base of the arista, suggesting an antenna-to-leg transformation (Fig. 3E,F). A similar transformation has been observed in hypomorphic Dll mutations (Sunkel and Whittle 1987; Cohen and Jürgens 1989a).
The loss of early Dll− clones in legs and antennae may suggest a Dll requirement for cell proliferation. To test this possibility, the sizes of Dll− and twin Dll+ clones in mature discs of genotype FRT arm–lacZ/FRT DllSA1 were compared. The Dll− clones, marked by lack of β-gal staining, only contain a few cells and are only detected occasionally, but the accompanying twin clone, labeled by the double intensity of β-gal, is much larger in size (Fig. 2B). This effect on proliferation of the leg Dll− cells was not observed in the wing imaginal cells (data not shown).
In contrast with that observed in the leg and antennal discs, both early and late y− (Dll−) clones were detected readily in the wing disc (Fig. 3G–I). These clones always affect the wing margin, eliminating the triple row of bristles in the anterior compartment (Fig. 3H) and the double row of long hairs in the posterior compartment (Fig. 3I). These were interpreted as Dll− clones because the majority of them were able to differentiate a few y− bristles. An important feature is that they affect both the dorsal and the ventral compartments, even if initiated during the third instar (72–96 hr AEL) after the D/V compartment boundary has been established (Morata and Lawrence 1979) and are therefore supposed to be confined to either compartment. This may indicate a nonautonomous effect or perhaps a transgression of the D/V border by the Dll− clones. In some experiments, Dll− clones were marked with forked36 (f36) to investigate the behavior of clones away from the margin. It was observed that these internal clones often affected vein differentiation in the vicinity of the wing margin, producing extra veins and sometimes eliminating parts of normal veins. This effect appears at times to be nonautonomous, as wild-type cells near Dll− cells are often affected (Fig. 3G). Another intriguing feature of Dll− clones is that they differentiate socketed bristles in the posterior compartment similar to those in the distal part of the anterior compartment (Fig. 3I) and also differentiate a halo of pigment, another feature of the wing margin in the anterior compartment. These observations suggest a late involvement of Dll in the maintenance of posterior identity.
Ectopic Dll expression
To assay the developmental potential of the Dll product, the GAL4/UAS system (Brand and Perrimon 1993) and a combination of the flip-out and GAL4 activation systems (Pignoni and Zipursky 1997) was used for expression in different body regions. We first checked the activity of the UAS–Dll construct by assaying its ability to rescue the Dll phenotype when expressed under Dll control. The line em212 carries the pGawB transposon inserted in the Dll locus and is a null mutant for Dll. The em212-GAL4/Df (2R)DllMP combination is lethal, but the lethality is rescued when the UAS–Dll construct is added. Consequently, em212-GAL4/Df (2R)DllMP;UAS–Dll flies survive and are of almost normal phenotype. In similar combinations, the UAS–Dll gene also rescues the phenotype of hypomorphic mutations such as Dll3 or DllIB.
Ectopic Dll expression in the leg and antennal discs produces duplications of the P/D axis
It was found that a general increase of the Dll product in the Dll domain, in a wild-type background, affects the more distal segments of the legs and antennal segments that are reduced in size (Fig. 4A) or missing. Therefore an excess of Dll product appears to result in a loss-of-function phenotype. Because the em212–GAL4/+ flies contain a normal dose of Dll, the implication is that the excess of Dll product in em212–GAL4/+; UAS–Dll flies suppresses the activity of endogenous Dll gene. Lower expression levels of the endogenous Dll were found in em212–GAL4/Dll–lacZ;UAS–Dll discs (data not shown).
Figure 4.
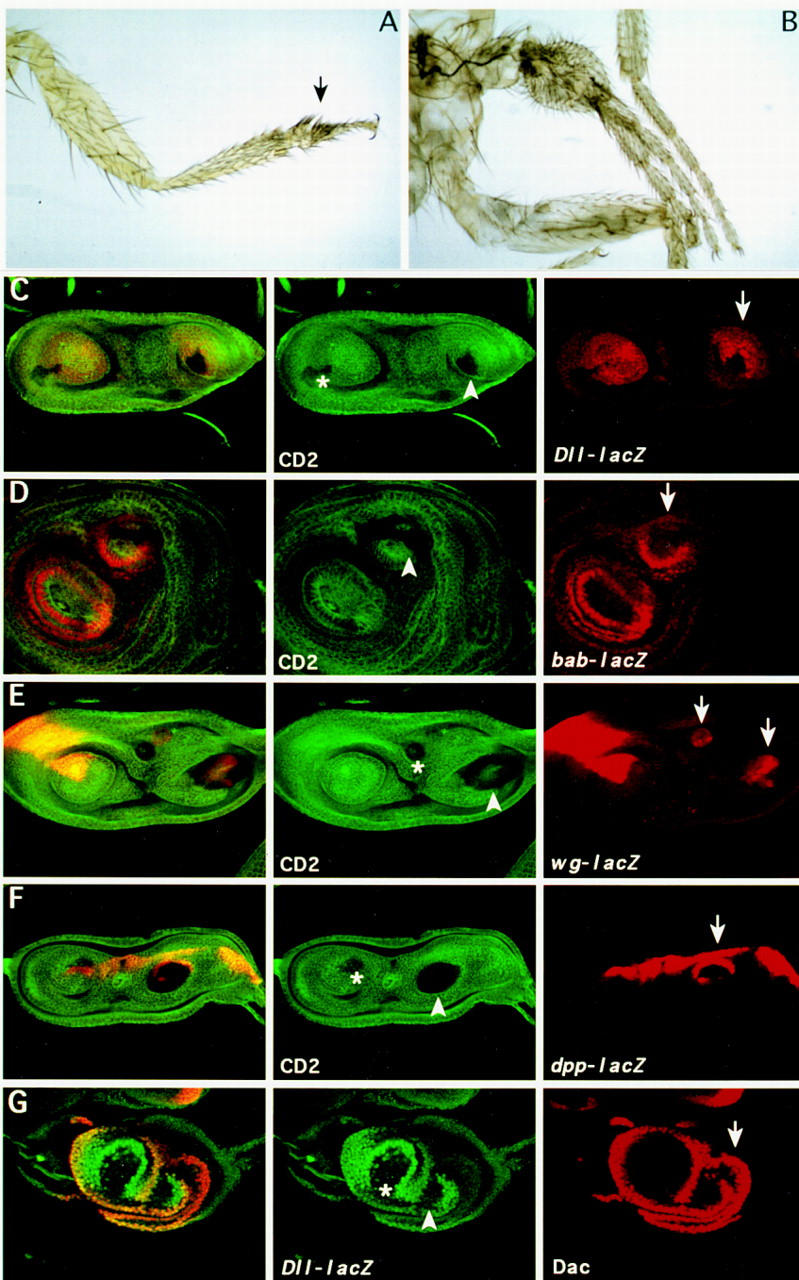
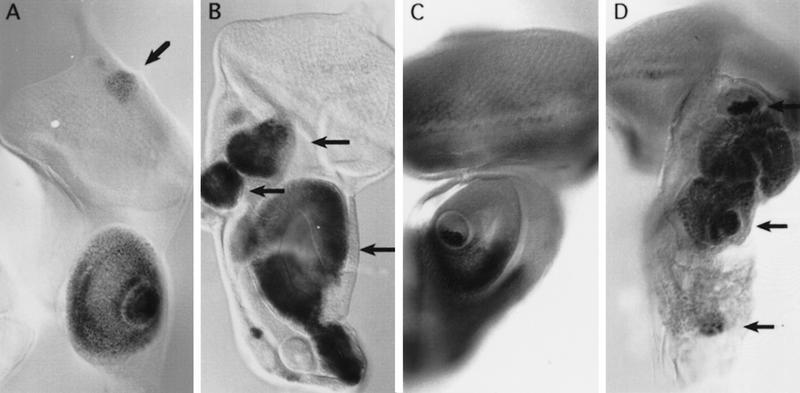
Effect of ectopic expression of Dll in the leg. (A) Phenotypic effect of the overexpression of Dll in its own domain. Distal part of the leg of em212–GAL4/UAS–Dll flies. The arrow indicates fusion of the tarsi. (B) Leg duplication induced by ectopic Dll+ clones (see Material and Methods). (C–G) Dll+ clones in leg imaginal discs induce duplication of the P/D axis. Clones were marked by the absence of the CD2 marker (except for the last panel) and were scored for the ectopic expression of Dll–lacZ (C), bab–lacZ (D), wg–lacZ (E), dpp–lacZ (F), and the Daschund (G) protein. Note the long-range effect of the Dll+ clones in the induction of gene expression. Arrowheads indicate clones that induce duplication of the P/D axis; asterisks indicate clones that do not induce duplication; arrows indicate ectopic expression of the different markers.
To assess the effect of ectopic Dll in the proximal leg and antennal regions where the gene is not expressed, Dll activity was induced in random patches by flip-out, using the same UAS–Dll (see Materials and Methods). Leg duplications were obtained when the clones were located in the proximal part of the leg (Fig. 4B). This was also seen in the disc as duplication of the growth cone (Fig. 4C–G). The induction of ectopic legs implicates a nonautonomous process and the marked clone is located in the distal part of the duplicated leg primordia. These ectopic Dll+ clones repress endogenous Dll expression (visualized by Dll–lacZ expression) autonomously, but induce Dll expression in cells outside of the clone (Fig. 4C). This nonautonomous effect can also be visualized using other ventral disc markers. bric à brac (bab) is a gene expressed in the leg and antennal discs in the presumptive region of the most distal segments (Godt et al. 1993; Fig. 7D, below) and is required for the proper segmentation of the tarsus. It has been suggested that it is regulated by Dll (Godt et al. 1993). We found that bab is activated in the marked Dll+ clone and also outside of the clone (Fig. 4D). dachshund (dac) is another gene expressed and required in the leg and antennae. It is expressed in the third antennal disc segment and in the presumptive trochanter, femur, tibia, and proximal tarsal segments of the leg disc (Mardon et al. 1994). dac is induced in the duplicated structure outside the labeled Dll+ clone (Fig. 4G).
Figure 7.
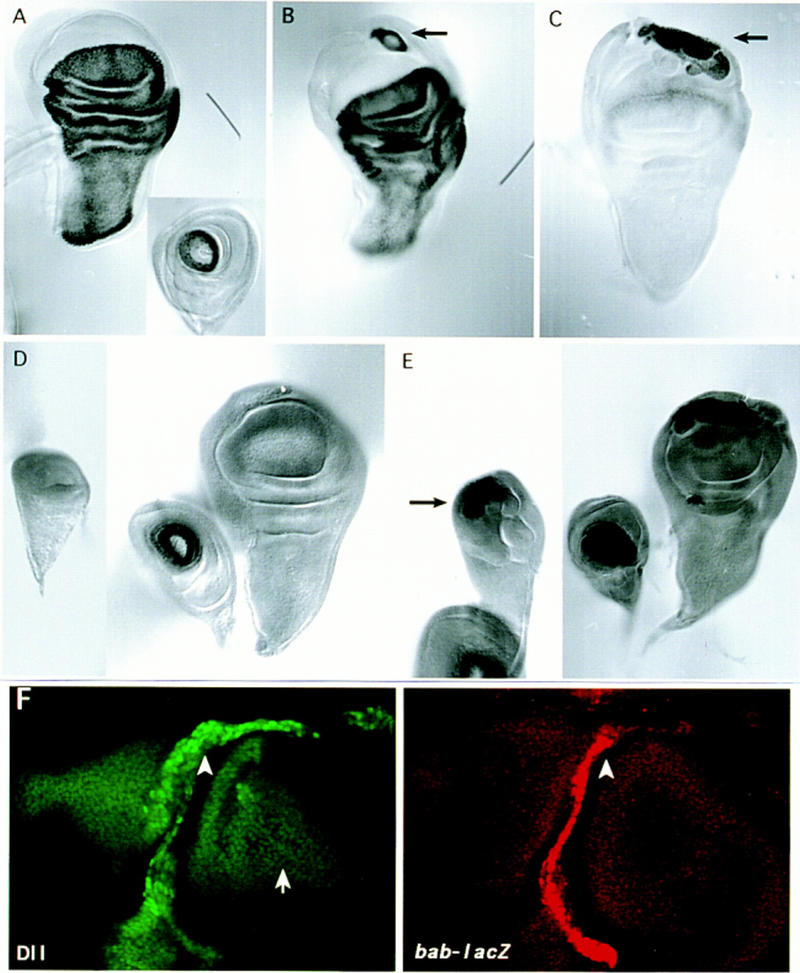
Induction of primordial legs in the wing is accompanied by the activation of the tarsal-specific expression of ap–lacZ and bab–lacZ. (A) ap–lacZ expression pattern in wing and leg (inset) discs. (B) Ectopic ap–lacZ expression in the ventral part of the wing disc of E132–GAL4/UAS–Dll/ap–lacZ flies. Note the ring pattern (arrow) similar to the wild-type ap–lacZ expression in the leg disc that corresponds to the fourth tarsus. (C) Sibling disc showing ectopic activation of endogenous Dll–lacZ (arrow) in the region where ectopic ap–lacZ appears. (D) Wild-type bab–lacZ expression pattern in wing, leg, and haltere discs. (E) Ectopic bab–lacZ expression (arrows) in the ventral part of the wing and haltere discs of E132–GAL4/UAS–Dll/bab–lacZ flies. (F) Ectopic bab–lacZ (red) and Dll (green) expression in a ptc–GAL4/UAS–Dll wing disc. Note the wild-type pattern of Dll expression at the presumptive wing margin (arrow) and the ectopic Dll and bab–lacZ expression driven by the ptc–GAL4 line (arrowheads).
Wg and Dpp have a long-range effect and are responsible for ventral and dorsal fates in the leg respectively (Peifer et al. 1991; Couso et al. 1993; Struhl and Basler 1993; Díaz-Benjumea and Cohen 1994; Held and Heup 1996). To explain the long-range effect of Dll+ clones we analyzed whether wg and dpp were also activated. As shown in Figure 4, E and F, there is wg and dpp expression in cells within (probably in complementary domains as in the normal leg) and outside the Dll+ clone. This is in accordance with the presence of ventral and dorsal structures in the duplicated legs.
Ectopic Dll expression in the wing and haltere discs produces ectopic legs
When the Dll product is expressed under the control of certain GAL4 lines that produce uniform Dll expression in the wing pouch, such as the C-68a and C-765 lines, it gives rise to rudimentary appendages lacking most structures. However, when GAL4 lines such as E132–GAL4, optomotor-blind (omb)–GAL4, apterous (ap)–GAL4, or patched (ptc)–GAL4 are used to induce localized expression in the wing, this structure is replaced partially by tissue containing bracted bristles and claws typical of the leg (Fig. 5; see legend for frequency). Rudimentary ectopic legs with claws formed at their distal ends are observed (Fig. 5B,C,E). In some cases, these ectopic legs include distal tarsal segments, the tibia, and part of the femur (Fig. 5E). These ectopic legs appear in the proximal part of the wing and at times present apical bristles, a marker of mid-leg identity (Fig. 5E). The halteres undergo very similar transformations to those described in the wings, presenting also ectopic leg structures (Fig. 5D). It is difficult to ascertain the identity of these legs, although apical bristles were not observed.
Figure 5.
Dorsal to ventral transformation in the thorax induced by ectopic Dll. (A) Wild-type wing. (Inset) Wild-type haltere. (B) Ectopic leg tissue emerging from the hinge region of E132-GAL4/UAS–Dll flies. (C) Magnification of B. (D) Ectopic leg tissue arising from the haltere of ap–GAL4/UAS–Dll flies. Arrows indicate the claws; arrowheads indicate bracted bristles. (E) Ectopic leg emerging from the proximal region of the wing of E132–GAL4/UAS–Dll flies. The arrows indicate the femur, tibia, and tarsi segments. Arrowheads indicate an apical bristle typical of the mesothoracic leg. These phenotypes occur at the following frequencies: 90% of the flies contained bracted bristles and 30% developed claws (n = 31) (using the E132–GAL4 line); 50% contained bracted bristles and 30% developed claws (n = 12) (using the ptc–GAL4); 80% (n = 12), and 85% (n = 34) contained bracted bristles (using the omb–GAL4 and ap–GAL4 lines, respectively). These frequencies are higher when looking at leg molecular markers in the imaginal discs.
The repression of wing development by the ectopic Dll product suggests that wing-forming genes should also be suppressed. The vg gene is likely to be affected specifically. This gene is activated in presumptive wing cells at the time of separation of the leg and wing primordia (Cohen et al. 1993) and is selectively required for wing cell proliferation (Williams et al. 1991). It has also been demonstrated that its expression is sufficient to induce outgrowths of wing tissue in other imaginal discs (Kim et al. 1996). The effect of the Dll protein on vg expression is illustrated in Figure 6: The Dll product suppresses vg expression.
Figure 6.
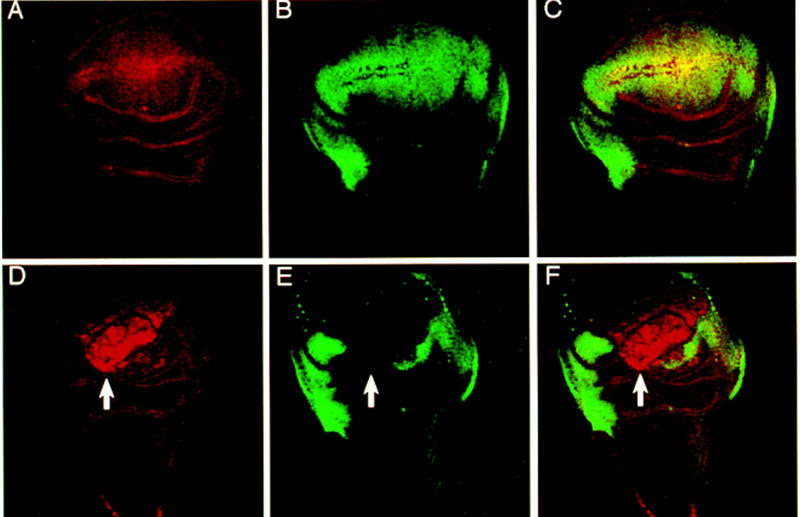
Ectopic expression of Dll in the wing disc activates the endogenous gene and represses the wing-specific gene vg. (A) Dll–lacZ expression pattern. (B) Vg wild-type expression pattern. (C) Merged channels. (D) Dll–lacZ expression pattern of omb–GAL4/UAS–Dll wing discs. (E) Vg expression pattern in the same disc. (F) Merge channels. Arrows indicate the repression of Vg and the activation of endogenous Dll. Here and in all remaining images of wing discs, ventral is at the top and anterior is to the left.
Because Dll shows positive autoregulation during embryonic development (Vachon et al. 1992; Castelli-Gair and Akam 1995), the possibility of the GAL4-driven Dll product inducing ectopic activation of the endogenous Dll gene during imaginal disc development was investigated. As shown in Figure 6D, exogenous Dll product activates endogenous Dll. The area of Dll activation corresponds to the part of the wing disc that is morphology altered and also corresponds to the area where vg activity is suppressed (Fig. 6E,F). Other characteristics of leg development are also reproduced in the ectopic legs. For example, the gene ap has restricted expression in the fourth tarsal segment of the leg (Cohen et al. 1992; Fig. 7A) and consequently a ring of ap expression is observed where the Dll product induces an ectopic leg (Fig. 7B,C). Another example is the activation of bab, a gene specific for ventral discs (Fig. 7D). Ectopic activation of bab in the wing and haltere discs as induced by Dll ectopic expression was found (Fig. 7E). This activation of bab may occur anywhere in the wing disc (Fig. 7F) even in the notal region suggesting that the fate of the leg can be induced anywhere, although adult ectopic rudimentary legs only appear in the hinge region. Figure 7F also shows that the level of ectopic Dll is higher than endogenous Dll as revealed by the Dll antibody. Only high levels of Dll repress Vg. This could explain the coexpression of Dll and Vg in wild-type wing imaginal discs. Dll only represses Vg when its expression is increased.
Ectopic Dll expression in the eye and head produces ectopic antennae
If the dorsal-to-ventral transformation of the wings and halteres to legs described above reflects an involvement of Dll in a general dorsal versus ventral decision concerning appendage organization, homologous transformations in other regions of the body would be expected. The eye–antennal disc contains dorsal and ventral components as suggested by the homeotic transformations described previously (see Morata and Lawrence 1979). Dll is expressed and required in the antennal region of the disc but it is not expressed in the eye or head capsule. The latter are considered to be dorsal derivatives (Cohen and Jürgens 1989b).
It was found that the ectopic expression of Dll in the eye precursor cells induces the formation of antennal structures. Figure 8, B and C, shows arista, aIII, and aII antennal segments emerging from the eye of a fly of genotype E132–GAL4/UAS–Dll. Using the ptc–GAL4 and C-68a–GAL4 lines ectopic antennae are observed in different regions of the head, such as the rostral membrane or its most dorsal posterior part (Fig. 8E–G). This may reflect the complex organization of the eye–antennal disc (see below) but the significant result is that the eye or head are transformed toward antennae.
Figure 8.
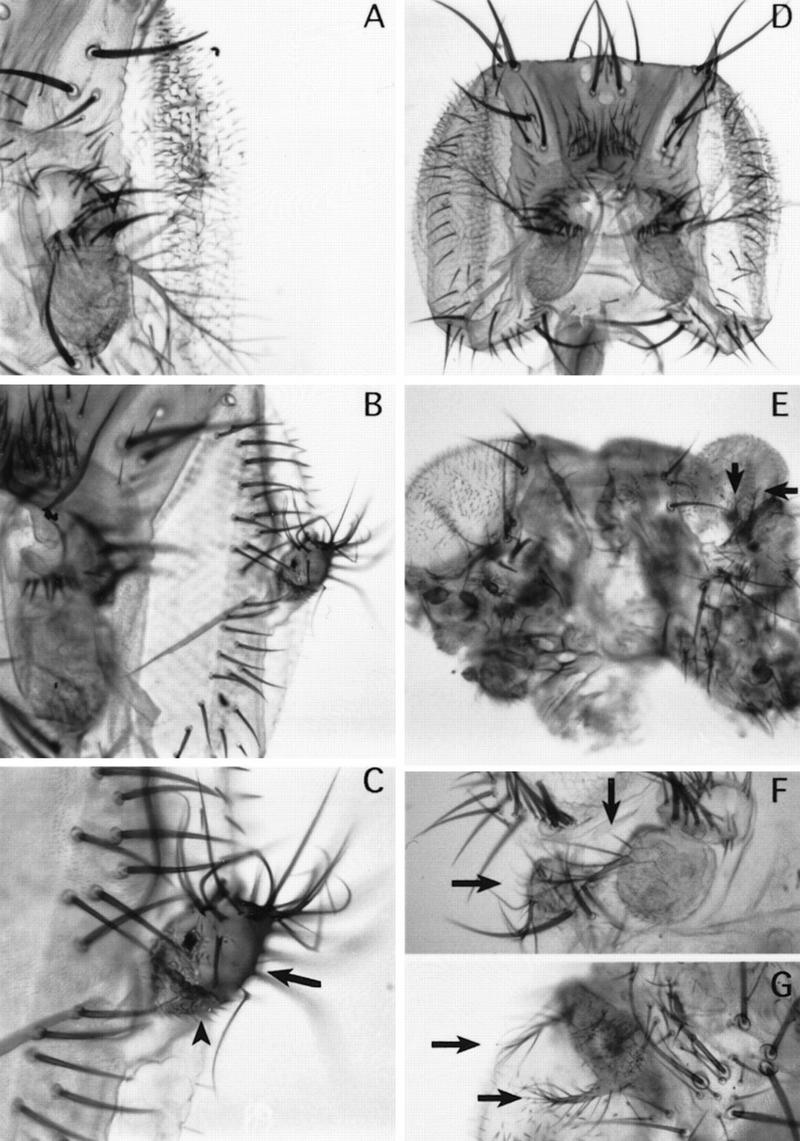
Dorsal to ventral transformation in the head induced by ectopic Dll. (A) Wild-type eye and antenna. (B) Ectopic antenna emerging from the eye of E132–GAL4/UAS–Dll flies. (C) Detail of the antennal outgrowth shown in B. Arrow indicates aII-like tissue emerging from the eye; arrowhead indicates aIII-like tissue. (D) Wild-type head. (E) Head from ptc–GAL4/UAS–Dll flies showing ectopic antennae in different locations. (F) Detail of a head of C-68a/UAS–Dll flies showing ectopic antenna, including aIII and arista. (G) Detail of a head of ptc–GAL4/UAS–Dll flies showing duplicated arista. Arrows indicate original and ectopic antennae. These phenotypes occur at the following frequencies: 46% of the flies developed ectopic antennal tissue in the eye (n = 26) (using the E132–GAL4 line); 90% (n = 10) and 30% (n = 27) developed ectopic antennae in different regions of the head (using the ptc–GAL4 and the C-68a lines, respectively).
As in the wing disc, it was found that the exogenous Dll product induces ectopic activation of endogenous Dll in the eye where it is not normally active (Fig. 9A). The ptc–GAL4 line (Fig. 9B) was also used to further show ectopic expression of endogenous Dll in defined areas of the antennal region of the disc. The induction of ectopic antennae by Dll expression in ptc–GAL4/UAS–Dll flies is also accompanied by the expression of genes such as en (Fig. 9D) and wg (data not shown) in a subset of cells of the ectopic appendages. In these mutant discs, en and wg expression appears in several separate patches, probably reflecting the composite nature of the disc.
Figure 9.
Ectopic expression of the endogenous Dll and En in the eye–antennal disc. (A) Ectopic activation of the endogenous Dll in the eye part (arrow) of the eye–antennal disc of E132–GAL4/UAS–Dll/Dll–lacZ flies. (B) Ectopic activation of the endogenous Dll in the eye-antennal disc of ptc–GAL4/UAS–Dll/Dll–lacZ flies. Arrows indicate the three areas with endogenous ectopic Dll that may correspond to the ectopic antennae observed in the adult head (Fig. 3). (C) En expression pattern in a wild-type disc. (D) Ectopic En expression (arrows) in different regions of the eye–antennal disc in those areas that might develop as ectopic antennae.
Discussion
Dll activity induces the formation of ventral appendages
Dll is expressed in the primordia of the larval and adult thoracic and cephalic appendages. In the adult legs, the Dll is domain extends from the trochanter to the tarsus and in the antennae it includes the second and third segments and the arista (see Fig. 1). The Dll domain probably represents the original leg appendage (see also Cohen and Jürgens 1989b; González-Crespo and Morata 1996). The proximal part of the leg, the pleura and the coxa, form part of the extradenticle (exd) domain. This domain is nearly complementary to that of Dll domain (González-Crespo and Morata 1996) and probably represents an expansion of the body trunk, the coxopodite (Snodgrass 1935). Although the argument for the antenna is not as compelling, the homology relationship between leg and antenna supports the idea of similar organization. For example, the aI segment is considered to be homologous to the coxa (Posthlewait and Schneiderman 1971) and the aII, aIII, and arista similar to the rest of the leg. In concordance to this, the aI segment (like the coxa) does not possess Dll function, whereas the rest of the antenna does. Therefore, Dll expression domains in legs and antennae are homologous.
These expression patterns reflect a functional requirement as loss of Dll function results in a corresponding loss of ventral appendages. In the viable Dll mutations the legs and antennae are defective; there is a gradual loss of structures depending on the strength of the mutation (Cohen and Jürgens 1989a). In the strongest viable mutations such as Dll3, most of the leg is lacking and only the pleura, coxa, trochanter, and part of the femur remain (Sunkel and Whittle 1987; Cohen and Jürgens 1989a). Moreover, clones of cells mutant for null Dll alleles generated in early larval development in either legs or antennae are unable to form the Dll domain structures. One reason for this is that Dll− clones do not proliferate in the Dll domain (Fig. 2B). The lack of growth observations suggests that, in the absence of Dll activity, the normal polarity of the appendage cannot be established and growth of the appendage is prevented. This suggestion is supported strongly by the present finding (Fig. 4B) that ectopic expression of Dll in the proximal regions of leg and antennal discs often results in the generation of a supernumerary appendage.
The induction of these additional appendages is of interest, for they require at least two extracellular signal molecules, Wg and Dpp, that during normal development act on downstream genes to control growth and pattern. The formation of the P/D axis appears to be initiated from the site where cells expressing wg are in close association with those expressing dpp (Basler and Struhl 1994; Díaz-Benjumea et al. 1994; Campbell and Tomlinson 1995). The combined action of these signals activates Dll (Díaz-Benjumea and Cohen 1994; Campbell and Tomlinson 1995). In this work it was demonstrated that Dll itself is able to induce this signaling process as shown by the observation that ectopic Dll+ clones produce a nonautonomous activation of wg and dpp. This new Wg and Dpp interaction in turn induces Dll expression nonautonomously and originates a new P/D axis. A similar positive feedback loop between a homeotic gene and Wg and Dpp also takes place in the embryonic midgut. The expression of Ubx is autoregulatory and requires cell communication involving Wg and Dpp signals (Bienz 1996).
However, these results do not explain the lack of proliferation of the Dll− cells in the leg and antennal discs, as Wg and Dpp are secreted by the surrounding cells. A possible explanation is that Dll− cells cannot respond to one or both of these signal molecules required for cell proliferation (Burke and Basler 1996; Penton and Hoffman 1996; Zecca et al. 1997). In this respect, it is worth pointing out that the late requirement of Dll in the wing could implicate the reception of Wg and Dpp. The wing margin and wing veins are affected in Dll− clones and both Wg and Dpp reception are required for the differentiation of these structures late in development (Phillips and Whittle 1993; Couso et al. 1994; de Celis 1997).
Dll is a component of the genetic address determining the identity of ventral appendages
In addition to its role in the induction of the appendage, these results indicate that Dll is also involved in the specification of the identity of ventral appendages. First, it is possible to recover late induced Dll− clones from legs and antennae, which are able to differentiate adult cuticular structures. These structures are unlike those corresponding to the region of the leg or antenna where the clone is located, indicating a change in the cell type. However, it was not possible to identify the type of structure formed by these clones with the exception of the base of the arista, where they are seen to differentiate leg bristles.
The second and stronger argument comes from the consideration that normal Dll activity is required for at least two distinct identities—legs and antennae. Moreover, when expressed ectopically, Dll activity induces the formation of the same two appendages depending on the context of the ectopic expression. In normal development, the genetic context appears to be provided by the activity of the homeotic gene Antp. The combination Dll-on-Antp-off specifies antennal development whereas Dll-on-Antp-on determines leg development. The ectopic expression of Antp (Schneuwly et al. 1987) transforms the antenna (Dll-on-Antp-off) into a mid-leg (Dll-on-Antp-on) and using the same rationale, lack of Antp transforms mid-leg into an antenna (Struhl 1981). This suggests that a combinatorial code (Struhl 1982) determines the type of ventral appendage. Induction of ectopic Dll activity in the eye shows that the combination Dll-on-Antp-off (Antp is not expressed in the head; Engström et al. 1992) produces antennal development, whereas in the wing disc that contains Antp function, especially in the proximal regions (Wirz et al. 1986), the Dll-on-Antp-on combination specifies leg development. It is also worth pointing out that ectopic Dll expression gives rise to the formation of ectopic leg structures not only in the wing but in the haltere. In the wing they develop with mid-leg identity, as indicated by specific markers. It also seems likely that they develop with hindleg identity in the haltere. The reason for this suggestion is that in the haltere as in the hindleg leg, there is Ubx activity that determines third leg identity in normal development. Leg development in the wing lacking Ubx product would result in mid-leg identity.
The results suggest that Dll is a component of a genetic address that determines the identity of ventral appendages. This identity is qualified by properties provided by the selector genes of the ANT-C and the BX-C along the A/P body axis. The role of Dll in specifying ventral identity is reflected at the molecular level by the expression of molecular markers in the ectopic primordia like the ringed expression of ap in the fourth tarsal segment and bab, which is leg specific (Fig. 7). Exogenous Dll activity also results in ectopic activation of the endogenous Dll gene indicating that the autocatalitic activity of Dll found in the embryo (Castelli-Gair and Akam 1995) also operates in the imaginal cells.
However, the mode of action of Dll differs significantly from that of other homeotic genes such as en, Ubx, or ap mutations (Morata and Lawrence 1975; Morata and García-Bellido 1976; Díaz-Benjumea and Cohen 1993; Guillén et al. 1995) involved in the specification of the identity of adult structures. The first difference is that the few late Dll− clones that survive do not produce a clear homeotic transformation. It is, however, possible that Dll is not the only contributor to the identity of the appendage and that the elimination of Dll results in a “nonsense codeword” of active selector genes (Struhl 1982). Examples of this type of situation exist, for example, the effect of Ubx or abd-A mutations in the posterior abdomen (Lewis 1978; Sánchez-Herrero et al. 1985; Tiong et al. 1985). The second and the more significant difference is that the Dll domain is not defined by a compartment border. This indicates that Dll activity is not maintained by cell heredity but possibly by cell interactions (Díaz-Benjumea et al. 1994). It is possible that segregation of the “coxopodite” and the “telopodite” (Snodgrass 1935; González-Crespo and Morata 1996) is achieved through mutual interactions between Dll and exd and/or tsh expressing cells.
The functional interaction of Dll with the wing determinant gene vg requires further study. Forcing Dll expression in the wing or haltere results in suppression of vg expression and consequently of dorsal appendage development. In the experiments reported by Kim et al. (1996), targeted vg expression produces ectopic wings, and presumably Dll suppression in legs and antennae. The rules governing these interactions are not yet understood fully. However, it is possible that the decisive factor involves relative amounts of products. In some of our experiments, targeted Dll expression resulted in the loss of the wing, probably as a consequence of vg repression. There are may be cases of unbalanced amounts of the two gene products that give rise a developmental conflict that arrests development.
A late Dll function is involved with the differentiation of the wing margin
Our results also indicate that there is a late requirement for Dll activity in the wing. The nature of this function is different from that in the leg and antenna; the Dll product appears later in the wing than in the leg discs and also the mutant phenotype is more discrete. Although hypomorphic Dll mutations do not detectably affect wing differentiation (Cohen and Jürgens 1989a), cells mutant for Dll− null mutations exhibit a phenotype in the wing. These Dll− clones, unlike those in the legs and antennae, proliferate normally even when induced in the first larval period and may occupy large portions of the wing. Dll− clones have a phenotype restricted to the wing margin and veins; the triple-row bristles and double-row posterior hairs are lacking or abnormal and the differentiation of the veins is also altered. One interesting aspect of the Dll− phenotype in the wing is that it is nonautonomous, suggesting that this Dll function involves a signaling mechanism.
Materials and methods
Fly stocks
The following Dll alleles were used: DllIB (Cohen and Jürgens 1989b), Dll3 (Sunkel and Whittle 1987), DllSA1 (Cohen and Jürgens 1989b) and Df (2R)DllMP(Cohen et al. 1989).
The reporter genes dpp–lacZ (Blackman et al. 1991), wg–lacZ (Kassis 1990), ap–lacZ (aprk568) (Cohen et al. 1992), Dll–lacZ (Dll01092) (Spradling et al. 1995; Zecca et al. 1997), bab–lacZ (babA128) (Godt et al. 1993) are expressed as their respective endogenous RNAs.
The following GAL4 drivers were used: three different insertions in the Dll gene (em212–GAL4, MD23–GAL4, MD728–GAL4), an insertion in ap (ap–GAL4) and another in omb (omb–GAL4) as described in Calleja et al. (1996). The MS-1096 line is described in Capdevila and Guerrero (1994) (gift from F. Jiménez and C. Parras). C-765, C-68a GAL4 lines were kindly provided by A. Brand (Brand and Perrimon 1993), dpp–GAL4 by M. Hoffman (Morimura et al. 1996), ptc–GAL4 by Campos-Ortega and Hinz (Hinz et al. 1994), and E132-GAL4 by W. Gehring (Halder et al. 1995). UAS–y+ (Calleja et al. 1996) was used to visualize the Dll expression pattern in the adult cuticle.
Clones of Dll mutant cells were generated by FLP-mediated mitotic recombination as described by Golic (1991) and Xu and Rubin (1993). The hsp70–flipase (FLP122) was obtained from G. Struhl (Struhl and Basler 1993). Males of the genotype y w FLP122; FRT42D DllSA1/CyO or y f36 FLP122; FRT42D DllSA1/CyO were crossed to y w; FRT42DP[ry+; y+]44B or y f36; FRT42DP[f44Cf52] females (kindly provided by D. Gubb). For lineage restriction analysis, males y w FLP122; FRT42D arm–lacZ (Chen and Struhl 1996) were crossed to y w; FRT42DP[ry+;y+]44B females. FLP-mediated recombination was induced by incubating larvae 24–120 hr AEL at 37°C for 60 min to produce Dll− clones and by incubating larvae 72–120 hr at 37°C for 10 min to generate arm–lacZ clones.
Ectopic expression of Dll using the GAL4 system
For the production of UAS–Dll transgenic fly lines, a fragment of 1.2 kb of the Dll c-DNA (Cohen et al. 1989) containing the entire Dll open reading frame (ORF) was cloned in the pUAST plasmid. The recombinant plasmid containing the Dll cDNA in the correct orientation was used to transform y w118 embryos by standard procedures of microinjection. Of the two independent lines that were obtained, only one showed the phenotypes described in this work. The other gave rise to lethal phenotypes when assayed using the different GAL4 lines.
To modify the levels of the UAS construct, we took advantage of the temperature sensitivity of the GAL4 system (Wilder and Perrimon 1993). Using the same GAL4 line, the effects of different levels of the protein at set temperatures were compared.
Generation of random Dll-expressing clones
To generate random clones of ectopic Dll a hybrid of the Flip-out and GAL4 activation systems (Pignoni and Zipursky 1997) was used. Clones expressing GAL4 were induced by flipping out an interruption cassette from an actin > CD2 > GAL4 transgene in a genetic background containing UAS–Dll. Females with the genotype FLP 122 [hsp70-flp]; UAS–Dll were mated to actin > CD2 > GAL4 males carrying dpp–lacZ, wg–lacZ, Dll–lacZ, or bab–lacZ reporters on the second chromosome. After one day of egg laying, adults were removed and the progeny aged for two days, heat-shocked (37°C for 30 min) and dissected three days later. The UAS–y+ (Calleja et al. 1996) was also introduced to analyze the Dll+ clones in the adult cuticle.
Whole-mount immunostaining of imaginal discs
X-Gal staining was performed following standard protocols (Ashburner 1989). Peroxidase and immunofluorescence staining were performed as described by Sánchez-Herrero et al. (1996). Anti-Vg (Williams et al. 1991), anti-En (Patel et al. 1989), anti-Dll (Vachon et al. 1992), and anti-Dac (Mardon et al. 1994) antisera were kindly provided by S. Carroll (University of Wisconsin, Madison), T. Kornberg (University of California, San Francisco), S. Cohen (EMBL, Heidelberg, Germany), and G. Mardon (Baylor College of Medicine, Houston, TX), respectively. Imaginal discs were examined under a Zeiss laser scan microscope.
Acknowledgments
We thank E. Sánchez-Herrero for daily discussions and suggestions during this work and also for comments on the manuscript; J.L. Mullor and S. González-Crespo for discussions; J.L. Mullor for unconditional help with the preparation of the photographic work; and R. González for her technical assistance. We also thank A. Brand, M. Calleja, J.A. Campos-Ortega, S. Carroll, S. Cohen, D. Gubb, U. Hinz, M. Hoffman, W. Gehring, F. Jiménez, T. Kornberg, G.F. Lasky, G. Mardon, E. Moreno, C. Parras, G. Struhl, and L. Zipursky for stocks, DNAs, and antibodies. We are supported by grants from the Dirección General Científica y Técnica, the International Human Frontier Science Program (372/94) and an institutional grant from Fundación Areces. N.G. was sponsored by a Mutis fellowship from the Instituto de Cooperación Iberoamericana.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL iguerrero@trasto.cbm.uam.es; FAX 34 1 3974799.
References
- Ashburner, M. 1989. Drosophila: A laboratory handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by Hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bienz M. Induction of the endoderm in Drosophila. Sem Cell Dev Biol. 1996;7:113–119. [Google Scholar]
- Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3′ cis-regulatory region directs the imaginal disc expression of decapentaplegic, a member of a TGF-β family in Drosophila. Development. 1991;111:657–665. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Blair SS. Mechanisms of compartment formation: Evidence that non proliferating cells do not play a critical role in defining the D/V lineage restriction in the developing wing of Drosophila. Development. 1993;119:339–351. doi: 10.1242/dev.119.2.339. [DOI] [PubMed] [Google Scholar]
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brook WJ, Cohen SM. Antagonistic interactions between Wingless and Decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science. 1996;273:1373–1376. doi: 10.1126/science.273.5280.1373. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122:2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Peláz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. Initiation of the proximodistal axis in insect legs. Development. 1995;121:619–628. doi: 10.1242/dev.121.3.619. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli-Gair J, Akam M. How the Hox gene Ultrabithorax specifies two different segments: The significance of spatial and temporal regulation within metameres. Development. 1995;121:2973–2982. doi: 10.1242/dev.121.9.2973. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for Patched in sequestering and transducing Hedhehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Imaginal disc development. In: Martinez-Arias A, Bate M, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 747–842. [Google Scholar]
- Cohen SM, Jürgens G. Proximal-distal pattern formation in Drosophila: Graded requirement for Dll gene activity during limb development. Wilhelm Roux’s Arch Dev Biol. 1989a;198:157–169. doi: 10.1007/BF02438941. [DOI] [PubMed] [Google Scholar]
- ————— Proximo-distal pattern formation in Drosophila: Cell autonomous requirements for Distal-less gene activity in limb development. EMBO J. 1989b;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Drosophila headlines. Trends Genet. 1991;7:262–267. doi: 10.1016/0168-9525(91)90327-M. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Brönner G, Küntter F, Jürgens G, Jäckle H. Distal-less encodes a homeodomain protein required for limb development in Drosophila. Nature. 1989;338:432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Cohen B, McGuffin ME, Pfeifle C, Segal D, Cohen SM. apterous: A gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes & Dev. 1992;6:715–729. doi: 10.1101/gad.6.5.715. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martínez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martínez-Arias A. The Wingless signalling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- de-Celis J F. Expression and function of decapentaplegic and thick veins during the differentation of the veins in the Drosophila wing. Development. 1997;124:1007–1018. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- Díaz-Benjumea FJ, Cohen SM. Interactions between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell. 1993;75:741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- ————— wingless acts through shaggy/zeste-white 3 kinases to direct dorsal-ventral axis formation in the Drosophila leg. Development. 1994b;120:1661–1670. doi: 10.1242/dev.120.6.1661. [DOI] [PubMed] [Google Scholar]
- ————— Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- Díaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- Engström YM, Schneuwly S, Gehring WJ. Spatial and temporal expression of an Antennapedia/lacZ gene construct integrated into the endogenous Antennapedia gene of Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1992;201:65–80. doi: 10.1007/BF00420417. [DOI] [PubMed] [Google Scholar]
- Gehring W. Bildung eines vollständigen Mittelbeines mit Sternopleura in der Antennenregion bei der Mutante Nasobemia (Ns) von Drosophila melanogaster. Arch Julius Klaus-Stift Vererbungsforch Sozialanthropol, Rassenhyg. 1966;41:44–54. [PubMed] [Google Scholar]
- Gehring W, Seippel S. Die Imaginalzellen des Clypeo-Labrums und die Bildung des Rüssels von Drosophila melanogaster. Rev Suisse Zool. 1967;74:589–596. [PubMed] [Google Scholar]
- Godt D, Couderc J-L, Cramton SE, Laski F. Pattern formation in the limbs of Drosophila: bric à brac is expressed in both a gradient and a wave like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119:799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- González-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development. 1995;121:2117–2125. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- ————— Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development. 1996;122:7539–7546. doi: 10.1242/dev.122.12.3921. [DOI] [PubMed] [Google Scholar]
- Guillén I, Mullor JL, Capdevilla J, Sánchez-Herrero E, Morata G, Guerrero I. The function of engrailed and the specification of Drosophila wing pattern. Development. 1995;121:3447–3456. doi: 10.1242/dev.121.10.3447. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Held LI., Jr Axes, boundaries and coordinates; the ABCs of fly leg development. BioEssays. 1995;17:721–732. doi: 10.1002/bies.950170809. [DOI] [PubMed] [Google Scholar]
- Held LI, Jr, Heup MA. Genetic mosaic analysis of decapentaplegic and wingless gene function in the Drosophila leg. Dev Genes Evol. 1996;206:180–194. doi: 10.1007/s004270050044. [DOI] [PubMed] [Google Scholar]
- Held LI, Jr, Heup MA, Sappington JM, Peters SD. Interactions of decapentaplegic, wingless, and Distal-less in the Drosophila leg. Wilhelm Roux’s Arch Dev Biol. 1994;203:310–319. doi: 10.1007/BF00457802. [DOI] [PubMed] [Google Scholar]
- Heslip TR, Theisen H, Walker H, Marsh LJ. Shaggy and Dishevelled exert effects on wingless and decapentaplegic expression and on positional identity in imaginal discs. Development. 1997;124:1069–1078. doi: 10.1242/dev.124.5.1069. [DOI] [PubMed] [Google Scholar]
- Hinz U, Giebel B, Campos-Ortega JA. The basic helix-loop-helix of Drosophila lethal of Scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994;76:77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell. 1996;86:401–409. doi: 10.1016/s0092-8674(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Shubiger G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development. 1996;122:3519–3529. doi: 10.1242/dev.122.11.3519. [DOI] [PubMed] [Google Scholar]
- Kassis JA. Spatial and temporal control elements of the Drosophila engrailed gene. Genes & Dev. 1990;4:433–443. doi: 10.1101/gad.4.3.433. [DOI] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Lawrence PA. The making of a fly: The genetics of animal design. Oxford, UK: Blackwell Scientific Publications; 1992. [Google Scholar]
- Lawrence PA, Morata G. Homeobox genes: Their function in segmentation and pattern formation in Drosophila. Cell. 1994;78:181–189. doi: 10.1016/0092-8674(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Mardon G, Salomon NM, Rubin MR. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255:614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- ————— The development of the eye-antennal disc of Drosophila. Dev Biol. 1979;99:27–33. [Google Scholar]
- Morata G, García-Bellido A. Developmental analysis of some mutants of the bithorax system of Drosophila. Wilhelm Roux’s Arch Dev Biol. 1976;179:125–143. doi: 10.1007/BF00848298. [DOI] [PubMed] [Google Scholar]
- Morimura S, Maves L, Chen Y, Hoffmann FM. decapentaplegic overexpression affects Drosophila wing and leg imaginal disc development and wingless expression. Dev Biol. 1996;177:136–151. doi: 10.1006/dbio.1996.0151. [DOI] [PubMed] [Google Scholar]
- Patel NH, Martín-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. Expression of Engrailed proteins in arthropods, annelids, and chordates. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Peifer M, Rauskolb C, Williams M, Riggleman B, Wieschaus E. The segment polarity gene armadillo interacts with the wingless signalling pathway in both embryonic and adult pattern formation. Development. 1991;111:1029–1043. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- Penton A, Hoffmann MF. Decapentaplegic restricts the domain of wingless during Drosophila limb patterning. Nature. 1996;382:162–165. doi: 10.1038/382162a0. [DOI] [PubMed] [Google Scholar]
- Phillips R, Whittle R. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development. 1993;118:427–436. doi: 10.1242/dev.118.2.427. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by Decapentaplegic. Development. 1997;124:271–278. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Postlethwait J, Schneiderman H. Pattern formation and determination in the antenna of the homeotic mutant Antennapedia of Drosophila melanogaster. Dev Biol. 1971;25:606–640. doi: 10.1016/0012-1606(71)90008-x. [DOI] [PubMed] [Google Scholar]
- Sánchez–Herrero E, Vernos I, Marco R, Morata G. Genetic organization of the Drosophila Bithorax complex. Nature. 1985;313:108–113. doi: 10.1038/313108a0. [DOI] [PubMed] [Google Scholar]
- Sánchez-Herrero E, Couso JP, Capdevila J, Guerrero I. The fu gene discriminates between pathways to control dpp expression in Drosophila imaginal discs. Mech Dev. 1996;55:159–170. doi: 10.1016/0925-4773(96)00498-4. [DOI] [PubMed] [Google Scholar]
- Schneuwly S, Klemenz R, Gehring WJ. Redesigning the body plan of Drosophila by ectopic expression of the homeotic gene Antennapedia. Nature. 1987;325:816. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass RE. Principles of insect morphology. New York, NY: McGraw-Hill; 1935. The segmental appendages of arthropods; pp. 83–99. [Google Scholar]
- Spradling AC, Stern DM, Kiss I, Roote J, Laverty T, Rubin GM. Gene disruptions using P transposable elements: An integral component of the Drosophila genome project. Proc Natl Acad Sci. 1995;92:10824–10830. doi: 10.1073/pnas.92.24.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E. Establishment of compartments in the developing leg imaginal disc of Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1976;180:9–30. doi: 10.1007/BF00848882. [DOI] [PubMed] [Google Scholar]
- Struhl G. A homeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292:635–638. doi: 10.1038/292635a0. [DOI] [PubMed] [Google Scholar]
- ————— Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of the Wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sunkel CE, Whittle JRS. Brista: A gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1987;196:124–132. doi: 10.1007/BF00402034. [DOI] [PubMed] [Google Scholar]
- Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development. 1995;121:3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- Theisen H, Haerry TE, O’Connor MB, Marsh L. Developmental territories created by mutual antagonism between Wingless and Decapentaplegic. Development. 1996;122:3939–3948. doi: 10.1242/dev.122.12.3939. [DOI] [PubMed] [Google Scholar]
- Tiong S, Bone LM, Whittle JR. Recessive lethal mutations within the bithorax complex in Drosophila. Mol & Gen Genet. 1985;200:335–342. doi: 10.1007/BF00425445. [DOI] [PubMed] [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen S. Homeotics genes of the bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- Wilder EL, Perrimon N. Dual functions of wingless in the Drosophila leg imaginal dis. Development. 1993;121:477–488. doi: 10.1242/dev.121.2.477. [DOI] [PubMed] [Google Scholar]
- Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes & Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Wirz J, Fessler L, Gehring WJ. Localization of the Antennapedia protein in Drosophila embryos and imaginal discs. EMBO J. 1986;5:3327–3334. doi: 10.1002/j.1460-2075.1986.tb04647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;121:2265–2278. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a Wingless morphogen gradient. Cell. 1997;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]