Abstract
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor (TGF)-β superfamily which regulates bone formation, haematopoiesis and development. While TGF-β is known to be a negative regulator of the immune system, the effect of BMPs on the immune system is largely unknown. Herein, the effect of BMP-6 on the innate immune system was investigated using the murine macrophage cell line RAW 264·7. BMP-6 altered cellular morphology, inhibited cellular proliferation, increased the fraction of subG1 phase cells, and decreased the fraction of cells in the S and G2M phases, without changing the percentage of apoptotic cells. In addition, BMP-6 induced expression of pro-inflammatory inducible nitric oxide synthase (iNOS) and the cytokine tumour necrosis factor (TNF)-α. Reverse transcription–polymerase chain reaction (RT-PCR) analysis demonstrated the expression of all three known type II BMP receptors [BMP-RII, activin (Act)-RIIA and Act-RIIB] and two of the three known type I receptors [activin receptor-like kinase 2 (ALK2) and ALK3]. Over-expression as well as knock-down studies using short hairpin RNA (shRNA) demonstrated that BMP-RII, ALK2 and ALK3 are the functional BMP-6 receptors in macrophages. Finally, the effect of BMP-6 was confirmed in murine peritoneal macrophages and the THP-1 human monocyte cell line. Taken together, these results demonstrate that BMP-6 regulates the proliferation and gene expression profile of macrophages.
Keywords: activation, bone morphogenetic proteins, macrophages
Introduction
Bone morphogenetic proteins (BMPs), with more than 20 subtypes, are the largest subfamily within the transforming growth factor (TGF)-β superfamily. They were initially characterized as factors that induce bone and cartilage formation.1,2 Since then, it has been demonstrated that BMPs are critical in gastrulation, mesoderm formation, left-right symmetry, neural patterning, skeletal and limb development, organogenesis, gametogenesis, cellular chemotaxis, and cellular differentiation.3,4 More recently, BMP-6 has been reported to inhibit T-cell proliferation.5 Like TGF-β, BMPs exist as large dimeric proproteins in the cytoplasm and are cleaved by proteases during secretion. Once secreted, the mature 21–25-kDa dimeric ligands bind to membrane receptors on target cells.
Once BMP receptors are activated, receptor-activated Smads (R-Smads) (Smads 1, 5 and 8) are phosphorylated. Subsequently, the phosphorylated R-Smads interact with the common mediator Smad (Co-Smad) (Smad4), translocate into the nucleus, and regulate specific gene expression. Although the precise function of Smad1, Smad5 and Smad8 in BMP signalling remains unclear, BMP receptors and Smads have differing affinities. In addition to the canonical Smad-dependent pathway, Smad-independent signalling pathways have been reported. Specifically, a mitogen-activated protein kinase (MAPK) pathway involving p38MAPK and c-Jun N-terminal kinase (JNK) has been identified.6 More recently, activation of the nuclear factor (NF)-κB pathway via the X-linked inhibitor of apoptosis (XIAP) has been shown to transduce BMP signalling.7 Currently, the biological roles of these various Smad-independent pathways remain unclear.
Macrophages are the key regulators of the innate immune system, and originate from stem cells located in the bone marrow. Monoblasts are the most immature type of cell to exhibit macrophage characteristics. Upon division, monoblasts give rise to promonocytes and monocytes in the bone marrow. In response to appropriate stimuli mediated by cytokines, these monocytes migrate into tissues and organs where they differentiate into macrophages. The principal effectors of macrophage activation are armed inflammatory T (CD4) cells, which secrete interferon (IFN)-γ, a critical cytokine for macrophage activation. Under experimental conditions, macrophages can be activated in a two-stage reaction: priming and triggering.8 Activated macrophages also express major histocompatibility complex (MHC) class I and class II antigens. Macrophages can be divided into two broad groups: resident tissue macrophages and inflammatory macrophages. Tissue macrophages are heterogeneous, and those isolated from different tissues differ in function, possibly as a result of adaptive responses to the local microenvironment.9 Inflammatory macrophages are derived largely from circulating monocytes, which infiltrate damaged tissues, but some can arise by local cell division.10
As BMPs belong to the TGF-β superfamily and TGF-β is a potent immune regulator, we predicted that BMPs would also regulate the immune response. Of the available BMPs, our initial effort focused on BMP-6, because BMP-6 has been reported to have an antiproliferative effect on B and T cells.5,11 We report for the first time that, in macrophages, BMP-6 changes morphology, inhibits cellular proliferation, and induces the expression of pro-inflammatory inducible nitric oxide synthase (iNOS) and the cytokine tumour necrosis factor (TNF)-α. The effects of BMP-6 are concentration-dependent and mediated through the BMP receptors. In addition, the effect of BMP-6 was further confirmed in murine primary peritoneal macrophages and the human monotype THP-1 cell line.
Materials and methods
Cell culture
The macrophage cell line RAW 264·7 and the human monocyte cell line THP-1 were purchased from the American Type Culture Collection (Manassas, VA) and routinely cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS). To obtain murine peritoneal macrophages, 0·9 g of thioglycollate was dissolved in 30 ml of dH2O and autoclaved. Then, 2 ml was injected intraperitoneally into 8-week-old C57BL/6 mice. Mice were killed 3 days later and peritoneal lavage was carried out using 10 ml of phosphate-buffered saline (PBS). The lavage fluid was centrifuged at 500 g for 5 min and seeded into a T25 flask in DMEM supplemented with 10% FBS. The medium was changed 4 hr later.
To determine the effect on cellular proliferation, RAW 264·7 cells, primary murine macrophages, and THP-1 cells (1 × 104 cells/well) were treated with BMP-6 (R&D Systems, Minneapolis, MN) for 48 and 96 hr in 24-well plates for 2 days at the indicated concentrations. Cells in each well were scraped and counted using a haemocytometer.
Apoptosis
The RAW 264·7 cells were incubated with 100-fold diluted fluorescein isothiocyanate (FITC)-VAD (Promega, Madison, WI) for 30 min at room temperature. Cells were washed twice with PBS and then diluted in PBS. Cell-associated fluorescence was then quantified by flow cytometry [fluorescence-activated cell sorting (FACS)] with Cytomics FC 500 Flow Cytometry Systems (Becton-Dickinson, San Jose, CA) using detectors: FL1 525 nm/40.
Cell cycle analysis
RAW 264·7 cells were grown in medium with 10% FBS and treated with BMP-6 for 48 hr. All cells were collected and washed once in PBS, and the cellular DNA was stained with propidium iodide. The cellular DNA content was analysed using flow cytometry.
Luciferase assay
A luciferase assay was carried out using the Dual-Luciferase Reporter Assay System (Promega). RAW 264·7 cells (2 × 105 cells/well) were plated in six-well plates and were transfected with BMP response element (BRE)-luciferase (1 μg) and CMV-Renilla luciferase plasmids (0·5 μg) using lipofectamine. Cells were cultured for 48 hr and treated with the indicated concentrations of BMP-6 for 48 and 96 hr. Subsequently, growth medium was removed from the cultured cells and 200 μl of cell lysis buffer was dispensed into each well and the plates were placed on a rocking platform for 15 min. The lysates were centrifuged for 30 seconds at top speed. Sixty μl of the supernatant was transferred to a 96-well plate and luciferase activity was measured according to the manufacturer’s protocol. Data are expressed as the normalized luciferase activity (firefly luciferase activity divided by Renilla luciferase activity) of transfected cells relative to that of control cells.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA), and RT-PCR was performed using the One Step SuperScript RT-PCR kit (Invitrogen). Reverse transcription was carried out at 50° for 30 min and 94° for 2 min. PCR conditions were as follows: 94° for 30 seconds, 55° for 30 seconds, and 68° for 1 min. The primers used are listed in Table 1.
Table 1.
Reverse transcription–polymerase chain reaction (RT-PCR) primer sequences
| BMP-6 forward primer | 5′-AAC TCG AGT CAC GCC AAG GGC CA |
| BMP-6 reverse primer | 5′-AAC TCG AGA ATG TGT GTC CCC AGC ATC T |
| mALK2 forward primer | 5′-ATG GTC GAT GGA GTA ATG ATC C-3′ |
| mALK2 reverse primer | 5′-AGC CCT TCA ATG GTA CCA TAC T-3′ |
| mAct-RIIA forward primer | 5′-ATG GGA GCT GCA AAG TTG G-3′ |
| mAct-RIIA reverse primer | 5′-ATG TTC TCA TGC TTC ATT CCA GGT-3′ |
| mAct-RIIB forward primer | 5′-ATC TAC TAC AAC GCC AAC TGG G-3′ |
| mAct-RIIB reverse primer | 5′-AGG TCG CTC TTC AGC AGT ACA T-3′ |
| mALK3 forward primer | 5′-ACG CGT GCG AAT CAG ACA ATG A-3′ |
| mALK3 reverse primer | 5′-AGA CAG CCA TGG AAA TGA GCA C-3′ |
| mALK6 forward primer | 5′-AAA GTT AAA GGA GCA ACC CGG C-3′ |
| mALK6 reverse primer | 5′-AAG AAC ACT TTC ACA GCC ACC TT-3′ |
| mBMP-RII forward primer | 5′-ACT ACG GCT GCT TCT CAG AAT C-3′ |
| mBMP-RII reverse primer | 5′-AGA CGA TTT CCA GTT AGC CTC A-3′ |
| miNOS forward primer | 5′-TAG TTT CCA GAA GCA GAA TGT GAC C-3′ |
| miNOS reverse primer | 5′-CCA AGA CTC TAA ATC GGA TCT CTC-3′ |
| mTNF-α forward primer | 5′-TAC CTT GTC TAC TCC CAG GTT CTC-3′ |
| mTNF-α reverse primer | 5′-AGAGCA ATG ACT CCA AAG TAG ACC-3′ |
| mβ actin forward primer | 5′-CTT CCT TAA TGT CAC GCA CGA TTT C-3′ |
| mβ actin reverse primer | 5′-GTG GGG CGC CCC AGG CAC CA-3′ |
| hALK2 forward primer | 5′-GCA TTC CCA GAG CAC CAA TC -3′ |
| hALK2 reverse primer | 5′-CTG TGA GTC TTG CGG ATG GA -3′ |
| hAct-RIIA forward primer | 5′-ATG GCT AGA GGA TTG GCA TAT T -3′ |
| hAct-RIIA reverse primer | 5′-TCT TCA ATG GTT TCA CAG AGC A -3′ |
| hAct-RIIB forward primer | 5′-ACA GGT AGG CAC GAG ACG GT -3′ |
| hAct-RIIB reverse primer | 5′-GTA GTG CCG TTG ACC GAC CT -3′ |
| hALK3 forward primer | 5′-GCA TAA CTA ATG GAC ATT GCT -3′ |
| hALK3 reverse primer | 5′-TAG AGT TTC TCC TCC GAT GG -3′ |
| hALK6 forward primer | 5′-GCA GCA CAG ACG GAT ATT GT -3′ |
| hALK6 reverse primer | 5′-TTT CAT GCC TCA TCA ACA CT -3′ |
| hBMP-RII forward primer | 5′-ACG GGA GAG AAG ACG AGC CT -3′ |
| hBMP-RII reverse primer | 5′-CTA GAT CAA GAG AGG GTT CG -3′ |
| hβ actin forward primer | 5′-GGC ATC GTG ATG GAC TCC -3′ |
| hβ actin reverse primer | 5′-GCT GGA AGG TGG ACA GCG -3′ |
Act, activin; ALK, activin receptor-like kinase; BMP, bone morphogenetic protein; h, human; iNOS, inducible nitric oxide synthase; m, murine; R, receptor; TNF, tumour necrosis factor.
Transient transfection of cells with BMP receptors
For transient transfections, RAW 264·7 cells were plated at 1 × 105 cells/well in a six-well plate. Lipofectamine (Invitrogen) at 1 μl/ml was then used to transfect cells with 1 μg/ml of the BMP receptors BRE-luc, activin receptor-like kinase (ALK2)-HA and ALK3-HA.
Short hairpin RNA (shRNA) vector construction and expression
shRNA sequences targeted to type II BMP receptors were: BMP-RII: forward primer CCG GGC AGT CCA TTC TAA ATC TAG CCT CGA GGC TAG ATT TAG AAT GGA CTG CTT TTT G; reverse primer AAT TCA AAA AGC AGT CCA TTC TAA ATC TAG CCT CGA GGC TAG ATT TAG AAT GGA CTG C; activin (Act)-RIIA: forward primer CCG GGC CAT TGC AGC TGT TAG AAG TCT CGA GAC TTC TAA CAG CTG CAA TGG CTT TTT G; reverse primer AAT TCA AAA AGC CAT TGC AGC TGT TAG AAG TCT CGA GAC TTC TAA CAG CTG CAA TGG C; Act-RIIB: forward primer CCG GGC ATC TAC TAC AAC GCC AAC TCT CGA GAG TTG GCG TTG TAG TAG ATG CTT TTT G; reverse primer AAT TCA AAA AGC ATC TAC TAC AAC GCC AAC TCT CGA GAG TTG GCG TTG TAG TAG ATG C. Oligonucleotides containing the specific sequences were cloned into the pLKO.1-puro lentiviral vector.12 Subsequently, all shRNA constructs were transformed using the ViraPower T-Rex Lentiviral System (Invitrogen). RAW 264·7 cells were infected with the resulting lentiviruses and stable cell lines were generated. Knockdown of the target gene was confirmed by RT-PCR.
Statistical analysis
For all analyses, Student t-tests were performed. A P-value of < 0·05 was considered to indicate significant differences between data sets.
Results
Effect of BMP-6 on RAW 246·7 cells
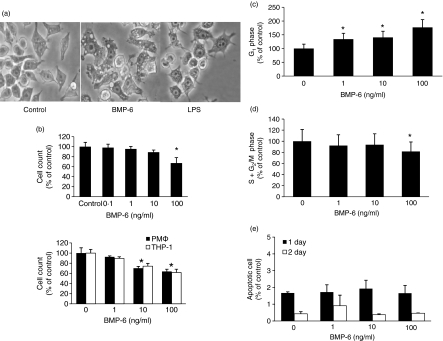
To investigate the effect of BMP-6 on macrophages, we used the murine macrophage cell line RAW 264·7. The results demonstrated that BMP-6 dramatically altered the cellular morphology (Fig. 1a). The morphology of cells treated with BMP-6 at 10 ng/ml was similar to that of macrophages activated with lipopolysaccharide (LPS). Simultaneously, there was suppression of cellular proliferation in a concentration-dependent manner (Fig. 1b, top panel). At 100 ng/ml, BMP-6 decreased the relative cell count of RAW 264·7 cells to 69·3% compared with the control after 96 hr. BMP-6 also inhibited proliferation of primary murine macrophages and the human monocyte cell line THP-1. Next, we performed cell cycle analysis with flow cytometry after treatment with BMP-6. BMP-6 increased the fraction of cells in the G1 phase and decreased that of cells in the S + G2M phase (Fig. 1c,d). Simultaneously, BMP-6 had no significant effect on the percentage of apoptotic cells (Fig. 1e).
Figure 1.
Effects of bone morphogenetic protein-6 (BMP-6) on RAW 264·7 cells. (a) BMP-6 dramatically altered the morphology of RAW 264·7 cells, similarly to lipopolysaccharide (LPS) treatment. (b) Cellular proliferation was determined by haemocytometer. Treatment with BMP-6 suppressed the proliferation of RAW 264·7 cells in a concentration-dependent manner (*P ≤ 0·05) (top panel). BMP-6 also inhibited cellular proliferation of primary murine peritoneal macrophages and the human monocyte/macrophage cell line THP-1 in a concentration-dependent manner (*P < 0·05) (bottom panel). Bars represent the average cell count ± standard deviation (SD). (c, d) Cell cycle analysis by flow cytometry showed an increased fraction of cells in the G1 phase and a decreased fraction in the S and G2/M phases after BMP-6 treatment (*P ≤ 0·05). Bars represent the average cell count ± SD. (e) RAW 264·7 cells were treated with 100 ng of BMP-6 for 24 and 48 hr and the apoptotic population was measured using fluorescence-activated cell sorting (FACS). PMφ, peritoneal macrophage φ.
Effect of BMP-6 on gene transcription activity and BMP receptor expression in RAW 264·7 cells
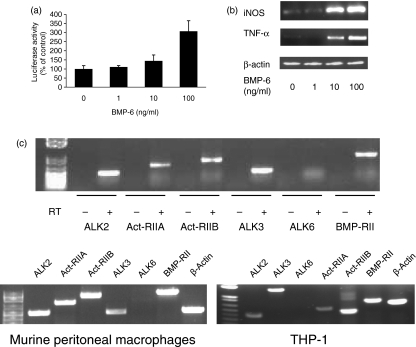
As an independent and complementary assay to demonstrate that RAW 264·7 cells are sensitive to BMP-6, cells were transiently transfected with a luciferase reporter construct containing the BMP response element (BRE-luc). When transfected cells were treated with BMP-6, luciferase activity increased in a concentration-dependent manner, indicating that BMP-6 signalling is active in RAW 264·7 cells (Fig. 2a). To determine the effect of BMP-6 on the function of macrophages, we next investigated markers that are expressed by activated macrophages, namely iNOS and TNF-α, using semiquantitative RT-PCR. The results demonstrated that BMP-6 induced the expression of iNOS and TNF-α in a concentration-dependent manner after 48 hr (Fig. 2b).
Figure 2.
Bone morphogenetic protein-6 (BMP-6) induced production of inducible nitric oxide synthase (iNOS) and tumour necrosis factor (TNF)-α. The expression of BMP receptors was increased in RAW 264·7 cells. (a) BMP response element (BRE) activity was increased by BMP treatment. Cells were transfected with the reporter construct BRE-luciferase (luc). As the concentration of BMP-6 increased, the levels of luciferase activity increased (*P ≤ 0·05). Bars represent the average cell count ± standard deviation (SD). (b) Effects of BMP-6 on the expression of iNOS and TNF-α in RAW 264·7 cells. Reverse transcription–polymerase chain reaction (RT-PCR) was carried out to determine the effect of BMP-6 on the expression of iNOS and TNF-α. The expression of iNOS and TNF-α increased with BMP-6 exposure in a dose-dependent manner. (c) In RT-PCR analysis for type I and type II BMP receptors, all BMP receptors with the exception of activin receptor-like kinase 6 (ALK6) were found to be highly expressed in RAW 264·7 cells (top panel). A similar expression pattern was found in primary murine peritoneal macrophages and the human monocyte/macrophage cell line THP-1 (bottom panel).
To initiate BMP signalling, a heteromeric complex composed of type I and type II receptors is required. There are three BMP type I receptors (Act-RIA/ALK2, BMP-RIA/ALK3 and ALK6/ALK6) and three BMP type II receptors (BMP-RII, Act-RIIA and Act-RIIB).13 Using RT-PCR, it was demonstrated that RAW 264·7 cells expressed detectable levels of all three type II BMP receptors, while, of the type I receptors, only ALK2 and ALK3 were detected (Fig. 2c, top panel). A similar BMP receptor profile was found in primary murine macrophages and the human monocyte cell line THP-1 (Fig. 2c, bottom panel). The authenticity of the RT-PCR products was confirmed by sequencing (data not shown).
BMP-6 type I receptor functions in RAW 264·7 cells
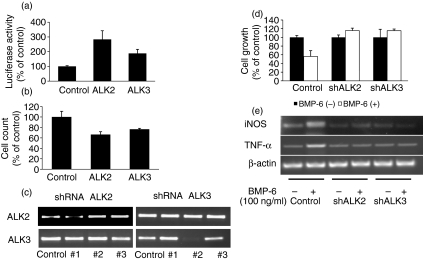
To determine which type I receptors are required in BMP-6 signalling, cells were co-transfected with constitutively active ALK2 or ALK3 (ALK2-CA or ALK3-CA, respectively) and BRE-luc. The results demonstrated that, 24 hr after the transfection of cells with either ALK2-CA or ALK3-CA, there was a significant increase in the level of luciferase activity (Fig. 3a). With respect to cellular proliferation, again both ALK2-CA and ALK3-CA over-expression led to a significant decrease in cell count 24 hr after transfection (Fig. 3b). To allow the results to be adjusted for variation in transfection efficiency, cells were co-transfected with the CMV-LacZ plasmid. As a complementary assay, cells were infected with lentivirus containing an shRNA sequence targeting ALK2 and ALK3 (Fig. 3c). The results demonstrated that shRNA of both ALK2 and ALK3 abolished BMP-6-induced growth inhibition in RAW 264·7 cells (Fig. 3d). Also, induction of iNOS and TNF-α by BMP-6 was blocked by shRNA of ALK2 and ALK3 (Fig. 3e). These results suggest that ALK2 and ALK3 are functional BMP-6 receptors in peritoneal macrophages.
Figure 3.
Functional type I bone morphogenetic protein (BMP) receptor in RAW 264·7 cells. (a) Cells were transfected with constitutively active activin receptor-like kinase 2 (ALK2) or ALK3 (ALK2-CA and ALK3-CA, respectively) along with BMP response element-luciferase (BRE-luc). Compared with the control (in which cells were transfected with the empty expression vector pcDAN3·1), there was a significant increase in luciferase activity level when cells were transfected with either ALK2-CA or ALK3-CA. (b) An experiment identical to that in (a) was carried out and the effect on cell count was determined 48 hr after transfection. Compared with the control (pcDAN3·1), cells transfected with either ALK2-CA or ALK3-CA demonstrated a significant decrease in cell count. (c) RAW 264·7 cells were transfected with a lentivirus-based short hairpin RNA (shRNA) construct, and expression of type I receptors by these cells was detected by RT-PCR. The mRNA level was decreased for clone 1 of ALK2 and clone 2 of ALK3. Con, control; #1, #2 and #3, clones 1, 2 and 3, respectively. (d) Effect of the shRNA construct on cell growth. The growth inhibitory effect of BMP-6 was abolished by shRNA. (e) Induction of inducible nitric oxide synthase (iNOS) and tumour necrosis factor (TNF)-α in shRNA-transfected cells. In ALK2- and shALK3-transfected cells, BMP-6 could not induce iNOS and TNF-α.
BMP-6 type II receptor functions in RAW 264·7 cells
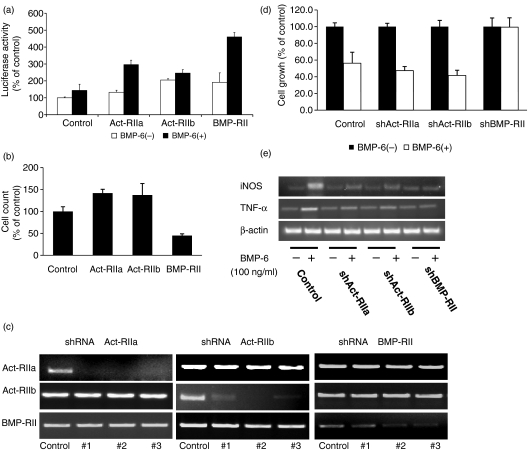
Type II BMP receptors are constitutively active serine/threonine kinases. Ligand binding results in cross-phosphorylation and activation of type I receptors, which in turn transduce the BMP signal. To determine the functional type II receptor for BMP-6 in RAW 264·7 cells, the cells were co-transfected with each type II receptor and BRE-luc. As shown in Fig. 4a, cells transfected with BMP-RII exhibited the highest level of luciferase activity following treatment with BMP-6 at 100 ng/ml. Transfection with Act-RIIA also increased the level of luciferase activity to a statistically significant but more modest level. Next, the effect of type II receptor over-expression on cellular proliferation was determined after treating cells with BMP-6 at 100 ng/ml for 3 days. Transfection with the reporter construct CMV-LacZ gave similar transfection rates for all three type II receptors. The results demonstrated that only transfection with BMP-RII led to a significant decrease in cell count (Fig. 4b). shRNA constructs of type II receptors were also tested to confirm the function of type II receptors. Cells were infected with lentivirus containing an shRNA sequence targeting each of the type II BMP receptors (Fig. 4c). Only the shRNA construct of BMP-RII significantly blocked BMP-6-induced cellular inhibition in RAW 264·7 cells (Fig. 4d). BMP-RII completely abolished iNOS and TNF-α induction, but Act-RIIa and Act-RIIb partially decreased the mRNA level (Fig. 4e). Thus, BMP-RII is mainly responsible for the effect of BMP-6 in the RAW 264·7 macrophage cell line.
Figure 4.
Functional type II bone morphogenetic protein (BMP) receptor in RAW 264·7 cells. (a) Cells were transfected with each of the three type II BMP receptors along with the reporter plasmid BMP response element-luciferase (BRE-luc). Following treatment with BMP-6, all three type II receptors increased the luciferase activity in transfected cells. However, cells transfected with BMP-RII demonstrated the highest level of luciferase activity. (b) Following transfection with each of the three type II BMP receptors, cells were treated with BMP-6 at 100 ng/ml. At the end of a 48-hr period, the effect on cell count was determined. As expected, only the proliferation of cells transfected with BMP-RII was inhibited with BMP-6. Cells transfected with the empty expression vector pcDAN3·1 and treated with BMP-6 were used as the control. To control for varying transfection efficiency, cells were co-transfected with the reporter construct CMV-LacZ. (c) RAW 264·7 cells were transfected with lentiviral constructs containing short hairpin RNA (shRNA) targeting each of the three BMP type II receptors. Three sequences were tested for each of the type II BMP receptors. The effect on the levels of expression of the target was determined by reverse transcription–polymerase chain reaction (RT-PCR). Con, control; #1, #2 and #3, clones 1, 2 and 3, respectively. (d) RAW 264·7 cells were transfected with lentivirus containing a shRNA sequence targeting each of the type II BMP receptors. Subsequently, cells were treated with 100 ng/ml of BMP-6 for 48 hr. The results demonstrated that BMP-6-induced growth inhibition was reversed only when the expression of BMP-RII was knocked down. (e) Induction of iNOS and TNF-α was investigated in shRNA transfected cells. ShBMP-RII completely blocked induction of inducible nitric oxide synthase (iNOS) and tumour necrosis factor (TNF)-α. However, the shRNA construct of activin (Act)-RIIa and Act-RIIb only partially blocked the induction of iNOS and TNF-α.
Discussion
In the present study, it was shown that BMP-6 induced morphological changes in association with decreased proliferation and induction of pro-inflammatory iNOS and TNF-α in peritoneal macrophages. Further investigation revealed that these effects of BMP-6 in macrophages were mediated through BMP-RII and ALK2/3. Taken together, these observations demonstrate that BMP-6 is a potential regulator of macrophages.
Previously, it has been reported that BMP-6 inhibits the proliferation of CD4+ T and B cells. Consistent with these previous findings, in the present study, the cell count of macrophages decreased after treatment with BMP-6. This negative effect on macrophage cell count was associated with cell cycle arrest, as indicated by an increased fraction of cells in the G1 phase and a decreased fraction of cells in the S2/M phase; no effect on apoptosis was seen. The mechanism underlying this apparent cell cycle arrest in macrophages is uncertain. However, BMP-2 has been shown to induce cell cycle arrest in the G1 phase via p21/WAF1/CIP1 in breast cancer, gastric cancer and aortic smooth muscle cells.14–16 As p21/WAF1/CIP1 inhibits CDK activity by binding to cyclin–CDK complexes in the G1 phase, BMP-6 may also inhibit the cell cycle via p21/WAF1/CIP1.
As many investigators have reported a negative effect of BMP-6 in a number of different systems,5,11,17 the inhibitory effect of BMP-6 on macrophages is not surprising. However, the negative effect of BMP-6 on the proliferation of macrophages was accompanied by induction of pro-inflammatory iNOS and the cytokine TNF-α. Moreover, morphological changes induced by BMP-6 were similar to those induced by LPS. Based on these observations, we propose that BMP-6 activates rather than inhibits macrophages. Indeed, the general phenotype of macrophages in response to BMP-6 reported herein is similar to that of macrophages exposed to LPS.18
The observation that BMP-6 modulates the behaviour of macrophages is in direct contrast to the reported effects of the prototype member of the BMP family, TGF-β. TGF-β has a profound effect on immune suppression.19 More specifically, TGF-β regulates both myeloid and lymphoid cells to suppress the immune response20 via growth inhibition, induction of apoptosis, inhibition of differentiation, and inhibition of pro-inflammatory iNOS21 and the cytokine TNF-α.22 In essence, BMP-6 in macrophages appears to counteract TGF-β. Currently, the mechanism underlying the differing effects of BMP-6 and TGF-β in macrophages remains unclear. Nevertheless, as the BMP-6 and TGF-β signalling pathways share Co-Smad but not R-Smads, we propose that R-Smads determine the target gene expression and fate of macrophages. Further investigation is underway in our laboratory to test this hypothesis.
The precise mechanism underlying the effect of BMP-6 on macrophages is unclear. RT-PCR as well as immunoblot analysis demonstrated that macrophages express ALK2 and ALK3 but not ALK6. Over-expression and knock-down studies revealed that both ALK2 and ALK3 are functional type I receptors involved in BMP-6 signalling in macrophages. With respect to type II receptors, macrophages express all three known receptors of this type – BMP-RII, Act-RIIA and Act-RIIB. Over-expression of BMP-RII, but not Act-RIIA and Act-RIIB, increased sensitivity to BMP-6-induced inhibition of cellular proliferation. In the context of iNOS expression and TNF-α, however, all three type II receptors appear to signal in response to BMP-6 treatment. Thus, varying effects of BMP-6 may be mediated by different type II receptors. Currently, the precise combination of intracellular BMP-6 signalling molecules – Smads – that are activated by each of the type II receptors in macrophages is under investigation.
In conclusion, we report that BMP-6 activates macrophages. This activation of macrophages by BMP-6 is in direct contrast to the inhibition of macrophages by the closely related protein TGF-β. In the future, the role of BMP-6 in local inflammation will be explored.
Acknowledgments
This work was supported in part by a generous grant from the Tanzman Foundation.
Glossary
Abbreviations:
- ALK
activin receptor-like kinase
- BMP
bone morphogenetic protein
- BRE
BMP response element
References
- 1.Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA. 1988;85:9484–8. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 3.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 4.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 5.Sivertsen EA, Huse K, Hystad ME, Kersten C, Smeland EB, Myklebust JH. Inhibitory effects and target genes of bone morphogenetic protein 6 in Jurkat TAg cells. Eur J Immunol. 2007;37:2937–48. doi: 10.1002/eji.200636759. [DOI] [PubMed] [Google Scholar]
- 6.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Lin SC, Huang Y, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruco LP, Meltzer MS. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978;121:2035–42. [PubMed] [Google Scholar]
- 9.Henson PM, Riches DW. Modulation of macrophage maturation by cytokines and lipid mediators: a potential role in resolution of pulmonary inflammation. Ann N Y Acad Sci. 1994;725:298–308. doi: 10.1111/j.1749-6632.1994.tb39813.x. [DOI] [PubMed] [Google Scholar]
- 10.Yang N, Isbel NM, Nikolic-Paterson DJ, Li Y, Ye R, Atkins RC, Lan HY. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 1998;54:143–51. doi: 10.1046/j.1523-1755.1998.00978.x. [DOI] [PubMed] [Google Scholar]
- 11.Kersten C, Sivertsen EA, Hystad ME, Forfang L, Smeland EB, Myklebust JH. BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1. BMC Immunol. 2005;6 doi: 10.1186/1471-2172-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. Rna. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–9. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Wen XZ, Miyake S, Akiyama Y, Yuasa Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316:100–6. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Wong GA, Tang V, El-Sabeawy F, Weiss RH. BMP-2 inhibits proliferation of human aortic smooth muscle cells via p21Cip1/Waf1. Am J Physiol Endocrinol Metab. 2003;284:E972–9. doi: 10.1152/ajpendo.00385.2002. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh Choudhury G, Kim YS, Simon M, Wozney J, Harris S, Ghosh-Choudhury N, Abboud HE. Bone morphogenetic protein 2 inhibits platelet-derived growth factor-induced c-fos gene transcription and DNA synthesis in mesangial cells. Involvement of mitogen-activated protein kinase. J Biol Chem. 1999;274:10897–902. doi: 10.1074/jbc.274.16.10897. [DOI] [PubMed] [Google Scholar]
- 17.Kim IY, Lee DH, Lee DK, et al. Decreased expression of bone morphogenetic protein (BMP) receptor type II correlates with insensitivity to BMP-6 in human renal cell carcinoma cells. Clin Cancer Res. 2003;1(16 Pt 1):6046–51. [PubMed] [Google Scholar]
- 18.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–94. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 20.Wahl SM, Wen J, Moutsopoulos N. TGF-beta: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–27. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsunawaki S, Sporn M, Nathan C. Comparison of transforming growth factor-beta and a macrophage- deactivating polypeptide from tumor cells. Differences in antigenicity and mechanism of action. J Immunol. 1989;142:3462–8. [PubMed] [Google Scholar]
- 22.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]