Abstract
Radiation therapy affects the immune system. In addition to killing radiosensitive immune cells, it can induce functional changes in those cells that survive. Our recent studies showed that the exposure of dendritic cells (DCs) to radiation in vitro influences their ability to present tumour antigen in vivo. Here we show that local radiation therapy of B16 melanoma tumours inhibits the development of systemic immunity to the melanoma antigen MART-1. This inhibition could not be overcome by intratumoral injection of DCs expressing human MART-1 after radiation therapy, suggesting that a form of immune suppression might have developed. On the other hand, injection of MART-expressing DCs prior to tumour irradiation was able to prevent inhibition from developing. These results suggest that local radiation therapy may block the generation of immunity under some circumstances and that strategies may be required to prevent this and allow radiation-induced cell death to translate fully into the development of systemic immunity.
Keywords: antigen presentation, dendritic cells, tumour antigens
Introduction
It is widely accepted that even local radiation therapy alters the balance of circulating immune cells and this is often ascribed to the depletion of radiosensitive subsets of cells.1–3 However, recently, attention has been focused on radiation-induced functional changes in immune cells and on approaches to use radiation as an immunological adjuvant to enhance tumour control. Radiation is more than simply a silent killer of immune cells. It affects various aspects of the immune system. It induces the expression of a myriad of cytokines, including tumour necrosis factor-α (TNF-α), interleukin (IL)-1α, IL-1β, IL-6, IL-8 and IL-10,4–8 up-regulates the expression of major histocompatibility complex (MHC) class I9 and the costimulatory molecules CD8010,11 and CD8612, and modulates the function of dendritic cells (DCs) to inhibit endogenous antigen processing while enhancing cross-presentation.13
Indeed, more recent studies have taken this knowledge a step further and revealed the value that might accrue from combining radiation therapy with immunotherapy in cancer treatment. In experimental models, DC-based vaccination against tumour-associated antigens appears to be superior when given with local tumour irradiation.14–17 Boosting tumour immunity with gene therapy approaches showed a similar benefit from combination with radiation therapy.18–20 However, whether local radiation therapy per se promotes or inhibits systemic tumour immunity is less clear, although understanding this may be critical for the development of optimal strategies aimed at combining these two therapies.
In this study, we used a B16 murine melanoma tumour model to investigate the effect of local irradiation on systemic tumour immunity. B16 tumours express the murine homologue of the human melanocyte lineage-specific tumour antigen, MART-1.21 We were therefore able to use this to monitor tumour-specific immune responses. The weak immune response that is normally generated in mice bearing B16 tumours was suppressed following local irradiation and rendered these mice unable to respond to a subsequent injection of intratumoral MART-1 gene-modified DCs [adenovirus MART-1 transduced DC (AdVMART-1/DC)]. This suggests that local radiation therapy may, on occasion, not only decrease systemic tumour immunity but also block its generation in response to immunotherapy. Vaccination prior to tumour irradiation, however, could prevent radiation-induced inhibition, indicating that the timing of immunotherapy with respect to radiation therapy may be critical for a favourable outcome.
Materials and methods
Mice and cell lines
Six- to 8-week-old C57BL/6 mice were bred and maintained in the defined-flora Association for Assessment and Accreditation of Laboratory Animal Care-accredited Animal Facility of the Department of Radiation Oncology, UCLA. All experimental protocols were approved by the UCLA Institutional Animal Care and Use Committee, and the care of animals was carried out according to local and national guidelines. The C57BL/6 B16 melanoma and EL4 lymphoma cell lines were obtained from the ATCC (Manassas, VA) and maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) (Mediatech, Herndon, VA) with 10% fetal bovine serum (FBS) (Sigma, St Louis, MO) and 1% antibiotic-antimycotic solution (Mediatech). The EL4(MART-1) cell line, an EL4 transfectant carrying the MART-1 cDNA and the neomycin-resistance gene, was generated as described previously22 and maintained in complete RPMI-1640 (Mediatech) with 0·5 mg/ml of G418. The 293 human embryonic renal cells (Qbiogene Inc., Carlsbad, CA) were used for amplification of adenoviral seed stocks.
Tumour implantation
Tumours were generated in vivo from 5 × 105 B16 tumour cells injected subcutaneously (s.c.) into the right thigh of mice. When the tumour size reached approximately 5–6 mm in diameter, tumours were irradiated with 0 or 10 Gy.
Irradiation
Mice were anaesthetized with an intraperioneal (i.p.) injection of ketamine/xylazine (80 mg/4 mg/kg of mouse body weight; pentobarbital) and positioned in a Lucite jig with lead shielding the body and only the leg bearing the tumour exposed for radiation treatment in the Gammacell 40 irradiator (Cs-137 source; Atomic Energy of Canada Ltd, Ottawa, Canada) at a dose rate of approximately 67 cGy/min. Irradiation of cells was performed using the MARK-1-30 irradiator (Cs-137 source, J.L. Shepherd & Associates, San Fernando, CA) at a dose rate of 4·5 Gy/min.
Generation of bone marrow-derived DCs
Murine DCs were generated from bone marrow cells as described previously.13,22 Briefly, bone marrow from femurs was cultured overnight in RPMI-1640 containing 2% FBS and 1% antibiotics. Non-adherent cells were resuspended in complete RPMI-1640 supplemented with 2 ng/ml of murine granulocyte–macrophage colony-stimulating factor (GM-CSF) and 10 ng/ml of murine IL-4 (Invitrogen, Carlsbad, CA) at 1–2 × 106 cells/ml. After 3 days of culture with cytokines, 80–90% of the medium was replaced with fresh medium containing GM-CSF and IL-4. Loosely adherent cells were harvested and used for experiments after 8 days in culture.
Adenovirus transduction of DCs
The E1-deleted replication-defective adenoviral vector containing human MART-1 (AdVMART-1)22 was amplified in 293 cells and then purified by centrifugation for 2 hr at 125 000 g in a 30–60% sucrose gradient. The virus titre was determined using the tissue infectious dose 50 (TCID50) method, as described in Quantum (Qbiogene Inc.). Irradiated (10 Gy) or unirradiated DCs were transduced with AdVMART-1 at a multiplicity of infection (MOI) of 100, washed and injected (5 × 105 cells) s.c. in the top of the inner leg of each mouse in a total volume of 100 μl of phosphate-buffered saline (PBS).13 In some experiments, mice were re-immunized 10 days after the first injection. Splenocytes were harvested 7–14 days after immunization and the enzyme-linked immunospot (ELISPOT) assay was used to measure MART-specific responses.
ELISPOT assay
The expression of interferon-γ (IFN-γ) and IL-4 by individual lymphocytes was used to assess MART-1-specific immune responses in an ELISPOT assay.13 Briefly, splenocytes were harvested on the indicated day after immunization, depleted of red blood cells in ammonium chloride-buffered (ACK) solution [0·83% (w/v) NH4Cl, 0·14% (w/v) KHCO3, 0·002% Na2EDTA, pH 7·3] and restimulated with heavily irradiated (50 Gy) EL4 or EL4(MART-1) cells in the presence of 10 U/ml of human IL-2 at 37° for 48 hr. Restimulated cells were added to anti-IFN-γ or anti-IL-4 antibody-coated MultiScreen-HA plates (Millipore, Bedford, MA) for a further 24-hr period. Released cytokines were then detected by adding biotinylated anti-IFN-γ or anti-IL-4 and horseradish peroxidase avidin D (1 : 2000 dilution; Vector Laboratories, Burlingame, CA). Red spots were developed by adding 0·4 mg/ml of 3-amino-9-ethyl-carbazole (AEC tablets; Sigma) in 0·05 m sodium acetate buffer (pH 5·0) and 0·012% hydrogen peroxide (Fisher Scientific, Pittsburgh, PA). Spots were counted using an ImmunoSpot Image Analyzer (Cellular Technology Ltd, Cleveland, OH).
Flow cytometric analysis
Splenocytes were obtained as described in the previous section and tumours were harvested and digested in 1 mg/ml of collagenase D (Roche, Nutley, NJ) and 227 U/ml of DNase I, type IV (Sigma), at 37° for 1 hr. Cells were stained with relevant fluorochrome-labelled mouse antibodies and analyzed using a FACSCalibur flow cytometry system (Becton Dickson, Mountain View, CA). The following monoclonal antibodies were used: phycoerythrin (PE)–anti-CD8a (CALTAG Laboratory/Invitrogen, Carlsbad, CA), fluorescein isothiocyanate (FITC)–anti-(pan-NK) cells (CD49b) (eBioscience, San Diego, CA), FITC–anti-CD4, FITC–anti-I-Ab, FITC–anti-CD11b (Mac-1) and PE–anti-CD11c (Pharmingen, San Diego, CA). To identify T regulatory (Treg), cells were stained using the Foxp3 staining buffer set (eBioscience). According to the manufacturer’s instructions, cells were first labelled with PE–Cy5–anti-CD4 and PE–anti-CD25 (Pharmingen), fixed overnight at 4°, washed twice with permeabilization buffer and then stained with FITC–anti-Foxp3 (eBioscience).
In vivo migration
Bone marrow-derived DCs were treated with 100 ng/ml of lipopolysaccharide (LPS) (Sigma) in cytokine-containing medium for 24 hr 1 day before harvest and labelled with the green fluorescent dye, PKH2 (Sigma), according to the manufacturer’s protocol. Briefly, DCs were washed three times with PBS to remove serum, resuspended in diluent A and 2 × 107 cells were added to 2 ml of PKH2-staining solution for 5 min at 20°. The reaction was stopped by adding complete medium containing 10% serum, followed by extensive washing with PBS. PKH2-stained DCs were irradiated, and 5 × 105 cells in 20 μl of PBS were injected s.c. into the left hind footpad of C57BL/6 mice. Regional lymph nodes (LNs) (popliteal and inguinal) were removed 48 hr later and single-cell suspensions with 1 mg/ml of collagenase D (Roche) and 227 U/ml of DNase I (Sigma) in PBS at 37° for 1 hr with continuous magnetic stirring. Flow cytometry was performed to detect fluorescent cells.
Statistical analysis
All P-values were obtained using the two-tailed Student’s t-test.
Results
Irradiated DCs are immunosuppressive
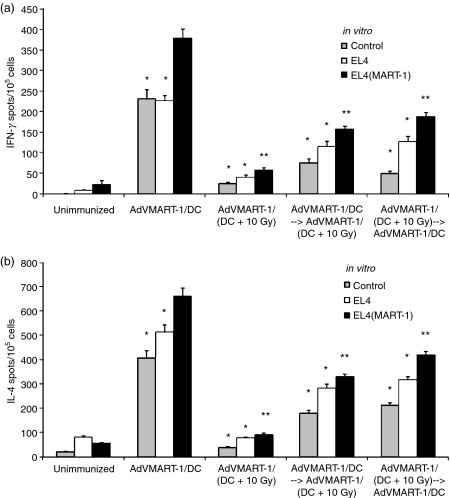
Our previous study demonstrated that in vitro irradiation of DCs could inhibit their ability to process MART-1 antigen through the endogenous pathway.13 In the present study we investigated whether DCs irradiated under the same conditions are immunologically ineffective or actively switch off immunity to MART-1, inducing a state of immune suppression. To test this, C57BL/6 mice were vaccinated with 5 × 105 irradiated (10 Gy) or non-irradiated DCs that had been transduced with AdVMART-1 and were vaccinated again 10 days later with the same vaccine or with the vaccine (irradiated or non-irradiated) that they did not receive the first time. One week after the last immunization, MART-1-specific IFN-γ and IL-4 T-cell responses were assessed using ELISPOT assays. Mice immunized with irradiated DCs alone showed the lowest numbers of IFN-γ- and IL-4-secreting cells. When irradiated DCs were given before or after non-irradiated DCs, both IFN-γ (Fig. 1a) and IL-4 (Fig. 1b) responses were suppressed to similar extents, suggesting that irradiated AdVMART-1/DCs are able to switch off MART-1-specific immunity and that both T helper 1 (Th1) and T helper 2 (Th2) T-cell subset responses are equally affected. This also confirms that the loss of DC function is an active process and is not caused by cell death.
Figure 1.
Radiation decreases immunity generated by adenovirus encoding MART-1 cDNA transduced dendritic cells (AdVMART-1/DC). C57BL/6 mice were treated with 5 × 105 irradiated (10 Gy) or non-irradiated AdVMART-1/DC. A second immunization was performed 10 days later with 5 × 105 irradiated or non-irradiated AdVMART-1/DC. One week after the last immunization, MART-1-specific (a) interferon-γ (IFN-γ) and (b) interleukin-4 (IL-4) responses were assessed using enzyme-linked immunospot (ELISPOT) assays. Splenocytes were restimulated for 48 hr with EL4(MART-1) cells (black bars) or EL4 cells (white bars), or were not restimulated (control; grey bars). Mice immunized with AdVMART-1/DC that had been irradiated showed a reduced number of IFN-γ- and IL-4-expressing cells compared to those injected with non-irradiated dendritic cells. Furthermore, irradiated AdVMART-1/DC, whether given before or after AdVMART-1/DC, decreased their ability to generate responses. Results are shown as mean ± 1 standard error of the mean (SEM) of triplicate data of one representative of three independent experiments [*P< 0·05 compared with the EL4(MART-1)-restimulated group; **P< 0·0001 as compared with the AdVMART-1/DC-injected group].
Local irradiation suppresses B16 tumour immunity
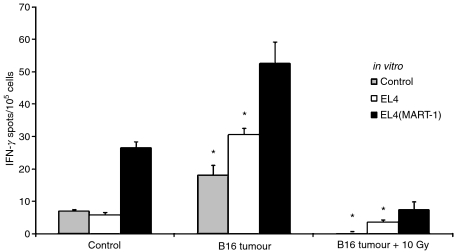
Radiation-induced inhibition of antigen processing/presentation by DC may be part of a general mechanism that prevents the generation of autoimmunity following damage to self-tissue. In a tumour microenvironment, it seems plausible that locally irradiated DCs could switch off tumour immunity. To test this, we irradiated (with 10 Gy) 5–6-mm-diameter B16 tumours grown in C57BL/6 mice and assessed MART-1-specific responses in the spleen 7 days later using an ELISPOT assay. In repeated experiments, spleens from untreated tumour-bearing mice showed reproducible, but relatively small, increases in the number of MART-specific IFN-γ-secreting cells, indicating that the tumour was weakly immunogenic (Fig. 2). Interestingly, mice bearing locally irradiated tumours had abrogated splenic immune responses, as assessed by IFN-γ expression.
Figure 2.
Radiation suppresses B16 anti-tumour immunity. C57BL/6 mice were implanted with viable B16 cells and irradiated with 0 or 10 Gy when the tumour size reached approximately 5–6 mm in diameter. One week after the treatment, the number of splenic interferon-γ (IFN-γ)-producing lymphocytes was detected using enzyme-linked immunospot (ELISPOT) assays 48 hr after restimulation with EL4(MART-1) cells (black bars) or EL4 cells (white bars), or without restimulation (control; grey bars). Three mice were used in each treatment group. Mice without any treatments served as controls. The results shown are the mean ± 1 standard error of the mean (SEM) of triplicate data from one representative of three experiments (*P< 0·05 compared with the EL4(MART-1)-restimulated group).
Tumour irradiation differentially affects a subpopulation of immune cells
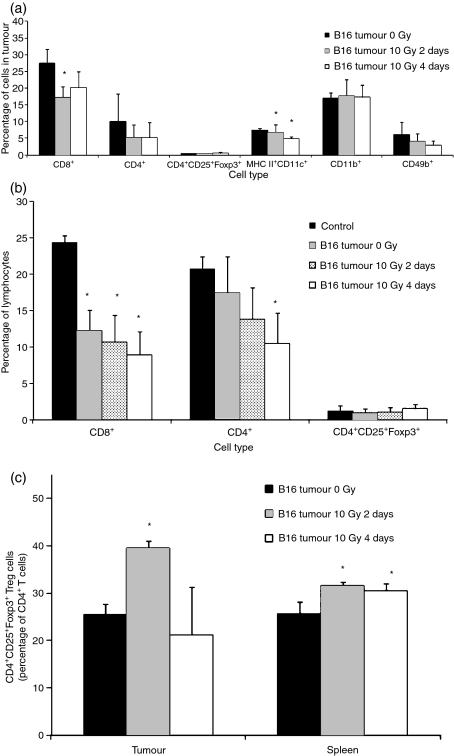
Flow cytometry was used to determine if alterations in immune-cell subsets in the spleen and in the tumour might account for the decreased response we observed after local tumour radiation therapy. B16 tumours and splenocytes were harvested from tumour-irradiated mice 2 or 4 days after treatment. CD4+, CD8+, CD11b+, CD49b+, MHC II+ CD11c+ and CD4+ CD25+ Foxp3+ subsets were enumerated (Fig. 3). In the tumour, the numbers of CD8+ T cells decreased by day 2 after 10 Gy irradiation and remained low on day 4 in three repeated experiments (Fig. 3a). The number of MHC II+ CD11c+ cells decreased on day 2 and continued to decrease with time. The number of CD11b+ cells was not reproducibly altered. The presence of tumour decreased the number of splenic CD8+ cells within the lymphocyte gate, and local tumour irradiation reproducibly decreased the number of splenic CD8+ cells further at 2 and 4 days (Fig. 3b). There was, however, a compensatory increase in the number of ‘null’ cells. The nature of these cells is still under investigation, but they were CD11b−, CD11c−/MHC class II− (not shown), in addition to lacking CD4 and CD8. Non-lymphocyte representation (CD11b+ and MHC II+ CD11c+) was not affected (data not shown). CD4+ CD25+ Foxp3+ Treg cell numbers in both tumour and spleen were generally unaffected by the radiation treatment. However, with CD8+ and CD4+ effector T-cell numbers decreasing, the fraction of CD4+ T cells that were CD4+ CD25+ Foxp3+ Tregs increased dramatically in both spleen and tumour following radiation (at day 2) (Fig. 3c).
Figure 3.
Flow cytometric analyses of cell populations in B16 tumour-bearing mice. C57BL/6 mice with 5–6-mm-diameter tumours were irradiated with 0 or 10 Gy. Cells were harvested from tumour (Fig. 3a) and spleens (Fig. 3b) and, either 2 or 4 days after the radiation treatment, were stained with antibodies to various immunecell subset markers and analyzed by flow cytometry. Mice without tumours are used as a control in Fig. 3b. Data in Fig. 3a,b represent the subpopulation in total tumour cells and in gated splenic lymphocytes respectively. Figure 3(c) shows CD4+ CD25+ Foxp3+ T-regulatory cells as a fraction of CD4+ cells in both tumour lymphocytes and splenic lymphocytes. Results are shown as mean ± 1 standard error of the mean (SEM) of three to five experiments. (*P< 0·05 as compared with the 0 Gy group in Fig. 3a,c and with the control group in Fig. 3b).
Combination of radiation treatment and DC vaccination
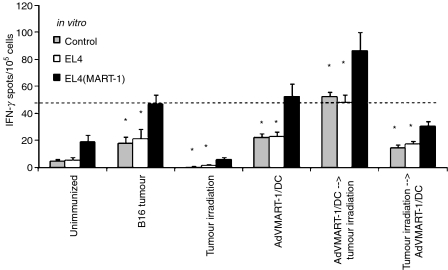
In order to test if irradiated DC induced-suppression could be overcome by combination treatment of radiation therapy and tumour vaccination, AdVMART-1/DCs (5 × 105 cells) were injected directly into B16 tumours and their ability to induce MART-specific responses was examined. As mentioned before, splenic responses in mice to growing B16 tumours were weak (Fig. 4) and remained low, even following intratumoral injection of AdVMART-1/DC. Subdued splenic T-cell responses to growing B16 tumours were decreased even further by local tumour irradiation of 10 Gy. Importantly, intratumoral injection of AdVMART-1/DC, when given 1 day prior to local tumour radiation therapy, not only rescued the treatment-related immune-suppression, but also generated higher T-cell responses than in untreated, tumour-bearing mice. In contrast, the combination of DC vaccination 1 day after local tumour radiation therapy failed to overcome immune suppression caused by tumour and/or radiation therapy alone, and the number of MART-1 responsive lymphocytes in the spleens of these mice was as low as in the unimmunized mice. This suggests that tumour irradiation can block the response to vaccination, whereas prior immunization may allow the immune system to resist the immunosuppressive effects of local radiation therapy.
Figure 4.
Intratumoral injection of adenovirus encoding MART-1 cDNA transduced dendritic cells (AdVMART-1/DCs) before, but not after, radiation therapy prevents radiation-induced immune suppression. C57BL/6 mice bearing 5–6-mm-diameter B16 tumours were irradiated (0 or 10 Gy) 1 day before or after the intratumoral delivery of AdVMART-1/DCs (5 × 105). Six groups of mice were used in this study: (i) without tumours or treatments; (ii) with tumours and no treatments; (iii) with B16 tumours irradiated with 10 Gy; (iv) with tumours injected with AdVMART-1/DCs; (v) with tumours and AdVMART-1/DCs injected 1 day prior to local tumour irradiation (10 Gy); and (vi) with tumours irradiated (10 Gy) followed 1 day later by AdVMART-1/DC injection. One week after the first treatment, interferon-γ (IFN-γ)-producing lymphocytes were detected using enzyme-linked immunospot (ELISPOT) assays after 48 hr of restimulation with EL4(MART-1) cells (black bars) or EL4 cells (white bars), or with no restimulation (control; grey bars). Three mice were used in each treatment group. The results shown are the mean ± 1 standard error of the mean (SEM) of triplicate data from one representative experiment of two carried out (*P< 0·05 as compared with the EL4(MART-1)-restimulated group).
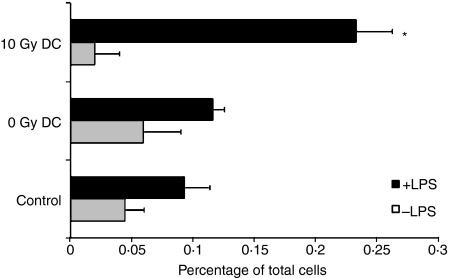
One possible explanation for these findings is that irradiation alters the ability of DCs to migrate to the draining LNs. To address this, DCs were treated with LPS to promote their migration to LNs,23 labelled with the green fluorescent dye, PKH2, irradiated or sham-irradiated and then injected into mouse footpads. Popliteal and inguinal LNs were harvested 48 hr after injection. Surprisingly, more 10 Gy-treated DCs migrated to LNs than non-irradiated DCs (Fig. 5). This rapid migration of irradiated DCs might explain why high T-cell responses could be generated after intratumoral DC injection when tumours were treated with radiation therapy 1 day later (Fig. 4).
Figure 5.
In vivo migration of irradiated or non-irradiated dendritic cells (DCs). Lipopolysaccharide (LPS)-treated and untreated DCs were labeled with the green fluorescent dye, PKH2, irradiated (0 or 10 Gy) and injected subcutaneously (s.c.) into the mouse foodpad. Mice without DC injection were used as controls. Fluorescent cells within the lymph node (LN) population were detected by flow cytometry 48 hr after injection of DC. Three mice were used in each treatment group. Results shown are the mean ± 1 standard error of the mean (SEM) of three experiments (*P< 0·05 compared with the 0 Gy DC group).
Discussion
In this study, we demonstrated that a non-curative radiation dose of 10 Gy delivered to the tumour site abolished splenic tumour-specific immune responses, suggesting that local radiation therapy can suppress systemic immunity. This is not a universal finding and cannot be true for all tumours. For example, local irradiation of ovalbumin (OVA) gene-transfected B16 melanoma tumours increased the numbers of OVA-specific lymphocytes within the draining LNs and tumours,24 and fractionated local radiation therapy of mouse prostate cancer TRAMP C1 tumours leads to a marginal increase in immunity.20 Others have reported slightly decreased or unchanged immune responses after irradiation of C3 or MethA sarcomas.15 Whether radiation increases or decreases tumour-specific immune responses may depend upon the immunogenicity of the tumour or even the immunodominant epitope. Many animal tumour models are immunogenic and it has been known for decades that curative radiation therapy given to such tumours will result in systemic immunity that assists in achieving local and micrometastatic tumour control. However, this bears little relevance to the clinical situation because most human tumours are weakly immunogenic. Our data suggest that under such circumstances radiation therapy might not behave as a sufficiently powerful adjuvant to enhance the generation of biologically relevant levels of anti-tumour immunity, and may indeed have the opposite effect.
One possible mechanism by which local radiation therapy might suppress systemic immunity is through the activation of immune-suppressor cell populations. Many cell populations have been detected following local radiation therapy, including regulatory/suppressor T cells with CD4+,25,26 CD8+27 and CD4− CD8−28 phenotypes. ‘Null’ or ‘natural’ suppressor lymphocytes,29 as well as suppressive DC subsets,30,31 have been described. In this study, we also observed a significant increase in the number of suppressive CD4+ CD25+ Foxp3+ Tregs in spleens and tumours within the CD4+ pool, although because of an increase in the number of‘null’ lymphocytes the overall percentage of Treg cells in the spleen was unaltered. This finding is consistent with our experience in colorectal cancer and prostate cancer patients who had elevated levels of CD4+ CD25+ Foxp3+ Treg cells in their circulation following radiation therapy.32 The relative radiosensitivity of different Treg subsets is not known, but it should be noted that the finding that radiosensitive CD8+ suppressor T cells could be eliminated by whole-body irradiation of tumour-bearing mice, leading to the rejection of immunogenic tumours,33 deals with a different subset than the one studied here.
Our study also showed that irradiation of DCs enables them to block immune responses or to induce immune suppression or tolerance. Tolerogenic DCs are normally associated with an immature phenotype and are known to play an important role in maintaining peripheral tolerance.34 As a result of insufficient costimulation, naïve T cells recognizing ligands on these immature DCs are deleted.35,36 The concept that this is a two-way interaction was introduced by Chang et al.,37 who observed that CD8+ CD28− T cells interfered with CD40–CD40 ligand-mediated signaling and consequently prevented functional DC maturation. Another mechanism by which DCs can induce antigen-specific tolerance is through the capture of ‘self’ antigens from dying cells.38
The mechanisms that confer immunosuppressive ability on DCs following radiation therapy seem somewhat different from the mechanisms described in previous studies. So far, we have been unable to ascribe the inhibitory effect of radiation on DC function to a lack of expression of costimulatory molecules, despite the fact that phenotypic changes are evident after exposure to radiation.13 Indeed, irradiation of DCs enhances their ability to present peptide antigen pulsed exogenously onto DCs,13 suggesting that antigen processing, not presentation, is the radiation target. Bearing in mind that tumour irradiation is likely to liberate a myriad of peptides ready to be captured by DCs, our current finding that intratumoral AdVMART1/DC vaccination was superior when given before radiation therapy may be yet another reflection of the increased exogenous peptide processing we observed in vitro following radiation.13 The fact that this is abolished when radiation therapy was given before DCs illustrates that there is a fine balance between immune-stimulatory and immune-inhibitory mechanisms that are differentially affected by radiation and time. Interestingly, irradiated DCs seemed to migrate faster than non-irradiated DCs, indicating another mechanism by which radiation therapy might affect DC function. It seems likely that in our study intratumorally injected DCs, given before radiation therapy, could have migrated to the lymph node and spleen by the time that radiation therapy was delivered, a suggestion that is supported by previous findings.39,40
Potential radiation effects on the tumour microenvironment are multifaceted, such as up-regulating the expression of inflammatory mediators [e.g. cyclooxygenase-2 (COX2) and prostaglandin E2 (PGE2)], heat shock proteins, immunomodulatory cytokines, adhesion molecules, costimulatory molecules, death receptors on tumour cells (e.g. Fas)41 and MHC class I molecules. Intratumoral DC administration has been clearly shown to enhance immune responses in a non-antigen-specific manner in several mouse models,14–16 and to have a better therapeutic outcome if the tumour was irradiated prior to intratumoral DC injection,15,16,40 which are different from the findings in our antigen-specific model. The outcome could ultimately depend on properties of the tumour and the microenvironment.
It is important to note that, in our model, intratumoral vaccination with AdVMART-1/DC in vivo, given even 1 day prior to tumour irradiation, resulted not only in the resistance to radiation-related immunosuppression but was also very effective at counteracting overall tumour-immune escape. This gives hope that combining radiation therapy and immunotherapy with the aim of increasing control of local tumours and distant micrometastases is feasible and worth pursuing, even if radiation therapy per se is often immunosuppressive. If the combination is to be effective, provision of more effective ‘danger’ signals than is provided by radiation therapy alone may be required,42–44 and the timing of immunization relative to irradiation might be critical.
Acknowledgments
This study was supported by National Institutes of Health/National Cancer Institute Grants RO1 CA87887:01A1 and RO1 CA101752:01 and United States Army Medical Research and Materiel Command W81XWH-0410126 and W81XWH-0710135.
Glossary
Abbreviations:
- AdV
adenovirus
- AdVMART-1
adenovirus encoding MART-1 cDNA
- AdVMART-1/DC
AdVMART-1 transduced DC
- AEC
3-amino-9-ethyl-carbazole
- DC
dendritic cell
- DMEM
Dulbecco’s modified Eagle’s minimal essential medium
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- IL
interleukin
- i.p.
intraperitoneally
- LN
lymph node
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- MOI
multiplicity of infection
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- s.c.
subcutaneously
- Th1
T-helper 1
- Th2
T-helper 2
- TNF-α
tumour necrosis factor-α
- Treg
T-regulatory
Disclosure
None.
References
- 1.Harrington NP, Chambers KA, Ross WM, Filion LG. Radiation damage and immune suppression in splenic mononuclear cell populations. Clin Exp Immunol. 1997;107:417–24. doi: 10.1111/j.1365-2249.1997.272-ce1158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JL, Patchen ML, Darden JH, Jackson WE. Effects of radiation on survival and recovery of T lymphocyte subsets in C3H/HeN mice. Exp Hematol. 1994;22:510–6. [PubMed] [Google Scholar]
- 3.Anderson RE, Warner NL. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- 4.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–7. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CM, Limanni A, Baker WH, Dobson ME, Kalinich JF, Patchen ML. Sublethal gamma irradiation increases IL-1alpha, IL-6, and TNF-alpha mRNA levels in murine hematopoietic tissues. J Interferon Cytokine Res. 1997;17:567–72. doi: 10.1089/jir.1997.17.567. [DOI] [PubMed] [Google Scholar]
- 6.Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys. 1995;33:619–26. doi: 10.1016/0360-3016(95)00279-8. [DOI] [PubMed] [Google Scholar]
- 7.Brach M, Gruss H, Kaisho T, Asano Y, Hirano T, Herrmann F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa B. J Biol Chem. 1993;268:8466–72. [PubMed] [Google Scholar]
- 8.Broski AP, Halloran PF. Tissue distribution of IL-10 mRNA in normal mice. Evidence that a component of IL-10 expression is T and B cell-independent and increased by irradiation. Transplantation. 1994;57:582–92. [PubMed] [Google Scholar]
- 9.Klein B, Loven D, Lurie H, Rakowsky E, Nyska A, Levin I, Klein T. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg. 1994;80:1074–7. doi: 10.3171/jns.1994.80.6.1074. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa F, Nakano H, Seo A, et al. Irradiation up-regulates CD80 expression through induction of tumour necrosis factor-alpha and CD40 ligand expression on B lymphoma cells. Immunology. 2002;106:354–62. doi: 10.1046/j.1365-2567.2002.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel A, Fernandez N, de La Coste A, Haddada H, Viguier M, Polla BS, Antoine B, Kahn A. Gamma-ray irradiation induces B7.1 costimulatory molecule neoexpression in various murine tumor cells. Cancer Immunol Immunother. 1998;46:277–82. doi: 10.1007/s002620050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu SZ, Jin SZ, Liu XD, Sun YM. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol. 2001;2:8. doi: 10.1186/1471-2172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao YP, Wang CC, Butterfield LH, Economou JS, Ribas A, Meng WS, Iwamoto KS, McBride WH. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–9. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 14.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, Mcginn CJ, Chang AE. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–75. [PubMed] [Google Scholar]
- 15.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–33. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004;109:685–90. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 17.Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129–35. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 18.Oh YT, Chen DW, Dougherty GJ, McBride WH. Adenoviral interleukin-3 gene-radiation therapy for prostate cancer in mouse model. Int J Radiat Oncol Biol Phys. 2004;59:579–83. doi: 10.1016/j.ijrobp.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CS, Syljuasen RG, Hong JH, Wallis A, Dougherty GJ, McBride WH. Effects of IL-3 gene expression on tumor response to irradiation in vitro and in vivo. Cancer Res. 1997;57:3899–903. [PubMed] [Google Scholar]
- 20.Tsai CH, Hong JH, Hsieh KF, Hsiao HW, Chuang WL, Lee CC, McBride WH, Chiang CS. Tetracycline-regulated intratumoral expression of interleukin-3 enhances the efficacy of radiation therapy for murine prostate cancer. Cancer Gene Ther. 2006;13:1082–92. doi: 10.1038/sj.cgt.7700977. [DOI] [PubMed] [Google Scholar]
- 21.Ribas A, Butterfield LH, Hu B, et al. Generation of T-cell immunity to a murine melanoma using MART-1-engineered dendritic cells. J Immunother. 2000;23:59–66. doi: 10.1097/00002371-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ribas A, Butterfield LH, McBride WH, et al. Genetic immunization for the melanoma antigen MART-1/Melan-A using recombinant adenovirus-transduced murine dendritic cells. Cancer Res. 1997;57:2865–9. [PubMed] [Google Scholar]
- 23.Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240–8. doi: 10.1007/s00262-003-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 25.Maeda H, Fujimoto S, Greene MI. Suppressor T cells regulate the nonanergic cell population that remains after peripheral tolerance is induced to the Mls-1 antigen in T cell receptor Vbeta 8.1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:13257–62. doi: 10.1073/pnas.230449097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. “Infectious” transplantation tolerance. Science. 1993;259:974–7. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 27.Inoue T, Asano Y, Matsuoka S, Furutani-Seiki M, Aizawa S, Nishimura H, Shirai T, Tada T. Distinction of mouse CD8+ suppressor effector T cell clones from cytotoxic T cell clones by cytokine production and CD45 isoforms. J Immunol. 1993;150:2121–8. [PubMed] [Google Scholar]
- 28.Cauley LS, Cauley KA, Shub F, Huston G, Swain SL. Transferable anergy: superantigen treatment induces CD4+ T cell tolerance that is reversible and requires CD4−CD8− cells and interferon gamma. J Exp Med. 1997;186:71–81. doi: 10.1084/jem.186.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–37. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- 30.Greene MI, Sy MS, Kripke M, Benacerraf B. Impairment of antigen-presenting cell function by ultraviolet radiation. Proc Natl Acad Sci USA. 1979;76:6591–5. doi: 10.1073/pnas.76.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao MD, Chen ZD, Xing Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction. Cell Biol Int. 2004;28:223–8. doi: 10.1016/j.cellbi.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Schaue D, Comin-Anduix B, Ribas A, et al. T cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14:4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellstrom I, Hellstrom KE. Antitumor effect of whole-body X-irradiation: possible role of an X-ray-sensitive T suppressor cell population. Transplant Proc. 1979;11:1073–6. [PubMed] [Google Scholar]
- 34.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 35.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 38.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–7. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappin MB, Weiss JM, Delattre V, et al. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology. 1999;98:181–8. doi: 10.1046/j.1365-2567.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Xia D, Bi X, Saxena A, Sidhu N, El-Gayed A, Xiang J. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J Gene Med. 2005;7:506–17. doi: 10.1002/jgm.692. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 42.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–80. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 43.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 44.McBride WH, Chiang CS, Olson JL, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]