Abstract
The outcome of hepatitis C virus (HCV) infection is determined by the interplay between the virus and the host immune response. Interleukin (IL)-18, an interferon-γ-inducing factor, plays a critical role in the T helper type 1 (Th1) response required for host defence against viruses, and antibodies to IL-18 have been found to prevent liver damage in a murine model. The present study was conducted to investigate the possible role of IL-18 in the pathogenesis and persistence of HCV. IL-18 levels were measured in sera of 50 patients at various stages of HCV infection (resolved, chronic and cirrhosis) and compared with those of normal controls. IL-18 gene expression was studied in peripheral blood mononuclear cells (PBMC) from each group, and in liver biopsy tissue from patients with chronic hepatitis C. The mean levels of IL-18 in sera were markedly elevated in patients with chronic hepatitis and cirrhosis, and were reduced in patients with resolved HCV infection. The serum IL-18 concentrations were related to the Child–Pugh severity of liver disease in cirrhotic patients. There also existed a strong positive correlation of IL-18 levels with histological activity score and necrosis. IL-18 mRNA expression was significantly up-regulated in the PBMC of cirrhotic patients when compared with other groups, while in the liver, higher levels of IL-18 transcripts were expressed in patients with chronic hepatitis C. The results of our study indicate that IL-18 levels reflect the severity and activity of HCV infection, and may contribute to the pathogenesis and progression of liver disease associated with HCV.
Keywords: cytokines, hepatitis, immunotherapeutics, inflammation
Introduction
Hepatitis C virus (HCV) is a major aetiological agent of chronic liver disease worldwide, affecting an estimated 3% of the population.1 Characteristic features of HCV infection include high incidences of persistence in the host and progression to chronic hepatitis, leading to cirrhosis, which is a strong risk factor for the development of hepatocellular carcinoma (HCC).2
Elucidation of the molecular virology of HCV and the response it elicits has emphasized the importance of host immunity in resolving infection and mediating liver damage. Suppression of viral replication and modulation of the immune responses can prevent disease progression, leading to an improvement in the severity of liver disease. As early events in the virus–host interaction are likely to determine the outcome of HCV infection, research has focused on the characterization of the strength and kinetics of the antiviral immune response at different stages of liver disease.
The main therapy currently available for patients with chronic hepatitis C is a 6–18-month course of pegylated interferon (IFN) and ribavirin,3 but it has limited efficacy, and only a subset of patients achieve a sustained virological response to the treatment. Improvements in existing therapies as well as development of new antiviral agents or vaccines for the treatment or prevention of chronic infection are thus highly desirable. The cytokines prompting development of the T helper type 1 (Th1) immune response are of particular interest for potential immunotherapy against HCV. However, cytokines regulating immune activity in HCV infection, and in particular cytokines expressed by the peripheral blood leucocytes, have not yet been thoroughly studied.
Interleukin (IL)-18, the IFN-γ-inducing cytokine, plays a critical role in the Th1 response,4 and administration of antibodies to IL-18 has been shown to prevent liver damage in an animal model.5 IL-18 is synthesized by different cell types, including Kupffer cells, activated macrophages, monocytes and dendritic cells. The importance of IL-18 in immunity and host defence is only beginning to be appreciated. As IL-18 appears to act at a very early stage of T-cell activation, triggering one of the earliest steps of the cytokine cascade should have the most pronounced and long-lasting effects on T-cell responses. IL-18 may also induce macrophages to produce tumour necrosis factor (TNF)-α and nitric oxide (NO) and account for the induction of cell death.6 Furthermore, it enhances Fas-ligand (FasL)-mediated killing by natural killer (NK) and T cells,7 and hence is expected to have positive effects against viral infections. IL-18 directly induces FasL expression in the liver8 and also exhibits antitumour effects through activation of NK cells.
Many reports suggest that IL-18 might play a role in viral infections. A positive effect of IL-18 has been shown in mouse models of herpes simplex and vaccinia virus infection,9–11 demonstrating that IL-18 inhibits human immunodeficiency virus (HIV) production in peripheral blood mononuclear cells (PBMC). However, the mechanism of this antiviral effect and its relationship to viral replication have not been determined. IL-18 has been shown to inhibit hepatitis B virus (HBV) replication in the livers of transgenic mice.12 In chronic hepatitis C and cirrhosis, an increase in the expression of proinflammatory cytokines, in particular IL-18, has been shown, which correlates with IFN-γ production.13 IL-18 is produced as an inactive precursor, so it needs to be clarified whether IL-18 is present in its active form to exert its effect and induce a Th1 response in hepatitis C infection, and whether IL-18 could be related to disease persistence. Another study by Abbate et al.14 revealed up-regulated expression of the IFN-related genes IFN-γ, IFN-α receptor-1, IFN regulatory factor-1, and IL-18, while expression of IFN-α and IFN-β was significantly lower in patients with HCV infection when compared with non-alcoholic steatohepatitis. Ludwiczek et al.15 found elevated levels of plasma IL-18 and IL-18 binding protein (IL-18BP) in patients with chronic liver disease compared with healthy controls.
There is a dearth of data on the nature of the involvement of IL-18 in HCV pathogenesis and host defence; this needs to be investigated so that therapeutic strategies can be developed to provide efficient defence against the virus by manipulating the immune response. The present study was therefore undertaken to determine IL-18 levels in patients at different stages of HCV infection and to assess the role of IL-18 in the persistence of HCV.
Materials and methods
Study population
A total of 50 patients (39 male and 11 female) with HCV infection attending the liver clinic at Pgimer, Chandigarh, India were recruited for the present study. Their mean age was 41·4 ± 11·7 (range 19–64) years. Liver function tests (LFTs) and anti-HCV tests using a third-generation enzyme-linked immunosorbent assay (ELISA) were performed for all patients. Detection of HCV RNA was performed with nested reverse transcriptase–polymerase chain reaction (RT-PCR) using primers from the conserved 5′-untranslated region of the HCV genome.16 The clinical features and demographics of the patients and normal controls are shown in Table 1.
Table 1.
Characteristics of patients with hepatitis C and controls
| Parameter | Group 1 (resolved) | Group 2 (chronic) | Group 3 (cirrhosis) | Group 4 (controls) |
|---|---|---|---|---|
| Number | 15 | 20 | 15 | 15 |
| Sex (male:female) | 11 : 4 | 17 : 3 | 9 : 6 | 7 : 8 |
| Age (years) | 35·2 ± 12·8 | 40·6 ± 8·0 | 48·0 ± 21·1 | 34·1 ± 9·8 |
| Bilirubin (mg/dl) | 0·5 ± 0·1 | 0·8 ± 0·5 | 2·1 ± 1·5 | 0·5 ± 0·1 |
| Albumin (g/dl) | 3·7 ± 0·6 | 4·0 ± 0·7 | 3·4 ± 0·6 | 4·1 ± 0·3 |
| Globulin (g/dl) | 3·1 ± 0·5 | 3·0 ± 0·7 | 3·1 ± 0·7 | 2·1 ± 0·5 |
| ALP (IU/l) | 147·4 ± 82·5 | 169·6 ± 91·8 | 186·8 ± 73·5 | 104·4 ± 21·7 |
| AST (IU/l) | 24·3 ± 7·6 | 67·3 ± 17·6 | 121·9 ± 93·7 | 30·1 ± 4·4 |
| ALT (IU/l) | 23·6 ± 8·7 | 99·3 ± 37·4 | 76·2 ± 49·3 | 21·1 ± 3·4 |
Values are given as mean ± standard deviation, unless otherwise indicated.
ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The patients were divided into three groups. Group 1 comprised 15 asymptomatic patients considered to have spontaneously resolved HCV infection, defined as persistently normal liver function tests on at least three occasions over 3 months, anti-HCV positive but HCV RNA-negative RT-PCR results on two separate occasions, and normal ultrasonography.17 The anti-HCV positivity and specificity of samples from these patients were confirmed by a supplemental recombinant immunoblot assay (RIBA) using LG HCD Confirm (LG Life Sciences Ltd, Seoul, Korea). Group 2 included 20 patients with chronic hepatitis C confirmed by biopsy. All were anti-HCV positive, had detectable HCV RNA in serum and alanine aminotransferase (ALT) levels ≥ 1·5× upper limit of normal (ULN) on at least three occasions. HCV genotype 3 was seen in 14 of these patients and six had infection with genotype 1. Group 3 included 15 patients with HCV-related cirrhosis of the liver (anti-HCV positive) as demonstrated by liver biochemistry, ultrasound, endoscopy findings and liver biopsy, if possible. The Child–Pugh score, an index of liver failure, was determined for each cirrhotic patient using values for all the three parameters of albumin, bilirubin and prothrombin time (blood test that measures the time it takes for the blood to clot), and the presence of ascites and hepatic encephalopathy. The stages of severity were classified as Child A, B and C with Child-Pugh scores of 5–6, 7–9 and ≥10, respectively. Group 4 comprised 15 normal healthy volunteers with normal LFT who were negative for anti-HCV and HBV surface antigen (HBsAg). Patients positive for HBsAg or antibodies to HIV, those with associated alcoholic liver disease, autoimmune hepatitis, Wilson’s disease, chronic renal failure, or cardiac or pulmonary dysfunction, and patients taking immunomodulatory drugs were excluded from the study.
The study protocol was approved by the ethical committee of the institute and informed consent was obtained from all the participants prior to inclusion in the study.
Measurement of IL-18
IL-18 levels in the sera of 50 patients and 15 controls were measured utilizing a solid-phase sandwich ELISA system (Diaclone, Besancon, France). The standard was reconstituted to a concentration of 2000 pg/ml and a row of IL-18 dilutions was created ranging from 62·5 to 2000 pg/ml. One hundred microlitres of the standard diluent was added to the blank well and 100 μl of serum was added to the sample wells. Fifty microlitres of diluted biotinylated anti-IL-18 was added to all the wells and the plate was incubated for 3 hr at room temperature. The plate was washed three times with the wash buffer provided. One hundred microlitres of streptavidin–horseradish peroxidase (HRP) conjugate was added followed by incubation for 30 min at room temperature. The plate was again washed as before, 100 μl of tetramethylbenzidine (TMB) substrate was added and incubation was carried out for 15 min at room temperature in the dark. The reaction was stopped by adding 100 μl of sulphuric acid. The absorbance was read at 450 nm. A linear standard curve was generated by plotting the absorbance values against standard concentrations.
mRNA expression of IL-18
Ten subjects randomly chosen from each of the four study groups were included in the investigation of IL-18 mRNA in PBMC. The intrahepatic expression of IL-18 mRNA was also determined in liver biopsy tissue from six patients with chronic hepatitis and three normal controls (obtained from a mortuary). The blood samples were added to acid citrate dextrose anticoagulant and PBMC were separated by standard Ficoll Hypaque density gradient centrifugation. The liver tissue was obtained by percutaneous needle biopsy as a part of the clinical diagnostic and pre-therapeutic evaluation. A portion of the specimen was fixed and saved for histological examination, and the remainder was processed for RNA extraction. The liver tissue was homogenized in lysis buffer, and RNA was isolated from the PBMC and homogenized liver tissue specimens by the acid phenol–chloroform extraction method of Chomcznski and Sacchi.18 The RNA concentration was quantified spectrophotometrically, and RNA degradation and purity were assessed by electrophoresis on a 1·5% agarose gel containing formaldehyde.
The RNA was denatured at 70° for 5 min and cDNA was synthesized using 200 units of murine Moloney leukaemia virus (MMLV) reverse transcriptase (MBI Fermentas, St. Leon-Rot, Germany) and 500 ng of oligo-dT primer (MBI Fermentas) in 20 μl of reaction mixture. Two separate PCR reactions were performed on the cDNA using primers specific for IL-18 and the housekeeping enzyme gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), in a final volume of 50 μl, with 35 cycles for IL-18 and 25 cycles for GAPDH, each comprising denaturation at 94° for 5 min, annealing at 55° for 1 min and extension at 72° for 1 min. PCR using GAPDH as a control was performed on each sample to ensure that differences between tubes were not the result of unequal concentrations of RNA or differential cDNA synthesis. A variable number of cycles was used so that amplification occurred in a linear phase, and the differences between control and experimental conditions were maintained by using a limited number of cycles. The cytokine primer pairs were designed to span exon–exon conjunctions and were therefore specific for mRNA/cDNA and non-reactive with genomic DNA. The controls were included in each set of reactions.
| Primer sequences | |
| IL-1819 | |
| Sense (nt 297–318) | 5′-GCTTGAATCTAAATTATCAGTC-3′ |
| Antisense (nt 617–638) | 5′-GAAGATTCAAATTGCATCTTAT-3′ |
| GAPDH | |
| Sense (nt 71–96) | 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ |
| Antisense (nt 1027–1050) | 5′-ATGTGGGCCATGAGGTCCACCAC-3′ |
IL, interleukin; nt, nucleotide.
The RT-PCR products were run on a 1·5% agarose gel and visualized by ultraviolet (UV) light illumination using ethidium bromide staining to yield amplicons of 342 bp (IL-18) and 983 bp (GAPDH). A DNA molecular size marker was also run alongside the RT-PCR products. Densitometric assessment of the PCR products was performed using scion image analysis software, a modified version for Windows available from the National Institutes of Health (NIH, Frederick, MD). The results are expressed semiquantitatively as the ratio of IL-18 to GAPDH.
Southern hybridization
The specificity of the IL-18 amplification products was confirmed by Southern hybridization using an IL-18-specific probe of the sequence 5′-ATGTATAAAGATAGCCAGCC-3′.13 The probe was prepared by end-labelling in a 20-μl reaction mixture containing 10 ng of the oligoprobe, 1·5 μl of polynucleotide kinase (PNK) enzyme, 1× PNK buffer and 30 μCi γ[32P]-ATP (Brit, Hyderabad, India). Southern hybridization was carried out at 50° overnight. Post-hybridization washings were performed with 2× saline sodium citrate (SSC) buffer containing 0·1% sodium dodecyl sulphate (SDS) twice at 55° and then twice at 37° for 15 min each. The membrane was finally rinsed in 250 ml of 2× SSC buffer for 10 min at room temperature. The membrane was air-dried, covered with saran wrap and autoradiographed overnight at −20° with a screen and X-ray film.
Histopathological evaluation of liver biopsies
The portion of the liver biopsy specimen fixed in 10% neutral buffered formalin and embedded in paraffin was used for histological examination. Thin sections of the biopsies (5 μm) were stained with haematoxylin and eosin, and evaluated by the modified Knodell system of Ishak et al.20 without knowledge of the patient’s biochemical or clinical data. A composite hepatitis activity index (HAI) score reflecting the severity was determined. The necro-inflammatory grade (0–18) and the stage of fibrosis (0–6) were also recorded separately.
Statistical analysis
The data were presented as mean ± standard deviation (SD). IL-18 concentrations were compared between groups using the Kruskal–Wallis test. The Pearson’s correlation coefficient test was used to find associations between parameters. The Mann–Whitney U-test was carried out to compare densitometric ratios, and values are expressed as median (range). A P-value < 0·05 was considered statistically significant.
Results
Serum IL-18 is elevated in chronic hepatitis C and cirrhosis
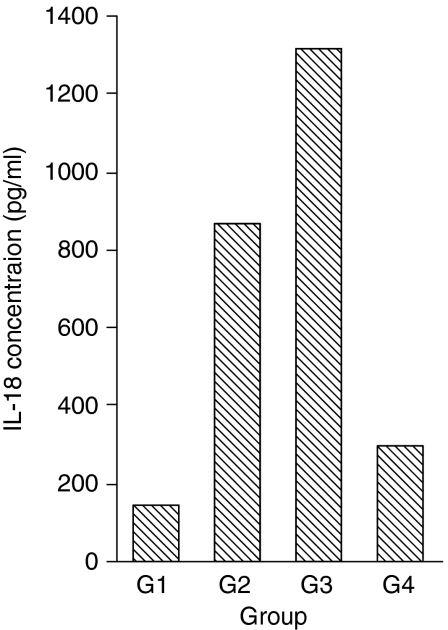
IL-18 protein production was quantified in serum samples from 50 patients in different stages of HCV infection and compared with that of normal healthy controls (Fig. 1). The mean levels of IL-18 were significantly elevated in patients with chronic hepatitis C (865·4 ± 283·4 pg/ml; P< 0·005) and HCV-related cirrhosis (1315·5 ± 714·1 pg/ml; P< 0·005) when compared with the normal controls (297·6 ± 88·6 pg/ml). The difference between the chronic hepatitis and cirrhotic groups was also significant (P< 0·005). In patients with self-limited hepatitis C, the IL-18 levels were reduced (140·9 ± 81·7 pg/ml), although not significantly, and were below the detection limit in one patient.
Figure 1.
Serum interleukin (IL)-18 levels are elevated in patients with chronic hepatitis C and cirrhosis. IL-18 production was measured in the sera of 50 patients at various stages of hepatitis C virus (HCV) infection and 15 controls using a commercial enzyme-linked immunosorbent assay (ELISA) system. The mean levels of IL-18 were 140·9 ± 81·7 pg/ml in the resolved HCV group (G 1), 865·4 ± 283·4 pg/ml in the chronic hepatitis C group (G 2), 1315·5 ± 714·1 pg/ml in the group of cirrhotic patients (G 3) and 297·6 ± 88·6 pg/ml in normal healthy controls (G 4). The increase in serum IL-18 was significant in group 2 (P< 0·005) and group 3 patients (P< 0·005) as compared with the control group 4.
Serum IL-18 levels correlate with Child–Pugh severity in cirrhosis
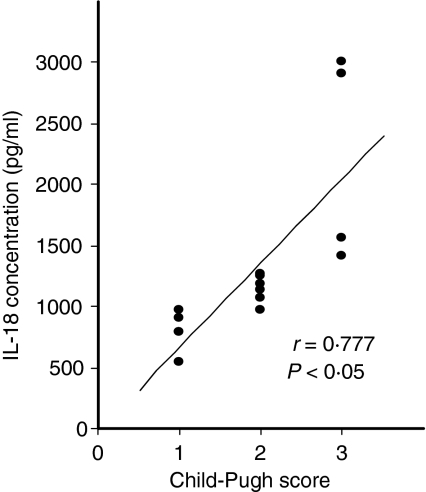
The correlations between serum IL-18 levels and various clinical parameters were analysed. IL-18 concentrations were associated neither with age nor with ALT levels in any of the patient groups. Also, comparable serum IL-18 production was observed in patients infected with HCV genotypes 1 and 3 (847·1 ± 382·7 and 873·2 ± 246·6 pg/ml, respectively), suggesting no significant relationship between HCV genotype and IL-18 production. However, IL-18 levels correlated with disease progression and severity of liver dysfunction according to the Child–Pugh classification (r= 0·777, P< 0·05) in 15 patients with cirrhosis (Fig. 2). IL-18 production was positively correlated with the Child–Pugh score, being higher in patients at stage Child C (2220·7 ± 852·7 pg/ml; n = 4) than in those at stage Child A (798·7 ± 162·9 pg/ml; n = 5) or Child B (1142·6 ± 109·2 pg/ml; n = 6).
Figure 2.
Interleukin (IL)-18 levels in serum correlate with Child–Pugh severity in cirrhosis. The patients with hepatitis C virus (HCV)-related cirrhosis were graded by calculating the Child–Pugh severity index based on their laboratory and clinical findings. The mean levels of IL-18 in patients with Child–Pugh stages A, B and C were 798·7 ± 162·9, 1142·6 ± 109·2 and 2220·7 ± 852·7 pg/ml, respectively. IL-18 levels correlated with the severity of liver dysfunction according to the Child–Pugh classification in patients with cirrhosis (r = 0·777; P< 0·05).
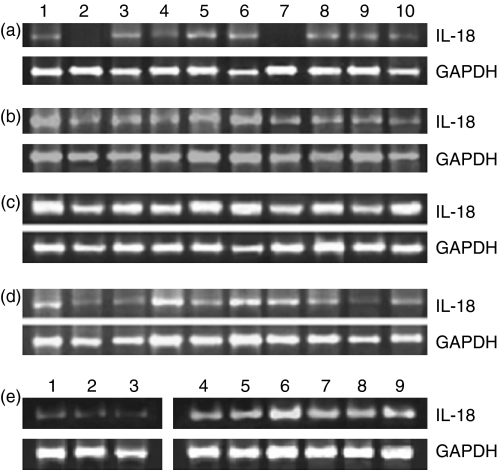
IL-18 mRNA expression is increased in the PBMC of patients with HCV-related cirrhosis
In order to determine the source of increased IL-18 in the sera of infected individuals, we investigated IL-18 gene expression in the PBMC. The median value (range) of the ratio of IL-18:GAPDH mRNA was 0·22 (0–0·46) for subjects with resolved HCV infection, 0·39 (0·21–0·75) for patients with chronic hepatitis C, 1·02 (0·53–1·67) for those with HCV-related cirrhosis, and 0·27 (0·16–0·51) for the normal controls. IL-18 mRNA expression was highest in the PBMC of patients with cirrhosis, as shown in Fig. 3(c), and was significantly up-regulated compared with the resolved hepatitis C (Fig. 3a; P< 0·001), chronic hepatitis C (Fig. 3b; P< 0·01) and control groups (Fig. 3d; P< 0·001). The bands in two of the samples in the resolved hepatitis C group (lanes 2 and 7; Fig. 3a) were too weak to be visualized on agarose gels. There was no significant difference in mRNA expression in the PBMC of patients with chronic hepatitis C and those of controls, although IL-18 levels in the sera from these groups differed significantly. The specificity of the IL-18 amplification products in samples from patients with chronic hepatitis C was confirmed by Southern hybridization using a radiolabelled IL-18-specific probe. All the samples showed positive signals corresponding to a size of 342 bp after autoradiography.
Figure 3.
Interleukin (IL)-18 mRNA expression is up-regulated in the peripheral blood mononuclear cells (PBMC) of cirrhotic patients and in the livers of patients with chronic hepatitis C. The gene expression of IL-18 was studied using semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) which yielded products of 342 bp for IL-18 (upper panel) and 983 bp for GAPDH (lower panel). The densitometric assessment was performed using scion image analysis software and the results are expressed as the ratio of IL-18:GAPDH mRNA in PBMC of (a) subjects with resolved hepatitis C virus (HCV) infection, (b) chronic hepatitis C patients, (c) patients with HCV-related cirrhosis, and (d) normal healthy controls. IL-18 mRNA expression in the PBMC of cirrhotic patients was significantly up-regulated compared with that in the resolved (P< 0·001), chronic hepatitis (P< 0·01) and control groups (P< 0·001). (e) The patients with chronic hepatitis C (lanes 4–9) expressed significantly higher levels of IL-18 transcripts in the liver (P< 0·025) compared with the controls (lanes 1–3).
IL-18 mRNA expression is up-regulated in the liver in chronic hepatitis C
To investigate the activation of the IL-18 gene in the liver, total RNA was obtained from liver biopsies and semiquantitative PCR was carried out. The production of transcripts of the GAPDH housekeeping gene was also determined for normalization. The results are shown in Fig. 3(e). The median (range) of the ratio of IL-18:GAPDH mRNA was 0·018 (0·016–0·036) for controls, and 0·059 (0·038–0·072) in chronic HCV-infected livers. Patients with chronic hepatitis C expressed higher levels of IL-18 transcripts in the liver compared with their normal control counterparts. Statistical evaluations showed that this difference was significant (P< 0·025). We did not investigate intrahepatic IL-18 expression in patients with resolved hepatitis C because of ethical considerations, and those with cirrhosis were also not included in this part of the study because the liver biopsy procedure in these cases often leads to complications.
IL-18 levels strongly correlate with liver histological activity and necroinflammation
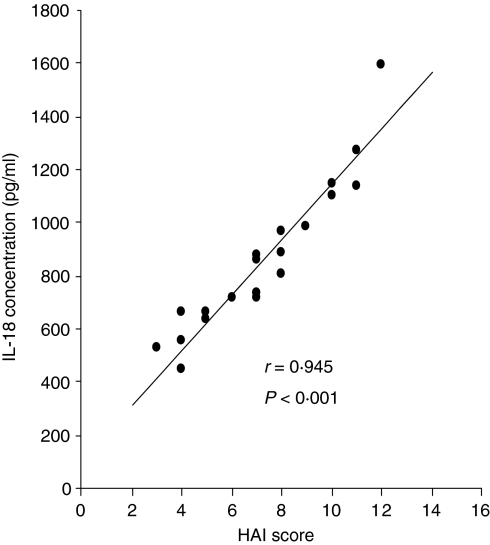
IL-18 production in sera from 20 patients with chronic hepatitis C was positively correlated with hepatic histological activity (correlation coefficient r= 0·945, P< 0·001) as shown in Fig. 4. The mean concentration of IL-18 was 672·5 ± 131·5 pg/ml in 11 patients with HAI scores ≤ 7, and tended to be higher (1101·1 ± 235·5 pg/ml) in the remaining nine patients with HAI values > 7. To assess the independent correlation of IL-18 with necrosis and fibrosis, six patients with the same necrosis score of 3, and another six patients with the same fibrosis score of 2 were selected. There was a positive correlation of IL-18 levels with necroinflammatory activity (correlation coefficient r = 0·853; P< 0·005). In contrast, no significant association was observed with fibrosis (r = 0·685; not significant).
Figure 4.
Interleukin (IL)-18 levels in sera show a strong positive correlation with hepatic histological activity in patients with chronic hepatitis C. The hepatitis activity index (HAI) score, reflecting severity, was determined in liver biopsies of 20 patients using the modified Knodell system. The mean IL-18 concentration in patients with HAI scores > 7 was higher than that in patients with HAI ≤ 7 (1101·1 ± 235·5 versus 672·5 ± 131·5 pg/ml, respectively), and IL-18 production was strongly associated with HAI (correlation coefficient r = 0·945; P< 0·001).
Discussion
The production of IFN-γ and the subsequent effect on T-cell activation are particularly important for protection against intracellular pathogens, including viruses. In addition to its potent induction of IFN-γ, IL-18 activates CD8+ T cells which play a central role in viral clearance. In the present study, we investigated whether this cytokine participates in the pathogenesis of HCV infection, as a thorough understanding of the immune response is important for the development of effective therapeutic strategies.
To obtain better characterization of the production of IL-18 in patients with HCV infection, we quantified this cytokine in sera from patients at different stages of HCV infection and correlated IL-18 concentrations with disease severity. Although a vigorous Th1 immune response and increased IFN-γ levels have been documented to cause eradication of HCV during the early phase of infection in about 15–20% of infected individuals, the role of the IFN-γ inducer IL-18 in resolution during the early phase of infection is not very clear from our study. The decreased levels of IL-18 in the present study could have been caused by negative feedback regulation through a parallel increase in IL-18BP21,22 or receptors, resulting in impairment of its bioactivity. Another possibility is that IL-18 levels increased in the early stages of infection, to achieve eradication, and by the time the study was performed levels had reduced again. The decrease in IL-18, however, did not reach statistical significance. As IL-18 has been shown to be a potent proinflammatory cytokine with the potential to exert deleterious effects, tight regulation is required. Regulation at the level of caspase 1 needs to be investigated. There is a clear dependence on the stage of HCV infection, as disease progression was accompanied by an increase in IL-18.
Our study showed a close relationship between elevated circulating plasma levels of IL-18 and the severity of HCV infection. IL-18 was positively correlated with hepatic histological activity in 20 patients with chronic hepatitis C. As necrosis and fibrosis are associated, independent correlations could be studied in only six patients, in whom IL-18 levels were strongly related to necroinflammation. Thus, the pathological consequences of infection mediated by IL-18 are clearly detrimental to the host and should be targets of therapeutic interventions. Our study did not show any association between serum levels of IL-18 and ALT values, whereas Hongyu et al.23 showed a positive correlation between these variables. The differential production of IL-18 during various stages of HCV infection could also be a result of polymorphism in the regulatory gene of IL-18, the effect of which has been investigated in several other diseases.24,25 These polymorphisms may be associated with protection against or susceptibility to infection. Giedraitis et al.26 reported that carriage of allele C (G→C polymorphism) at position −137 of the promoter of the IL-18 gene was associated with high production of IL-18. Recent studies have shown that polymorphism in the IL-18 gene at position −607 could be associated with the pathogenesis of hepatitis virus infection.27,28 Our study showed a higher concentration of IL-18 in patients with cirrhosis. Ludwiczek et al.15 have also shown increased plasma levels of IL-18 in chronic liver disease, but cytokine levels were analysed in cirrhotic and non-cirrhotic patients without any regard to the aetiology of the underlying liver disease. The levels of IL-18BP in cirrhosis correlated with severity, but decreased in cirrhosis class C. Elevation of serum IL-18 is an indicator of an overwhelming proinflammatory response and poor liver function in chronic liver diseases. However, the precise cellular and molecular mechanisms and mediators underlying the liver damage and also the protective antiviral effect may differ. Schvoerer et al.29 reported lower levels of IL-18 in plasma and supernatants of stimulated PBMC from patients with genotype 1 HCV infection than in those from normal controls, in contrast to our study. Thus the relationship among genotype, IL-18 production and HCV progression warrants further study.
The present investigation suggests a proinflammatory role of IL-18, especially during the late and terminal stages of HCV infection. The results of our study suggest a pathogenic role for IL-18 in HCV infection, through the induction of symptoms and the acceleration of progression of the disease. In our opinion, during the early and asymptomatic stage of infection, decreased levels of IL-18 stimulate and maintain high levels of hepatitis C virions, resulting in persistence. During the later stages of infection, an increase in IL-18 might result in prevention of progression to end-stage liver disease. IL-18 may directly modulate the viability of the cells supporting HCV replication, favouring their elimination and dissemination of the viral particles. This could be attributed to increased Fas-mediated apoptosis or cell death involving T cells and infected hepatocytes, which is inhibited by the core protein of HCV in persistent infection.
Studies in murine models30 and cell lines31 indicate that IL-18 has potent antitumour effects that are mediated by enhancement of cytotoxic T lymphocyte activity, induction of apoptosis and reduction of tumorigenesis. Hence it can be hypothesized that a significant increase in IL-18 could have a role in prevention of HCC. A study by Abiru et al.32 also showed spontaneous regression of HCC associated with elevated levels of IL-18. IL-18 could therefore be tested as a prognostic marker for cirrhotic patients who are likely to develop HCC. Cirrhotic patients who present low levels of IL-18 may perhaps progress to HCC, as was seen in one case in this study on prospective follow-up. However, as cirrhosis leads to liver carcinoma in only 10% of patients, and most of our patients with cirrhosis showed raised levels of IL-18, further longitudinal studies are required in this regard. It also needs to be determined whether HCV or any of its gene products plays a direct role in the increased concentration of this cytokine in sera from infected individuals.
To clarify the cellular sources of increased serum IL-18 in chronic hepatitis C and cirrhosis, we studied IL-18 gene expression in the liver and PBMC. In chronic hepatitis C and related cirrhosis, a significant up-regulation of intrahepatic IL-18 mRNA and IFN-γ expression was demonstrated in a previous study using semiquantitative PCR and a dot blot assay, and IL-18 mRNA expression was found to correlate with IFN-γ expression.13 Our study confirms these findings, revealing a transcriptional increase in IL-18 in the liver in chronic hepatitis C, although cirrhotic patients were not studied to avoid potential complications resulting from the biopsy procedure. Our data suggest a role of this cytokine in the cellular immune response to hepatocytes in the course of the disease. Furthermore, as the infection progresses to cirrhosis, even the PBMC may contribute to elevation of IL-18 in the circulation. Thus the increased IL-18 serum levels in cirrhosis may be derived partially from PBMC. In two patients with self-limited infection, expression of IL-18 mRNA could not be detected by RT-PCR, although the GAPDH control gene was amplified. In one of these patients, IL-18 was also undetectable in the serum. We cannot therefore rule out the possibility of feedback inhibition of IL-18 gene expression being caused by decreased serum concentrations of IL-18.
Intrahepatic mRNA levels for a number of genes involved in activation of the IFN system in chronic hepatitis C have been assessed by semiquantitative RT-PCR.14 In comparison with patients with non-alcoholic steatohepatitis, in HCV-infected subjects, IFN-α and IFN-β mRNA levels were significantly lower whereas IFN-γ, signal transducer and activator of transcription (STAT)-1α, interferon regulatory factor (IRF)-1, interferon-alpha receptor (IFNAR)-1 and IL-18 mRNA levels were up-regulated. IL-18 mRNA up-regulation is consistent with the known role of this cytokine in inflammation and with the increased amount of IL-18 in HCV-infected livers.33 The apparent discrepancy in mRNA levels may suggest that, after initial activation of the IFN-α and IFN-β response during acute infection, in the chronic phase, a general silencing of this response may occur. IL-18 transcript expression was analysed with regard to hepatic inflammatory activity, assessed using both histological grading and biochemical ALT activity in the serum. There was a lack of association between IL-18 mRNA expression and degree of hepatic inflammatory activity within the chronic HCV-infected group. IL-18 mRNA correlated with IFN-γ up-regulation but not with ALT. Our results, in contrast, show a strong association of IL-18 production with histological activity in the liver. Mihm et al.33 also showed that expression of interferon-gamma-inducible protein-10 (IP-10), a chemokine that recruits activated T lymphocytes, was associated strongly with the accumulation of IFN-γ and IL-18 mRNA in the liver in patients with chronic hepatitis C, but not in those with chronic hepatitis B or those with liver diseases unrelated to viral infections such as non-alcoholic steatohepatitis. Intrahepatic IL-18 production seems to be regulated not at the transcriptional but at the protein level, as mature IL-18 protein secretion is dependent on processing of the inactive precursor into a biologically active protein by caspase 1.34
Thus, the present study attempts to gain a better understanding of the involvement of IL-18 in HCV infection. The magnitude and timing of its production with regard to the stage of viral infection, and its levels in separate compartments such as the plasma, PBMC and liver should be dynamically evaluated in order to clarify the Th1/Th2 balance in HCV infection. Measurement of IL-18 production in accessible compartments could potentially help to establish the prognosis of HCV infection in patients. Our data confirm the proinflammatory role of IL-18 in HCV infection, and IL-18 levels were found to reflect HCV-induced inflammation and hepatic injury. It is possible that up-regulation of IL-18 production has a role in the development of chronicity and accelerates the evolution of chronic hepatitis towards cirrhosis. As Th1 cells have been implicated in the pathogenesis of HCV infection, IL-18 may be involved in mechanisms of tissue injury in hepatitis C, and IL-18 antagonists are promising candidates for therapeutic interventions to combat the pathological consequences of HCV infection.
Acknowledgments
The authors gratefully acknowledge Professor S. Majumdar for helpful discussions and for providing the facilities of his department. This work was supported by a research grant from the Postgraduate Institute of Medical Education and Research, India.
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(Suppl. 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 3.Shiffman ML. Optimizing the current therapy for chronic hepatitis C virus: peginterferon and ribavirin dosing and the utility of growth factors. Clin Liver Dis. 2008;12:487–505. doi: 10.1016/j.cld.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. IL-18: a Th1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 5.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961–7. [PubMed] [Google Scholar]
- 7.Dao T, Mehal WZ, Crispe IN. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J Immunol. 1998;161:2217–22. [PubMed] [Google Scholar]
- 8.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157:3967–73. [PubMed] [Google Scholar]
- 9.Fujioka N, Akazawa R, Ohashi K, Fujii M, Ikeda M, Kurimoto M. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J Virol. 1999;73:2401–9. doi: 10.1128/jvi.73.3.2401-2409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka-Kataoka M, Kunikata T, Takayama S, Iwaki K, Ohashi K, Ikeda M, Kurimoto M. In vivo antiviral effect of interleukin 18 in a mouse model of vaccinia virus infection. Cytokine. 1999;11:593–9. doi: 10.1006/cyto.1998.0453. [DOI] [PubMed] [Google Scholar]
- 11.Choi HJ, Dinarello CA, Shapiro L. Interleukin-18 inhibits human immunodeficiency virus type 1 production in peripheral blood mononuclear cells. J Infect Dis. 2001;184:560–8. doi: 10.1086/322805. [DOI] [PubMed] [Google Scholar]
- 12.Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702–7. doi: 10.1128/JVI.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260–9. doi: 10.1136/gut.46.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbate I, Romano M, Longo R, et al. Endogenous levels of mRNA for IFNs and IFN-related genes in hepatic biopsies of chronic HCV-infected and non-alcoholic steatohepatitis patients. J Med Virol. 2003;70:581–7. doi: 10.1002/jmv.10433. [DOI] [PubMed] [Google Scholar]
- 15.Ludwiczek O, Kaser A, Novick D, Dinarello CA, Rubinstein M, Vogel W, Tilg H. Plasma levels of interleukin-18 and interleukin-18 binding protein are elevated in patients with chronic liver disease. J Clin Immunol. 2002;22:331–7. doi: 10.1023/a:1020600230977. [DOI] [PubMed] [Google Scholar]
- 16.Chan SW, McOmish F, Holmes EC, Dow B, Peutherer JF, Follett E, Yap PL, Simmonds P. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J Gen Virol. 1992;73:1131–41. doi: 10.1099/0022-1317-73-5-1131. [DOI] [PubMed] [Google Scholar]
- 17.Cramp ME, Carucci P, Rossol S, Chokshi S, Maertens G, Williams R, Naoumov NV. Hepatitis C virus (HCV) specific immune responses in anti-HCV positive patients without hepatitis C viraemia. Gut. 1999;44:424–9. doi: 10.1136/gut.44.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomcznski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Hanck C, Manigold T, Bocker U, Kurimoto M, Kolbel CB, Singer MV, Rossol S. Gene expression of interleukin 18 in unstimulated peripheral blood mononuclear cells of patients with alcoholic cirrhosis. Gut. 2001;49:106–11. doi: 10.1136/gut.49.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 21.Muhl H, Kampfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. Interferon-gamma mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem Biophys Res Commun. 2000;267:960–3. doi: 10.1006/bbrc.1999.2064. [DOI] [PubMed] [Google Scholar]
- 22.Paulukat J, Bosmann M, Nold M, et al. Expression and release of Il-18 binding protein in response to IFN-gamma. J Immunol. 2001;167:7038–43. doi: 10.4049/jimmunol.167.12.7038. [DOI] [PubMed] [Google Scholar]
- 23.Hongyu J, Jie D, Sihe Z, Yingji M, Huafeng C. Clinical observation of serum IL-18, IL-10 and sIL-2R levels in patients with chronic hepatitis C pre- and post antiviral treatment. Chin Med J. 2003;116:605–8. [PubMed] [Google Scholar]
- 24.Tamura K, Fukuda H, Sashio H, et al. IL18 polymorphism is associated with an increased risk of Crohn’s disease. J Gastroenterol. 2002;37:111–6. doi: 10.1007/BF03326428. [DOI] [PubMed] [Google Scholar]
- 25.Zhang PA, Wu JM, Li Y, Yang XS. Association of polymorphisms of interleukin-18 gene promoter region with chronic hepatitis B in Chinese Han population. World J Gastroenterol. 2005;11:1594–8. doi: 10.3748/wjg.v11.i11.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146–52. doi: 10.1016/s0165-5728(00)00407-0. [DOI] [PubMed] [Google Scholar]
- 27.Hirankarn N, Manonom C, Tangkijvanich P, Poovorawan Y. Association of interleukin-18 gene polymorphism (−607A/A genotype) with susceptibility to chronic hepatitis B virus infection. Tissue Antigens. 2007;70:160–3. doi: 10.1111/j.1399-0039.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 28.Bouzgarrou N, Hassen E, Schvoerer E, et al. Association of IL-18 polymorphisms and plasma level with the outcome of chronic HCV infection. J Med Virol. 2008;80:607–14. doi: 10.1002/jmv.21079. [DOI] [PubMed] [Google Scholar]
- 29.Schvoerer E, Navas MC, Thumann C, Fuchs A, Meyer N, Habersetzer F, Stoll-Keller F. Production of interleukin-18 and interleukin-12 in patients suffering from chronic hepatitis C virus infection before antiviral therapy. J Med Virol. 2003;70:588–93. doi: 10.1002/jmv.10434. [DOI] [PubMed] [Google Scholar]
- 30.Micallef MJ, Yoshida K, Kawai S, et al. In vivo antitumor effects of murine interferon-γ-inducing factor/interleukin-18 in mice bearing syngeneic Meth A sarcoma malignant ascites. Cancer Immunol Immunother. 1997;43:361–7. doi: 10.1007/s002620050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jianhang L, Lihuang Z, Hangping Y, Xuetao C. Antitumor effects of interleukin-18 gene-modified hepatocyte cell line on implanted liver carcinoma. Chin Med J. 2003;116:1475–9. [PubMed] [Google Scholar]
- 32.Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of interleukin 18. Am J Gastroenterol. 2002;97:774–5. doi: 10.1111/j.1572-0241.2002.05580.x. [DOI] [PubMed] [Google Scholar]
- 33.Mihm S, Schweyer S, Ramadori G. Expression of the chemokine IP-10 correlates with the accumulation of hepatic IFN-γ and IL-18 mRNA in chronic hepatitis C but not in hepatitis B. J Med Virol. 2003;70:562–70. doi: 10.1002/jmv.10431. [DOI] [PubMed] [Google Scholar]
- 34.Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-γ- inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]